Abstract
Purpose
At the beginning of the Coronavirus disease (COVID-19) epidemic, physicians paid close attention to children with chronic diseases to prevent transmission or a severe course of infection. We aimed to measure the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody levels in children with chronic gastrointestinal and liver diseases to analyze the risk factors for infection and its interaction with their primary disease.
Methods
This cross-sectional study analyzed SARS-CoV-2 antibody levels in patients with gastrointestinal and liver diseases (n=141) and in healthy children (n=48) between January and February 2021.
Results
During the pandemic, 10 patients (7%) and 1 child (2%) had confirmed COVID-19 infection (p=0.2). The SARS-CoV-2 antibody test was positive in 36 patients (25.5%) and 11 children (22.9%) (p=0.7). SARS-CoV-2 antibody positivity was found in 20.4%, 26.6%, 33.3%, and 33.3% of patients with chronic liver diseases, chronic gastrointestinal tract diseases, cystic fibrosis, and liver transplantation recipients, respectively (p>0.05, patients vs. healthy children). Risk factors for SARS-CoV-2 antibody positivity were COVID-19-related symptoms (47.2% vs. 14.2%, p=0.00004) and close contact with SARS-CoV-2 polymerase chain reaction-positive patients (69.4% vs. 9%, p<0.00001). The use, number, and type of immunosuppressants and primary diagnosis were not associated with SARS-CoV-2 antibody positivity. The frequency of disease activation/flare was not significant in patients with (8.3%) or without (14.2%) antibody positivity (p=0.35).
Conclusion
SARS-CoV-2 antibodies in children with chronic gastrointestinal and liver diseases are similar to that in healthy children. Close follow-up is important to understand the long-term effects of past COVID-19 infection in these children.
Keywords: SARS-CoV-2, Antibodies, Chronic diseases, Liver diseases
INTRODUCTION
In December 2019, a novel outbreak of a new strain of coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) infection emerged in Wuhan, China and was declared a pandemic in early March 2020 [1]. Children accounted for approximately 2% of all confirmed cases. Although SARS-CoV-2 infection is generally milder in children than in adults, approximately 1% of children develop severe disease requiring admission to intensive care units. Common symptoms in children include cough, fever, sore throat, rhinorrhea, and to a lesser extent headache, myalgia, rash, and pink eyes [2].
Gastrointestinal symptoms, such as diarrhea, nausea, vomiting, abdominal pain, and feeding difficulties, have been reported as the initial symptoms of SARS-CoV-2 infection in approximately 20% of pediatric cases [3]. Meanwhile, ileus, mesenteric lymphadenopathy, terminal ileitis, necrotizing enterocolitis-like disease, and atypical appendicitis have been reported in a minority of patients [4]. Gastrointestinal symptoms in SARS-CoV-2 infection are associated with more severe disease and intensive care unit admissions [5]; however, they may mimic disease activation or flare in patients with chronic gastrointestinal disorders such as inflammatory bowel diseases [6]. Liver involvement in SARS-CoV-2 infection is less common in children, and mild elevation of liver enzymes, especially in children under the age of 3 years, has been reported in 13% to 20% of cases. However, children with chronic liver diseases (CLD) do not seem to have a greater risk of contracting SARS-CoV-2 or an increased risk of severe disease [6].
In this study, we aimed to analyze (i) the frequency of SARS-CoV-2 antibody positivity, (ii) risk factors for SARS-CoV-2 infection, and (iii) the relationship between past SARS-CoV-2 infection and disease flares in children with chronic diseases in the outpatient clinic of the pediatric gastroenterology unit.
MATERIALS AND METHODS
The study was conducted in the outpatient clinic of the pediatric gastroenterology unit between January 15 and February 15, 2021. All patients (aged <18 years) with chronic diseases (definite diagnosis) who were seen at the outpatient clinic during the study period were included in the study (n=141). A control group without any known chronic disease was randomly selected (4 boys and 4 girls, each aged 3 years) from a healthy child outpatient clinic for the study (n=48). Vaccination for Coronavirus disease (COVID-19) in children did not begin during the study period in our country. Verbal and written informed consent were obtained from all patients, healthy children, and their parents for the study.
Data on demographic characteristics, chronic disease-related data (primary diagnosis, time of diagnosis, and medications), and COVID-19-related parameters (with proven infection) were collected from hospital files of the patients with chronic gastrointestinal disease. A questionnaire containing items about COVID-19 was completed by the patients/healthy children/parents. The items included: (i) “Do you have COVID-19 related symptoms?”; (ii) “Did you perform a COVID-19 diagnostic test?” (nasopharyngeal swab for SARS-CoV-2 nucleic acid using reverse-transcriptase polymerase chain reaction [RT-PCR]); (iii) “Do you have any positive PCR test results for COVID-19?”; and (iv) “Have you had close contact with a SARS-CoV-2 PCR-positive patient?” Patients with COVID-19-related symptoms and/or close contact with a SARS-CoV-2 PCR-positive patient and/or any positive PCR test for COVID-19 were considered to have a high likelihood of SARS-CoV-2 antibody formation (HL+ subjects). Asymptomatic infection was defined as a positive SARS-CoV-2 antibody result without any COVID-19 related symptoms. Flare or reactivation of diseases was defined as recurrence of disease-related symptoms or abnormal laboratory parameters, such as diarrhea (±blood) for inflammatory bowel disease and elevation of liver enzymes for CLD/liver transplantation recipients, respectively.
For SARS-CoV-2 spike antibody measurement, 3–4 cm3 of peripheral venous blood was collected from the participants. Roche Diagnostic® kits (Roche Diagnostics International AG, Rotkreuz, Switzerland) were used, and analysis was performed using electrochemiluminescence immunoassay principles. Assays with an anti-SARS-CoV-2 spike antibody level of <0.8 U/mL and ≥0.80 U/mL were considered negative and positive, respectively [7].
The study was conducted according to the Declaration of Helsinki, and ethical approval was obtained from the Ethics Committee of the KTU Institutional Ethics Committee (study number: 2021/45). This study was supported by a research grant from Karadeniz Technical University Pediatric Association.
Statistical analyses were performed using SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean±standard deviation, and categorical variables are expressed as number (n) and percentage (%). Comparison of continuous variables between the groups was performed using Student’s t-test for normally distributed variables and the Mann–Whitney U-test for non-normally distributed variables. Categorical variables were compared using the chi-square test (or Fisher’s exact test when needed). The Shapiro–Wilk test was used to assess normal distribution. Statistical significance was set at p<0.05.
RESULTS
Demographic and clinical features
The study included 141 patients and 48 healthy children. No significant difference was found between the patients and healthy children in terms of age (11.2±4.6 [range, 0.5–17.5] years vs. 10.5±5.1 [range, 0.25–17] years, p>0.05) and sex (males 57.4% vs. females 50%, p>0.05).
CLD, mainly autoimmune hepatitis, metabolic liver disease, and Wilson’s disease, was noted in 49 patients (34.7%). Six patients had decompensated cirrhosis, and three patients were awaiting liver transplantation. Meanwhile, 68 patients (48.2%) had chronic gastrointestinal tract disease; 42 had celiac disease and were on a gluten-free diet and 17 had inflammatory bowel disease (7 Crohn’s disease, 10 ulcerative colitis). Nine (6.3%) patients had cystic fibrosis, and 15 (10.6%) patients underwent liver transplantation. The patients were followed up for 4.6±3.1 (range, 0.5–15) years. Overall, 49 patients received immunosuppressive treatment (30 monodrugs and 19 polydrugs), including antimetabolites in 30 patients (azathioprine in 26 and mycophenolate mofetil in 4), selective cytokine production inhibitors in 14 patients (tacrolimus in 10, everolimus in 2, cyclosporine in 1, and rapamycin in 1), biological agents in 5 patients (infliximab), and corticosteroids in 5 patients. Eighteen patients (12.7%) experienced disease activation since the pandemic began (Table 1).
Table 1. Chronic disease-related parameters of patients.
| Parameter | Value (n=141) | |
|---|---|---|
| Chronic liver disease | 49 (34.7) | |
| Autoimmune hepatitis | 19 | |
| Wilson’s disease | 6 | |
| Chronic viral hepatitis B | 2 | |
| Vascular liver disease | 1 | |
| Metabolic liver disease | 10 | |
| Cholestatic liver disease | 5 | |
| Fibrocystic liver disease | 5 | |
| Recurrent acute liver failure | 1 | |
| Chronic gastrointestinal tract diseases | 68 (48.2) | |
| Celiac disease | 42 | |
| Inflammatory bowel disease | 17 | |
| Short-bowel syndrome | 3 | |
| Intestinal polyposis | 2 | |
| Intestinal lymphangiectasia | 1 | |
| Eosinophilic esophagitis | 3 | |
| Cystic fibrosis | 9 (6.3) | |
| Post-liver transplantation | 15 (10.6) | |
| Duration of follow-up (yr) | 4.6±3.1 (0.5–15) | |
| Immunosuppressive treatment | 49 (34.7) | |
| Primary disease activation/flare during the pandemic | 18 (12.7) | |
Values are presented as number (%), number only, or mean±standard deviation (range).
SARS-CoV-2 infection-related findings
During the pandemic, 32 (22.6%) of the 141 patients developed COVID-19-like symptoms. Additionally, 45 patients (31.9%) were in close contact with SARS-CoV-2 PCR-positive patients. Overall, 43 patients (30.4%) underwent nasopharyngeal PCR test (for COVID-19-like symptoms, with close contact, or screening before interventional surgery). Ten patients (7%) had positive PCR results: three had inflammatory bowel disease, three were liver transplant recipients, two had celiac disease, one had Wilson’s disease, and one had short bowel syndrome. All patients were hospitalized at our center and discharged without any complications during the follow-up period (Table 2). In the control group, three (6.3%) of the 48 children had COVID-19 like symptoms, eight children (16.6%) had close contact, six children (12.5%) underwent nasopharyngeal PCR, and one (2.0%) had positive PCR results.
Table 2. Characteristics of patients with proven COVID-19.
| Parameter | Value (n=10) |
|---|---|
| Age (yr) | 8.5 (4.5–11) |
| Sex (male/female) | 4/6 |
| Primary diagnosis | Inflammatory bowel disease: 3, Post-liver transplantation: 3, Celiac disease: 2, Wilson’s disease: 1, Short bowel syndrome: 1 |
| Time from diagnosis to infection (mo) | 28 (6–80) |
| Close contact with a SARS-CoV-2 PCR-positive patient | 9 |
| Immunosuppressive treatment | 6 |
| Fever/cough | 8/3 |
| Lymphopenia (<1,500/mm3) | 4 |
| Thrombocytopenia (<150,000/mm3) | 1 |
| High liver enzymes | 2 |
| Hyperferritinemia (>1,000 µg/L) | 2 |
| Pneumonia | 1 |
| Disease severity | Mild: 9, Moderate: 1 |
| Intensive care unit admission | 1 |
| Treatment for COVID-19 | Azitromycine: 2, Azitromycine/Favipiravir: 1, Teicoplanin/Meropenem/Favipiravir: 1 |
| Outcome | All alive |
| Presence of SARS-CoV-2 IgG | 10 |
| Value (U/mL) | 178.7 (4.7–576) |
| Time from infection to analysis (mo) | 4 (2–11) |
Values are presented as median (range) or number only.
COVID-19: Coronavirus disease, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, PCR: polymerase chain reaction, IgG: immunoglobulin G.
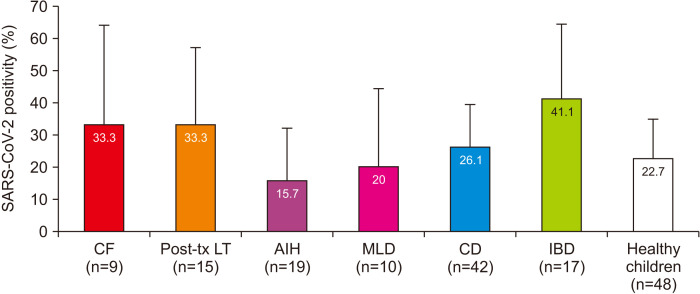
The SARS-CoV-2 antibody was positive in 36 patients (25.5%) and in 11 children (22.9%) in the control group (p=0.7). COVID-19-related symptoms, close contact with SARS-CoV-2 PCR-positive patients, and patients who underwent COVID-19 diagnostic tests were more common in patients with chronic diseases than in healthy children (p<0.05), but no significant difference was found between SARS-CoV-2 PCR positivity, SARS-CoV-2 antibody positivity, and asymptomatic infection (Table 3). SARS-CoV-2 antibody positivity was found in 20.4%, 26.6%, 33.3%, and 33.3% of patients with CLD, chronic gastrointestinal tract diseases, cystic fibrosis, and liver transplant recipients, respectively. No significant differences were found when the patient subgroups were compared with the healthy children (Fig. 1).
Table 3. COVID-19 related parameters.
| Parameter | Patients with chronic disease (n=141) | Healthy children (n=48) | p-value |
|---|---|---|---|
| COVID-19-related symptoms | 32 (22.6) | 3 (6.2) | 0.011 |
| Close contact with a SARS-CoV-2 PCR- positive patient | 45 (31.9) | 8 (16.6) | 0.04 |
| Patients in whom COVID-19 diagnostic test was performed | 43 (30.4) | 6 (12.5) | 0.01 |
| SARS-CoV-2 PCR positivity | 10 (7.0) | 1 (2.0) | 0.2 |
| HL+ subjects | 53 (37.5) | 11 (22.9) | 0.06 |
| Asymptomatic infection | 19 (13.4) | 8 (16.6) | 0.58 |
| SARS-CoV-2 antibody positivity | 36 (25.5) | 11 (22.9) | 0.7 |
Values are presented as number (%).
COVID-19: Coronavirus disease, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, PCR: polymerase chain reaction, HL: high likelihood.
Fig. 1. SARS-CoV-2 antibody positivity among the subgroups according to primary diagnosis. No significant difference was found between the patients and healthy controls. Bar indicates 95% confidence interval.
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, CF: cystic fibrosis, MLD: metabolic liver diseases, CD: celiac disease, AIH: autoimmune hepatitis, IBD: inflammatory bowel disease, tx: transplantation, LT: liver transplantation.
Risk factors for SARS-CoV-2 antibody positivity in patients with chronic gastrointestinal diseases
Risk factors for SARS-CoV-2 antibody positivity were analyzed in patients with chronic diseases. COVID-19 related symptoms (47.2% vs. 14.2%, p=0.00004, odds ratio [OR]: 5.3, 95% confidence interval [CI]: 2.2–12.5), close contact with a SARS-CoV-2 PCR-positive patient (69.4% vs. 19.0%, p<0.00001, OR: 9.6, 95% CI: 4–22.8), and HL+ subjects (72.2% vs. 25.7%, p<0.00001, OR: 7.51, 95% CI: 3.2–17.5) were associated with SARS-CoV-2 antibody positivity. The use, number, or type of immunosuppressants, and primary diagnosis were not associated with SARS-CoV-2 antibody positivity (Table 4).
Table 4. Risk factors for SARS-CoV-2 antibody positivity.
| Parameter | SARS-CoV-2 antibody positive (n=36) | SARS-CoV-2 antibody negative (n=105) | p-value | |
|---|---|---|---|---|
| Age (yr) | 11.6±4.5 | 11.1±4.5 | 0.97 | |
| Sex, female | 21 (58.3) | 60 (57.1) | 0.9 | |
| Duration of disease (yr) | 4.6±3.0 | 4.6±3.2 | 0.89 | |
| COVID-19 related symptoms | 17 (47.2) | 15 (14.2) | 0.00004 | |
| Close contact with a SARS-CoV-2 PCR-positive patient | 25 (69.4) | 20 (19.0) | <0.00001 | |
| HL+ subjects | 26 (72.2) | 27 (25.7) | <0.00001 | |
| Immunosuppressive usage | 14 (38.8) | 35 (33.3) | 0.54 | |
| Number of immunosuppressives | ||||
| Polydrug | 4 (11.1) | 13 (12.3) | 0.83 | |
| Type of immunosuppression | 0.27 | |||
| Antimetabolites | 6 (16.6) | 24 (22.8) | ||
| Selective cytokine inhibitors | 4 (11.1) | 10 (9.5) | ||
| Biological agents | 2 (5.5) | 3 (2.8) | ||
| Corticosteroids | 3 (8.3) | 2 (1.9) | ||
| Primary diagnosis | 0.68 | |||
| Hepatic disorders | 10 (27.7) | 39 (37.1) | ||
| Gastrointestinal tractus diseases | 18 (50.0) | 50 (47.6) | ||
| Cystic fibrosis | 3 (8.3) | 6 (5.7) | ||
| Post-liver transplantation | 5 (13.8) | 10 (9.5) | ||
| Primary disease activation/flare | 3 (8.3) | 15 (14.2) | 0.35 | |
Values are presented as mean±standard deviation or number (%).
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, HL: high likelihood.
During the pandemic, 18 patients had disease activation/flare, 13 developed inflammatory bowel disease (5 Crohn’s disease, 8 ulcerative colitis), 2 developed autoimmune hepatitis, 2 underwent liver transplantation, and 1 developed cholestatic liver disease. The frequency of disease activation/flare was not significant in patients with (n=3, 8.3%) or without (n=15, 14.2%) antibody positivity (p=0.35). All three patients in the antibody-positive group had inflammatory bowel disease, and PCR analysis for SARS-CoV-2 was negative during the flare period.
DISCUSSION
In this study, we analyzed the frequency of SARS-CoV-2 antibody positivity in children with chronic gastrointestinal and liver diseases and found that (i) the frequency and clinical outcomes of SARS-CoV-2 infection in children with chronic gastrointestinal/liver diseases were similar to those in healthy children, (ii) HL+ patients had higher frequency of SARS-CoV-2 antibody positivity, and (iii) no relationship was found between disease flare/activation and past infection.
Literature on the clinical features and outcomes of SARS-CoV-2 infection in children with chronic gastrointestinal and liver diseases has mainly focused on three diseases: CLD, liver transplantation recipients, and inflammatory bowel disease [8,9,10,11].
In adult studies, CLD was associated with a worse outcome, especially when associated with decompensated cirrhosis [12]. In contrast, the results of studies on the clinical progression of SARS-CoV-2 infection in children with CLD are inconsistent. According to the European Society for Paediatric Gastroenterology, Hepatology and Nutrition committee, children with CLD are at a higher risk of developing severe COVID-19 and may experience decompensation of end-stage liver disease during SARS-CoV-2 infection. Children with CLD associated with suspected or documented non-alcoholic fatty liver disease or autoimmune hepatitis may have a higher risk of developing severe COVID-19 [13]. Kehar et al. [14] compared children with CLD with liver transplant recipients and found that children with CLD required more intensive care unit management and had higher mortality than liver transplant recipients. In a multicenter study based on a phone survey questionnaire exploring the characteristics of COVID-19 in children with CLD, the observed incidence of COVID-19 infection was higher in children with CLD, but symptoms were mild with favorable outcomes, suggesting that preexisting CLD does not represent an additional risk factor for severe disease [15].
As mentioned above, COVID-19 in children undergoing liver transplantation seems to be mild compared to that in patients with CLD. Additionally, Tannuri et al. [16] reported a low incidence of COVID-19 in children and adolescents undergoing liver transplantation. Patients undergoing liver transplantation have some risk factors for severe COVID-19, such as immunosuppression with multiple drugs, underlying unknown immunological disturbances, and chemotherapy in some patients with hepatoblastoma. In contrast to the study by Kehar et al. [14], Buescher et al. [17] reported that patients with liver transplantation require hospitalization more frequently and present with more complications compared with patients with CLD. Sin et al. [18] reported a high rate of acute liver injury during a COVID-19 infection and worse outcomes in patients infected in the early period after liver transplantation. In contrast, Yuksel et al. [10] reported that being immunocompromised did not affect disease severity or survival. Despite immunosuppressive treatment, all patients developed antibodies against COVID-19 in their study.
The incidence rate of COVID-19 in children with inflammatory bowel disease appears to be lower than that in the general population, and they do not have an increased risk of severe disease. Chronic corticosteroid treatment alone may represent a risk factor for infection and severe disease, and treatment of inflammatory bowel disease should not be interrupted because of the flare/exacerbation risk [11]. Additionally, patients with celiac disease did not have an increased risk of COVID-19 or a severe clinical course; only delayed diagnosis and problems adhering to a gluten-free diet were reported during the lockdowns. However, an increased incidence of celiac disease and celiac disease associated with type 1 diabetes mellitus was reported during the pandemic [19].
Based on the symptom questionnaire and antibody analysis, we did not find any difference between children with chronic gastrointestinal/liver disease and healthy children in terms of disease severity and frequency in our study. Most of our patients had high-risk factors for both exposure and severe COVID-19 infection: (i) use of immunosuppressants and (ii) underlying autoimmune disturbances such as inflammatory bowel disease and autoimmune hepatitis. However, some immunosuppressants have negative effects, whereas others have positive effects on the course of COVID-19 infection. Immunosuppression with mycophenolate mofetil is an independent predictor of severe COVID-19 infection at higher doses (>1,000 mg/day) [20]. On the other hand, calcineurin inhibitors inhibit virus replication through their inhibitory effect on interleukin-6 production/secretion [21]. In our study, we could not find any effect of the type and number of immunosuppressants used on the clinical course and antibody formation. It is recommended that immunosuppressive drugs, except mycophenolate, be continued during COVID-19 infection.
The main source of SARS-CoV-2 infection in our patient group was close contact with a SARS-CoV-2 PCR-positive patient. Similar findings were reported in previous studies; Yuksel et al. [10] reported that among patients liver transplant recipients, the main source of infection was parents/siblings, and Sansotta et al. [9] reported that among patients with inflammatory bowel disease, the source of infection was close contact with a positive patient. It is important to inform patients with chronic disease in close contact with a positive patient about the virus transmission risk.
Exacerbation/flare of chronic diseases during the COVID-19 pandemic was first reported at the beginning of the pandemic owing to delayed or interrupted treatment in patients with inflammatory bowel disease. The treatments were mainly modified because of the increased risk of severe infection [9,11]. According to current scientific data, patients receiving biological agents do not have an increased risk of infection or severe disease course. Furthermore, biological agents have been used to treat severe COVID-19 and COVID-19-related multisystem inflammatory syndrome in children. On the other hand, COVID-19 may induce or trigger autoimmunity in the long term; therefore, exacerbation of chronic autoimmune diseases may be observed [22]. In our study, we did not find any relationship between disease activation/flares and past COVID-19 infection. Similar findings were reported by Sansotta et al. [9] in children with inflammatory bowel disease. It is recommended that these patients be closely followed-up.
The limitations of our study are (i) the dynamic process of the pandemic, such as differences in the virulence of novel variants, may prevent us to make a general comment about the progression and outcome of COVID-19 infection in children with chronic gastrointestinal/liver disease, (ii) lack of measurement of neutralizing antibodies, (iii) lack of longer follow-up of the patients to monitor levels of antibodies (decreased or maintained) and long-term effect of past COVID-19 infection, and (iv) heterogeneity in the primary diagnosis of the patients, which would require a larger sample size for a more suitable subgroup analysis.
In conclusion, we reported our experience regarding the presence and outcome of SARS-CoV-2 antibodies in children with chronic gastrointestinal and liver diseases. Our study was performed during the delta wave. The epidemic has a dynamic process, and novel variants associated with milder but highly contagious disease (Omicron) have recently been defined. However, the amount and quality of neutralizing antibodies are more important in commenting on the protective role of these antibodies against further infection. Close follow-up of patients with chronic diseases is important to understand the long-term effects of past COVID-19 infection in these children. Our results may provide vaccination strategies for children with chronic diseases in countries where vaccination programs have not yet been initiated.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akobeng AK, Grafton-Clarke C, Abdelgadir I, Twum-Barimah E, Gordon M. Gastrointestinal manifestations of COVID-19 in children: a systematic review and meta-analysis. Frontline Gastroenterol. 2020;12:332–337. doi: 10.1136/flgastro-2020-101529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tullie L, Ford K, Bisharat M, Watson T, Thakkar H, Mullassery D, et al. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Health. 2020;4:e19–e20. doi: 10.1016/S2352-4642(20)30165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assa A, Benninga MA, Borrelli O, Broekaert I, de Carpi JM, Saccomani MD, et al. Gastrointestinal Committee of ESPGHAN. Gastrointestinal perspective of coronavirus disease 2019 in children-an updated review. J Pediatr Gastroenterol Nutr. 2021;73:299–305. doi: 10.1097/MPG.0000000000003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche Diagnostics GmbH. Elecsys® Anti-SARS-CoV-2 S assay [Internet] Burgess Hill: Roche Diagnostics GmbH; 2022. [cited 2022 May 4]. Available from: https://diagnostics.Roche.com/gb/en/products/params/elecsys-anti-sars-cov-2-s.html. [Google Scholar]
- 8.Di Giorgio A, Hartleif S, Warner S, Kelly D. COVID-19 in children with liver disease. Front Pediatr. 2021;9:616381. doi: 10.3389/fped.2021.616381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sansotta N, Norsa L, Zuin G, Panceri R, Dilillo D, Pozzi E, et al. Children with inflammatory bowel disease in the COVID-19 main endemic focus: the Lombardy experience. Front Pediatr. 2021;9:607285. doi: 10.3389/fped.2021.607285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuksel M, Akturk H, Mizikoglu O, Toroslu E, Arikan C. A single-center report of COVID-19 disease course and management in liver transplanted pediatric patients. Pediatr Transplant. 2021;25:e14061. doi: 10.1111/petr.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrias A, Cortes GM, Bardanzellu F, Melis A, Fanos V, Marcialis MA. Risk, course, and effect of SARS-CoV-2 infection in children and adults with chronic inflammatory bowel diseases. Children (Basel) 2021;8:753. doi: 10.3390/children8090753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marjot T, Webb GJ, Barritt AS, 4th, Moon AM, Stamataki Z, Wong VW, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicastro E, Ebel NH, Kehar M, Czubkowski P, Ng VL, Michaels MG, et al. The impact of severe acute respiratory syndrome coronavirus type 2 on children with liver diseases: a joint European Society for Pediatric Gastroenterology, Hepatology and Nutrition and Society of Pediatric Liver Transplantation position paper. J Pediatr Gastroenterol Nutr. 2022;74:159–170. doi: 10.1097/MPG.0000000000003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehar M, Ebel NH, Ng VL, Baquero JER, Leung DH, Slowik V, et al. Severe Acute respiratory syndrome coronavirus-2 infection in children with liver transplant and native liver disease: an international observational registry study. J Pediatr Gastroenterol Nutr. 2021;72:807–814. doi: 10.1097/MPG.0000000000003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Giorgio A, Nicastro E, Arnaboldi S, Montini O, Di Stasio F, D’Antiga L, et al. “Health status of children with chronic liver disease during the SARS-CoV-2 outbreak: results from a multicentre study”. Clin Res Hepatol Gastroenterol. 2021;45:101610. doi: 10.1016/j.clinre.2020.101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tannuri U, Tannuri ACA, Cordon MNA, Miyatani HT. Low incidence of COVID-19 in children and adolescent post-liver transplant at a Latin American reference center. Clinics (Sao Paulo) 2020;75:e1986. doi: 10.6061/clinics/2020/e1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buescher G, Sebode M, Marjot T, Webb GJ, Moon AM, Barnes E, et al. SARS-CoV-2 in pediatric liver transplant recipients: the European experience. J Pediatr Gastroenterol Nutr. 2022;74:e41–e42. doi: 10.1097/MPG.0000000000003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sin P, Díaz LA, Martínez M, Vizcaya C, D’Agostino D, Gana JC. Acute liver injury among pediatric liver transplantation recipients with coronavirus disease 2019: an international collaborative study. J Pediatr Gastroenterol Nutr. 2021;73:391–394. doi: 10.1097/MPG.0000000000003213. Erratum in: J Pediatr Gastroenterol Nutr 2022;74:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cakir M, Guven B, Issi F, Ozkaya E. New-onset celiac disease in children during COVID-19 pandemic. Acta Paediatr. 2022;111:383–388. doi: 10.1111/apa.16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romanelli A, Mascolo S. Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020;20:1947–1948. doi: 10.1111/ajt.15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner D, Huang Y, Martín-de-Carpi J, Aloi M, Focht G, Kang B, et al. Paediatric IBD Porto group of ESPGHAN. Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2020;70:727–733. doi: 10.1097/MPG.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]