Abstract
Background aim
The Omicron COVID-19 variants BA.1* and BA.2* evade immune system leading to increased transmissibility and breakthrough infections. We aim to test the hypothesis that immunity achieved post COVID-19 infection combined with vaccination (hybrid immunity), is more effective against Omicron infection than vaccination alone in a health-care setting.
Methods
Data on regular pre-emptive PCR testing from all Health-Care Workers (HCWs) at Laiko University Hospital from 29th December 2020, date on which the national COVID-19 immunization program began in Greece, until 24th May 2022, were retrospectively collected and recorded. The infection rate was calculated after December 21st, 2021, when Omicron was the predominant circulating variant in Greece, as the total number of infections (positive PCR COVID-19 test regardless of symptoms) divided by the total person-months at risk.
Results
Of 1,305 vaccinated HCWs who were included in the analysis [median age of 47 (IQR: 36, 56) years, 66.7 % women], 13 % and 87 % had received 2 or 3 vaccine doses (full and booster vaccination), respectively. A COVID-19 infection had occurred in 135 of 1,305 of participants prior to Omicron predominance. Of those 135 HCWs with hybrid immunity only 13 (9.6 %) were re-infected. Of the 154 and 1,016 HCWs with full and booster vaccination-induced immunity, respectively, 71 (46.1 %, infection rate 13.4/100 person-months) and 448 (44.1 %, infection rate 12.2/100 person-months) were infected during the follow up period. No association between gender or age and COVID-19 infection was found and none of the participants had a severe infection or died.
Conclusions
Hybrid immunity confers higher protection by almost 5-fold compared to full or booster vaccination for COVID-19 infection with the Omicron variant among HCWs who are at high risk of exposure. This may inform public health policies on how to achieve optimal immunity in terms of the timing and mode of vaccination.
Keywords: COVID-19, Vaccine, Infection rate, Hybrid immunity, Health-Care Workers (HCWs)
Abbreviations: ACE2, Angiotensin-converting enzyme 2; CDC, Centers for Disease Control and Prevention; CI, Confidence Interval; COVID-19, Coronavirus Disease 2019; HCWs, Health-Care Workers; IDSA, Infectious Diseases Society of America; IQR, interquartile range; PCR, Polymerase Chain Reaction; RBD, Receptor-Binding Domain; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; VOCs, Variants of Concern
1. Introduction
During the Coronavirus Disease 2019 (COVID-19) pandemic several factors had an impact on the number of newly diagnosed patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. The overall COVID-19 pattern so far includes waves causing an increase in COVID-19 cases followed by a decline, often associated with specific mutated variants of concern (VOCs). Factors that showed to have had an impact on the infection rate during each “wave” include the appropriate use of personal protective equipments, implementation of public health measures (i.e., lock-down, school and business closure, bans on mass gatherings, social-distancing), immunization programs, the waning effect of vaccination over time, diverse individual behaviors, infectiousness, and immune escape capacity of each dominant circulating COVID-19 variant [1], [2], [3]. As new COVID-19 variants of concern appear and since immunity wanes over time, there is a need for continuous monitoring of vaccine effectiveness. A previous COVID-19 infection with the initial SARS-CoV-2 strains had a time-dependent protective effect against re-infection with the B.1.1.7 (alpha), B.1.351 (beta), and B.1.617.2 (delta) variants [4], [5], [6].
Since winter 2021–2022 the predominant circulating Omicron COVID-19 variants BA.1* and BA.2* behave differently to previous variants, evading the immune system due to multiple mutations in the receptor-binding domain (RBD) of the spike protein that have been associated with increased transmissibility and immune evasion both after natural infection and vaccination, leading to breakthrough infections at a higher rate [7]. Concerns have recently been raised about the effectiveness of the currently available COVID-19 vaccines, due to the rapid increase in COVID-19 cases related with the spread of the Omicron COVID-19 variants even among vaccinated populations with booster dose(s) [8]. Findings of studies based on neutralizing activity of antibodies showed a reduced activity against the Omicron variants compared to the initial SARS-CoV-2 strains, or subsequent VOCs other than the Omicron. Although a booster COVID-19 vaccine dose increased protection against infection, the protective effect also waned over time [9], [10].
Re-infections seemed to be uncommon among previously infected persons including health-care Workers (HCWs) over a period before the emergence of Omicron variants [11]. A recent national case-control study found no discernable differences in protection against symptomatic Omicron infection with previous infection, vaccination and hybrid immunity, i.e., immunity achieved post natural COVID-19 infection combined with completion of appropriate immunization [12]. Herein, we aim to test the hypothesis that hybrid immunity is more effective against Omicron infection than vaccination alone in a health-care setting.
2. Material and methods
2.1. Study design
Data from all 1,780 HCWs working at Laiko University Hospital between 29th December 2020 and 24th May 2022 were retrospectively collected and recorded. On the 29th of December 2020 the national COVID-19 immunization program began in Greece. As of this date the mRNA COVID-19 vaccines have become available for vaccinating HCWs in priority, albeit on a voluntary basis. Since vaccine hesitancy is a well-established phenomenon, even among HCWs, as of the 1st of September 2021 vaccination has become mandatory by State law for all HCWs in our country, and therefore our study population consists of fully or boosted vaccinated personnel only [2]. Data with regards to age, sex, occupation, number of COVID-19 vaccine doses, date of each COVID-19 vaccine dose, and history of COVID-19 infection were collected.
2.2. Inclusion and exclusion criteria
HCWs working at Laiko University Hospital were assessed for eligibility to be included in the study. Participants were excluded if they: i) were temporarily moved to other services/locations during the study period or had been on a long-term leave due to medical conditions (n = 17), ii) had been vaccinated for COVID-19 abroad and dates of vaccination were not available (n = 7), iii) had not been vaccinated with any of the available COVID-19 vaccines (n = 270), iv) had received only one dose (partial vaccination) of any of the available COVID-19 vaccines (n = 95), v) had been COVID-19 infected before the initiation of the national COVID-19 immunization program in Greece i.e., before the 29th December 2020 (n = 50), vi) had been COVID-19 infected before having the first dose of any of the available COVID-19 vaccines (n = 23), vii) had been COVID-19 infected before having the second dose of a mRNA COVID-19 vaccine (Pfizer-BioNTech or Moderna) plus 14 days (n = 12), and viii) had been COVID-19 infected before having the first dose of a viral vector COVID-19 vaccine (Johnson and Johnson/Janssen) plus 14 days (n = 1).
2.3. Case definitions
A periodic pre-emptive infection control testing policy has been applied by the Infection Control Committee of Laiko University Hospital involving a COVID-19 PCR rhinopharyngeal test every 2 weeks for all HCWs. HCWs with a positive PCR test result were considered as infected. In case a participant had more than one positive COVID-19 PCRs we followed the CDC recommendations for defining re-infection i.e., for persons with or without COVID-19-like symptoms the time window was set at ≥ 90 days.
Participants were divided in fully and booster vaccinated. HCWs were considered as fully vaccinated when they had received 2 doses of any of the available mRNA vaccines (Pfizer-BioNTech or Moderna) or 1 dose of the single dose vaccine (Johnson and Johnson/Janssen), whereas as booster vaccinated were considered those who had received 3 doses of any mRNA vaccine or the single dose vaccine plus a booster dose of Johnson and Johnson/Janssen. Fully and booster vaccinated participants were further divided in two subgroups, namely, those who had and those who had not been infected with COVID-19 before the follow up. The starting date of the follow up period was after the 21st of December 2021 since according to data from the National Public Health Organization of Greece from that date onwards, Omicron variants (BA.1* and BA.2*) predominated in our country. Regarding the proportion of different omicron subvariants, based on the national genomic surveillance results, BA.1*, BA.2* and BA.5* were the dominant variants during the periods: end of December 2021-end of February 2022, beginning of March 2022-end of May 2022 and begging of June 2022 until currently (end of August 2022), respectively (https://eody.gov.gr). For this reason, the starting date of the follow up was set to the 21st December 2021 for those who have completed their vaccination plus 14 days. Otherwise, it was set to the date of the 3rd or 2nd COVID-19 vaccine dose plus 14 days. For participants who had not been COVID-19 infected after the starting date of the follow up, the follow up ended on the 24th May 2022, that was the last date of data collection, while for those who had been COVID-19 infected, it was ended on the date of the positive COVID-19 PCR test.
2.4. Data collection
Data with regards to age, sex, occupation, COVID-19 infection, possible hospital admission and outcome were collected. As severe COVID-19 was considered the infection if the patients had SpO2 ≤ 94 % on room air, including patients on supplemental oxygen, in accordance with the Infectious Diseases Society of America (IDSA) guidelines [13]. Dates of each administered COVID-19 vaccine dose, as well as dates of positive PCR COVID-19 test, defined as date of COVID-19 infection, were additionally retrieved and recorded through the National COVID-19 Registry by authorized users.
2.5. Statistical analysis
We used median (interquartile range, IQR) and frequencies (percentages) to describe the continuous and categorical variables, respectively, of our dataset. Shapiro-Wilk test was used to examine continues variables for normality. Statistical analysis was carried out using the Pearson’s chi-squared test and Fisher’s exact test to compare the characteristics of COVID-19 infected and non-infected participants in the four subgroups of HCWs. Infection rate was calculated as the total number of infections divided by the total person-months at risk (per 100 person-months). Relative vaccine effectiveness is reported as a percentage and was defined as (1 – IRR) × 100, where IRR denotes the infection rate ratio for vaccinated HCWs with hybrid immunity versus vaccinated HCWs without hybrid immunity. Statistical significance was assumed at p < 0.05 and all the analyses were performed on STATA 16.0 program.
3. Results
3.1. Population characteristics
The demographics of 1305 vaccinated HCWs participating in the study, with or without hybrid immunity by a COVID-19 infection before the follow up period, are summarized in Table 1 . The median age of all participants was 47 (IQR: 36, 56) years. The median age of fully vaccinated HCWs without and with previous COVID-19 infection was 47 (IQR: 36, 57) and 49 (IQR: 33.5, 51) years, respectively, whereas the median age of booster vaccinated HCWs without and with previous COVID-19 infection was 47 (IQR: 37, 56) and 40 (IQR: 32, 52) years, respectively. The distribution of HCWs according to gender and occupation in the four subgroups is shown in Table 1. Of the 1,305 HCWs participating in the study 870 (66.7 %) were females.
Table 1.
Demographics of vaccinated Health-Care Workers with or without hybrid immunity (by a previous COVID-19 infection).
| Fully vaccinated without hybrid immunity | Fully vaccinated with hybrid immunity | Booster vaccinated without hybrid immunity | Booster vaccinated with hybrid immunity | |
|---|---|---|---|---|
| Number (N) | 154 | 16 | 1,016 | 119 |
| Median age (IQR) | 47 (36, 57) | 49 (33.5, 51) | 47 (37, 56) | 40 (32, 52) |
| Gender (%) | ||||
| Male | 55 (35.7) | 4 (25.0) | 345 (34.0) | 31 (26.0) |
| Female | 99 (64.3) | 12 (75.0) | 671 (66.0) | 88 (74.0) |
| Occupation (%) | ||||
| Physician | 38 (24.7) | 3 (18.8) | 407 (40.1) | 40 (33.6) |
| Nurse | 44 (28.6) | 7 (43.7) | 298 (29.3) | 43 (36.1) |
| Other | 72 (46.7) | 6 (37.5) | 311 (30.6) | 36 (30.3) |
3.2. Outcomes
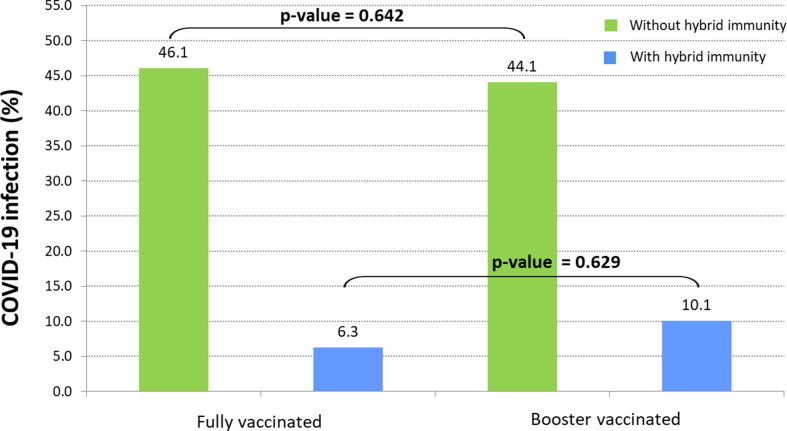
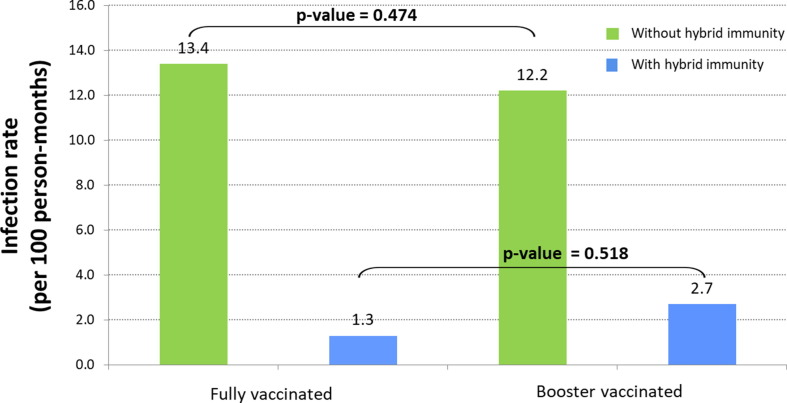
Of 1,305 vaccinated HCWs who were included in the analysis, 170 (13 %) and 1,135 (87 %) had received 2 or 3 vaccine doses (full and booster vaccination), respectively. In more detail, all the fully-vaccinated HCWs (N = 170, 100 %) had received 2 doses of any of the available mRNA vaccines (Pfizer-BioNTech or Moderna) and among the 1,135 booster-vaccinated HCWs, 1,107 (97.5 %) had received 3 doses of any mRNA vaccine and 28 (2.5 %) the single dose vaccine (Johnson and Johnson/Janssen) plus a booster dose of Johnson and Johnson/Janssen. A COVID-19 infection had occurred in 135 of 1,305 (10 %) participants before the initiation of the follow up period (during which Omicron was the predominant circulating variant in Greece), thus developing hybrid immunity. Of those 135 HCWs with hybrid immunity 13 (9.6 %) were re-infected. Of the 154 and 1,016 HCWs with full and booster vaccination-induced immunity, respectively, 71 (46.1 %, infection rate 13.4/100 person-months) and 448 (44.1 %, infection rate 12.2/100 person-months) were infected during the follow up period. As shown in Fig. 1 , further comparison of fully vs. booster vaccinated COVID-19 infected HCWs without and with hybrid immunity against COVID-19 infection showed no significant difference (46.1 % vs. 44.1 %, p-value = 0.642 and 6.3 % vs. 10.1 %, p-value = 0.629, respectively). Infection rate per 100 person-months for fully and booster vaccinated without or with hybrid immunity is shown in Fig. 2 . When the infection rate between fully vaccinated HCWs with hybrid immunity was compared with that of fully vaccinated HCWs without hybrid immunity, the relative vaccine effectiveness showed to be 90.3 % (95 % CI: 44.1–99.8). The relative vaccine effectiveness was 77.9 % (95 % CI: 60.2–88.4) when the infection rate between booster vaccinated HCWs with hybrid immunity was compared with that of booster vaccinated HCWs without hybrid immunity. Further analysis to compare the characteristics of COVID-19 infected and non-infected participants in the four subgroups of HCWs showed no significant differences and no association between gender or age and COVID-19 infection (Table 2 ). None of the participants had a severe SARS-CoV-2 infection, required hospitalization, or died.
Fig. 1.
Comparison of COVID-19 proportion of Omicron infections between fully and booster vaccinated Health-Care Workers with or without hybrid immunity (by a previous COVID-19 infection) during the follow up period.
Fig. 2.
Omicron infection rate per 100 person-months for fully and booster vaccinated Health-Care Workers with or without hybrid immunity (by a previous COVID-19 infection) during the follow up period.
Table 2.
Characteristics of COVID-19 infected and non-infected participants in the four subgroups of vaccinated Health-Care Workers with or without hybrid immunity (by a previous COVID-19 infection).
| Fully vaccinated without hybrid immunity | Fully vaccinated with hybrid immunity | Booster vaccinated without hybrid immunity | Booster vaccinated with hybrid immunity | |||||
|---|---|---|---|---|---|---|---|---|
| Number (N) | 154 | 16 | 1,016 | 119 | ||||
| Median age (IQR) | 47 (36, 57) | 49 (33.5, 51) | 47 (37, 56) | 40 (32, 52) | ||||
| Infected | Non-infected | Infected | Non-infected | Infected | Non-infected | Infected | Non-infected | |
| Age (%) | ||||||||
| Age < median | 40 (52.6) | 36 (47.4) | 1 (14.3) | 6 (85.7) | 248 (51.7) | 232 (48.3) | 6 (10.2) | 53 (89.8) |
| Age ≥ median | 31 (39.7) | 47 (60.3) | 0 (0.0) | 9 (100.0) | 200 (37.3) | 336 (62.7) | 6 (10.0) | 54 (90.0) |
| p-value | 0.109 | 0.437 | <0.001 | 0.976 | ||||
| Gender (%) | ||||||||
| Male | 17 (30.9) | 38 (69.1) | 0 (0.0) | 4 (100.0) | 138 (40.0) | 207 (60.0) | 2 (6.4) | 29 (93.6) |
| Female | 54 (54.6) | 45 (45.4) | 1 (8.3) | 11 (91.7) | 310 (46.2) | 361 (53.8) | 10(11.4) | 78 (88.6) |
| p-value | 0.005 | 0.999 | 0.059 | 0.435 | ||||
4. Discussion
To our knowledge, this is the first study addressing the effect of hybrid immunity in preventing COVID-19 re-infection compared to vaccination alone among HCWs who are at high risk of exposure through vicinity with confirmed COVID-19 cases at the working environment. Our results show that hybrid immunity confers higher protection by almost 5-fold compared to vaccination alone for COVID-19 re-infection with the Omicron variant, regardless of whether immunization was full or boosted. Although older ages (>70 years old) with impaired immunity were not included in our study, no differences were seen when age or gender were taken into account. Due to the fact that HCWs at our Hospital are tested preemptively on a regular basis we managed to identify and include even asymptomatic infections due to Omicron variant. Based on our findings, Omicron is a hypertransmissive strain, leading to COVID-19, despite vaccination, in almost half of HCWs during a 5-month period. Importantly, both hybrid and vaccine-induced immunity prevented severe disease as shown by the fact that none of the participants was hospitalized or died. Notably, initial COVID-19 infection did not require hospitalization in any of the vaccinated HCWs described herein investigation on the potential association between the severity of initial infection and the outcome of reinfection was not possible in our population [2].
It has been well established that COVID-19 vaccination results in strong protection against COVID-19-related hospitalization and death [1], [2], [14]. Although a remarkably similar pattern of immune response to build protection against COVID-19 infection has been described, even when neutralizing antibody titers were taken into account, the nature of vaccine induced immunity remains unclear and wanes over time. According to the published evidence, the third dose of the COVID-19 vaccine improves both humoral and cellular immunity against SARS-CoV-2, with greater neutralizing activity against different COVID-19 variants of concern [8]. Despite the fact that the COVID-19 vaccine-induced protection against infection appears to wane overtime, the protective effect against hospitalization and death remains robust, with no evidence of waning for several months after the additional vaccine dose [15], [16], [17], [18].
Prior infection with an initial COVID-19 variant, regardless of whether this prior infection was ascertained by PCR or antibody testing, was associated with low infection incidence by other circulating variants [5]. Consecutive studies showed that, while natural infection was protective from re-infection at a higher rate compared to vaccine protection, immediately after the second COVID-19 vaccine dose the difference was minimal and even at 4 months after vaccination there was no statistically significant difference between protection of natural infection and that of vaccine-induced protection [4], [19], [20], [21]. However, Goel et al. showed that the B and T-cell immune responses after SARS-CoV-2 mRNA vaccination remained for long, even as antibodies decline. This immune memory was resilient to VOCs and generated an efficient recall response upon antigen re-exposure [22]. Thus, the cellular immunity may have an impact in limiting progression and the durable T-memory cells may be responsible for preserving the protection against severe disease in vaccinated individuals [23]. Indeed, as most recently shown, prior to Omicron predominance, natural infection protection waned over time reaching a 70 % at 16 months after the primary infection, more likely attributed to the genuine waning in immunity than the viral immune evasion [24]. Along this line, hybrid immunity which is presumably associated with strong cellular immunity should confer an additive protective effect. In fact, it has been shown that the highest protection against COVID-19 re-infection was that of hybrid immunity that resulted from prior infection combined with a recent booster vaccination, even when the Omicron variants BA.1 and BA.2 were taken into account [25]. This protective effect, however, was less apparent during the Omicron variants BA.4 and BA.5 emergence if the prior infection involved a pre-Omicron variant, but increased when involved a BA.1 or BA.2 Omicron variant, reflecting the immune system evasion capacity of the newly circulating Omicron strains [26]. Finally, the advantages of hybrid immunity are also supported by, as yet unpublished, findings showing that hybrid immunity reduces by almost 2-fold the transmissibility of COVID-19 re-infections, comparing to either vaccination induced-immunity alone or natural immunity alone [27].
Kumar et al. shed light to the increased transmissibility of the Omicron variant with the discovery of mutations, many of which occurring in the spike protein’s RBD. The discovery of multiple unidentified Omicron mutations inside dominant antibody epitopes has raised concerns that vaccination effectiveness and therapeutic antibody approaches may be influenced and decreased. These mutations lead to an altered binding pocket volume for the Omicron variants that have the potential to affect viral transmissibility, infectivity and pathogenicity through enhancement of the ACE2 receptor’s affinity [28]. However, COVID-19 mRNA vaccine effectiveness against COVID-19 hospitalization and death due to Omicron infection remained high at 70 % after the second dose increasing to 90 % after the booster dose, thus justifying the need for a booster vaccine dose to increase robust protection by immune response activation against severe infection and death from Omicron variant infection [21].
Our study is not without limitations. The study population is HCWs of working age without many comorbidities and therefore our results do not apply to immunocompromized and people at older age who may respond diversely to an Omicron infection. Moreover, we should acknowledge that the statistical power of the presented data is relatively low and that we have no data with regards to the specific Omicron variants responsible for each of the re-infections, but we are assuming that HCWs have been infected by the same strains as those circulating at the general population reported as part of the SARS-CoV-2 genomic surveillance (https://www.eody.gov.gr). During the follow up period, starting after the 21st of December 2021 (Omicron predominance in Greece), we experienced circulation of BA.1* and BA.2* variants, thus suggesting that the study population was exposed to different variants. Strengths of our study include the fact that the study population was tested continuously over almost 2.5 years. In addition, our results have incorporated an important factor that is person time of the exposure period during the follow up.
5. Conclusions
We have shown that hybrid immunity confers far higher protection compared to vaccination alone in preventing COVID-19 re-infection with the Omicron variant among HCWs. Omicron variant was hypertransmissive even in a vaccinated population such as the HCWs, but did not result in severe SARS-CoV-2 disease or death. Because our study included participants that reflect a healthy working population of a productive age, with fewer immunocompromizing conditions in general, our results are not generalizable. However, our findings may inform public health policies on how to achieve optimal immunity in terms of the timing and mode of immunization. Health policy makers should address the real challenges by optimizing vaccination recommendations and encouraging further developments of vaccines that will incorporate the novel VOCs in their design.
Ethical statement
The study was conducted according to Declaration of Helsinki guidelines and approved by the Institutional Ethics Committee of the participating hospital (Laiko University Hospital Ethics Committee Approval ID: 605/6-10-2021). Due to the anonymized and non-interventional nature of the survey, patient consent was not required.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
None.
Funding statement
This work was partly supported by the Kleon Tsetis Foundation, in the context of promoting academic research initiatives within its COVID-19 response framework
References
- 1.Altarawneh H.N., Chemaitelly H., Hasan M.R., Ayoub H.H., Qassim S., AlMukdad S., et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N Engl J Med. 2022;386(13):1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ntziora F, Kostaki EG, Grigoropoulos I, Karapanou A, Kliani I, Mylona M, et al. Vaccination hesitancy among Health-Care-Workers in Academic Hospitals is associated with a 12-fold increase in the risk of COVID-19 infection: a nine-month Greek cohort study. Viruses 2021 Dec 24;14(1):26. Doi:10.3390/v14010026. PMID: 35062230; PMCID: PMC8779273. [DOI] [PMC free article] [PubMed]
- 3.World Health Organization (WHO), Tracking SARS-CoV-2 variants, https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed 8/7/2022).
- 4.Chemaitelly H., Bertollini R., Abu-Raddad L.J. National Study Group for COVID-19 Epidemiology. Efficacy of natural immunity against SARS-CoV-2 reinfection with the beta variant. N Engl J Med. 2021;385(27):2585–2586. doi: 10.1056/NEJMc2110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Coyle P, Malek JA, Ahmed AA, et al. Introduction and expansion of the SARS-CoV-2 B.1.1.7 variant and reinfections in Qatar: A nationally representative cohort study. PLoS Med. 2021 Dec 16;18(12):e1003879. doi: 10.1371/journal.pmed.1003879. PMID: 34914711; PMCID: PMC8726501. [DOI] [PMC free article] [PubMed]
- 6.Kim P, Gordon SM, Sheehan MM, Rothberg MB. Duration of SARS-CoV-2 Natural Immunity and Protection against the Delta Variant: A Retrospective Cohort Study. Clin Infect Dis. 2021 Dec 3:ciab999. doi: 10.1093/cid/ciab999. Epub ahead of print. PMID: 34864907; PMCID: PMC8690283. [DOI] [PMC free article] [PubMed]
- 7.European Centre for Disease Prevention and Control. Implications of the further emergence and spread of the SARSCoV-2 B.1.1.529 variant of concern (omicron) for the EU/EEA — first update. December 2, 2021 (https://www .ecdc.europa.eu/sites/default/files/documents/threat-assessment-covid-19-emergence-sars-cov-2-variant-omicron-december-2021.pdf).
- 8.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejnirattisai W, Huo J, Zhou D, Zahradník J, Supasa P, Liu C, et al. Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. bioRxiv [Preprint]. 2021 Dec 22:2021.12.03.471045. doi: 10.1101/2021.12.03.471045. Update in: Cell. 2022 Feb 3;185(3):467-484.e15. PMID: 34981049; PMCID: PMC8722586. [DOI] [PMC free article] [PubMed]
- 10.Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, et al. Effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. doi: https://doi.org/10.1101/2021.12.30.21268565 (accessed 8/7/2022). [DOI] [PMC free article] [PubMed]
- 11.Akinbami LJ, Biggerstaff BJ, Chan PA, McGibbon E, Pathela P, Petersen LR. Reinfection with SARS-CoV-2 among previously infected healthcare personnel and first responders. Clin Infect Dis. 2021 Nov 15:ciab952. doi: 10.1093/cid/ciab952. Epub ahead of print. PMID: 34791108; PMCID: PMC8767877. [DOI] [PMC free article] [PubMed]
- 12.Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N Engl J Med. 2022;387(1):21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VCC, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. www.idsociety.org/COVID19guidelines (accessed 24/7/2022). [DOI] [PMC free article] [PubMed]
- 14.Abu-Raddad L.J., Chemaitelly H., Bertollini R. Waning mRNA-1273 Vaccine Effectiveness against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2022;386(11):1091–1093. doi: 10.1056/NEJMc2119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Raddad L.J., Chemaitelly H., Bertollini R. Effectiveness of mRNA-1273 and BNT162b2 Vaccines in Qatar. N Engl J Med. 2022;386(8):799–800. doi: 10.1056/NEJMc2117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., et al. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. N Engl J Med. 2022;386(4):340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon S.K., Hegmann K.T., Thiese M.S., Burgess J.L., Ellingson K., Lutrick K., et al. Protection with a Third Dose of mRNA Vaccine against SARS-CoV-2 Variants in Frontline Workers. N Engl J Med. 2022;386(19):1855–1857. doi: 10.1056/NEJMc2201821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Šmíd M., Berec L., Přibylová L., Májek O., Pavlík T., Jarkovský J., et al. Protection by Vaccines and Previous Infection Against the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2. J Infect Dis. 2022;jiac161 doi: 10.1093/infdis/jiac161. accessed 8/7/2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, et al. Protection of prior natural infection compared to mRNA vaccination against SARS-CoV-2 infection and severe COVID-19 in Qatar. medRxiv 2022.03.17.22272529; doi: https://doi.org/10.1101/2022.03.17.22272529 (accessed 8/7/2022). [DOI] [PMC free article] [PubMed]
- 20.Chemaitelly H., Tang P., Hasan M.R., AlMukdad S., Yassine H.M., Benslimane F.M., et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chemaitelly H., Ayoub H.H., AlMukdad S., Coyle P., Tang P., Yassine H.M., et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13(1) doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374(6572) doi: 10.1126/science:abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 Feb 5;371(6529):eabf4063. doi: 10.1126/science.abf4063. Epub 2021 Jan 6. PMID: 33408181; PMCID: PMC7919858. [DOI] [PMC free article] [PubMed]
- 24.Chemaitelly H, Nagelkerke N, Ayoub HH, Coyle P, Tang P, Yassine HM, et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection in Qatar. medRxiv 2022.07.06.22277306; doi: https://doi.org/10.1101/2022.07.06.22277306 (accessed 24/7/2022). [DOI] [PMC free article] [PubMed]
- 25.Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effect of prior infection, vaccination, and hybrid immunity against symptomatic BA.1 and BA.2 Omicron infections and severe COVID-19 in Qatar. medRxiv 2022.03.22.22272745; doi: https://doi.org/10.1101/2022.03.22.22272745 (accessed 24/7/2022).
- 26.Altarawneh HN, Chemaitelly H, Ayoub HH, Hasan MR, Coyle P, Yassine HM, et al. Protection of SARS-CoV-2 natural infection against reinfection with the Omicron BA.4 or BA.5 subvariants. medRxiv 2022.07.11.22277448; doi: https://doi.org/10.1101/2022.07.11.22277448 (accessed 24/7/2022).
- 27.Tan ST, Kwan AT, Rodríguez-Barraquer I, Singer BJ, Park HJ, Lewnard JA, et al. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. medRxiv 2022.08.08.22278547; doi: https://doi.org/10.1101/2022.08.08.22278547 (accessed 30/08/2022). [DOI] [PMC free article] [PubMed]
- 28.Kumar S, Karuppanan K, Subramaniam G. Omicron (BA.1) and Sub-Variants (BA.1, BA.2 and BA.3) of SARS-CoV-2 Spike Infectivity and Pathogenicity: A Comparative Sequence and Structural-based Computational Assessment. bioRxiv 2022.02.11.480029; doi: https://doi.org/10.1101/2022.02.11.480029 (accessed 8/7/2022). [DOI] [PMC free article] [PubMed]