Abstract
Purpose
To assess whether mRNA and viral vector coronavirus disease 2019 (COVID-19) vaccines detrimentally affected semen parameters.
Materials and Methods
In this prospective study, we enrolled 101 men vaccinated for COVID-19 (76% received mRNA vaccines, 20% viral vector vaccines, 2% a mixed formulation, and for 2 men no information about vaccine xlink:type was available) in 2021 and with a previous semen analysis. For each man we compared semen parameters before and after vaccination.
Results
Post-vaccine samples were obtained at a median of 2.3±1.5 months after the second dose. After vaccination, the median sample volume significantly decreased (from 3.0 to 2.6 mL, p=0.036), whereas the median sperm concentration, the progressive motility, and total motile sperm count increased (from 25.0 to 43.0 million/mL, p<0.0001; from 50% to 56%, p=0.022; from 34.8 to 54.6 million, p<0.0001, respectively). Thirty-four patients were oligospermic before the vaccine, and also in these patients we observed a significant increase of sperm parameters after vaccine. Finally, we confirmed the aforementioned results in men who received a mRNA or a viral vector vaccine.
Conclusions
The semen parameters following COVID-19 vaccination did not reflect any causative detrimental effect from vaccination, and for the first time we demonstrated that this applies to both mRNA and viral-vector vaccines. The known individual variation in semen and the reduced abstinence time before the post-vaccine sample collection may explain the increases in sperm parameters.
Keywords: COVID-19; Infertility, male; Semen analysis; Vaccines, synthetic
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a single-stranded RNA coronavirus and it causes coronavirus disease 2019 (COVID-19). There are currently four COVID-19 vaccines validated for use by the World Health Organization (WHO): Pfizer-BioNTech, Moderna, Johnson & Johnson, and Oxford-AstraZeneca. The Oxford-AstraZeneca vaccine has received authorization for use in the United Kingdom and European counties, while the first three have also been approved in the United States. The Pfizer-BioNTech and Moderna vaccines are mRNA vaccines, which are composed of nucleoside-modified mRNA encoding the viral spike glycoprotein. Human cells take up the mRNA and transcribe it into spike proteins, which lead to the immune recognition of these proteins and the production of antibodies against these proteins. The Johnson & Johnson and Oxford-AstraZeneca vaccines use traditional viral vector-based technology: a stabilized variant of the SARS-CoV-2 spike protein is placed in a recombinant, replication-incompetent adenovirus (viral vector), to deliver it to the human cells that produce spike protein, thus leading to immune recognition.
Due to the exceptionality of the situation, all the experimental steps necessary for clinical use were carried out in a short period of time and the first vaccine, Pfizer-BioNTech, was approved for Emergency Use Authorization (EUA) in the US at the end of November 2020 and in Europe after a month, less than one year from the first COVID-19 case. While clinical trials demonstrated the safety and effictiveness of these vaccines, their impact on male fertility has not yet been investigated [1]. The most common side effects reported in clinical trials were pain/redness/swelling in the injection site, fatigue, headache, muscle pain, chills, fever, and nausea. Serious side effects were rare and included axillary lymphadenopathy, paroxysmal ventricular arrhythmia, arm/leg paresthesia, and urologic symptoms. There is currently no evidence that the vaccines can cause infertility in women, damage to the placenta, or miscarriages [2,3,4,5]. No one reported symptoms related to erectile, ejaculatory or sexual function. However, following evidence of impaired reproductive function after COVID-19 infection, public concerns emerged over unfounded claims in social media suggesting a potential negative impact of COVID-19 vaccines on sperm and fertility. This hesitation in the uptake of the vaccine was largely motivated by the lack of knowledge of newly developed mRNA-based vaccines and the misconception that spike proteins produced after vaccination may allow the virus to enter cells, including gametes, and modifying their genome.
Some of these fears also stem from the fact that pregnant women were excluded from clinical trials, and men and women of reproductive age enrolled in studies were required to avoid conception. But this was due to the strict protocols of clinical trials, not because of concerns that COVID-19 vaccines would affect fertility/offspring.
To date, only two reports on the possible effects of COVID-19 mRNA vaccines on semen quality are available. First, a study on semen samples from 75 fertile men tested once 1 to 2 months after vaccination concluded that semen parameters following vaccine were predominantly within the normal WHO reference ranges [6]. Secondly, in 45 healthy men comparison of semen before and after COVID-19 mRNA vaccine showed no significant decreases in any sperm parameter [7]. Finally, another study found that COVID-19 vaccination had no impact on fertility treatment outcomes [8].
Due to the limited data available collected in small studies and the lack of evidence concerning the effect of the different xlink:types of COVID-19 vaccines on male fertility, in this study we prospectively evaluated the semen parameters of 101 men before and after two doses of a COVID-19 mRNA or viral vector vaccine.
MATERIALS AND METHODS
1. Study population and study design
This prospective study was performed at a tertiary level public fertility center. Semen samples were obtained from 101 vaccinated men undergoing fertility treatments at the Physiopathology of Human Reproduction, IRCCS Ospedale Policlinico San Martino, Genova, Italy, from February to December 2021, that had undergone semen analysis at least once also before vaccination. Seventy-six percent (77/101) of men received mRNA vaccines (80% Pfizer-BioNTech, 20% Moderna), 20% (20/101) viral vector vaccines (70% AstraZeneca, 30% Johnson & Johnson), 2% (2/101) a mixed formulation, and for 2 men information about xlink:type of vaccine was not available. For each man we compared semen parameters before and after the COVID-19 vaccination.
2. Ethics statement
The present study protocol was reviewed and approved by the Ethics Committee of Regione Liguria (approval number: 407/2022 – DB id 12559). Written informed consent was obtained from all participants.
3. Semen analysis
Semen samples were collected by masturbation into a sterile container after 2 to 5 days of abstinence. After semen liquefaction at room temperature for 30 to 60 minutes, basic analysis, including semen volume, sperm concentration and motility, was performed in accordance with WHO guidelines [9]. Briefly, the volume was assessed using a graduated disposable pipette. Sperm concentration and motility were assessed using a Makler chamber. Sperm score for motility evaluation was expressed as follows: a=rapid progressive motility, b=slow progressive motility, c=local motility, and d=immobility. Progressive motility rate was calculated as the percentage of a+b. Total motile sperm count (TMSC) was calculated as product of sperm count, motility and semen volume.
4. Statistical analysis
Descriptive statistics are reported as mean±standard deviation or median and interquartile range (IQR) for continuous variables, and as absolute frequencies and percentages for categorical variables. The impact of COVID-19 vaccine on endpoints (semen volume, concentration, motility) was evaluated by Wilcoxon rank sum test to perform paired comparison of pre- and post-vaccination semen parameters. An analysis of covariance (ANCOVA) was also performed to evaluate the association between sperm parameters and vaccine after adjustment for relevant confounders (type of vaccine used [mRNA vs. viral vectors], body mass index [BMI], presence of urological factors, male infertility, age, smoke habit, alcohol consumption, frequency of ejaculations, and days of sexual abstinence).
The coefficients of variation within-subject and between-subject (CVw and CVb, respectively) were calculated for semen volume, sperm concentration, progressive motility, and TMSC. Each CVw was calculated by root mean square method [10] as the square root of Σ(d/m)2/2n, where ‘d’ was the difference between two paired measurements and ‘m’ was the mean of paired measurements, and expressed as a percentage. For each semen parameter the CVb was calculated as the square root of the mean squares between divided by the grand mean and expressed as a percentage.
Analyses were carried out by MedCalc® software (Ostend, Belgium) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
The characteristics of the men enrolled in the study are shown in Table 1. There were no differences in BMI, urological disease, male infertility and lifestyle factors (smoking habit and alcohol consumption) between patients who received a mRNA or a viral vector vaccine.
Table 1. Characteristics of the men enrolled in the study.
| Characteristic | Patients who received mRNA vaccine (n=77) | Patients who received viral vector vaccine (n=20) | p-value | |
|---|---|---|---|---|
| BMI, kg/m2 | ||||
| 18.5–24.9 | 39 (50.6) | 9 (45.0) | 0.820 | |
| 25.0–30.0 | 31 (40.3) | 9 (45.0) | 0.881 | |
| 30.1–34.9 | 4 (5.2) | 1 (5.0) | 0.565 | |
| 35.0–40.0 | 3 (3.9) | 1 (5.0) | 0.669 | |
| Urological diseases | ||||
| Yes | 17 (22.1) | 4 (20.0) | 0.911 | |
| No | 60 (77.9) | 16 (80.0) | 0.911 | |
| Male infertility | ||||
| Yes | 17 (22.1) | 6 (30.0) | 0.649 | |
| No | 60 (77.9) | 14 (70.0) | 0.649 | |
| Smoke habit | ||||
| Yes | 23 (29.9) | 7 (35.0) | 0.873 | |
| No | 54 (70.1) | 13 (65.0) | 0.873 | |
| Alcohol consumption | ||||
| Yes | 10 (13.0) | 3 (15.0) | 0.893 | |
| No | 67 (87.0) | 17 (85.0) | 0.637 | |
Values are presented as number (%).
The clinic characteristics of the patients and the semen parameters of the samples collected before and after the vaccine are shown in Table 2. Pre-vaccination samples were obtained after a median abstinence period of 4 days (IQR, 3–5 d) and post-vaccination samples after a median of 3 days (IQR, 3–4 d; p=0.004). Post-vaccine samples were obtained at a median of 2.3±1.5 months after the second dose. The median volume of the samples before vaccination was 3.0 mL (IQR, 2.2–4.0 mL) and it significantly decreased to 2.6 mL (IQR, 1.9–3.5 mL; p=0.036) after the second dose of vaccine. All other semen parameters, including sperm concentration, progressive motility, and TMSC, significantly increased (p<0.0001, p=0.022, p<0.0001, respectively) after vaccination compared to pre-vaccination values. The ANCOVA analysis, with semen parameters as dependent variable and xlink:type of vaccine used, BMI, alcohol and tobacco consumption, presence of urological factors, male infertility, age, frequency of ejaculations and days of sexual abstinence as confounders, indicate that vaccine had a significant effect on sperm concentration (p<0.0001) and TMSC (p=0.0004). Vaccine was not significantly associated with semen volume (p=0.291) and progressive motility (p=0.125).
Table 2. Demographic and clinic characteristics of the men enrolled in the study and semen parameters before and after vaccine.
| Parameter | Pre-vaccine | Post-vaccine | p-value | CVw (%) | CVb (%) | |
|---|---|---|---|---|---|---|
| All patients (n=101) | ||||||
| Age, y | 37.5±5.5 | 38.5±5.6 | 0.202 | - | - | |
| Sexual abstinence, d | 4 (3–5) | 3 (3–4) | 0.004 | - | - | |
| Frequency of ejaculations, times/wk | 2 (1–3) | 2 (1–3) | 0.694 | - | - | |
| Volume, mL | 3.0 (2.2–4.0) | 2.6 (1.9–3.5) | 0.036 | 19.5 | 44.1 | |
| Sperm concentration, million/mL | 25.0 (11.4–38.0) | 43.0 (17.0–86.5) | <0.0001 | 45.3 | 90.7 | |
| Progressive motility, % | 50 (40–60) | 56 (40–65) | 0.022 | 14.6 | 33.1 | |
| TMSC, million | 34.8 (11.6–68.8) | 54.6 (18.9–105.6) | <0.0001 | 53.8 | 111.5 | |
| Patients with oligospermia (n=34) | ||||||
| Age, y | 37.1±5.4 | 38.2±5.6 | 0.413 | - | - | |
| Sexual abstinence, d | 4 (4–5) | 3 (3–4) | 0.087 | - | - | |
| Frequency of ejaculations, times/wk | 2 (2–3) | 2 (2–2) | 0.519 | - | - | |
| Volume, mL | 4.0 (3.5–4.4) | 2.0 (1.9–3.5) | 0.153 | 13.6 | 38.0 | |
| Sperm concentration, million/mL | 5.0 (4.5–8.6) | 19.0 (5.0–20.0) | 0.001 | 51.8 | 82.7 | |
| Progressive motility, % | 35 (26– 45) | 45 (35–60) | 0.002 | 25.0 | 45.1 | |
| TMSC, million | 8.4 (4.8–14.9) | 17.2 (5.2–35.5) | 0.001 | 68.6 | 230.6 | |
Values are presented as mean±standard deviation or median (interquartile range).
CVw: coefficients of variation within subjects, CVb: coefficients of variation between subjects, TMSC: total motile sperm count, -: not available.
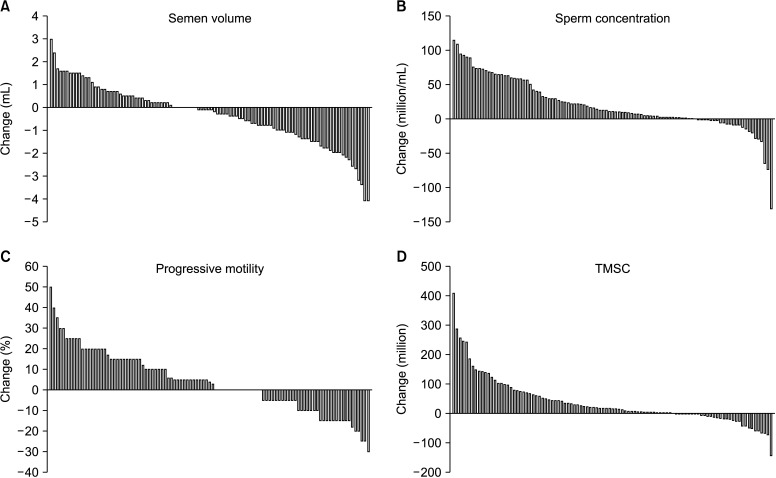
Fig. 1 shows for each man the within-individual changes in semen parameters in relation to the pre-vaccine value. We observed that 39% (39/101) of men increased their semen volume, whereas 24% (24/101) of patients recorded a decrease in sperm concentration (median percentage decrease: 41.2%; IQR, 26.5%–72.5%) and 34% (34/101) in progressive motility (median percentage decrease: 10%; IQR, 5%–15%).
Fig. 1. Bar graph showing changes in semen volume (A), sperm concentration (B), progressive motility (C), and TMSC (D) from pre-vaccine values for each man. Each bar represents a patient. TMSC: total motile sperm count.
Thirty-four patients were oligospermic before the vaccine (mean concentration 7.8±4.3 million/mL). Among them, 71% (24/34) received a mRNA vaccine, 26% (9/34) a viral vector vaccine, and 3% (1/34) a mixed formulation. Also in this subset of patients we observed a significant increase of the median sperm concentration, progressive motility, and TMSC (p=0.001, p=0.002, p=0.001, respectively) in post-vaccine semen samples compared to the pre-vaccine ones (Table 2). Of them, 17 patients (50%) increased sperm concentration at normozoospermia after vaccine (median concentration: 20.0 million/mL; IQR, 19.5–22.0 million/mL).
Finally, we separately analyzed patients who received a mRNA vaccine and those who received a viral vector vaccine, and we confirmed that there were no significant decreases in any sperm parameter again in these two groups (Table 3).
Table 3. Demographic and clinic characteristics of men who received a mRNA or a viral vector vaccine, and semen parameters before and after vaccine.
| Parameter | Pre-vaccine | Post-vaccine | p-value | CVw (%) | CVb (%) | |
|---|---|---|---|---|---|---|
| Patients who received mRNA vaccine (n=77) | ||||||
| Age, y | 37.6±5.4 | 38.6±5.5 | 0.257 | - | - | |
| Sexual abstinence, d | 4 (3–5) | 3 (3–4) | 0.033 | - | - | |
| Frequency of ejaculations, times/wk | 2 (1–3) | 2 (1–2) | 0.446 | - | - | |
| Volume, mL | 3 (2.0–3.9) | 2 (1.6–2.4) | 0.008 | 18.4 | 47.2 | |
| Sperm concentration, million/mL | 27.0 (13.3–60.5) | 67.0 (19.0–102.5) | <0.0001 | 45.1 | 86.4 | |
| Progressive motility, % | 50 (40–60) | 55 (40–65) | 0.398 | 12.9 | 34.2 | |
| TMSC, million | 37.5 (11.7–68.4) | 54.1 (17.3–105.0) | 0.0002 | 51.4 | 112.6 | |
| Patients who received viral vector vaccine (n=20) | ||||||
| Age, y | 36.4±6.1 | 37.4±6.3 | 0.613 | - | - | |
| Sexual abstinence, d | 5 (4–5) | 4 (3–4) | 0.042 | - | - | |
| Frequency of ejaculations, times/wk | 2 (2–3) | 2 (2–3) | 0.625 | - | - | |
| Volume, mL | 2.9 (2.5–3.6) | 2.0 (2.4–4.1) | 0.275 | 19.5 | 28.9 | |
| Sperm concentration, million/mL | 27.0 (5.0–48.0) | 36.0 (16.0–69.0) | 0.027 | 40.3 | 88.9 | |
| Progressive motility, % | 47 (38–60) | 58 (50–65) | 0.018 | 20.1 | 28.0 | |
| TMSC, million | 20.0 (6.2–116.1) | 55.0 (21.8–114.3) | 0.036 | 64.3 | 109.7 | |
Values are presented as mean±standard deviation or median (interquartile range).
CVw: coefficients of variation within subjects, CVb: coefficients of variation between subjects, TMSC: total motile sperm count, -: not available.
CVw and CVb in the whole cohort and in the various categories considered are shown in Table 2 and 3. For all parameters measured, the variation within the subjects was less than the relative variation observed between the subjects. Semen volume and sperm motility had the smallest degree of variation, both within and between subjects. The largest variations were observed for sperm concentration and TMSC. In particular, for semen volume we observed CVw ranging from 13.6% in oligospermic patients to 19.5% in the entire cohort and in the subgroup of patients who received a viral vector vaccine. For sperm count the CVw was between 40.3% in patients who received a viral vector vaccine and 51.8% in patients with oligospermia. Minimum CVw for progressive motility and TMSC were in patients who received a mRNA vaccine (12.9% and 51.4, respectively), whereas the maximum values were in oligospermic patients (25.0% and 68.6, respectively).
DISCUSSION
In this study we confirmed that the semen parameters do not reflect any causal detrimental effect from COVID-19 vaccines in a larger cohort of men than in a previous report with the same study design [7]. Even the semen of men who were oligospermic before the vaccine showed no further decline.
The SARS-CoV-2 is comprised of a nucleoprotein surrounding the RNA genome, a viral envelope, and a spike protein. The spike protein plays a crucial role in cell attachment and fusion during viral infection [11]. Viral entry into cells requires expression of both angiotensin-converting enzyme 2 (ACE2) and the transmembrane protease serine 2 (TMPRSS2) on the surface of the host cell: once the spike protein binds to ACE2, the TMPRSS2 activates cellular proteases to cleave the spike protein into two subunits. This allows the virus entry, so that viral RNA is released, and genome replication and transcription begin [12]. Therefore, SARS-CoV-2 affects cells based on their expression of ACE2 and TMPRSS2.
The ability of SARS-CoV-2 to cross the blood-testis barrier and affect male fertility is still a controversial topic. On one hand, the male reproductive system may be a potential target for SARS-CoV-2 since ACE2 is highly expressed in testis, both in Sertoli and Leydig cells, and spermatogonia, as well as TMPRSS2A is expressed in Leydig cells [13,14,15,16]. In support of this, Li et al [17] detected SARS-CoV-2 in the semen of COVID-19 patients. However, subsequent studies published so far have not found the virus in the semen of both men with active disease and those who recovered [18,19,20,21,22,23]. A recent review concluded that the probability that SARS-CoV-2 is present in the semen of COVID-19 patients is very small [24].
Regardless of a putative local infection in the testis, COVID-19 disease has a harmful, probably temporary, effect on semen parameters, due to fever or through a severe cytokine storm with an acute immune response and autoimmune orchitis that could damage spermatogenesis [17,25,26,27]. Moreover, an independent association has been observed between the status of SARS-CoV-2 infection and secondary hypogonadism, with low testosterone levels [28], a condition that can last several months [29].
Given the aforementioned potential negative effect of COVID-19 infection on male fertility, vaccination against COVID-19 can prevent many infertility cases, so couples wishing to conceive should vaccinate. In support of this observation, on 9th January 2021 a joint statement of the Society for Male Reproduction and Urology and the Society for the Study of Male Reproduction recommended that the COVID-19 vaccine should be offered to all men, including those who desire fertility and emphasized that “patients undergoing fertility treatment and pregnant patients should be encouraged to receive vaccination based on eligibility criteria” [30].
It should be noted that side effects of the COVID-19 vaccine are usually mild [31,32] and only about 16% of men in the Pfizer/BioNtech COVID-19 vaccine clinical trial had fever after the second dose. Fever can cause temporary declines in sperm production that would be similar to or lower than if the individual experienced fever for other reason. As a result, none of the men in our cohort had serious side effects from the vaccine.
Moreover, mRNA COVID-19 vaccines are unlikely to affect sperm parameters because they contain mRNA and not the live virus, and it is plausible that even the viral vector vaccines would not cause any harmful effect on male fertility. In fact, in the case of the viral vector vaccine, the adenovirus is modified to not replicate and cannot cause a real infection in any xlink:type of organ or cell. It simply acts as a driver for the code of the SARS-CoV-2spike protein.
Our study corroborates the results previously reported by Gonzalez et al [7], as we have shown that COVID-19 vaccination has not affected the sperm quality of men, thus reassuring the young male population undergoing vaccination.
Each patient served as its own control, thus making the comparison self-controlled and eliminating variability among individuals, which most likely would not be attributable only to vaccination. At the same time, the known individual variation according to which sperm parameters may vary greatly in a patient’s semen over time [32,33,34,35,36,37], can explain the overall increases in sperm parameters and the decrease in semen quality experienced by approximately 30% of patients. Despite this individual variation, all the CVb were markedly greater than their respective CVw, thus reassuring that the intra-individual variation in semen was much smaller, in agreement with previous data [37,38,39,40]. Moreover, it is noteworthy that the magnitude of change for most CVw were within the ranges of observations published in infertile men, i.e., 24% to 29% for semen volume, 27% to 48% for sperm count, and 9% to 26% for motility [38].
Last but not least, the reduced abstinence time before collection of the post-vaccine sample may explain the better semen quality, in particular the lower semen volume and the higher sperm count and motility, compared to the pre-vaccine samples. In fact, the length of abstinence is known to be related to the within-subject variability of human semen with regard to sperm count and volume [33,41]. Accordingly, TMSC showed the greatest variation, both within and between subjects. Since TMSC is a variable calculated as a product of sperm count, motility and semen volume, it is conceivable that these individual parameters can be expanded into the combined value of TMSC.
There are several strengths of our study. First, this study was the first to assess the impact of different xlink:types of COVID-19 vaccination on sperm parameters. Secondly, this is the largest study specifically designed to investigate the difference of pre-vaccination against post COVID-19 vaccination in sperm parameters in infertile men. Conversely, other authors did not include a pre-vaccination examination, thus limiting the validity of their results [6] or have analyzed fertile men [7]. Third, the study’s time frame encompassed the entire life cycle of sperms: the spermatozoa mature for 72 days and most subjects had a semen sample collected 72 days or more after the second dose of the vaccine.
Likewise, we are aware that some weaknesses of our study should be considered. Firstly, the small sample size; secondly, the short follow-up without long-term results, therefore later sequalae is still possible; thirdly, limited generalizability due to the lack of a healthy control group; fourth, no evaluation of male reproductive potential i.e., comparing the outcomes of ART cycles before and after vaccination.
CONCLUSIONS
As a relatively large cohort of 101 men (double the number of patients enrolled in the other two reports published on this topic [6,7]) is being reported, and considering that the duration of the study encompassed the full life cycle of sperms in most cases, we are confident in suggesting that any xlink:type of COVID-19 vaccination is safe without any evident negative effects on sperm parameters even in men who were oligospermic.
Acknowledgements
The authors thank our nurses Roberta Accinelli, Laura Bacigalupo, Martino Borra, Antonella Santi, Patrizia Sanguinetti for their assistance for this study.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Conceptualization: CM, SS, PS.
- Data curation: SS, EM, MFF, IG.
- Formal analysis: FB.
- Investigation: CM, SS, PS.
- Methodology: EM.
- Supervision: PA.
- Visualization: FB, PS.
- Writing – original draft: PS.
- Writing – review & editing: CM, SS, FB, PA, PS.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kumar V, Kaur M. COVID-19 Vaccine and Male Fertility. Urol J. 2021 doi: 10.22037/uj.v18i.6897. [Epub] [DOI] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris RS. SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F S Rep. 2021;2:253–255. doi: 10.1016/j.xfre.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H, Souders C, Carmel M, Anger JT. Low rates of urologic side effects following coronavirus disease vaccination: an analysis of the food and drug administration vaccine adverse event reporting system. Urology. 2021;153:11–13. doi: 10.1016/j.urology.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lifshitz D, Haas J, Lebovitz O, Raviv G, Orvieto R, Aizer A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod Biomed Online. 2022;44:145–149. doi: 10.1016/j.rbmo.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, Ory J, et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA. 2021;326:273–274. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orvieto R, Noach-Hirsh M, Segev-Zahav A, Haas J, Nahum R, Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients' performance during IVF-ET cycle? Reprod Biol Endocrinol. 2021;19:69. doi: 10.1186/s12958-021-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) WHO laboratory manual for the examination and processing of human semen. 6th ed. Geneva: WHO; 2021. [Google Scholar]
- 10.Hyslop NP, White WH. Estimating precision using duplicate measurements. J Air Waste Manag Assoc. 2009;59:1032–1039. doi: 10.3155/1047-3289.59.9.1032. [DOI] [PubMed] [Google Scholar]
- 11.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas GC, O'Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 14.Younis JS, Abassi Z, Skorecki K. Is there an impact of the COVID-19 pandemic on male fertility? The ACE2 connection. Am J Physiol Endocrinol Metab. 2020;318:E878–E880. doi: 10.1152/ajpendo.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma S, Saksena S, Sadri-Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis. Biol Reprod. 2020;103:449–451. doi: 10.1093/biolre/ioaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayaaslan B, Korukluoglu G, Hasanoglu I, Kalem AK, Eser F, Akinci E, et al. Investigation of SARS-CoV-2 in semen of patients in the acute stage of COVID-19 infection. Urol Int. 2020;104:678–683. doi: 10.1159/000510531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoli D, Pallotti F, Colangelo S, Basilico F, Mazzuti L, Turriziani O, et al. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest. 2020;43:1819–1822. doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song C, Wang Y, Li W, Hu B, Chen G, Xia P, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod. 2020;103:4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo L, Zhao S, Li W, Wang Y, Li L, Jiang S, et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2021;9:42–47. doi: 10.1111/andr.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y, Wang J, Ren J, Zhao Y, Chen J, Chen X. Effect of COVID-19 on male reproductive system - a systematic review. Front Endocrinol (Lausanne) 2021;12:677701. doi: 10.3389/fendo.2021.677701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarra A, Albani E, Castellano S, Arruzzolo L, Levi-Setti PE. Coronavirus disease-19 infection: implications on male fertility and reproduction. Front Physiol. 2020;11:574761. doi: 10.3389/fphys.2020.574761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod. 2021;36:1520–1529. doi: 10.1093/humrep/deab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orvieto R, Segev-Zahav A, Aizer A. Does COVID-19 infection influence patients' performance during IVF-ET cycle?: an observational study. Gynecol Endocrinol. 2021;37:895–897. doi: 10.1080/09513590.2021.1918080. [DOI] [PubMed] [Google Scholar]
- 28.Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, et al. Severely low testosterone in males with COVID-19: a case-control study. Andrology. 2021;9:1043–1052. doi: 10.1111/andr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salonia A, Pontillo M, Capogrosso P, Gregori S, Carenzi C, Ferrara AM, et al. Testosterone in males with COVID-19: a 7-month cohort study. Andrology. 2022;10:34–41. doi: 10.1111/andr.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Society for Reproductive Medicine (ASRM) COVID-19 updates and resources: statements [Internet] Washington, D.C.: ASRM; c2021. [cited 2021 Jan 9]. Available from: https://www.asrm.org/news-and-publications/covid-19/#Statements . [Google Scholar]
- 31.Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10:1428. doi: 10.3390/jcm10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riad A, Hocková B, Kantorová L, Slávik R, Spurná L, Stebel A, et al. Side effects of mRNA-based COVID-19 vaccine: nationwide phase IV study among healthcare workers in Slovakia. Pharmaceuticals (Basel) 2021;14:873. doi: 10.3390/ph14090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz D, Laplanche A, Jouannet P, David G. Within-subject variability of human semen in regard to sperm count, volume, total number of spermatozoa and length of abstinence. J Reprod Fertil. 1979;57:391–395. doi: 10.1530/jrf.0.0570391. [DOI] [PubMed] [Google Scholar]
- 34.Poland ML, Moghissi KS, Giblin PT, Ager JW, Olson JM. Variation of semen measures within normal men. Fertil Steril. 1985;44:396–400. doi: 10.1016/s0015-0282(16)48866-7. [DOI] [PubMed] [Google Scholar]
- 35.Knuth UA, Kühne J, Bals-Pratsch M, Nieschlag E. Intra-individual variation of sperm velocity, linearity, lateral head displacement and beat frequency in healthy volunteers. Andrologia. 1988;20:243–248. [PubMed] [Google Scholar]
- 36.Cooper TG, Jockenhövel F, Nieschlag E. Variations in semen parameters from fathers. Hum Reprod. 1991;6:859–866. doi: 10.1093/oxfordjournals.humrep.a137441. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez C, Castilla JA, Martínez L, Ramírez JP, Vergara F, Gaforio JJ. Biological variation of seminal parameters in healthy subjects. Hum Reprod. 2003;18:2082–2088. doi: 10.1093/humrep/deg430. [DOI] [PubMed] [Google Scholar]
- 38.Keel BA. Within- and between-subject variation in semen parameters in infertile men and normal semen donors. Fertil Steril. 2006;85:128–134. doi: 10.1016/j.fertnstert.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 39.Schrader SM, Turner TW, Simon SD. Longitudinal study of semen quality of unexposed workers. Sperm motility characteristics. J Androl. 1991;12:126–131. [PubMed] [Google Scholar]
- 40.Wijchman JG, de Wolf BT, Graafe R, Arts EG. Variation in semen parameters derived from computer-aided semen analysis, within donors and between donors. J Androl. 2001;22:773–780. [PubMed] [Google Scholar]
- 41.Sokol P, Drakopoulos P, Polyzos NP. The effect of ejaculatory abstinence interval on sperm parameters and clinical outcome of ART. A systematic review of the literature. J Clin Med. 2021;10:3213. doi: 10.3390/jcm10153213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.