Abstract
Introduction
Total pancreatectomy with en-bloc celiac axis resection (TP-CAR) and interposition graft placement between the aorta and the proper hepatic artery is a technically demanding, very uncommonly performed operation, even in high-volume pancreatic centers.
Presentation of case
We present, in clinical and technical detail, a patient with locally advanced adenocarcinoma of the pancreatic body and neck involving the celiac and common hepatic arteries and portal vein, who underwent neoadjuvant chemotherapy and radiation with very good response, followed by TP-CAR and aorto-proper hepatic artery bypass using saphenous vein graft. The patient had an uneventful intraoperative and postoperative course, short hospital stay, and histology consistent with a curative resection.
Discussion
TP-CAR with common hepatic artery resection and proper hepatic artery reconstruction in patients with locally advanced pancreatic body cancer after appropriate neoadjuvant therapy can be performed safely and be potentially curative in centers with an established track record in advanced pancreatic surgery involving major peripancreatic vessels.
Conclusion
TP-CAR with proper hepatic artery reconstruction is a rare but potentially curative operation for selected patients with otherwise unresectable pancreatic adenocarcinoma.
Abbreviations: CA, celiac axis; CHA, common hepatic artery; CT, computed tomography; DP-CAR, distal pancreatectomy with celiac axis resection; EBL, estimated blood loss; GDA, gastroduodenal artery; ICU, intensive care unit; LFTs, liver function tests; NAC, neoadjuvant chemotherapy; PC, pancreatic cancer; PHA, proper hepatic artery; PV, portal vein; SMA, superior mesenteric artery; SMV, superior mesenteric vein; TP-CAR, total pancreatectomy with celiac artery resection
Keywords: Case report, Celiac artery resection, Hepatic artery resection, Proper hepatic artery, DP-CAR
Highlights
-
•
TP-CAR and aorto-PHA bypass graft: A very rare, technically demanding operation with significant morbidity & mortality
-
•
This operation may provide curative (R0) resection of an otherwise unresectable cancer
-
•
Our patient had optimal perioperative outcome and excellent histology result
-
•
To the best of our knowledge this is the first TP-CAR with aorto-PHA bypass graft case in Greece
1. Introduction
Celiac artery resection (CAR) in the context of major pancreatectomy for locally advanced pancreatic cancer (PC), according to the NCCN criteria (Version 1.2020, 11/26/19), is still a rather uncommon operation, even in well-established, high-volume pancreatic centers. In two [1], [2] recent multi-institutional studies over 18 and 38 years respectively, the annual number of these cases per pancreatic center is calculated at 0.4 and 0.5. CAR is most often performed in the course of distal pancreatectomy (DP), where the arterial supply to the liver relies on the integrity of the gastroduodenal (GDA) and proper hepatic artery (PHA) pathway. However, when the tumor extends to and involves the whole length of the common hepatic artery (CHA) to its bifurcation to GDA and PHA, or even the GDA itself, then, not only a more extended pancreatectomy is necessary, but also arterial blood should be rerouted to the PHA more distally. Such cases are very rare in the literature. They appear as reports of a single patient [3], [4], [5], [6], [7], [8], or two patients [9]. Even in series with a significant number of arterial resections, these particular types of cases remain rare: one [10], two [11], five [12], seven [13], or eight patients [14]. Only very recently, a series of at least 33 patients from the Mayo Clinic [15] was published, in which a rational classification of various types of CAR procedures was suggested, depending on the extent of pancreatectomy and the additional to CAR arterial resection(s) and reconstruction(s) required. According to this classification, CAR associated with CHA resection requiring an interposition graft between the aorta and the PHA forms Class 2; when associated with total pancreatectomy (TP), it forms Class 2B.
“Classic” DP-CAR (without PHA revascularization) is generally associated with significant mortality and morbidity, much higher than “straightforward” pancreatoduodenectomy, or DP, even in experienced hands [1], [2]. However, with increasing expertise, improved patient selection, universal administration of neoadjuvant chemotherapy (NAC) and even neoadjuvant radiation, not only perioperative results have significantly improved, but also survival may extend to 2–3 years [16], [17], [18], or even longer [15].
We hereby present a case of TP-CAR and interposition vein graft placement between the aorta and the PHA (Mayo Clinic class 2B) with very good perioperative outcome. To the best of our knowledge, this is the first such case in our country and one of the very few in the English literature with the exception of the Mayo Clinic. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. This work is exempt from approval from our institution's ethics committee and has been reported in line with the SCARE criteria [19].
2. Case report
A 50-year-old woman presented with a four-month history of gradually increasing back pain and a three-month history of new onset non-insulin dependent diabetes mellitus. She denied abdominal pain, weight loss, decreased appetite, or other symptoms. She was a nonsmoker, active, high school teacher with excellent performance status and a body mass index of 25.2 kg/m2. Her past medical history included only Hashimoto's thyroiditis, managed with thyroxine. She had no family history of cancer. Physical exam and blood work including liver function tests (LFTs) and bilirubin were normal. Ultrasonography showed a tumor in the body of the pancreas. CA 19–9 was 476 u/mL and CEA 10.8 u/mL. Computed tomography (CT) demonstrated a 5 cm low-density tumor in the neck and body of the pancreas with retropancreatic extension encasing the common hepatic artery (CHA) for 3600 at all its length, as well as the celiac axis (CA) for 3600 sparing 0.7 cm of its origin from the aorta. In addition, it abutted the anterior and right aspect of the superior mesenteric artery (SMA) for 2100 and 3 cm. It also infiltrated the splenomesenteric vein junction and the left aspect of the portal vein (PV) for 2 cm. There was no evidence of liver or peritoneal metastases. Endoscopic ultrasonography with fine needle aspiration documented ductal adenocarcinoma. NAC with FOLFIRINOX was initiated and she completed a six-cycle protocol (twice a month for six months), without any significant side effects. With NAC completion, CA 19-9 had decreased to 17 u/mL and CEA to 0.7 u/mL. A month later, neoadjuvant radiation was administered with further decrease of the CA 19–9 and CEA to 14 u/mL and 0.4 u/mL respectively. Two months later, CT documented only slight decrease in the tumor size, but the pattern and degree of both CA and CHA involvement was unchanged (Fig. 1, Fig. 2). Magnetic resonance imaging ruled out liver involvement and all imaging was negative for any distant metastatic disease.
Fig. 1.
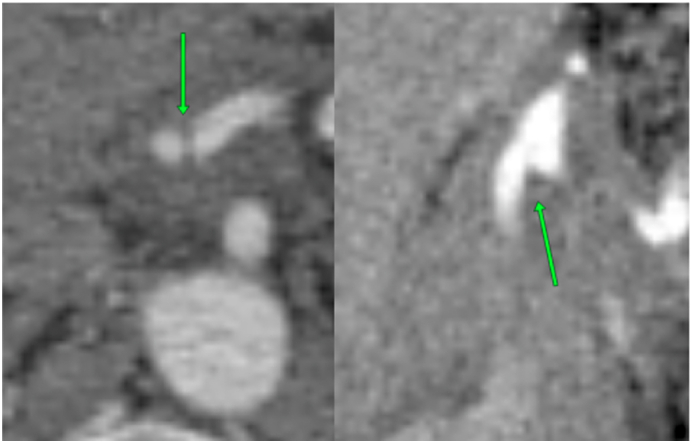
CT (axial and coronal planes): CA bifurcation within tumor.
Fig. 2.
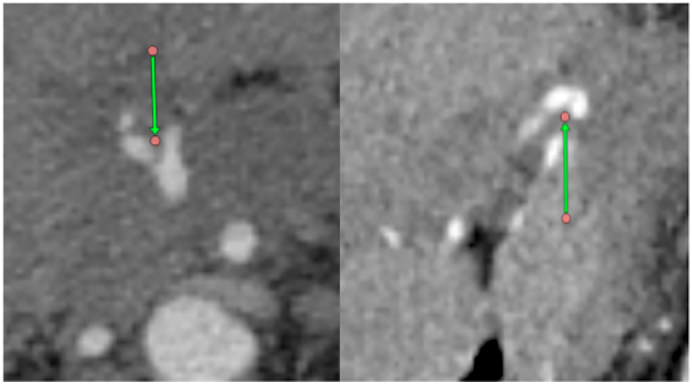
CT (axial and coronal planes): CHA bifurcation within tumor.
Given the overall good condition of the patient, the completion of every possible neoadjuvant treatment modality, the absence of metastatic disease, and the pattern of peripancreatic arterial involvement, a pancreatectomy with arterial resection and reconstruction was planned (by the first author).
A month later (i.e: 3 months from completion of neoadjuvant radiation and 10 months from diagnosis), the patient was laparoscopically explored and absence of peritoneal, or omental seeding was secured. Inraoperative ultrasound ruled out intraparenchymal liver lesions. A midline incision was then made. The tumor involved the body and neck of the pancreas extending to the right of the GDA towards the head and incorporated the CHA, its bifurcation to GDA and PHA, and the GDA. It also involved CA's trifurcation, as well as the CA itself circumferentially sort of 5 mm from its origin. The immediate preaortic space between CA and SMA origins was free of disease, as was the posterior 1800 of the SMA from its origin down. Given the pattern of arterial and parenchymal involvement, the decision was made to proceed with total pancreatectomy, CAR, resection of all the CHA including the first portion of the PHA. Arterial blood supply to the liver would be re-established with a bypass from the aorta to the PHA.
The pancreas, duodenum and spleen were mobilized, the PHA was extensively dissected, as were the PV and SMV above and below the pancreas. The supraceliac aorta was dissected for 6 cm and 2700, so a vascular side clamp could be safely and comfortably applied. The SMA rostral to the lower edge of the pancreas was circumferentially dissected, as was the origin of the CA. The right major saphenous vein was then retrieved and a proximal aorto-saphenous vein anastomosis was performed. The PHA was transected 1 cm from its origin and a distal anastomosis between the vein segment and the PHA followed (Fig. 3). Ultrasonography confirmed excellent liver arterial flow. The CA was then ligated and transected at its origin and the pancreas was dissected off the anterior aspect of the SMA for 3 cm and 2100. Finally, a 3 cm circumferential segment of the SMV-PV around the splenomesenteric junction was resected and a primary venous anastomosis was made. The specimen, including a subtotal gastrectomy, was removed. Dissection around the gastroesophageal junction had been kept to a minimum, so that blood supply to the proximal stomach could be enough to prevent a total gastrectomy. Continuity of the gastrointestinal tract was reestablished with an end-to-side hepaticojejunostomy and an antecolic, isoperistaltic side-to-side gastrojejunostomy 40 cm distal to the former.
Fig. 3.
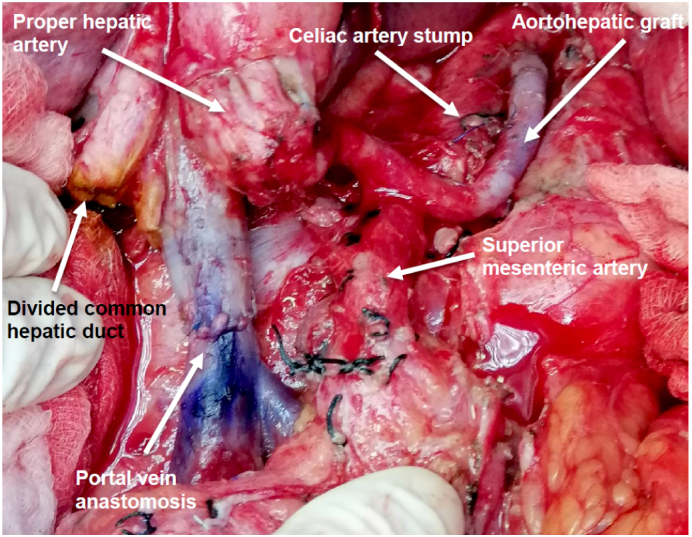
Saphenous vein graft between the supraceliac aorta and the proper hepatic artery.
Estimated blood loss (EBL) was 1800 mL and total operative time (including anesthesia time) was 710 min. The patient tolerated the operation very well, was intraoperatively transfused with 3 units of packed red blood cells, was transferred to the ICU, where she stayed for two days and then moved to the her room. Next day's hemoglobin was 8.9 g/dL and increased to 9.6 g/dL by the day of discharge. Her postoperative course was uneventful, she tolerated full liquids from the third postoperative day, bowel function resumed in the third day, and progressed to general diet in the fifth day. Her abdominal drain was removed and was discharged in the 12th postoperative day in good condition. At discharge LFTs and bilirubin were normal and CT angiogram demonstrated normal patency of the saphenous vein graft. Pathology documented R0 resection (margin>1 mm), ypT1cN0 ductal adenocarcinoma, with near-total pathologic response to neoadjuvant therapy (Tumor Regression Score:1 in Ryan's modified scale 0–3). Two months postoperatively, she has no abdominal symptoms and tolerates general diet being dependent on loperamide and insulin. LFTs are normal. She is fully cognizant that she underwent a very uncommon operation, which had a very good overall outcome and is about to return to her previous level of activity.
3. Discussion
Recently, an increasing number of major peripancreatic arterial resections for locally advanced PC, as defined by the NCCN criteria (Version 1.2020, 11/26/19), especially after NAC, are being reported [14], [20], [21], [22], [23]. Among them, CAR has gained the most interest [1], [2], [10], [13], [15], [18], [24], although, it is still rarely performed, even in well-established pancreatic centers, where the annual number of CARs varies between 0.4 and 3.8 [1], [2], [13], [16]. It is only in the last 7 years that the annual mean number of CARs, in the most prominent of those centers, has increased to 7.3 [10], or 11.3 [15]. CAR is most commonly performed in the context of DP (DP-CAR) for a locally advanced PC of the body of the pancreas involving the CA posteriorly. Since the CHA is resected, hepatic arterial blood supply relies on retrograde flow via the SMA, pancreaticoduodenal, GDA, PHA route, which has to remain intact. However, when the body tumor extends more to the right and involves the CHA bifurcation (to GDA and PHA), the head of the pancreas and its arterial blood supply ensuring retrograde hepatic arterial flow cannot be preserved. Arterial blood can then reach the liver only through an alternative route, which can be provided with a bypass from the aorta to the PHA.
Such cases represent a very slim subgroup in the published experience with CARs in the English literature: Eight appear as case reports [3], [4], [5], [6], [7], [8], [9], and 23 as small subgroups within larger studies [10], [11], [12], [13], [14], summing up 31 patients altogether. Recently, the first attempt at CAR classification was published [15]. It was based on the largest (90 patients) single-institution experience with CAR. The classification depends on the pattern of arterial involvement, resection(s) and reconstruction(s), as well as the extent of pancreatectomy required for a curative operation. Thus, patients in whom the CHA is resected including the GDA/PHA take-off and who require an aorto-PHA bypass are categorized as Class 2 (if the SMA is not involved), or Class 3B (if the SMA also has to be resected and reconstructed). The authors stated that they had 33 Class 2 patients, but they did not specify the number of Class 3B patients. This experience of >33 patients is thus higher than all the rest world experience combined.
Our division has a long-standing dedication to pancreatic surgery and performs >30 pancreas resections annually. We have been performing pancreatectomies with SMV-PV resection and reconstruction for several years [25] for borderline and locally advanced PC and we have moved on to major peripancreatic arterial resections [26]. The case presented is our first TP-CAR with aorto-PHA bypass (Mayo Clinic Class 2B) and, to the best of our knowledge, the first in Greece. Our patient was carefully selected: she had excellent performance status, her tumor and peripancreatic vascular anatomy were scrutinized and had completed all possible neoadjuvant therapy with very good biologic response. Her intraoperative course was smooth and her postoperative course was without complications, such as hemorrhage, gastropathy, delayed gastric emptying, chylous ascites, or failure to thrive. Steatorrhea and diabetes were well controlled.
The limited published experience on perioperative mortality of pancreatectomy, CAR, and aorto-PHA by-pass provides evidence that it is not high, at 0–7 % [1], [2], [3], [4], [5], [6], [8], [9], [10], [11], [12], [13], [15]. EBL may vary from 105 mL to 1250 mL [6], [7], [11], OR time can be long (660–1035 min) [6], [7], [11], and hospitalization extended: 16 [4], 19 [3], 30 [8], 49 [12], 73 [6], or 92 [7] days. Major complications, such as liver infarction [7], chylous ascites [4], [11], and others are common (38–53 %) [13], [14], [15], often necessitating a reoperation [4], [11], [13], [14]. Although our patient had a higher EBL, her operative time, hospital stay and total absence of complications compare favorably to the literature. Concomitant SMV-PV resection and reconstruction, as with our patient, is common (66–73 %) [13], [15] due to the locoregional extent of the tumor.
Various types of conduits have been used for the aorto-PHA by-pass: saphenous vein [4], [6], [11], [14], as in our patient, middle colic artery [3], [8], jejunal artery [7], [12], iliac artery [10], transposed splenic artery [9], cadaveric cryopreserved arterial graft [14], [15], or native femoral artery [15], but there is no comparative evidence among them. With TP-CAR, where the left and right gastric arteries and the gastroepiploic artery are all resected, it seems safer to proceed with total gastrectomy, as is always the case in the largest such series [15]. However, if the dissection around the gastroesophageal junction is kept to a minimum and the lower esophageal vasculature preserved, a subtotal gastrectomy is feasible [4], [9], [11], [12], as in our patient. This gastric remnant preservation may prove nutritionally significant, especially in patients with exocrine insufficiency after TP. Our patient never complained postoperatively of any symptom that might allude to gastric pathology and upper endoscopy was not performed.
With NAC, survival has been reported at 2 [4], [6], [11], 3 [7], [11], or 5 years [8]. Granted these patients are very highly selected and there is an inherent positive bias in their outcome, there is accumulating evidence, from larger cohorts, that survival after major arterial resection may actually reach a little sort of 4 years (44 months), especially in patients with good biologic response to neoadjuvant treatment modalities, i.e. normalization of CA 19–9 preoperatively and “major pathologic response” [15]. This is in distinction to the limited survival of 14, or 18 months when NAC is scarce [14], or not uniform [13] respectively.
In summary, we presented a patient with locally advanced PC of the body of the pancreas involving the CA, CHA and GDA, who underwent TP-CAR with a saphenous vein graft between the supraceliac aorta and the PHA, an operation very rarely performed because of its unique oncologic, anatomic and technical features. Our patient had a curative resection, excellent postoperative course and recovery, and very good pathologic response reflecting the optimal possible outcome in published experience.
Sources of funding
No funding was received for this work.
Ethical approval
Research studies involving patients require ethical approval. Please state whether approval or exemption has been given, name the relevant ethics committee and the state the reference number for their judgement. Please give a statement regarding ethnical approval that will be included in the publication of your article, if the study is exempt from ethnical approval in your institution please state this.
Consent
The patient's written and signed consent was obtained for publication of the case, as well as of all relevant imaging and operative photographs.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Gregory G. Tsiotos: Performed the operation, study concept, wrote the paper.
Nikiforos Ballian: Assisted in the operation, data collection, reviewed and edited paper.
Fotios Milas and Evangelia Peraki: Assisted in the operation.
Georgia Kostopanagiotou: Provided anesthesia and postoperative management
Konstantinos Tsigaridas: Administered neoadjuvant chemotherapy, reviewed and edited paper.
Registration of research studies
|
Guarantor
The Guarantor is the one or more people who accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Gregory G. Tsiotos, M.D.
Declaration of competing interest
None.
References
- 1.Klompmaker S., Peters N.A., van Hilst J., Bassi C., Boggi U., Busch O.R., Niesen W., Van Gulik T., Javed A.A., Kleeff J., Kawai M., Lesurtel M., Lombardo C., Moser A.J., Okada K., Popescu I., Prasad R., Salvia R., Sauvanet A., Sturesson C., Weiss M.J., Zeh H.J., Zureikat A.J., Yamaue H., Wolfgang C.L., Hogg M.E., Besselink M.G., the E-AHPBA DP-CAR study group Outcomes and risk score for distal pancreatectomy with celiac axis resection (DP-CAR): an international multicenter analysis. Ann. Surg. Oncol. 2019;26(3):772–781. doi: 10.1245/s10434-018-07101-0. Published online 2019 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong H., Ma R., Gong J., Cai C., Song Z., Xu B. Distal pancreatectomy with en-block celiac axis resection for locally advanced pancreatic cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2016 Mar;95(10) doi: 10.1097/MD.0000000000003061. Published online 2016 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki H., Hosouchi Y., Sasaki S., Araki K., Kubo N., Watanabe A., Kuwano H. Reconstruction of the hepatic artery with the middle colic artery is feasible in distal pancreatectomy with celiac artery resection: a case report. WorldJ. Gastrointest. Surg. 2013 Jul;5(7):224–228. doi: 10.4240/wjgs.v5.i7.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latona J.A., Lamb K.M., Pucci M.J., et al. Modified Appleby procedure with arterial reconstruction for locally advanced pancreatic body cancer: a literature review and report of three unusual cases. J. Gastrointest. Surg. 2016;20:300–306. doi: 10.1007/s11605-015-3001-2. [DOI] [PubMed] [Google Scholar]
- 5.Glebova N.O., Piazza K.M., Wolfgang C.L., Abularrage C.J. Proper and left hepatic artery bypass for resection of pancreatic mass involving the celiac axis. J. Vasc. Surg. 2017 Mar;65(3):865–866. doi: 10.1016/j.jvs.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Murase Y., Ban D., Maekawa A., Watanabe S., Ishikawa Y., Akahoshi K., Ogawa K., Ono H., Kudo A., Kudo T., Tanaka S., Tanabe M. Successful conversion surgery of distal pancreatectomy with celiac artery resection (DP-CAR) with double arterial reconstrvction using saphenous vein grafting for locally advanced pancreatic cancer: a case report. Surg. Case Rep. 2020 Dec 1;6(1):302. doi: 10.1186/s40792-020-01082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura Y., Imamura M., Itoh T., Yotsuyanagi T., Kawaharada N., Takemasa I. Conversion pancreaticoduodenectomy with dual arterial reconstructions for locally advanced pancreatic cancer: case report and literature rewiew. Int. J. Surg. Case Rep. 2021 Mar;80 doi: 10.1016/j.ijscr.2021.105692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata Y., Uemura K., Kondo N., Sumiyoshi T., Okada K., Seo S., Otsuka H., Murakami Y., Arihiro K., Takahashi S. Long-term survival after distal pancreatectomy with celiac artery resection and hepatic artery reconstruction in the setting of locally advanced unresectable pancreatic cancer. Clin. J. Gastroenterol. 2022 doi: 10.1007/s12328-022-01621-9. Mar 29. [DOI] [PubMed] [Google Scholar]
- 9.Aosasa S., Nishikawa M., Noro T., Yamamoto J. Total pancreatectomy with celiac artery resection and hepatic artery reconstruction using splenic artery autograft interposition. J. Gastrointest. Surg. 2016;20:644–647. doi: 10.1007/s11605-015-2991-0. [DOI] [PubMed] [Google Scholar]
- 10.Schmocker R.K., Wright M.J., Ding D., Beckman M.J., Javed A.A., Cameron J.L., Lafaro K.J., Burns W.R., Weiss M.J., He J., Wolfgang C.L., Burkhart R.A. An aggressive approach to locally confined pancreatic cancer: defining surgical and oncologic outcomes unique to pancreatectomy with celiac artery resection (DP-CAR) Ann. Surg. Oncol. 2021 Jun;28(6):3125–3134. doi: 10.1245/s10434-020-09201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stitzenberg K.B., Watson J.C., Roberts A., Kagan S.A., Cohen S.J., Konski A.A., Hoffman J.P. Survival after pancreatectomy with major arterial resection and reconstruction. Ann. Surg. Oncol. 2008;15(5):1399–1406. doi: 10.1245/s10434-008-9844-y. [DOI] [PubMed] [Google Scholar]
- 12.Amano R., Kimura K., Nakata B., Yamazoe S., Motomura H., Yamamoto A., Tanaka S., Hirakawa K. Pancreatectomy with major arterial resectionafter neoadjuvant chemoradiotherapy gemcitabine and S-1 and concurrent radiotherapy for locally advanced unresectable pancreatic cancer. Surgery. 2015 Jul;158(1):191–200. doi: 10.1016/j.surg.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Addeo P., Guerra M., Bachellier P. Distal pancreatectomy with en-bloc celiac axis resection (DP-CAR) and arterial reconstruction: techniques and outcomes. J. Surg. Oncol. 2021 Jun;123(7):1592–1598. doi: 10.1002/jso.26424. [DOI] [PubMed] [Google Scholar]
- 14.Bockhorn M., Burdelski C., Bogoevski D., Sgourakis G., Yekebas E.F., Izbicki J.R. Arterial en bloc resection for pancreatic carcinoma. Br. J. Surg. 2011;98:86–92. doi: 10.1002/bjs.7270. [DOI] [PubMed] [Google Scholar]
- 15.Truty M.J., Colglazier J.J., Mendes B.C., Nagorney D.M., Bower T.C., Smoot R.L., DeMarino R.R., Cleary S.P., Oderich G.S., Kendrick M.L. En block celiac axis resection for pancreatic cancer: classification of anatomical variants based on tumor extent. J. Am. Coll. Surg. 2020 Jul;231(1):8–29. doi: 10.1016/j.jamcollsurg.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Ocuin L.M., Miller-Ocuin J.L., Novak S.M., Bartlett D.L., Marsh J.W., Tsung A., Lee K.K., Hogg M.E., Zeh H.J., Zureikat A.H. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic tumors: a single institutional assessment of perioperative outcomes and survival. HPB (Oxford) 2016 Oct;18(10):835–842. doi: 10.1016/j.hpb.2016.05.003. Published online 2016 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueda A., Sakai N., Yoshitomi H., Furukawa K., Takayashiki T., Kuboki S., Takano S., Suzuki D., Kagawa S., Mishima T., Nakadai E., Miyazaki M., Ohtsuka M. Is hepatic artery coil embolization useful in distal pancreatectomy with en-block celiac artery resection for locally advanced pancreatic cancer? World J. Surg. Oncol. 2019;17:124. doi: 10.1186/s12957-019-1667-8. Published online 2019 Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T., Hirano S., Noji T., et al. Distal pancreatectomy with en bloc celiac axis resection (modified Appleby procedure) for locally advanced pancreatic body cancer: a single-center review of 80 consecutive patients. Ann. Surg. Oncol. 2016;23(Suppl. 5):969–975. doi: 10.1245/s10434-016-5493-8. [DOI] [PubMed] [Google Scholar]
- 19.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Loos M., Kester T., Klaiber U., Mihaljevic A.L., Mehrabi A., Muller-Stich B.M., Diener M.K., Schneider M.A., Berchtold C., Hinz U., Feisst M., Strobel O., Hackert T., Buchler M.W. Arterial resection in pancreatic cancer surgery: effective after a learning curve. Ann. Surg. 2022 Apr;275(4):759–768. doi: 10.1097/SLA.0000000000004054. [DOI] [PubMed] [Google Scholar]
- 21.Tee M.C., Krajewski A.C., Groeschl R.T., Farnell M.B., Nagorney D.M., Kendrick M.L., Cleary S.P., Smoot R.L., Croome K.P., Truty M.J. Indications and perioperative outcomes for pancreatectomy with arterial resection. J. Am. Coll. Surg. 2018 Aug;227(2):255–269. doi: 10.1016/j.jamcollsurg.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Del Chiaro M., Rangelova E., Halimi A., Ateeb Z., Scandavini C., Valente R., Seversvard R., Arnelo U., Verbeke C.S. Pancreatectomy with arterial resection is superior to palliation with borderline resectable or locally advanced pancreatic cancer. HPB (Oxford) 2019 Feb;21(2):219–225. doi: 10.1016/j.hpb.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Kwon J., Shin S.A., Yoo D., Hong S., Lee J.W., Youn W.Y., Hwang K., Lee S.J., Park G., Park Y., Lee W., Song K.B., Lee J.H., Hwang D.W., Kim S.C. Arterial resection during pancreatectomy for pancreatic ductal adenocarcinoma with arterial resection: a single-center experience with 109 patients. Medicine (Baltimore) 2020 Sep;99(37) doi: 10.1097/MD.0000000000022115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumgartner J.M., Krasinskas A., Daouadi M., et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic adenocarcinoma following neoadjuvant therapy. J. Gastrointest. Surg. 2012;16:1152–1159. doi: 10.1007/s11605-012-1839-0. [DOI] [PubMed] [Google Scholar]
- 25.Tsiotos G.G., Ballian N., Michelakos T., Milas F., Ziogou P., Papaioannou D., Salla C., Athanasiadis I., Razis E., Stavridi F., Psomas M. Portal-Mesenteric vein resection in borderline pancreatic cancer: 33 month-survival in patients with good performance status. J. Pancreat Cancer. 2019 Sep 26;5(1):43–50. doi: 10.1089/pancan.2019.0013. eCollection 2019. PMID: 31559380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsiotos G.G., Ballian N., Milas F., Ziogou P., Athanasiadis I. Distal pancreatectomy with celiac artery resection (DP-CAR): optimal perioperative outcome in a patient with locally advanced pancreas adenocarcinoma. Int. J. Surg. Case Rep. 2020;76:399–403. doi: 10.1016/j.ijscr.2020.09.194. [DOI] [PMC free article] [PubMed] [Google Scholar]


