Abstract
The National Partnership to Improve Dementia Care in Nursing Homes (i.e. the National Partnership) was launched in March 2012. Using national Medicare, Minimum Data Set, and Nursing Home Compare data in CY2010-2014, we examined changes in hospital readmissions for older post-acute skilled nursing facility (SNF) residents with Alzheimer’s disease or related dementias (ADRD) following the National Partnership. Using residents without ADRD as reference group to control for concurrent policy and SNF quality changes, we estimated linear probability models to examine the relationship between readmissions and the National Partnership for residents with ADRD, and also stratified the analysis by quality of SNFs. We found a decreasing trend in hospital readmissions over time. The risk of readmissions in residents with ADRD decreased additional 0.3 percentage-points (p<0.01) after the launch of the National Partnership. This relationship varied across SNFs with different quality, as it was stronger in high-quality compared to low-quality SNFs.
Keywords: nursing homes, antipsychotics, dementia, hospital readmission, the National Partnership to Improve Dementia Care in Nursing Homes
Introduction
In skilled nursing facilities (SNFs), half of post-acute care residents have the diagnosis of Alzheimer’s disease or related dementias (ADRD) (CMS, 2020a). Individuals with ADRD are likely to develop behavioral and psychological symptoms of dementia (BPSD) as the disease advances, such as wandering, agitation, or verbal/physical aggression (Sink, Covinsky, Newcomer, & Yaffe, 2004). BPSD present great challenges for nursing facilities as they may need to invest in staff and resources to properly manage them effectively and, if not well managed, BPSD can result in adverse outcomes, such as injuries, poor quality of life, and increased risks of hospitalizations (Aud, 2004; Hersch & Falzgraf, 2007).
While antipsychotic medications are indicated for treatment of psychosis in serious mental illnesses such as schizophrenia or bipolar disorders, they are often used to manage BPSD among nursing home (NH) residents (Hughes, Lapane, & Mor, 2000; Kamble, Chen, Sherer, & Aparasu, 2008). However, there are serious concerns about potentially inappropriate prescription of antipsychotics to older adults with ADRD, in whom their use has been associated with increased risks of cerebrovascular accidents (CVAs), fractures, and other conditions (Kate, Pawar, Parkar, & Sawant, 2015), as well as increased rate of hospitalizations and mortality (Gill et al., 2007; Johnell et al., 2017). For example, the use of antipsychotics among nursing home residents with ADRD is known to increase the risk of 30-day hospitalizations by 2.4 times (Rochon et al., 2008).
With the concerns regarding the adverse outcomes of antipsychotic use among older adults with ADRD, the Food and Drug Administration issued “black-box warnings” in 2005 and 2008, first for second-generation antipsychotics and then extended to all antipsychotics (FDA, 2005, 2008). However, the prevalence of antipsychotic use among older NH residents has remained high since these warnings were issued: a 2015 report published by Government Accountability Office indicated that one third of NH residents with ADRD were prescribed antipsychotics in 2012 (GAO, 2015).
In March 2012, the Centers for Medicare and Medicaid Services (CMS) collaborated with federal and state agencies, NHs, and other stakeholders and launched the National Partnership to Improve Dementia Care in Nursing Homes (referred to as “the National Partnership”), with the goals to reduce inappropriate antipsychotic use and to promote goal-directed, person-centered dementia care in NHs (CMS, 2020b). The National Partnership implemented multi-dimensional interventions, including public antipsychotic use reporting at the facility level, setting up state-based coalitions, conducting research, and offering training for providers. Specifically, in July 2012, the CMS started to publish each facility’s antipsychotics use rates for all residents on the Nursing Home Compare website, regardless of ADRD status but excluding those diagnosed with schizophrenia, Tourette’s syndrome, or Huntington’s disease. The public reporting on NH antipsychotic use may be one of the most important components of the National Partnership as this measure is relatively clearly defined and uniformly implemented across the country.
There have been significant reductions in the prevalence of antipsychotic use in NHs since the launch of the National Partnership – the prevalence of antipsychotic use among NH residents declined from 23.9% in the fourth quarter of 2011 to 14.0% in the fourth quarter of 2019 (CMS, 2021). However, despite the reduction in the prevalence of antipsychotic use in NHs, there is no empirical evidence on care quality among NH residents with ADRD associated with this reduction. While it is expected that the reduction of antipsychotics may lead to better dementia care and better health outcomes among residents with ADRD, the achievement of such improvement may rely on the provision of alternative approaches to effective dementia care. For example, NHs may need to invest in non-pharmacological interventions to manage behavioral issues among residents with ADRD (Ballard et al., 2015). Otherwise, simply withdrawing antipsychotic use may not achieve the goal of promoting person-centered dementia care and may lead to adverse outcomes. On the other hand, there might be unintended consequences related to the motivation of reducing antipsychotic use. For example, NHs may be motivated to hospitalize residents with ADRD and have their behavioral problems managed in the hospital in order to avoid the prescription of antipsychotics in NHs. Indeed, a recent study found that the launch of the National Partnership was associated with increased risks of hospitalization among NH long-stay residents with ADRD (Wang, Temkin-Greener, Conwell, & Cai, 2020). However, the evidence regarding the impact of National Partnership on health outcomes among post-acute SNF residents is scarce.
To address this gap in the literature, we examined the association between the introduction of the National Partnership and 30-day hospital readmission rates among post-acute SNF residents (i.e. those who receive skilled nursing facility care) with ADRD, using 2010-2014 national data (including Medicare claims, the Minimum Data Set [MDS] 3.0, and Nursing Home Compare data [NHC]). We focused on the measure of 30-day readmissions because it is likely to have negative effects on the health status of frail and older residents in nursing facilities and many of such readmissions are potentially avoidable (Ouslander et al., 2010). Furthermore, we examined the variation in the relationship between the introduction of the National Partnership and 30-day readmission across SNFs with high- versus low-quality, because these SNFs may respond differently to the national Partnership. For example, lower-quality NHs are more likely to be resource-poor facilities and thus less likely to invest in non-pharmacological approaches compared to higher-quality SNFs.
Methods
Data
We employed national data sets that included the MDS 3.0, Medicare Master Beneficiary Summary File (MBSF), Medicare Provider and Analysis Review (MedPAR) file, and NHC for CY 2010-2014. The MDS 3.0 is a comprehensive assessment tool that is required to be conducted for all residents in Medicare- or Medicaid-certified NHs. The assessments contain detailed information on individual health status (e.g. physical and cognitive functional status) at the time of admission, at least every quarter after that if a resident remains in the NH, if there are significant changes in individual’s health status, and at the time of discharge. The MBSF contains information on individual Medicare enrollment status, Medicare-Medicaid dual status, and Medicare Advantage plan enrollment status. MedPAR contains information on inpatient stays, such as the admission and discharge date, International Classification of Diseases version 9 (ICD-9) based diagnosis, diagnosis related groups (DRGs), and the use of intensive care units (ICU).
Cohort
We identified Medicare fee-for-service (FFS) enrollees 65 years of age or older who were newly admitted to free-standing SNFs following an acute inpatient event between January 1, 2011 and June 30, 2014 (we used 2010 data to track their prior NH use and hospitalizations). We refer to the acute inpatient event that led to the qualified SNF stay as “index hospitalization” in this study. Newly admitted residents were identified as those who did not have any NH/SNF stays during the 180 days preceding the identified NH admission. To build a cleaner FFS cohort, individuals who were enrolled in Medicare Advantage plans anytime between 12 months before and 1 month after the identified SNF admission were not included in this study (2,648,741 Medicare FFS beneficiaries were in the cohort). We then excluded individuals who died within 180 days after SNF admission (22.6% of the cohort). We consider persons with life expectancy shorter than 6 month as approaching end-of-life state (for example, 6-month life expectancy is used to determine Medicare eligibility for hospice care)(CMS, 2018a), and these residents may have unique care needs and different patterns of using hospital service. If an individual had multiple qualified new SNF admissions, only the first admission was selected. And we further excluded records with missing values in covariates (2% of remaining sample). Our final analytical sample included 2,011,351 individuals who were newly admitted to 14,707 SNFs for post-acute care.
Variables
The outcome measure was defined as dichotomous, indicating whether a SNF resident was readmitted to hospitals within 30-days of SNF admission (based on Medicare claims). The explanatory variables of interest included the diagnosis of ADRD at SNF admission (dichotomous variable based on checkboxes of Alzheimer’s disease and dementia and the ICD 9 codes in the MDS), an indicator of the initiation of the National Partnership (i.e. before or after March 2012), and an interaction term between these two variables.
We controlled for a comprehensive set of individual characteristics, such as socio-demographics (e.g. age, gender, race, Medicaid coverage), functional status (Activities of Daily Living [ADL] dependency score ranging 0-28 (Morris, Fries, & Morris, 1999)), cognitive status (4-category Cognitive Function Scale [CFS] score (Thomas, Dosa, Wysocki, & Mor, 2017)), diagnoses of selected medical and mental conditions, and other health conditions that may relate to hospital readmission. Furthermore, we accounted for the characteristics of index hospitalizations (e.g. type of index hospitalization [surgical vs medical], DRG weights, length of stay, ICU use), as they can be related to the complexity of individuals’ conditions and affect the readmission risks. We also accounted for whether the resident used any inpatients services within 30, 31-180, or 181-365 days prior to the index hospitalizations with dichotomous indicators.
To examine whether the relationship between the National Partnership and hospital readmission varies with the quality of SNFs, we used the 5-star rating system to determine high and low quality SNFs. The quality star ratings are published by the CMS on its NHC website for all Medicare- or Medicaid- certified NH/SNFs, where a 5-star overall rating indicates the quality much above average and a 1-star overall rating indicates the quality much below average.
Statistical analyses
We first compared the individual characteristics between residents with and without ADRD, before and after the initiation of the National Partnership. We also described the overall trend of 30-day hospital readmission among SNF residents with and without ADRD between 2011 and 2014.
We then estimated a set of linear probability models with facility fixed effects and robust standard errors to explore the relationship between the National Partnership and the risks of readmissions among residents with ADRD, accounting for individual socio-demographic status and health conditions. The linear probability model is commonly used in the field as it approximates the nonlinear model (e.g. logit) and eases the interpretation for interaction terms (Wooldridge, 2010). Residents without ADRD were chosen as the reference group, and this design allowed us to account for changes in SNF quality of care during the study period, as well as the effect from other concurrent policy interventions that may have affected hospital readmissions among SNF residents. Facility fixed effects account for time-invariant characteristics of SNFs, such as the overall quality of care, that can affect both residents with and without ADRD.
The regression model includes the indicator of ADRD, indicator of the National Partnership, and the interaction term between these two variables. The coefficient of ADRD indicator captures the differences in readmissions between residents with and without ADRD at the baseline, and indicator of the National Partnership represents the overall change in readmissions in post-Partnership period. We were mainly interested in the interaction term, which represents the impact of the National Partnership on ADRD residents, accounting for concurrent changes among residents without ADRD within a facility.
We further performed stratified analyses by quality of SNFs, as SNFs with different quality may respond to the National Partnership differently. We focused on SNFs with 5-star rating and SNFs with 1-star rating as ratings of a SNF may fluctuate and 5-star SNFs are more likely to be high quality SNFs, and 1-star ones are more likely to be low quality SNFs.
Lastly, we performed a set of sensitivity analyses to check the robustness of the findings. First, we repeated our analyses with a more restricted sample by only including those who survived 365 days after SNF admission, and a broader sample that included those who died within 180 days of SNF admission. Furthermore, we used a different cut-off date to define the initiation of the National Partnership. While the National Partnership was launched in March 2012, the public reporting of NH antipsychotic use was implemented on July 1st, 2012.
Therefore, considering the potentially important effect of the public reporting, we defined the alternative post-partnership period starting at July 2012, and repeated the main analyses. Lastly, to reduce the ambiguity of the policy initiation time, we excluded SNF residents who were admitted between April and June 2012 and repeated the main analyses.
Results
The overall prevalence of ADRD was 22.5% among the newly admitted SNF residents. Table 1 and appendix Table 1 presents characteristics of residents with and without ADRD at the time of SNF admission, before and after the launch of the National Partnership. Compared to residents without ADRD, those with ADRD were older, more likely to be black, more likely to be dually enrolled in Medicare and Medicaid, and more physically and cognitively impaired. The distribution of chronic conditions was mixed between the two groups: for example, residents with ADRD had higher prevalence of mental conditions (e.g. anxiety, depression, bipolar disorder, schizophrenia, and psychotic disorders), stroke, or pneumonia as compared to those without ADRD, but had lower prevalence of diabetes and asthma/chronic obstructive pulmonary disease (COPD).
Table 1.
Individual characteristics for ADRD and non-ADRD residents, before and after the launch of the National Partnership
| Residents with ADRD | Residents without ADRD | |||
|---|---|---|---|---|
| Variable | Pre (n=174,319) |
Post (n=277,576) |
Pre (n= 549,870) |
Post (n= 1,009,586) |
| Rehospitalization | ||||
| 30-day readmission from NHs | 11.3% | 9.8% | 11.3% | 10.1% |
| Socio-demographics | ||||
| Race | ||||
| white | 84.0% | 82.6% | 86.5% | 85.2% |
| black | 8.7% | 8.8% | 6.6% | 6.7% |
| other | 7.3% | 8.6% | 6.9% | 8.1% |
| Age | 83.90 | 83.80 | 80.03 | 79.66 |
| Male | 32.1% | 33.1% | 31.9% | 33.6% |
| Medicare-Medicaid dual status | 24.9% | 24.1% | 18.8% | 17.3% |
| Functional Status | ||||
| ADL | 17.81 | 18.04 | 16.03 | 16.38 |
| CFS | ||||
| intact | 20.6% | 21.5% | 71.5% | 73.4% |
| mild | 29.2% | 29.0% | 19.9% | 18.7% |
| moderate | 41.7% | 41.9% | 5.9% | 5.6% |
| severe | 6.8% | 6.4% | 0.8% | 0.7% |
| missing | 1.7% | 1.3% | 2.0% | 1.5% |
| Major Diagnoses | ||||
| cancer | ||||
| Yes | 5.2% | 5.2% | 6.7% | 6.9% |
| No | 86.2% | 86.3% | 75.6% | 75.8% |
| missing | 8.6% | 8.4% | 17.7% | 17.3% |
| CAD | ||||
| Yes | 23.6% | 23.3% | 22.0% | 21.8% |
| No | 67.8% | 68.3% | 60.3% | 60.9% |
| missing | 8.6% | 8.4% | 17.7% | 17.3% |
| Heart failure | 18.5% | 17.2% | 20.0% | 18.9% |
| Hypertension | 79.1% | 79.0% | 79.3% | 79.1% |
| Diabetes | 27.4% | 27.0% | 32.1% | 31.8% |
| Asthma/COPD | 18.3% | 17.5% | 23.4% | 22.6% |
| PVD | ||||
| Yes | 5.1% | 6.1% | 5.7% | 7.1% |
| No | 86.4% | 93.9% | 76.7% | 92.9% |
| missing | 8.5% | 17.6% | ||
| Pneumonia | 12.1% | 11.5% | 10.4% | 9.8% |
| UTI | 28.6% | 26.6% | 17.9% | 16.1% |
| Stroke | 15.3% | 14.1% | 10.2% | 9.3% |
| Anxiety | 21.2% | 22.1% | 16.2% | 16.9% |
| Depression | 39.3% | 38.0% | 25.8% | 24.8% |
| Bipolar disorder | 1.9% | 2.1% | 1.2% | 1.3% |
| Psychotic disorder | 7.7% | 8.3% | 1.1% | 1.1% |
| Schizophrenia | 1.3% | 1.4% | 0.7% | 0.8% |
| Anemia | 25.5% | 24.7% | 27.6% | 27.0% |
Note: ADRD (Alzheimer’s disease or related dementias); NH (nursing home); ADL (activities of daily living); CFS (cognitive function scale); CAD (Coronary artery disease); COPD (Chronic obstructive pulmonary disease); PVD (Peripheral Vascular Disease); UTI (urinary tract infection); DRG (diagnosis related group); ICU (intensive care unit); IV (intravenous)
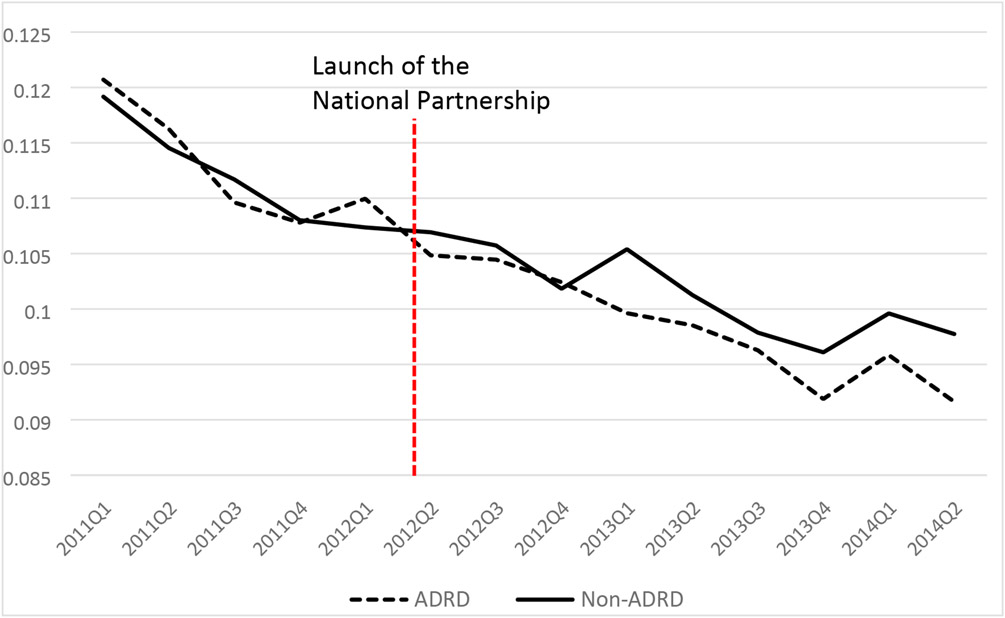
Figure 1 illustrates the trends of unadjusted 30-day hospital readmission rates for residents with and without ADRD over time. There was a declining trend in hospital readmissions for both groups over time. Readmission rates declined from 12.2% in 2011Q1 to 9.1% in 2014Q2 for residents with ADRD and from 12.0% to 9.8% among residents without ADRD after the launch of the National Partnership.
Figure 1.
30-day NH originated hospitalization readmission among residents with and without ADRD
Table 2 shows the main findings from the linear probability model with facility fixed effects and robust standard errors, accounting for all other covariates (full results in Appendix Table 2). On average, residents with ADRD had a 1.6 percentage-point lower likelihood (i.e. −0.016 with P<0.001) of being readmitted to hospitals within 30-days of SNF admission prior to the launch of the National Partnership, compared to those without ADRD. There was a decreasing trend in 30-day readmissions among all SNF residents (i.e. −0.006 with P<0.001) after the launch of the National Partnership. The post-Partnership reduction in 30-day readmissions appeared to be greater among residents with ADRD compared to residents without ADRD – residents with ADRD had an additional 0.3 percentage-point decrease in 30-day readmission rates after the launch of the National Partnership (the coefficient of the interaction term was −0.003 with P=0.005).
Table 2.
Main findings from linear probability models with facility fixed effects and robust standard errors, after adjusting for individual covariates
| Full sample (n=2,011,351) |
5-star SNFs (n=493,699) |
1-star SNFs (n=195,755) |
|
|---|---|---|---|
| ADRD | −0.016*** (.001) |
−0.011*** (.002) |
−0.012*** (.003) |
| Post-Partnership | −0.006*** (.001) |
−0.006*** (.002) |
−0.004 (.003) |
| ADRD post-Partnership | −0.003*** (.001) |
−0.010*** (.003) |
−0.005 (.003) |
Note: ADRD (Alzheimer’s disease or related dementias)
Standard errors in the parentheses
Difference statistically significant at the 1% level
In addition to the main analysis that included all SNFs, we then examined the relationship between the National Partnership and readmissions in 5-star and 1-star SNFs separately. We observed decreasing trends in the prevalence of antipsychotic use in both 5- and 1-star SNFs. Specifically, based on antipsychotic use rates published on NHC website, the prevalence of antipsychotics in nursing facilities decreased from 21.9% to 17.3% in 5-star NHs, and from 25.2% to 20.9% in 1-star NHs between 2011 and 2014. Despite the similar decline in the prevalence of antipsychotics use in these two types of nursing facilities, the relationship between the National Partnership and 30-day hospital readmission appeared to vary, as presented in Table 2. Specifically, among high quality SNFs (i.e. 5-star SNFs), in addition to the overall reduction in 30-day readmissions after the launch of the National Partnership (i.e. 0.6 percentage point reduction, P=0.006), there was an additional 1.0 percentage point reduction in readmissions for residents with ADRD (i.e. −0.010 with P<0.001). However, such relationship was not detected in 1-star SNFs – the interaction term was negative but was not statistically significant (i.e. −0.005 with P=0.13).
The findings from the sensitivity analyses (Table 3, full results in Appendix Table 3) were consistent with the main findings. The change of study cohort to those who survived 365 days or by including those who died did not change the main findings. The analyses with alternative pre- and post-Partnership periods, one with the post- period starting at July 2012 and the other excluding admissions between April and June 2012, were also consistent with the main findings.
Table 3.
Sensitivity Analyses on the association between the National Partnership and hospital readmission among ADRD residents, with restriction in the sample or alternative time thresholds
| Residents survived 365 days (n=1,529,803) |
All residents including those died (n=2,588,995) |
Post start at 2012Q3 (n=2,011,351) |
Exclude 2012Q2 (n=1,866,937) |
|
|---|---|---|---|---|
| ADRD | −0.018*** (0.001) |
−0.020*** (0.001) |
−0.017*** (0.001) |
−0.016*** (0.001) |
| Post-Partnership | −0.006*** (0.001) |
−0.007 (0.001) |
−0.003*** (0.001) |
−0.006*** (0.001) |
| ADRD * post-Partnership | −0.002* (0.001) |
−0.004 (0.001) |
−0.003*** (0.001) |
−0.003*** (0.001) |
Note: ADRD (Alzheimer’s disease or related dementias)
Standard errors in the parentheses
All models with facility fixed effects and robust standard errors
Difference statistically significant at the 10% level
Difference statistically significant at the 5% level
Difference statistically significant at the 1% level
Discussion
In this study we examined the association between the National Partnership and 30-day hospital readmissions among newly admitted post-acute SNF residents with ADRD. We found that the risk of hospital readmissions in residents with ADRD decreased after the launch of the National Partnership, adjusting for individual covariates, facility fixed effects, and concurrent changes in 30-day readmissions among all SNF residents. The relationship between the National Partnership and reduced risks of readmission appeared to be more robust in high-quality than in low-quality SNFs.
One step further from published studies focusing on the reductions in NH antipsychotic use following the launch of the National Partnership (Bowblis, Lucas, & Brunt, 2015; Gurwitz, Bonner, & Berwick, 2017), we examined the change in an important health outcome, i.e. hospital readmission, associated with the launch of this policy intervention. We did not find evidence that the National Partnership increased the readmission rates among older adults with ADRD who were newly admitted to SNFs for post-acute care. Instead, in addition to the decrease in hospital readmissions for all post-acute care SNF residents after the start of the National Partnership, we found an additional reduction in hospital readmissions among SNF residents with ADRD. While the effect size is modest, the avoided readmissions could still be meaningful as they can potentially reduce negative health outcomes related to hospitalizations, such as further deterioration of functional status or impaired quality of life (Phelan, Borson, Grothaus, Balch, & Larson, 2012).
However, the effect of the National Partnership on hospital readmissions varied with the overall NH quality. We detected a stronger and more robust effect of the National Partnership on hospital readmissions among residents with ADRD in highest-quality SNFs (e.g. 1 percentage point decrease in 5-star SNFs, which is 3 times the overall effect). Although we observed similar declines in the prevalence of antipsychotic use in both 5-star and 1-star SNFs, the National Partnership was not clearly associated with significant reductions in hospital readmissions among SNF residents with ADRD in 1-star SNFs. These findings suggest that while the reduction of antipsychotic use in nursing facilities may lead to decreased hospital readmissions, such a relationship may not hold in all facilities. Interestingly, a recent study found increased hospitalization risks among NH long-stay residents with ADRD after the National Partnership also reported the unequal effect of the National Partnership among NHs: residents with ADRD who residing in facilities with high antipsychotic use rate prior to the National Partnership experienced greater increase in hospitalization risks after the launch of National Partnership, compared to those resided in facilities with low prior antipsychotic use (Wang et al., 2020). While our findings are not directly comparable to this above study as the study populations are different (post-acute SNF residents v.s. NH long-stayer residents) and outcomes are different (30-day readmission v.s. any hospitalization within 180 days), our analyses about the different effect among higher- and lower-quality nursing homes support the finding of this recent study.
While this study did not further examine the factors that contribute to the different impact of the National Partnership in high- and low-quality SNFs, this difference may relate to the level of resources that facilities have. Antipsychotics are often referred to as “chemical restraints” and are used in NHs to manage residents’ behavioral issues and to relieve the stress and burden of care on NH staff (Hughes, Lapane, & Mor, 2000; Kamble, Chen, Sherer, & Aparasu, 2008). To avoid the use of antipsychotics and to improve the quality of dementia care, NHs need to adopt non-pharmacological approaches, such as offering behavioral therapy and validation therapy, and preparing the environment that causes less confusion for ADRD patients, which are generally recommended to manage behavioral problems among residents with ADRD (Douglas, James, & Ballard, 2004; Gitlin, Kales, & Lyketsos, 2012). However, the adoption of these approaches can be costly. For example, NHs may need to invest in staffing, provide training on dementia care, or even modify the physical environment or infrastructure. While high quality NHs may have more resources (Grabowski et al., 2016) and higher staffing levels (Spilsbury, Hewitt, Stirk, & Bowman, 2011) that allow them to adopt recommended non-pharmacological approaches to improve their dementia care under the National Partnership, low quality NHs (e.g. 1-star NHs) may not be able to afford doing so. Low-quality NHs generally have fewer financial resources and lower staffing levels (Grabowski et al., 2016; Park & Werner, 2011; Spilsbury et al., 2011), and thus may not be able to invest in dementia care, such as allocating extra staff time to monitor residents’ behavior or their care needs. Indeed, we found that 1-star SNFs generally were more likely to have more Medicaid residents and more likely to be for-profit facilities compared to 5-star SNFs. While these low-quality SNFs may be able to simply reduce the use of antipsychotics to achieve the goal set by the CMS, they may not be able to or may not be motivated enough to consistently adopt safer but costly non-pharmacological approaches to improve dementia care, and therefore may not experience the improved patient outcome as the high quality NHs do.
During our study period, there were concurrent policy changes aimed at reducing hospital readmissions, such as the Hospital Readmission Reduction Program (HRRP)(CMS, 2018b) or the INTERACT quality improvement program (Ouslander et al., 2011). However, these policy interventions targeted the general population and may have affected readmissions for all SNF residents, while the National Partnership was more likely to have had a direct effect on residents with ADRD. By accounting for the change in hospital readmissions among residents without ADRD, our analytical strategy largely obviated any concern about concurrent policy changes occurring during the study period. We acknowledge that there is a possibility that the National Partnership had an indirect impact on the outcome of residents without ADRD, e.g. through improved infrastructure or safer SNF physical environment. If this was the case, we would have underestimated the potential effects of the National Partnership among residents with ADRD.
Our study has several limitations. First, we were not able to investigate whether there were changes in procedure or process of dementia care in NHs other than the reduction in antipsychotic use, and the extent to which these other procedures or processes may have contributed to readmission decline. In addition, the National Partnership included multi-dimensional interventions, and we were not able to disentangle the components of the National Partnership that contributed to the reduction of hospital readmissions among ADRD residents. Second, the National Partnership as well as its main component, public reporting of antipsychotics, was implemented nationwide. Thus, we could not find a comparison group that was not subject to this policy change. However, our analytical strategy of using NH residents without ADRD as reference group somewhat alleviated this concern, as residents without ADRD were not likely be directly affected by the National Partnership, which specifically targeted ADRD patients. Third, we excluded SNF residents who died within 180 days of SNF admission as people approaching the end of life are likely to have different needs than post-acute SNF residents in general. By doing so, however, this study was not able to detect the change in mortality after the launch of the National Partnership. Fourth, for the outcome 30-day readmission, we included all-cause hospitalizations and did not specify the reasons for the readmission. While the measure we used is consistent with the CMS’ quality measure, examining the reason for readmission will help gain understanding about the role of the National Partnership and will be important for future studies. And lastly, the prevalence of ADRD (22.5%) in our cohort is lower than the reported prevalence among general post-acute SNF residents (51%, (CMS, 2020a), possibly because we excluded people who had any NH use within 180 days prior to the target SNF admission and the ADRD prevalence is likely to be higher among these repeated NH users. Given that we only included individuals who were newly admitted to SNFs, our results may not be generalized to all post-acute SNF residents.
Our findings have important policy implications for future legislative interventions to improve dementia care in NHs. Although the National Partnership is a multi-dimensional intervention, the direct measurable outcome for the intervention is the reduction of the prevalence in antipsychotic use. However, the extent to which NHs reduce antipsychotic use does not necessarily indicate the improvement in dementia care and patient outcomes. Incorporating more outcome-orientated measures, such as hospital readmission rate, into the policy goals and the public reporting could better align NHs’ interests with CMS’s mission to improve patient outcomes through better dementia care. In addition, appropriate incentives may be needed, especially for NHs with different level of resources or baseline quality.
In conclusion, our study found that the National Partnership was associated with a decrease in the risk of hospital readmission among SNF post-acute residents with ADRD, but this relationship was detected only among high-quality SNFs when the analysis is stratified by SNF quality rating. Given the high prevalence of ADRD patients in SNFs and the remaining concerns about the quality of dementia care in this care setting, future research is needed to understand and develop effective and efficient interventions to improve dementia care in nursing facilities.
What this paper adds:
Our study found that the risk of hospital readmission in skilled nursing facility (SNF) residents with Alzheimer’s disease or related dementias (ADRD) has decreased after the launch of the National Partnership compared to residents without ADRD.
The association between the National Partnership and decreased hospital readmission among SNF residents with ADRD was stronger in high-quality SNFs and was statistically insignificant in low-quality SNFs.
Applications of study findings:
As the direct measurable outcome of the National Partnership, e.g., antipsychotic use in nursing facilities, does not necessarily indicate the improvement in dementia care and patient outcomes, incorporating more outcome-orientated measures into policy goals is necessary.
Interventions to improve care in nursing facilities, such as the National Partnership, may have uneven impact on SNFs of different qualities and may exacerbate disparities among SNFs. Stakeholders need to consider how facilities with lower quality at the baseline can benefit more from these interventions.
Acknowledgments
This study has been funded by the National Institute on Aging Grant R01AG052451 (PI: Cai).
This study has been approved by University of Rochester IRB (STUDY ID: STUDY00001025).
Appendix
Appendix Table 1.
Individual characteristics for ADRD and non-ADRD residents, before and after the launch of the National Partnership
| Residents with ADRD | Residents without ADRD | |||
|---|---|---|---|---|
| Variable | Pre (n=174,319) |
Post (n=277,576) |
Pre (n= 549,870) |
Post (n= 1,009,586) |
| Rehospitalization | ||||
| 30-day readmission from NHs | 11.3% | 9.8% | 11.3% | 10.1% |
| Socio-demographics | ||||
| Race | ||||
| white | 84.0% | 82.6% | 86.5% | 85.2% |
| black | 8.7% | 8.8% | 6.6% | 6.7% |
| other | 7.3% | 8.6% | 6.9% | 8.1% |
| Age | 83.90 | 83.80 | 80.03 | 79.66 |
| Male | 32.1% | 33.1% | 31.9% | 33.6% |
| Medicare-Medicaid dual status | 24.9% | 24.1% | 18.8% | 17.3% |
| Functional Status | ||||
| ADL | 17.81 | 18.04 | 16.03 | 16.38 |
| CFS | ||||
| intact | 20.6% | 21.5% | 71.5% | 73.4% |
| mild | 29.2% | 29.0% | 19.9% | 18.7% |
| moderate | 41.7% | 41.9% | 5.9% | 5.6% |
| severe | 6.8% | 6.4% | 0.8% | 0.7% |
| missing | 1.7% | 1.3% | 2.0% | 1.5% |
| Aggressive behavior | 18.1% | 17.3% | 0.1% | 0.1% |
| Major Diagnoses | ||||
| cancer | ||||
| Yes | 5.2% | 5.2% | 6.7% | 6.9% |
| No | 86.2% | 86.3% | 75.6% | 75.8% |
| missing | 8.6% | 8.4% | 17.7% | 17.3% |
| CAD | ||||
| Yes | 23.6% | 23.3% | 22.0% | 21.8% |
| No | 67.8% | 68.3% | 60.3% | 60.9% |
| missing | 8.6% | 8.4% | 17.7% | 17.3% |
| Heart failure | 18.5% | 17.2% | 20.0% | 18.9% |
| Hypertension | 79.1% | 79.0% | 79.3% | 79.1% |
| Diabetes | 27.4% | 27.0% | 32.1% | 31.8% |
| Asthma/COPD | 18.3% | 17.5% | 23.4% | 22.6% |
| PVD | ||||
| Yes | 5.1% | 6.1% | 5.7% | 7.1% |
| No | 86.4% | 93.9% | 76.7% | 92.9% |
| missing | 8.5% | 17.6% | ||
| Pneumonia | 12.1% | 11.5% | 10.4% | 9.8% |
| Septicemia | 1.9% | 2.0% | 1.8% | 1.8% |
| UTI | 28.6% | 26.6% | 17.9% | 16.1% |
| Wound infect | 1.9% | 1.6% | 3.2% | 2.6% |
| Hip fracture | 10.8% | 11.4% | 8.9% | 8.8% |
| Other fracture | 11.3% | 11.5% | 11.3% | 11.3% |
| Stroke | 15.3% | 14.1% | 10.2% | 9.3% |
| Respiratory failure | 1.4% | 1.7% | 2.2% | 2.7% |
| Anxiety | 21.2% | 22.1% | 16.2% | 16.9% |
| Depression | 39.3% | 38.0% | 25.8% | 24.8% |
| Bipolar disorder | 1.9% | 2.1% | 1.2% | 1.3% |
| Psychotic disorder | 7.7% | 8.3% | 1.1% | 1.1% |
| Schizophrenia | 1.3% | 1.4% | 0.7% | 0.8% |
| Anemia | 25.5% | 24.7% | 27.6% | 27.0% |
| Ulcerative Colitis/Crohn’s disease | ||||
| Yes | 0.8% | 0.7% | 0.9% | 0.9% |
| No | 78.7% | 84.6% | 71.6% | 76.7% |
| Missing | 20.5% | 14.7% | 27.5% | 22.4% |
| Viral hepatitis | 0.1% | 0.1% | 0.2% | 0.2% |
| Seizure disorder or epilepsy | 4.7% | 4.8% | 2.9% | 2.9% |
| Index Hospitalization | ||||
| DRG weights | 1.42 | 1.47 | 1.96 | 2.04 |
| Length of stay | ||||
| ≤ 3 days | 25.2% | 26.1% | 29.1% | 30.8% |
| Between 4 and 7 days | 48.4% | 48.2% | 43.4% | 42.5% |
| ≥ 8 days | 26.3% | 25.7% | 27.5% | 26.7% |
| ICU | 22.7% | 23.5% | 27.1% | 27.8% |
| Prior hospitalization 30day | 12.3% | 11.5% | 13.6% | 12.8% |
| Prior hospitalization 31-180day | 16.7% | 15.2% | 17.0% | 15.6% |
| Prior hospitalization 181-365day | 18.1% | 15.9% | 17.6% | 15.5% |
| Surgical index hospitalization | 22.3% | 23.8% | 47.3% | 49.5% |
| MDS-based Other Conditions | ||||
| End-stage prognosis | 0.5% | 0.4% | 0.4% | 0.3% |
| fall_30day | ||||
| Yes | 47.2% | 48.4% | 34.5% | 34.8% |
| No | 43.1% | 43.5% | 59.1% | 60.2% |
| Unable to answer | 7.2% | 6.5% | 3.8% | 3.4% |
| missing | 2.5% | 1.5% | 2.6% | 1.5% |
| fall_31_180day | ||||
| Yes | 18.5% | 20.0% | 12.3% | 13.3% |
| No | 59.8% | 61.5% | 75.1% | 76.5% |
| Unable to answer | 18.1% | 16.2% | 9.3% | 8.1% |
| missing | 3.5% | 2.2% | 3.3% | 2.1% |
| Venous/Arterial ulcer present | 1.4% | 1.3% | 2.0% | 1.8% |
| Infection of the foot | 0.8% | 0.7% | 1.1% | 1.0% |
| Diabetic foot ulcer | 0.3% | 0.3% | 0.6% | 0.6% |
| Dehydrate | 0.4% | 0.4% | 0.3% | 0.2% |
| Internal bleeding | 0.9% | 0.8% | 0.9% | 0.8% |
| Pain Frequency | ||||
| 0 No pain | 62.1% | 63.3% | 36.0% | 37.0% |
| 1 Almost constant | 3.4% | 2.7% | 6.9% | 5.7% |
| 2 Frequently | 11.6% | 10.1% | 25.9% | 23.4% |
| 3 Occasionally | 18.0% | 19.0% | 26.7% | 29.4% |
| 4 Rarely | 3.9% | 3.8% | 4.3% | 4.3% |
| Unable to answer | 1.0% | 1.1% | 0.2% | 0.2% |
| Surgical wound | 18.9% | 20.7% | 42.4% | 44.9% |
| Bowel continent | ||||
| 1 Always continent | 48.9% | 46.8% | 74.9% | 72.8% |
| 2 occasionally incontinent | 13.3% | 13.7% | 8.8% | 9.6% |
| 3 frequently incontinent | 18.8% | 21.5% | 9.0% | 10.4% |
| 4 always incontinent | 17.7% | 16.6% | 5.4% | 5.1% |
| 9 not rated | 1.3% | 1.4% | 1.9% | 2.1% |
| Shortness of breath with exertion | 10.7% | 10.3% | 15.9% | 15.2% |
| Shortness of breath - sitting | 3.2% | 2.9% | 4.8% | 4.4% |
| Shortness of breath - lying | 5.4% | 5.6% | 7.9% | 8.1% |
| Parenteral/IV feeding | 1.4% | 0.8% | 1.2% | 0.7% |
| Feeding tube | 2.3% | 2.3% | 2.1% | 2.1% |
| Ostomy care | 1.1% | 1.1% | 2.0% | 2.1% |
| Antibiotic received | ||||
| Yes | 29.1% | 37.2% | 25.9% | 35.0% |
| No | 50.4% | 62.8% | 46.6% | 65.0% |
| Missing | 20.5% | 27.5% | ||
| Insulin injection | 14.9% | 14.1% | 19.3% | 18.2% |
Note: ADRD (Alzheimer’s disease or related dementias); NH (nursing home); ADL (activities of daily living); CFS (cognitive function scale); ABS (aggressive behavior scale); CAD (Coronary artery disease); COPD (Chronic obstructive pulmonary disease); PVD (Peripheral Vascular Disease); UTI (urinary tract infection); DRG (diagnosis related group); ICU (intensive care unit); IV (intravenous)
Appendix Table 2.
Main findings (full), with facility fixed effect and robust standard error
| VARIABLES | Full sample | 5-star SNFs | 1-star SNFs |
|---|---|---|---|
| ADRD = 1 | −0.0163*** (0.000955) |
−0.0105*** (0.00224) |
−0.0125*** (0.00268) |
| post = 1 | −0.00644*** (0.00101) |
−0.00606*** (0.00219) |
−0.00357 (0.00319) |
| ADRD * post | −0.00312*** (0.00108) |
−0.0102*** (0.00251) |
−0.00501 (0.00327) |
| Demographics | |||
| Age | −0.000631*** (3.11e-05) |
−0.000506*** (6.04e-05) |
−0.000814*** (9.98e-05) |
| Male | 0.00711*** (0.000465) |
0.00578*** (0.000891) |
0.0101*** (0.00151) |
| Race = black | 0.0104*** (0.00103) |
0.00869*** (0.00218) |
0.0131*** (0.00298) |
| Race = other | 0.00176* (0.000949) |
−0.000244 (0.00178) |
0.00659** (0.00311) |
| Dual status | −1.58e-05 (0.000616) |
0.000907 (0.00131) |
−0.00356** (0.00177) |
| Functional/cognitive/behavioral Status | |||
| CFS = 2 | 0.0356*** (0.000700) |
0.0384*** (0.00148) |
0.0311*** (0.00187) |
| CFS = 3 | 0.0256*** (0.000857) |
0.0240*** (0.00179) |
0.0252*** (0.00253) |
| CFS = 4 | 0.0340*** (0.00196) |
0.0290*** (0.00437) |
0.0391*** (0.00558) |
| CFS, missing | 0.231*** (0.00356) |
0.256*** (0.00799) |
0.191*** (0.00830) |
| ADL | 0.00544*** (6.69e-05) |
0.00570*** (0.000148) |
0.00542*** (0.000193) |
| Chronic Conditions | |||
| Cancer = Yes | 0.0104*** (0.000892) |
0.0104*** (0.00174) |
0.00629** (0.00295) |
| Cancer = missing | −0.0605*** (0.0160) |
−0.0441* (0.0260) |
−0.0987*** (0.0250) |
| CAD = Yes | 0.00415*** (0.000557) |
0.00357*** (0.00112) |
0.00811*** (0.00181) |
| CAD = missing | −0.202*** (0.0168) |
−0.205*** (0.0274) |
−0.177*** (0.0278) |
| Heart failure | 0.0300*** (0.000630) |
0.0307*** (0.00130) |
0.0299*** (0.00208) |
| Hypertension | 0.0124*** (0.000506) |
0.0135*** (0.000969) |
0.0124*** (0.00170) |
| Diabetes | 0.00775*** (0.000602) |
0.00519*** (0.00116) |
0.00906*** (0.00202) |
| Asthma/COPD | 0.00759*** (0.000578) |
0.00721*** (0.00116) |
0.00856*** (0.00189) |
| PVD = Yes | 0.0185*** (0.000975) |
0.0190*** (0.00193) |
0.0181*** (0.00316) |
| PVD = missing | 0.145*** (0.00686) |
0.135*** (0.0153) |
0.134*** (0.0177) |
| Pneumonia | 0.0323*** (0.000872) |
0.0293*** (0.00175) |
0.0341*** (0.00277) |
| Septicemia | 0.0435*** (0.00195) |
0.0402*** (0.00420) |
0.0389*** (0.00605) |
| UTI | 0.0283*** (0.000649) |
0.0277*** (0.00131) |
0.0283*** (0.00202) |
| Wound infect | 0.0689*** (0.00169) |
0.0691*** (0.00369) |
0.0633*** (0.00517) |
| Hip fracture | 0.0129*** (0.000867) |
0.0100*** (0.00162) |
0.0139*** (0.00296) |
| Other fracture | 0.0140*** (0.000708) |
0.0134*** (0.00141) |
0.0108*** (0.00241) |
| Stroke | 0.00679*** (0.000740) |
0.00858*** (0.00159) |
0.00338 (0.00232) |
| Anxiety | 0.00977*** (0.000584) |
0.00941*** (0.00117) |
0.0126*** (0.00193) |
| Depression | 0.00187*** (0.000500) |
0.00324*** (0.000964) |
0.00339* (0.00174) |
| Bipolar disorder | 0.00751*** (0.00181) |
0.00613 (0.00396) |
0.0112** (0.00556) |
| Psychotic disorder | 0.0118*** (0.00148) |
0.0110*** (0.00320) |
0.0197*** (0.00417) |
| Schizophrenia | −0.00501** (0.00237) |
−0.00992* (0.00557) |
−0.00335 (0.00612) |
| Respiratory failure | 0.0123*** (0.00183) |
0.00972*** (0.00376) |
0.0115** (0.00571) |
| Anemia | 0.00734*** (0.000494) |
0.00784*** (0.000973) |
0.00693*** (0.00172) |
| Ulcerative Colitis/Crohn’s disease = 1 | 0.00859*** (0.00218) |
0.0158*** (0.00441) |
−0.00478 (0.00700) |
| Ulcerative Colitis/Crohn’s disease = missing | 0.384*** (0.00712) |
0.369*** (0.0146) |
0.403*** (0.0143) |
| Viral hepatitis | 0.0115** (0.00513) |
0.0198* (0.0107) |
−0.00326 (0.0149) |
| Seizure disorder or epilepsy | 0.000686 (0.00121) |
0.00158 (0.00260) |
−0.00399 (0.00371) |
| Index Hospitalization | |||
| DRG Weights | 0.00405*** (0.000241) |
0.00392*** (0.000494) |
0.00380*** (0.000783) |
| Hospital LOS: less than 3 days | −0.0111*** (0.000474) |
−0.0121*** (0.000916) |
−0.0103*** (0.00161) |
| Hospital LOS: more than 8 days | 0.0293*** (0.000613) |
0.0291*** (0.00127) |
0.0290*** (0.00191) |
| ICU use | 0.0122*** (0.000569) |
0.0119*** (0.00115) |
0.0129*** (0.00187) |
| Prior hosp_30 | 0.0321*** (0.000746) |
0.0324*** (0.00157) |
0.0337*** (0.00230) |
| Prior hosp_180 | 0.0152*** (0.000638) |
0.0144*** (0.00132) |
0.0197*** (0.00200) |
| Prior hosp_365 | 0.00949*** (0.000616) |
0.00993*** (0.00122) |
0.00634*** (0.00188) |
| Surgical DRG | 0.000524 (0.000900) |
0.000266 (0.00177) |
0.00167 (0.00297) |
| MDS-based Other Conditions | |||
| End-stage prognosis | −0.0191*** (0.00323) |
−0.0188*** (0.00620) |
−0.0300*** (0.0104) |
| Fall(30day) = Yes | 0.000310 (0.000549) |
0.000391 (0.00109) |
−0.00122 (0.00186) |
| Fall(30day) = Unable to answer | 0.0131*** (0.00157) |
0.0184*** (0.00373) |
0.00554 (0.00430) |
| Fall(30day) = missing | −0.0213*** (0.00392) |
−0.00703 (0.00815) |
−0.0342*** (0.0102) |
| Fall(31-180day) = Yes | −0.00106 (0.000647) |
−0.000720 (0.00131) |
−0.00112 (0.00219) |
| Fall(31-180day) = Unable to answer | 0.00852*** (0.00104) |
0.00817*** (0.00219) |
0.0154*** (0.00315) |
| Fall(31-180day) = missing | 0.0164*** (0.00307) |
0.0113* (0.00616) |
0.0246*** (0.00816) |
| Venous/Arterial ulcer present | 0.0174*** (0.00202) |
0.0152*** (0.00395) |
0.0195*** (0.00592) |
| Infection of the foot | 0.0222*** (0.00254) |
0.0206*** (0.00549) |
0.0130* (0.00750) |
| Diabetic foot ulcer | 0.0274*** (0.00354) |
0.0203*** (0.00765) |
0.0247** (0.0106) |
| Dehydrate | 0.0377*** (0.00516) |
0.0463*** (0.0118) |
0.0253* (0.0150) |
| Internal bleeding | 0.140*** (0.00334) |
0.129*** (0.00672) |
0.140*** (0.0104) |
| Pain freq: 1 Almost constant | 0.000705 (0.00105) |
−0.000594 (0.00218) |
0.00112 (0.00328) |
| Pain freq: 2 Frequently | −0.00357*** (0.000702) |
−0.00218 (0.00138) |
−0.00226 (0.00216) |
| Pain freq: 3 Occasionally | −0.0103*** (0.000614) |
−0.00948*** (0.00121) |
−0.00916*** (0.00196) |
| Pain freq: 4 Rarely | −0.0141*** (0.00105) |
−0.0129*** (0.00195) |
−0.0139*** (0.00362) |
| Pain freq: Unable to answer | −0.00634* (0.00366) |
0.00622 (0.00801) |
0.00631 (0.0115) |
| Surgical wound | 0.000265 (0.000820) |
0.00135 (0.00158) |
−7.89e-05 (0.00264) |
| Bowel continent: 2 occasionally incontinent | 0.00901*** (0.000737) |
0.00937*** (0.00148) |
0.0113*** (0.00240) |
| Bowel continent: 3 frequently incontinent | 0.00872*** (0.000771) |
0.00908*** (0.00155) |
0.0144*** (0.00243) |
| Bowel continent: 4 always incontinent | 0.0151*** (0.00108) |
0.0184*** (0.00237) |
0.0115*** (0.00311) |
| Bowel continent: 9 not rated | 0.0511*** (0.00301) |
0.0448*** (0.00647) |
0.0525*** (0.00890) |
| Shortness of breath with exertion | 0.00998*** (0.000822) |
0.0102*** (0.00170) |
0.00736*** (0.00261) |
| Shortness of breath sitting | 0.0700*** (0.00169) |
0.0765*** (0.00358) |
0.0592*** (0.00484) |
| Shortness of breath lying | 0.00976*** (0.00120) |
0.0130*** (0.00249) |
0.0118*** (0.00377) |
| Parenteral/IV feeding | 0.0852*** (0.00322) |
0.0869*** (0.00653) |
0.0690*** (0.00820) |
| Feeding tube | 0.0173*** (0.00203) |
0.0156*** (0.00452) |
0.0239*** (0.00569) |
| Ostomy care | 0.00802*** (0.00294) |
0.00840 (0.00606) |
−0.00245 (0.00908) |
| Antibiotic received = YES | −0.00664*** (0.000524) |
−0.00358*** (0.00105) |
−0.00699*** (0.00172) |
| Antibiotic received = missing | −0.140*** (0.00656) |
−0.123*** (0.0145) |
−0.125*** (0.0161) |
| Insulin injection | 0.00950*** (0.000780) |
0.0119*** (0.00159) |
0.0102*** (0.00254) |
| Year= 2012 | 0.00704*** (0.00100) |
0.0114*** (0.00263) |
0.00658* (0.00346) |
| Year = 2013 | 0.00473*** (0.00116) |
0.00865*** (0.00288) |
0.00704 (0.00437) |
| Year= 2014 | 0.00418*** (0.00125) |
0.0102*** (0.00302) |
−0.000567 (0.00496) |
| Constant | −0.0656*** (0.00296) |
−0.0904*** (0.00600) |
−0.0524*** (0.00912) |
| Observations | 2,011,351 | 493,699 | 195,755 |
| R-squared | 0.134 | 0.137 | 0.130 |
| Number of facilities | 14,704 | 5,640 | 3,983 |
Note: ADRD (Alzheimer’s disease or related dementias); NH (nursing home); ADL (activities of daily living); CFS (cognitive function scale); CAD (Coronary artery disease); COPD (Chronic obstructive pulmonary disease); PVD (Peripheral Vascular Disease); UTI (urinary tract infection); DRG (diagnosis related group); ICU (intensive care unit); IV (intravenous)
Difference statistically significant at the 10% level
Difference statistically significant at the 5% level
Difference statistically significant at the 1% level
Appendix Table 3.
Sensitivity analyses (full)
| VARIABLES | Residents survived 365 days (2011-2013) |
Including residents who died (2011-2014) |
Different time threshold | |
|---|---|---|---|---|
| Post start at 2012Q3 | Exclude 2012Q2 | |||
| ADRD = 1 | −0.0170*** (0.00101) |
−0.0195*** (0.000952) |
−0.0167*** (0.000884) |
−0.0162*** (0.000959) |
| post = 1 | −0.00625*** (0.00103) |
−0.00724*** (0.00105) |
−0.00325*** (0.000860) |
−0.00631*** (0.00101) |
| ADRD * post | −0.00228* (0.00118) |
−0.00410*** (0.00109) |
−0.00282*** (0.00103) |
−0.00290*** (0.00110) |
| Demographics | ||||
| Age | −0.000578*** (3.49e-05) |
−0.000317*** (3.21e-05) |
−0.000631*** (3.11e-05) |
−0.000627*** (3.25e-05) |
| Male | 0.00666*** (0.000522) |
0.0141*** (0.000482) |
0.00711*** (0.000465) |
0.00736*** (0.000483) |
| Race = black | 0.00859*** (0.00114) |
0.00991*** (0.00103) |
0.0104*** (0.00103) |
0.0105*** (0.00106) |
| Race = other | 0.000499 (0.00109) |
0.00291*** (0.000967) |
0.00175* (0.000949) |
0.00192* (0.001000) |
| Dual status | −0.000108 (0.000699) |
−0.00601*** (0.000624) |
−1.49e-05 (0.000616) |
−0.000135 (0.000642) |
| Functional/cognitive/behavioral Status | ||||
| CFS = 2 | 0.0360*** (0.000788) |
0.0439*** (0.000707) |
0.0356*** (0.000700) |
0.0359*** (0.000725) |
| CFS = 3 | 0.0258*** (0.000974) |
0.0358*** (0.000855) |
0.0255*** (0.000857) |
0.0255*** (0.000889) |
| CFS = 4 | 0.0347*** (0.00238) |
0.0161*** (0.00167) |
0.0340*** (0.00196) |
0.0343*** (0.00205) |
| CFS, missing | 0.236*** (0.00398) |
0.205*** (0.00274) |
0.231*** (0.00356) |
0.234*** (0.00365) |
| ADL | 0.00532*** (7.32e-05) |
0.00694*** (7.00e-05) |
0.00544*** (6.69e-05) |
0.00544*** (6.91e-05) |
| Chronic Conditions | ||||
| Cancer = Yes | 0.0103*** (0.00105) |
0.0282*** (0.000866) |
0.0104*** (0.000892) |
0.0104*** (0.000928) |
| Cancer = missing | −0.0594*** (0.0188) |
−0.0365** (0.0156) |
−0.0604*** (0.0160) |
−0.0607*** (0.0163) |
| CAD = Yes | 0.00421*** (0.000629) |
0.00362*** (0.000568) |
0.00416*** (0.000557) |
0.00417*** (0.000581) |
| CAD = missing | −0.201*** (0.0195) |
−0.174*** (0.0164) |
−0.202*** (0.0168) |
−0.200*** (0.0171) |
| Heart failure | 0.0289*** (0.000724) |
0.0412*** (0.000623) |
0.0300*** (0.000630) |
0.0303*** (0.000654) |
| Hypertension | 0.0123*** (0.000567) |
0.0127*** (0.000528) |
0.0124*** (0.000506) |
0.0126*** (0.000531) |
| Diabetes | 0.00788*** (0.000677) |
0.00774*** (0.000614) |
0.00775*** (0.000602) |
0.00801*** (0.000631) |
| Asthma/COPD | 0.00717*** (0.000653) |
0.0136*** (0.000586) |
0.00760*** (0.000578) |
0.00776*** (0.000602) |
| PVD = Yes | 0.0173*** (0.00112) |
0.0220*** (0.000971) |
0.0184*** (0.000975) |
0.0181*** (0.00102) |
| PVD = missing | 0.138*** (0.00700) |
0.138*** (0.00627) |
0.145*** (0.00686) |
0.141*** (0.00679) |
| Pneumonia | 0.0328*** (0.00101) |
0.0444*** (0.000844) |
0.0323*** (0.000872) |
0.0334*** (0.000910) |
| Septicemia | 0.0439*** (0.00225) |
0.0442*** (0.00186) |
0.0435*** (0.00195) |
0.0436*** (0.00201) |
| UTI | 0.0280*** (0.000729) |
0.0310*** (0.000648) |
0.0283*** (0.000649) |
0.0288*** (0.000671) |
| Wound infect | 0.0715*** (0.00190) |
0.0651*** (0.00165) |
0.0690*** (0.00169) |
0.0695*** (0.00174) |
| Hip fracture | 0.0128*** (0.000972) |
0.0191*** (0.000904) |
0.0129*** (0.000867) |
0.0131*** (0.000902) |
| Other fracture | 0.0148*** (0.000798) |
0.0116*** (0.000747) |
0.0140*** (0.000708) |
0.0142*** (0.000737) |
| Stroke | 0.00637*** (0.000836) |
0.00326*** (0.000739) |
0.00680*** (0.000740) |
0.00709*** |
| Anxiety | 0.00952*** (0.000663) |
0.00965*** (0.000584) |
0.00977*** (0.000584) |
0.00981*** (0.000606) |
| Depression | 0.00251*** (0.000555) |
−0.000515 (0.000503) |
0.00187*** (0.000500) |
0.00199*** (0.000522) |
| Bipolar disorder | 0.00848*** (0.00202) |
−0.000800 (0.00183) |
0.00748*** (0.00181) |
0.00762*** (0.00188) |
| Psychotic disorder | 0.0118*** (0.00171) |
0.0121*** (0.00146) |
0.0118*** (0.00148) |
0.0113*** (0.00153) |
| Schizophrenia | −0.00470* (0.00268) |
−0.0136*** (0.00239) |
−0.00500** (0.00237) |
−0.00443* (0.00248) |
| Respiratory failure | 0.0109*** (0.00222) |
0.0151*** (0.00167) |
0.0123*** (0.00183) |
0.0122*** (0.00193) |
| Anemia | 0.00640*** (0.000557) |
0.0141*** (0.000508) |
0.00734*** (0.000494) |
0.00759*** (0.000514) |
| Ulcerative Colitis/Crohn’s disease = 1 | 0.00670*** (0.00240) |
0.00881*** (0.00228) |
0.00856*** (0.00218) |
0.00820*** (0.00225) |
| Ulcerative Colitis/Crohn’s disease = missing | 0.375*** (0.00742) |
0.378*** (0.00644) |
0.383*** (0.00711) |
0.382*** (0.00714) |
| Viral hepatitis | 0.00995* (0.00589) |
0.0226*** (0.00498) |
0.0115** (0.00513) |
0.0106** (0.00535) |
| Seizure disorder or epilepsy | −0.000119 (0.00140) |
−0.00554*** (0.00121) |
0.000681 (0.00121) |
0.00110 (0.00127) |
| Index Hospitalization | ||||
| DRG Weights | 0.00417*** (0.000270) |
0.00294*** (0.000236) |
0.00405*** (0.000241) |
0.00405*** (0.000250) |
| Hospital LOS: less than 3 days | −0.0107*** (0.000524) |
−0.0157*** (0.000501) |
−0.0111*** (0.000474) |
−0.0111*** (0.000490) |
| Hospital LOS: more than 8 days | 0.0286*** (0.000696) |
0.0398*** (0.000614) |
0.0293*** (0.000613) |
0.0290*** (0.000637) |
| ICU use | 0.0122*** (0.000639) |
0.0144*** (0.000565) |
0.0122*** (0.000569) |
0.0122*** (0.000593) |
| Prior hosp_30 | 0.0316*** (0.000859) |
0.0413*** (0.000715) |
0.0321*** (0.000746) |
0.0327*** (0.000781) |
| Prior hosp_180 | 0.0147*** (0.000729) |
0.0217*** (0.000625) |
0.0152*** (0.000638) |
0.0153*** (0.000665) |
| Prior hosp_365 | 0.00881*** (0.000688) |
0.0111*** (0.000608) |
0.00951*** (0.000616) |
0.00949*** (0.000636) |
| Surgical DRG | 0.000712 (0.00102) |
−0.00469*** (0.000895) |
0.000529 (0.000900) |
0.000353 (0.000942) |
| MDS-based Other Conditions | ||||
| End-stage prognosis | −0.0132*** (0.00386) |
−0.156*** (0.00229) |
−0.0191*** (0.00323) |
−0.0201*** (0.00336) |
| Fall(30day) = Yes | −0.000316 (0.000623) |
0.000932 (0.000569) |
0.000309 (0.000549) |
0.000136 (0.000573) |
| Fall(30day) = Unable to answer | 0.0130*** (0.00178) |
0.00951*** (0.00152) |
0.0131*** (0.00157) |
0.0128*** (0.00163) |
| Fall(30day) = missing | −0.0244*** (0.00424) |
−0.0244*** (0.00387) |
−0.0214*** (0.00392) |
−0.0217*** (0.00403) |
| Fall(31-180day) = Yes | −0.000825 (0.000735) |
−0.000525 (0.000665) |
−0.00106* (0.000647) |
−0.00115* (0.000671) |
| Fall(31-180day) = Unable toAnswer | 0.00979*** (0.00116) |
0.0111*** (0.00104) |
0.00853*** (0.00104) |
0.00873*** (0.00108) |
| Fall(31-180day) = missing | 0.0190*** (0.00340) |
0.0207*** (0.00316) |
0.0165*** (0.00307) |
0.0164*** (0.00318) |
| Venous/Arterial ulcer present | 0.0168*** (0.00230) |
0.0278*** (0.00187) |
0.0174*** (0.00202) |
0.0180*** (0.00210) |
| Infection of the foot | 0.0223*** (0.00287) |
0.0231*** (0.00252) |
0.0222*** (0.00254) |
0.0223*** (0.00263) |
| Diabetic foot ulcer | 0.0295*** (0.00421) |
0.0260*** (0.00342) |
0.0274*** (0.00354) |
0.0282*** (0.00370) |
| Dehydrate | 0.0353*** (0.00604) |
0.0218*** (0.00420) |
0.0377*** (0.00516) |
0.0368*** (0.00540) |
| Internal bleeding | 0.142*** (0.00384) |
0.136*** (0.00284) |
0.140*** (0.00334) |
0.139*** (0.00345) |
| Pain freq: 1 Almost constant | 0.00146 (0.00117) |
0.000431 (0.00109) |
0.000689 (0.00105) |
0.000842 (0.00109) |
| Pain freq: 2 Frequently | −0.00408*** (0.000784) |
−0.00355*** (0.000720) |
−0.00358*** (0.000702) |
−0.00387*** (0.000730) |
| Pain freq: 3 Occasionally | −0.0112*** (0.000692) |
−0.0110*** (0.000636) |
−0.0103*** (0.000614) |
−0.0105*** (0.000636) |
| Pain freq: 4 Rarely | −0.0146*** (0.00117) |
−0.0141*** (0.00113) |
−0.0141*** (0.00105) |
−0.0142*** (0.00109) |
| Pain freq: Unable to answer | −0.00815* (0.00422) |
−0.00609* (0.00338) |
−0.00635* (0.00366) |
−0.00719* (0.00377) |
| Surgical wound | 0.00113 (0.000921) |
−0.00658*** (0.000818) |
0.000246 (0.000820) |
0.000857 (0.000856) |
| Bowel continent: 2 occasionally incontinent | 0.00803*** (0.000849) |
0.0154*** (0.000761) |
0.00902*** (0.000737) |
0.00880*** (0.000771) |
| Bowel continent: 3 frequently incontinent | 0.00836*** (0.000876) |
0.0207*** (0.000759) |
0.00872*** (0.000771) |
0.00846*** (0.000803) |
| Bowel continent: 4 always incontinent | 0.0152*** (0.00127) |
0.0216*** (0.00103) |
0.0151*** (0.00108) |
0.0153*** (0.00112) |
| Bowel continent: 9 not rated | 0.0511*** (0.00354) |
0.0276*** (0.00259) |
0.0511*** (0.00301) |
0.0514*** (0.00314) |
| Shortness of breath with exertion | 0.00964*** (0.000934) |
0.0231*** (0.000825) |
0.00998*** (0.000822) |
0.00956*** (0.000851) |
| Shortness of breath sitting | 0.0702*** (0.00195) |
0.0734*** (0.00144) |
0.0700*** (0.00169) |
0.0694*** (0.00174) |
| Shortness of breath lying | 0.00872*** (0.00137) |
0.0110*** (0.00117) |
0.00976*** (0.00120) |
0.00999*** (0.00125) |
| Parenteral/IV feeding | 0.0847*** (0.00362) |
0.0772*** (0.00268) |
0.0853*** (0.00322) |
0.0846*** (0.00327) |
| Feeding tube | 0.0150*** (0.00243) |
0.0267*** (0.00175) |
0.0173*** (0.00203) |
0.0171*** (0.00210) |
| Ostomy care | 0.0102*** (0.00343) |
0.0252*** (0.00262) |
0.00802*** (0.00294) |
0.00777** (0.00306) |
| Antibiotic received = YES | −0.00698*** (0.000596) |
−0.00391*** (0.000534) |
−0.00665*** (0.000524) |
−0.00711*** (0.000543) |
| Antibiotic received = missing | −0.133*** (0.00668) |
−0.132*** (0.00600) |
−0.139*** (0.00653) |
−0.137*** (0.00650) |
| Insulin injection | 0.00808*** (0.000874) |
0.0144*** (0.000780) |
0.00951*** (0.000780) |
0.00950*** (0.000810) |
| Year = 2012 | 0.00752*** (0.00103) |
0.00687*** (0.00102) |
0.00388*** (0.000813) |
0.00712*** (0.00101) |
| Year = 2013 | 0.00538*** (0.00119) |
0.00398*** (0.00119) |
0.00177* (0.00103) |
0.00475*** (0.00116) |
| Year = 2014 | 0.00311** (0.00129) |
0.00121 (0.00113) |
0.00476*** (0.00135) |
|
| Constant | −0.0689*** (0.00329) |
−0.107*** (0.00313) |
−0.0658*** (0.00296) |
−0.0669*** (0.00308) |
| Observations | 1,529,803 | 2,588,995 | 2,011,351 | 1,866,937 |
| R-squared | 0.132 | 0.145 | 0.134 | 0.134 |
| Number of facilities | 14,454 | 14,765 | 14,704 | 14,647 |
Note: ADRD (Alzheimer’s disease or related dementias); NH (nursing home); ADL (activities of daily living); CFS (cognitive function scale); CAD (Coronary artery disease); COPD (Chronic obstructive pulmonary disease); PVD (Peripheral Vascular Disease); UTI (urinary tract infection); DRG (diagnosis related group); ICU (intensive care unit); IV (intravenous)
Difference statistically significant at the 10% level
Difference statistically significant at the 5% level
Difference statistically significant at the 1% level
Footnotes
All authors report no conflicts of interest relevant to this article.
Reference
- Aud MA (2004). Dangerous wandering: elopements of older adults with dementia from long-term care facilities. American Journal of Alzheimer's Disease & Other Dementias®, 19(6), 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Orrell M, YongZhong S, Moniz-Cook E, Stafford J, Whittaker R, … Khan Z (2015). Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. American Journal of Psychiatry, 173(3), 252–262. [DOI] [PubMed] [Google Scholar]
- Bowblis JR, Lucas JA, & Brunt CS (2015). The effects of antipsychotic quality reporting on antipsychotic and psychoactive medication use. Health services research, 50(4), 1069–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMS. (2018a). How hospice works. Retrieved from https://www.medicare.gov/what-medicare-covers/part-a/how-hospice-works.html
- CMS. (2018b, Sept). Readmissions Reduction Program (HRRP). Retrieved from https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html
- CMS. (2020a). Medicare Post-Acute Care & Hospice - by Provider and Service. Retrieved from https://data.cms.gov/provider-summary-by-type-of-service/medicare-post-acute-care-hospice/medicare-post-acute-care-hospice-by-provider-and-service
- CMS. (2020b). National Partnership - Dementia Care Resources. Retrieved from https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/National-Partnership-Dementia-Care-Resources
- CMS. (2021, April). National Partnership to Improve Dementia Care in Nursing Homes: Antipsychotic Medication Use Data Report. Retrieved from https://www.cms.gov/files/document/antipsychotic-medication-use-data-report-2020q4-updated-07302021.pdf
- Douglas S, James I, & Ballard C (2004). Non-pharmacological interventions in dementia. Advances in psychiatric treatment, 10(3), 171–177. [Google Scholar]
- FDA. (2005). Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. Retrieved from https://wayback.archive-it.org/7993/20170113112252/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm
- FDA. (2008). Information for healthcare professionals: conventional antipsychotics. Retrieved from https://wayback.archive-it.org/7993/20170722190727/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm
- GAO. (2015, Jan). Report to Congressional Requesters: Antipsychotic drug use. Retrieved from https://www.gao.gov/assets/670/668221.pdf
- Gill SS, Bronskill SE, Normand S-LT, Anderson GM, Sykora K, Lam K, … Herrmann N (2007). Antipsychotic drug use and mortality in older adults with dementia. Annals of internal medicine, 146(11), 775–786. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Kales HC, & Lyketsos CG (2012). Nonpharmacologic management of behavioral symptoms in dementia. Jama, 305(19), 2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski DC, Hirth RA, Intrator O, Li Y, Richardson J, Stevenson DG, … Banaszak-Holl J (2016). Low-quality nursing homes were more likely than other nursing homes to be bought or sold by chains in 1993–2010. Health Affairs, 35(5), 907–914. [DOI] [PubMed] [Google Scholar]
- Gurwitz JH, Bonner A, & Berwick DM (2017). Reducing excessive use of antipsychotic agents in nursing homes. Jama, 318(2), 118–119. [DOI] [PubMed] [Google Scholar]
- Hersch EC, & Falzgraf S (2007). Management of the behavioral and psychological symptoms of dementia. Clinical Interventions in Aging, 2(4), 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CM, Lapane KL, & Mor V (2000). Influence of facility characteristics on use of antipsychotic medications in nursing homes. Medical care, 1164–1173. [DOI] [PubMed] [Google Scholar]
- Johnell K, Jonasdottir Bergman G, Fastbom J, Danielsson B, Borg N, & Salmi P (2017). Psychotropic drugs and the risk of fall injuries, hospitalisations and mortality among older adults. International journal of geriatric psychiatry, 32(4), 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble P, Chen H, Sherer J, & Aparasu RR (2008). Antipsychotic drug use among elderly nursing home residents in the United States. The American journal of geriatric pharmacotherapy, 6(4), 187–197. [DOI] [PubMed] [Google Scholar]
- Kate NS, Pawar SS, Parkar SR, & Sawant NS (2015). Adverse drug reactions due to antipsychotics and sedative-hypnotics in the elderly. Journal of Geriatric Mental Health, 2(1), 16. [Google Scholar]
- Morris JN, Fries BE, & Morris SA (1999). Scaling ADLs within the MDS. The Journals of Gerontology: Series A, 54(11), M546–M553. [DOI] [PubMed] [Google Scholar]
- Ouslander JG, Lamb G, Perloe M, Givens JH, Kluge L, Rutland T, … Saliba D (2010). Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs. Journal of the American Geriatrics Society, 55(4), 627–635. [DOI] [PubMed] [Google Scholar]
- Ouslander JG, Lamb G, Tappen R, Herndon L, Diaz S, Roos BA, … Bonner A (2011). Interventions to reduce hospitalizations from nursing homes: evaluation of the INTERACT II collaborative quality improvement project. Journal of the American Geriatrics Society, 59(4), 745–753. [DOI] [PubMed] [Google Scholar]
- Park J, & Werner RM (2011). Changes in the relationship between nursing home financial performance and quality of care under public reporting. Health economics, 20(7), 783–801. [DOI] [PubMed] [Google Scholar]
- Perlman CM, & Hirdes JP (2008). The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. Journal of the American Geriatrics Society, 56(12), 2298–2303. [DOI] [PubMed] [Google Scholar]
- Phelan EA, Borson S, Grothaus L, Balch S, & Larson EB (2012). Association of incident dementia with hospitalizations. Jama, 307(2), 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon PA, Normand S-L, Gomes T, Gill SS, Anderson GM, Melo M, … Gurwitz JH (2008). Antipsychotic therapy and short-term serious events in older adults with dementia. Archives of internal medicine, 168(10), 1090–1096. [DOI] [PubMed] [Google Scholar]
- Sink KM, Covinsky KE, Newcomer R, & Yaffe K (2004). Ethnic differences in the prevalence and pattern of dementia-related behaviors. Journal of the American Geriatrics Society, 52(8), 1277–1283. [DOI] [PubMed] [Google Scholar]
- Spilsbury K, Hewitt C, Stirk L, & Bowman C (2011). The relationship between nurse staffing and quality of care in nursing homes: a systematic review. International journal of nursing studies, 48(6), 732–750. [DOI] [PubMed] [Google Scholar]
- Thomas KS, Dosa D, Wysocki A, & Mor V (2017). The minimum data set 3.0 cognitive function scale. Medical care, 55(9), e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Temkin-Greener H, Conwell Y, & Cai S (2020). Policy to reduce antipsychotic use and hospitalization of nursing home residents with dementia. Journal of the American Medical Directors Association, 21(11), 1617–1622. e1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge JM (2010). Econometric analysis of cross section and panel data: MIT press. [Google Scholar]