Abstract
Background:
Feasible, scalable, and cost-effective approaches to ensure viral suppression (VS) among children living with HIV (CLHIV) are urgently needed. The goal of the Opt4Kids study was to determine the impact of point-of-care (POC) viral load (VL) and targeted drug resistance mutation (DRM) testing in improving VS among children on antiretroviral therapy (ART) in Kenya.
Methods:
We conducted a randomized controlled trial to evaluate the use of POC VL and targeted DRM testing among children aged 1–14 years on ART at health facilities in western Kenya 2019–2020. Children were randomized 1:1 to intervention (POC VL every 3 months, targeted DRM testing, and clinical management support) vs. control (standard-of-care, centralized VL every 6 months) groups. Our primary outcome was VS (VL <1000 copies/mL) 12 months after enrollment.
Findings:
Of the 704 participants enrolled, the median age at enrollment was 9 years (interquartile range [IQR] 7, 12), 344 (49%) were female, the median time on ART was 5.8 years (IQR 3.1, 8.6), and 536 (76%) had documented VS at enrollment. At 12 months after enrollment, the proportion of participants achieving VS in the intervention group 283/313 (90%) was very similar to that in the control group 289/315 (92%, risk ratio (RR) 0.99, 95% confidence interval [CI] 0.94, 1.03). We identified 122 episodes of viremia in intervention participants, of which 107 (89%) samples successfully underwent DRM testing and 91 (85%) had major DRMs. The median turnaround time for VL results was one (IQR 0, 1) and 15 (IQR 10, 21) days in the intervention and control groups, respectively.
Interpretation:
POC VL markedly decreased turnaround time and targeted DRM testing identified a high prevalence of HIV drug resistance mutations in CLHIV, but did not increase rates of VS. Further research in combination interventions, including POC VL and DRM testing coupled with psychosocial support, is needed to optimize VS for CLHIV.
Keywords: HIV, children, antiretroviral therapy, point-of-care (POC) testing, viral load, drug resistance mutations (DRM)
INTRODUCTION
Among the nearly 1 million children living with HIV (CLHIV) on antiretroviral treatment (ART), less than half achieve virologic suppression (VS), lagging well behind the estimated 67% VS for adults. While the causes of virologic failure among CLHIV are multifactorial, lack of timely viral load (VL) and drug resistance monitoring to facilitate early and appropriate clinical decision-making are likely contributors. To meet the UNAIDS 95-95-95 targets for population VS among CLHIV, novel approaches to such monitoring are urgently needed.
Kenya has a high burden of pediatric HIV, with an estimated 111,500 CLHIV and 5,200 newly infected children under the age of 14 in 2020. Increasing availability of routine VL testing, which began in Kenya in 2014 per 2013 World Health Organization (WHO) recommendations, has raised awareness of treatment failure in children but has not resulted in higher VS rates. Numerous challenges, such as lengthy turnaround times for test results, the cost of transporting samples to centralized laboratories, and the inability to monitor VL more frequently than national guidelines allow, reduce the potential benefits of laboratory-based VL testing.1,2 Inadequate supply chains for key VL reagents, poor utilization and lack of understanding of VL results among patients and providers, and failure to switch ART despite persistent virologic failure further reduce the potential impact of VL monitoring.3–7 Point-of-care (POC), or even near POC, VL assessments have been shown to be feasible, accurate, and less expensive than laboratory-based VL assays.7–10 Kenya has launched a nation-wide POC tuberculosis testing platform using GeneXpert® technology which has been used to pilot test HIV early infant diagnosis and limited POC VL testing and can be leveraged to carry out greater POC VL testing.11–13
HIV drug resistance is also expected to have a significant role in virologic failure among CLHIV. Recent WHO reports warn that HIV drug resistance mutations (DRMs) could jeopardize the attainment of the global targets for HIV. Existing data in sub-Saharan Africa suggest that DRMs are prevalent among children who do not achieve VS and those newly diagnosed with HIV.14–18 Incorporating DRM testing and results into clinical decision making for CLHIV may be an important component of effectively managing CLHIV with virologic failure.
Thus, we conducted Opt4Kids, a randomized controlled trial, to evaluate if using higher frequency POC VL with targeted DRM testing and clinical decision support for children aged 1–14 years on ART in Kenya could improve VS rates. We hypothesized that POC VL and targeted DRM testing would facilitate earlier and more appropriate clinical decision-making, resulting in improved treatment outcomes among CLHIV.
METHODS
Study setting and population
The study was conducted in Kisumu County, western Kenya, with the second-highest prevalence of pediatric HIV infections in Kenya. The study was implemented at five low-resource, high-HIV burden public sector facilities from March 2019 to December 2020. Comprehensive pediatric HIV care and treatment services were provided per Kenyan ART guidelines, including ART for all children diagnosed with HIV.19 First-line ART regimens in children under three years of age during the study period included combinations of two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) lamivudine, abacavir, or zidovudine with: (1) non-nucleos(t)ide reverse transcriptase inhibitors (NNRTIs) efavirenz, (2) protease inhibitors (PIs) lopinavir/ritonavir, or (3) integrase inhibitors (INSTIs) dolutegravir.20 For children 3–15 years of age, efavirenz was preferred over lopinavir/ritonavir, and in 2020, the guidelines were updated to recommend dolutegravir for those weighing at least 20 kilograms for both treatment initiation or switch.21,22 Routine VL monitoring was offered through a network of public-sector, centralized laboratories and was recommended for children after six months of continuous ART and every six months thereafter for those with VL <1000 copies/mL (Supplemental Figure 1). Management of children with VL ≥ 1000 copies/mL included enhanced adherence counseling and repeat VL testing after three months of good adherence followed by switch of ART to second line regimens if still not virologically suppressed. DRM testing at national reference laboratories was restricted to children with virologic failure on a protease inhibitor (PI)-containing 1st line or on 2nd or 3rd line ART who continue to have viremia after adherence optimization. DRM testing became accessible in 2018 though it requires approval by the Kenyan Ministry of Health (MOH) HIV ART treatment committees, who also guide local providers on clinical management.
The study population was recruited from CLHIV ages 1–14 years newly initiating or already receiving ART at the study facilities.
Study design
All study methods are described in detail elsewhere.23 In brief, we conducted an open-label, individually randomized controlled trial over 12 months allocating children on ART 1–14 years of age 1:1 to intervention (POC VL testing every three months, targeted DRM testing, and clinical decision support; abbreviated to POC hereafter) or control (standard of care (SOC)) groups in five facilities in western Kenya (Figure 1, Supplemental Table 1). We chose the study facilities to leverage existing POC technologies, specifically the GeneXpert® platform. Our initial eligibility criteria excluded children on 2nd, 3rd, or salvage ART regimens; however, given the overall high usage of PI-containing ART for all ART regimens in Kenya, we removed type of ART as an exclusion criterion.
Figure 1:
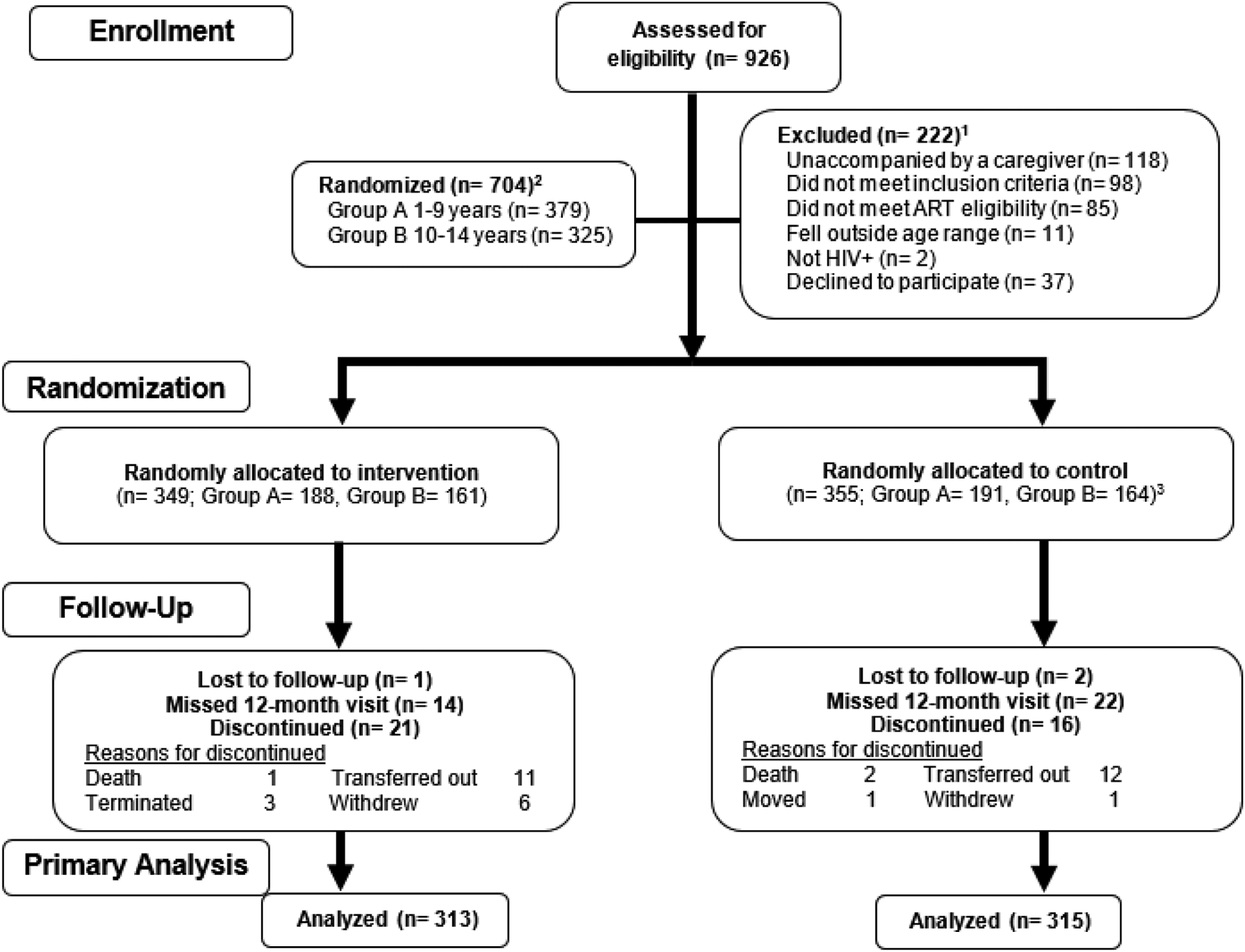
Flow diagram of study participants
1Reasons fur exclusion are not mutually exclusive; ART eligibility changed from only including children on 1st line ART regimens to include all ART regimens (change made in September 2019).
2A total of 17 protocol deviations occurred: in n=4 instances, participants were mistakenly enrolled again in the study, n=4 were allocated to the control group but received intervention condition (more below), n=3 participants were randomized within the wrong age group, n=3 were not on 1st line antiretroviral therapy prior to change in eligibility criteria and participation was terminated, n=1 each for study procedures conducted on a previously terminated participant, sample collected on a non-participant, and drug resistance testing result reported erroneously on the incorrect study participant.
3N=351 received control condition while n=4 were mistakenly allocated to intervention condition; our intent-to-treat analysis considered these 4 individuals as members of the control group.
Study procedures
Randomization, allocation, and blinding
We conducted randomization using sequentially numbered, sealed, opaque envelopes containing allocation assignment using a balanced blocked randomization scheme with varying block sizes of 6, 8 or 10, stratified by facility site and age groups (Group A: 1–9 or Group B: 10–14 years of age). Study staff at each facility approached potential participants’ primary caregivers for study participation at routine clinical visits and obtained informed consent, with additional assent only for participants 13–14 years of age. Study staff selected sequential envelopes to assign randomized allocation. Thus, participants, including for the SOC group, could be enrolled at any timepoint in their routine care. Investigators were blinded to the randomized group, while participants, site coordinators (who also implemented POC VL testing for the primary outcome), and the data team were not. A total of 17 protocol deviations occurred, including four instances each of participants being enrolled again and not being allocated assigned group (Figure 1).
Intervention- POC VL testing
Our POC VL testing approach utilized the existing GeneXpert® system at four of the five facilities which can simultaneously perform TB and HIV diagnostics. For the one facility that did not have a GeneXpert® system on site, samples were transported daily to nearest facility less than 2km away. Fidelity to the group allocations was maintained by restricting POC VL test ordering to study staff.
We conducted POC VL testing at study enrollment and every three months through 12 months of follow-up with results delivered via text message or phone to caregivers (and paper results for providers) for the intervention group. This VL testing schedule is more frequent than the SOC schedule in Kenya already described (Supplemental Figure 1) and was intended to inform more rapid clinical management and participant/caregiver adherence behavior through VL result counseling leading to improved VS. SOC VL testing per routine care was continued by clinical facility staff in intervention group participants.
Intervention- targeted HIV DRM testing
Targeted HIV DRM testing on plasma or DBS samples with VL ≥ 1000 copies/ml using consensus sequencing using Applied Biosystems 3130xl Genetic Analyzers at the KEMRI-CDC HIV Research and Sanger 3730xl at the Kenya National HIV Reference Laboratories. Subsequent episodes of VL ≥ 1000 copies/ml did not necessarily trigger a DRM test. Integrase inhibitor testing was not conducted. The laboratories conduct batch testing at periodic intervals and return results to the study staff within 24 hours of assay result. Study staff then forwarded the DRM results to the clinical providers within 24 hours of their receipt.
Intervention- clinical decision support for management of children on ART with drug resistance
Overall, clinical providers were instructed to follow current Kenyan national guidelines for managing any child with VL ≥ 1000 copies/mL. A Clinical Management Committee (CMC) was formed based on the existing MOH regional technical working group. The CMC included the co-chairs of the regional technical working group, facility clinical and psychosocial service providers, study staff, principal investigators, MOH and other country HIV experts, and HIV implementing partner technical advisors. It met at least monthly to conduct case reviews of every participant with a DRM result in the intervention group using a standardized case review form prepared by facility and study staff. Recommendations regarding ART regimen and case management were agreed on by consensus and summarized using a standardized CMC recommendation form.
Retention activities
The study followed clinic-based retention activities, which include text message reminders, phone calls, home visits, and loss-to-follow-up tracing by clinic staff, which site coordinators supplemented with additional phone calls or text messaging as needed. We provided 500 Kenyan Shillings (approximately $5) to the participant’s caregiver for enrollment and primary endpoint (12 months after enrollment) study visits.
Data collection
Our data collection included in-person (or via phone during periods of the COVID-19 pandemic) questionnaires, review of routine MOH standardized patient records, including paper or electronic medical records, for clinical and laboratory information, and sample collection when applicable.
Outcomes
Our primary outcome VS (defined as VL <1000 copies/mL, as per country guidelines) by POC VL testing at 12 months after enrollment (defined as 48 weeks +/− 16 weeks, a wide window intended to maximize our ability to obtain a VS measurement in spite of COVID interruptions). If a POC VL test was not available at 12 months, any available SOC test in the same window was used instead. Our secondary outcomes included VS defined by lower VL cutoffs and a set of process outcomes, such as uptake of and turnaround time of VL testing results. We define major, minor, and accessory classification for HIV drug resistance according to the Stanford HIV Resistance Database. All primary and secondary outcomes defined in our protocol are reported here.
Statistical analysis
Power for the trial was based on the primary outcome of the proportion of children with VS in the intervention vs. control groups 12 months. Historical facility counts showed 700 children potentially eligible at study sites; if 90% enrolled, with 10% lost to follow-up, we calculated that the remaining 567 children (284 per group) would provide 80% power to detect a difference in VS between groups of at least 11.05% if VS in the control group is 65% (from historical facility data). Calculations were based on continuity-corrected Chi-squared test with two-sided α=0.05.
We provide descriptive statistics for participant baseline characteristics by group. We describe VS by group at enrollment (any blood draw 0–90 days prior to enrollment), 3, 6, and 9 months (any blood draw +/− 6 weeks within visit target except for the 3-month visit which additionally included blood draws from day after enrollment to 6 weeks after the 3-month visit). The primary analysis was intention-to-treat, comparing the proportion of children with VS at 12 months (primary outcome) in the intervention vs. control groups. We estimated the relative risk (RR) using a modified Poisson regression model with robust standard error estimation, adjusting for facility and age group strata.24 We conducted an a priori sensitivity analysis defining VS as VL<40 copies/mL as well as a post hoc analysis of VL<400 copies/mL. We examined potential effect modification of the intervention effect on the primary outcome in a priori subgroups (sex, age (1–9 or 10–14 year groups), time of ART (<2 years, 2–5 years, or >5 years groups), VS of caregiver (yes, no, or unknown groups) and a post hoc subgroup (biological parent caregiver), by including the main effect of subgroup and the interaction between subgroup and randomization group in the model. We performed a secondary analysis among children who were newly initiating ART or initially unsuppressed (VL ≥400 copies/mL at first VL result within study visits 0–6 months using group-specific testing modality) and compared VS (<400 copies/mL) at 12 months by the randomized group. Subgroup analyses were performed for a priori subgroups (age group and whether newly initiating ART). We conducted two post hoc sensitivity analyses of the primary analysis, one adjusting for sex (since sex distribution was slightly different by group) and a second using inverse probability weighting (IPW, to potentially better address missing outcomes).
Ethics review and safety monitoring
AMREF in Kenya and University of Washington and University of Colorado in the U.S. provided ethical approval for this study. Given the determination of this trial as a minimal risk study, a formal data and safety monitoring board was not assembled. Instead, the study investigators conducted periodic data and safety monitoring activities to ensure participant safety. The trial is registered with registration number NCT03820323.
Role of the funding source
Support for this study is provided by the National Institutes of Mental Health of the U.S. National Institutes of Health (NIH, R34MH115769) and the Thrasher Research Fund. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Washington Institute of Translational Health Sciences and supported by the National Center for Advancing Translational Sciences of the NIH (UL1 TR002319). The funding sources or study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
RESULTS
Enrollment characteristics
A total of 926 potential participants were assessed for study eligibility, of which 222 (24%) participants were excluded (53% because the child was unaccompanied by a caregiver who could provide informed consent, 38% did not meet the initial more restrictive ART criteria limited to 1st line regimens only, and 17% declined participation; Figure 1). Thus, 704 participants were randomized, with 349 allocated to the intervention group and 355 to the control group. Four significant adverse events occurred (3 deaths and 1 hospitalization, all deemed unrelated to study intervention).
The median age at enrollment for Group A (1–9 years) was 7 years (interquartile range [IQR] 5, 8), and Group B (10–14 years) was 12 years (IQR 11, 13). A total of 344 (49%) participants were females, with 45% of control group and 53% of intervention group being female. (Table 1). All participants were on either already on ART or initiated ART within 30 days of enrollment, 382 (54%) were on non-nucleoside reverse transcriptase inhibitors (NNRTI)-, 294 (42%) on PI-, and 27 (4%) on integrase-containing ART at enrollment, and the median time on ART was 5.8 years (IQR 3.1, 8.6). Among 613 (87%) of the participants with a VL documented in the 24 months prior to enrollment, 536 (76%) had VS. Additional facility characteristics and ART regimen changes during the course of the study can be found in Supplemental Tables 1 and 2, respectively.
Table 1:
Baseline characteristics of enrolled children and their caregivers in the Opt4Kids study, March 2019- December 2020
| Variable | Total study group (n=704) | Intervention group (POC VL; n=349) | Control group (SOC VL; n=355) |
|---|---|---|---|
| Child characteristics | |||
| Age at enrollment | |||
| 1–9 years | 379 (54%) | 188 (54%) | 191 (54%) |
| 10–14 years | 325 (46%) | 161 (46%) | 164 (46%) |
| Median age (IQR) | 9 (7,12) | 9 (6, 12) | 9 (7, 12) |
| Sex | |||
| Female | 344 (49%) | 184 (53%) | 160 (45%) |
| Male | 360 (51%) | 165 (47%) | 195 (55%) |
| Age at ART initiation in years | |||
| <2 | 302 (43%) | 156 (45%) | 146 (41%) |
| 2 to <5 | 269 (38%) | 137 (39%) | 132 (37%) |
| 5 to <10 | 118 (17%) | 51 (15%) | 67 (19%) |
| 10 to 14 | 13 (2%) | 4 (1%) | 9 (3%) |
| Unknown/missing | 2 (<1%) | 1 (<1%) | 1 (<1%) |
| Median age at ART initiation (IQR) | 2 (1, 4) | 2 (2, 4) | 2 (1, 5) |
| Time on ART in years | |||
| Newly initiating | 20 (3%) | 11 (3%) | 9 (3%) |
| 1 month to <2 years | 90 (13%) | 46 (13%) | 44 (12%) |
| 2 to <5 years | 188 (27%) | 91 (26%) | 97 (27%) |
| 5 to < 10 years | 306 (43%) | 145 (42%) | 161 (45%) |
| ≥10 years | 98 (14%) | 55 (16%) | 43 (12%) |
| Unknown/missing | 2 (<1%) | 1 (<1%) | 1 (<1%) |
| Median time on ART in years (IQR) | 5.8 (3.1, 8.6) | 6.0 (3.1, 8.9) | 5.6 (3.1, 8.4) |
| ART regimen at study enrollment containing | |||
| NNRTI | 382 (54%) | 182 (52%) | 200 (56%) |
| PI | 294 (42%) | 148 (42%) | 146 (41%) |
| Integrase | 27 (4%) | 19 (5%) | 8 (2%) |
| PI and integrase | 1 (<1%) | 0 (0%) | 1 (<1%) |
| WHO clinical stage, prior to two years to or on day of enrollment | |||
| I | 207 (29%) | 100 (29%) | 107 (30%) |
| II | 281 (40%) | 141 (40%) | 140 (39%) |
| III | 129 (18%) | 61 (17%) | 68 (19%) |
| IV | 23 (3%) | 13 (4%) | 10 (3%) |
| Not indicated or missing | 64 (9%) | 34 (10%) | 30 (8%) |
| Viral suppression (VL<1000 copies/mL) at enrollment1 | |||
| No | 77 (11%) | 38 (11%) | 39 (11%) |
| Yes | 536 (76%) | 262 (75%) | 274 (77%) |
| No VL recorded/missing | 91 (13%) | 49 (14%) | 42 (12%) |
| Primary caregiver or household characteristics | |||
| Type of caregiver | |||
| Father | 59 (8%) | 26 (7%) | 33 (9%) |
| Mother | 482 (68%) | 244 (70%) | 238 (67%) |
| Other biological | 149 (21%) | 73 (21%) | 76 (21%) |
| Non-biological | 14 (2%) | 6 (2%) | 8 (2%) |
| Unknown | 0 (0%) | 0 (0%) | 0 (0%) |
| Marital status2 | |||
| Married or cohabitating | 444 (63%) | 224 (64%) | 220 (62%) |
| Not Married | 259 (37%) | 125 (36%) | 134 (38%) |
| Unknown | 1 (<1%) | 0 (0%) | 1 (<1%) |
| Maternal HIV status among n=482 enrolled children with maternal caregiver | |||
| Positive | 475 (99%) | 240 (98%) | 235 (99%) |
| Negative | 7 (1%) | 4 (2%) | 3 (1%) |
| Unknown | 0 (0%) | 0 (0%) | 0 (0%) |
| Maternal ART status among n=475 maternal caregivers living with HIV | |||
| Yes | 472 (99%) | 239 (100%) | 233 (99%) |
| No | 2 (<1%) | 1 (<1%) | 1 (<1%) |
| Unknown/missing | 1 (<1%) | 0 (0%) | 1 (<1%) |
| Paternal HIV status among n=59 enrolled children with paternal caregiver | |||
| Positive | 49 (83%) | 23 (88%) | 26 (79%) |
| Negative | 10 (17%) | 3 (12%) | 7 (21%) |
| Unknown | 0 (0%) | 0 (0%) | 0 (0%) |
| Paternal ART status among n=49 paternal caregivers living with HIV | |||
| Yes | 48 (98%) | 23 (100%) | 25 (96%) |
| No | 0 (0%) | 0 (0%) | 0 (0%) |
| Unknown | 1 (2%) | 0 (0%) | 1 (4%) |
| Viral suppression of primary caregiver | |||
| Yes | 431 (61%) | 212 (61%) | 219 (62%) |
| No | 39 (6%) | 26 (7%) | 13 (4%) |
| Not living with HIV | 135 (19%) | 62 (18%) | 73 (21%) |
| Unknown | 99 (14%) | 49 (14%) | 50 (14%) |
| Caregiver educational attainment | |||
| No primary | 29 (4%) | 14 (4%) | 15 (4%) |
| Primary education | 398 (57%) | 198 (57%) | 200 (56%) |
| More than primary | 277 (39%) | 137 (39%) | 140 (39%) |
| Having household commodities | |||
| Electricity | 403 (57%) | 206 (59%) | 197 (55%) |
| Radio | 554 (79%) | 270 (77%) | 284 (80%) |
| Television | 375 (53%) | 191 (55%) | 184 (52%) |
| Phone | 679 (96%) | 336 (96%) | 343 (97%) |
| Higher quality floor | 411 (58%) | 204 (58%) | 207 (58%) |
| Higher quality roofing material | 696 (99%) | 347 (99%) | 349 (98%) |
| More than one room | 580 (82%) | 284 (81%) | 296 (83%) |
| Higher quality cooking material | 160 (23%) | 87 (25%) | 73 (21%) |
| Reporting food insecurity (n, %) | |||
| None 0 | 140 (20%) | 64 (18%) | 76 (21%) |
| Mild 1–9 | 351 (50%) | 177 (51%) | 174 (49%) |
| Moderate 10–18 | 186 (26%) | 90 (26%) | 96 (27%) |
| Severe 19–27 | 27 (4%) | 18 (5%) | 9 (3%) |
Abbreviations: IQR = interquartile range; VL=viral load; POC=point-of-care; SOC=standard-of-care; ART=antiretroviral therapy
Initiating ART within 30 days of study enrollment
SOC viral load at enrollment or most recent up to 2 years prior.
The majority, or 482 (68%), of primary caregivers, were reported as the biological mother of the participant, of which 99% were known to be living with HIV and on ART. Among all types of primary caregivers who identified as living with HIV, 153 (71%) self-reported VS at the time of enrollment. Most caregivers, 444 (63%), were married and 277 (39%) had attained more than primary education. Neither participant nor caregiver characteristics differed significantly between the intervention and control groups, except more participants in the intervention group (184, 53%) were females compared to control group (160, 45%).
The proportion of children with VS at enrollment in the intervention group was 286/342 (84%) by POC VL testing, and the proportion of children with VS at enrollment in the control group was 145/167 (87%) by SOC VL testing (Table 2). Among those in the intervention group who also had SOC VL testing, it was 113/131 (86%).
Table 2:
Comparison of viral suppression by study group over time by subgroups and varying threshold of VL cutoffs among the Opt4Kids study participants (n=704), March 2019- December 2020
| Variable | Intervention group (POC VL; n=349) | Control group (SOC VL; n=355) | Risk Ratio1 (95% CI) | p-value1 |
|---|---|---|---|---|
| Viral suppression < 1000 copies/mL | ||||
| By testing interval 2 | ||||
| Enrollment | 286/342 (83.6%) | 145/167 (86.8%) | - | - |
| 3 months | 211/241 (87.6%) | 159/185 (86.0%) | - | - |
| 6 months | 110/124 (88.7%) | 122/131 (93.1%) | - | - |
| 9 months | 130/149 (87.3%) | 128/143 (89.5%) | - | - |
| Primary outcome: viral suppression <1000 copies/mL 12 months after enrollment 3 | 283/313 (90.4%) | 289/315 (91.8%) | 0.99 (0.94, 1.03) | 0.55 |
| Viral suppression < 1000 copies/mL at 12 months by baseline subgroup | ||||
| Male | 137/149 (92.0%) | 160/171 (93.6%) | 0.98 (0.93, 1.05) | |
| 10–14 years | 114/126 (90.5%) | 111/117 (94.9%) | 0.95 (0.89, 1.02) | |
| > 5 years | 173/187 (92.5%) | 176/187 (94.1%) | 0.98 (0.93, 1.04) | |
| HIV-1 negative or unknown | 47/52 (90.4%) | 65/69 (94.2%) | 0.96 (0.86, 1.07) | |
| No | 61/67 (91.0%) | 68/73 (93.2%) | 0.97 (0.88, 1.07) | |
| Viral suppression <400 copies/mL | ||||
| By testing interval 2 | ||||
| Enrollment | 273/342 (79.8%) | 132/167 (79.0%) | - | - |
| 3 months | 205/241 (85.1%) | 148/185 (80.0%) | - | - |
| 6 months | 109/124 (87.9%) | 114/131 (87.0%) | - | - |
| 9 months | 127/149 (85.2%) | 124/143 (86.7%) | - | - |
| Viral suppression<400 copies/mL at 12 months after enrollment 3 | 277/313 (88.5%) | 281/315 (89.2%) | 0.99 (0.94, 1.05) | 0.76 |
| Viral suppression <40 copies/mL | ||||
| By testing interval 2 | ||||
| Enrollment | 225/342 (65.8%) | 80/167 (47.9%) | - | - |
| 3 months | 178/241 (73.9%) | 87/185 (47.0%) | - | - |
| 6 months | 95/124 (76.6%) | 75/131 (57.3%) | - | - |
| 9 months | 111/149 (74.5%) | 87/143 (60.8%) | - | - |
| Viral suppression<40 copies/mL at 12 months after enrollment 3 | 235/313 (75.1%) | 231/315 (73.3%) | 1.02 (0.93, 1.12) | 0.67 |
Abbreviations: CI = confidence interval; VL=viral load; POC=point-of-care; SOC=standard-of-care; ART=antiretroviral therapy
Risk ratios, confidence intervals, and p-values generated by Poisson regression modified with robust standard error estimation, and adjusted for facility and age group strata. Models generating RRs by subgroup also include the subgroup variable as a main effect and the interaction term between the subgroup variable and the intervention. P-values indicate statistical significance of the effect of the intervention, except for p-values for subgroups which are the p-value for the interaction term between subgroup and intervention effect and indicate whether the estimates for the RR differ by subgroup status. For example, the p-value for sex indicates whether the RR for the effect of the intervention is statistically significantly different in males vs. females. Models reporting associations of baseline subgroups with viral suppression are reported in supplemental Table 4,
POC VL results are shown for the intervention group and standard lab-based VL results are shown for the control group
VL results documented at 12 months +/− 16 weeks were included in the analysis
Primary outcome of VS proportions
At 12 months after enrollment, 283/313 (90%) of participants in the intervention group and 289/315 (92%) in the control group achieved VS by POC VL testing (RR 0.99, 95% confidence interval [CI] 0.94, 1.03; Table 2). VS proportions by various subgroups, including by sex, age at enrollment, VS status of caregiver, and whether the caregiver was a biologic parent or not, did not show modification of the relationship between the intervention and VS (Table 2 and main effects in Supplemental Table 3). However, we see some evidence that the effect of the intervention may have varied by subgroups for time on ART (p=0.07), with children on ART for <2 years having VS 77% in intervention vs. 90% in control groups (RR 0.84, 95% CI 0.70, 1.02). When assessing VS using a lower threshold of <400 or <40 copies/mL, the VS proportion at 12 months after enrollment was lower overall than seen with the higher threshold but still similar between the two groups. Findings from the two sensitivity analyses, one for adjusting by sex and second accounting for IPW, demonstrated similar findings as our primary outcome analysis (Supplemental Table 4).
DRM testing, resistance identified, and ART change recommendations
In the intervention group, we identified 138 episodes of viremia, among 81 participants, from enrollment to 9-months post-enrollment (Table 3). We requested 120 DRM tests, of which 107 (89%) were successfully conducted (13 samples failed to amplify). Among successful DRM tests, 107 (100%) identified at least one DRM, of which 91 (85%) tests were classified as major DRMs. The distribution for all DRM test results by HIV drug class were: 61 (57%) NRTI, 88 (82%) NNRTI, and 9 (8%) PI. Our Clinical Management Committee recommended that 33 (31%) children undergo an ART change, of which all 33 (100%) had an ART change documented by 12-months post-enrollment. In contrast, we recorded 72 episodes of viremia, among 56 participants, only 5 DRM tests were requested, 2 (2.8%) of which received a successful DRM test result, and both results documented major DRMs.
Table 3:
Description of drug resistance testing up until 12 months of study follow-up and outcomes by study arm among Opt4Kids study participants (n=704), March 2019-December 2020.1
| Variable | Intervention group (POC VL; n=349) | Control group (SOC VL; n=355) |
|---|---|---|
| Episodes of viremia (may be >1 per participant) | ||
| Participants with viremia (≥ 1000 copies/ml)1 | 81 | 56 |
| Episodes of viremia (≥ 1000 copies/ml)1 | 138 | 72 |
| DRM tests requested | 1202 | 57 |
| DRM tests performed successfully | 107/120 (89%)3 | 2/5 (40%)4 |
| Any DRM identified | 107/107 (100%) | 2/2 (100%) |
| Any major DRM identified | 91/107 (85%) | 2/2 (100%) |
| Any DRM type by HIV drug classes | ||
| NRTI | 61/107 (57%) | 1/2 (50%) |
| NNRTI | 88/107 (82%) | 2/2 (100%) |
| PI | 9/107 (8%) | 1/2 (50%) |
| Major DRM type by HIV drug classes | ||
| NRTI | 61/91 (67%) | 1/2 (50%) |
| NNRTI | 88/91 (97%) | 2/2 (100%) |
| PI | 9/91 (10%) | 1/2 (50%) |
| ART change recommended per each DRM test successfully conducted | 33/107 (31%) | N/A |
| Recommended ART change made by 12-months post-enrollment | 33/33 (100%) | N/A |
Abbreviations: VL=viral load; POC=point-of-care; SOC=standard-of-care; ART=antiretroviral therapy; DRM=drug resistance mutation; NRTI=nucleos(t)ide reverse transcriptase inhibitor; NNRTI= non-nucleos(t)ide reverse transcriptase inhibitor; PI=protease inhibitor
From study enrollment through last date prior to the 12-month study visit (e.g., data excludes results obtained as part of 12-month study visit)
DRM testing was not requested for 16 samples which were from a participant’s second viremic episode, occurring prior to our study’s protocol change requiring DRM testing for all episodes. Two of the remaining 122 had insufficient sample for a DRM test.
Of 120 samples where we requested DRM test, 13 (11%) failed to amplify
We are not able to elucidate reasons why 5 of the samples were selected for DRM testing or why 3 of the 5 requested DRM test were not performed successfully
Secondary process outcomes
Of the participants attending each study visit in the intervention group, 100%, 91%, 55%, 56%, and 100% had a POC VL conducted at 0, 3, 6, 9, and 12 months after enrollment, respectively (Supplemental Table 5); the 6- and 9-month after enrollment visits were heavily impacted by inability to conduct in-person visits during COVID-19 related restrictions in 2020. Among participants in the control group, 47%, 52%, 37%, 40%, and 38% had a SOC VL conducted at 0, 3, 6, 9, and 12 months after enrollment.
Of the POC VL tests conducted in the intervention group during the entire study period, >95% were returned to the participant/caregiver, and ≥80% were returned within 24 hours of the blood draw, excluding the 12-month visit in which only 57% of VL results were returned in 24 hours due to marked disruptions in the global supply of POC VL cartridges for GeneXpert® systems; Supplemental Table 5). Return of results to providers followed similar patterns. Neither the number of total VL tests requests nor the turnaround time from sample collection to participant/caregiver was not available in the control group. From sample collection to result return to providers, the median turnaround time was one day (IQR 0, 1) for POC VL testing and 15 days (IQR 10, 21) for SOC VL testing in the intervention and control groups, respectively.
Viral suppression among unsuppressed or newly initiating ART
VS at 12 months among those initially unsuppressed early in the study or newly initiating ART (n=71 in each group) were similar between the randomized groups (60% in intervention vs. 71% in control groups, RR 0.86, 95% CI 0.66, 1.13; Table 4), and some evidence for those newly initiating ART having better VS than those already on ART (interaction term p-value 0.053).
Table 4:
Viral suppression at 12 months among children newly initiating ART or initially virally unsuppressed
| Intervention group (POC VL) | Control group (SOC VL) | Risk ratio (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Initially unsuppressed, n | Suppressed at 12 months, n (%) | Initially unsuppressed, n | Suppressed at 12 months, n (%) | |||
| Virally suppressed at 12 months (<400 copies/mL | 71 | 36/60 (60.0%) | 71 | 42/59 (71.2%) | 0.86 (0.66, 1.13) | 0.28 |
| By age at enrollment | 0.911 | |||||
| 1–9 years | 49 | 22/38 (57.9%) | 49 | 28/40 (70.0%) | 0.86 (0.61, 1.20) | |
| 10–14 years | 22 | 14/22 (63.6%) | 22 | 14/19 (73.7%) | 0.88 (0.58, 1.33) | |
| By recent ART initiation (within 30 days of enrollment) | 0.051 | |||||
| Newly initiating ART | 10 | 3/7 (42.9%) | 9 | 6/6 (100%) | 0.45 (0.19, 1.05) | |
| On ART | 61 | 33/53 (62.3%) | 62 | 26/53 (67.9%) | 0.94 (0.71, 1.24) | |
Abbreviations: VL=viral load; POC=point-of-care; SOC=standard-of-care; ART=antiretroviral therapy.
interaction term p-value (tests whether effect of intervention is modified by subgroup status)
DISCUSSION
In the first randomized control trial of POC VL testing among children on ART, we did not find greater VS rates among children receiving the intervention vs. SOC 12 months after study enrollment, either overall or when limited to those newly initiating ART or initially virally unsuppressed. CLHIV enrolled in both groups demonstrated similar VS, defined as <1000 copies/mL, at enrollment (84–87%) and 12 months later (90–92%). However, a sizable proportion failed to achieve VS, particularly at lower thresholds of VS of 400 (89%) or 40 (73–75%) copies/mL, and major DRMs were common among viremic children. As expected, turnaround time was markedly faster with POC vs. SOC VL testing. Our study demonstrates the high overall feasibility of utilizing POC VL testing for treatment monitoring among CLHIV.
Our intervention demonstrated the feasibility, but not efficacy, of the use of POC VL testing for CLHIV, a population which experiences lower rates of VS and more rapid clinical progression than adults with virologic failure.7,25 Results on the impact of POC VL in various populations of people living with HIV have been mixed. For example, more frequent POC VL testing had no impact on VS among South African postpartum WLHIV. Preliminary analysis of a Ugandan study suggests modest improvement in VS (7%) among some subsets of people living with HIV, including children, receiving POC VL. POC VL testing combined with a differentiated service delivery strategy enhanced VS by 10.3% and retention by 7.7% among adults living with HIV in South Africa.26 Our failure to demonstrate improved VS may be due to higher-than-expected VS in the SOC group. Compared to historical data from the study facilities showing approximately 65% VS in CLHIV prior to the start of the study, enrolled CLHIV had higher VS at enrollment (84–87%). This could be due to overall improvements in VS among children over time and it is possible that our study enrolled CLHIV who were more likely to be retained in care and have higher rates of VS. For instance, we could not identify a caregiver to give consent for the majority (53%) of eligible participants screened but not enrolled, despite intense efforts. This subgroup of children unaccompanied by caregivers may have worse HIV outcomes, including VS rates, than those with caregivers who could enroll in our study.27–29 Second, it is possible the impact of POC VL testing would become more apparent after a longer duration of follow-up. Although VS can be achieved within three months with improved adherence or after switch to a more effective ART regimen, it is also clear that children with virologic failure often have multiple barriers to adequate adherence and may require a longer time to achieve VS.25,29 Third, our overall focus on the biomedical aspects of HIV care, with the heavy emphasis on the laboratory components of HIV treatment monitoring, did not address the behavioral or structural barriers that exist to adherence to ART among CLHIV. CLHIV with virologic failure likely need additional interventions that address the psychosocial and behavioral issues facing these children and their families. However, considerable work remains to develop effective interventions that work for CLHIV and their families.
Our study showed high uptake of VL testing during non-COVID impacted timepoints, demonstrating the feasibility of 6-monthly VL testing via POC or SOC. While our trial did not demonstrate the efficacy of POC VL testing in improving VS rates among CLHIV, the scale-up of POC VL testing in national testing systems in LMIC can serve a complementary role for centralized testing. The WHO/UNITAID already supports the integration of POC VL testing for treatment monitoring, and international funders and partners have also endorsed POC VL testing. For instance, Kenya already has a strategic plan for integrating POC VL testing in its treatment monitoring guidelines.13 If POC VL testing can be achieved at the same or lower costs, perceived to be more accessible, and have a faster turnaround time than SOC VL testing, POC VL testing may still have the potential to improve HIV care, especially for vulnerable subpopulations like CLHIV. Additional benefits of more accessible POC VL testing may be that it can support differentiated service delivery models, with rapid identification of CLHIV in need of intensified support versus those who may benefit from less frequent visits while maintaining VS.
We also demonstrated the feasibility of implementing DRM testing for children with virologic failure and identified high rates (>80%) of major DRMs in viremic children. This resulted in over a third of children with DRM testing having a recommendation for ART regimen change, though some recommendations for drug changes were related to treatment simplification or optimization and not solely due to drug resistance. Even for children without DRMs that require regimen changes, DRT may guide decisions about whether to continue adherence efforts with the current ART regimen or switch to another regimen. Given that both primary and secondary drug resistance is a growing issue among CLHIV, it is even more important that DRT become more widely available in LMIC. Additional studies may inform how best to use this resource, including modeling and costing studies.
Limitations
We have conducted one of the first studies utilizing POC VL testing combined with DRT to optimize VS among CLHIV; however, our study had limitations. First, as noted above, it is possible we had a sample biased towards higher levels of VS given we only enrolled CLHIV already in care and excluded those in whom we could not contact a caregiver for consent. Second, though our package of POC VL testing, targeted DRM testing, and clinical decision support was only offered to intervention group participants, it was the same providers caring for participants in intervention and control groups. We observed greater fidelity in conducting SOC VL tests in the SOC group than expected and anecdotally, increased confidence in facility staff over time in managing CLHIV with non-VS; therefore, some spillover effect in control participants is possible. Alternative study designs, such as facility-level cluster randomization, may avoid potential spillover effects though would require greater sample sizes. Third, we note that VS was assessed on different schedules for the intervention group (3-monthly) versus the control group (6-monthly unless unsuppressed and then 3-monthly until suppressed), so, theoretically, the intervention group participants had a greater number of opportunities to act on their test results but also greater opportunities to detect viremia. Ultimately, restrictions and reagent stock outs related to COVID-19 greatly affected our ability to conduct POC VL testing every 3 months as planned, which while making our comparisons between groups more reliable, arguably negated our ability to offer our intervention package to the intervention group effectively. While the impacts of COVID-19 may not be as significant in the future, it is likely programs that implement POC VL testing may find similar challenges to ensuring compliance to any VL testing schedule or platform. Fourth, potential measurement bias in ITT estimates and selection bias due to missing outcome data (on approximately 11% of our enrolled participants) are limitations; however, our sensitivity analysis using IPW substantiated our primary outcome analysis findings. Lastly, while we conducted quarterly external quality control testing with our participating laboratories for POC VL testing, formal laboratory validation and verification for the POC VL testing before the start of the study limited local technical partners’ support to substitute study POC VL testing over SOC VL testing. Greater partnerships and coordination at national and local levels will be needed to realize POC VL testing full potential.
Conclusion
In a randomized control trial of POC VL testing among children on ART in western Kenya, VS was not different among children receiving the intervention of POC VL with targeted DRT and clinical decision support vs. SOC at 12 months after study enrollment. Nonetheless, POC VL was highly feasible to implement leveraging existing TB testing systems and resulted in increased access to VL testing and a faster turnaround time for results return than SOC. Among children without VS, rates of drug resistance were concerningly high. Further research is needed to evaluate combination interventions that best utilize POC VL and DRM testing coupled with behavioral support to optimize VS and care for CLHIV.
Supplementary Material
Research in context (no references).
Evidence before this study
We searched online journal databases, such as PubMed, for randomized controlled trials evaluating the effects of point-of-care (POC) HIV viral load testing in people living with HIV and receiving antiretroviral therapy (ART). We included trials published up to February 5, 2022, in any language. Our search included several combinations of key terms, such as: “HIV,” “point-of-care,” “viral load,” “adolescents,” “children,” and “point-of-care viral load (“POC VL”) testing.” We screened abstracts for relevance and then retrieved the full-text articles if deemed relevant; we examined the reference lists from these articles for any additional relevant studies. We also searched conference abstracts of leading HIV conferences to find data that has not yet been published and archived in PubMed.
We identified a few pertinent studies with mixed evidence regarding the impact of POC VL on viral suppression (VS). A randomized controlled trial among adults in South Africa, STREAM, found that paired POC VL testing with task shifting enhanced VS and retention in HIV care. In contrast, preliminary analysis from a study among postpartum women in South Africa did not find that POC VL testing improved VS rates. Another trial in South Africa, the POwER study, aims to assess the feasibility of POC VL testing to manage viremia among people living with HIV ≥18 years, though its findings are not yet published. Preliminary analysis from a study conducted in Uganda demonstrated modest improvements (7%) in VS among subsets of people living with HIV undergoing POC VL testing. The Clinton Health Access Initiative evaluated the impact of near POC VL testing across seven countries in sub‐Saharan Africa. Their findings suggest that near POC VL testing enabled rapid test result delivery for high-risk populations and significantly improved turnaround time for results compared to centralized testing. Finally, a study evaluating POC VL testing among adolescents and young adults with HIV in Haiti identified no difference in VL outcomes between their study groups. Thus, while POC VL testing improves timeliness of result return, it does not consistently improve VS. However, there are no studies assessing impact of POC VL on VS in children with HIV (CLHIV).
Added value of this study
Our study is the first study to integrate POC VL testing with HIV drug resistance testing (DRT) among CLHIV and to evaluate this combination’s impact on VS. We found no difference in VS between CLHIV who underwent POC VL testing combined with DRT vs. those who received standard care. POC VL was feasible to implement in clinics with GeneXpert® tuberculosis testing systems and resulted in significant improvements in turnaround time for HIV VL results. DRT identified a high prevalence of HIV drug resistance among CLHIV on ART with viremia.
Implications of all the available evidence
Our findings, alongside all available evidence, suggest that POC VL testing significantly decreases turnaround time but does not necessarily improve VS among all subsets of people living with HIV, including CLHIV. Nonetheless, a significant portion of CLHIV did not achieve VS and these children face high rates of HIV drug resistance. More comprehensive interventions that couple biomedical testing with psychosocial support may be necessary to improve VS in the remaining CLHIV with viremia.
Acknowledgements
We recognize the study participants, caregivers, and facility staff participating in and supporting the Opt4Kids study. We acknowledge the support of the Kisumu County Health Management Team, Ministry of Health, and Family AIDS Care and Education Services. We also thank members of our Scientific Advisory Committee.
Funding:
National Institutes of Mental Health of the U.S. National Institutes of Health, Thrasher Research Fund
Role of the funding source
Support for this study is provided by the National Institutes of Mental Health of the U.S. National Institutes of Health (NIH, R34MH115769) and the Thrasher Research Fund. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Washington Institute of Translational Health Sciences and supported by the National Center for Advancing Translational Sciences of the NIH (UL1 TR002319). The funding sources or study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials registration: NCT03820323
Ethical review
Ethical approval for this study has been obtained from the African Medical and Research Foundation (AMREF) and Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH) Institutional Review Boards (IRBs) in Kenya, as well as the University of Washington and the University of Colorado Denver IRBs in the United States.
Data sharing
De-identified participant data, data dictionary, or other specified data sets may be made available to others requesting them upon communication with corresponding author, demonstration of appropriate ethic reviews, and establishment of data sharing agreements. Study protocol, statistical analysis plan, informed consent forms, analysis code, or other documents can be made available upon request with the corresponding author.
References
- 1.Lecher SWJ, Fonjungo PN, et al. Progress with scale-up of HIV viral load monitoring—seven sub-Saharan African countries. Morbidity and Mortality Weekly Report 2016; 65(47): 1332–5 January 2015–June 2016. [DOI] [PubMed] [Google Scholar]
- 2.Lecher SED, Kim AA, et al. Scale-up of HIV viral load monitoring—seven sub-Saharan African countries. Morbidity and Mortality Weekly Report 2015; 64(46): 1287–90 2015. [DOI] [PubMed] [Google Scholar]
- 3.Glass TR, Motaboli L, Nsakala B, et al. The viral load monitoring cascade in a resource-limited setting: A prospective multicentre cohort study after introduction of routine viral load monitoring in rural Lesotho. PLoS One 2019; 14(8): e0220337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens WS, Marshall TM. Challenges in implenting HIV load testing in South Africa. Journal of Infectious Diseases 2010; 201(Supplement_1): S78–S84. [DOI] [PubMed] [Google Scholar]
- 5.Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. The Lancet Infectious diseases 2018; 18(2): 188–97. [DOI] [PubMed] [Google Scholar]
- 6.Etoori D, Ciglenecki I, Ndlangamandla M, et al. Successes and challenges in optimizing the viral load cascade to improve antiretroviral therapy adherence and rationalize second‐line switches in Swaziland. Journal of the International AIDS Society 2018; 21(10): e25194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadima J, Patterson E, Mburu M, et al. Adoption of routine virologic testing and predictors of virologic failure among HIV-infected children on antiretroviral treatment in western Kenya. PLoS One 2018; 13(11): e0200242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutstein SE, Golin CE, Wheeler SB, et al. On the front line of HIV virological monitoring: barriers and facilitators from a provider perspective in resource-limited settings. AIDS Care 2016; 28(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simeon KSM, Dorward J, et al. Comparative cost analysis of point-of-care versus laboratory-based testing to initiate and monitor HIV treatment in South Africa. PLoS One 2019; 14(10): e0223669 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulterys MA, Oyaro P, Brown E, et al. Costs of Point-of-Care Viral Load Testing for Adults and Children Living with HIV in Kenya. Diagnostics (Basel) 2021; 11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandbulte MR, Gautney BJ, Maloba M, et al. Infant HIV testing at birth using point-of-care and conventional HIV DNA PCR: an implementation feasibility pilot study in Kenya. Pilot Feasibility Stud 2019; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bwana P, Ageng’o J, Mwau M. Performance and usability of Cepheid GeneXpert HIV-1 qualitative and quantitative assay in Kenya. PLoS One 2019; 14(3): e0213865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Health KMo. National Point of Care Testing Implementation Roadmap in Kenya 2019 Edition. Nairobi, Kenya: June 2019. Print, 2019. [Google Scholar]
- 14.Huibers MHWMP, Cornelissen M, et al. High prevalence of virological failure and HIV drug mutations in a first-line cohort of Malawian children. . J Antimicrob Chemother 2018; 73(12): 3471–5 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paula Vaz WCB, Bhatt Nilesh, Bila Dulce, Auld Andrew, Houston James, Cossa Loide, Alfredo Charity, Jobarteh Kebba, Sabatier Jennifer, Macassa Eugénia, Sousa Amina, DeVos Josh, Jani Ilesh, Yang Chunfu, . Compromise of Second-Line Antiretroviral Therapy Due to High Rates of Human Immunodeficiency Virus Drug Resistance in Mozambican Treatment-Experienced Children With Virologic Failure, . Journal of the Pediatric Infectious Diseases Society, Volume 9, Issue 1, March 2020, Pages 6–13, https://doiorg/101093/jpids/piy102 2020. [DOI] [PubMed] [Google Scholar]
- 16.Hunt GMLJ, Salimo A, Kalimashe M, Dinh TH, Jackson D, Sherman G, Puren A, Ngandu NK, Lombard C, Morris L, Goga A. . Prevalence of HIV-1 drug resistance amongst newly diagnosed HIV-infected infants age 4–8 weeks, enrolled in three nationally representative PMTCT effectiveness surveys, South Africa: 2010, 2011–12 and 2012–13. . BMC Infect Dis 2019 Sep 16;19(Suppl 1):787 doi: 101186/s12879-019-4339-y 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inzaule SCOS, Akinbiyi G, Emeka A, Khamofu H, Mpazanje R, Ilesanmi O, Ndembi N, Odafe S, Sigaloff KCE, Rinke de Wit TF, Akanmu S. . High Prevalence of HIV Drug Resistance Among Newly Diagnosed Infants Aged <18 Months: Results From a Nationwide Surveillance in Nigeria. J Acquir Immune Defic Syndr 2018 Jan 1;77(1):e1–e7 doi: 101097/QAI0000000000001553 2018. [DOI] [PubMed] [Google Scholar]
- 18.Jordan MR PM, Cournil A, Vubil A, Jani I, Hunt G, Carmona S, Maphalala G, Mthethwa N, Watera C, Kaleebu P, Musanhu CC, Mtapuri-Zinyowera S, Dzangare J, Peeters M, Yang C, Parkin N, Bertagnolio S. . Human Immunodeficiency Virus (HIV) Drug Resistance in African Infants and Young Children Newly Diagnosed With HIV: A Multicountry Analysis. . Clin Infect Dis 2017 Nov 29;65(12):2018–2025 doi: 101093/cid/cix698 2017. [DOI] [PubMed] [Google Scholar]
- 19.NASCOP. Ministry of Health, National AIDS & STI Control Program. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018 Edition, Print. Nairobi, Kenya, August 2018. . [Google Scholar]
- 20.NASCOP. Ministry of Health, National AIDS & STI Control Program. New guidance on ART transition for children and adolescents less than 15 years of age living with HIV (CALHIV) in Kenya. 2019. Print. Nairobi, Kenya, August 2018. [Google Scholar]
- 21.Ministry of Health NASCP. DTG Use of Dolutegravir based Regimen in Adolescent Girls & Women Living with HIV. Nairobi, Kenya: NASCOP, 2020. Print 2020 Edition. [Google Scholar]
- 22.Ministry of Health NASCP. Use of Dolutegravir based regimen in Adolescent Girls and Women Living with HIV Nairobi, Kenya: . NASCOP, Print. [Google Scholar]
- 23.Patel RC, Oyaro P, Odeny B, et al. Optimizing viral load suppression in Kenyan children on antiretroviral therapy (Opt4Kids). Contemp Clin Trials Commun 2020; 20: 100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Z. G A modified poisson regression approach to prospective studies with binary data. . Am J Epidemiol 2004;159(7):702–6 2004. [DOI] [PubMed] [Google Scholar]
- 25.Boerma RSBT, Bussink AP, et al. Suboptimal Viral Suppression Rates Among HIV-Infected Children in Low- and Middle-Income Countries: A Meta-analysis. . Clin Infect Dis 2016;63(12):1645–1654 doi:101093/cid/ciw645 2016. [DOI] [PubMed] [Google Scholar]
- 26.Drain PK, Dorward J, Violette LR, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV 2020; 7(4): e229–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasuuna E, Kigozi J., Muwanguzi PA et al. Challenges faced by caregivers of virally non-suppressed children on the intensive adherence counselling program in Uganda: a qualitative study. . BMC Health Serv Res 19, 150 (2019) https://doiorg/101186/s12913-019-3963-y 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross RBT, Langhaug L, Mujuru H, Lowenthal E, Ferrand R. . Factors associated with self-reported adherence among adolescents on antiretroviral therapy in Zimbabwe. . AIDS Care 2015; 27: 322–26 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowenthal ED MT, Tshume O, et al. Parental absence from clinic predicts human immunodeficiency virus treatment failure in adolescents. . JAMA Pediatr 2015; 169: 498–500 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data, data dictionary, or other specified data sets may be made available to others requesting them upon communication with corresponding author, demonstration of appropriate ethic reviews, and establishment of data sharing agreements. Study protocol, statistical analysis plan, informed consent forms, analysis code, or other documents can be made available upon request with the corresponding author.


