Summary
Background
Although the American Academy of Sleep Medicine suggests at least 9 hours of sleep per day for 6- to 12-year-olds, children in recent generations often report sleeping less than this amount. As early adolescence is a critical period for neurocognitive development, we investigated how insufficient sleep has impacted children’s mental health, cognition, brain function and structure over two years.
Methods
We obtained large-scale data from the ongoing Adolescent Brain Cognitive Development study, and included 8,323 eligible participants aged 9–10 years from 21 U.S. study sites. Participants were separated into two groups, namely sufficient sleep (SS) versus insufficient sleep (IS) groups based on a cutoff of 9 hours of sleep. Using propensity score matching (PSM), we matched these two groups of participants on 11 key covariates, including sex, socioeconomic status, puberty status, etc. The outcome measures are behavioral problems, mental health, cognition, structural and resting-state functional brain measures, assessed at baseline and at two-year follow-up (FL2). We examined group differences on these outcomes over those 2 years.
Findings
We identified 3,021 matched SS-IS pairs at baseline and 749 matched pairs at FL2, and observed similar SS-IS differences in behavior and neural measures at both points in time. For example, the effect sizes of SS-IS differences in behavioral measures at these two timepoints were significantly correlated with each other (r = 0.85, 95% CI 0.73–0.92, p < 0.0001). A similar pattern was observed in resting-state functional connectivity (r = 0.54, 95% CI 0.45–0.61, p < 0.0001) and in structural measures (r = 0.52, 95% CI 0.40–0.61, p < 0.0001). These results suggest the temporal stability of compromised neurocognitive development is associated with insufficient sleep. We then performed mediation analyses to reveal the neural correlates of behavioral changes induced by insufficient sleep. We found that cortico-basal ganglia functional connections mediate the effects of insufficient sleep on depression, thought problems, and crystallized intelligence, and that structural properties of the anterior temporal lobe mediate the impact of insufficient sleep on crystallized intelligence.
Interpretation
These results provide population-level evidence for the long-lasting impact of insufficient sleep on neurocognitive development in early adolescence. These findings highlight the value of early sleep intervention to improve early adolescents’ long-term developmental outcomes.
Funding
This study is supported by NIH grants: R01AG060054, R01AG070227, R01EB031080-01A1, P41EB029460-01A1, 1UL1TR003098.
Introduction
Early adolescents frequently report insufficient and poor sleep.1 As the brain maturation process is vulnerable to sleep loss,2 young adolescents with insufficient sleep often show compromised neurocognitive functions, manifested as poorer academic performance and reduced social-emotional skills.3 However, the neural mechanisms underlying the adverse effects of insufficient sleep on adolescent development remain poorly understood.4 This could be due to many reasons. First, several covariates, such as socioeconomic status,5 sex,6 and pubertal status,7 can substantially influence adolescents’ sleep patterns and their neurocognitive functions. For example, a child’s sleep duration and household income are often highly correlated (see Table S1), which would make it difficult to isolate the direct impact of insufficient sleep on neurocognitive development. Second, as prior work has primarily employed cross-sectional designs,3,8 it remains unknown whether insufficient sleep has a temporary or long-lasting impact on early adolescent development. For instance, although the American Academy of Sleep Medicine has suggested 9–12 hours of sleep per day for 6–12 years old,9 it is unclear how less than 9 hours of sleep would affect children’s behavior, brain structure and function over time. Clarification of these issues is critical for understanding neurocognitive vulnerability and resilience to insufficient sleep in the developing brain.10 It will also inform early sleep interventions to improve long-term developmental outcomes in adolescents.
In this study, therefore, we applied propensity score matching (PSM) to investigate how insufficient sleep impacts the brain and behavior in early adolescents across two years after controlling for various key covariates, such as age, sex, race, socioeconomic status, and pubertal status.11 We leveraged data from a population-based sample of US 9–10-year-olds in the Adolescent Brain Cognitive Development (ABCD) study.12 We hypothesized that insufficient sleep would have long-lasting adverse impacts on participants’ neurocognitive developmental outcomes captured by behavioral, brain functional and structural measures. In addition, motivated by previous research,13 we predicted that brain function and structure should mediate the long-term effects of sleep duration on early adolescents’ cognitive and affective functions.
Methods
Data source.
Data used in the current study were from the ABCD data release 3.0 (2021) (https://abcdstudy.org), which includes behavioral and neural data from 11,878 9–10-year-olds collected at baseline, 1-year (FL1), and 2-year follow-ups (FL2). Detailed protocols and designs have been described previously.12 Informed consent from the primary caregiver and assent from the children were obtained before the study. This project was approved by the Institutional Review Boards of local study sites (21 in total). Participants were recruited using stratified sampling to reflect the diversity of the U.S. population. Participants were excluded from data analysis if they did not pass the baseline resting-state fMRI quality check (n = 2,491) or had missing data for the covariates involved in PSM (n = 1064). After exclusion, 8,323 (4153 female, 49.9%) out of the entire 11,878 participants were included in this study (see Figure S1 for details).
Measurements.
We estimated a child’s sleep duration as an independent variable based on the parent-reported Sleep Disturbance Scale for Children administered at baseline, FL1, and FL2. We used the data from the question, “How many hours of sleep does your child get on most nights in the past six months?”, which provide estimates of a child’s sleep duration in 5 categories (see Table S1). Based on the recommendation by American Academy of Sleep Medicine, we identified children with < 9-hours of sleep per day at baseline as the insufficient sleep group (IS, n = 4,181) and children with >= 9-hours of sleep per day as the sufficient sleep group (SS, n = 4,142).9
We then included dependent variables of interest from the following four categories: (1) Behavioral problems. We used the parent-reported Child Behavior Checklist (cbcl) at baseline, FL1, and FL2 to capture a child’s behaviors in emotional, social, and behavioral domains. (2) Cognition. We used scores from the NIH Cognition Battery Toolbox (nihtbx) at baseline and FL2 to assess a child’s general cognitive function. (3) Mental Health. We estimated a child’s overall mental health at baseline and FL2 based on scores from the Prodromal Psychosis Scale (pps), UPPS-P impulsive behavior scale, Behavioral inhibition scale (BIS). Table S3 summarizes these variable names used in the ABCD dataset. (4) Brain Measures. All children underwent standardized resting-state fMRI, structural MRI, and diffusion tensor imaging scans at baseline and FL2. Acquired images were processed and quality controlled at the Data Analysis, Informatics and Resource Center of the ABCD study.14 Resting-state functional connectivity (rs-FC) of cortical networks was calculated as the average Fisher-transformed correlation between the time courses of each pair of regions within or between 12 cortical networks defined by the Gordon atlas. In addition, rs-FC between the 12 cortical networks and 19 subcortical regions were also calculated (Figure 3). Structural measures, including cortical area, gray matter volume (GMV), and cortical thickness from 148 regions were extracted based on the Destrieux Parcellation. Subcortical volumes of 36 regions and fractional anisotropy (FA) of 35 tracts from diffusion tensor imaging were also calculated based on protocols in the ABCD study.
Figure 3. The effects of insufficient sleep on resting-state functional connectivity.
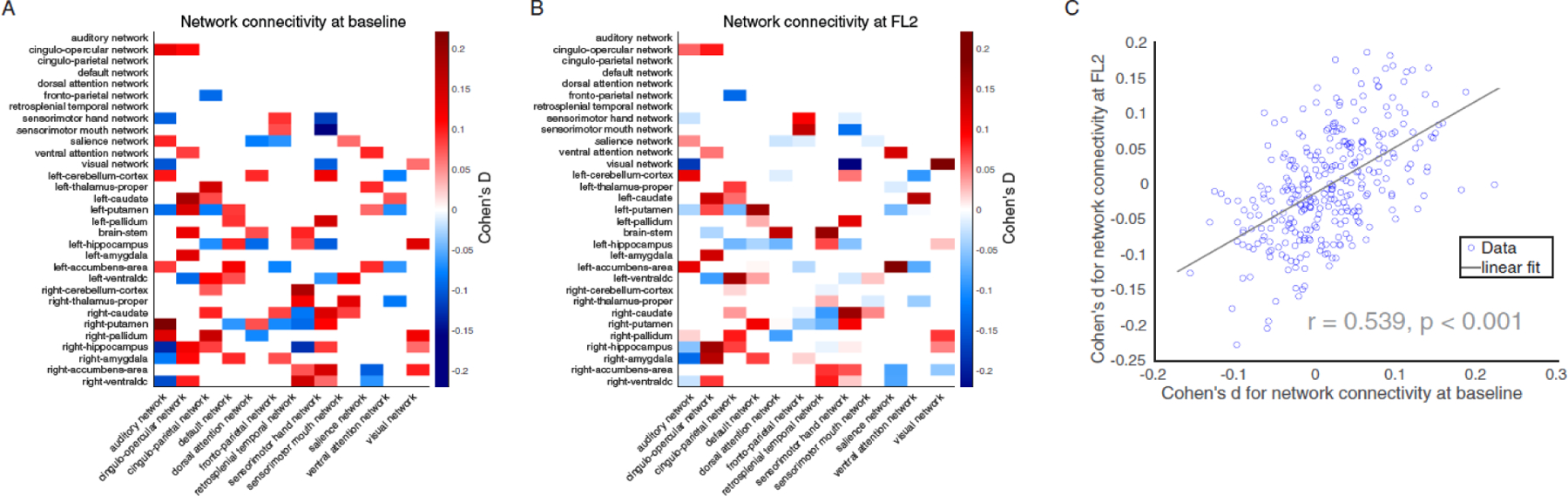
Cohen’s d for resting-state functional connectivity measures in the comparisons between SS and IS group at baseline (A) and at FL2 (B). Red denotes SS group have higher connectivity than IS group, while blue means SS group have lower connectivity compared to IS group. (C) The impact of insufficient sleep on resting-state functional connectivity at baseline are significantly correlated with that on FL2. Note: SS = sufficient sleep; IS = insufficient sleep; FL2 = 2-year follow-up.
We considered the following covariates included in PSM: (1) basic demographic characteristics of a participant (age in months, sex, the interaction between age and sex, race, and study sites; See Supplement); (2) theoretically relevant factors in adolescent sleep patterns, including parent education level and household income;5 pubertal status7 (assessed by ABCD Youth Pubertal Development Scale and Menstrual Cycle Survey History), and body mass index (BMI);15 (3) fMRI data quality indices, including average motion during resting scans (mean framewise-displacement, FD) and the number of fMRI time points that remained after preprocessing, as both head motion and image quality are critical for interpreting pediatric structural and functional neuroimaging.16
Data Analysis.
At the outset we performed PSM to control for covariates in these observational data so that the causal impacts of insufficient sleep on the outcome measures could be estimated. We proceeded with this analysis using the MatchIt R package.11 In this procedure, participants were matched based on the probability of being in a comparison group conditioned on observed covariates using logistic regression. As previously mentioned, these covariates included demographic variables, theoretically relevant factors, and data quality indices. We then matched participants with sufficient versus insufficient sleep based on one-to-one matching without replacement within a predefined propensity score radius (i.e., caliper = 0.1). To check for matching quality, we compared the standardized mean difference of covariates between the SS and IS groups and found that all the covariates were well balanced between groups after matching (Figure S2). Hence, additional group differences could not be attributed to these covariates. Ultimately, we identified 3,021 matching pairs at baseline with both behavioral and neuroimaging data, of which 2,762 pairs had FL1 (behavioral) data and 749 pairs had FL2 (neuroimaging) data. An independent-sample t-test was used to assess these group differences, because it tends to yield a more conservative effect size estimate as compared with paired-sample t-test.17 For neuroimaging data, we focused on available participant pairs that had passed quality control for each corresponding brain measure.14 Multiple comparisons were corrected for baseline measures based on false discovery rate (FDR). To test the extent to which sleep duration has similar effects on behavioral and neural outcome measures over time, we directly compared the effect sizes of sleep duration on outcome measures at baseline and FL2.18
Second, motivated by the conceptual framework that brain measures would mediate the relationship between sleep patterns and development outcomes,13 we tested whether baseline brain measures can mediate the effect of baseline sleep duration on behavioral measures at baseline and FL2 after controlling for the 11 covariates that were used in PSM (see Supplement for details). For the analyses of FL2 data, the corresponding baseline behavioral measure was added as an additional covariate. Of primary interest, we focused on brain rs-FC and behavior assessments that showed a Cohen’s d greater than 0.15 between SS and IS groups, which is 50% higher than a typical effect size identified based on the ABCD dataset (Cohen’s d = 0.03–0.09).19 This application of effect size as a threshold measure may improve replicability in neuroimaging findings.20 We implemented these analyses via an established neuroimaging mediation toolbox.21 The significance of these mediation analyses was bootstrapped with 100,000 random-generated samples.
Role of the funding source.
Funders played no role in study design, data collection, analysis, interpretation, writing of the manuscript, and the decision to submit for publication.
Results
Sleep patterns over 2 years
We first examined how participants’ sleep patterns changed over time. Based on baseline measures, we matched participants in the sufficient sleep (SS) group with those in the insufficient sleep (IS) group on 11 key covariates using PSM (Figure S2). In follow-up assessments, we found that participants’ sleep patterns in the IS group have remained relatively stable (<20% change), whereas participants in the SS group tended to sleep less and less over time (Figure 1). Because the impact of insufficient sleep on neurocognitive functions may be cumulative and even irreversible as suggested by animal studies,22 we expected that participants with initial insufficient sleep would show adverse effects on behavioral and brain measures at follow-up assessments as compared with the SS group. Hence, we retained the group assignment for these well-matched pairs in subsequent analyses to investigate the long-term effects of insufficient sleep on adolescent neurocognitive development.
Figure 1. Sleep duration changes in SS and IS groups over two years.
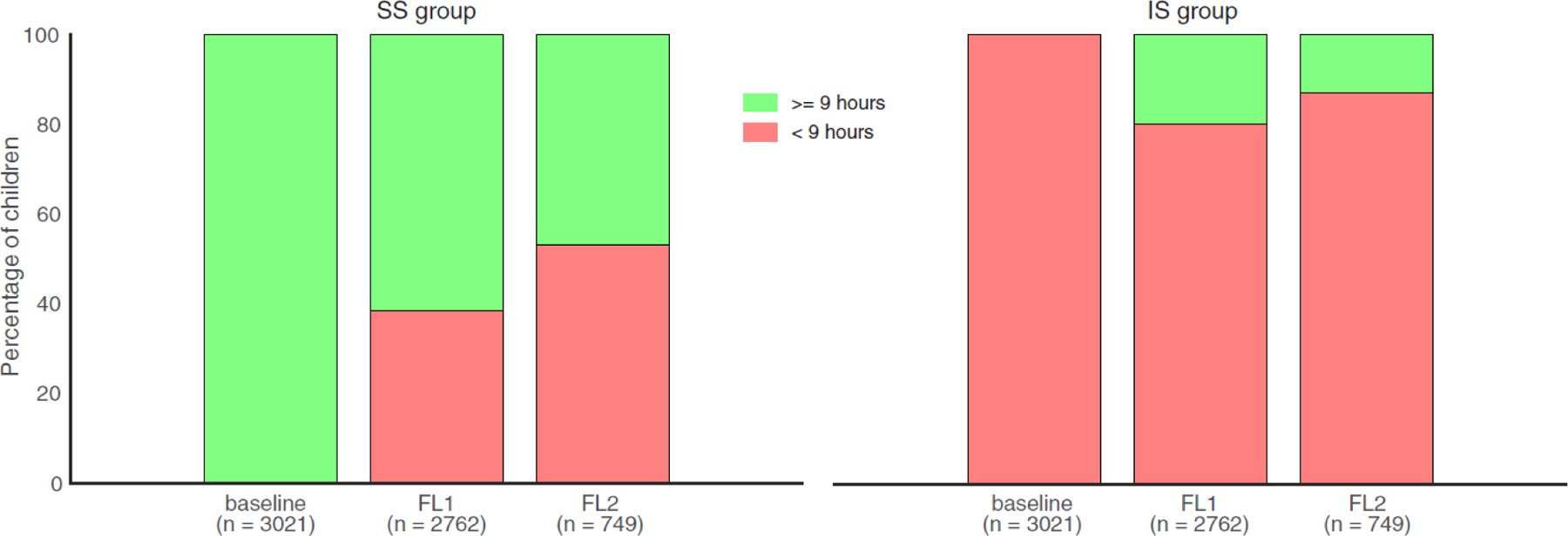
SS (left) and IS (right) groups were identified based on the baseline assessment of sleep duration (9 hours as a cutoff) with well-matched covariates. Over two years, participants’ sleep pattern in the IS group remained relatively stable, whereas participants in the SS group slept less and less. In addition, we performed a visual illustration of how sleep duration changes with crystallized intelligence, see Figure S6. Note: SS = sufficient sleep; IS = insufficient sleep; FL1 = 1-year follow-up; FL2 = 2-year follow-up.
Stable effects of insufficient sleep on behavioral measures over two years
We next tested how insufficient sleep influenced the 42 behavioral measurements that capture adolescent behavioral problems (e.g., aggression and rule-breaking, 20 items), cognitive functions (e.g., fluid and crystallized intelligence, 10 items), and mental health (e.g., psychosis and impulsivity, 12 items) at baseline and at FL2. Consistent with previous findings,8 we found that insufficient sleep had widespread impacts on baseline behavioral measures. Specifically, 32 of these 42 assessments showed a significant difference at baseline between SS and IS groups after propensity score matching on covariates (Figure 2A; p < .05, FDR corrected). Among these effects, four behavioral measures had an absolute Cohen’s d value higher than 0.15, including depression, thought problems, picture-vocabulary test performance, and crystallized intelligence. We observed similar patterns at FL2, with comparable effect sizes as those at baseline (Figure 2B). To quantify the extent to which insufficient sleep had similar impacts on these behavioral measures at both time points,18 we correlated the Cohen’s ds of the difference between SS and IS groups at FL2 and that at baseline for all 42 behavioral measures. We found that these effects were significantly correlated with one another (Figure 2C; r = 0.85, 95% CI 0.73–0.92, p < 0.0001), suggesting that insufficient sleep has stable impacts on adolescents’ behavioral problems, neurocognition, and mental health over two years. Although FL1 only included measures of behavioral problems, we observed similar findings between baseline and FL1 assessments (Figure S3).
Figure 2. The effects of insufficient sleep on behavioral measures.
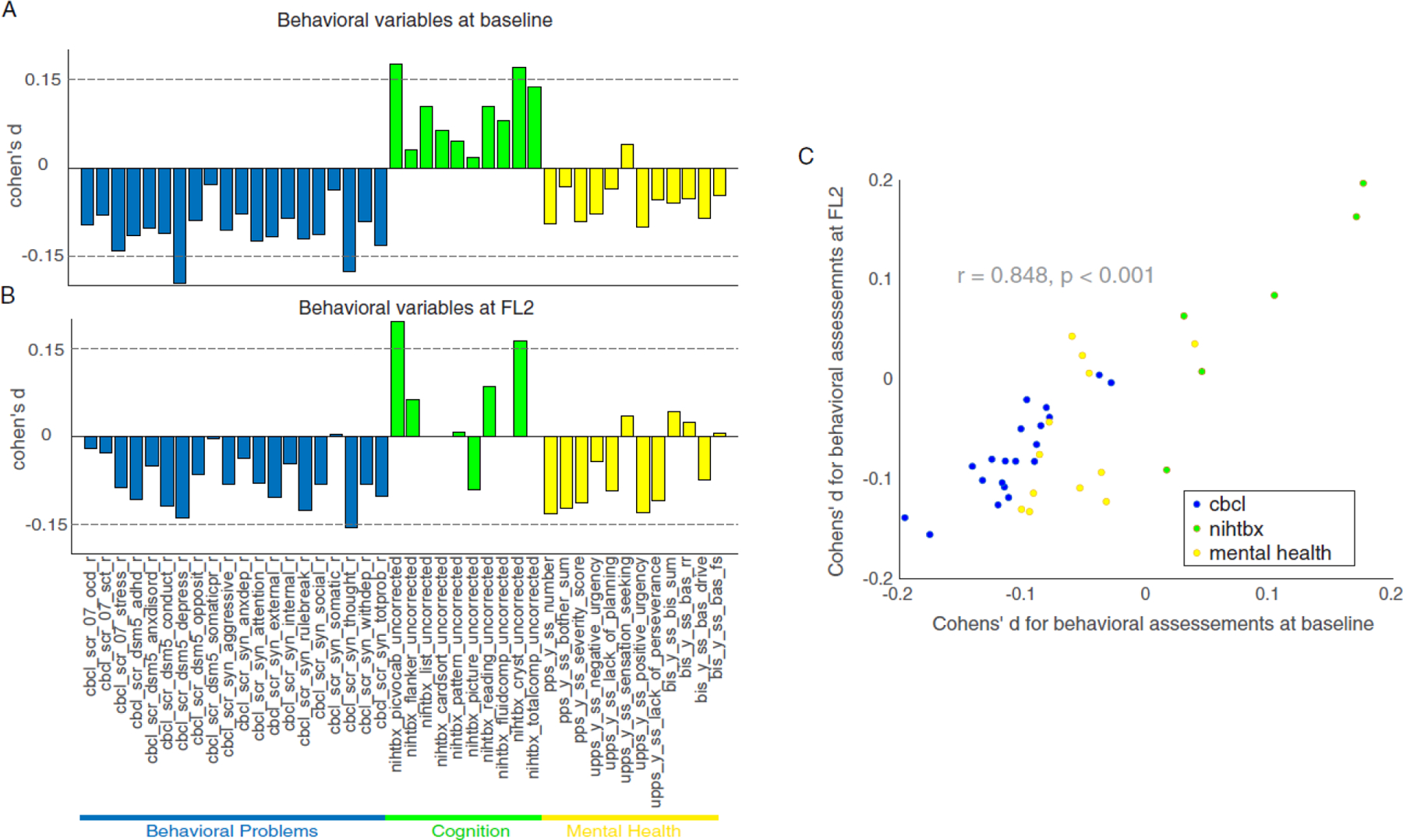
Cohen’s d for behavior problems (blue), cognition (green), and mental health (yellow) in the comparions between SS and IS groups at baseline (A) and at FL2 (B). (C) The impact of insufficient sleep on behavioral measures at baseline are significantly correlated with that on FL2. Note: SS = sufficient sleep; IS = insufficient sleep; FL2 = 2-year follow-up; see Table S3 for variable names used in the figure. At FL2, four measures of cognition were not available: nihtbx_list_uncorrected, nihtbx_cardsort_uncorrected, nihtbx_fluidcomp_uncorrected, and nihtbx_totalcomp_uncorrected.
Stable effects of insufficient sleep on resting-state functional connectivity over two years
We next examined how sleep duration affects the intrinsic functional organization of brain networks in the developing brain at baseline and at FL2. At baseline, we found that 93 out of the 306 unique functional connections showed a significant difference between the SS and IS groups (Figure 3A; p < 0.05, FDR corrected). Noticeably, four out of the five connectivities that had a Cohen’s d value higher than 0.15 involved regions of the basal ganglia, which plays a core role in regulating the sleep-wake cycle.23 Additionally, the effect sizes of sleep duration on all 306 available functional connectivities were significantly correlated with that in FL2 (r = 0.54, 95% CI 0.45–0.61, p < 0.0001, Figure 3C). These results suggest that certain functional connectivity measures are consistently vulnerable to insufficient sleep over time.
Stable effects of insufficient sleep on brain structures over 2 years
We further investigated how sleep duration affects the development of brain structures at baseline and at FL2. At baseline, we found that GMV in 12 out of 184 regions showed a statistically significant difference between the SS and IS groups (Figure 4A; p < 0.05, FDR corrected). A similar pattern was found in FL2 (Figure 4B). The pattern of Cohen’s d on GMV in the 184 regions at baseline was significantly correlated with that at FL2 (Figure 4C; r = 0.52, 95% CI 0.40–0.61, p < 0.0001), suggesting that certain structural measures are consistently susceptible to insufficient sleep over time. While we obtained similar results based on cortical areas (Figure 4D–4F), we did not observe statistically significant differences between SS and IS groups in measures of cortical thickness or FA (all p > 0.05, FDR corrected, see Supplement).
Figure 4. The effects of insufficient sleep on brain structural measurements.
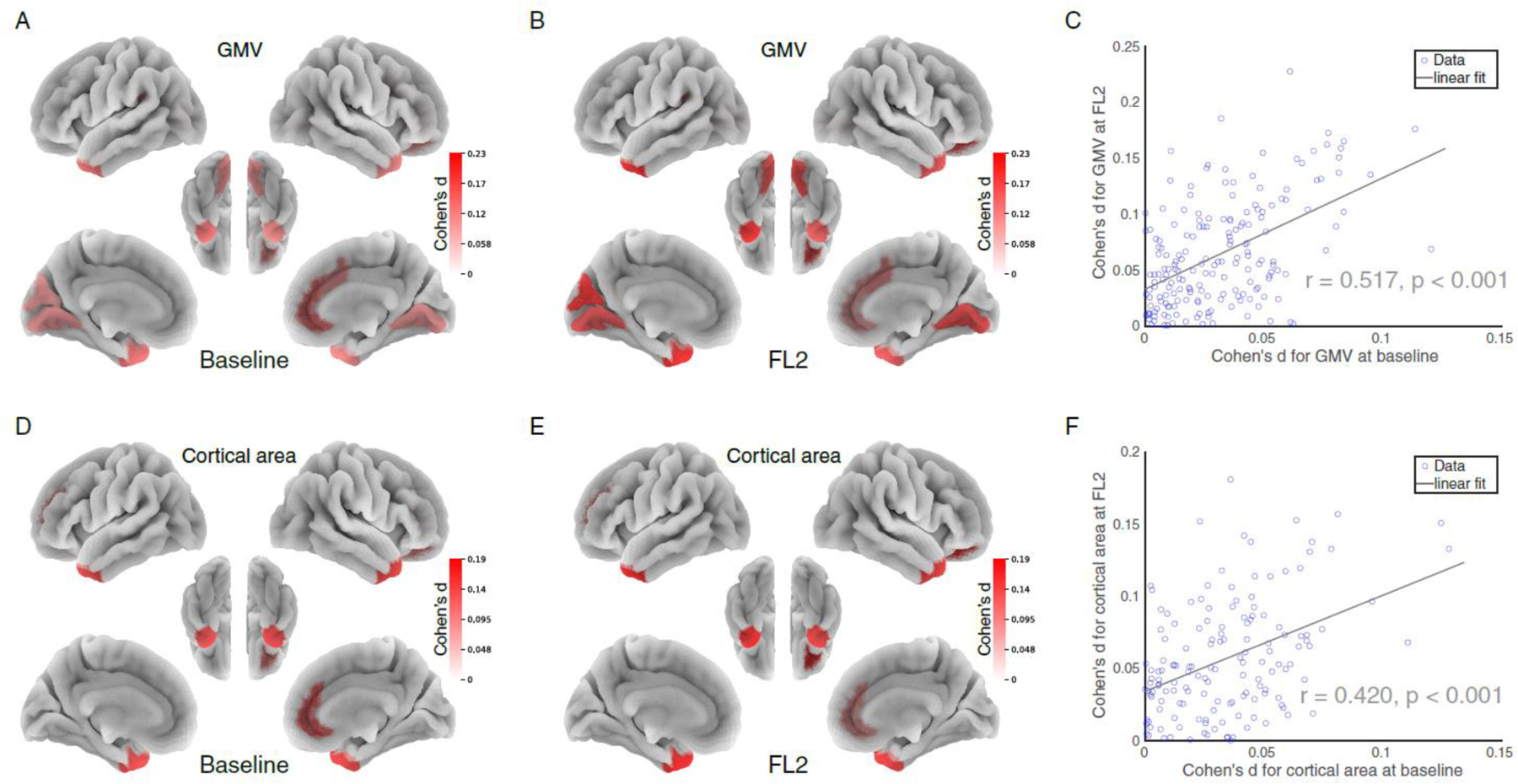
Cohen’s d for GMV (A, B) and cortical areas (D, E) in the comparions between SS and IS groups at baseline and at FL2. (C, F) The impact of insufficient sleep on brain structure measure at baseline are significantly correlated with that on FL2. Note: SS = sufficient sleep; IS = insufficient sleep; FL2 = 2-year follow-up.
Brain measures mediate the effects of sleep duration on behavior
Finally, to reveal how brain measures mediate the relationship between sleep patterns and behavioral outcomes, we tested whether the brain measures identified with larger effect sizes in respective categories at baseline (i.e. five rs-FC measurements, three cortical areas, and three GMV, see Figure 5C & 5D Y-axis) mediated the effects of sleep duration on the four behavioral measures identified with a Cohen’s d greater 0.15 (i.e., depression, thought problems, picture-vocabulary test performance, and crystallized intelligence). In this analysis, we regressed out covariances used in the PSM. At baseline, we found that the identified functional and structural brain measures robustly mediated the effects of sleep duration on picture vocabulary test performance and crystallized intelligence (Figure 5C; bootstrapped p <.05). Three out of five rs-FC measures also mediated the effect of insufficient sleep on depression and thought problems (Figure 5C; bootstrapped p < .05). In addition, we identified distributed mediation patterns across all behavioral measures (See Figure S5 for details).
Figure 5. Baseline brain measures mediated the effect of insufficient sleep on behavioral problems and cognition at baseline (A & C) and FL2 (B & D).
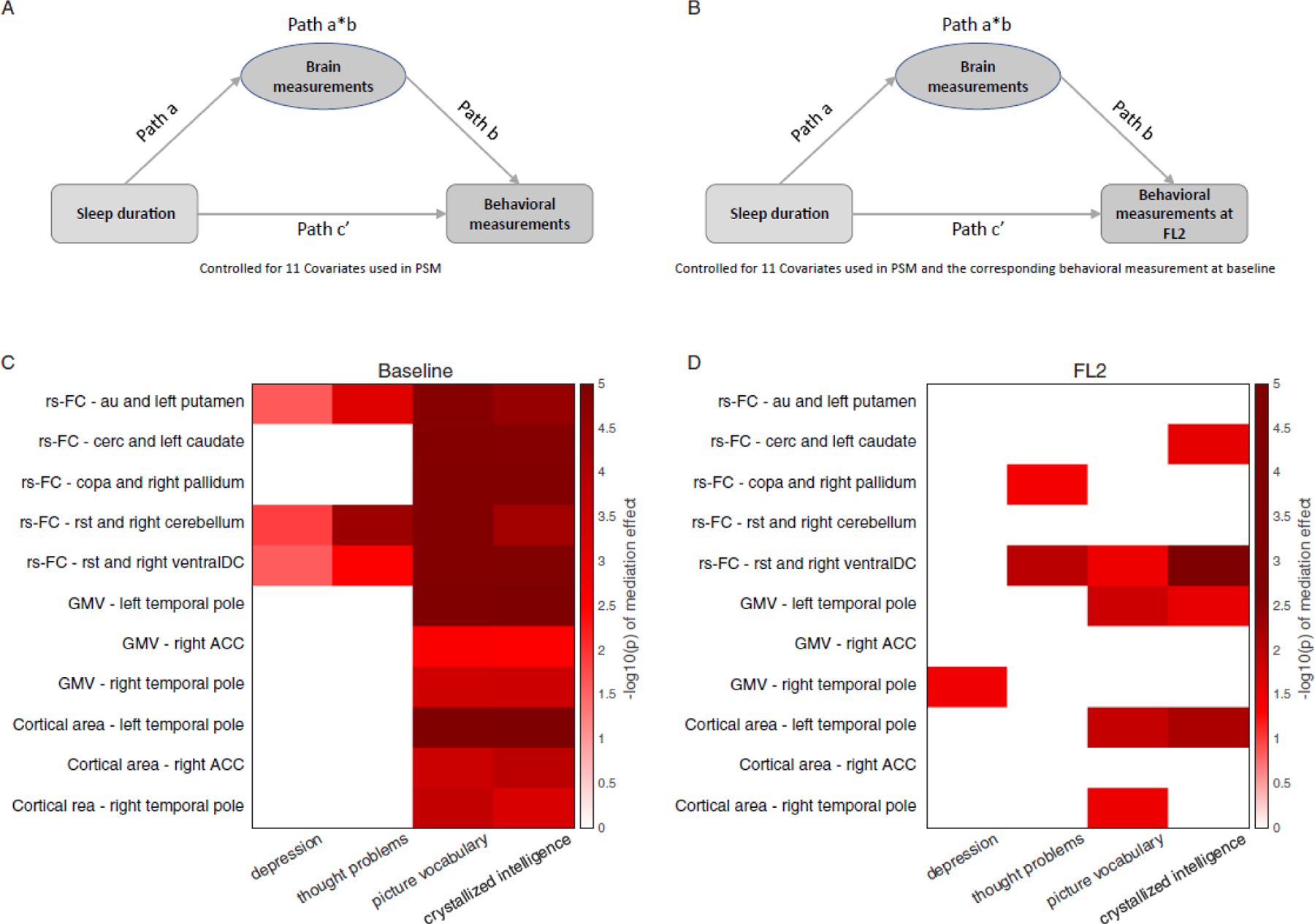
Based on matched group comparisons, we have examined how brain measures identified with larger effect sizes in respective categories (i.e.,rs 5 rs-FC, 3 cortical areas, and 3 GMV; Y-axis) would mediate the effects of sleep duration on the 4 behavioral measures identified with a Cohen’s d greater 0.15 (i.e., depression, thought problems, picture-vocabulary test performance, and crystallized intelligence; Y-axis). Also see Figure S5 for analyses based on all behavioral variables. Color bars are coded based on log-transferred bootstrapped p-values of the mediation effects. Only the effects reached statistical significance (p < .05) using bootstrap sampling with 100,000 random-generated samples shown in the figure. Note: rs-FC: resting-state functional connectivity; au: auditory network; cerc: cingulo-opercular network; copa: cingulo-parietal network; rst: retrosplenial-temporal network; ventralDC: ventral diencephalon; ACC: anterior cingulate cortex; FL2: 2-year follow-up.
To investigate the robustness of these observations over time, we performed a longitudinal mediation analysis,24 in which we tested whether baseline brain functional and structural measures would mediate the effects of sleep duration on behavioral measures at FL2, after partialling covariates and baseline behavioral measures. This time-lag analysis further reveals whether the identified brain measures can serve as biomarkers of behavioral changes over time.24 We found that some of the identified functional and structural measures mediated the effects of sleep duration on thought problems (i.e., retrosplenial-temporal network, rst – right ventral diencephalon connectivity), picture-vocabulary test performance, and crystallized intelligence (i.e., rst – right ventral diencephalon connectivity, left and right temporal pole structural measures; See Figure 5D and Figure S5).
Discussion
By controlling for various key covariates of sleep duration, this study estimated the long-term impact of insufficient sleep on neurocognitive development in early adolescence. In contrast to recent adolescent sleep research,3,8 this study identified two important neural mechanisms of insufficient sleep on neurocognitive development that have not previously been examined systematically in the literature. First, changes in rs-FC between the basal ganglia and cortical regions may underlie the widespread adverse behavioral effects induced by insufficient sleep. Second, structural properties of the temporal pole mediate the effects of sleep duration on crystallized intelligence. These effects lasted for at least two years, suggesting long-lasting consequences of insufficient sleep on adolescents’ neurocognitive development. If further confirmed, our findings may provide empirical and theoretical groundings for early sleep intervention programs to improve long-term developmental outcomes in adolescence.
Long-lasting impacts of insufficient sleep on brain development and behaviors
The effects of insufficient sleep on rs-FC converged on the connections between the basal ganglia and cortical regions (see Supplement for the effects on structural connectivities in the corticostriate tracts). The basal ganglia, along with the prefrontal cortex, undergoes the greatest structural changes across the whole brain during adolescence.23 The basal ganglia plays a key role in regulating sleep-wake behavior through cortical activation.23 Insufficient sleep may reciprocally disrupt normal functioning of the basal ganglia, such as through the dopamine or adenosine signaling pathway, and subsequently impair the cortex-basal ganglia-thalamus-cortex circuit.25 This disruption may result in weakened attention and limited information processing, leading to impairment in cognitive and affective functions.25 However, the durability of these detrimental impacts has been unknown until now.4 Here, using well-matched samples, we show that disrupted basal ganglia-cortex connections could last for at least two years. These connections also mediate the impact of insufficient sleep on depressed mood, thought problems, and crystallized intelligence.
Furthermore, given the increased statistical power and well-balanced covariates using PSM, our results resolve the previously mixed findings in the literature on the relationship between insufficient sleep and intelligence in the literature.3 We find that insufficient sleep has a long-lasting impact on crystallized intelligence; the magnitude of this effect is about twice that of fluid intelligence (Cohen’s d = 0.17 vs. 0.08, respectively). Our mediation analyses show that this behavioral effect is associated with changes in core neural substrates underlying the representation (temporal pole in the anterior temporal lobe, ATL)26,27 and retrieval (rst, retrosplenial-temporal network)28 of structuralized knowledge. In particular, we find that structural properties of the ATL and the retrosplenial-temporal network mediate the long-term detrimental effects of insufficient sleep on picture-vocabulary test performance and crystallized intelligence. These findings raise the possibility that insufficient sleep may disrupt memory consolidation by delaying temporal lobe maturation in the adolescent brain.2,29,30 Future research may examine how insufficient sleep influences the formation of crystallized knowledge at even younger ages.31
Another important contribution of this study is the estimated impact of insufficient sleep on neurocognitive development based on PSM that resembles randomized experiments.32 Because key covariates that may influence sleep duration and neurocognitive measures are well balanced between the SS and IS groups, the group comparison cannot be accounted for by covariates, such as socioeconomic status5, sex,6 pubertal status7, and urbanity approximated by study sites. This approach, therefore, resolves the uncertainty on whether sleep-related effects on neurocognitive development can be attributed to these individual differences. In addition, although >= 9 hours was selected by pediatricians and sleep experts as a reasonable amount of sleep for early adolescents, evidence supporting this recommendation has been mixed.33 Our data provided clear evidence that 9 hours or more of sleep is beneficial for neurocognitive development in early adolescents, and hence substantiate this recommended sleep duration for this age group.
Limitations
A few limitations should be noted. First, one caveat of PSM is that it can only control for observed covariates, leaving unobserved variances that may influence the selection of matched pairs. As shown in Table S2, a subset of participants could not be matched based on the current method, suggesting that additional factors may introduce further variability. For example, different school programs have been employed to improve students’ sleep habits,34 but the ABCD dataset has limited information related to these variables. Second, although PSM offers an approach to draw causal inferences based on observational data, the causal relationship needs to be confirmed with experimental approaches. For example, future research could examine whether school programs designed to prolong adolescents’ sleep duration could improve adolescents’ long-term development outcomes.32 Third, with data from only two time points for most measures, the current dataset is not adequate to distinguish within- and between-person effects in the impact of sleep duration on behavioral and neural measures. As the ABCD study is ongoing, future research incorporating more waves of data could better address this issue. Fourth, other sleep measures, such as architectural and microstructural features of sleep, as well as circadian rhythms, might also contribute to the relationship between sleep, brain, and behavior. Future studies need to investigate how these features of sleep (based on wearable devices, such as the Fitbit watch) are related to neurocognitive development in a comprehensive model.
Although our findings showed that more than 9 hours of sleep is beneficial for neurocognitive development, these results by no means indicate the longer sleep duration the better. Both short and long sleep duration could be associated with compromised mental health in adults.35 As the current ABCD dataset only provides coarse estimates of sleep duration (see Table S1), it remains to be tested how a prolonged sleep duration (e.g., >12 hours)9 would impact neurocognitive development in adolescents. Given that insufficient sleep has become a pronounced global issue for early adolescents,1 our current findings highlight the critical need for early sleep intervention in the young generation.
Conclusion
This study estimates the long-term effects of insufficient sleep on neurocognitive development in early adolescence while controlling for key covariates, including sex, socioeconomic status, puberty status, physical health indicators, etc. Our findings suggest that (1) cortico-basal ganglia connections play an important role in mediating the effect of insufficient sleep on cognitive and affective functions, and (2) structural properties of the anterior temporal lobe might contribute to the effect of insufficient sleep on crystallized intelligence. These effects can last at least two years, highlighting the importance of early sleep intervention at young ages to improve long-term neurocognitive development outcomes.
Supplementary Material
Research in context.
Evidence before this study
This study was designed in September, 2021. Few studies have estimated the long-lasting impact of insufficient sleep on the developing brain. Of those few publications, most reported data have not simultaneously controlled for several key covariates, such as sex, socioeconomic status, and pubertal status. A search of PubMed and Google Scholar was performed on March 28, 2022 using the terms “sleep,” “sleep duration,” “brain,” “matched/matching,” “control,” “child/children,” “adolescent,” “youth,” “young,” and “longitudinal” from inception and with no language restrictions. We did not identify any matched cohort studies with a longitudinal design that estimated the long-lasting impact of insufficient sleep on early adolescents’ neurocognitive development. We also did not find any studies that have considered both structural and functional brain measures in a longitudinal design.
Added value of this study
This is a large cohort study of early adolescents with well-matched covariates based on propensity score matching. This approach controls for several key covariables that may influence the relationship between sleep duration and neurocognitive development, thus better clarifies the direct impact of insufficient sleep on adolescent development. Furthermore, by examining these matched group differences at baseline and at 2-year follow-up, our data provide much-needed insights into the long-lasting impact of insufficient sleep on early adolescents’ neurocognitive development.
Implications of all the available evidence
We provide evidence for the long-lasting impact of insufficient sleep on adolescents’ neurocognitive development across two years in a population-based early adolescent cohort. Our findings have implications for parents, social services, researchers, educators, and clinicians in understanding the critical need for early intervention in sleep duration to improve long-term developmental outcomes in adolescents.
Acknowledgment
Research efforts (data analysis and interpretation) in this work were supported by NIH grants: R01AG060054, R01AG070227, R01EB031080-01A1, P41EB029460-01A1, 1UL1TR003098.
The preprint of this manuscript is available at medRxiv (DOI: https://doi.org/10.1101/2022.01.10.22269013). We thank the ABCD consortium and NIH for providing the data for performing the research in this work. Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org/) and are held in the NIMH Data Archive. This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health (NIH) and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners/. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. we are not paid to write this article by a pharmaceutical company or other agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing interests.
Ethics approval statement
ABCD study received ethical approval in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Data sharing statement
The ABCD data used by this study are available in the National Institutes of Mental Health Data Archive (NDA): https://nda.nih.gov/abcd. For the ABCD consortium, see https://abcdstudy.org/principal-investigators.html.
References
- 1.Gariepy G, Danna S, Gobiņa I, et al. How Are Adolescents Sleeping? Adolescent Sleep Patterns and Sociodemographic Differences in 24 European and North American Countries. J Adolesc Health 2020; 66: S81–8. [DOI] [PubMed] [Google Scholar]
- 2.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev 2006; 10: 49–62. [DOI] [PubMed] [Google Scholar]
- 3.de Bruin EJ, van Run C, Staaks J, Meijer AM. Effects of sleep manipulation on cognitive functioning of adolescents: A systematic review. Sleep Med Rev 2017; 32: 45–57. [DOI] [PubMed] [Google Scholar]
- 4.Dutil C, Walsh JJ, Featherstone RB, et al. Influence of sleep on developing brain functions and structures in children and adolescents: A systematic review. Sleep Med Rev 2018; 42: 184–201. [DOI] [PubMed] [Google Scholar]
- 5.Felden ÉPG, Leite CR, Rebelatto CF, Andrade RD, Beltrame TS. Sleep in adolescents of different socioeconomic status: a systematic review. Rev Paul Pediatr 2015; 33: 467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KA, McEnany G, Weekes D. Gender differences in sleep patterns for early adolescents. J Adolesc Health Off Publ Soc Adolesc Med 1999; 24: 16–20. [DOI] [PubMed] [Google Scholar]
- 7.Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the Transition to Adolescence: A Longitudinal Study. Sleep 2009; 32: 1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng W, Rolls E, Gong W, et al. Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol Psychiatry 2020; : 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paruthi S, Brooks LJ, D’Ambrosio C, et al. Consensus Statement of the American Academy of Sleep Medicine on the Recommended Amount of Sleep for Healthy Children: Methodology and Discussion. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med 2016; 12: 1549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause AJ, Ben Simon E, Mander BA, et al. The sleep-deprived human brain. Nat Rev Neurosci 2017; 18: 404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho D, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw 2011; 42: 1–28. [Google Scholar]
- 12.Casey BJ, Cannonier T, Conley MI, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci 2018; 32: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang FN, Liu TT, Wang Z. Functional connectome mediates the association between sleep disturbance and mental health in preadolescence: A longitudinal mediation study. Hum Brain Mapp 2022; 43: 2041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagler DJ, Hatton SeanN, Cornejo MD, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage 2019; 202: 116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer KA, Wall MM, Larson NI, Laska MN, Neumark-Sztainer D. Sleep Duration and BMI in a Sample of Young Adults. Obesity 2012; 20: 1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makowski C, Lepage M, Evans AC. Head motion: the dirty little secret of neuroimaging in psychiatry. J Psychiatry Neurosci JPN 2019; 44: 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan RCK, Xie W, Geng F, et al. Clinical Utility and Lifespan Profiling of Neurological Soft Signs in Schizophrenia Spectrum Disorders. Schizophr Bull 2016; 42: 560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salthouse TA. Item Analyses of Memory Differences. J Clin Exp Neuropsychol 2017; 39: 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens MM, Potter A, Hyatt CS, et al. Recalibrating expectations about effect size: A multi-method survey of effect sizes in the ABCD study. PloS One 2021; 16: e0257535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandekar SN, Stephens J. Improving the replicability of neuroimaging findings by thresholding effect sizes instead of p-values. Hum Brain Mapp 2021; 42: 2393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage 2009; 47: 821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z, Zhao X, Veasey SC. Neural Consequences of Chronic Short Sleep: Reversible or Lasting? Front Neurol 2017; 8. https://www.frontiersin.org/article/10.3389/fneur.2017.00235 (accessed Jan 24, 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa H, Selway R, Gnoni V, et al. The subcortical belly of sleep: New possibilities in neuromodulation of basal ganglia? Sleep Med Rev 2020; 52: 101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang FN, Liu TT, Wang Z. Functional connectome mediates the association between sleep disturbance and mental health in preadolescence: A longitudinal mediation study. Hum Brain Mapp; n/a. DOI: 10.1002/hbm.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sil’kis IG. Possible Mechanisms for Impairments to Learning, Memory, and Attention due to Sleep Deprivation. Neurosci Behav Physiol 2014; 44: 576–83. [Google Scholar]
- 26.Xie W, Bainbridge WA, Inati SK, Baker CI, Zaghloul KA. Memorability of words in arbitrary verbal associations modulates memory retrieval in the anterior temporal lobe. Nat Hum Behav 2020; 4: 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herlin B, Navarro V, Dupont S. The temporal pole: From anatomy to function—A literature appraisal. J Chem Neuroanat 2021; 113: 101925. [DOI] [PubMed] [Google Scholar]
- 28.Kaboodvand N, Bäckman L, Nyberg L, Salami A. The retrosplenial cortex: A memory gateway between the cortical default mode network and the medial temporal lobe. Hum Brain Mapp 2018; 39: 2020–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res 2012; 76: 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tononi G, Cirelli C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 2014; 81: 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngo CT, Benear SL, Popal H, Olson IR, Newcombe NS. Contingency of semantic generalization on episodic specificity varies across development. Curr Biol 2021; 31: 2690–2697.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albrecht JN, Werner H, Rieger N, et al. Association Between Homeschooling and Adolescent Sleep Duration and Health During COVID-19 Pandemic High School Closures. JAMA Netw Open 2022; 5: e2142100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Short MA, Blunden S, Rigney G, et al. Cognition and objectively measured sleep duration in children: a systematic review and meta-analysis. Sleep Health 2018; 4: 292–300. [DOI] [PubMed] [Google Scholar]
- 34.Cassoff J, Knäuper B, Michaelsen S, Gruber R. School-based sleep promotion programs: Effectiveness, feasibility and insights for future research. Sleep Med Rev 2013; 17: 207–14. [DOI] [PubMed] [Google Scholar]
- 35.Zhai L, Zhang H, Zhang D. Sleep Duration and Depression Among Adults: A Meta-Analysis of Prospective Studies. Depress Anxiety 2015; 32: 664–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ABCD data used by this study are available in the National Institutes of Mental Health Data Archive (NDA): https://nda.nih.gov/abcd. For the ABCD consortium, see https://abcdstudy.org/principal-investigators.html.


