Abstract
Objectives:
Studies of race-specific colon cancer (CC) survival differences between right- vs. left-sided CC typically focus on Black and White persons and often consider all CC stages as one group. To more completely examine potential racial and ethnic disparities in side- and stage-specific survival, we evaluated 5-year CC cause-specific survival probabilities for five racial/ethnic groups by anatomic site (right or left colon) and stage (local, regional, distant).
Methods:
We obtained cause-specific survival probability estimates from National Cancer Institute’s population-based Surveillance, Epidemiology, and End Results (SEER) for CC patients grouped by five racial/ethnic groups (Non-Hispanic American Indian/Alaska Native [AIAN], Non-Hispanic Asian/Pacific Islander [API], Hispanic, Non-Hispanic Black [NHB], and Non-Hispanic White [NHW]), anatomic site, stage, and other patient and SEER registry characteristics. We used meta-regression approaches to identify factors that explained differences in cause-specific survival.
Results:
Diagnoses of distant-stage CC were more common among NHB and AIAN persons (>22 %) than among NHW and API persons (< 20 %). Large disparities in anatomic site-specific survival were not apparent. Those with right-sided distant-stage CC had a one-year cause-specific survival probability that was 16.4 % points lower (99 % CI: 12.2–20.6) than those with left-sided distant-stage CC; this difference decreased over follow-up. Cause-specific survival probabilities were highest for API, and lowest for NHB, persons, though these differences varied substantially by stage at diagnosis. AIAN persons with localized-stage CC, and NHB persons with regional- and distant-stage CC, had significantly lower survival probabilities across follow-up.
Conclusions:
There are differences in CC presentation according to anatomic site and disease stage among patients of distinct racial and ethnic backgrounds. This, coupled with the reality that there are persistent survival disparities, with NHB and AIAN persons experiencing worse prognosis, suggests that there are social or structural determinants of these disparities. Further research is needed to confirm whether these CC cause-specific survival disparities are due to differences in risk factors, screening patterns, cancer treatment, or surveillance, in order to overcome the existing differences in outcome.
Keywords: Colon cancer, Survival, Minority health, Disparities
1. Introduction
Over 100,000 cases of colon cancer (CC) diagnosed annually, and with a five-year relative survival of 65 % for patients diagnosed between 2015 and 2018 it is the second leading cause of cancer-related deaths in the United States [1,2]. Although recent decades have demonstrated improvements in CC survival, these gains have not erased differences in mortality across races/ethnicities [3,4]. From 2010 to 2019, sex- and age-adjusted colorectal cancer mortality rates per 100,000 decreased from 15.6 to 13.0 in non-Hispanic White (NHW), from 23.3 to 17.6 in non-Hispanic Black (NHB), from 11.6 to 9.2 in non-Hispanic Asian/Pacific Islander (API), from 19.2 to 16.1 in non-Hispanic American Indian/Alaska Native (AIAN), and from 12.6 to 10.6 in Hispanic persons [5]. It is notable that the 2019 mortality rates among NHB and AIAN persons are higher than those for NHW individuals in 2010, and 2019 rates for NHW persons are higher than those for API and Hispanic individuals in 2010.
Current differences among mortality rates by race/ethnicity have similar patterns in the incidence of CC in the right vs. left anatomic sites, with NHB persons displaying the highest right to left CC incidence ratio (1.87) and API persons the lowest (0.99) [6]. Understanding differences in survival corresponding to the location where the cancer develops may have important implications for screening recommendations. CCs that develop on the right side of the colon typically have worse prognosis even after accounting for survival differences arising due to being diagnosed at different stages of the disease [7–17]. Screening-related factors such as poor right-sided preps, incomplete colonoscopy, and anatomic configurations compromising visibility [18,19] may contribute to these differences. Biologically-driven factors may also contribute. For instance, serrated adenomas are flatter and more difficult to visualize endoscopically, characteristically carry BRAF V600E mutations, give rise to microsatellite unstable CCs, and are more common in the right colon [20]. Recent data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program suggest the risk differential between CC of different anatomic sites may not be large [21,22], suggesting more remains to be understood regarding site-specific CC survival outcomes.
Disparities in CC outcomes may contribute to reported differences in survival between right- and left-sided CC: different racial/ethnic groups experience different CC survival outcomes [1,3–5] as well as differences in the incidence of right- vs. left-sided CC and in the stage of CC at diagnosis [6,23,24]. Relatively little has been reported about site- and stage-specific CC survival outcomes for all different racial/ethnic groups, particularly for AIAN persons. Appropriately quantifying survival following CC diagnosis among persons of distinct racial and ethnic backgrounds by anatomic site of the lesion and its summary stage has a variety of important implications. For instance, a particular racial/ethnic group may experience poorer CC survival due to any of a number of reasons. Some of these include higher general CC incidence, higher site-specific CC incidence, lower overall CC screening adherence, CC screening using a sub-optimal modality (e.g., colonoscopy demonstrates superiority over FIT in detecting right-sided neoplasia [25–27]), lower quality and less timely CC treatment and follow-up care, or some combination thereof. Understanding the different survival probabilities by race/ethnicity that correspond to the location where the cancer develops may have important implications for improving strategies for prevention, screening, treatment, or follow-up care for CC.
We sought to enhance understanding of CC survival probabilities and their differences between anatomic sites, within the context of existing racial/ethnic disparities. Because of the impact of disease stage on CC survival, we estimated and compared CC survival probabilities among racial/ethnic groups by anatomic site and stage at diagnosis over five years of follow-up.
2. Methods
2.1. Study design
We conducted a retrospective cohort study of persons diagnosed with CC from 1992 through 2018. The University of New Mexico Health Sciences Center’s Human Research Review Committee deemed the research protocol exempt from review.
2.2. Data source and study population
We used data from SEER, the SEER*Stat 8.4.0 Database: “Incidence – SEER Research Plus Data, 12 Registries, Nov 2021”, for cancers diagnosed from 1992 to 2018. We included all persons who received their first diagnosis of adenocarcinoma of the colon, excluding appendiceal cancers, included in this database. We excluded individuals with missing data for age, year of diagnosis, race, stage, CC anatomical location, and if the CC involved both right and left anatomic sites.
2.3. Study variables
Our primary outcome was the probability of survival after CC diagnosis. We identified eligible participants and estimated survival probabilities at one through five years post-diagnosis within combinations of: sex, age at diagnosis, year of diagnosis (within five-year categories), race/ethnicity (AIAN, API, Hispanic, NHB, and NHW), CC side (Right: cecum, ascending colon, hepatic flexure and transverse colon; and Left: splenic flexure, descending colon, sigmoid colon and rectosigmoid junction), grade (I–IV), and summary stage (Localized, Regional, and Distant). The county-level descriptors of median household income (quartiles), and Metropolitan-Urban-Rural categorization of Rural-Urban Continuum Codes (RUCC) were also tabulated, combining rural and small urban (population < 20,000) counties according to whether or not they were adjacent to metropolitan counties.
2.4. Statistical analysis
We summarized the numbers and percentages of individuals with eligible CC diagnoses within categories of the factors of interest. Due to restrictions on extracting individual-level data, we used SEER*Stat to estimate one- through five-year cause-specific survival probabilities, and their standard errors, for persons aggregated into groups simultaneously cross-classified by all of the factors outlined above. When SEER*Stat’s variance estimate was zero, we used the variance of a binomial proportion computed after adding 0.5 to the numerator and denominator counts. These SEER-estimated survival probabilities and standard errors accounted for censoring of individuals for loss to follow-up, or death due to other causes. After confirming distributional assumptions of the cause-specific survival probability outcome variable across the cross-classified groups, we used linear regression models in a meta-regression framework, with weights corresponding to the inverse of the squared standard errors of the cause-specific survival probabilities, to assess the degree to which factors of interest explained differences in cause-specific survival probabilities across aggregated groups of patients sharing the same characteristics while simultaneously adjusting for other explanatory factors. We modeled interactions among race, CC side, CC stage, and follow-up period while adjusting for sex, age, ethnicity, year of diagnosis, and CC grade as fixed effects to accomplish the goals of this analysis. We used generalized estimating equations to account for within-group correlations among cause-specific survival probabilities estimated over time within analysis subgroups. We tested the significance of the four-way interaction among race, anatomic site, stage, and follow-up year, and removed non-significant interactions via backward elimination. We retained interactions that were statistically significant, and the interactions nested within them, while adjusting for the main effects of sex, age at diagnosis, and tumor grade. We reported model-based estimates of average cause-specific survival probabilities, and 99 % confidence intervals (CI) to reflect their precision, for patient groups defined by each of the highest-order interactions included in the final model, as these were the terms that explained differences in cause-specific survival. We also reported estimates of pairwise differences, and their 99 % CI, between groups defined by combinations of the factors in significant interactions. Statistical significance was set at a two-sided p < 0.001 to account for multiple comparisons. Analyses were performed using SEER*Stat (https://seer.cancer.gov/seerstat_version_8.4.0) and SAS 9.4 (SAS Institute Inc, Cary, NC).
3. Results
3.1. Sample and tumor characteristics
The SEER Research Pluse Data, 12 Registries, Nov 2020 in SEER*Stat 8.4.0 contained data from 321,433 CC diagnoses. 309,061 (96.2 %) of these met inclusion/exclusion criteria (Table 1). Proportions of men and women with CC were approximately equal, and most persons were 65 years and older. NHW and NHB persons more often presented with right-sided CC, 54.5 % and 55.8 %, respectively, and API, AIAN, and Hispanic persons more often presented with left-sided CC, 59.3 %, 52.2 %, and 51.0 % respectively. 25.1 % of NHB, 22.4 % of AIAN, and 21.6 % of Hispanic persons were diagnosed with distant-stage CC; while distant-stage CC diagnoses were received by 19.2 % of NHW and 19.4 % of API.
Table 1.
Characteristics of patients with colorectal cancer in the left and right anatomic side from 1992 to 2019, by race and ethnicity.
| AIAN |
API |
Hispanic |
NHB |
NHW |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Total | 2968 | 1.0 | 34,546 | 11.2 | 30,959 | 10.0 | 27,277 | 8.8 | 213,311 | 69.0 | 309,061 | 100.0 | |
| Sex | Male | 1435 | 48.3 | 17,690 | 51.2 | 15,952 | 51.5 | 12,997 | 47.6 | 105,951 | 49.7 | 154,025 | 49.8 |
| Female | 1533 | 51.7 | 16,856 | 48.8 | 15,007 | 48.5 | 14,280 | 52.4 | 107,360 | 50.3 | 155,036 | 50.2 | |
| Age | < 40 years | 135 | 4.5 | 951 | 2.8 | 1441 | 4.7 | 769 | 2.8 | 3458 | 1.6 | 6754 | 2.2 |
| 40–49 years | 284 | 9.6 | 2728 | 7.9 | 3053 | 9.9 | 2470 | 9.1 | 10,870 | 5.1 | 19,405 | 6.3 | |
| 50–59 years | 647 | 21.8 | 6289 | 18.2 | 6460 | 20.9 | 5913 | 21.7 | 28,608 | 13.4 | 47,917 | 15.5 | |
| 60–69 years | 805 | 27.1 | 8601 | 24.9 | 7941 | 25.7 | 7452 | 27.3 | 46,409 | 21.8 | 71,208 | 23.0 | |
| 70–79 years | 727 | 24.5 | 9092 | 26.3 | 7260 | 23.5 | 6557 | 24.0 | 64,008 | 30.0 | 87,644 | 28.4 | |
| 80 + years | 370 | 12.5 | 6885 | 19.9 | 4804 | 15.5 | 4116 | 15.1 | 59,958 | 28.1 | 76,133 | 24.6 | |
| Year of Diagnosis | 1992–1996 | 340 | 11.5 | 4471 | 12.9 | 3549 | 11.5 | 4182 | 15.3 | 43,450 | 20.4 | 55,992 | 18.1 |
| 1997–2001 | 455 | 15.3 | 5646 | 16.3 | 4445 | 14.4 | 4715 | 17.3 | 44,231 | 20.7 | 59,492 | 19.2 | |
| 2002–2006 | 526 | 17.7 | 6445 | 18.7 | 5290 | 17.1 | 5156 | 18.9 | 40,474 | 19.0 | 57,891 | 18.7 | |
| 2007–2011 | 568 | 19.1 | 6956 | 20.1 | 6340 | 20.5 | 5271 | 19.3 | 36,090 | 16.9 | 55,225 | 17.9 | |
| 2012–2016 | 688 | 23.2 | 6923 | 20.0 | 6896 | 22.3 | 4937 | 18.1 | 31,554 | 14.8 | 50,998 | 16.5 | |
| 2017–2019 | 391 | 13.2 | 4105 | 11.9 | 4439 | 14.3 | 3016 | 11.1 | 17,512 | 8.2 | 29,463 | 9.5 | |
| Site | Right | 1419 | 47.8 | 14,071 | 40.7 | 15,169 | 49.0 | 15,214 | 55.8 | 116,353 | 54.5 | 162,226 | 52.5 |
| Left | 1549 | 52.2 | 20,475 | 59.3 | 15,790 | 51.0 | 12,063 | 44.2 | 96,958 | 45.5 | 146,835 | 47.5 | |
| Stage | Localized | 1097 | 37.0 | 13,559 | 39.2 | 11,573 | 37.4 | 10,186 | 37.3 | 87,836 | 41.2 | 124,251 | 40.2 |
| Regional | 1207 | 40.7 | 14,273 | 41.3 | 12,689 | 41.0 | 10,252 | 37.6 | 84,560 | 39.6 | 122,981 | 39.8 | |
| Distant | 664 | 22.4 | 6714 | 19.4 | 6697 | 21.6 | 6839 | 25.1 | 40,915 | 19.2 | 61,829 | 20.0 | |
| Grade | I | 252 | 8.5 | 2219 | 6.4 | 2691 | 8.7 | 2239 | 8.2 | 17,305 | 8.1 | 24,706 | 8.0 |
| II | 1761 | 59.3 | 21,408 | 62.0 | 17,689 | 57.1 | 16,038 | 58.8 | 122,611 | 57.5 | 179,507 | 58.1 | |
| III | 342 | 11.5 | 4858 | 14.1 | 4450 | 14.4 | 3508 | 12.9 | 38,320 | 18.0 | 51,478 | 16.7 | |
| IV | 45 | 1.5 | 363 | 1.1 | 437 | 1.4 | 327 | 1.2 | 3659 | 1.7 | 4831 | 1.6 | |
| Unknown | 568 | 19.1 | 5698 | 16.5 | 5692 | 18.4 | 5165 | 18.9 | 31,416 | 14.7 | 48,539 | 15.7 | |
| Rural-Urban Continuum Code (RUCC) County Categories | Metropolitan: population > 1,000,000 | 546 | 18.4 | 23,781 | 68.8 | 22,725 | 73.4 | 23,869 | 87.5 | 118,776 | 55.7 | 189,697 | 61.4 |
| Metropolitan: population 250,000–1,000,000 | 304 | 10.2 | 6208 | 18.0 | 4646 | 15.0 | 2427 | 8.9 | 44,309 | 20.8 | 57,894 | 18.7 | |
| Metropolitan: population < 250,000 | 272 | 9.2 | 561 | 1.6 | 1312 | 4.2 | 219 | 0.8 | 15,043 | 7.1 | 17,407 | 5.6 | |
| Non-Metro, Metro adjacent | 272 | 9.2 | 75 | 0.2 | 929 | 3.0 | 417 | 1.5 | 18,424 | 8.6 | 20,117 | 6.5 | |
| Non-Metro, not Metro adjacent | 278 | 9.4 | 1229 | 3.6 | 1239 | 4.0 | 324 | 1.2 | 15,875 | 7.4 | 18,945 | 6.1 | |
| Unknown (or AK/HI entire state) | 1296 | 43.7 | 2692 | 7.8 | 108 | 0.3 | 21 | 0.1 | 884 | 0.4 | 5001 | 1.6 | |
| Cause-specific Survival Probability (%): Age and Sex Adjusted Estimate (99 % CI) | 1 year | 84.7 (81.3–88.1) | 95.5 (94.8–96.1) | 93.9 (93.2–94.6) | 92.2 (91.4–93.0) | 95.0 (94.6–95.3) | 92.8 (92.4–93.3) | ||||||
| 2 years | 75.4 (70.0–80.8) | 89.1 (88.0–90.3) | 86.7 (85.4–88.0) | 81.0 (79.4–82.5) | 88.3 (87.7–89.0) | 87.9 (87.4–88.4) | |||||||
| 3 years | 68.5 (61.5–75.3) | 82.1 (80.5–83.8) | 76.8 (75.0–78.7) | 69.2 (67.0–71.3) | 81.2 (80.3–82.1) | 80.6 (80.1–81.2) | |||||||
| 4 years | 63.3 (55.7–70.9) | 77.2 (75.3–79.1) | 70.9 (68.7–73.0) | 59.2 (56.8–61.7) | 74.9 (73.8–75.9) | 73.8 (73.2–74.4) | |||||||
| 5 years | 59.7 (51.6–67.7) | 73.1 (71.0–75.2) | 65.8 (63.5–68.2) | 52.5 (49.8–55.1) | 69.1 (68.0–70.2) | 67.8 (67.2–68.5) | |||||||
AIAN: Non-Hispanic American Indian/Alaska Native, API: Non-Hispanic Asian/Pacific Islander, NHB: Non-Hispanic Black, NHW: Non-Hispanic White.
3.2. Deriving the model describing differences in cause-specific survival probabilities
Of the 309,061 individuals who met inclusion/exclusion criteria, at least one year of follow-up data was available for 243,660 (78.8 %). While adjusting for sex, age, ethnicity, year of diagnosis, and CC grade, the four-way interaction among race, CC stage, anatomic site, and follow-up was not statistically significant (p = 0.20). After removal of this interaction, and the non-significant three-way interaction among race, anatomic site, and follow-up year (p = 0.51), we evaluated the three-way interaction among race, stage at diagnosis, and anatomic site. Although not statistically significant (p = 0.08), we summarized site-specific differences within groups by race/ethnicity and stage, as this was a comparison of primary interest. Our final simplified model contained two meaningful three-way interactions, CC stage by anatomic site by follow-up period and race/ethnicity by CC stage by follow-up period and their nested interactions and main effects, along with the adjusting factors noted above.
3.3. Differences in cause-specific survival between left- and right-sided CC by race/ethnicity and stage
Differences in cause-specific survival probabilities between left- and right-sided CC within combinations of a person’s race/ethnicity and CC stage are shown in Table 2. The left-minus-right differences were smallest for those diagnosed with localized-stage CC, and largest for those diagnosed with distant-stage CC. The left-minus-right cause-specific survival differences were negligible for all race/ethnicity groups when CC was diagnosed at localized stage. For those diagnosed with regional-stage CC, the left-minus-right differences were greater than 2 % for all but NHB persons (1.7 %, 99 % CI = − 0.05 to 3.8). For those diagnosed with distant-stage CC, left-minus-right differences in cause-specific survival were greater than five percentage points for all but Hispanic persons (2.1 %, 99 % CI = − 6.7 % to 11.0 %). However, precision was low when estimating these patterns of left-minus-right differences within CC stages and we cannot conclude that they differ significantly by race/ethnicity (p = 0.08).
Table 2.
Differences in adjusted survival probabilities between left- and right-sided CC for persons of different race/ethnicity diagnosed with CC at different summary stages.
| Left-sided minus right-sided difference in survival (%) |
||||
|---|---|---|---|---|
| Race/Ethnicity | Stage | Difference | Lower 99 % confidence limit | Upper 99 % confidence limit |
| AIAN | Localized | −0.5 | −4.2 | 3.1 |
| Regional | 5.2 | −1.9 | 12.2 | |
| Distant | 11.7 | 1.6 | 21.8 | |
| All | 5.4 | 1.2 | 9.7 | |
| API | Localized | −0.2 | −1.2 | 0.7 |
| Regional | 2.5 | 0.9 | 4.0 | |
| Distant | 5.7 | −4.5 | 15.9 | |
| All | 2.6 | −0.8 | 6.1 | |
| Hispanic | Localized | 0.3 | −0.8 | 1.3 |
| Regional | 3.1 | 1.0 | 5.2 | |
| Distant | 2.1 | −6.7 | 11.0 | |
| All | 0.3 | −0.8 | 1.3 | |
| NHB | Localized | 0.7 | −0.3 | 1.7 |
| Regional | 1.7 | −0.5 | 3.8 | |
| Distant | 5.1 | 0.1 | 10.2 | |
| All | 2.5 | 0.6 | 4.4 | |
| NHW | Localized | 0.2 | −0.4 | 0.8 |
| Regional | 3.4 | 2.0 | 4.8 | |
| Distant | 9.1 | 6.2 | 12.1 | |
| All | 4.2 | 3.1 | 5.3 | |
Estimates of differences and lower and upper 99 % confidence intervals (CI) at each year post diagnosis are shown. Estimates were obtained while controlling for sex, age at diagnosis, tumor grade, year of diagnosis, and RUCC categories. Two significant (p < 0.001) three-way interactions were included: race/ethnicity by stage by follow-up year, and stage by side by follow-up year. One non-significant (p = 0.076) three-way interaction was also included to enable estimation of these differences of interest: stage by side by race/ethnicity. The model also included all two-way interactions required to build each three-way interaction.
AIAN: Non-Hispanic American Indian/Alaska Native, API: Non-Hispanic Asian/Pacific Islander, NHB: Non-Hispanic Black, NHW: Non-Hispanic White.
3.4. Differences in cause-specific survival between anatomic sites by CC stage over follow-up
We observed the largest differences among cause-specific survival probabilities within groups defined by combinations of stage, anatomic site, and follow-up (Fig. 1). Those with localized-stage CC had five-year survival probabilities above 95 %; those with regional disease had five-year survival probabilities above 75 %; and those with distant disease had five-year survival probabilities below 10 %. Table 3 illustrates the left-minus-right differences in cause-specific CC survival probabilities by stage over follow-up. For those with localized-stage CC, differences in cause-specific survival between anatomic sites were less than 1 % point. For those with regional-stage CC, there was a small difference in survival between left- and right-sided lesions after one year (1 %, 99 % CI = 0.4–1.7 %); in subsequent years the differences were all greater than 3, and lower confidence bounds of the 99 % CI were greater than 2 % for all but the five-year follow-up estimate. For those with distant-stage CC, those with left-sided lesions had better survival than those with right-sided lesions. This left-sided survival advantage was greatest at one-year (16.4 %, 99 % CI = 12.2–20.6 %) post-diagnosis, and declined to a 2.0 % difference (99 % CI = 0.3–3.8 %) at five years post diagnosis.
Fig. 1.
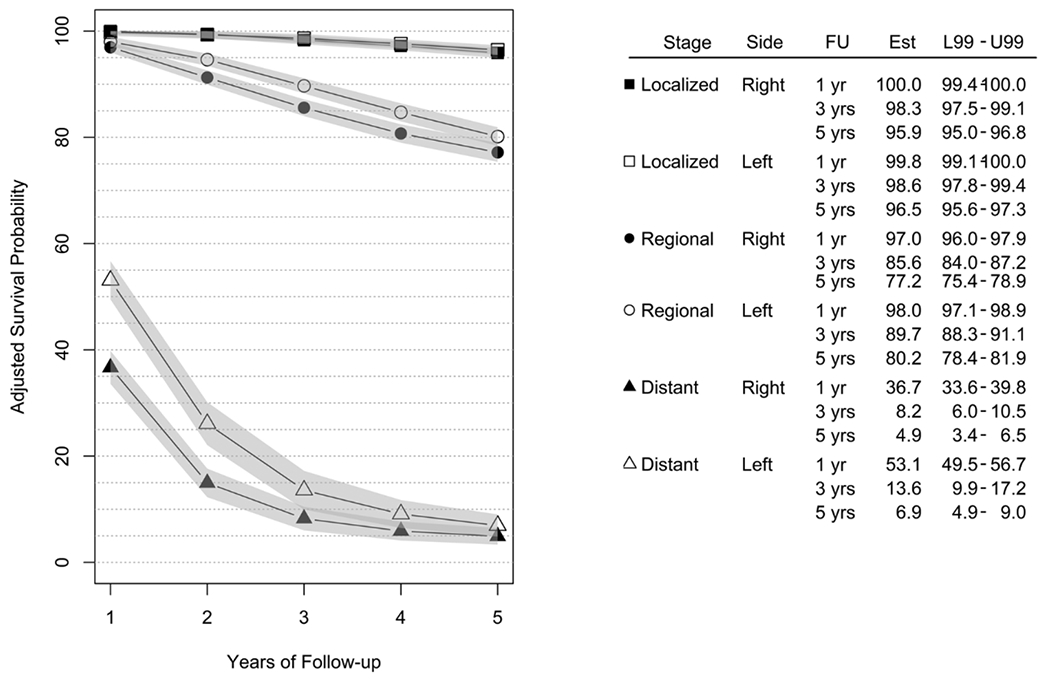
CC cause-specific survival probabilities, expressed as percentages, by stage of disease, anatomic site, and follow-up period (FU). Estimated survival probabilities (Est) and 99 % confidence intervals are shown (L99 - U99). Estimates were obtained while controlling for race/ethnicity, sex, age at diagnosis, tumor grade, year of diagnosis, and RUCC categories. Two significant three-way interactions were also included: race/ethnicity by stage by follow-up year, and stage by side by follow-up year, along with all two-way interactions required to build each three-way interaction.
Table 3.
Differences in adjusted survival probabilities for persons diagnosed with colon cancer arising in the left vs. right anatomic sites.
| Left-sided: minus right-sided difference in survival (%) |
||||
|---|---|---|---|---|
| Stage | Year of follow-up | Difference | Lower 99 % Confidence Limit | Upper 99 % Confidence Limit |
| Localized | 1 | −0.4 | −0.9 | 0.1 |
| 2 | −0.1 | −0.6 | 0.4 | |
| 3 | 0.3 | −0.2 | 0.8 | |
| 4 | 0.4 | −0.2 | 1.0 | |
| 5 | 0.6 | −0.1 | 1.2 | |
| Regional | 1 | 1.0 | 0.4 | 1.7 |
| 2 | 3.4 | 2.3 | 4.5 | |
| 3 | 4.1 | 2.6 | 5.6 | |
| 4 | 4.0 | 2.3 | 5.7 | |
| 5 | 3.0 | 1.1 | 4.8 | |
| Distant | 1 | 16.4 | 12.2 | 20.6 |
| 2 | 11.2 | 7.1 | 15.2 | |
| 3 | 5.3 | 2.4 | 8.3 | |
| 4 | 3.2 | 1.0 | 5.4 | |
| 5 | 2.0 | 0.3 | 3.8 | |
Estimates of differences and lower and upper 99 % confidence intervals (CI) at each year post diagnosis are shown. Estimates were obtained while controlling for sex, age at diagnosis, tumor grade, year of diagnosis, and RUCC categories. Two significant (p < 0.001) three-way interactions were also included: race/ethnicity by stage by follow-up year, and stage by side by follow-up year, along with all two-way interactions required to build each three-way interaction.
AIAN: Non-Hispanic American Indian/Alaska Native, API: Non-Hispanic Asian/Pacific Islander, NHB: Non-Hispanic Black, NHW: Non-Hispanic White.
3.5. Racial and ethnic differences in cause-specific survival probabilities by stage at diagnosis over follow-up
Stage-specific CC survival probabilities varied over follow-up according to a person’s race and ethnicity (Table 4). For those with localized-stage CC, cause-specific survival probabilities at one year post-diagnosis are all above 95 %, and remained above 90 % for all groups up to five-years post diagnosis. However, AIAN persons experienced persistently lower survival than all other racial/ethnic groups across the follow-up period (Table 5). For those with regional-stage CC, cause-specific survival probabilities were above 94 % for all groups at one year, and declined differentially among different racial/ethnic groups. By five-years post diagnosis, AIAN and NHB persons had lower survival than the other groups (Table 5). For those with distant-stage CC, one-year cause-specific survival was above 40 %, but declined sharply for all racial/ethnic groups. NHB persons consistently had the worst prognosis. It is notable that cause-specific survival in distant-stage CC tended to be higher for AIAN persons, and lower for API persons, than for other groups (Tables 4 and 5).
Table 4.
Adjusted cause-specific survival probabilities, expressed as percentages, for 1–5 years of follow-up post-diagnosis. Race- and ethnicity-specific estimates are shown for each summary stage (Localized, Regional, and Distant) of colon cancer. 99 % confidence intervals for the adjusted survival probabilities are also shown. Estimates were obtained while controlling for sex, age at diagnosis, tumor grade, year of diagnosis, and RUCC categories. Two significant three-way interactions were also included: race/ethnicity by stage by follow-up year, and stage by side by follow-up year, along with all two-way interactions required to build each three-way interaction.
| Localized |
Regional |
Distant |
|||||
|---|---|---|---|---|---|---|---|
| Years of follow-up | Race/Ethnicity | Estimate | 99 % CI | Estimate | 99 % CI | Estimate | 99 % CI |
| 1 | AIAN | 98.1 | (96.4–99.8) | 94.7 | (91.5–97.8) | 44.4 | (37.6–51.3) |
| API | 99.1 | (99.2–100.0) | 99.0 | (98.2–99.9) | 45.4 | (38.5–52.2) | |
| Hispanic | 100.0 | (99.2–100.0) | 98.7 | (97.6–99.7) | 48.2 | (41.5–54.9) | |
| NHB | 100.0 | (99.2–100.0) | 97.8 | (96.7–98.9) | 42.0 | (37.1–46.8) | |
| NHW | 100.0 | (99.3–100.7) | 97.2 | (96.5–97.9) | 44.5 | (41.7–47.3) | |
| 2 | AIAN | 97.0 | (95.0–99.0) | 89.0 | (85.1–92.9) | 26.9 | (20.5–33.4) |
| API | 100.6 | (99.2–100.0) | 95.5 | (94.4–96.6) | 16.5 | (8.1–24.9) | |
| Hispanic | 100.1 | (99.1–100.0) | 94.8 | (93.0–96.6) | 22.2 | (14.8–29.5) | |
| NHB | 99.9 | (99.0–100.0) | 92.6 | (90.8–94.3) | 16.5 | (12.1–20.8) | |
| NHW | 99.4 | (98.7–100.0) | 92.9 | (92.0–93.8) | 20.6 | (18.2–23.0) | |
| 3 | AIAN | 95.5 | (93.2–97.9) | 83.7 | (79.1–88.3) | 18.3 | (12.1–24.6) |
| API | 99.9 | (99.1–100.0) | 91.4 | (89.8–92.9) | 7.5 | (0.8–14.1) | |
| Hispanic | 99.3 | (98.5–100.0) | 89.5 | (87.2–91.8) | 9.1 | (0.3–17.9) | |
| NHB | 98.9 | (98.0–99.9) | 85.6 | (83.3–88.0) | 7.6 | (4.8–10.3) | |
| NHW | 98.7 | (98.0–99.3) | 87.9 | (86.8–89.0) | 12.1 | (10.5–13.8) | |
| 4 | AIAN | 94.1 | (91.7–96.6) | 78.5 | (73.3–83.8) | 13.7 | (8.3–19.1) |
| API | 99.2 | (98.4–100.0) | 86.7 | (84.7–88.6) | 5.0 | (0.1–9.9) | |
| Hispanic | 98.4 | (97.6–99.2) | 85.1 | (82.3–87.9) | 5.5 | (0.0–11.4) | |
| NHB | 97.5 | (96.5–98.5) | 79.8 | (77.2–82.3) | 4.4 | (2.3–6.5) | |
| NHW | 97.8 | (97.1–98.5) | 83.5 | (82.3–84.8) | 9.0 | (7.6–10.3) | |
| 5 | AIAN | 92.4 | (89.7–95.1) | 75.5 | (70.2–80.8) | 11.0 | (6.2–15.8) |
| API | 98.4 | (97.5–99.2) | 82.6 | (80.6–84.6) | 4.0 | (0.1–7.8) | |
| Hispanic | 97.4 | (96.5–98.2) | 80.6 | (77.7–83.4) | 3.9 | (0.0–8.3) | |
| NHB | 96.0 | (94.8–97.2) | 74.9 | (72.1–77.7) | 3.2 | (1.4–5.0) | |
| NHW | 96.7 | (96.0–97.5) | 79.7 | (78.4–81.1) | 7.6 | (6.3–8.8) | |
AIAN: Non-Hispanic American Indian/Alaska Native, API: Non-Hispanic Asian/Pacific Islander, NHB: Non-Hispanic Black, NHW: Non-Hispanic White.
Table 5.
Differences in adjusted cause-specific survival probabilities (%) among persons of different racial and ethnic groups who were diagnosed with colon cancer at distinct summary stages.
| Stage | Years post-diagnosis: Comparison | 1 Diff (99 % CI) |
2 Diff (99 % CI) |
3 Diff (99 % CI) |
4 Diff (99 % CI) |
5 Diff (99 % CI) |
||
|---|---|---|---|---|---|---|---|---|
| Localized | AIAN | – | API | −2.8 (−4.6 – −1.1) | −3.6 (−5.6 – −1.6) | −4.4 (−6.7 – −2.0) | −5.1 (−7.6 – −2.6) | −6.0 (−8.7 – −3.2) |
| AIAN | – | Hispanic | −2.4 (−4.2 – −0.6) | −3.1 (−5.1 – −1.0) | −3.8 (−6.2 – −1.4) | −4.3 (−6.8 – −1.8) | −5.0 (−7.8 – −2.2) | |
| AIAN | – | NHB | −2.4 (−4.2 – −0.7) | −2.9 (−4.9 – −0.8) | −3.4 (−5.8 – −1.0) | −3.4 (−6.0 – −0.8) | −3.6 (−6.5 – −0.7) | |
| AIAN | – | NHW | −1.9 (−3.6 – −0.1) | −2.4 (−4.4 – −0.4) | −3.1 (−5.4 – −0.8) | −3.7 (−6.1 – −1.2) | −4.3 (−7.1 – −1.6) | |
| API | – | Hispanic | 0.4 (−0.3 – 1.1) | 0.5 (−0.2 – 1.2) | 0.6 (−0.1 – 1.3) | 0.8 (0.1–1.6) | 1.0 (0.2–1.9) | |
| API | – | NHB | 0.4 (−0.3 – 1.1) | 0.7 (0.0–1.4) | 1.0 (0.2–1.8) | 1.7 (0.8–2.7) | 2.4 (1.2–3.6) | |
| API | – | NHW | 1.0 (0.4–1.5) | 1.2 (0.7–1.8) | 1.3 (0.7–1.8) | 1.5 (0.8–2.1) | 1.7 (0.9–2.4) | |
| Hispanic | – | NHB | 0.0 (−0.7 – 0.7) | 0.2 (−0.6 – 1.0) | 0.4 (−0.4 – 1.3) | 0.9 (−0.1 – 1.8) | 1.4 (0.2–2.6) | |
| Hispanic | – | NHW | 0.5 (−0.1 – 1.2) | 0.7 (0.1–1.3) | 0.7 (0.1–1.3) | 0.6 (0.0–1.3) | 0.6 (−0.1 – 1.4) | |
| NHB | – | NHW | 0.6 (−0.1 – 1.2) | 0.5 (−0.1 – 1.1) | 0.3 (−0.5 – 1.0) | −0.3 (−1.1 – 0.6) | −0.7 (−1.8 – 0.4) | |
| Regional | AIAN | – | API | −4.4 (−7.5 – −1.2) | −6.5 (−10.5 – −2.5) | −7.6 (−12.4 – −2.9) | −8.1 (−13.7 – −2.6) | −7.1 (−12.7 – −1.5) |
| AIAN | – | Hispanic | −4.0 (−7.2 – −0.8) | −5.8 (−10.0 – −1.6) | −5.8 (−10.8 – −0.7) | −6.6 (−12.5 – −0.7) | −5.1 (−11.0 – 0.8) | |
| AIAN | – | NHB | −3.1 (−6.4 to 0.1) | −3.5 (−7.8 – 0.7) | −1.9 (−7.0 – 3.2) | −1.2 (−7.0 – 4.6) | 0.6 (−5.3 – 6.5) | |
| AIAN | – | NHW | −2.6 (−5.7 – 0.6) | −3.9 (−7.8 – 0.1) | −4.2 (−8.9 – 0.5) | −5.0 (−10.4 – 0.3) | −4.2 (−9.6 – 1.1) | |
| API | – | Hispanic | 0.4 (−0.5 – 1.3) | 0.7 (−1.1 – 2.5) | 1.9 (−0.7 – 4.4) | 1.6 (−1.7 – 4.9) | 2.0 (−1.3 – 5.4) | |
| API | – | NHB | 1.2 (0.2–2.3) | 3.0 (1.1–4.9) | 5.7 (3.1–8.4) | 6.9 (3.8–10.0) | 7.7 (4.4–11.1) | |
| API | – | NHW | 1.8 (1.2–2.5) | 2.6 (1.5–3.8) | 3.4 (1.8–5.1) | 3.1 (0.9–5.3) | 2.9 (0.6–5.1) | |
| Hispanic | – | NHB | 0.9 (−0.4 – 2.1) | 2.3 (0.0–4.5) | 3.9 (0.8–7.0) | 5.3 (1.7–9.0) | 5.7 (1.8–9.5) | |
| Hispanic | – | NHW | 1.4 (0.6–2.3) | 1.9 (0.3–3.6) | 1.6 (−0.8 – 3.9) | 1.5 (−1.4 – 4.5) | 0.8 (−2.1 – 3.8) | |
| NHB | – | NHW | 0.6 (−0.5 – 1.6) | −0.3 (−2.1 – −1.5) | −2.3 (−4.7 – 0.1) | −3.8 (−6.5 – −1.1) | −4.9 (−7.8 – −1.9) | |
| Distant | AIAN | – | API | −1.0 (−10.6 – 8.7) | 10.4 (−0.1 – 21.0) | 10.8 (1.7–19.9) | 8.7 (1.5–15.9) | 7.0 (0.9–13.2) |
| AIAN | – | Hispanic | −3.8 (−13.4 – 5.9) | 4.8 (−5.1 – 14.6) | 9.2 (−1.7 – 20.1) | 8.2 (0.2–16.3) | 7.1 (0.5–13.6) | |
| AIAN | – | NHB | 2.5 (−5.9 – 10.9) | 10.5 (2.7–18.2) | 10.7 (3.9–17.6) | 9.3 (3.5–15.0) | 7.8 (2.7–12.9) | |
| AIAN | – | NHW | −0.1 (−7.5 – 7.3) | 6.3 (−0.6 – 13.2) | 6.2 (−0.3 – 12.6) | 4.7 (−0.8 – 10.2) | 3.4 (−1.5 – 8.4) | |
| API | – | Hispanic | −2.8 (−12.7 – 7.0) | −5.6 (−17.1 – 5.8) | −1.6 (−13.0 – 9.8) | −0.4 (−8.4 – 7.5) | 0.0 (−6.0 – 6.1) | |
| API | – | NHB | 3.4 (−5.0 – 11.8) | 0.0 (−9.3 – 9.4) | −0.1 (−7.2 – 7.0) | 0.6 (−4.6 – 5.8) | 0.7 (−3.4 – 4.9) | |
| API | – | NHW | 0.8 (−6.5 – 8.2) | −4.1 (−12.7 – 4.4) | −4.7 (−11.4 – 2.0) | −4.0 (−8.9 – 1.0) | −3.6 (−7.5 – 0.3) | |
| Hispanic | – | NHB | 6.2 (−2.0 – 14.5) | 5.7 (−2.8 – 14.2) | 1.5 (−7.6 – 10.7) | 1.0 (−5.1 – 7.2) | 0.7 (−3.9 – 5.4) | |
| Hispanic | – | NHW | 3.7 (−3.6 – 10.9) | 1.5 (−6.1 – 9.2) | −3.0 (−11.8 – 5.8) | −3.5 (−9.5 – 2.4) | −3.6 (−8.0 – 0.8) | |
| NHB | – | NHW | −2.6 (−8.1 – 2.9) | −4.2 (−8.9 – 0.6) | −4.6 (−7.6 – −1.5) | −4.6 (−6.9 – −2.3) | −4.3 (−6.3 – −2.4) | |
Estimates of differences (Diff) and lower and upper 99 % confidence intervals (CI) at each year post diagnosis are shown. Estimates were obtained while controlling for sex, age at diagnosis, tumor grade, year of diagnosis, and RUCC categories. Two significant three-way interactions were also included: race/ethnicity by stage by follow-up year, and stage by side by follow-up year, along with all two-way interactions required to build each three-way interaction.
AIAN: Non-Hispanic American Indian/Alaska Native, API: Non-Hispanic Asian/Pacific Islander, NHB: Non-Hispanic Black, NHW: Non-Hispanic White.
4. Discussion
We sought to explore the degree to which differences in CC cause-specific survival among different racial and ethnic groups, particularly with respect to the stage of the disease at diagnosis, might enhance current understanding regarding differences in CC cause-specific survival between the anatomical sites of the lesion. We compared CC cause-specific survival probabilities among racial/ethnic groups for anatomic sites and stages at diagnosis over five years of follow-up in the SEER Registries. Our study uniquely adds to the knowledge base by assessing interaction terms among our primary variables of interest—race, anatomic site, stage, and time of follow-up. This approach offers stronger evidence for some of the marginal associations already established [8,11,16,19,28] while expanding the knowledge base by simultaneously examining the joint impacts of the key factors of race, stage, and anatomic site on differences in survival probabilities.
After accounting for patient-related factors, and while modeling relationships among CC cause-specific survival probabilities within groups defined by combinations of summary stage, anatomic site, follow-up period, and race/ethnicity, we found that the pattern of left vs. right anatomic site differences within stage at diagnosis did not differ significantly among racial/ethnic groups. However, we did find that across all three stages of disease, right-sided CC exhibited generally lower cause-specific survival probabilities—an outcome observed elsewhere in the literature [11,12]. However, the magnitude of these differences were was not consistent among disease stages nor over all follow-up. There were no meaningful differences noted among those diagnosed with localized-stage CC. For those diagnosed with regional-stage CC, right-sided lesions had lower cause-specific survival probability than left-sided CC by 3 % points or more after two years of follow-up, with lower 99 % confidence limits consistently above 1 % point. The pattern was most striking for distant-stage CC, where right-sided lesions had much lower survival probabilities in earlier years post diagnosis, with the difference decreasing over time, from 16.4 % points (99 % CI = 12.2–20.6) at one year to 2.0 (99 % CI: 0.3–3.8) at five years following diagnosis. This finding runs counter to those of He et al. [22] who found improved survival outcomes for overall and distant left-sided CC at 5 years, but not for localized or regional-stage CC. Our results overlap partially with others [15,21], who found increasing differences between left- and right-sided lesions for regional-stage CC over time, but not for local CC. However, we note that no prior studies included interaction terms to explicitly test these differences. Our identification of a significant interaction among CC stage, anatomic site, and follow-up length suggests that these variables need to be considered simultaneously to fully evaluate their impacts on cause-specific survival.
Our findings underscore persistent racial and ethnic disparities in stage-specific CC survival. These disparities are often neglected by research that combines smaller categories of race/ethnicity, resulting in the oft-reported, over-simplified categories of White, Black, and “other”. This last group combines API and AIAN even though these two groups exhibit idiosyncratic cancer incidence rates, and cancer treatment behaviors and resources. Our analysis suggests that outcomes do differ between these groups. We found that survival probabilities were highest for API, and lowest for AIAN and NHB, persons, but that patterns of racial/ethnic differences were distinct among those diagnosed with different stages of CC. For instance, although API persons have higher survival probabilities when diagnosed with localized or regional-stage CC, their longer-term survival when diagnosed at distant-stage CC is among the lowest of the groups studied here. Although AIAN persons have notably lower cause-specific survival when diagnosed with localized or regional-stage CC, they have the highest cause-specific survival following distant-stage CC. For NHB, who have the lowest cause-specific survival when diagnosed with distant-stage CC, and nearly the lowest when diagnosed with regional-stage CC, their cause-specific survival probabilities approach those seen for NHW and Hispanic persons when diagnosed with localized stage disease.
There are several potential explanations for these differences. As reported here and elsewhere [4,11,29–32], AIAN and NHB persons are more often diagnosed with distant-stage CC. Although CC screening can prevent or detect CC early [33,34], access-to-care obstacles prevent many AIAN and Black persons from receiving guideline-concordant screening compared to other racial/ethnic groups [4,9,35–38]. Slower adoption of colonoscopy may also account for a preponderance for right-sided CC and poorer survival. Differences in access to and utilization of quality health care may contribute to the observed survival differences [30,39,40]. Some have reported that Black persons were less likely than White persons to receive surgical treatment and adjuvant chemotherapy [39–42]. Differences in posttreatment surveillance and comorbidities among AIAN and NHB persons may also influence survival disparities [6,36]. That AIAN persons’ cause-specific survival probabilities decrease faster than all other races for localized-stage CCs over five years suggests suboptimal CC treatment relative to that of non-AIAN persons: the average per capita healthcare expenditures for the IHS population were $4078 in 2019, less than half the corresponding expenditures for the general US population [43].
Prior studies demonstrated that lower incidence and mortality in left-sided CCs may be explained in part by the earlier diagnoses achieved with colonoscopy, as well as the propensity for left-sided CC to present with symptoms that lead individuals to seek earlier care, while right-sided CC are more challenging to detect with colonoscopy and present with more subtle symptoms [11,18,44]. Right-sided CC are also more often mucinous (10.7 % vs. 5.0 %) or signet cell ring carcinomas (1.4 % vs. 0.7 %), portending poorer prognosis regardless of detection [19]. Additionally, genetic mutations and microsatellite instability (MSI) can differ among racial groups and can affect CC prognosis [20,45–47]. For instance, KRAS mutations suggest poor prognosis and possibly resistance to treatment, and appear to be more prevalent in Black than White persons [48]. MSI tumors, more common among Black persons, are more prevalent in right-sided tumors and are less likely to be screen-detected [46]. These factors may explain the likelihood of right-sided CC among Black persons [4,9,49]. Genetic differences also cannot be excluded for survival differences among AIAN persons [50].
The differences in CC presentation by anatomic site and disease stage among racial/ethnic groups points to the likelihood that there are social or structural determinants contributing to the disparities in CC survival. Such systems-level barriers as lower screening, a focus on acute care over preventive services, lower per capita expenditure on healthcare, and higher provider turnover may explain much of the CC survival disparities that we report on here, particularly for AIAN persons. Other contributing factors could include transportation barriers, cultural beliefs, fear and stigma about screening and about cancer, and concerns over privacy [51–54]. The documentation of early-onset CC in Black persons has led to changes in screening recommendations; the USPTF (United States Preventive Task Force) and others now recommend beginning colorectal cancer screening at age 45 rather than 50 in such groups of individuals [55].
In spite of our study’s strengths, we must acknowledge several limitations. First, although we adjusted for key factors associated with cause-specific CC survival, including age at diagnosis, tumor grade, and so forth, the number of key factors we were able to extract was relatively limited. For instance SEER has relatively little information on comorbidities, access to care, and insurance status. Second, we included individuals with a CC diagnosis, regardless of age at onset. We therefore performed a sensitivity analysis where we excluded those diagnosed before age 50. The age-adjusted probability estimates shifted down slightly in this sensitivity analysis, but the overall patterns of differences were essentially unchanged from those observed in the full data set. Third, racial and ethnic classifications in medical records, such as those most commonly available in large data sources such as SEER, may reflect misclassification [56]. Finally, SEER registries represent a subset of AIAN persons in the United States; they do not capture data from Oklahoma, Arizona, or the Northern Plains and Great Lakes [57]. Even with these limitations, we are able to provide new insights into cause-specific CC survival for multiple racial/ethnic groups, according to stage at diagnosis, anatomic site, and length of follow-up.
5. Conclusion
We have identified significant differences in the presentation of CC among racial and ethnic groups, and described notable differences in their cause-specific survival probabilities over five years of follow-up according to the anatomic site of and stage at diagnosis. Differences in CC survival probabilities between anatomic side are present across five years of follow-up, and they differ by stage. Future efforts should implement and evaluate multi-level interventions at the individual, structural, and policy levels to address the persistent disparities in CC survival among AIAN and NHB persons. Future research should also continue to capture information from all key racial and ethnic subgroups to further understand those disparities that are present, and to identify ways in which they may be corrected.
Funding statement
This work was supported by the National Cancer Institute of the National Institutes of Health, USA, Grants R01CA192967 (Mishra, PI) and 3P30CA118100 (University of New Mexico Comprehensive Center Support Grant, Tomkinson, PI), and the Biostatistics Shared Resource of the University of New Mexico Comprehensive Cancer Center, USA.
Footnotes
CRediT authorship contribution statement
V. Shane Pankratz: Conceptualization, Methodology, Software, Formal analysis, Writing – original draft. Mikaela Kosich: Investigation, Writing – review & editing. Nicholas Edwardson: Investigation, Writing – original draft. Kevin English: Conceptualization, Writing – review & editing. Prajakta Adsul: Writing – review & editing. Yiting Li: Writing – review & editing. Gulshan Parasher: Investigation, Writing – review & editing. Shiraz I. Mishra: Conceptualization, Writing – review & editing, Funding acquisition.
Conflict of Interest Statement
All authors declare that they have no competing interests relating to the materials presented in the current manuscript.
References
- [1].CDC Cancer, An Update on Cancer Deaths in the United States [Internet], Centers for Disease Control and Prevention, 2021. [cited 2021 Aug 14]. Available from: ⟨https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm⟩. [Google Scholar]
- [2].SEER Program, Cancer Stat Facts: Colorectal Cancer [Internet], National Cancer Institute, 2022. [cited 2022 Jul 1]. Available from: ⟨https://seer.cancer.gov/statfacts/html/colorect.html⟩. [Google Scholar]
- [3].Howlander N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. , SEER Cancer Statistics Review, 1975–2018 [Internet], 2021. Available from: ⟨https://seer.cancer.gov/csr/1975_2018/⟩, based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
- [4].Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020, CA Cancer J. Clin 70 (3) (2020) 145–164. [DOI] [PubMed] [Google Scholar]
- [5].SEER Program, Cancer Stat Facts: Cancer Disparities [Internet], National Cancer Institute, 2022. [cited 2022 Jul 1]. Available from: ⟨https://seer.cancer.gov/statfacts/html/disparities.html⟩. [Google Scholar]
- [6].Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB, Sex disparities in colorectal cancer incidence by anatomic subsite, race and age, Int. J. Cancer 128 (7) (2011) 1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hansen IO, Jess P, Possible better long-term survival in left versus right-sided colon cancer–a systematic review, Dan. Med. J 59 (6) (2012) A4444. [PubMed] [Google Scholar]
- [8].Hemminki K, Santi I, Weires M, Thomsen H, Sundquist J, Bermejo JL, Tumor location and patient characteristics of colon and rectal adenocarcinomas in relation to survival and TNM classes, BMC Cancer 10 (1) (2010) 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116 (3) (2010) 544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weiss JM, Pfau PR, O’Connor ES, King J, LaConte N, Kennedy G, et al. Mortality by stage for right-versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results–medicare data, J. Clin. Oncol 29 (33) (2011) 4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis, JAMA Oncol. 3 (2) (2017) 211. [DOI] [PubMed] [Google Scholar]
- [12].Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N, Is there a difference in survival between right- versus left-sided colon cancers? Ann. Surg. Oncol 15 (9) (2008) 2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Christodoulidis G, Spyridakis M, Symeonidis D, Kapatou K, Manolakis A, Tepetes K, Clinicopathological differences between right- and left-sided colonic tumors and impact upon survival. Tech. Coloproctol 14 (S1) (2010) 45–47. [DOI] [PubMed] [Google Scholar]
- [14].Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival, Dis. Colon Rectum 53 (1) (2010) 57–64. [DOI] [PubMed] [Google Scholar]
- [15].Huang CW, Tsai HL, Huang MY, Huang CM, Yeh YS, Ma CJ, et al. Different clinicopathologic features and favorable outcomes of patients with stage III left-sided colon cancer. World J. Surg. Oncol 13 (1) (2015) 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Warschkow R, Sulz MC, Marti L, Tarantino I, Schmied BM, Cerny T, et al. Better survival in right-sided versus left-sided stage I–III colon cancer patients, BMC Cancer 16 (1) (2016) 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lim DR, Kuk JK, Kim T, Shin EJ, Comparison of oncological outcomes of right-sided colon cancer versus left-sided colon cancer after curative resection: which side is better outcome? Medicine 96 (42) (2017), e8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, et al. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology, J. Gastroenterol. Hepatol 23 (3) (2008) 418–423. [DOI] [PubMed] [Google Scholar]
- [19].Ulanja MB, Rishi M, Beutler BD, Sharma M, Patterson DR, Gullapalli N, et al. Colon cancer sidedness, presentation, and survival at different stages, J. Oncol (2019), e4315032 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VEPP, Rutten HJT, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients, Ann. Oncol 21 (12) (2010) 2396–2402. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Yang L, Zhou M, Shen L, Zhang J, Deng W, et al. Disparities in survival for right-sided vs. left-sided colon cancers in young patients: a study based on the Surveillance, Epidemiology, and End Results database (1990–2014), Cancer Manag. Res 10 (2018) 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kang He X, Wu W, Ding YE, Li Y, Min Sun L, Si J, Different anatomical subsites of colon cancer and mortality: a population-based study, Gastroenterol. Res. Pract 2018 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Murphy CC, Wallace K, Sandler RS, Baron JA, Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology 156 (4) (2019) 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berry J, Bumpers K, Ogunlade V, Glover R, Davis S, Counts-Spriggs M, et al. Examining racial disparities in colorectal cancer care, J. Psychosoc. Oncol 27 (1) (2009) 59–83. [DOI] [PubMed] [Google Scholar]
- [25].Zorzi M, Hassan C, Capodaglio G, Narne E, Turrin A, Baracco M, et al. Divergent long-term detection rates of proximal and distal advanced neoplasia in fecal immunochemical test screening programs: a retrospective cohort study, Ann. Intern. Med 169 (9) (2018) 602. [DOI] [PubMed] [Google Scholar]
- [26].Brenner H, Niedermaier T, Chen H, Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin: specific sensitivity of fits, Int. J. Cancer 140 (9) (2017) 2015–2022. [DOI] [PubMed] [Google Scholar]
- [27].Wong MCS, Ching JYL, Chan VCW, Lam TYT, Shum JP, Luk AKC, et al. Diagnostic accuracy of a qualitative fecal immunochemical test varies with location of neoplasia but not number of specimens, Clin. Gastroenterol. Hepatol 13 (8)(2015) 1472–1479. [DOI] [PubMed] [Google Scholar]
- [28].Wong RJ, Marked variations in proximal colon cancer survival by race/ethnicity within the United States, J. Clin. Gastroenterol 44 (9) (2010) 625–630. [DOI] [PubMed] [Google Scholar]
- [29].Chien C, Morimoto LM, Tom J, Li CI, Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer 104 (3) (2005) 629–639. [DOI] [PubMed] [Google Scholar]
- [30].Gomez SL, O’Malley CD, Stroup A, Shema SJ, Satariano WA, Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: impact of neighborhood socioeconomic status, treatment and comorbidity, BMC Cancer 7 (1) (2007) 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Haverkamp D, Melkonian SC, Jim MA, Growing Disparity in the Incidence of Colorectal Cancer among Non-Hispanic American Indian and Alaska Native Populations—United States, 2013–2017, vol. 30(no. 10), 2021, pp. 1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Silber JH, Rosenbaum PR, Ross RN, Niknam BA, Ludwig JM, Wang W, et al. Racial disparities in colon cancer survival: a matched cohort study, Ann. Intern. Med 161 (12) (2014) 845. [DOI] [PubMed] [Google Scholar]
- [33].Elmunzer BJ, Singal AG, Sussman JB, Deshpande AR, Sussman DA, Conte ML, et al. Comparing the effectiveness of competing tests for reducing colorectal cancer mortality: a network meta-analysis, Gastrointest. Endosc 81 (3) (2015) 700–709 (.e3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pan J, Xin L, Ma YF, Hu LH, Li ZS, Colonoscopy reduces colorectal cancer incidence and mortality in patients with non-malignant findings: a meta-analysis. Am. J. Gastroenterol 111 (3) (2016) 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].American Cancer Society, Cancer Facts and Figures 2022 [Internet], 2022. [cited 2022 Mar 10]. Available from: ⟨https://www.cancer.org/research/cancer-facts-statistics.html⟩.
- [36].Haverkamp D, English K, Jacobs-Wingo J, Tjemsland A, Espey D, Effectiveness of interventions to increase colorectal cancer screening among American Indians and Alaska natives, Prev. Chronic Dis 17 (2020), E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch. Intern. Med 172 (7) (2012) 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, et al. Cancer screening test use — United States, 2015, Morb. Mortal. Wkly. Rep 66 (8) (2017) 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gross CP, Smith BD, Wolf E, Andersen M, Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer 112 (4) (2008) 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF, Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer, J. Clin. Oncol 20 (5) (2002) 1192–1202. [DOI] [PubMed] [Google Scholar]
- [41].Baldwin LM, Dobie SA, Billingsley K, Cai Y, Wright GE, Dominitz JA, et al. Explaining black–white differences in receipt of recommended colon cancer treatment, JNCI J. Natl. Cancer Inst 97 (16) (2005) 1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Demissie K, Oluwole OO, Balasubramanian BA, Osinubi OO, August D, Rhoads GG, Racial differences in the treatment of colorectal cancer: a comparison of surgical and radiation therapy between Whites and Blacks, Ann. Epidemiol 14 (3) (2004) 215–221. [DOI] [PubMed] [Google Scholar]
- [43].Indian Health Service, IHS Profile [Internet], 2020. Available from: ⟨https://www.ihs.gov/sites/newsroom/themes/responsive2017/display_objects/documents/factsheets/IHSProfile.pdf⟩.
- [44].Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y, The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis, J. Gastrointest. Surg 20 (3) (2016) 648–655. [DOI] [PubMed] [Google Scholar]
- [45].Gavin P, Colangelo LH, Fumagalli D, Tanaka N, Remillard MY, Yothers G, et al. , Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value, vol. 18 (no. 23), 2012, pp. 6531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lindblom A, Different mechanisms in the tumorigenesis of proximal and distal colon cancers, vol. 13(no. 1), 2001, pp. 63–9. [DOI] [PubMed] [Google Scholar]
- [47].Nayani R, Ashktorab H, Brim H, Laiyemo AO, Genetic basis for colorectal cancer disparities, Curr. Colorectal Cancer Rep 11 (6) (2015) 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yoon HH, Shi Q, Alberts SR, Goldberg RM, Thibodeau SN, Sargent DJ, et al. Racial differences in BRAF/KRAS mutation rates and survival in stage III colon cancer patients, JNCI J. Natl. Cancer Inst [Internet] 107 (10) (2015) (Available from: ⟨ 10.1093/jnci/djv186⟩). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Troisi RJ, Freedman AN, Devesa SS, Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975–1994, Cancer 85 (8) (1999) 1670–1676. [PubMed] [Google Scholar]
- [50].Whitworth A, New research suggests access, genetic differences play role in high minority cancer death rate, J. Natl. Cancer Inst 98 (10) (2006) 669. [DOI] [PubMed] [Google Scholar]
- [51].Filippi MK, James AS, Brokenleg S, Talawyma M, Perdue DG, Choi WS, et al. Views, barriers, and suggestions for colorectal cancer screening among American Indian women older than 50 years in the midwest, J. Prim. Care Community Health 4 (3) (2013) 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].James AS, Filippi MK, Pacheco CM, Cully L, Perdue D, Choi WS, et al. Barriers to colorectal cancer screening among american indian men aged 50 or older, Kansas and Missouri, 2006–2008, Prev. Chronic Dis 10 (2013), E170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pandhi N, Guadagnolo BA, Kanekar S, Petereit DG, Smith MA, Cancer screening in native Americans from the northern plains. Am. J. Prev. Med 38 (4) (2010) 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schumacher MC, Slattery ML, Lanier AP, Ma KN, Edwards S, Ferucci ED, et al. Prevalence and predictors of cancer screening among American Indian and Alaska native people: the EARTH study. Cancer Causes Control 19 (7) (2008) 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Screening for colorectal cancer: US preventive services task force recommendation statement, JAMA 325 (19) (2021) 1965. [DOI] [PubMed] [Google Scholar]
- [56].Atekruse SF, Cosgrove C, Cronin K, Yu M, Comparing cancer registry abstracted and self-reported data on race and ethnicity, J. Regist. Manag 44 (1) (2017) 30–33. [PubMed] [Google Scholar]
- [57].Melkonian SC, Weir HK, Jim MA, Preikschat B, Haverkamp D, White MC, Incidence of and Trends in the Leading Cancers with Elevated Incidence Among American Indian and Alaska Native Populations, 2012–2016, vol. 190(no. 4), 2020, pp. 528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]


