Abstract
Cancer stem cells (CSCs) lead to the occurrence and progression of cancer due to their strong tumorigenic, self-renewal, and multidirectional differentiation abilities. Existing cancer treatment methods cannot effectively kill or inhibit CSCs but instead enrich them and produce stronger proliferation, invasion, and metastasis capabilities, resulting in cancer recurrence and treatment resistance, which has become a difficult problem in clinical treatment. Therefore, targeting CSCs may be the most promising approach for comprehensive cancer therapy in the future. A variety of natural products (NP) have significant antitumor effects and have been identified to target and inhibit CSCs. However, pharmacokinetic defects and off-target effects have greatly hindered their clinical translation. NP-based nanoformulations (NPNs) have tremendous potential to overcome the disadvantages of NP against CSCs through site-specific delivery and by improving their pharmacokinetic parameters. In this review, we summarize the recent progress of NPNs targeting CSCs in cancer therapy, looking forward to transforming preclinical research results into clinical applications and bringing new prospects for cancer treatment.
Keywords: cancer, cancer stem cells, nanoformulations, natural products, targeted therapy
Cancer remains a major public health problem worldwide, causing a huge disease burden. It is not only one of the leading causes of death globally but is also an important factor hindering the extension of human life expectancy, about 80% of tumor types derives from some solid cancers including prostate, breast, ovary, pancreas and lung.1 With an estimated 19.3 million new cancer cases and nearly 10 million cancer deaths globally in 2020, the cancer death rate is expected to nearly double by 2040 due to the adverse effects of the COVID-19 pandemic.2
The lives of cancer patients have been significantly prolonged, the overall risk of cancer death has decreased by 32%, and approximately 3.5 million cancer deaths have been avoided owing to a series of revolutionary breakthroughs in early screening, surgical treatment, and immunotherapy in the past decade. Nonetheless, malignant cancer is still formidable, and the overall mortality rate is still high. Metastasis, recurrence, and multidrug resistance (MDR) are major challenges to current cancer treatment.3,4
Conventional cancer treatment has multiple drawbacks, including damage to healthy tissues, serious side effects, and MDR. Cancer stem cells (CSCs) are specialized cell subsets in tumor tissue with high self-renewal, multidirectional differentiation potential and tumorigenic ability and are the driving force of malignant proliferation, invasion, metastasis, drug resistance and recurrence of tumors.5,6 As CSCs play a key role in treatment resistance, it has been difficult to significantly improve the overall clinical efficacy of Traditional oncology therapies from their current level. Undoubtedly, novel therapeutic strategies targeting CSCs will bring new approaches for cancer treatment.7 By targeting the surface markers, signaling pathways, microenvironment, metabolic features and differentiation of CSCs, various inhibitors of CSCs have been investigated in preclinical studies or clinical trials.8–10 Meanwhile, some existing inhibitors inevitably have certain side effects, such as dizziness and gastrointestinal reactions, which severely limit the clinical drug development of cancer therapies targeting CSCs.11
Increasing evidence has shown that many natural products (NP), such as curcumin, sulforaphane, quercetin, and berberine, have promising anticancer activity by targeting CSCs.12,13 Although the incredible health benefits of NP have been elucidated, their widespread use in cancer therapy is severely restricted by limitations in terms of their water solubility, absorption, bioavailability, and targeting.14 By specifically targeting the drug-resistant and aggressive CSCs in tumor tissues, nanoparticle-loaded drugs can improve patient survival while minimizing their side effects and alleviating patient suffering.15 Therefore, the integration of NP and nanotechnology into cancer therapy could result in novel NP-based nanoformulations (NPNs) to target CSCs, which could improve the pharmacological response of patients and achieve better clinical benefits.16,17 Accordingly, we aimed to systematically summarize the current new trends and development challenges of NPNs targeting CSCs for the treatment of cancer in this review.
CSCs
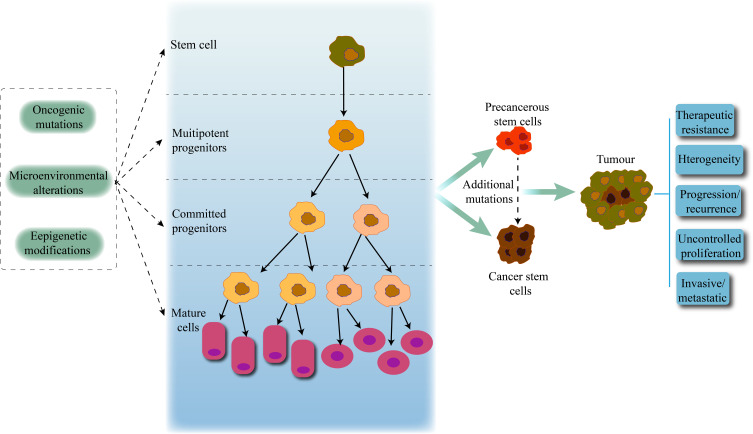
Cancer is a highly heterogeneous tissue, and different mechanisms contribute to its heterogeneity, such as genetic mutations, the microenvironment, and the presence of CSCs. CSCs maintain tumors in an immortalized or malignant clonal manner and differentiate into heterogeneous cancer cells, leading to the progression of primary tumors and the development of new tumors.18,19 CSCs can be derived from self-renewing normal stem cells or progenitor cells that have acquired self-renewal capacity due to mutation or dedifferentiation of mature tumor cells. Studies have found that normal stem cells gradually develop into precancerous stem cells and CSCs after the first oncogenic mutation, and then further accumulate mutations under the effect of mutagens and the microenvironment to increase the tumor’s heterogeneity, resulting in uncontrolled cell growth and promoting tumor development, metastasis, treatment resistance, and recurrence20,21 (Figure 1).
Figure 1.
CSCs and tumor progression. Normal stem cells give rise to multipotent progenitor cells, committed progenitor cells, and mature differentiated cells. Oncogenic mutations, microenvironmental alterations, and epigenetic modifications lead to the emergence and abnormal proliferation of CSCs, promoting the development and progression of malignant tumors.
The earliest evidence for CSC models came from acute granulocytic leukemia. Dick et al isolated human acute myeloid leukemia stem cells with an immunophenotype of CD34+/CD38− and confirmed the self-renewal ability of CSCs in immunodeficient mice.22 The presence of CSCs in other solid tumors was then established, such as in breast, lung, pancreatic, prostate, and brain cancers.23–25
The most important feature of CSCs is their ability to continuously self-renew and maintain multidirectional tumor differentiation.26 The self-renewal and differentiation pathways of normal stem cells are tightly regulated by Wingless-related integration site (Wnt), Notch, Hedgehog, Janus kinase–signal transducer and activator of transcription (JAK-STAT), Transforming growth factor (TGF)-β and other signaling pathways, and these pathways are significantly dysregulated in CSCs.27–29 These signaling pathways do not individually act as single regulators but instead form an intertwined signaling network that together regulates the stemness of CSCs, resulting in the unlimited potential of CSCs for self-renewal, proliferation, and multidirectional differentiation, as well as initiating tumor formation, reconstituting tumor heterogeneity, and providing resistance to chemoradiotherapy.13
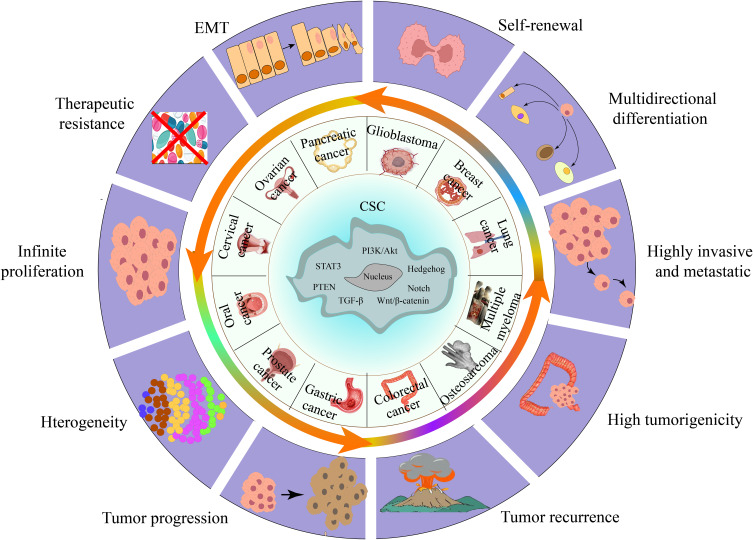
CSCs specifically express various stemness-related genes and markers, such as Krüppel-like factor 4 (Klf4), Nanog, Notch, CD24, CD26, CD44, CD133, CD166, aldehyde dehydrogenase (ALDH), and epithelial cell adhesion molecule (EpCAM). The expression of these stem cell-related genes and surface markers can significantly promote tumorigenesis and facilitate the isolation and identification of stem cells.30,31 In addition, CSCs jointly maintain stemness characteristics by virtue of their biological characteristics (cell cycle arrest, DNA damage repair, drug efflux, epithelial-mesenchymal transition (EMT), etc.) and the protective effect of the tumor microenvironment (hypoxic environment, cancer-associated fibroblasts, chronic inflammation, etc.), which increases the difficulty of tumor treatment.19,32 Moreover, CSCs have efficient DNA repair systems and represent a core-to-edge transition profile for enabling resistance and being protected within tumor microenvironment stem niches.33–35 In addition, epigenetic mechanisms play critical roles in the formation and function of CSCs, tumor heterogeneity, tumorigenicity, tumor development, and metastasis. The interaction between epigenetic modifications and the tumor-surviving microenvironment modulates the plasticity of CSCs and shapes the architecture of tumors.36–38 Therefore, the regulatory modes involved in CSCs interact and constitute a complex regulatory network of CSCs, which increases the difficulty of cancer treatment (Figure 2).
Figure 2.
CSC-related malignant tumor behavior. CSCs are cells in tumors that can self-renew and generate heterogeneous tumor cells, such as breast cancer and gastric cancer. The presence of CSCs promotes tumorigenesis, growth, metastasis, EMT, drug resistance, and recurrence.
Due to the complex characteristics of the CSCs described above, conventional cancer treatment options can only kill cancer cells with limited proliferative potential, while enriched CSCs lead to tumor reconstruction.39 The presence of CSCs has inspired the design of innovative therapeutic strategies against cancer aimed at eliminating CSCs. Therefore, targeting CSCs is considered a more promising approach to improve therapeutic outcomes, whether it is the development of monoclonal antibodies against surface antigens, self-renewal pathways of CSCs, or the induction of differentiation and modulation of the CSC microenvironment, as possible new therapeutic strategies targeting CSCs.40,41
NanoMaterials
Nanomaterials are defined as particles with particle sizes <100 nm or materials with particle sizes of 100 nm to 1000 nm but exhibiting nanoparticle properties. Due to their specificity in spatial dimensions, nanomaterials possess properties that are different from those of macroscopic materials, such as surface effects and small size effects.42 These properties not only endow nanomaterials with high catalytic activity to enhance the bioavailability of drugs but also endow nanomaterials with good protection and penetration, which can protect drugs from enzymatic degradation and facilitate drug absorption, thus enabling targeted delivery to tumor tissues and improved drug delivery efficiency. Nanomaterials as delivery carriers can increase drug loading, prolong their circulation time, improve their bioavailability and achieve drug enrichment in specific organs or tissues due to their unique physicochemical properties, pharmacokinetic characteristics and modifiable biodistribution.43–45 In particular, nanomaterials deliver drugs mainly to malignant regions rather than healthy ones, thus minimizing toxic off-target effects and maximizing efficacy.46 Therefore, nanoMedicine plays an important role in tumor therapy.47
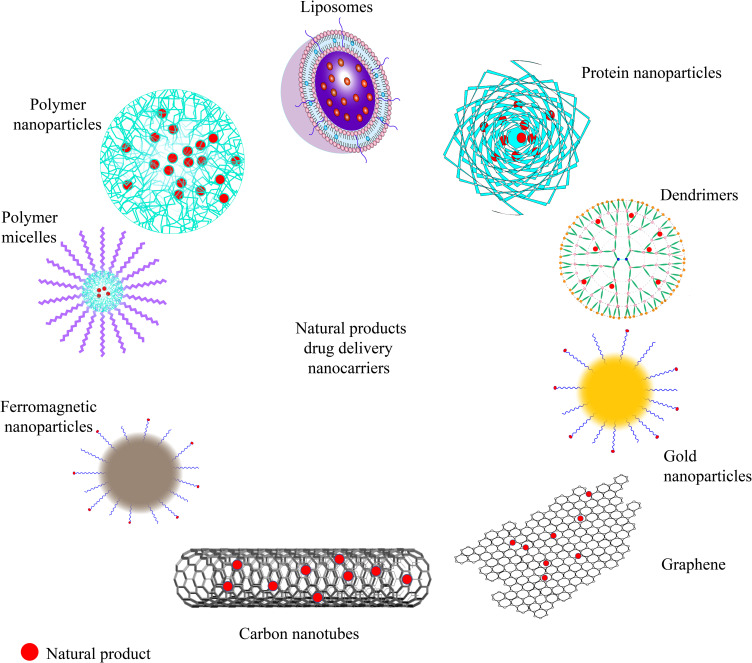
The particle size, shape, arrangement, surface charge distribution, and surface chemistry of nanomedicine carriers are the key factors that determine their physicochemical properties and interactions with the biological environment (molecules, cells, tissues, etc.).48,49 The first generation of nanoMedicines was based on the enhanced permeability and retention (EPR) effect of the tumor vasculature to load traditional small-molecule antitumor drugs with liposomes as carriers to achieve drug enrichment in tumors. Liposomal doxorubicin (Doxil) became the first nanomedicine approved by the Food and Drug Administration(FDA), achieving a breakthrough in the field of nanomedicine applied as tumor therapy.50 The preparation of subsequent nanomedicines is more diversified in terms of carrier types and drug type selection, with albumin-bound paclitaxel (Abraxane) and paclitaxel polymer micelles (Genexol-PM) being the most representative.51,52 In recent years, a variety of novel nanocarriers have also become a hotspot in the field of nanomedicines, showing stronger specificity than Traditional carriers.53,54 The choice of nanocarriers loaded with NP affects the strength of antitumor effects and the potential to target CSCs (Figure 3).
Figure 3.
Drug delivery nanocarriers for NP.
Liposomes
Liposomes are hollow spherical vesicles formed by the self-assembly of amphiphilic molecules containing a lipid bilayer and an internal aqueous core that allow liposomes to carry and target the delivery of hydrophilic and hydrophobic drugs, respectively, and protect the activity of the drug during slow drug release. Liposomes are inherently biocompatible and biodegradable because their main components are phospholipids and cholesterol, naturally present in cell membranes. Ordinary liposomes are rapidly cleared by the liver and spleen, resulting in a short half-life and circulation time in the body. Modification of liposomes with hydrophilic polymers such as polyethylene glycol (PEG) and chitosan can prolong their in vivo circulation time and enhance their tumor enrichment. In addition, liposomes modified with sugar residues, receptor ligands, antibodies, hormones and other ligands can bind specifically to tumor cell target proteins and enter the cells through receptor-mediated endocytosis, accelerating the application of nanomedicine delivery to tumors.41–44
Polymeric Nanoparticles
Polymeric nanoparticles are colloidal nanoparticles composed of natural or synthetic polymeric materials, mainly classified as nanocapsules (cavities surrounded by polymeric membranes or shells) or nanospheres (solid matrix systems) according to their morphology and further classified as polymeric vesicles, micelles and dendrimers. The drug can be encapsulated inside the polymer or coupled to the polymer surface, and further modification of ligands on the surface can achieve targeted drug delivery. However, polymeric nanoparticles have the potential risk of increased toxicity and particle aggregation, so polymers with high biocompatibility and degradability, such as poly (lactic acid) (PLA), poly(lactic acid-hydroxyacetic acid) (PLGA), and polycaprolactone (PCL), are being developed.45–47
Proteins
Proteins of plant and animal origin or recombinant proteins are attractive nanocarriers that are important in clinical therapy, especially in targeted tumor therapy, because of their good biocompatibility, biodegradability, nontoxicity, high stability and drug delivery.48 Protein-based drug carriers such as albumin and ferritin are commonly used. Albumin is an ideal carrier for delivering hydrophobic drugs, and paclitaxel albumin nanoconjugated particles (Abraxane) use human serum albumin as a carrier to rapidly distribute and aggregate in tumor tissues through the natural transport pathway of albumin in the body, which increases the efficacy of chemotherapy and reduces toxicity at the same time.49,50 Ferritin is the major iron storage protein in the body, and its specific affinity for transferrin receptor 1 (TfR1) allows for drug encapsulation and specific targeting of tumors, especially brain tumors, without additional modifications.51 However, high temperature, light, strong acid, strong alkali and other factors in the environment can denature and inactivate proteins, and the limitations of proteins as a carrier have limited their further development in clinical applications.52
Inorganic Nanoparticles
Inorganic nanoparticles, which have the advantages of simple preparation, a high drug loading rate, good stability, good photothermal and photodynamic effects, and easy surface modification, mainly include carbon nanomaterials (carbon nanotubes, fullerenes, graphene and graphene oxide), gold nanoparticles, ferromagnetic oxide nanoparticles, silica nanoparticles, calcium nanomaterials, etc.53,54 Typical representatives such as Aurmine (CYT6091), a gold nanoparticle carrying tumor necrosis factor (TNF), carry toxic but highly effective doses of the anticancer agent TNF into tumor tissues to destroy their blood vessels, rather than releasing it into healthy tissues, allowing subsequent chemotherapy to penetrate the tumor and kill the cancer cells inside.55 However, the low water solubility and high toxicity of inorganic materials are still important issues that cannot be ignored when considering clinical applications, such as oxidative stress and DNA damage induced by iron oxide nanomaterials. In contrast, carbon quantum dots, as emerging carbon-based nanomaterials with good biocompatibility and low cytotoxicity, are widely used in biofluorescence imaging, tumor diagnosis and treatment and may be the most ideal nanomedicine or carrier for the integration of tumor diagnosis and treatment.56–58
NPNs Targeting CSCs
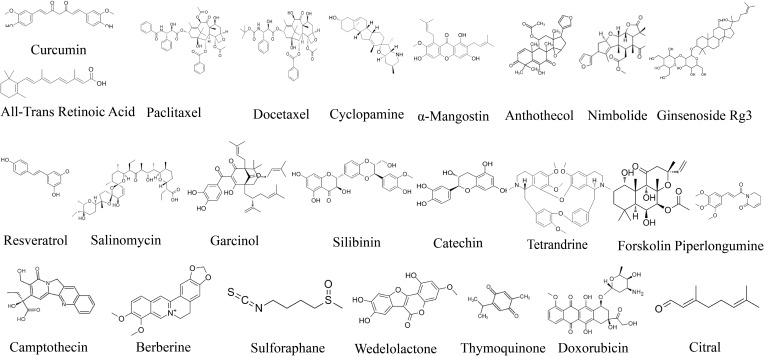
Numerous clinical trials have shown that NPNs can improve the antitumor efficacy of NP.55–58 Nanocarriers enable NP, such as curcumin, paclitaxel, cyclopamine, all-trans retinoic acid, resveratrol, and silibinin (Figure 4), to maintain a stable form before reaching their target organs, and their controllable release of NP to precisely target CSCs significantly improves their therapeutic effect against tumors.
Figure 4.
Chemical structure of NPs targeting CSCs.
Curcumin
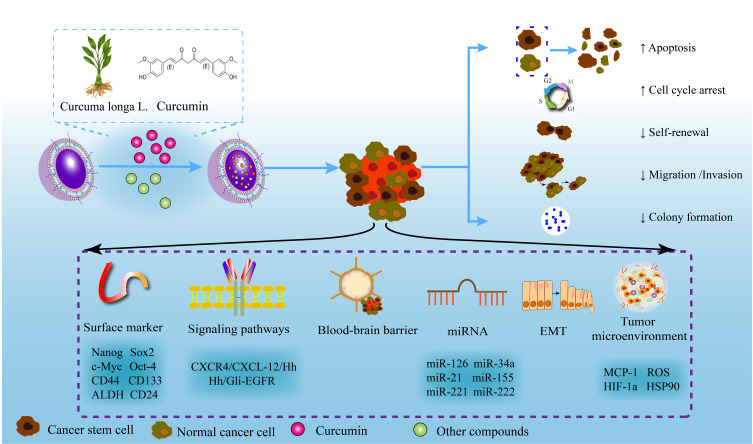
Curcumin contained in Curcuma longa L. is a natural polyphenolic compound. With the increasing amount of research on curcumin, it has been found that curcumin has a wide range of pharmacological activities, such as antitumor, anti-inflammatory, antioxidant, antiviral, and anti-infection activities.59,60 Curcumin exerts excellent antitumor effects by inhibiting proliferation, inducing apoptosis, and reversing MDR.61,62 Curcumin can inhibit CSCs in numerous types of cancers, such as glioma and breast, colorectal, pancreatic, brain, and esophageal cancers.63–66 Even so, its therapeutic potential is limited by its poor bioavailability. Nanotechnology-based drug delivery systems, such as nanoparticles and liposomes, could facilitate curcumin targeting CSCs, thereby improving its bioavailability, cellular uptake, and antitumor activity (Figure 5, Table 1).
Figure 5.
Mechanism of targeting CSCs by curcumin nanoformulations. A nanotechnology-based drug delivery system can improve the bioavailability and antitumor activity of curcumin and promote the targeting of curcumin to CSCs. By regulating surface markers, signaling pathways, the tumor microenvironment, EMT, and other mechanisms of CSCs, curcumin nanoformulations have shown excellent efficacy against pancreatic cancer, breast cancer, colorectal cancer, glioma, and other cancers.
Table 1.
Characteristics of NP-Based Nanoformulations with Curcumin
| Nanocarrier | Co-Loaded | Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) | Drug Loading (%) | Target CSCs | Mechanism | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Superparamagnetic iron oxide nanoparticle | - | 120-140 | 0.244 ± 0.03 | -7 | - | - | Pancreatic cancer | ↓ Tumorsphere formation, self-renewal, C-X-C chemokine receptor type 4/C-X-C motif chemokine ligand 12 /sonic hedgehog signaling pathway ↑ Gemcitabine uptake and efficacy |
[65] |
| Polymeric nanoparticle | - | - | - | - | - | 1.5 | Glioblastoma | ↓ CD133+ stem-like population, STAT3 | [70, 71] |
| Niosome nanoparticle | - | 90 | 0.2 ± 0.002. | -35 | 80 | - | Glioblastoma | ↓ Proliferation, viability, migration, invasion and colony forming ↑ Apoptosis and necrosis |
[72] |
| P-aminophenyl-α-D-mannopyranoside-targeting curcumin plus quinacrine liposomes | Quinacrine | 119.7 ± 0.17 | 0.22 ± 0.01 | −2.73 ± 0.74 | 94.32 ± 0.71 | - | Glioblastoma | ↑ Apoptosis, uptake and endocytic effects ↑ Cross the blood-brain barrier |
[73] |
| Chitosan- PLGA nanoparticles | - | 187.50 ± 5.08 | - | 21.57 ± 3.73 | 82.67 ± 2.02 | 2.5 | Glioblastoma | ↓ Proliferation ↑ Cross the blood-brain barrier |
[74] |
| PH-sensitive core-shell nanoparticle | Doxorubicin | 160.8 ± 8.64 | - | -30.6 ± 4.98 | 85.07 ± 2.86 | 4.46 ± 0.87 | Glioma | ↓ CD133+ stem-like population | [75] |
| Hybrid lipid capsules | - | Three different sizes: 27 ± 3/78 ± 5/149 ± 8 | 0.247 ± 0.016/0.271 ± 0.017/0.247 ± 0.024 | -10 ± 2 /-14 ± 2/-19 ± 1 | 90 | 4 | Breast Cancer | ↓ Mammosphere size/number and stemness | [76] |
| Sterically stabilized phospholipid nanomicelles | - | 11.5 ± 2.0 | - | - | 86.0 ± 4.8 | - | Breast Cancer | ↓ Tumorsphere formation ↑ Uptake, water solubility and stability of curcumin |
[77] |
| Folic acid functionalized nanoliposomes | - | 83 ± 17 | <0.2 | -27 ± 2 | 68 ± 4 | 3.3 ± 0.3 | Breast cancer | ↓ Enrichment, growth, proliferation, mammosphere growth and epithelial-mesenchymal transition | [78] |
| Phosphorylated amphiphilic calixarene micelles | - | 3.86 ± 0.32 | 0.125 ± 0.078 | −25.18 ± 5.74 | 95.40 ± 4.50 | 17.10 ± 1.25 | Triple-negative breast cancer | ↓ Tumorsphere formation, proliferation, invasion and migration ↓ CD44+ /CD133+ breast CSCs |
[80] |
| Glucose nanogold particles | - | 15 | - | - | - | - | Breast cancer | ↓ Tumorsphere formation, proliferation, hypoxia-inducible factor 1α and heat shock protein 90 ↑ Apoptosis and reactive oxygen species |
[82] |
| HA-PLGA hybrid NPs | Paclitaxel | 347.6 | 0.12 | -26.5 | 44.6 | - | Breast cancer | ↓ Mammosphere formation, population and migration of CSCs | [83] |
| PH multistage responsive micellar | Paclitaxel | 79.4 ± 3.4 | 0.112 ± 0.15 | -2.26 ± 3.9 | 30.9 ± 2.4 | 37.1 ± 2.9 | Breast cancer | ↑ Cellular uptake and deep tumor penetration ↓ Formation and growth of mammospheres |
[84] |
| Oligosaccharides of hyaluronan -histidine-menthone 1,2-glycerol ketal | Paclitaxel | 120.6 | - | - | - | - | Breast cancer | ↓ CD44+ breast CSCs | [85] |
| PH-sensitive polymeric nanoparticles of mPEG-PLGA-PGlu with hybrid core | Doxorubicin | 103.4 ± 0.3 | - | -11.7 ± 0.1 | 80.30 ± 1.82 | 1.91 ± 0.13 | Breast cancer | ↓ Percentage of CSCs | [86] |
| Hyaluronic acid conjugatedPLGA-PEG-NH2 co-polymer | Salinomycin | 153.4 ± 4.6 | - | −32.6 ± 2.5 | 82 | - | Breast cancer | ↑ G1 cell cycle arrest ↓ Epithelial-mesenchymal transition |
[87] |
| PLGA nanoparticles | GANT61 | 347.4 ± 2.75 | 0.318 ± 0.02 | -21.3 ± 0.23 | 99.97 ± 0.09 | 28.6 ± 2.05 | Breast cancer | ↓ Self-renewal ↑ Autophagy and apoptosis |
[88] |
| Stearic acid-g-chitosan oligosaccharide | - | 114.7 ± 16.9 | 0.57 ± 0.02 | 18.5 ± 0.4 | 29.9 ± 2.9 | - | Colorectal cancer | ↓ CD44+/CD24+ CSCs, tumorsphere formation and proliferation ↑ Uptake of curcumin |
[89] |
| Polymersome nanoparticles | - | 259.5 ± 1.5 | 0.465 ± 0.012 | -8.74 ± 0.2 | 97.18 ± 0.05 | 16.08 ± 0.07 | Colorectal cancer | ↓ CD44+/CD24+ /CD133+ CSCs ↑ Apoptosis and S cell cycle arrest |
[90] |
Abbreviations: PDI, polydispersity index; ↑, increase or promote; ↓, decrease or inhibit; -, no data available.
Although gemcitabine is the first-line chemotherapy regimen for pancreatic cancer, drug resistance invariably develops, significantly limiting its clinical efficacy.67 Khan et al prepared a superparamagnetic iron oxide nanoparticle formulation of curcumin (SP-CUR) that could effectively deliver curcumin to pancreatic tumors, target the tumor microenvironment, and improve gemcitabine uptake and efficacy by inhibiting the C-X-C chemokine receptor type 4 (CXCR4)/C-X-C motif chemokine ligand 12 (CXCL-12)/sonic hedgehog signaling pathway. More importantly, the combination therapy of SP-CUR and gemcitabine inhibited CSC growth and self-renewal by regulating pluripotent maintenance stem cell factors and limiting tumor sphere formation.65 These results indicated that SP-CUR has great potential for the clinical treatment and management of pancreatic cancer.
Glioblastoma is the most common and fatal central nervous system malignancy, originating from glial cells and accounting for one-third of all central nervous system tumors. Glioblastoma is almost impossible to cure, prone to recurrence and has a high lethality rate. Even with surgery plus postoperative chemoradiotherapy, the median survival of patients is only approximately 15 months, and the 5-year survival rate is less than 10%.68,69 The poor transport of drugs across the blood‒brain barrier (BBB) and glioblastoma stem cells play key roles in the occurrence, invasion and recurrence of the disease. Curcumin nanoparticles have been found to inhibit the growth of multiple glioblastoma cell lines by reducing CSCs.70,71 Negah et al designed a curcumin-loaded niosomal nanoparticle (CM-NP) to target curcumin delivery to CSCs, showing stronger anti-CSC ability than free curcumin. CM-NP have good physicochemical stability and effectively target and inhibit the survival, proliferation, migration, invasion and colony formation of CSCs by regulating cell cycle arrest, apoptosis, reactive oxygen species (ROS) generation, and monocyte chemoattractant protein-1.72 An ability to penetrate the BBB is an essential characteristic of the anti-glioblastoma efficacy of NPNs. Wang et al designed liposomes coloaded with curcumin and quinacrine modified by mannose, which contributed to curcumin and quinacrine crossing the BBB and significantly enhanced the curcumin uptake effect, endocytosis effect and induction of apoptosis in glioblastoma cells and CSCs.73 Sialic acid can improve the hydrophilicity of curcumin-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles and inhibit the proliferation of CSCs by allowing it to cross the BBB.74 In addition, curcumin combined with chemotherapy drugs is also an effective method for the treatment of GBM. Xu et al developed a pH-sensitive core-shell nanoparticle for the proportional delivery of curcumin/doxorubicin to target both CD133+ CSCs and differentiated cancer cells, showing a synergistic therapeutic effect between curcumin and doxorubicin.75 These data suggest that nanocarriers can efficiently deliver curcumin across the BBB to inhibit glioblastoma CSCs, thereby improving the clinical efficacy of glioblastoma treatment.
Breast cancer is the leading cancer affecting women in terms of both incidence and mortality, and chemotherapy resistance and disease recurrence associated with breast CSCs remain serious challenges.2 Yadava et al developed curcumin-loaded nanostructure hybrid lipid capsules (CMN-nHLCs) to optimize their physicochemical properties and anticancer efficacy for the co-elimination of CSCs and cancer cells. CMN-nHLCs effectively prevented the enrichment, growth, and proliferation of CSCs by downregulating the expression of ALDH-1 and led to the disintegration or size/number reduction of mammospheres with an anticancer activity 2.5 times higher than that of free curcumin.76 Gülçür et al found that the vasoactive intestinal peptide (VIP) receptor is an attractive molecular target overexpressed in breast CSCs, and VIP-modified curcumin sterically stabilized phospholipid nanomicelles (C-SSM-VIP) were designed to enhance cell-selective and intracellular drug uptake, actively targeting VIP receptors to inhibit CSCs.77 Similarly, folate receptor (FR)-targeted nanoliposomes promoted curcumin internalization into FR-positive CSCs, thereby preventing CSC enrichment, growth, proliferation, spheroid formation, and epithelial-mesenchymal transition.78
Triple-negative breast cancer (TNBC) is a heterogeneous and difficult-to-treat type of breast cancer for which there are currently no effective targeted therapies.79 Phosphorylated calixarene POCA4C6 is not only an excellent carrier for the delivery of curcumin, but itself has good anticancer activity. Studies have shown that curcumin-loaded POCA4C6 micelles (CPMs) can induce cell cycle arrest and apoptosis and decrease β-catenin nuclear activity and androgen receptor levels. More importantly, these NPNs can significantly destroy the formation of CD44+/CD133+ breast CSCs and tumor spheroids without causing obvious systemic toxicity,80 which provides new hope for the targeted therapy of TNBC.
It was also found that gold nanoparticles showed good radiosensitizing ability.81 Yang et al prepared curcumin combined with glucose gold nanoparticles (Glu-GNPs), which showed great potential in alleviating the hypoxic tumor microenvironment and improving the radiosensitivity of breast CSCs by inhibiting the expression of hypoxia-inducible factor 1α (HIF-1α) and heat shock protein 90 (HSP90) and increasing the level of ROS.82 Surprisingly, the nanoformulations prepared by combining curcumin with chemotherapeutic drugs such as paclitaxel,83–85 doxorubicin,86 salinomycin,87 and the Hh/Gli-EGFR signaling pathway inhibitor GANT6188 also showed superior tumor suppressive activity and CSC killing ability, which provides a potential strategy for drugs combined with targeted therapy to improve the treatment of breast cancer.
Colorectal cancer is the third most common type of gastrointestinal cancer in the world and the second leading cause of cancer-related mortality. Targeting the inhibition of the malignant biological behavior of CSCs is an important strategy for colorectal cancer treatment.2 Wang et al prepared curcumin encapsulated in stearic acid-g-chitosan oligosaccharide polymeric micelles to increase the accumulation of curcumin in cancer cells through endocytosis, and the NPNs not only reduced the tumor volume but also inhibited the expression of the colorectal CSC marker CD44+/CD24+.89 Pakizehkar et al encapsulated curcumin with polyribosomal nanoparticles, which significantly inhibited the proliferation of CSCs and induced apoptosis by modulating colorectal CSC surface markers (CD133, CD24, and CD44), miRNAs (miR-126, miR-34a, miR-21, miR-155, miR-221, and miR-222) and the expression of apoptosis targets such as P53, CASP9, CASP8, CASP3, BAX and BCl-2.90 This suggests that nanocarrier-loaded curcumin can eliminate CSCs as well as bulk cancer cells, which has significant advantages in cancer therapy.
Paclitaxel
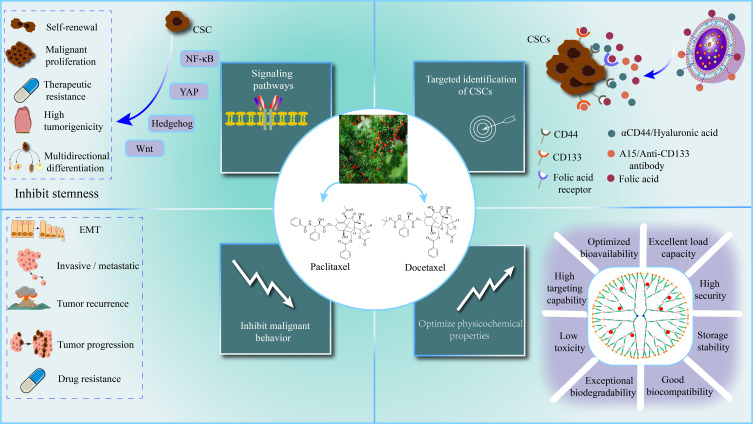
Paclitaxel (PTX) is a natural secondary metabolite isolated and purified from the bark of Taxus chinensis.91 PTX has been proven to have good antitumor effects, especially on ovarian cancer, uterine cancer, and breast cancer. The anticancer mechanism of PTX is mainly to inhibit the depolymerization of tubulin and promote its polymerization, inhibit cell division and proliferation, and eventually lead to tumor cell apoptosis. Although PTX can eliminate most tumor cells and reduce the size of tumors, it also enriches the CSC population, leading to acquired drug resistance, recurrence, metastasis, and progression.92,93 Therefore, reducing tumor cells while targeting CSCs is a key strategy to improve the anticancer efficiency of PTX (Figure 6, Table 2).
Figure 6.
Mechanism and advantages of paclitaxel and docetaxel nanoformulations. Nanoformulations of paclitaxel and docetaxel significantly improved their physicochemical properties. By modulating key signaling pathways, surface markers and other mechanisms of CSCs, nanoformulations of paclitaxel and docetaxel significantly inhibited cancer migration, invasion, progression, recurrence, and drug resistance.
Table 2.
Characteristics of NP-based nanoformulations with paclitaxel or docetaxel
| Natural Products | Nanomaterials | Co-loaded | Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) | Drug Loading (%) | Target CSCs | Mechanism | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Paclitaxel | Multifunctional tandem peptide modified Liposomes | - | 100.8 ± 1.5 | 0.201 ± 0.018 | −7.36 ± 0.32 | 92.37 ± 4.91 | - | Glioma | ↓ Proliferation ↑ Apoptosis and destruction of VM channels |
[95] |
| PLGA nanoparticles, conjugated with folic acid | - | 294.7 | - | - | 73 | 1.46 | Ovarian cancer | ↓ ATP-binding cassette sub-family G-2 (ABCG2) and multidrug resistance-1 (MDR1) ↑ Caspase-3 and p53 |
[96] | |
| Anti-CD44-Conjugated Olive Oil Liquid Nanocapsules | - | 110 ± 20 | 0.25 ± 0.01 | -35 | 81.1 | 2.2 | Pancreatic Cancer | ↓ CD44 ↑ Uptake of paclitaxel |
[98] | |
| CD133-targeted nanoparticles | - | 318.6 | 0.228 | -8.3 | 91 | 11.9 ± 0.6 | Breast cancer | ↓ Formation of tumor spheres, CD133 | [99] | |
| Albumin nanoparticle | - | - | - | - | - | - | Triple-negative breast cancer | ↑ Uptake of CSCs | [101] | |
| Lipid nanocapsules | Salinomycin | 89 ± 3 | 0.08 ± 0.002 | -6 ± 1 | 98 | 0.202 | Breast cancer | ↓ Formation of tumor spheres, CD44 ↑ Apoptosis |
[104] | |
| PLGA nanoparticles | Salinomycin | 116.71 ± 4.31 | 0.257 ± 0.08 | 68.2 ± 2.2 | 59.7 ± 5.7 | 5 | Breast cancer | ↑ Cellular uptake ↓ Population of CD44 + cells |
[105] | |
| Liquid crystal nanoparticles | Forskolin | 90 | - | -15.3 | 60-90 | - | Breast cancer | ↑ Epithelial-mesenchymal transition ↓ Wnt/β-catenin pathway, stemness |
[107] | |
| Lipid-polymer hybrid nanoparticles | Verteporfin | 80-100 | - | -3 | 67 | 0.56 | Triple-negative breast cancer | ↓ NF-κB, Wnt, and YAP signaling pathways ↓ CD44+/CD24− and ALDH+ CSCs |
[111] | |
| Dual-modified cationic liposomes | Survivin-siRNA | 118.7 ± 6.3 | 0.134 ± 0.082 | 11.5 ± 0.6 | 98.2 ± 0.6 | 2.7 ± 0.4 | Glioma | ↑ Apoptosis and differentiation of CSCs | [113] | |
| Docetaxel | Gelatinase-stimuli nanoparticles | Salinomycin | 214.5 ± 2.6 | 0.186 ± 0.013 | - | 10.26 ± 3.9 | 61.57 ± 3.9 | Cervical cancer | ↓ CD133/CD44 ↓ Epithelial-mesenchymal transition, tumorigenicity and tumor growth rate |
[117] |
| Polylactide-co-glycolide/D-alpha-tocopherol polyethylene glycol 1000 succinate | Salinomycin | 73.83 ± 3.59 | 0.193 ± 0.021 | −25.7 ± 2.03 | 53.28 ± 8.96 | 4.08 ± 0.86 | Breast cancer | ↑ The circulation time ↓ Mammospheres |
[118] | |
| Hyaluronan modified mesoporous silica nanoparticles -supported lipid bilayers | 8-Hydroxyquinoline | 189.9 ± 3.428 | 0.092 ± 0.015 | -54.2 ± 0.372 | 8.23 ± 0.91 | 8.51 ± 0.15 | Breast cancer | ↓ CD44 ↑ Uptake |
[121] | |
| HA-modified polymeric nanoparticles | Photosensitizer mesotetraphenyl chlorine disulfonate | 205 ± 3 | 0.2 | −37.2 ± 3 | 96 ± 4 | 4.8 | Breast cancer | ↓ Self-renewal capacity, CD44 | [122] | |
| Liposomes | Telmisartan | 133.2 ± 11.7 | 0.207 ± 0.0113 | - | 96.4 ± 2.45 | - | Lung cancer | ↑ Apoptosis and reactive oxygen species ↓ CD44, SOX2, ABCC1 and ABCG2 |
[126] |
Notes: PDI, polydispersity index;↑, increase or promote;↓, decrease or inhibit; -, no data available.
PTX Nanoformulations
Researchers have found that the presence of vasculogenic mimicry (VM) and CSCs inevitably leads to the malignant progression of gliomas. VM transports nutrients and blood to the extravascular areas of tumors, while CSCs are associated with drug resistance and glioma recurrence. Therefore, the key to glioma treatment is to inhibit VM and CSCs.94 Liu et al prepared multifunctional tandem peptide R8-c (RGD)-modified PTX liposomes for targeted inhibition of VM and CSCs. RGD specifically promotes the contact of CSCs with liposomes, thereby increasing the uptake of liposomes by CSCs. Both in vitro and in vivo experiments demonstrated that PTX-loaded liposomes effectively inhibited the proliferation and induced apoptosis of CSCs and induced the destruction of VM channels, thereby cutting off the nutrient transport channels and preventing glioma recurrence.95 ElNaga et al prepared PTX-loaded PLGA nanoparticles to target ovarian CSCs by recognizing FR. In vitro experiments showed that the inhibition efficiency of the NPNs on ovarian CSCs was much higher than that of free PTX. A xenograft model was established by subcutaneous injection of CSCs in the backs of nude mice, and PLGA nanoparticles enhanced the targeted antitumor ability of PTX and minimized systemic toxicity. Surprisingly, the tumors in the xenograft mice completely disappeared after injection of PTX-loaded folic acid (FA)/PLGA nanoparticles, the possible mechanism being the appearance of reactive lymphoid follicles, high expression of caspase-3 and P53 promoting apoptosis and suppression of the expression of chemoresistant genes.96
Lipid liquids are also excellent nanocarriers for drug delivery.97 Marchal et al developed olive oil liquid nanocapsules (O2LNCs), which covalently linked specific immuno-γ-globulin to the surface of O2LNCs, thereby preparing a new immunonanoformulation. Its internal hydrophobic domain was used to encapsulate PTX, and the outer shell was covalently conjugated with an anti-CD44-fluorescein isothiocyanate antibody (αCD44) to specifically deliver PTX to CD44-overexpressing pancreatic CSCs. Both in vitro and in vivo experiments showed that pancreatic CSCs with high uptake of PTX-loaded αCD44-O2LNCs were four times more effective than free PTX against cancer.98 This immuno-nanoformulation innovatively optimizes the ability of conventional nanomaterials to target CSCs with IgG, providing a new idea for the advancement of nanodrug delivery systems. Similarly, Swaminathan et al combined an anti-CD133 monoclonal antibody with nanoparticles formulated with poly (D, L-lactide-co-glycolide) polymers to load PTX to achieve targeted killing of breast CD133+ CSCs.99
Abraxane, an albumin nanoparticle of PTX, has been approved by the FDA as a first-line treatment for metastatic breast cancer, advanced non-small cell lung cancer, and advanced pancreatic cancer.100 In patients with metastatic breast cancer, Abraxane was significantly more effective than free PTX. Yuan et al found that the plasma concentration of Abraxane was much lower than that of paclitaxel, but the tumor/plasma drug concentration ratio of Abraxane was 10 times higher, explaining its targeting and high efficacy. PTX enriches CSCs in residual tumors while eliminating breast cancer cells. In contrast, Abraxane increased drug uptake by CSCs 3- to 15-fold and dramatically improved its anticancer activity by eliminating CSCs,101 suggesting that NPNs can optimize the efficacy of existing chemotherapy drugs and reverse drug resistance and cancer recurrence.
Nanocarriers Coloaded with PTX and Other Molecules
Many small-molecule compounds (such as salinomycin and doxorubicin) and plant-derived compounds (such as curcumin, piperine, epigallocatechin gallate, and sulforaphane) have been shown to be effective in eliminating CSCs.102 Therefore, the establishment of nanoformulations coloaded with PTX and other natural anti-CSC compounds, may ameliorate their respective limitations, reduce their side effects, and enhance their anticancer efficacy due to their different modes of action.
Among the various anti-CSC natural compounds screened, salinomycin isolated from Streptomyces albicans has proven to be a perfect candidate for killing CSCs.103 Basu et al developed lipid nanocapsules (LNCs) coloaded with PTX and salinomycin, which showed superior drug loading capacity and storage stability. Its lipid properties facilitate efficient cellular uptake, delivering drugs to breast cancer cells and CSCs simultaneously. The intervention of free PTX led to an increase in the number of CSCs (increased expression of CD44+/CD24− and ALDH), while the presence of salinomycin reduced the stemness of CSCs. The combined application of these two natural compounds significantly induced CSC apoptosis and tumor mammosphere growth, showing superior cytotoxicity and anti-CSC properties.104 Hyaluronan was coated on the surface of polymeric nanoparticles coloaded with PTX and salinomycin, which actively targeted CD44 receptors overexpressed on CSCs and eradicated tumors by killing the cancer cells and CSCs.105 This suggests that combination therapy with conventional chemotherapeutic agents and CSC inhibitors may be a promising approach to overcome cancer recurrence caused by drug-resistant cell populations.
Forskolin, a diterpene extracted from the roots of Coleus forskohlii, induces the differentiation of CSCs through cAMP signaling, causing them to lose their mesenchymal state and acquire a nonstem cell-like epithelial state.106,107 Singh et al prepared liquid crystal nanoparticles coloaded with forskolin and PTX, and they could target both differentiated CSCs and bulk tumor cells, resulting in overall tumor targeting, thus significantly improving the therapeutic efficacy.107 Conventional PTX therapy has been shown to upregulate the NF-κB, YAP, and Wnt signaling pathways, while inhibition of these signaling pathways by the photosensitizer verteporfin may abolish the PTX-induced enrichment of CSCs.108–110 Sulaiman et al developed coloaded PTX and verteporfin hybrid nanoparticles (PV-NPs) targeting TNBC patient-derived xenografts and CSCs. PV-NPs accumulated in tumors, coinhibited the NF-κB, Wnt, and YAP signaling pathways, and exhibited synergistic effects in inhibiting tumor growth and CD44+/CD24− and ALDH+ CSC populations.111
Survivin, an important protein involved in the regulation of apoptosis, is strongly expressed in glioma tissues (especially CSCs) and is an ideal molecular target for glioma therapy. The combination of PTX with survivin-siRNA represents a potentially useful chemotherapeutic gene therapy strategy.112 Sun et al designed dual-modified cationic liposomes (DP-CLPs) linking two receptor-specific ligands, angiopep-2 and A15. DP-CLPs durably and stably bound glioma cells and brain microvascular endothelial cells and delivered drugs (survivin-siRNA/PTX) to CD133+ glioma CSCs. In vivo experiments showed that DP-CLPs significantly inhibited tumorigenesis and improved survival of tumor-bearing nude mice by inducing apoptosis of CD133+ CSCs without therapeutic toxicity.113 This suggests that dual-targeting ligands targeting CD133 can be used to develop safe and efficient nanoformulations.
Docetaxel
By enhancing tubulin polymerization and inhibiting microtubule depolymerization, docetaxel leads to the formation of stable nonfunctional microtubule bundles and destroys the process of mitosis in tumor cells.114 Docetaxel is one of the first-line chemotherapeutic drugs for the treatment of recurrent or metastatic cervical cancer. However, it leads to the enrichment of CSCs and the decreased expression of E-cadherin associated with EMT, resulting in treatment failure.115 The preparation of docetaxel nanoparticles targeting CSCs could address the above problems (Figure 6, Table 2).
Salinomycin is one of the key NP that could be used to change this situation. Wang et al synthesized salinomycin nanoparticles, showing their advantages of specific aggregation in tumors, anti-CSCs and low toxicity.116 Then, salinomycin-docetaxel-loaded gelatinase-stimuli nanoparticles (Sal-Doc-SE-NP) were designed. Sal-Doc-SE-NP improves the therapeutic efficacy while minimizing side effects and recurrence, not only enhancing the antitumor effect of docetaxel but also significantly inhibiting CSCs and non-CSCs in cervical cancer xenograft mice by suppressing EMT and CD133/CD44 expression.117 The nanoparticles codelivered salinomycin and docetaxel to tumor tissues to inhibit breast cancer cells and CSCs with better tumor targeting and antitumor activity.118
8-Hydroxyquinoline (8-HQ), an organic compound with preferential activity against CSCs, showed promising anticancer activity in combination with docetaxel.119,120 Wang et al combined 8-HQ-loaded HA-modified mesoporous silica nanoparticles-supported lipid bilayer (HA-MSS) and docetaxel-loaded MSS. In vivo and in vitro experiments demonstrated that docetaxel-loaded MSS was more cytotoxic to MCF-7 cells, whereas 8-HQ-loaded HA-MSS was more cytotoxic to CSCs. Therefore, the combined treatment with the two nanoformulations killed both breast cancer cells and CSCs, showing the best antitumor activity.121 Combining chemotherapy and photodynamic therapy, Gaio et al made HA-modified polymeric nanoparticles to simultaneously deliver docetaxel and the photosensitizer mesotetraphenyl chlorine disulfonate, showing excellent ability to target and kill CD44+ breast CSCs.122
Telmisartan, as an antifibrotic agent, was found to disrupt the tumor interstitial barrier and promote the distribution of docetaxel nanoparticles within lung cancer tissues and to penetrate CSCs to enhance the anticancer effect.123–125 Pretreatment with telmisartan significantly increased the uptake of docetaxel liposomes by lung CSCs and enhanced the anticancer effect by improving the hypoxic conditions of the tumor microenvironment, inducing ROS generation and apoptosis, and downregulating drug resistance genes and marker expression of CSCs.126
Cyclopamine
Cyclopamine (CYP), a steroidal alkaloid extracted from veratrum, can inhibit the proliferation, invasion and metastasis of cancer cells by suppressing the hedgehog signaling pathway. The hedgehog signaling pathway is closely related to the self-renewal and maintenance of stem cells, so the targeted inhibition of the pathway by cyclopamine can eliminate CSCs, and its nanoformulation can improve its high hydrophobicity, systemic toxicity and poor pharmacokinetics to expand its clinical applications127–129 (Table 3).
Table 3.
Characteristics of NP-Based Nanoformulations with Cyclopamine or All-Trans Retinoic Acid
| Natural Products | Nanomaterials | Co-Loaded | Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) | Drug Loading (%) | Target CSCs | Mechanism | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyclopamine | Hyaluronic acid functional amphipathic and redox-responsive polymer particles | Doxorubicin | 245.3 | 0.11 | - | 58.2/70.6 | - | Breast cancer | ↓ The number and size of tumor spheres | [130] |
| N-(2-hydroxypropyl)methacrylamide copolymer | - | - | - | - | - | - | Prostate cancer | ↓ CD133 | [131] | |
| N-(2-hydroxypropyl)methacrylamide copolymer-cyclopamine/docetaxel conjugate | - | - | - | - | - | 6.6/7.2 | Prostate cancer | ↑ Apoptosis ↓ CD133+CSCs |
[132] | |
| mPEG-b-PCC-g-PTX/CYP-g-DC | Paclitaxel | 76.37 ± 0.15 | 0.273 | - | 14.50 ± 1.60 | 5.36 ± 0.07 | Prostate cancer | ↓ Hedgehog signaling and colony formation ↑ Tumor suppressor miRNA expression |
[133] | |
| All-trans retinoic acid | Nanoparticles | Doxorubicin | 151.6 | - | 1.8 | 40.01 | 3.05 | Breast cancer | ↑ Differentiation of CSCs | [137] |
| Albumin nanoparticles | - | 180.63 ± 0.38 | 0.180 ± 0.007 | 32.1 ± 0.42 | 93 | 8.37 | Lung cancer | ↑ Uptake of ATRA, apoptosis ↓ Cell growth |
[138] | |
| Lipid-polymer nanoparticles | - | 125.2 ± 9.9 | 0.18 ± 0.08 | -16.3 ± 7.2 | 86.4 ± 5.6 | 10.5 ± 4.5 | Osteosarcoma | ↓ CD44+ CSCs | [139] | |
| Nanoparticles | - | 106.7 ± 8.7 | 0.17 ± 0.18 | -11.5 ± 5.3 | 84.5 ± 6.9 | 8.4 ± 3.4 | Gastric cancer | ↓ CD44+ and CD133+ gastric CSCs | [140] | |
| FA-modified chitosan (CSO)-derived polymer micelles | Doxorubicin | 73.34 | 0.209 | 25.4 | - | - | Breast cancer | ↑ Apoptosis ↓ Stemness and metastasis |
[142] | |
| Stealth liposomes | - | 81.1 ± 0.8 nm | 0.18 ± 0.01 | -6.1± 1.4 | >90 | 9.3 ± 0.1 | Breast cancer | ↑ Differentiation and cell cycle arrest ↓ Proliferation |
[143] | |
| Electrospun polycaprolactone nanofibers | - | 929 | - | - | 86.2 | 12.17 | Glioblastoma | ↑ Differentiation ↑ Effect of photothermal therapy |
[144] | |
| Gold nanostars-dendritic polyglycerol | - | 68.1 | - | 13.9 | - | 54.5 | Breast cancer | ↓ Stemness gene expression, tumor sphere formation, self-renewal and tumor growth | [145] |
Notes: PDI, polydispersity index;↑, increase or promote;↓, decrease or inhibit; -, no data available.
Hu et al synthesized HA-cystamine-polylactic-co-glycolic acid (HA-SS-PLGA) dual drug-loaded particles loaded with cyclopamine and the chemotherapeutic drug doxorubicin. The dual drug-loaded particles with hyaluronic acid targeting showed redox-responsive drug release characteristics, releasing cyclopamine and doxorubicin on demand, targeting CD44-overexpressing breast CSCs and breast cancer cells, significantly reducing the number and size of tumor spheres, and almost completely inhibiting tumor growth, showing an excellent synergistic antitumor effect.130
The selective inhibitory effect of N-(2-hydroxypropyl)methacrylamide(HPMA) copolymer cyclopamine conjugate (P-CYP) on prostate CSCs was stronger than its effect on ordinary tumor cells, and the proportion of CD133+ cells among the surviving cancer cells was significantly reduced by attenuating CD133 expression and CSCs activity.131 Zhou’s team then engineered an HPMA copolymer docetaxel conjugate (P-DTX) to kill bulk tumor cells, but not CSCs. P-DTX and P-CYP together showed superior synergistic effects against prostate tumors.132 Likewise, the combined use of cyclopamine and PTX polymer-drug conjugates alleviated PTX resistance and inhibited prostate cancer colony formation by inhibiting Hh signaling and upregulating tumor suppressor miRNA expression.133 This suggests that the use of two or more drugs with independent mechanisms of action on cancer cells can achieve synergistic therapeutic effects.
All-Trans Retinoic Acid
All-trans retinoic acid (ATRA) is the major natural metabolite of vitamin A. As a low toxicity cell differentiation agent, the anticancer efficacy of ATRA has been extensively studied in various malignancies. By activating retinoic acid receptors and retinoid X receptors to regulate gene transcription, induce stem cell differentiation and regulate stem cell maintenance-related signaling pathways, ATRA has shown excellent ability to target CSCs.134–136 However, due to the low solubility and stability of ATRA, it is rapidly cleared, resulting in a rapid decrease in the concentration of ATRA in plasma and serious dose-dependent side effects. ATRA encapsulated in nanoparticles is expected to protect it from degradation, and targeting ATRA to CSCs may stimulate CSCs to shift to a more differentiated state, resulting in a better response to chemotherapy137 (Table 3).
Li et al developed an HA-functionalized cationic albumin-based targeted nanoparticle-delivered ATRA to target CD44-overexpressing CSCs. Due to the HA modification, CD44+ CSCs promoted the uptake of ATRA and exhibited a strong inhibitory effect on cell growth and induction of apoptosis. In vivo imaging revealed that drug-loaded nanoparticles inhibited the tumorigenicity of CSCs, showed targeted accumulation in mouse tumor-bearing lungs and significantly inhibited tumor growth.138 Similarly, the use of an anti-CD133 antibody can increase the efficient and specific delivery of ATRA-loaded lipid-polymer nanoparticles to osteosarcoma stem cells for higher therapeutic efficacy.139 However, cancers often have multiple CSCs populations with different phenotypes, suggesting that targeting just one CSCs population is not enough to eliminate CSCs completely, and therefore targeting multiple CSCs subpopulations simultaneously would yield better outcomes. Chen et al prepared ATRA-loaded poly(lactide-co-glycolide)-lecithin-PEG nanoparticles (ATRA-PLPN), and combined anti-CD44 and anti-CD133 antibodies with the nanoparticles to transport ATRA specifically to CD44+ and CD133+ gastric CSCs, thereby enhancing the growth inhibition of gastric CSCs. In contrast, ATRA-PLPN cannot target any CSCs population due to a lack of anti-CD44 or anti-CD133 antibodies.140 It can be expected that better efficacy will be achieved by simultaneously targeting more phenotypic populations of CSCs.
The chemotherapeutic drug doxorubicin has been used to treat a variety of cancers, but CSCs in many solid tumors are resistant to it, which may also further enrich CSCs after treatment, leading to chemoresistance, tumor recurrence, and metastasis.141 Sun et al prepared nanoparticles simultaneously encapsulating ATRA and doxorubicin, which effectively increased the drug’s enrichment in tumor tissues and CSCs and reduced CSCs in breast tumors in a synergistic manner. ATRA and doxorubicin were simultaneously delivered to the CSCs. ATRA induced differentiation of the CSCs, thereby attenuating their tumor-initiating ability and subsequently enhancing the cytotoxicity of doxorubicin, without triggering CSCs enrichment after treatment. At the same time, in vivo experiments also confirmed the significant synergistic inhibitory effect of the combined administration of ATRA and doxorubicin on tumor growth.137 Liu et al developed an FA-modified chitosan (CSO)-derived polymer (FA-CSOSA). FA modification can promote the uptake of nanoparticles by cancer cells through FA receptor-mediated cellular internalization, and thus they synthesized ATRA and doxorubicin-loaded micelles (FA-CSOSA-DOX/ATRA). Simultaneous use of these two micelles induces cancer cell apoptosis and inhibits the breast cancer stemness and metastasis induced by doxorubicin treatment.142 Li et al encapsulated ATRA in pegylated liposomes (stealth liposomes) through the EPR effect, resulting in better accumulation in tumors and significant inhibition of tumor formation and growth. Compared with regular cancer cells, breast CSCs are more sensitive to ATRA stealth liposomes and they prevent rapid proliferation of CSCs in the mitotic stage by inducing CSCs differentiation and cell cycle arrest in G0/G1 phase. At the same time, the combination of ATRA stealth liposomes and low-concentration vinorelbine stealth liposomes showed a stronger ability to kill CSCs, providing a new strategy for the treatment and prevention of the recurrence of breast cancer.143
In addition, a combination of ATRA and heat therapy is also an effective strategy for the removal of CSCs. The combination of hydroxylated multiwalled carbon nanotubes (MWCNTs-OH) and ATRA in an electrospun polycaprolactone (PCL) nanofiber system can disrupt the stemness of CSCs and reduce their tolerance to heat therapy to improve its effectiveness. Both in vivo and in vitro experiments showed that after ATRA induced CSCs differentiation, multiwalled carbon nanotubes generated heat under near-infrared, significantly inhibited the activity of glioma stem cells, and killed more CSCs.144 Similarly, Pan et al developed a gold nanostars-dendritic polyglycerol nanoplatform loaded with retinoic acid (RA) to specifically and multivalently target breast CSCs. RA induces CSCs differentiation, combined with photothermal therapy in a synergistic inhibitory manner to inhibit stemness gene expression, CSCs-driven tumor sphere formation, CSCs self-renewal, and tumor growth.145 This points the way to improving the efficacy of existing cancer treatments, including photothermal therapy, to specifically eliminate CSCs.
Flavonoids
Flavonoids are widely found in natural plants and are produced by the secondary metabolism of natural polyphenolic compounds. Studies have shown that dietary intake of rich flavonoids will reduce the risk of colon cancer, prostate cancer, breast cancer and other cancers, and play an effective role in inhibiting the growth and spread of tumor cells at multiple stages.146–148 The current main research direction is focused on the structural modification and optimization of natural flavonoids, and targeting cancer cells and CSCs through drug delivery systems may achieve more clinically meaningful effects (Table 4).
Table 4.
Characteristics of NP-Based Nanoformulations with Flavonoids or Alkaloids
| Natural Products | Nanomaterials | Co-loaded | Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) | Drug Loading (%) | Target CSCs | Mechanism | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| α-Mangostin | PLGA nanoparticle | - | 186.3 ± 6.42 | - | 0.03 ± 0.005 | 51.16 ± 2.61 | - | Pancreatic cancer | ↓ Sonic hedgehog pathway, epithelial-mesenchymal transition, self-renewal, proliferation, colony formation ↑ Apoptosis |
[150] |
| PLGA nanoparticles | - | - | - | - | - | - | Colorectal cancer | ↓ Notch pathway ↓ Epithelial-mesenchymal transition and self-renewal capacity |
[151] | |
| Garcinol | HA-coated GA-loaded PLGA nanoparticles |
- | 158.8 | 0.192 | 47.4 | 60.31 | 5.02 | Breast cancer | ↓ Hypoxia-inducible factors (HIF-1α and HIF-2α) ↓ Notch pathway |
[152] |
| Silibinin | Liposomes | Carbazole | 63.0 ± 1.0 | 0.26 ± 0.007 | 10.7 ± 0.3 | 99.1 ± 0.02 | 10 | Prostate cancer | ↓ Colony formation and migration ↑ Apoptosis and G2/M phase arrest |
[156] |
| Polymersome | - | 221.7 ± 59.23 | 0.32 | - | 94.86 ± 0.07 | 15.81 ± 0.57 | Pancreatic cancer | ↑ Apoptosis ↓ Migration and proliferation |
[159] | |
| Polymersome | - | 219.2 | 0.32 | -12.15 | 94.86 | 15.81 | Pancreatic cancer | ↑ Apoptosis ↓ Migration and proliferation |
[160] | |
| Catechin | Carbon nanotubes | - | 11 | 0.354 | - | - | - | Prostate cancer | ↑ Radiosensitivity ↓ Nanog, Oct4 and β-catenin |
[162] |
| Resveratrol | Liposomes | - | - | - | - | - | - | Glioblastoma | ↑ Caspases 3/7 | [163] |
| Liposomes | - | 200 | - | - | - | - | Glioblastoma | ↑ Apoptosis | [164] | |
| Nanoparticle. | - | 198.5 ± 0.28 | 0.196 ± 0.020 | 3.40 ± 0.976 | - | - | Oral cancer | ↑ Cytokines ↓ Invasion, proliferation and growth of CSCs |
[165] | |
| Camptothecin | Nanoparticle | - | - | - | - | - | - | Breast Cancer | ↓ Hypoxia-inducible factor 1α | [169] |
| Nanoparticle | All-trans retinoic acid | 150 | - | - | - | 6.7 | Breast Cancer | ↓ Hypoxia-inducible factor 1α ↑ Differentiation of CSCs into non-CSCs, reactive oxygen species |
[170] | |
| Nanocapsules | Fluorouridine and lovastatin | 107.72 ± 10.78 | 0.152 ± 0.013 | -26.45 ± 5.33 | 67.6 | 68.3 | Triple-negative Breast Cancer | ↓ Growth and metastasis of CSCs | [171] | |
| Berberine | Liposomes | - | 96.88 ± 1.81 | 0.20 ± 0.01 | -8.98 ± 0.96 | 93.5 ± 3.14 | - | Breast Cancer | ↓ ABC transporters (ABCC1, ABCC2, ABCC3, ABCG2) and Bcl-2 ↑ Apoptosis |
[174] |
| Piperlongumine | PLGA based nanoparticle | - | 251 | 0.3 | - | 95 | 9.5 | Triple-negative Breast Cancer | ↓ Self-renewal, stemness, chemoresistance, epithelial-mesenchymal transition and aggressiveness ↓ STAT3 |
[175] |
| Tetrandrine | Liposomes | Vinorelbine | 102.05 ± 0.99 | 0.193 ± 0.003 | 24.35 ± 4.76 | 89.453 ± 1.86 | - | glioma | ↑ Apoptosis | [176] |
Note: ↑, increase or promote;↓, decrease or inhibit; -, no data available.
Abbreviation: PDI, polydispersity index
α-Mangostin is a bioactive flavonoid in Garcinia mangostana, and it has been proven to have good antitumor properties.149 α-Mangostin-coated PLGA nanoparticles (Mang-NPs) were readily taken up by CSCs and cancer cells to inhibit cell viability, proliferation, colony formation, EMT, and induce apoptosis without affecting normal epithelial cells. More importantly, by inhibiting the expression of Notch, sonic hedgehog pathways and their downstream targets, stem cell markers (CD24, CD133, CD44, Musashi and Lgr5) and pluripotent maintenance factors (Oct4, Sox-2, Klf-4, c-myc and Nanog), Mang-NPs significantly inhibited the self-renewal ability of CSCs, suggesting the great potential of nanotechnology targeting CSCs signaling pathways in blocking cancer progression, metastasis, drug resistance and recurrence.150,151 Likewise, garcinol is a polyisoprenylated benzophenone derivative that is highly abundant in the genus Garcinia. Hyaluronic acid-modified PLGA nanoparticles loaded with garcinol reduced its toxicity to normal tissues and improved drug accumulation in tumors. By downregulating hypoxia-inducible factors (HIF-1α and HIF-2α) and the Notch pathway to induce apoptosis and inhibit CSCs proliferation, garcinol nanoformulations were able to inhibit CD44+ breast CSCs growing in a hypoxic microenvironment.152
Silibinin is a flavonoid compound isolated from the fruit and seeds of milk thistle (Silybum marianum L. Gaertn), and it has been widely used in clinical practice as a hepatoprotective drug.153 In recent years, the anticancer and tumor preventive effects exhibited by silibinin have shown great potential for development and improved bioavailability with nanomaterials.154,155 Coloaded carbazole and silibinin cationic liposomes can kill prostate cancer cells and CSCs simultaneously.156 Furthermore, aberrant up-/down- regulation of microRNAs (miRNA or miR) has been identified in different cancers and it plays an important role in the self-renewal and differentiation of stem cells.157,158 Therefore, upregulation of tumor suppressor miR and downregulation of tumor miR expression by NP with anticancer effects may be a novel approach to target CSCs. Tehrani et al found that by inhibiting tumor miR (miR-21, miR-155, and miR-221) and inducing tumor suppressor miR (miR-34a, miR-126, and miR- let7b) and their targeted expression, a nanoformulation of silibinin induced apoptosis and inhibited the migration and proliferation of pancreatic cells and CSCs.159,160 This suggests that targeting CSCs by modulating the function of miRNAs associated with stem cells is a feasible and promising approach.
Catechin, a flavonoid found in various plants, including green tea, is widely considered an adjuvant in cancer treatment.161 Carbon nanotubes deliver catechin specifically targeting CSCs, inhibit the expression of related transcription factors and regulators (including Nanog, Oct4, and β-catenin), and significantly increase the radiosensitivity of cancer cells, which is expected to eradicate prostate CSCs through synergistic effects and radiosensiticity.162 In addition, resveratrol is a well-known natural polyphenolic organic compound that is widely found in grapes, peanuts, knotweed, blueberries, and other plants, and has multiple health benefits such as anti-inflammatory, anticancer, cardiovascular and cerebrovascular protection, and anti-aging.66 The targeted delivery of resveratrol encapsulated in nanocarriers to CSCs can induce apoptosis (regulate caspases 3/7, P53), regulate the production of inflammatory cytokines (such as TNF-α, IL-6, IL-1β, etc.), and decrease the expression of metastasis (CD133, ALDH1, CXCR4, etc.) and angiogenic markers (matrix metalloproteinases, inducible nitric oxide synthase, vascular endothelial growth factor -A, etc.) in xenograft mouse model systems.163–165
Alkaloids
In recent years, alkaloids such as camptothecin, berberine, piperlongumine, and tetrandrine have been found to have good therapeutic effects on multiple types of cancer166 (Table 4). The combination of camptothecin with anti-angiogenic drugs and differentiation inducers is an effective therapeutic strategy against CSCs-derived tumor heterogeneity. The induction of hypoxia and concomitant upregulation of HIF-1a stimulates tumor angiogenesis, invasion, metastasis, resistance to anti-angiogenic drugs and self-renewal of CSCs, and the alkaloid camptothecin is a powerful inhibitor of HIF-1a activity.167,168 The camptothecin nanoformulation (CRLX101) blocks hypoxia-induced accumulation of CSCs and HIF-1a in breast cancer cells while enhancing the efficacy of anti-angiogenic drugs.169 Shen et al prepared nanoparticles for the combined delivery of ATRA and camptothecin. The nanoparticles differentially released the two drugs to maximize their synergistic anticancer efficacy and eliminate both CSCs and bulk tumor cells.170 In addition, Zhang et al innovatively combined statins with chemotherapeutic agents to design nanocapsules loaded with a lovastatin-camptothecin-fluorouridine conjugate for simultaneous drug delivery to tumor sites showing encouraging synergistic anticancer and antimetastatic potential.171 This nanodelivery system provides a simple and synergistic strategy to significantly reduce chemotherapy resistance, recurrence, and metastasis associated with CSCs.
Conventional chemotherapeutic drugs will enrich CSCs while destroying cancer cells and generate drug resistance, which in turn leads to cancer recurrence and metastasis. The overexpression of cell membrane ATP-binding cassette (ABC) transporter proteins and mitochondrial apoptosis-related proteins is the main cause of drug resistance in CSCs.172,173 Berberine liposomes can cross the membranes of CSCs, downregulate the expression of ABC transporter proteins (ABCC1, ABCC2, ABCC3, ABCG2) and selectively accumulate in mitochondria, which in turn induces the death of CSCs due to acute cytotoxic injury and the induction of apoptosis.174 PLGA-based piperlongumine nanoparticles (PL-NPs) similarly induced apoptosis in CSCs via the mitochondria pathway and inhibited self-renewal, stemness, chemoresistance, EMT, and invasiveness of CSCs by downregulating STAT3.175 The multifunctional targeting of vinorelbine plus tetrandrine liposomes enhances drug targeting across the BBB to aggregate in brain tumor sites, penetrate and destroy CSCs, and induce apoptosis in CSCs by activating relevant apoptotic proteins.176 These results suggest that targeted modulation of CSCs apoptosis may improve chemotherapy drug resistance and reduce the consequent risk of cancer recurrence and metastasis.
Other NP
Sulforaphane (SFN), a natural isothiocyanate, can inhibit CSCs and CSCs-like properties through a variety of mechanisms, such as blocking self-renewal signaling (Wnt/b-catenin, Hedgehog, and Notch signaling.), activating apoptotic and autophagic pathways, and altering miRNAs (miR-140, 21 and 29).66,177,178 However, SFN is highly hydrophobic and has poor stability to light and oxygen, which limit its efficacy and wide application.179 Gu et al developed mineralized hyaluronic acid-SS-tetradecyl nanocarriers that are responsive to highly reducing and mildly acidic tumor microenvironments, and could rapidly deliver SFN and target CD44+ breast CSCs via HA, enhancing the efficacy of SFN in inhibiting CSCs-like properties and significantly inhibit CSCs invasion, self-renewal and tumor growth.180 Likewise, by targeting the Wnt/β-catenin signaling pathway, the SFN-loaded nanoparticles significantly inhibited the self-renewal of breast CSCs and improved their chemotherapy sensitivity, and its combination with doxorubicin can eliminate both cancer cells and CSCs, thus effectively eradicating breast cancer.181
ALDHs maintain intracellular environmental homeostasis by catalyzing the conversion of toxic aldehydes to nontoxic carboxylic acids, which are essential for maintaining the self-renewal of normal and cancer stem cells. Members of the ALDH1A family (ALDH1A1, ALDH1A3) are closely associated with cancer development, metastasis, and drug resistance, hence targeting ALDH1A associated with CSCs may be an effective adjuvant cancer therapy.182–184 Natural coumestan wedelolactone-encapsulated PLGA nanoparticles enhanced drug retention and sustained release in CSCs by downregulating the expression of drug resistance genes SOX2 and ABCG2, thereby significantly reducing self-renewal, pluripotency, invasiveness and increasing sensitivity to PTX in ALDH1A1+ breast CSCs (which are known to be resistant to breast CSCs).185 CaCO3 nanoparticles codeliver thymoquinone and doxorubicin to significantly eliminate breast CSCs by inhibiting ALDH activity.186 In addition, citral extracted from lemongrass oil is a natural inhibitor of ALDH1A1 and ALDH1A3, and the nanodelivery system improves the stability of citral and maintains its ability to specifically inhibit ALDH1A1 and ALDH1A3 activity to significantly block CSCs self-renewal, colony formation, drug resistance, and metastatic potential, while its combination with PTX shows strong synergistic effects.187,188
Realgar is a mineral that has been used in China for more than 3000 years and its main active ingredient is tetraarsenic tetrasulfide (As4S4). In recent years, many studies have shown that As4S4 can induce apoptosis in various cell lines such as leukemia cell lines and lung cancer cell lines.189–191 Grinding the realgar coarse powder into nanoparticles showed higher efficacy and less toxicity, significantly eliminating CSCs and reducing their clonogenic ability.192 Moreover, realgar nanoparticles inhibited glucose metabolism, lung CSCs viability, and tumor growth by inhibiting metabolic reprogramming, which may be associated with the downregulation of HIF-1α expression via the PI3K/Akt/mTOR pathway.193
Nanomaterials delivering triterpenoids such as anthothecol, nimbolide, and ginsenoside Rg3 similarly showed excellent ability to target CSCs. The use of nanoparticle graphene oxide loaded with ginsenoside Rg3 significantly inhibited the malignant progression of osteosarcoma, inhibited the sphere formation of CSCs, and improved the effect of photodynamic therapy.194 Anthothecol-coated PLGA nanoparticles (Antho-NPs) inhibited the proliferation, colony formation, and induced apoptosis of pancreatic CSCs and suppressed the self-renewal ability of CSCs by targeting the sonic hedgehog pathway and genes regulating cell survival and the cell cycle with no effect on human normal pancreatic ductal epithelial cells. Moreover, nimbolide-encapsulated PLGA nanoparticles (Nim NPs) are similar to Antho-NPs, both of which can induce the transition of pancreatic CSCs from mesenchyme to epithelium, thereby inhibiting their movement, migration, invasion, MDR, and self-renewal capacity195,196 (Table 5).
Table 5.
Characteristics of NP-Based Nanoformulations with Other Natural Products
| Natural Products | Nanomaterials | Co-loaded | Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) | Drug Loading (%) | Target CSCs | Mechanism | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Sulforaphane | Mineralized hyaluronic acid-SS-tetradecyl nano-carrier | - | 85.90 ± 3.49 | 0.13 ± 0.01 | -13.81 ± 0.36 | 92.36 ± 2.17 | 33.64 ± 1.33 | Breast cancer | ↓ Invasion, self-renewal of CSC ↓ Tumor growth |
[180] |
| Self-Assembled Poly(D, L-lactide-co-glycolide)/Hyaluronic Acid Block Copolymer-Based Nanoparticles | Doxorubicin | 179.3 ± 2.8 | 0.113 ± 0.023 | -26.3 ± 1.5 | - | 5.7 ± 1.0 | Breast cancer | ↓ Wnt/β-catenin signaling pathway ↓ Self-renewal of breast CSCs |
[181] | |
| Wedelolactone | PLGA nanoparticles | Doxorubicin | 95 ± 0.34 | 0.77 ± 0.065 | -8.5 ± 2.35 | 83.3 ± 2.15 | - | Breast cancer | ↓ Self-renewal, pluripotency, invasiveness ↑ Sensitivity to paclitaxel |
[185] |
| Thymoquinone | Cockle Shell-derived aragonite CaCO3 nanoparticles | - | - | - | - | - | - | Breast cancer | ↓ALDH, CD44 and CD24 ↓ Migration, invasion and sphere formation |
[186] |
| Citral | Polymeric micelles | Paclitaxel | 26.51 | 0.1 | -13.87 | 99.73 | 9.95 | Breast cancer | ↓ Self-renewal, differentiation and migration, aldehyde dehydrogenase family (ALDH1A1) | [187] |
| Nanoparticle | Paclitaxel | - | - | - | - | - | Breast cancer | ↓ Colony forming, aldehyde dehydrogenase family(ALDH1A3) | [188] | |
| Realgar | Realgar nanoparticles | - | 131 | - | - | - | - | Multiple myeloma | ↓ Clonogenic capacity | [192] |
| Nano-realgar particles | - | 72.79 | - | - | - | - | Lung cancer | ↓ Glucose metabolism, CSCs viability and tumor growth | [193] | |
| Ginsenoside Rg3 | Graphene Oxide Nanoparticle | - | - | - | - | - | - | Osteosarcoma | ↓ Sphere formation ↑ Photodynamic therapy |
[194] |
| Anthothecol | PLGA-nanoparticles | - | 275 | 0.3 | -20 | - | - | Pancreatic cancer | ↓ Epithelial-mesenchymal transition; ↓ AKT and mTOR |
[195] |
| PLGA-nanoparticles | - | 190.52 ± 5.39 | 0.02 ± 0.01 | - | 45.25 ± 3.55 | - | Pancreatic cancer | ↓ Proliferation, colony formation, self-renewal, sonic hedgehog pathway, epithelial-mesenchymal transition ↑ Apoptosis |
[196] |
Notes: PDI, polydispersity index;↑, increase or promote;↓, decrease or inhibit; -, no data available.
Conclusions and Future Perspectives
Cancer remains a serious global health problem. With more in-depth research on cancer, the continuous emergence of new anticancer drugs and technical means, and the early screening of the disease, the treatment of cancer has made great progress, but serious treatment-related side effects, drug resistance, recurrence, and metastasis lead to high cancer mortality and a major social burden.197 CSCs present in tumor tissues control the occurrence and development of tumors, chemotherapy resistance, recurrence, and metastasis due to their characteristics of high proliferation, self-renewal, multidirectional differentiation, high tumorigenicity, and multidrug resistance. CSCs are highly resistant to conventional chemotherapeutic drugs, and the application of chemotherapeutic drugs will greatly enrich CSCs.40 Therefore, finding drugs that target and clear CSCs is a new strategy to improve the cure rate of cancer. A growing body of epidemiological and clinical research suggests that ingestion of natural plant compounds has health benefits, both in reducing cancer incidence and the risk of recurrence, and they exhibit synergistic effects with traditional anticancer drugs. More importantly, compared with most chemotherapeutic agents, plant compounds from abundant and safe sources directly or indirectly target key signaling pathways, self-renewal pathways, metabolism, epigenetic modifications, and the tumor microenvironment of CSCs, and this may become a new therapeutic strategy for targeting CSCs.198,199 However, despite the tremendous health benefits of NP, their clinical use is severely limited by their poor water solubility, low absorption rate, low bioavailability, and nonspecific targeting. Nanocarriers, due to their superior storage stability, tissue permeability, and biocompatibility, target the delivery of biologically active compounds of natural origin to improve their bioavailability, prolong the drug circulation time in vivo, and enhance drug efficacy.16 Therefore, the development of nanocarrier systems for targeted delivery and controlled drug release provides a possibility to overcome the toxic and side effects of traditional chemotherapeutic drugs and kill CSCs.
Furthermore, non-CSCs can be spontaneously and randomly transformed into CSCs.31,200 Therefore, better therapeutic outcomes can be achieved using a combination of conventional chemotherapy and antitumor stem cell agents to simultaneously remove cancer cells and CSCs. Currently, clinical combination therapies are mainly based on the combined administration of conventional agents, which may have synergistic, additive, or even antagonistic effects. Nanoformulations are powerful tools whose pharmacokinetics and distribution in vivo depend on the nanocarriers themselves, independent of the characteristics of the drug.201 Currently, coadministration regimens for nanoformulations are divided into the combined use of two single-drug delivery systems and the combination of two drugs in a single-drug delivery system. The use of two separate nanoformulations allows the flexibility to administer drugs at different doses and times, but it is difficult to achieve synchronization of their pharmacokinetics and biodistribution to maintain a synergistic ratio of drugs at the tumor site. In contrast, drug delivery systems that coload NP (killing CSCs) and conventional chemotherapeutic agents (killing cancer cells) can deliver them simultaneously to the tumor site in synergistic ratios, thus synchronizing tumor treatment in time and space and ensuring the elimination of different cancer cell subpopulations to achieve synergistic therapeutic effects.202–204 However, maintaining synergistic drug ratios in the same nanodelivery system is not easy because each drug exhibits different release rates according to its properties, which also poses new challenges for the design of nanodelivery systems.
In this review, we summarize the great potential of nanocarrier-delivered natural product agents in targeting CSCs to overcome cancer recurrence, metastasis, and drug resistance. Nanocarriers improve the bioavailability of natural active compounds such as curcumin, PTX, doxorubicin, cyclopamine, and all-trans retinoic acid, which are passively or actively targeted by ligand modification for delivery to CSCs to inhibit their stemness and they show excellent synergistic effects in combination with chemotherapeutic agents, providing more options for the development of novel, safe, and effective antitumor drugs. Although breakthroughs have been made in the experimental research of nanomedicines in the preclinical stage, they are still far from the core goal of achieving clinical therapeutic effects. Currently, moving from basic laboratory research to clinical applications is a key issue in the development of the nanomedicine field. With significant advances in nanotechnology, targeted drug delivery, and cancer biology, this work is expected to achieve a breakthrough soon.
Acknowledgments
This work was supported by the Key R&D project of Sichuan Province (No. 2022YFG0145), National Natural Science Foundation of China (No. 82074397), and “Hundred Talents Program” of the Hospital of Chengdu University of Traditional Chinese Medicine (No. 20-L01).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Najafi M, Majidpoor J, Toolee H, Mortezaee K. The current knowledge concerning solid cancer and therapy. J Biochem Mol Toxicol. 2021;35:e22900. doi: 10.1002/jbt.22900 [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27:34–44. doi: 10.1038/s41591-020-01195-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 5.Zhu P, Lu T, Chen Z, et al. 5-hydroxytryptamine produced by enteric serotonergic neurons initiates colorectal cancer stem cell self-renewal and tumorigenesis. Neuron. 2022;110:2268–2282.e4. doi: 10.1016/j.neuron.2022.04.024 [DOI] [PubMed] [Google Scholar]
- 6.Corgnac S, Damei I, Gros G, et al. Cancer stem-like cells evade CD8(+)CD103(+) tumor-resident memory T (T(RM)) lymphocytes by initiating an epithelial-to-mesenchymal transition program in a human lung tumor model. J Immunother Cancer. 2022;10:e004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam KH, Ma S. Noncellular components in the liver cancer stem cell niche: biology and potential clinical implications. Hepatology. 2022. doi: 10.1002/hep.32629 [DOI] [PubMed] [Google Scholar]
- 8.Song S, Chen Q, Li Y, et al. Targeting cancer stem cells with a pan-BCL-2 inhibitor in preclinical and clinical settings in patients with gastroesophageal carcinoma. Gut. 2021;70:2238–2248. doi: 10.1136/gutjnl-2020-321175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuhmacher J, Heidu S, Balchen T, et al. Vaccination against RhoC induces long-lasting immune responses in patients with prostate cancer: results from a Phase I/II clinical trial. J Immunother Cancer. 2020;8:e001157. doi: 10.1136/jitc-2020-001157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi MY, Widhopf GF, Ghia EM, et al. Phase I trial: cirmtuzumab inhibits ROR1 signaling and stemness signatures in patients with chronic lymphocytic leukemia. Cell Stem Cell. 2018;22:951–959.e953. doi: 10.1016/j.stem.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao K, Du Y, Bao X, et al. Glutathione-bioimprinted nanoparticles targeting of N6-methyladenosine FTO demethylase as a strategy against leukemic stem cells. Small. 2022;18:e2106558. doi: 10.1002/smll.202106558 [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Verwilst P, Won M, et al. A small molecule strategy for targeting cancer stem cells in hypoxic microenvironments and preventing tumorigenesis. J Am Chem Soc. 2021;143:14115–14124. doi: 10.1021/jacs.1c03875 [DOI] [PubMed] [Google Scholar]
- 13.Dandawate PR, Subramaniam D, Jensen RA, Anant S. Targeting cancer stem cells and signaling pathways by phytochemicals: novel approach for breast cancer therapy. Semin Cancer Biol. 2016;40–41:192–208. doi: 10.1016/j.semcancer.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Li SJ, Liu Y, et al. Bioengineered nanogels for cancer immunotherapy. Chem Soc Rev. 2022;51:5136–5174. doi: 10.1039/d2cs00247g [DOI] [PubMed] [Google Scholar]
- 16.Kashyap D, Tuli HS, Yerer MB, et al. Natural product-based nanoformulations for cancer therapy: opportunities and challenges. Semin Cancer Biol. 2021;69:5–23. doi: 10.1016/j.semcancer.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 17.Shen S, Xia JX, Wang J. Nanomedicine-mediated cancer stem cell therapy. Biomaterials. 2016;74:1–18. doi: 10.1016/j.biomaterials.2015.09.037 [DOI] [PubMed] [Google Scholar]
- 18.Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2022;19:26–44. doi: 10.1038/s41575-021-00508-3 [DOI] [PubMed] [Google Scholar]
- 19.Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer. 2021;21:526–536. doi: 10.1038/s41568-021-00366-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Egeren D, Escabi J, Nguyen M, et al. Reconstructing the lineage histories and differentiation trajectories of individual cancer cells in myeloproliferative neoplasms. Cell Stem Cell. 2021;28:514–523.e519. doi: 10.1016/j.stem.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bu W, Liu Z, Jiang W, et al. Mammary precancerous stem and non-stem cells evolve into cancers of distinct subtypes. Cancer Res. 2019;79:61–71. doi: 10.1158/0008-5472.CAN-18-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730 [DOI] [PubMed] [Google Scholar]
- 23.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26:2871–2875. doi: 10.1200/JCO.2007.15.1613 [DOI] [PubMed] [Google Scholar]
- 24.Oikawa T. Cancer stem cells and their cellular origins in primary liver and biliary tract cancers. Hepatology. 2016;64:645–651. doi: 10.1002/hep.28485 [DOI] [PubMed] [Google Scholar]
- 25.Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806–2812. doi: 10.1200/JCO.2008.16.6702 [DOI] [PubMed] [Google Scholar]
- 26.Prager BC, Xie Q, Bao S, Rich JN. Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell. 2019;24:41–53. doi: 10.1016/j.stem.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - A clinical update. Nat Rev Clin Oncol. 2020;17:204–232. doi: 10.1038/s41571-019-0293-2 [DOI] [PubMed] [Google Scholar]
- 28.Zhou HM, Zhang JG, Zhang X, Li Q. Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents. Signal Transduct Target Ther. 2021;6:62. doi: 10.1038/s41392-020-00430-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortezaee K, Majidpoor J. Key promoters of tumor hallmarks. Int J Clin Oncol. 2022;27:45–58. doi: 10.1007/s10147-021-02074-9 [DOI] [PubMed] [Google Scholar]
- 30.Han J, Won M, Kim JH, et al. Cancer stem cell-targeted bio-imaging and chemotherapeutic perspective. Chem Soc Rev. 2020;49:7856–7878. doi: 10.1039/D0CS00379D [DOI] [PubMed] [Google Scholar]
- 31.Walcher L, Kistenmacher AK, Suo H, et al. Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. 2020;11:1280. doi: 10.3389/fimmu.2020.01280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nallasamy P, Nimmakayala RK, Karmakar S, et al. Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1-CD44 axis. Gastroenterology. 2021;161:1998–2013.e1997. doi: 10.1053/j.gastro.2021.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farhood B, Najafi M, Salehi E, et al. Disruption of the redox balance with either oxidative or anti-oxidative overloading as a promising target for cancer therapy. J Cell Biochem. 2019;120:71–76. doi: 10.1002/jcb.27594 [DOI] [PubMed] [Google Scholar]
- 34.Mortezaee K. Hypoxia induces core-to-edge transition of progressive tumoral cells: a critical review on differential yet corroborative roles for HIF-1α and HIF-2α. Life Sci. 2020;242:117145. doi: 10.1016/j.lfs.2019.117145 [DOI] [PubMed] [Google Scholar]
- 35.Mortezaee K, Majidpoor J. (Im)maturity in tumor ecosystem. Front Oncol. 2021;11:813897. doi: 10.3389/fonc.2021.813897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukha A, Kahya U, Dubrovska A. Targeting glutamine metabolism and autophagy: the combination for prostate cancer radiosensitization. Autophagy. 2021;17:3879–3881. doi: 10.1080/15548627.2021.1962682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bi Z, Zhang Q, Fu Y, et al. Cooperation between NRF2-mediated transcription and MDIG-dependent epigenetic modifications in arsenic-induced carcinogenesis and cancer stem cells. Semin Cancer Biol. 2021;76:310–318. doi: 10.1016/j.semcancer.2021.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohan-Codeço M, Barambo-Wagner ML, Nasciutti LE, Ribeiro Pinto LF, Meireles Da Costa N, Palumbo A. Molecular mechanisms associated with chemoresistance in esophageal cancer. Cell Mol Life Sci. 2022;79:116. doi: 10.1007/s00018-022-04131-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia D, Li L, Andrew S, et al. An autocrine inflammatory forward-feedback loop after chemotherapy withdrawal facilitates the repopulation of drug-resistant breast cancer cells. Cell Death Dis. 2017;8:e2932. doi: 10.1038/cddis.2017.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank MH, Wilson BJ, Gold JS, Frank NY. Clinical implications of colorectal cancer stem cells in the age of single-cell omics and targeted therapies. Gastroenterology. 2021;160:1947–1960. doi: 10.1053/j.gastro.2020.12.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaimal R, Vinoth V, Shrikrishna Salunke A, et al. Highly sensitive and selective detection of glutathione using ultrasonic aided synthesis of graphene quantum dots embedded over amine-functionalized silica nanoparticles. Ultrason Sonochem. 2022;82:105868. doi: 10.1016/j.ultsonch.2021.105868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Sun S, Zhang Z, Shi D. Nanomaterials for cancer precision medicine. Adv Mater. 2018;30:e1705660. doi: 10.1002/adma.201705660 [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Zhong X, Li J, Liu Z, Cheng L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem Soc Rev. 2021;50:8669–8742. doi: 10.1039/d0cs00461h [DOI] [PubMed] [Google Scholar]
- 45.Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85. doi: 10.1186/s13045-021-01096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pellico J, Gawne PJ, T M de Rosales R. Radiolabelling of nanomaterials for medical imaging and therapy. Chem Soc Rev. 2021;50:3355–3423. doi: 10.1039/d0cs00384k [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Deng Q, Xiao C, Li Z, Yang X. Rational design of nanotherapeutics based on the five features principle for potent elimination of cancer stem cells. Acc Chem Res. 2022;55:526–536. doi: 10.1021/acs.accounts.1c00635 [DOI] [PubMed] [Google Scholar]
- 48.De R, Mahata MK, Kim KT. Structure-based varieties of polymeric nanocarriers and influences of their physicochemical properties on drug delivery profiles. Adv Sci. 2022;9:e2105373. doi: 10.1002/advs.202105373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Z, Ukidve A, Krishnan V, Mitragotri S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv Drug Deliv Rev. 2019;143:3–21. doi: 10.1016/j.addr.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 50.He H, Liu L, Morin EE, Liu M, Schwendeman A. Survey of clinical translation of cancer nanomedicines-lessons learned from successes and failures. Acc Chem Res. 2019;52:2445–2461. doi: 10.1021/acs.accounts.9b00228 [DOI] [PubMed] [Google Scholar]
- 51.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937 [DOI] [PubMed] [Google Scholar]
- 52.Kim DW, Kim SY, Kim HK, et al. Multicenter Phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007;18:2009–2014. doi: 10.1093/annonc/mdm374 [DOI] [PubMed] [Google Scholar]
- 53.Cui W, Li J, Decher G. Self-assembled smart nanocarriers for targeted drug delivery. Adv Mater. 2016;28:1302–1311. doi: 10.1002/adma.201502479 [DOI] [PubMed] [Google Scholar]
- 54.Thanuja MY, Anupama C, Ranganath SH. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: so near and yet so far. Adv Drug Deliv Rev. 2018;132:57–80. doi: 10.1016/j.addr.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 55.Azmi AS, Khan HY, Muqbil I, et al. Preclinical assessment with clinical validation of selinexor with gemcitabine and nab-paclitaxel for the treatment of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2020;26:1338–1348. doi: 10.1158/1078-0432.CCR-19-1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bockorny B, Macarulla T, Semenisty V, et al. Motixafortide and pembrolizumab combined to nanoliposomal irinotecan, fluorouracil, and folinic acid in metastatic pancreatic cancer: the COMBAT/KEYNOTE-202 trial. Clin Cancer Res. 2021;27:5020–5027. doi: 10.1158/1078-0432.CCR-21-0929 [DOI] [PubMed] [Google Scholar]
- 57.Shi M, Gu A, Tu H, et al. Comparing nanoparticle polymeric micellar paclitaxel and solvent-based paclitaxel as first-line treatment of advanced non-small-cell lung cancer: an open-label, randomized, multicenter, phase III trial. Ann Oncol. 2021;32:85–96. doi: 10.1016/j.annonc.2020.10.479 [DOI] [PubMed] [Google Scholar]
- 58.Piha-Paul SA, Thein KZ, De Souza P, et al. First-in-human, phase I/IIa study of CRLX301, a nanoparticle drug conjugate containing docetaxel, in patients with advanced or metastatic solid malignancies. Invest New Drugs. 2021;39:1047–1056. [DOI] [PubMed] [Google Scholar]
- 59.Kavimughil M, Leena MM, Moses JA, Anandharamakrishnan C. 3D printed MCT oleogel as a co-delivery carrier for curcumin and resveratrol. Biomaterials. 2022;287:121616. [DOI] [PubMed] [Google Scholar]
- 60.Zheng P, Ding B, Zhu G, Li C, Lin J. Biodegradable Ca2+ nanomodulators activate pyroptosis through mitochondrial Ca2+ overload for cancer immunotherapy. Angew Chem Int Ed Engl. 2022. doi: 10.1002/ange.202204904 [DOI] [PubMed] [Google Scholar]
- 61.Weng W, Goel A. Curcumin and colorectal cancer: an update and current perspective on this natural medicine. Semin Cancer Biol. 2022;80:73–86. doi: 10.1016/j.semcancer.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Zhang T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014;346:197–205. [DOI] [PubMed] [Google Scholar]
- 63.Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, et al. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63:661–669. doi: 10.1016/j.jhep.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kantara C, O’Connell M, Sarkar S, Moya S, Ullrich R, Singh P. Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA. Cancer Res. 2014;74:2487–2498. doi: 10.1158/0008-5472.CAN-13-3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khan S, Setua S, Kumari S, et al. Superparamagnetic iron oxide nanoparticles of curcumin enhance gemcitabine therapeutic response in pancreatic cancer. Biomaterials. 2019;208:83–97. doi: 10.1016/j.biomaterials.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 66.Chu M, Zheng C, Chen C, Song G, Hu X, Wang ZW. Targeting cancer stem cells by nutraceuticals for cancer therapy. Semin Cancer Biol. 2021. doi: 10.1016/j.semcancer.2021.07.008 [DOI] [PubMed] [Google Scholar]
- 67.Zhou C, Yi C, Yi Y, et al. LncRNA PVT1 promotes gemcitabine resistance of pancreatic cancer via activating Wnt/β-catenin and autophagy pathway through modulating the miR-619-5p/Pygo2 and miR-619-5p/ATG14 axes. Mol Cancer. 2020;19:118. doi: 10.1186/s12943-020-01237-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Solinge TS, Nieland L, Chiocca EA, Broekman MLD. Advances in local therapy for glioblastoma - taking the fight to the tumour. Nat Rev Neurol. 2022;18:221–236. doi: 10.1038/s41582-022-00621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shea A, Harish V, Afzal Z, et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016;5:1917–1946. doi: 10.1002/cam4.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lim KJ, Bisht S, Bar EE, Maitra A, Eberhart CG. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther. 2011;11:464–473. doi: 10.4161/cbt.11.5.14410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim K, Jing M, Anirban B, et al. Abstract 4440: using nanocurcumin to treat medulloblastoma and glioblastoma. Cancer Res. 2010;70:4440–4440. doi: 10.1158/1538-7445.AM10-4440 [DOI] [Google Scholar]
- 72.Sahab-Negah S, Ariakia F, Jalili-Nik M, et al. Curcumin loaded in niosomal nanoparticles improved the anti-tumor effects of free curcumin on glioblastoma stem-like cells: an in vitro study. Mol Neurobiol. 2020;57:3391–3411. doi: 10.1007/s12035-020-01922-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Ying X, Xu H, Yan H, Li X, Tang H. The functional curcumin liposomes induce apoptosis in C6 glioblastoma cells and C6 glioblastoma stem cells in vitro and in animals. Int J Nanomedicine. 2017;12:1369–1384. doi: 10.2147/IJN.S124276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuo YC, Wang LJ, Rajesh R. Targeting human brain cancer stem cells by curcumin-loaded nanoparticles grafted with anti-aldehyde dehydrogenase and sialic acid: colocalization of ALDH and CD44. Mater Sci Eng C Mater Biol Appl. 2019;102:362–372. doi: 10.1016/j.msec.2019.04.065 [DOI] [PubMed] [Google Scholar]
- 75.Xu HL, Fan ZL, ZhuGe DL, et al. Ratiometric delivery of two therapeutic candidates with inherently dissimilar physicochemical property through pH-sensitive core-shell nanoparticles targeting the heterogeneous tumor cells of glioma. Drug Deliv. 2018;25:1302–1318. doi: 10.1080/10717544.2018.1474974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yadava SK, Basu SM, Valsalakumari R, Chauhan M, Singhania M, Giri J. Curcumin-loaded nanostructure hybrid lipid capsules for co-eradication of breast cancer and cancer stem cells with enhanced anticancer efficacy. ACS Appl Bio Mater. 2020;3:6811–6822. doi: 10.1021/acsabm.0c00764 [DOI] [PubMed] [Google Scholar]
- 77.Gülçür E, Thaqi M, Khaja F, Kuzmis A, Önyüksel H. Curcumin in VIP-targeted sterically stabilized phospholipid nanomicelles: a novel therapeutic approach for breast cancer and breast cancer stem cells. Drug Deliv Transl Res. 2013;3:562–574. doi: 10.1007/s13346-013-0167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pradhan A, Mishra S, Basu SM, et al. Targeted nanoformulation of C1 inhibits the growth of KB spheroids and cancer stem cell-enriched MCF-7 mammospheres. Colloids Surf B Biointerfaces. 2021;202:111702. doi: 10.1016/j.colsurfb.2021.111702 [DOI] [PubMed] [Google Scholar]
- 79.Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19:91–113. doi: 10.1038/s41571-021-00565-2 [DOI] [PubMed] [Google Scholar]
- 80.Chen W, Li L, Zhang X, et al. Curcumin: a calixarene derivative micelle potentiates anti-breast cancer stem cells effects in xenografted, triple-negative breast cancer mouse models. Drug Deliv. 2017;24:1470–1481. doi: 10.1080/10717544.2017.1381198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xia D, Hang D, Li Y, et al. Au-hemoglobin loaded platelet alleviating tumor hypoxia and enhancing the radiotherapy effect with low-dose X-ray. ACS Nano. 2020;14:15654–15668. doi: 10.1021/acsnano.0c06541 [DOI] [PubMed] [Google Scholar]
- 82.Yang K, Liao Z, Wu Y, et al. Curcumin and Glu-GNPs induce radiosensitivity against breast cancer stem-like cells. Biomed Res Int. 2020;2020:3189217. doi: 10.1155/2020/3189217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Z, Sun N, Cheng R, Zhao C, Liu J, Tian Z. Hybrid nanoparticles coated with hyaluronic acid lipoid for targeted co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. J Mater Chem B. 2017;5:6762–6775. doi: 10.1039/C7TB01510K [DOI] [PubMed] [Google Scholar]
- 84.Yang Z, Sun N, Cheng R, et al. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials. 2017;147:53–67. doi: 10.1016/j.biomaterials.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 85.Chen DQ, Wang GH, Song WG, Zhang Q. Novel CD44 receptor targeting multifunctional “nano-eggs” based on double pH-sensitive nanoparticles for co-delivery of curcumin and paclitaxel to cancer cells and cancer stem cells. J Nanoparticle Res. 2015;17. doi: 10.1007/s11051-015-3217-9 [DOI] [Google Scholar]
- 86.Yuan JD, ZhuGe DL, Tong MQ, et al. pH-sensitive polymeric nanoparticles of mPEG-PLGA-PGlu with hybrid core for simultaneous encapsulation of curcumin and doxorubicin to kill the heterogeneous tumour cells in breast cancer. Artif Cells Nanomed Biotechnol. 2018;46:302–313. doi: 10.1080/21691401.2017.1423495 [DOI] [PubMed] [Google Scholar]
- 87.Zhao Y, Wang K, Zheng Y, Zeng X, Lim YC, Liu T. Co-delivery of salinomycin and curcumin for cancer stem cell treatment by inhibition of cell proliferation, cell cycle arrest, and epithelial-mesenchymal transition. Front Chem. 2020;8:601649. doi: 10.3389/fchem.2020.601649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borah A, Pillai SC, Rochani AK, et al. GANT61 and curcumin-loaded PLGA nanoparticles for GLI1 and PI3K/Akt-mediated inhibition in breast adenocarcinoma. Nanotechnology. 2020;31:185102. doi: 10.1088/1361-6528/ab6d20 [DOI] [PubMed] [Google Scholar]
- 89.Wang K, Zhang T, Liu L, et al. Novel micelle formulation of curcumin for enhancing antitumor activity and inhibiting colorectal cancer stem cells. Int J Nanomedicine. 2012;7:4487–4497. doi: 10.2147/IJN.S34702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pakizehkar S, Ranji N, Sohi AN, et al. Polymersome‐assisted delivery of curcumin: a suitable approach to decrease cancer stemness markers and regulate miRNAs expression in HT29 colorectal cancer cells. Polym Adv Technol. 2020;31(1):160–177. doi: 10.1002/pat.4759 [DOI] [Google Scholar]
- 91.Schneider F, Pan L, Ottenbruch M, List T, Gaich T. The chemistry of nonclassical taxane diterpene. Acc Chem Res. 2021;54:2347–2360. doi: 10.1021/acs.accounts.0c00873 [DOI] [PubMed] [Google Scholar]
- 92.Sharma A, Cao EY, Kumar V, et al. Longitudinal single-cell RNA sequencing of patient-derived primary cells reveals drug-induced infidelity in stem cell hierarchy. Nat Commun. 2018;9:4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blohmer JU, Link T, Reinisch M, et al. Effect of denosumab added to 2 different nab-paclitaxel regimens as neoadjuvant therapy in patients with primary breast cancer: the GeparX 2 × 2 randomized clinical trial. JAMA Oncol. 2022;8:1010. doi: 10.1001/jamaoncol.2022.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai H, Liu W, Liu X, et al. Advances and prospects of vasculogenic mimicry in glioma: a potential new therapeutic target? Onco Targets Ther. 2020;13:4473–4483. doi: 10.2147/OTT.S247855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y, Mei L, Yu Q, et al. Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl Mater Interfaces. 2015;7:16792–16801. doi: 10.1021/acsami.5b04596 [DOI] [PubMed] [Google Scholar]
- 96.Abou-ElNaga A, Mutawa G, El-Sherbiny IM, et al. Novel nano-therapeutic approach actively targets human ovarian cancer stem cells after xenograft into nude mice. Int J Mol Sci. 2017;18:813. doi: 10.3390/ijms18040813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yaghmur A, Mu H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm Sin B. 2021;11:871–885. doi: 10.1016/j.apsb.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Navarro-Marchal SA, Griñán-Lisón C, Entrena JM, et al. Anti-CD44-conjugated olive oil liquid nanocapsules for targeting pancreatic cancer stem cells. Biomacromolecules. 2021;22:1374–1388. doi: 10.1021/acs.biomac.0c01546 [DOI] [PubMed] [Google Scholar]
- 99.Swaminathan SK, Roger E, Toti U, Niu L, Ohlfest JR, Panyam J. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J Control Release. 2013;171:280–287. doi: 10.1016/j.jconrel.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 100.Di Cosimo S. Advancing immunotherapy for early-stage triple-negative breast cancer. Lancet. 2020;396:1046–1048. doi: 10.1016/S0140-6736(20)31962-0 [DOI] [PubMed] [Google Scholar]
- 101.Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17:2275–2286. doi: 10.1021/acs.molpharmaceut.9b01221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh AK, Sharma N, Ghosh M, Park YH, Jeong DK. Emerging importance of dietary phytochemicals in fight against cancer: role in targeting cancer stem cells. Crit Rev Food Sci Nutr. 2017;57:3449–3463. doi: 10.1080/10408398.2015.1129310 [DOI] [PubMed] [Google Scholar]
- 103.Qi D, Liu Y, Li J, Huang JH, Hu X, Wu E. Salinomycin as a potent anticancer stem cell agent: state of the art and future directions. Med Res Rev. 2022;42:1037–1063. doi: 10.1002/med.21870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Basu SM, Yadava SK, Singh R, Giri J. Lipid nanocapsules co-encapsulating paclitaxel and salinomycin for eradicating breast cancer and cancer stem cells. Colloids Surf B Biointerfaces. 2021;204:111775. doi: 10.1016/j.colsurfb.2021.111775 [DOI] [PubMed] [Google Scholar]
- 105.Muntimadugu E, Kumar R, Saladi S, Rafeeqi TA, Khan W. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf B Biointerfaces. 2016;143:532–546. doi: 10.1016/j.colsurfb.2016.03.075 [DOI] [PubMed] [Google Scholar]
- 106.Cardon T, Franck J, Coyaud E, et al. Alternative proteins are functional regulators in cell reprogramming by PKA activation. Nucleic Acids Res. 2020;48:7864–7882. doi: 10.1093/nar/gkaa277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh D, Singh P, Pradhan A, Srivastava R, Sahoo SK. Reprogramming cancer stem-like cells with nanoforskolin enhances the efficacy of paclitaxel in targeting breast cancer. ACS Appl Bio Mater. 2021;4:3670–3685. doi: 10.1021/acsabm.1c00141 [DOI] [PubMed] [Google Scholar]
- 108.Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2:78–91. doi: 10.1016/j.stemcr.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sulaiman A, Sulaiman B, Khouri L, et al. Both bulk and cancer stem cell subpopulations in triple-negative breast cancer are susceptible to Wnt, HDAC, and ERα coinhibition. FEBS Lett. 2016;590:4606–4616. doi: 10.1002/1873-3468.12496 [DOI] [PubMed] [Google Scholar]
- 110.Sulaiman A, McGarry S, Li L, et al. Dual inhibition of Wnt and Yes-associated protein signaling retards the growth of triple-negative breast cancer in both mesenchymal and epithelial states. Mol Oncol. 2018;12:423–440. doi: 10.1002/1878-0261.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sulaiman A, McGarry S, El-Sahli S, et al. Co-targeting bulk tumor and CSCs in clinically translatable TNBC patient-derived xenografts via combination nanotherapy. Mol Cancer Ther. 2019;18:1755–1764. doi: 10.1158/1535-7163.MCT-18-0873 [DOI] [PubMed] [Google Scholar]
- 112.Tang JH, Yang L, Chen JX, et al. Bortezomib inhibits growth and sensitizes glioma to temozolomide (TMZ) via down-regulating the FOXM1-Survivin axis. Cancer Commun. 2019;39:81. doi: 10.1186/s40880-019-0424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun X, Chen Y, Zhao H, et al. Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma. Drug Deliv. 2018;25:1718–1727. doi: 10.1080/10717544.2018.1494225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mo DC, Qin L, Ye LJ. Neoadjuvant docetaxel, oxaliplatin, and S-1 in resectable advanced gastric cancer. J Clin Oncol. 2021;39:3883–3884. doi: 10.1200/JCO.21.01528 [DOI] [PubMed] [Google Scholar]
- 115.Ashrafizadeh M, Mirzaei S, Hashemi F, et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed Pharmacother. 2021;141:111824. doi: 10.1016/j.biopha.2021.111824 [DOI] [PubMed] [Google Scholar]
- 116.Wang Q, Liu F, Wang L, et al. Enhanced and prolonged antitumor effect of salinomycin-loaded gelatinase-responsive nanoparticles via targeted drug delivery and inhibition of cervical cancer stem cells. Int J Nanomedicine. 2020;15:1283–1295. doi: 10.2147/IJN.S234679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Q, Yen YT, Xie C, et al. Combined delivery of salinomycin and docetaxel by dual-targeting gelatinase nanoparticles effectively inhibits cervical cancer cells and cancer stem cells. Drug Deliv. 2021;28:510–519. doi: 10.1080/10717544.2021.1886378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao J, Liu J, Xie F, Lu Y, Yin C, Shen X. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomedicine. 2019;14:9199–9216. doi: 10.2147/IJN.S230376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou J, Zhang H, Gu P, Margolick JB, Yin D, Zhang Y. Cancer stem/progenitor cell active compound 8-quinolinol in combination with paclitaxel achieves an improved cure of breast cancer in the mouse model. Breast Cancer Res Treat. 2009;115:269–277. doi: 10.1007/s10549-008-0072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Türk D, Hall MD, Chu BF, et al. Identification of compounds selectively killing multidrug-resistant cancer cells. Cancer Res. 2009;69:8293–8301. doi: 10.1158/0008-5472.CAN-09-2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang D, Huang J, Wang X, et al. The eradication of breast cancer cells and stem cells by 8-hydroxyquinoline-loaded hyaluronan modified mesoporous silica nanoparticle-supported lipid bilayers containing docetaxel. Biomaterials. 2013;34:7662–7673. doi: 10.1016/j.biomaterials.2013.06.042 [DOI] [PubMed] [Google Scholar]
- 122.Gaio E, Conte C, Esposito D, Reddi E, Quaglia F, Moret F. CD44 targeting mediated by polymeric nanoparticles and combination of chlorine TPCS(2a)-PDT and docetaxel-chemotherapy for efficient killing of breast differentiated and stem cancer cells in vitro. Cancers. 2020;12:278. doi: 10.3390/cancers12020278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Godugu C, Patel AR, Doddapaneni R, Marepally S, Jackson T, Singh M. Inhalation delivery of Telmisartan enhances intratumoral distribution of nanoparticles in lung cancer models. J Control Release. 2013;172:86–95. doi: 10.1016/j.jconrel.2013.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rasheduzzaman M, Moon JH, Lee JH, Nazim UM, Park SY. Telmisartan generates ROS-dependent upregulation of death receptor 5 to sensitize TRAIL in lung cancer via inhibition of autophagy flux. Int J Biochem Cell Biol. 2018;102:20–30. doi: 10.1016/j.biocel.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 125.Green R, Howell M, Khalil R, et al. Actinomycin D and telmisartan combination targets lung cancer stem cells through the Wnt/Beta catenin pathway. Sci Rep. 2019;9:18177. doi: 10.1038/s41598-019-54266-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arthur P, Patel N, Surapaneni SK, et al. Targeting lung cancer stem cells using combination of Tel and Docetaxel liposomes in 3D cultures and tumor xenografts. Toxicol Appl Pharmacol. 2020;401:115112. doi: 10.1016/j.taap.2020.115112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miyazaki Y, Matsubara S, Ding Q, et al. Efficient elimination of pancreatic cancer stem cells by hedgehog/GLI inhibitor GANT61 in combination with mTOR inhibition. Mol Cancer. 2016;15:49. doi: 10.1186/s12943-016-0534-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Infante P, Alfonsi R, Ingallina C, et al. Inhibition of Hedgehog-dependent tumors and cancer stem cells by a newly identified naturally occurring chemotype. Cell Death Dis. 2016;7:e2376. doi: 10.1038/cddis.2016.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu K, Zhou H, Liu Y, et al. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale. 2015;7:8607–8618. doi: 10.1039/C5NR01084E [DOI] [PubMed] [Google Scholar]
- 131.Zhou Y, Yang J, Kopeček J. Selective inhibitory effect of HPMA copolymer-cyclopamine conjugate on prostate cancer stem cells. Biomaterials. 2012;33:1863–1872. doi: 10.1016/j.biomaterials.2011.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Y, Yang J, Rhim JS, Kopeček J. HPMA copolymer-based combination therapy toxic to both prostate cancer stem/progenitor cells and differentiated cells induces durable anti-tumor effects. J Control Release. 2013;172:946–953. doi: 10.1016/j.jconrel.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang R, Mondal G, Wen D, Mahato RI. Combination therapy of paclitaxel and cyclopamine polymer-drug conjugates to treat advanced prostate cancer. Nanomedicine. 2017;13:391–401. doi: 10.1016/j.nano.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 134.Nguyen PH, Giraud J, Staedel C, et al. All-trans retinoic acid targets gastric cancer stem cells and inhibits patient-derived gastric carcinoma tumor growth. Oncogene. 2016;35:5619–5628. doi: 10.1038/onc.2016.87 [DOI] [PubMed] [Google Scholar]
- 135.Lim YC, Kang HJ, Kim YS, Choi EC. All-trans-retinoic acid inhibits growth of head and neck cancer stem cells by suppression of Wnt/β-catenin pathway. Eur J Cancer. 2012;48:3310–3318. doi: 10.1016/j.ejca.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 136.Kim D, Choi BH, Ryoo IG, Kwak MK. High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis. 2018;9:896. doi: 10.1038/s41419-018-0903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun R, Liu Y, Li SY, et al. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials. 2015;37:405–414. doi: 10.1016/j.biomaterials.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 138.Li Y, Shi S, Ming Y, et al. Specific cancer stem cell-therapy by albumin nanoparticles functionalized with CD44-mediated targeting. J Nanobiotechnology. 2018;16:99. doi: 10.1186/s12951-018-0424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gui K, Zhang X, Chen F, et al. Lipid-polymer nanoparticles with CD133 aptamers for targeted delivery of all-trans retinoic acid to osteosarcoma initiating cells. Biomed Pharmacother. 2019;111:751–764. doi: 10.1016/j.biopha.2018.11.118 [DOI] [PubMed] [Google Scholar]
- 140.Chen H, Lin J, Shan Y, Zhengmao L. The promotion of nanoparticle delivery to two populations of gastric cancer stem cells by CD133 and CD44 antibodies. Biomed Pharmacother. 2019;115:108857. doi: 10.1016/j.biopha.2019.108857 [DOI] [PubMed] [Google Scholar]
- 141.Paramanantham A, Jung EJ, Kim HJ, et al. Doxorubicin-resistant TNBC cells exhibit rapid growth with cancer stem cell-like properties and EMT phenotype, which can be transferred to parental cells through autocrine signaling. Int J Mol Sci. 2021;22:12438. doi: 10.3390/ijms222212438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Y, Yu F, Dai S, et al. All-trans retinoic acid and doxorubicin delivery by folic acid modified polymeric micelles for the modulation of Pin1-mediated DOX-induced breast cancer stemness and metastasis. Mol Pharm. 2021;18:3966–3978. doi: 10.1021/acs.molpharmaceut.1c00220 [DOI] [PubMed] [Google Scholar]
- 143.Li RJ, Ying X, Zhang Y, et al. All-trans retinoic acid stealth liposomes prevent the relapse of breast cancer arising from the cancer stem cells. J Control Release. 2011;149:281–291. doi: 10.1016/j.jconrel.2010.10.019 [DOI] [PubMed] [Google Scholar]
- 144.Chen H, Shi Y, Sun L, Ni S. Electrospun composite nanofibers with all-trans retinoic acid and MWCNTs-OH against cancer stem cells. Life Sci. 2020;258:118152. doi: 10.1016/j.lfs.2020.118152 [DOI] [PubMed] [Google Scholar]
- 145.Pan Y, Ma X, Liu C, et al. Retinoic acid-loaded dendritic polyglycerol-conjugated gold nanostars for targeted photothermal therapy in breast cancer stem cells. ACS Nano. 2021;15:15069–15084. doi: 10.1021/acsnano.1c05452 [DOI] [PubMed] [Google Scholar]
- 146.Martínez-Rodríguez OP, Thompson-Bonilla MDR, Jaramillo-Flores ME. Association between obesity and breast cancer: molecular bases and the effect of flavonoids in signaling pathways. Crit Rev Food Sci Nutr. 2020;60:3770–3792. doi: 10.1080/10408398.2019.1708262 [DOI] [PubMed] [Google Scholar]
- 147.Khan H, Belwal T, Efferth T, et al. Targeting epigenetics in cancer: therapeutic potential of flavonoids. Crit Rev Food Sci Nutr. 2021;61:1616–1639. doi: 10.1080/10408398.2020.1763910 [DOI] [PubMed] [Google Scholar]
- 148.Bondonno NP, Dalgaard F, Kyrø C, et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat Commun. 2019;10:3651. doi: 10.1038/s41467-019-11622-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bonafè F, Pazzini C, Marchionni S, Guarnieri C, Muscari C. Complete disaggregation of MCF-7-derived breast tumour spheroids with very low concentrations of α-mangostin loaded in CD44 thioaptamer-tagged nanoparticles. Int J Med Sci. 2019;16:33–42. doi: 10.7150/ijms.28135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Verma RK, Yu W, Shrivastava A, Shankar S, Srivastava RK. α-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (Kras(G12D), and Kras(G12D)/tp53R270H) mice. Sci Rep. 2016;6:32743. doi: 10.1038/srep32743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chandra Boinpelly V, Verma RK, Srivastav S, Srivastava RK, Shankar S. α-Mangostin-encapsulated PLGA nanoparticles inhibit colorectal cancer growth by inhibiting Notch pathway. J Cell Mol Med. 2020;24:11343–11354. doi: 10.1111/jcmm.15731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hulangamuwa AC, Ediriweera MK, Rajagopalan U, Karunaratne DN, Samarakoon SR. Development of a new nanocarrier for dietary garcinol: characterization and in vitro efficacy evaluation using breast cancer stem cells grown in hypoxia. J Food Qual. 2021;2021:1–10. doi: 10.1155/2021/6654211 [DOI] [Google Scholar]
- 153.Tuli HS, Mittal S, Aggarwal D, et al. Path of Silibinin from diet to medicine: a dietary polyphenolic flavonoid having potential anti-cancer therapeutic significance. Semin Cancer Biol. 2021;73:196–218. doi: 10.1016/j.semcancer.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 154.Huo M, Wang H, Zhang Y, et al. Co-delivery of silybin and paclitaxel by dextran-based nanoparticles for effective anti-tumor treatment through chemotherapy sensitization and microenvironment modulation. J Control Release. 2020;321:198–210. doi: 10.1016/j.jconrel.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 155.Liu Y, Xie X, Hou X, et al. Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis. J Nanobiotechnology. 2020;18:83. doi: 10.1186/s12951-020-00638-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mahira S, Kommineni N, Husain GM, Khan W. Cabazitaxel and silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: a new insight into nanomedicine based combinational chemotherapy for prostate cancer. Biomed Pharmacother. 2019;110:803–817. doi: 10.1016/j.biopha.2018.11.145 [DOI] [PubMed] [Google Scholar]
- 157.Khan AQ, Ahmed EI, Elareer NR, Junejo K, Steinhoff M, Uddin S. Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies. Cells. 2019;8:840. doi: 10.3390/cells8080840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Asadzadeh Z, Mansoori B, Mohammadi A, et al. microRNAs in cancer stem cells: biology, pathways, and therapeutic opportunities. J Cell Physiol. 2019;234:10002–10017. doi: 10.1002/jcp.27885 [DOI] [PubMed] [Google Scholar]
- 159.Khakinezhad Tehrani F, Ranji N, Kouhkan F, Hosseinzadeh S. PANC-1 cancer stem-like cell death with silybin encapsulated in polymersomes and deregulation of stemness-related miRNAs and their potential targets. Iran J Basic Med Sci. 2021;24:514–523. doi: 10.22038/ijbms.2021.54001.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khakinezhad Tehrani F, Ranji N, Kouhkan F, Hosseinzadeh S. Apoptosis induction and proliferation inhibition by silibinin encapsulated in nanoparticles in MIA PaCa-2 cancer cells and deregulation of some miRNAs. Iran J Basic Med Sci. 2020;23:469–482. doi: 10.22038/ijbms.2020.39427.9349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Samynathan R, Thiruvengadam M, Nile SH, et al. Recent insights on tea metabolites, their biosynthesis and chemo-preventing effects: a review. Crit Rev Food Sci Nutr. 2021;1–20. doi: 10.1080/10408398.2021.1984871 [DOI] [PubMed] [Google Scholar]
- 162.Castro Nava A, Cojoc M, Peitzsch C, et al. Development of novel radiochemotherapy approaches targeting prostate tumor progenitor cells using nanohybrids. Int J Cancer. 2015;137:2492–2503. doi: 10.1002/ijc.29614 [DOI] [PubMed] [Google Scholar]
- 163.Jhaveri A, Luther E, Torchilin V. The effect of transferrin-targeted, resveratrol-loaded liposomes on neurosphere cultures of glioblastoma: implications for targeting tumour-initiating cells. J Drug Target. 2019;27:601–613. doi: 10.1080/1061186X.2018.1550647 [DOI] [PubMed] [Google Scholar]
- 164.Mukherjee S, Baidoo JNE, Sampat S, et al. Liposomal TriCurin, a synergistic combination of curcumin, epicatechin gallate and resveratrol, repolarizes tumor-associated microglia/macrophages, and eliminates glioblastoma (GBM) and GBM stem cells. Molecules. 2018;23:201. doi: 10.3390/molecules23010201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pradhan R, Chatterjee S, Hembram KC, Sethy C, Mandal M, Kundu CN. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J Nutr Biochem. 2021;92:108624. doi: 10.1016/j.jnutbio.2021.108624 [DOI] [PubMed] [Google Scholar]
- 166.Efferth T, Oesch F. Repurposing of plant alkaloids for cancer therapy: pharmacology and toxicology. Semin Cancer Biol. 2021;68:143–163. doi: 10.1016/j.semcancer.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 167.Zhao J, Xiao A, Liu C, et al. The HIF-1A/miR-17-5p/PDCD4 axis contributes to the tumor growth and metastasis of gastric cancer. Signal Transduct Target Ther. 2020;5:46. doi: 10.1038/s41392-020-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wan J, Wu W. Hyperthermia induced HIF-1a expression of lung cancer through AKT and ERK signaling pathways. J Exp Clin Cancer Res. 2016;35:119. doi: 10.1186/s13046-016-0399-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Conley SJ, Baker TL, Burnett JP, et al. CRLX101, an investigational camptothecin-containing nanoparticle-drug conjugate, targets cancer stem cells and impedes resistance to antiangiogenic therapy in mouse models of breast cancer. Breast Cancer Res Treat. 2015;150:559–567. doi: 10.1007/s10549-015-3349-8 [DOI] [PubMed] [Google Scholar]
- 170.Shen S, Xu X, Lin S, et al. A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nat Nanotechnol. 2021;16:104–113. doi: 10.1038/s41565-020-00793-0 [DOI] [PubMed] [Google Scholar]
- 171.Zhang N, Liang X, Gao C, et al. Loading lovastatin into camptothecin-floxuridine conjugate nanocapsules for enhancing anti-metastatic efficacy of cocktail chemotherapy on triple-negative breast cancer. ACS Appl Mater Interfaces. 2018;10:29385–29397. doi: 10.1021/acsami.8b11723 [DOI] [PubMed] [Google Scholar]
- 172.Garcia-Mayea Y, Mir C, Masson F, Paciucci R, LLeonart ME. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin Cancer Biol. 2020;60:166–180. doi: 10.1016/j.semcancer.2019.07.022 [DOI] [PubMed] [Google Scholar]
- 173.Fulda S. Regulation of apoptosis pathways in cancer stem cells. Cancer Lett. 2013;338:168–173. doi: 10.1016/j.canlet.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 174.Ma X, Zhou J, Zhang CX, et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34:4452–4465. doi: 10.1016/j.biomaterials.2013.02.066 [DOI] [PubMed] [Google Scholar]
- 175.Singh P, Sahoo SK. Piperlongumine loaded PLGA nanoparticles inhibit cancer stem-like cells through modulation of STAT3 in mammosphere model of triple negative breast cancer. Int J Pharm. 2022;616:121526. doi: 10.1016/j.ijpharm.2022.121526 [DOI] [PubMed] [Google Scholar]
- 176.Li XT, Tang W, Jiang Y, et al. Multifunctional targeting vinorelbine plus tetrandrine liposomes for treating brain glioma along with eliminating glioma stem cells. Oncotarget. 2016;7:24604–24622. doi: 10.18632/oncotarget.8360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang F, Sun Y, Huang X, et al. Sulforaphane inhibits self-renewal of lung cancer stem cells through the modulation of sonic Hedgehog signaling pathway and polyhomeotic homolog 3. AMB Express. 2021;11:121. doi: 10.1186/s13568-021-01281-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gu HF, Mao XY, Du M. Metabolism, absorption, and anti-cancer effects of sulforaphane: an update. Crit Rev Food Sci Nutr. 2021;2021:1–17. [DOI] [PubMed] [Google Scholar]
- 179.McClements DJ, Xiao H. Designing food structure and composition to enhance nutraceutical bioactivity to support cancer inhibition. Semin Cancer Biol. 2017;46:215–226. doi: 10.1016/j.semcancer.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 180.Gu HF, Ren F, Mao XY, Du M. Mineralized and GSH-responsive hyaluronic acid based nano-carriers for potentiating repressive effects of sulforaphane on breast cancer stem cells-like properties. Carbohydr Polym. 2021;269:118294. doi: 10.1016/j.carbpol.2021.118294 [DOI] [PubMed] [Google Scholar]
- 181.Huang J, Tao C, Yu Y, et al. Simultaneous targeting of differentiated breast cancer cells and breast cancer stem cells by combination of docetaxel- and sulforaphane-loaded self-assembled poly(D, L-lactide-co-glycolide)/hyaluronic acid block copolymer-based nanoparticles. J Biomed Nanotechnol. 2016;12:1463–1477. doi: 10.1166/jbn.2016.2234 [DOI] [PubMed] [Google Scholar]
- 182.Zhou L, Sheng D, Wang D, et al. Identification of cancer-type specific expression patterns for active aldehyde dehydrogenase (ALDH) isoforms in ALDEFLUOR assay. Cell Biol Toxicol. 2019;35:161–177. doi: 10.1007/s10565-018-9444-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huddle BC, Grimley E, Buchman CD, et al. Structure-based optimization of a novel class of aldehyde dehydrogenase 1A (ALDH1A) subfamily-selective inhibitors as potential adjuncts to ovarian cancer chemotherapy. J Med Chem. 2018;61:8754–8773. doi: 10.1021/acs.jmedchem.8b00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jiménez R, Pequerul R, Amor A, et al. Inhibitors of aldehyde dehydrogenases of the 1A subfamily as putative anticancer agents: kinetic characterization and effect on human cancer cells. Chem Biol Interact. 2019;306:123–130. doi: 10.1016/j.cbi.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 185.Das S, Mukherjee P, Chatterjee R, Jamal Z, Chatterji U. Enhancing chemosensitivity of breast cancer stem cells by downregulating SOX2 and ABCG2 using wedelolactone-encapsulated nanoparticles. Mol Cancer Ther. 2019;18:680–692. doi: 10.1158/1535-7163.MCT-18-0409 [DOI] [PubMed] [Google Scholar]
- 186.Ibiyeye KM, Zuki ABZ. Cockle shell-derived aragonite CaCO(3) nanoparticles for co-delivery of doxorubicin and thymoquinone eliminates cancer stem cells. Int J Mol Sci. 2020;21:1900. doi: 10.3390/ijms21051900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Abu-Serie MM, Andrade F, Cámara-Sánchez P, et al. Pluronic F127 micelles improve the stability and enhance the anticancer stem cell efficacy of citral in breast cancer. Nanomedicine. 2021;16:1471–1485. doi: 10.2217/nnm-2021-0013 [DOI] [PubMed] [Google Scholar]
- 188.Thomas ML, de Antueno R, Coyle KM, et al. Citral reduces breast tumor growth by inhibiting the cancer stem cell marker ALDH1A3. Mol Oncol. 2016;10:1485–1496. doi: 10.1016/j.molonc.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhu HH, Wu DP, Du X, et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: a non-inferiority, randomised Phase 3 trial. Lancet Oncol. 2018;19:871–879. doi: 10.1016/S1470-2045(18)30295-X [DOI] [PubMed] [Google Scholar]
- 190.Zhu HH, Wu DP, Jin J, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31:4215–4221. doi: 10.1200/JCO.2013.48.8312 [DOI] [PubMed] [Google Scholar]
- 191.Bujňáková Z, Kello M, Kováč J, et al. Preparation of As(4)S(4)/Fe(3)O(4) nanosuspensions and in-vitro verification of their anticancer activity. Mater Sci Eng C Mater Biol Appl. 2020;110:110683. doi: 10.1016/j.msec.2020.110683 [DOI] [PubMed] [Google Scholar]
- 192.Cholujova D, Bujnakova Z, Dutkova E, et al. Realgar nanoparticles versus ATO arsenic compounds induce in vitro and in vivo activity against multiple myeloma. Br J Haematol. 2017;179:756–771. doi: 10.1111/bjh.14974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang FR, Zhao YF, Hu XW, et al. Nano-realgar suppresses lung cancer stem cell growth by repressing metabolic reprogramming. Gene. 2021;788:145666. doi: 10.1016/j.gene.2021.145666 [DOI] [PubMed] [Google Scholar]
- 194.Lu SL, Wang YH, Liu GF, et al. Graphene oxide nanoparticle-loaded ginsenoside rg3 improves photodynamic therapy in inhibiting malignant progression and stemness of osteosarcoma. Front Mol Biosci. 2021;8:663089. doi: 10.3389/fmolb.2021.663089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Singh D, Mohapatra P, Kumar S, Behera S, Dixit A, Sahoo SK. Nimbolide-encapsulated PLGA nanoparticles induces mesenchymal-to-epithelial transition by dual inhibition of AKT and mTOR in pancreatic cancer stem cells. Toxicol In Vitro. 2022;79:105293. doi: 10.1016/j.tiv.2021.105293 [DOI] [PubMed] [Google Scholar]
- 196.Verma RK, Yu W, Singh SP, Shankar S, Srivastava RK. Anthothecol-encapsulated PLGA nanoparticles inhibit pancreatic cancer stem cell growth by modulating sonic hedgehog pathway. Nanomedicine. 2015;11:2061–2070. doi: 10.1016/j.nano.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 197.Mullard A. Addressing cancer’s grand challenges. Nat Rev Drug Discov. 2020;19:825–826. doi: 10.1038/d41573-020-00202-0 [DOI] [PubMed] [Google Scholar]
- 198.Clarke MF. Clinical and Therapeutic Implications of Cancer Stem Cells. N Engl J Med. 2019;380:2237–2245. doi: 10.1056/NEJMra1804280 [DOI] [PubMed] [Google Scholar]
- 199.Deng LJ, Deng WQ, Fan SR, et al. m6A modification: recent advances, anticancer targeted drug discovery and beyond. Mol Cancer. 2022;21:52. doi: 10.1186/s12943-022-01510-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Safa AR, Saadatzadeh MR, Cohen-Gadol AA, Pollok KE, Bijangi-Vishehsaraei K. Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes Dis. 2015;2:152–163. doi: 10.1016/j.gendis.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Raj S, Khurana S, Choudhari R, et al. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin Cancer Biol. 2021;69:166–177. doi: 10.1016/j.semcancer.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 202.Zhu F, Tan G, Zhong Y, et al. Smart nanoplatform for sequential drug release and enhanced chemo-thermal effect of dual drug loaded gold nanorod vesicles for cancer therapy. J Nanobiotechnology. 2019;17:44. doi: 10.1186/s12951-019-0473-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jiang J, Shen YY, Li J, Lin YH, Luo CX, Zhu DY. (+)-Borneol alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Eur J Pharmacol. 2015;757:53–58. doi: 10.1016/j.ejphar.2015.03.056 [DOI] [PubMed] [Google Scholar]
- 204.Abedi F, Davaran S, Hekmati M, Akbarzadeh A, Baradaran B, Moghaddam SV. An improved method in fabrication of smart dual-responsive nanogels for controlled release of doxorubicin and curcumin in HT-29 colon cancer cells. J Nanobiotechnology. 2021;19:18. doi: 10.1186/s12951-020-00764-6 [DOI] [PMC free article] [PubMed] [Google Scholar]