Abstract
Plants produce and accumulate triacylglycerol (TAG) in their seeds as an energy reservoir to support the processes of seed germination and seedling development. Plant seed oils are vital not only for the human diet but also as renewable feedstocks for industrial use. TAG biosynthesis consists of two major steps: de novo fatty acid biosynthesis in the plastids and TAG assembly in the endoplasmic reticulum. The latest advances in unraveling transcriptional regulation have shed light on the molecular mechanisms of plant oil biosynthesis. We summarize recent progress in understanding the regulatory mechanisms of well-characterized and newly discovered transcription factors and other types of regulators that control plant fatty acid biosynthesis. The emerging picture shows that plant oil biosynthesis responds to developmental and environmental cues that stimulate a network of interacting transcriptional activators and repressors, which in turn fine-tune the spatiotemporal regulation of the pathway genes.
Keywords: plant oil biosynthesis, oil accumulation, seed development, environmental and developmental signals, transcription factor, transcriptional regulation
This review summarizes progress on understanding the molecular mechanisms that underlie the control of oil biosynthesis in seed plants, as well as the response of oil biosynthesis to developmental and environmental cues. The translational applications of oil regulators in bioengineering plant oil production are also discussed.
Introduction
Plant oils (often referred to as vegetable oils) are generated and stored mainly in the form of triacylglycerol (TAG) in seeds. For plants, TAG serves as a major carbon and energy source to support seed germination and seedling development. For humans, plant oils are not only essential as a component of the diet, providing about one-fourth of dietary calories in developed countries, but also important as a source of carbon-neutral fuels and raw materials for industrial products, such as lubricants, detergents, and nylon (Chapman and Ohlrogge, 2012; Covas et al., 2015; Kong et al., 2020a; Song et al., 2021b). Global demand for vegetable oils is increasing rapidly and is projected to double by 2030 (Chapman and Ohlrogge, 2012), incentivizing a tremendous amount of research effort to enhance plant oil biosynthesis.
The mechanism of fatty acid (FA) biosynthesis has been extensively studied and reviewed (Chapman and Ohlrogge, 2012; Bates et al., 2013; Li-Beisson et al., 2013; Allen, 2016; Bates, 2016; Xu and Shanklin, 2016; Kong et al., 2020a; Song et al., 2021b; Miray et al., 2021). In brief, FA synthesis starts with the provision of carbon from glycolysis. After glycolysis, pyruvate dehydrogenase converts pyruvate into acetyl-CoA, the initial substrate for de novo FA biosynthesis. Acetyl-CoA carboxylase (ACCase) catalyzes the first committed step, converting acetyl-CoA and bicarbonate into malonyl-CoA. A key co-factor, acyl-carrier protein (ACP), then accepts the malonyl group to form malonyl-ACP. After the first cycle of FA synthesis produces an acetoacetyl-ACP, additional cycles, facilitated by the fatty acid synthase complex, elongate the FA chain in a two-carbon increment, whereby acetyl units provided from malonyl-CoA are added successively to a lengthening acyl chain esterified to an ACP. During this process, 3-ketoacyl-ACP synthase (KAS) catalyzes the condensation reaction to connect new carbons to the growing acyl chain. KAS III initiates FA biosynthesis by combining acetyl-CoA and malonyl-ACP. KAS I subsequently extends the acyl chain to C12–C16. Eventually, KAS II completes the biosynthesis to C18. The acyl chains are released from acyl-ACPs by hydrolysis reactions catalyzed by thioesterases. The products of thioesterases are activated to acyl-CoA molecules prior to export to the endoplasmic reticulum. Currently, there are still some ambiguities regarding the specific steps and the sequence of events that occur during fatty acyl activation and export out of plastid envelopes (Bates et al., 2013; Bates, 2016). The pathway to TAG assembly from glycerol-3-phosphate and acyl-CoAs consists of multiple enzymatic steps (Chapman and Ohlrogge, 2012; Li-Beisson et al., 2013; Allen, 2016; Bates, 2016). First, glycerol-3-phosphate acyltransferase generates lysophosphatidic acid by transfer of an FA from acyl-CoA to the sn-1 hydroxyl of glycerol-3-phosphate. Then lysophosphatidic acid acyltransferase (LPAAT) esterifies a second FA to the sn-2 hydroxyl of lysophosphatidic acid to produce phosphatidic acid. Third, phosphatidic acid phosphatase mediates the removal of the sn-3 phosphate to produce de novo diacylglycerol (DAG). The de novo DAG pool is vital, as it can be used for biosynthesis of membrane lipids, such as phosphatidylcholine (PC) and phosphatidylethanolamine, or for TAG production. The DAG for TAG biosynthesis can also be obtained from PC. Acyl flux of the de novo DAG into PC and the ensuing acyl flux of PC-derived DAG out of PC that generates the DAG substrate for producing TAG may occur through a phosphocholine headgroup exchange mechanism. In the final stage, diacylglycerol acyltransferase (DGAT) or phospholipid:diacylglycerol acyltransferase (PDAT) catalyzes the conversion of DAG to TAG (Zou et al., 1999; Dahlqvist et al., 2000; Zhang et al., 2009). TAG is then coated with oleosins and stored as lipid droplets (Chapman et al., 2012).
TAG biosynthesis is a complex biological process that involves three main metabolic pathways: glycolysis, FA de novo synthesis and elongation, and TAG assembly. Glycolysis provides carbons that can be esterified with glycerol to produce TAG. Hence, seed oil accumulation requires the balanced transcriptional regulation of multiple branch pathways (Chapman and Ohlrogge, 2012; Lu et al., 2018; Kong et al., 2019). Transcription factors are proteins that recognize and bind, directly or indirectly, to specific DNA sequences in gene promoters to control gene expression (Lambert et al., 2018; Zhu et al., 2018). Numerous transcription factors have been shown to participate in regulating the expression of TAG biosynthetic genes (Cernac and Benning, 2004; Baud et al., 2007; Mu et al., 2008; Yamamoto et al., 2010; Ma et al., 2013; Li et al., 2017; Pelletier et al., 2017; Kong and Ma, 2018b; Tian et al., 2020). Among them, the APETALA2 transcription factor WRINKLED1 (WRI1) directly regulates the expression of various genes involved in glycolysis and FA biosynthesis, functioning to “push” FA de novo synthesis from upstream (Vanhercke et al., 2014, 2019). Other transcriptional regulators also participate in regulation of the FA biosynthetic pathway by mediating the expression of WRI1 (Baud et al., 2007; Yamamoto et al., 2010; Li et al., 2017; Kong et al., 2020a; Tian et al., 2020). Unlike animals, plants are sessile organisms that are incapable of escaping from various environmental hazards. Plants have therefore evolved sophisticated systems to translate environmental cues into metabolic modulation to mitigate environmental impacts. FA biosynthesis is part of seed development, which responds to various environmental signals, including light, humidity, and temperature. Transcriptional and other regulatory networks integrate the balanced regulation of environmental signal perception/transduction, seed development, and FA biosynthesis (Figure 1). In this review, we focus on understanding some of the key regulators in these networks.
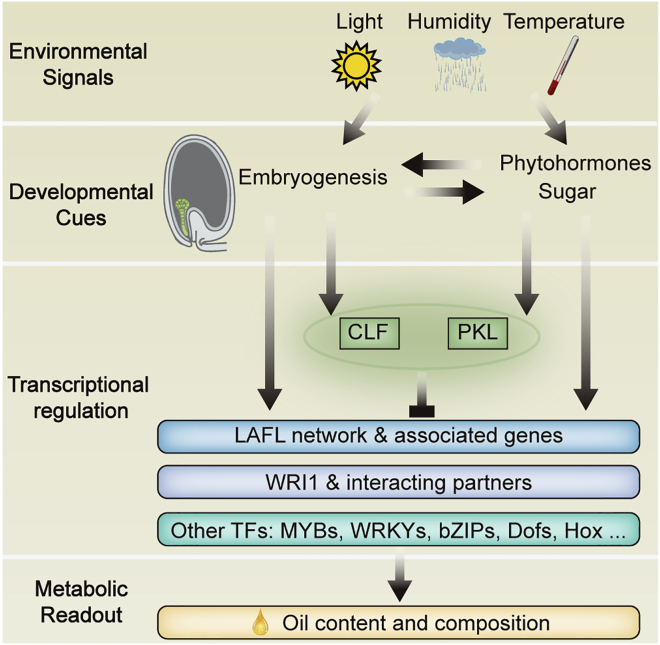
Figure 1.
Environmental and developmental cues mediate gene expression and transcriptional activity during seed development.
Environmental signals affect embryogenesis as well as phytohormones and sugar levels, which also control embryogenesis. Developmental signals significantly affect the LAFL network (which includes LEC1, LEC2, L1L, ABI3, and FUS3), WRI1 and its interacting partners, and other transcription factors, leading to dynamic transcriptional regulation of plant oil biosynthetic pathways. Epigenetic regulators such as CURLY LEAF (CLF) and PICKLE (PKL) also determine the transcription levels of several key regulators in the LAFL network and WRI1. The combined transcriptional regulation leads to responsive metabolic readout, resulting in alteration of oil content and composition.
The essential roles of key regulators of seed development in transcriptional regulation of seed oil accumulation
Numerous studies suggest that four transcriptional regulators, LEAFY COTYLEDON1 (LEC1), LEAFY COTYLEDON2 (LEC2), FUSCA3 (FUS3), and ABSCISIC ACID INSENSITIVE3 (ABI3), play central roles in the process of seed development and maturation (Giraudat et al., 1992; Parcy et al., 1997; Lotan et al., 1998; Luerssen et al., 1998; Stone et al., 2001; Verdier and Thompson, 2008; Yamamoto et al., 2010; Pelletier et al., 2017; Jo et al., 2019; Song et al., 2021a). Recent evidence shows that LEC1, LEC2, FUS3, and ABI3 are also involved in the transcriptional regulation of seed oil production (Baud et al., 2007; Mu et al., 2008; Shen et al., 2010; Tan et al., 2011; Zhang et al., 2016; Manan et al., 2017; Tian et al., 2020; Yang et al., 2021). Genes encoding these regulators are expressed predominantly during seed development and have a considerable effect on seed oil accumulation.
LEC1 encodes a HAP3 subunit of the CCAAT-binding transcription factor. Inducible transgenic lines of Arabidopsis overexpressing AtLEC1 (AtLEC1-OXi) showed enhanced FA production (Mu et al., 2008). Global transcriptomic analysis of AtLEC1-OXi lines revealed that LEC1 regulates the expression of genes involved in FA metabolism, including key reactions of condensation, chain elongation, and desaturation of FA biosynthesis (Mu et al., 2008). Elevation of LEC1 enhanced seed oil content. Overexpression of AtLEC1, BnLEC1 (Brassica napus LEC1), and ZmLEC1 (maize LEC1) increased oil content in transgenic plants (Mu et al., 2008; Shen et al., 2010; Tan et al., 2011). LEC1-LIKE (L1L), a close homolog of LEC1, displays a similar function as LEC1 in the regulation of FA biosynthesis in Arabidopsis and B. napus (Mu et al., 2008; Tan et al., 2011). Notably, a recent study illustrated the significance of the endosperm-produced AtLEC1 in facilitating the seed developmental process, including seed maturation (Song et al., 2021a). LEC1 expression occurs in the endosperm, followed by LEC1 trafficking to the embryo to regulate seed maturation, indicating the complex roles of LEC1 in mediating seed development and controlling metabolic activities, such as FA production (Song et al., 2021a).
LEC2 is a B3 domain transcription factor vital for embryo development (Stone et al., 2001). In addition to its essential function in controlling the developmental process, LEC2 is involved in transcriptional regulation of FA biosynthesis and has therefore been used in engineering plants to enhance oil production (Santos Mendoza et al., 2005; Baud et al., 2007; Vanhercke et al., 2014, 2019; Kim et al., 2015). Transgenic Arabidopsis seedlings expressing an inducible LEC2 showed oil accumulation in leaves upon induction, accompanied by increased expression of genes encoding key transcription factors, such as LEC1, ABI3, and FUS3 (Santos Mendoza et al., 2005).
In the transcriptional network, LEC1 and LEC2 positively regulate the expression of WRI1 (Baud et al., 2007; Mu et al., 2008; Pelletier et al., 2017; Kong et al., 2019). WRI1 expression is elevated in an Arabidopsis gain-of-function mutant of LEC1, tnp, as well as inducible LEC1 overexpression lines (Casson and Lindsey, 2006; Mu et al., 2008). Inducible LEC2 overexpression after cycloheximide treatment (to block translation) showed the activation of AtWRI1 without the participation of other intermediate proteins, and AtWRI1 expression is decreased in the lec2 mutant compared with that in the wild-type (WT) control (Baud et al., 2007). Chromatin immunoprecipitation (ChIP) experiments confirmed WRI1 as a direct target of LEC1 (Pelletier et al., 2017). Effects of LEC1 and LEC2 on WRI1 expression are conserved across other plant species, such as maize and soybean (Shen et al., 2010; Manan et al., 2017).
The B3 domain transcription factor FUSCA3 (FUS3) triggers the expression of various FA biosynthetic genes, such as FATTY ACID DESATURASE3 (FAD3), KAS I, and FATTY ACID ELONGATION1 (FAE1) (Yamamoto et al., 2010; Wang and Perry, 2013). FUS3 also binds directly to the promoters of LEC1, L1L, and ABI3 to mediate their expression (Wang and Perry, 2013). Transcriptomic analysis revealed that the expression of WRI1 is decreased in the fus3 mutant compared with the WT (Yamamoto et al., 2010). WRI1 was identified as a direct target of FUS3 in a genome-wide ChIP-chip analysis (Wang and Perry, 2013). The fus3 mutant displays fewer oil droplets in the cotyledons of developing seeds, as well as reduced seed oil content compared with the WT (Meinke et al., 1994; Tiedemann et al., 2008; Roscoe et al., 2015). Transgenic overexpression of FUS3 in Arabidopsis significantly triggers TAG accumulation in vegetative tissues, suggesting a useful role for FUS3 in the bioengineering of vegetable oil production (Zhang et al., 2016).
The B3 domain transcription factor ABI3 plays an important role in the abscisic acid (ABA) signaling pathway that mediates physiological processes such as seed maturation and dormancy (Rohde et al., 2000; Monke et al., 2012; Boulard et al., 2017; Tian et al., 2020). Recent ChIP-chip experiments identified direct targets of ABI3; they included WRI1 and other genes encoding FA biosynthetic genes, including FAD3 and FATTY ACID BIOSYNTHESIS 2 (FAB2) (Tian et al., 2020). The combined results of ChIP-chip and comparative transcriptomic analysis of developing Arabidopsis seeds of abi3 and WT showed that 317 genes, including WRI1, were directly activated by ABI3 (Tian et al., 2020). Furthermore, a recent study indicated that transgenic tobacco BY2 cell lines or transgenic Arabidopsis seedlings expressing an inducible ABI3 showed activation of oil production, further supporting a role for ABI3 in the regulation of oil biosynthesis. In the fus3 mutant, induction of ABI3 triggers strong TAG production in transgenic seedlings, suggesting that ABI3 activates oil biosynthesis in a FUS3-independent manner. Further transcriptomic analysis showed that LIPID DROPLET PROTEIN (LDP) genes and WRI1 are activated by ABI3 in transgenic fus3 plants (Yang et al., 2021).
AGAMOUS-Like15 (AGL15) is a well-characterized MADS transcription factor (Thakare et al., 2008; Zheng et al., 2009; Serivichyaswat et al., 2015; Cosio et al., 2017; Joshi et al., 2021). ChIP-chip analysis detected various direct targets of AGL15, including LEC2, FUS3, and ABI3 (Zheng et al., 2009). Recent ChIP analysis revealed that HIGH-LEVEL EXPRESSION OF SUGAR INDUCIBLE GENE2 (HSI2)/VP1/ABI3-LIKE1 (VAL1) directly binds to the AGL15 promoter via the RY element to repress the expression of AGL15. In the hsi2-2 mutant, the expression of AGL15 (as well as LEC1, LEC2, ABI3, and FUS3) was significantly increased compared with the WT (Chen et al., 2018b). BABY BOOM (BBM), encoding an APETALA2 transcription factor, is essential for transcriptional regulation of plant cell totipotency (Horstman et al., 2014, 2017; Lutz et al., 2015). Functional analysis revealed that BBM binds directly to the promoters of LEC1, LEC2, and ABI3. In BBM-overexpressing transgenic plants, expression of LEC1, LEC2, FUS3, and ABI3 was upregulated, suggesting a positive role for BBM in the activation of these FA-biosynthesis-related genes in planta (Horstman et al., 2017). The direct involvement of AGL15 or BBM in oil production remains to be explored.
The pivotal role of WRI1 in transcriptional control of plant oil biosynthesis
The AtWRI1 loss-of-function mutant wri1-1 displays a near 80% reduction in seed oil content compared with the WT (Focks and Benning, 1998). Transcriptomic analysis of developing wri1-1 seeds showed that most genes with decreased expression encoded key enzymes in late glycolysis and the FA pathways (Ruuska et al., 2002). Subsequent studies confirmed that AtWRI1 directly regulates many of these genes (Baud et al., 2007; Maeo et al., 2009), including pyruvate kinase beta subunit 1 (PKP-β1), biotin carboxyl carrier protein (BCCP2; encoding a subunit of the ACCase), ACP1, and KAS I. WRI1 binds directly to the promoter region and activates the expression of the GLB1 gene, which encodes a PII protein, a key integrator of central metabolism (Baud et al., 2010). PII displays inhibitory effect on ACCase activity and is involved in fine-tuning FA biosynthesis in developing seeds (Baud et al., 2010). Several BIOTIN ATTACHMENT DOMAIN-CONTAINING (BADC) genes that repress FA biosynthesis are activated by AtWRI1 (Liu et al., 2019), suggesting another level of mediation of WRI1-regulated oil biosynthesis. Furthermore, a recent discovery showed that carboxyltransferase interactor (CTI), encoding an envelope membrane regulator of ACCase, is also positively regulated by WRI1 (Ye et al., 2020). In contrast to the positive regulation by LEC1/LEC2/FUS3, the Arabidopsis R2R3-MYB transcription factor MYB89 represses AtWRI1 expression (Li et al., 2017) (Figure 2A). It should be noted that alternative transcriptional regulators may mediate WRI1 expression in other plant species. In oil palm mesocarp (where most palm oil is generated), EgWRI1 was highly repressed during fruit ripening. However, no transcripts of orthologs of known WRI1-regulator genes (e.g., LEC1, LEC2, ABI3, FUS3) were detected, suggesting that additional upstream transcriptional regulators (possibly fruit-specific factors) control the expression of EgWRI1 (Bourgis et al., 2011). Transcriptional regulators, including EgNF-YA3, EgNF-YC2, and EgABI5, participate in the transcriptional control of EgWRI1 by binding to the EgWRI1 promoter (Yeap et al., 2017).
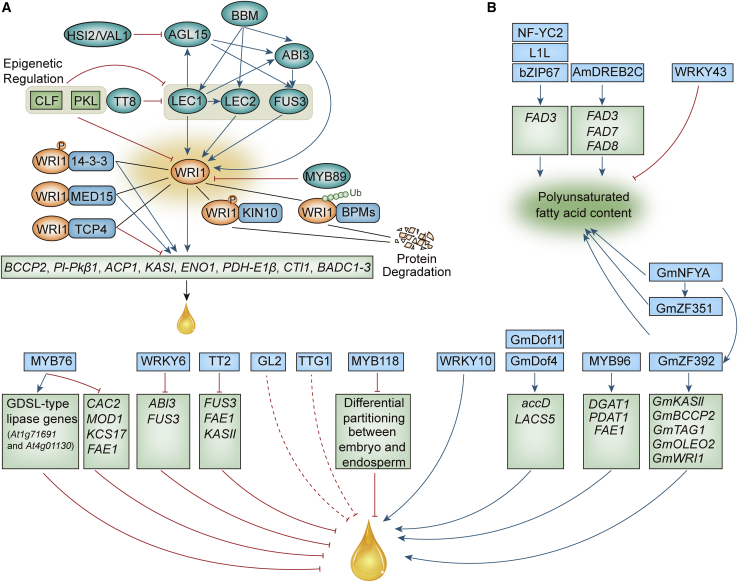
Figure 2.
The complex and multi-level regulatory mechanisms for the control of plant seed oil accumulation.
(A) LEC1, LEC2, FUS3, and ABI3 are the major upstream transcriptional regulators that mediate embryo development, seed maturation, and metabolism. Together with HSI2/VAL1, AGL15, BBM, TT8, MYB89, they form a network that tightly controls the expression of WRI1. These upstream regulators also mutually regulate one another. The upstream regulators are affected by epigenetic factors, including CLF and PKL. WRI1 physically interacts with several transcription factors, such as BPMs, 14-3-3s, MED15, and TCP4, as well as post-translational modifiers, such as KIN10 kinase. WRI1 is subject to post-translational modifications, such as phosphorylation (circled P) and ubiquitination (Ub), that result in protein degradation. The targets of WRI1 include genes involved in the FA biosynthetic pathway and late glycolysis. The positive (arrows) and negative (T-bars) regulatory mechanisms, together with post-translational modifications, form a network that fine-tunes oil biosynthesis.
(B) Other transcriptional regulators that participate in the regulation of plant oil biosynthesis by upregulating oil pathway genes. MYB96, DOF4, DOF11, TZF, WRKY10, WRKY43, DREB2C, and bZIP67 positively regulate FA accumulation in seeds. Other factors, including TT2, TTG1, GL2, MYB76, MYB118, and WRKY6, negatively affect seed oil accumulation. The crosstalk among the positive and negative regulatory modules enables balanced and fine-tuned responses to environmental and developmental signals during seed oil accumulation. The green arrows indicate positive regulation. The red T-bars represent negative regulation. Black lines indicate the formation of protein–protein interacting complexes. Genes in light blue shaded circles are downstream targets of the transcription factors.
To prevent hyperactivation of essential biological processes, which might result in metabolic imbalance, the activities of transcriptional regulators are frequently calibrated through interactions with other regulators (Hafner et al., 2019; Memelink and Gantet, 2007; Mouchiroud et al., 2014; Pireyre and Burow, 2015; Sui et al., 2018). As the protein–protein interaction network of WRI1 has been unfolded, various interacting partners of AtWRI1 have been found to modulate its activity (summarized in Figure 2A). The CULLIN3 (CUL3)-based E3 ligase adaptor BTB/POZMATH (BPM) interacts with AtWRI1 to bridge AtWRI1 to CUL3, which mediates AtWRI1 degradation by the 26S proteasome (Chen et al., 2013). A recent study showed that BPM mRNA stability is mediated by Arabidopsis PUMILIO PROTEIN24 (APUM24, encoding a Pumilio homology domain-containing protein that is involved in rRNA processing and mRNA degradation), subsequently fine-tuning the function of the BPM-WRI1 module (Huang et al., 2021). The 14-3-3 proteins, a family of phosphopeptide-binding proteins, physically interact with AtWRI1 in yeast two-hybrid and bimolecular fluorescence complementation assays (Ma et al., 2016). Transient overproduction of 14-3-3 with AtWRI1 results in increased oil biosynthesis in Nicotiana benthamiana leaves by enhancing the protein stability and transcriptional activity of AtWRI1 (Ma et al., 2016). BPM1 and 14-3-3 independently interact with AtWRI1 protein in an overlapping region, leading to the hypothesis that 14-3-3 interacts with AtWRI1 at the expense of the AtWRI1–BPM interaction (Ma et al., 2016; Kong and Ma, 2018a). The SNF1-related protein kinase KIN10 is another AtWRI1-interacting partner that phosphorylates AtWRI1, resulting in the degradation of AtWRI1 protein (Zhai et al., 2017). In addition, phosphorylation-deficient mutagenesis at amino acid residues T70 and S166 of AtWRI1 abolished KIN10-mediated phosphorylation and improved AtWRI1 stability (Zhai et al., 2017). Trehalose 6-phosphate is involved in stabilizing AtWRI1 and boosting FA biosynthesis by suppressing KIN10 activity (Zhai et al., 2018).
Furthermore, interacting partners may modulate the assembly of the WRI1 transcriptional complex. In eukaryotic cells, recruitment of mediator (MED) subunits by transcription factors to initiate transcription is a conserved regulatory mechanism (Taatjes, 2010). The MED15 subunit of the Arabidopsis mediator complex has been shown to physically interact with WRI1 both in vitro and in vivo (Kim et al., 2016). Transgenic Arabidopsis overexpressing MED15 displays upregulation of the FA biosynthetic genes targeted by AtWRI1. ChIP experiments also revealed that MED15 binds to the promoters of the AtWRI1 target genes. However, the transgenic wri1 mutant that overproduces MED15 also displays enhanced expression of AtWRI1 target genes, suggesting that alternative transcription factors interact with MED15 to modulate the expression of the oil biosynthetic genes (Kim et al., 2016).
Intra- and inter-family interactions of transcriptional regulators significantly increase the complexity of combinatorial transcriptional regulation. In plants, protein complexes formed by members of a transcription factor family are well documented; nonetheless, the importance of cross-family interactions of transcriptional regulators in gene regulation is increasingly recognized (Bemer et al., 2017). Members of a transcription factor family are generally a group of cis-element-specific DNA-binding factors with overlapping or divergent functions. Different transcription factor families vary in their DNA binding properties, transactivation activities (e.g., functions as repressors or activators), and responses to environmental/developmental stimuli. As such, cross-family transcription factor interactions are a key molecular mechanism for fine-tuning gene expression in response to diverse environmental and developmental cues. Screening of an Arabidopsis transcription factor library led to the identification of TCP4 as a cross-family interacting regulator of AtWRI1 (Kong et al., 2020c). Functional analyses revealed that TCP4 reduces AtWRI1-regulated oil accumulation by repressing AtWRI1 transactivation activity. The tcp4 loss-of-function mutants exhibit elevated seed oil content compared with the WT (Kong et al., 2020c). Until recently, little was known about the transcriptional repressors of WRI1 activity. This work uncovered the potential function of TCP4 as a transcriptional repressor of AtWRI1-regulated oil biosynthesis. However, the molecular mechanisms of the WRI1–TCP4 regulatory module remain to be elucidated. For instance, phosphorylation of WRI1 or TCP4 individually affects their activities and functions (Ma et al., 2015; Kubota et al., 2017; Zhai et al., 2017; Kong et al., 2020b, 2020c); however, it is unclear how phosphorylation affects the DNA binding, subcellular localization, protein stability, protein complex formation, and transcriptional activity of the WRI1–TCP4 module. In addition, TCPs are a plant-specific transcription factor family with diverse functions, and it will be interesting to investigate the interactions between WRI1 and other TCP members and their roles in the regulation of FA biosynthesis.
Although the WRI1 binding sequence AW-box has been established as a key cis-regulatory element (Maeo et al., 2009), AtWRI1 is capable of binding to the promoter of GH3.3 (involved in auxin degradation) at a non-AW-box element (Kong et al., 2017). Because AtWRI1 recognizes and binds to the AW-box in the promoters of the auxin carrier-protein PIN genes, a role for AtWRI1 in auxin homeostasis has been suggested (Kong et al., 2017). This hypothesis is supported in soybean, as overexpression of GmWRI1 elevates, and knockdown of GmWRI1 decreases, the expression of auxin-related genes (Chen et al., 2020). How WRI1 modulates alternative targets to affect plant growth and development is currently being explored. Recent interesting work showed that AtWRI1 itself neither activates nor suppresses the activity of the GH3.3 promoter (Kong et al., 2022). Yeast two-hybrid screening identified TCP20 as an interacting regulator of AtWRI1 (Kong et al., 2022). TCP20 was found to regulate the expression of GH3.3 by binding to a cis-acting element. Intriguingly, AtWRI1 physically interacts with TCP20 to mitigate GH3.3 expression, highlighting the role of a WRI1–TCP20 regulatory module in fine-tuning auxin homeostasis (Kong et al., 2022).
The complex transcriptional network involved in controlling seed oil accumulation
In addition to LEC1, LEC2, FUS3, ABI3, and WRI1, significant progress has been made in understanding other transcriptional regulators that control seed oil biosynthesis (Figure 2B). The transcription factor bZIP67 binds to the G-box of the FAD3 promoter and controls the biosynthesis of α-linolenic acid in Arabidopsis seeds in a L1L- and NF-YC2-dependent manner (Mendes et al., 2013). In addition, overproduction of a soybean bZIP transcription factor (GmbZIP123) elevates seed oil content in transgenic Arabidopsis plants (Song et al., 2013). In this case, the expression of genes involved in de novo FA biosynthesis is not affected, but rather several sucrose transport-related genes are upregulated, suggesting that GmbZIP123 mediates FA biosynthesis by regulating sucrose metabolism (Song et al., 2013).
In addition to MYB89, other MYB transcription factors regulate seed oil biosynthesis. MYB96 regulates seed oil accumulation by controlling the expression of DGAT1 and PDAT1 (Lee et al., 2018) and controls the biosynthesis of very long chain fatty acids by regulating the expression of FAE1 by directly binding to its promoter (Lee et al., 2015). MYB76, MYB118, and MYB123 (TRANSPARENT TESTA2 [TT2]) also affect seed oil biosynthesis (Chen et al., 2012a; Barthole et al., 2014; Duan et al., 2017). However, their functions seem to be distinct from that of MYB96. For example, MYB118 acts as a repressor of FA biosynthesis in the endosperm by repressing maturation-associated genes (Barthole et al., 2014). TT2, known for regulating proanthocyanidin production (Nesi et al., 2001), also modulates seed oil content. The tt2 mutant displays increased seed oil content and increased expression of genes involved in FA biosynthesis, such as FUS3, FAE1, and KAS II, suggesting that TT2 negatively regulates seed oil accumulation (Chen et al., 2012a). Moreover, TT8 (bHLH42) and TRANSPARENT TESTA GLABRA1 (TTG1), known to mediate the biosynthesis of proanthocyanidin and anthocyanin, respectively (Baudry et al., 2004), play negative roles in regulating seed FA biosynthesis. TT8 represses seed FA production by binding to the promoters of LEC1, LEC2, and FUS3 (Chen et al., 2014). TTG1, encoding a WD40 protein, is involved in various physiological processes, including anthocyanin biosynthesis, embryogenesis, seed coat mucilage production, and formation of trichomes and root hairs in Arabidopsis (Walker et al., 1999; Debeaujon et al., 2000; Western et al., 2001; Tsuchiya et al., 2004; Ramsay and Glover, 2005; Tominaga-Wada et al., 2011). The seeds of the ttg1 mutant contain higher total proteins, starch, and fatty acids (Chen et al., 2015). Further analysis revealed that TTG1 indirectly represses the expression of various genes involved in seed developmental processes and FA biosynthesis (Chen et al., 2015). Overproduction of soybean GmMYB73 resulted in increased lipid accumulation in seeds of Arabidopsis and Lotus, possibly through downregulation of GLABRA2 (GL2) expression (Liu et al., 2014).
Other transcriptional regulators, including GL2, WRKY, Dof (DNA binding one finger), and tandem CCCH zinc finger (TZF) proteins, are also involved in controlling seed oil accumulation. GL2 encodes a homeobox protein that plays key roles in root development, trichome formation, and seed coat mucilage biosynthesis (Cheng et al., 2014; Hung et al., 1998; Masucci et al., 1996; Rerie et al., 1994; Western et al., 2001). The gl2 loss-of-function mutant displays an 8% increase in seed oil content compared with the WT (Shen et al., 2006). Further study revealed that GL2 is expressed in both embryo and seed coat, and its target gene MUCILAGE MODIFIED 4 (MUM4) is involved in mucilage biosynthesis. The mum4 mutant displays a similar increased seed oil phenotype as the gl2 mutant, possibly because the gl2 mutant generates more oil with elevated carbon allocation to the embryo at the expense of seed coat mucilage production (Shi et al., 2012). WRKY6 was previously reported to play diverse roles in leaf senescence and responses to low phosphate stress (Chen et al., 2009; Ye et al., 2018b; Zhang et al., 2018). A recent study revealed a new role of WRKY6 in mediating plant seed oil accumulation. The wrky6 loss-of-function mutant displays enhanced seed oil content compared with the WT (Song et al., 2020). The expression levels of FUS3 and DGAT1 are upregulated in the seeds of wrky6; however, how WRKY6 controls the expression of the oil biosynthetic genes remains unclear (Song et al., 2020). Mutation of the MINISEED3 gene, encoding WRKY10, which controls seed development in Arabidopsis, leads to reduced total FA content, as well as decreased seed size and seed weight (Fatihi et al., 2013). The disruption of WRKY43 in Arabidopsis did not change total FA content in seeds but significantly altered the ratio of polyunsaturated fatty acids (including linoleic acid and linolenic acid), suggesting a potential role for WRKY43 in modulating seed FA desaturation (Geilen et al., 2017). Dofs are plant-specific transcription factors that regulate diverse plant physiological processes, such as light response, seed germination, plant defense, vascular development, and phytohormone signaling (Yanagisawa, 2002; Le Hir and Bellini, 2013; Ruta et al., 2020). Overexpression of two soybean Dofs (GmDof4 and GmDof11) in Arabidopsis increased seed oil content (Wang et al., 2007). The two GmDofs activate the expression of ACCase and long-chain-acyl CoA synthetase (LACS) genes while repressing CRA1 (encoding a seed storage protein) by binding to the Dof cis-element in their promoters (Wang et al., 2007). Plant TZF proteins participate in a variety of phytohormone signaling events and stress responses to regulate gene expression (Kim et al., 2008; Lin et al., 2011; Bogamuwa and Jang, 2013). A recent work revealed that a soybean TZF protein, GmZF392, functions as a transcriptional activator in a seed-specific manner (Lu et al., 2021). Overexpression of GmZF392 enhanced seed oil accumulation in transgenic Arabidopsis and soybean plants. GmZF392 controls the expression of FA biosynthetic genes by binding to the bipartite elements in their promoters (Lu et al., 2021).
Epigenetic regulators involved in mediating seed oil accumulation
Epigenetic regulators affect transcriptional regulation by altering the regional state of chromatin. Recent evidence suggests that epigenetic regulators participate in controlling seed oil accumulation (Figure 2A). CURLY LEAF (CLF), a histone methyltransferase of Polycomb Repressive Complex 2 (PRC2), which modulates the trimethylation of histone H3 Lys 27 (H3K27me3), functions as a key regulator in repressing gene expression during embryonic development (Liu et al., 2016). The clf mutant produces larger seeds with increased seed mass, greater seed oil content, and modified long-chain FA composition compared with the WT (Liu et al., 2016). Genes encoding transcriptional regulators involved in controlling FA biosynthesis, such as LEC1, LEC2, FUS3, WRI1, as well as various FA biosynthetic genes, are upregulated in developing seeds of the clf mutant, suggesting a role for CLF in the transcriptional repression of FA biosynthesis (Liu et al., 2016). PICKLE (PKL) is a nuclear-localized ATP-dependent chromatin remodeler from the chromodomain helicase DNA-binding domain (CHD3) family (Ogas et al., 1999; Dean Rider et al., 2003; Zhang et al., 2012; Park et al., 2017; Zha et al., 2020). PKL represses the expression of LEC1, LEC2, and FUS3. Expression levels of LEC1/LEC2/FUS3 significantly increased in roots of the pkl mutant compared with the WT (Ogas et al., 1999; Dean Rider et al., 2003), correlated with the upregulation of storage lipid biosynthesis (Ogas et al., 1997, 1999).
Environmental and developmental cues that affect seed oil biosynthesis
Light potentially affects seed oil accumulation (Table 1). Most Brassicaceae family members, including Arabidopsis, produce green seeds with photosynthetic activity (Eastmond et al., 1996; Eastmond and Rawsthorne, 1998). The light passing through the silique wall is an important driving force for FA production (Ruuska et al., 2004; Schwender et al., 2004; Goffman et al., 2005). Analysis of Arabidopsis plants grown under different light conditions revealed a positive correlation between seed oil content (as well as seed yield and weight) and light intensity (Li et al., 2006; Karki and Bates, 2018). Other studies have also indicated that reduced sunlight during seed filling leads to decreased oil content in oil crops (Andrade and Ferreiro, 1996; de la Vega et al., 2001; Singer et al., 2016). Increased light intensity also alters seed FA composition, increasing the oleic acid to linoleic acid ratio (Seiler, 1986). Although the molecular mechanism by which light affects seed lipid metabolism remains to be elucidated, it is rational to hypothesize that photosynthesis affected by light conditions modifies carbon supply, which eventually influences FA biosynthesis.
Table 1.
Effects of environmental and developmental cues on seed oil biosynthesis.
| Environmental and developmental cues | Effect on seed oil biosynthesis | Species | Reference(s) |
|---|---|---|---|
| High light intensity | Increases seed oil content Increases oleic acid content |
Brassica napus, Arabidopsis thaliana, Zea mays, Helianthus annuus | Goffman et al., 2005; Ruuska et al., 2004; Schwender et al., 2004; Karki and Bates, 2018; Li et al., 2006; Andrade and Ferreiro, 1996; de la Vega et al., 2001; Singer et al., 2016; Seiler, 1986 |
| High temperature | Reduces seed oil content Reduces unsaturated fatty acid content |
Glycine max, Helianthus annuus, Brassica napus, Linum usitatissimum, Glycine max | Canvin, 1965; Carrera et al., 2009; Harris et al., 1978; Singer et al., 2016; Schulte et al., 2013; Wolf et al., 1982 |
| Drought | Reduces seed oil content Increases oleic acid content, reduces linoleic acid content |
Helianthus annuus, Glycine max, Brassica napus | Singer et al., 2016 |
| Salinity | Reduces seed oil content Increases oleic acid content, reduces linoleic acid and/or linolenic acid content |
Helianthus annuus, Carthamus tinctorius L., Gossypium hirsutum | Singer et al., 2016 |
| Gibberellins | Alter seed oil content Modify fatty acid composition |
Arabidopsis thaliana | Cao et al., 2006; Chen et al., 2012b; Zentella et al., 2007 |
| Auxin and jasmonates | Alter seed oil content Modify fatty acid composition |
Brassica napus | Niu et al., 2009 |
Temperature affects seed oil accumulation and composition. The influence of higher global temperatures due to climate change on FA biosynthesis may change crop oil yield and/or FA composition. For example, when Canada experienced a season of abnormally warm temperatures in 2012, B. napus seed oil yield was significantly reduced from the normal average of 44.4% to 43.5% (Singer et al., 2016). For certain B. napus varieties, cooler growing temperatures, particularly during the seed filling period, tend to result in greater oil contents (Harris et al., 1978; Carrera et al., 2009; Singer et al., 2016). Similar to seed oil content, seed FA composition is also affected by temperature. The FA unsaturation level tends to be inversely correlated with increasing environmental temperature. Plant species such as B. napus and sunflower show reduced levels of linoleic acid and α-linolenic acid when grown at higher temperatures, and the decrease in these polyunsaturated FAs is compensated by an increase in oleic acid (Canvin, 1965; Wolf et al., 1982; Schulte et al., 2013; Singer et al., 2016). The molecular mechanism that underlies temperature effects on seed oil yield and composition remains unclear. Nonetheless, it has been proposed that higher temperature increases nitrogen bioavailability, which leads to a competition for the carbon backbone required for storage lipid production, consistent with the inverse correlation between FA and protein yields under heat stress (Canvin, 1965; Singer et al., 2016). Lower FA desaturation at elevated temperatures can also be due to lower oxygen solubility, as has been demonstrated in Acanthamoeba (Jones et al., 1991; Rutter et al., 2002), although such a mechanism has not been reported in higher plants.
Drought generally leads to a substantial reduction in seed oil content and modification of FA composition in plants (Singer et al., 2016) (Table 1). It is proposed that drought leads to reduced seed filling but faster embryo development, which affects FA biosynthesis (Singer et al., 2016). In addition, salinity stress causes reductions in seed oil and changes in FA composition (Singer et al., 2016). Salinity generates reactive oxygen species, which damage cellular redox homeostasis and trigger oxidative stress, negatively affecting metabolic enzymes, including those involved in FA biosynthesis. Interestingly, overproduction of DGAT1 in B. napus reduces the adverse effects of drought stress on seed oil production (Weselake et al., 2008). Transgenic Camelina co-producing CsMYB96A and CsDGAT1C also shows increased resistance to drought stress with improved seed oil content (Kim et al., 2019). Furthermore, the expression of DGAT1 is upregulated under stresses such as salinity (Lu and Hills, 2002; Kong et al., 2013), suggesting a wider role for DGAT1 in response to environmental stresses. Transgenic plants overexpressing DGAT1 are ideal materials for gaining an in-depth understanding of the role of DGAT1 under various stresses.
FA biosynthesis is associated with phytohormone signaling. Gibberellins (GAs) are important phytohormones involved in regulating numerous plant developmental processes, including stem elongation, germination, dormancy, flowering, flower development, and leaf and fruit senescence (Sasaki et al., 2003; Fleet and Sun, 2005; Swain and Singh, 2005). GAs potentially affect FA metabolism in plant cells. The DELLA proteins are essential players in the complex GA signal transduction cascade (Thomas and Sun, 2004; Jiang and Fu, 2007; Zentella et al., 2007). A transcriptomic analysis showed that DELLAs mediate the downregulation of various GDSL-type lipase genes in seeds and flower buds, indicating a possible function of DELLAs in affecting seed FA metabolism (Cao et al., 2006). Further study showed that exogenous GA treatment or DELLA mutations caused alterations in total seed FA content, modification of FA composition, and various morphological changes in seeds (Chen et al., 2012b). A global transcriptomic analysis revealed that GA3 treatment of ga1–3 (a severe GA-deficient mutant) significantly changes the expression of WRI1 (Zentella et al., 2007). As essential phytohormones, auxin and jasmonate (JA) participate in mediating various developmental processes (Woodward and Bartel, 2005; Zhao, 2010; Lavy and Estelle, 2016; Huang et al., 2017; Guo et al., 2018). Transcriptomic analysis of developing B. napus seeds revealed that multiple auxin de novo biosynthesis-associated genes and a JA-inducible gene are co-expressed with FA biosynthetic genes, implying a potential role for auxin and JA in FA biosynthesis in B. napus seed development (Niu et al., 2009) (Table 1). These studies shed light on the relationships among FA biosynthesis, phytohormone signaling, and seed development; however, the detailed molecular mechanisms by which phytohormone signaling modulates FA metabolism during embryogenesis remain to be fully elucidated.
Earlier studies provided evidence for the involvement of WRI1 in ABA and sugar signaling, particularly in developing seedlings (Masaki et al., 2005; Cernac et al., 2006). The interaction between ABA and sugar signaling pathways fine-tunes gene expression during plant developmental processes (Finkelstein and Gibson, 2002; Dekkers et al., 2008; Sakr et al., 2018). ABA broadly participates in various stress-related responses and developmental regulation. Unraveling the position of WRI1 in phytohormone crosstalk, particularly related to seed filling under stress conditions, is of agricultural importance. Plant-fungi interactions have been crucial for initial land plant colonization and vascular plant evolution. The arbuscular mycorrhizal (AM) symbiosis is a close evolutionary relationship between land plants and Glomeromycotina fungi (Remy et al., 1994; Bonfante and Genre, 2010). Recent studies demonstrated that WRI-like proteins, including CTTC MOTIF-BINDING TRANSCRIPTION FACTOR1 (CBX1) and WRI5a (from Lotus japonicus and Medicago truncatula, respectively), play essential roles in FA production and phosphate uptake that are necessary for the AM symbiosis (Jiang et al., 2018; Xue et al., 2018). These findings shed light on the molecular mechanism underlying bidirectional nutrient exchanges during the AM symbiosis, suggesting that, evolutionarily, WRI-like proteins are ancient transcriptional regulators. A recent study showed a conserved transcriptional change in land plants during the AM symbiosis. A WRI-like transcriptional regulator identified from Marchantia paleacea functions in the transcriptional regulation of FA biosynthesis and transport, which are vital for mutualistic interactions in M. paleacea symbiosis (Rich et al., 2021).
Translational applications of plant transcriptional regulators for bioengineering plant oil accumulation
The function of WRI1 seems to be conserved across various angiosperms, both dicots and monocots. The orthologs of WRI1 have been characterized in a variety of plant species. Transgenic plants overproducing AtWRI1 or WRI1 orthologs display substantially enhanced seed oil content (An and Suh, 2015; Cernac and Benning, 2004; Chen et al., 2020; Chen et al., 2018a; Liu et al., 2010; Shen et al., 2010; Sun et al., 2017; Yang et al., 2015; Ye et al., 2018a) (Table 2). The shared regulatory network controlling both development and FA biosynthesis should be considered when using these transcriptional regulators to engineer plant oil production. Choosing appropriate promoters to drive WRI1 expression is crucial for oil bioengineering. For instance, transgenic maize showed considerably elevated oil accumulation when ZmWRI1 expression was driven by the embryo-preferred OLEOSIN (OLE) promoter but not by the starch endosperm-specific 19 KD ZEIN promoter (Shen et al., 2010). Another study showed that use of the FUS3 promoter to drive AtWRI1 expression, aiming to extend the oil accumulation period during the mid-phase of seed development, is an efficient strategy for improving seed oil accumulation (Kanai et al., 2016) (Table 2).
Table 2.
Translational applications of plant transcriptional regulators in bioengineering plant oil.
| Promoter | Gene | Host species | Phenotype | Reference(s) |
|---|---|---|---|---|
| AtWRI1 and WRI1 orthologs | ||||
| CaMV 35S | AtWRI1 | Arabidopsis thaliana | Increases seed oil content | Cernac and Benning, 2004 |
| SiW6 promoter | AtWRI1 | Camelina sativa | Enhances seed oil content and seed mass | An and Suh, 2015 |
| CaMV 35S | GmWRI1a/GmWRI1b | Arabidopsis thaliana | Enhance total TAG content | Chen et al., 2020 |
| Napin promoter | GmWRI1a | Glycine max | Increases the content of total oil and total fatty acids in seeds | Chen et al., 2018a |
| CaMV 35S | BnWRI1 | Arabidopsis thaliana | Increases seed oil content, seed weight, and size | Liu et al., 2010 |
| OLE promoter | ZmWRI1 | Zea mays | Increases seed oil content with no significant difference in embryo size | Shen et al., 2010 |
| EnP2 promoter | CoWRI1 | Oryza sativa | Increases seed oil content and alters lipid composition | Sun et al., 2017 |
| EnP2 promoter | CoWRI1 | Arabidopsis thaliana | Increases seed oil content and alters lipid composition | Sun et al., 2017 |
| ZmUBI1 promoter | BdWRI1 | Brachypodium distachyon | Increases grain dry weight and leads to higher TAG content in endosperm and leaf blades | Yang et al., 2015 |
| Glycinin promoter | HaWRI1 | Arabidopsis thaliana | Increases seed oil content, seed weight, and size | Lim et al., 2022 |
| CaMV 35S | JcWRI1 | Jatropha curcas | Elevates seed lipid content and increases seed mass | Ye et al., 2018a |
| CaMV 35S | StWRI1/PtWRI1/AsWRI1/CeWRI1 | Nicotiana benthamiana leaves | Cause significant increases in oil accumulation | Grimberg et al., 2015 |
| FUS3 promoter | AtWRI1 | Arabidopsis thaliana | Prolonged expression of AtWRI1 during seed development leads to increased oil content in seeds | Kanai et al., 2016 |
| AtWRI1 variants | ||||
| CaMV 35S | AtWRI11−397 | Nicotiana benthamiana leaves | Deletion of the IDR-PEST motif of AtWRI1 leads to increased AtWRI1 protein stability and higher TAG content | Ma et al., 2015 |
| CaMV 35S | AtWRI1PEST/AA | Nicotiana benthamiana leaves, Arabidopsis thaliana (wri1-1) | Mutation of multiple putative phosphorylation sites in PEST motif of AtWRI1 (AtWRI1S398A/S401A/S402A/S407A/S415A/S416A/T420A/T421A/T422A/S423A) leads to increased stability of AtWRI1 and higher TAG content | Ma et al., 2015 |
| CaMV 35S | AtWRI14SA | Nicotiana benthamiana leaves | Mutation of four residues (AtWRI1S398A/S401A/S402A/S407A) in PEST motif of AtWRI1 leads to increased stability and higher TAG content | Ma et al., 2015 |
| CaMV 35S | AtWRI1RR | Nicotiana benthamiana leaves | The lysine to arginine mutation in the dilysine motif (AtWRI1K2R/K3R) in AtWRI1 results in increased stability and TAG accumulation | Zhai et al., 2017 |
| WRI1-interacting partners | ||||
| CaMV 35S | 14-3-3κ or 14-3-3λ | Nicotiana benthamiana leaves, Arabidopsis thaliana | Enhancement of AtWRI1 stability and transcriptional activity leads to increased oil production | Ma et al., 2016 |
| CaMV 35S | MED15 | Arabidopsis thaliana | Increases total fatty acid content in seeds | Kim et al., 2016 |
| CaMV 35S | KIN10 | Nicotiana benthamiana leaves | Significantly reduces TAG production; KIN10 negatively regulates AtWRI1 by triggering phosphorylation of sites in the AP2 DNA binding domains, leading to its degradation | Zhai et al., 2017 |
| CaMV 35S | TCP4 | Nicotiana benthamiana leaves | TCP4 negatively regulates the activity of AtWRI1, leading to reduced oil biosynthesis | Kong et al., 2020b |
| CaMV 35S | BPM-microRNA | Arabidopsis thaliana | Increases total fatty acid content in seeds; AtWRI1 interacts with E3 ligase adaptor BPMs for degradation through the 26S proteasome | Chen et al., 2013 |
| Combination of WRI1 and other genes | ||||
| CaMV 35S | AtWRI1, DGAT1 | Nicotiana benthamiana leaves | Increase oil accumulation due to the upregulation of late glycolysis and fatty acid biosynthesis by WRI1 and TAG synthesis by DGAT1 | Vanhercke et al., 2013 |
| CaMV 35S | AtWRI1 | Arabidopsis thaliana | Lead to significant accumulation of oil in vegetative tissues due to the downregulation of starch formation by APS1 and directing carbon to fatty acid synthesis mediated by AtWRI1 | Sanjaya et al., 2011 |
| Patatin B33 promoter | AtAPS1-RNAi | |||
| Rubi3 promoter | AtWRI1 | Sugarcane (Saccharum spp. hybrids) | Elevate TAG accumulation in leaves and stems | Zale et al., 2016 |
| Mubi promoter | ZmDGAT1-2 | |||
| CaMV 35S | AtOLE1 | |||
| CaMV 35S | AGPase-RNAi | |||
| CaMV 35S | PXA1-RNAi | |||
Transient overexpression of AtWRI1 or other WRI1 orthologs effectively activates TAG production in tobacco leaves (Grimberg et al., 2015; Ma et al., 2015; Vanhercke et al., 2013) (Table 2). Transient co-expression of AtWRI1 and DGAT1 in tobacco leaves leads to markedly elevated oil production compared with the expression of AtWRI1 alone, indicating a synergistic effect of the two genes (Vanhercke et al., 2013). Transient overproduction of the more stable AtWRI1 variants, or transient co-expression of AtWRI1 with AtWRI1-interacting regulators that stabilize AtWRI1, results in enhanced oil production compared with expression of the native WRI1 (Ma et al., 2015, 2016; Zhai et al., 2017). In an effort to improve oil biosynthesis in vegetative tissues, transgenic Arabidopsis were designed to overexpress AtWRI1 and repress AGPase (the small subunit of ADP-glucose pyrophosphorylase) by RNAi, and they showed increased TAG accumulation in the leaves (Sanjaya et al., 2011). In sugarcane, co-expression of WRI1, DGAT1, and oleosin 1 (OLE1), together with the repression of AGPase and PXA1 (encoding a subunit of the peroxisomal ABC transporter) by RNAi, considerably increased TAG generation in vegetative tissues (Zale et al., 2016) (Table 2). The engineering scheme of “push, pull, package, and protect” involves concurrently augmenting (pushing) de novo FA biosynthesis by activating a key transcriptional regulator, pulling the precursors into the final products by rate-limiting enzymes, packaging TAG molecules into oil droplets, and shielding the TAG from decay by deactivating lipases (Vanhercke et al., 2014, 2019).
Enhancement of seed oil accumulation can also be achieved by the manipulation of other transcriptional regulators that mediate the expression of genes involved in alternative metabolic pathways (e.g., seed storage and carbohydrate metabolism). Transgenic Arabidopsis overproducing the soybean transcription factors GmbZIP123, GmDof4, and GmDof7 showed increased seed oil content, possibly because of the upregulation of genes involved in sucrose metabolism and seed storage (Wang et al., 2007; Song et al., 2013).
Plants produce diverse unusual fatty acids, such as hydroxy fatty acids (HFAs), that are highly valuable for industrial applications because of their unique physical and chemical characteristics. Ectopic expression of a hydroxylase gene in Arabidopsis, while producing a small amount of HFAs, significantly decreases seed oil content (Bates and Browse, 2011; Bates et al., 2014), perhaps because of feedback inhibition of de novo FA synthesis in the plastids (Bates et al., 2014). Using an improved strategy in which AtWRI1 and RcFAH12 (a castor fatty acid hydroxylase) are co-expressed, both HFA and seed oil content are considerably increased, indicating that AtWRI1 efficiently overcomes the feedback inhibition by the FA hydroxylase (Adhikari et al., 2016).
Concluding remarks and perspectives
Plants are sessile organisms that consistently face environmental challenges. Plants have developed elegant systems to respond to environmental signals and modify their metabolic activities to overcome these stresses. The available evidence clearly shows that an integrated regulatory network balances environmental responses, seed development, and FA biosynthesis. Although it has been recognized that various environmental cues affect seed oil biosynthesis and FA composition (Li et al., 2006; Singer et al., 2016; Karki and Bates, 2018), the molecular mechanisms that orchestrate these responses require further investigation. For instance, although many individual transcription factors in the regulatory network have been characterized, how they work in concert to perceive, transduce, and respond to environmental and developmental signals is poorly understood.
Manipulation of transcriptional regulators is a proven strategy for improving vegetable oil yield compared with the single-enzyme approach (Broun, 2004; Grotewold, 2008; Shen et al., 2010). Although various degrees of success have been achieved, a deeper understanding of the transcriptional regulation of plant FA biosynthesis is required to overcome the remaining challenges and obstacles. Since the identification of AtWRI1 more than 15 years ago, a wealth of information suggests that WRI1 plays an essential role in the transcriptional control of plant oil biosynthesis. However, how the key seed development regulators (LEC1, LEC2, FUS3, and ABI3) mediate WRI1 expression remains to be fully elucidated. Whether additional upstream transcriptional regulators modulate WRI1 expression, particularly in response to different environmental and developmental cues, is an important question to be addressed in the future. The recently discovered non-seed-specific transcription factors in oil palm that mediate EgWRI1 expression beg the question of whether similar regulators exist in oil-producing tissues of other plant species (Yeap et al., 2017). To tackle this question, it is necessary to conduct more thorough analyses of WRI1 promoters from diverse plant species; the resulting identification of transcription factor binding sites may shed light on additional regulators of WRI1. Recent functional and structural analyses of different WRI1 orthologs and variants have concluded that the phosphorylation residues, IDR3-PEST motif, interacting motif of BPMs, and 14-3-3s are pertinent to the stability and transcriptional activity of WRI1 that affect plant oil accumulation (Ma et al., 2013, 2015, 2016; Zhai et al., 2017). The missing “VYL” motif in AsWRI1c, RcWRI1-B, and OsWRI1-1 casts doubt on the functional significance of “VYL” (Ji et al., 2018; Mano et al., 2019; Yang et al., 2019). Therefore, solving the three-dimensional structure, together with computational modeling and simulation, will facilitate our understanding of the structure/function relationship of WRI1.
Recent advances suggest that the crosstalk between WRI1-interacting partners is regulated at the post-translational level, underlining the collaborative nature of the WRI1 transcriptional apparatus. Although dual roles of phosphorylation in modulating the activity of WRI1 have been proposed (Ma et al., 2016; Kong and Ma, 2018a), future work will focus on illustrating the molecular mechanisms by which WRI1 activity is fine-tuned through phosphorylation and dephosphorylation by diverse kinases and phosphatases in response to a variety of developmental and environmental stimuli. The recently established WRI1 interactome has contributed to the discovery of novel WRI1-interacting regulators (Figure 2A), opening new doors to the investigation of the molecular action of the regulatory network.
Alternative targets of WRI1 beyond the oil biosynthetic pathway might be factors to consider in translational application of WRI1 for oil bioengineering in crops. Nevertheless, numerous studies have demonstrated that WRI1 is one of the most effective transcriptional regulators for the metabolic engineering of vegetable oil production. The use of protein engineering and genome editing approaches to improve WRI1 binding specificity to genes in the oil biosynthetic pathway should be considered in future work on oil bioengineering.
The AtWRI gene family remains to be explored. AtWRI3 and AtWRI4 are WRI1-like genes capable of complementing the phenotype of the wri1 mutant (To et al., 2012). The functions of AtWRI3 and AtWRI4 in transcriptional control of seed oil accumulation are not certain, as their genes are preferentially expressed in non-seed tissues (e.g., stems and flowers), despite their overlapping roles with AtWRI1 in supplying acyl chains for cutin production (To et al., 2012). No obvious changes in plant growth were observed in the wri1 wri3 wri4 triple mutant, and the expression of FA biosynthetic genes remained at a basal level in the triple mutant, leading to speculation that alternative transcriptional regulators may control FA biosynthetic genes in vegetative tissues (To et al., 2012; Marchive et al., 2014). Dissecting the distinct and overlapping roles of the WRI1-like proteins will contribute to our understanding of the complex transcriptional regulation of FA biosynthesis, particularly in vegetative tissues.
Seed oil biosynthesis involves hundreds of genes in multiple regulatory and metabolic pathways. How this dynamic process is regulated during seed development remains to be explored at the whole-genome and transcriptome levels. The development of population transcriptome analysis in recent years has led to the exploration of genes that regulate lipid metabolism. Using population transcriptome data from maize, expression quantitative trait locusi analysis revealed an intergenic locus that affected three oil-related genes, long-chain fatty alcohol dehydrogenase (FADD), glycosylphosphatidylinositol (GPI), and glycolipid transfer protein 1 (GLTP1), resulting in variation in kernel oil concentration (Liu et al., 2017). Combining expression quantitative trait locus with co-expression networks helped to identify some key regulatory genes of lipid metabolism. The transcription factor BrMD-2 was identified using transcriptomic data from a B. rapa doubled haploid population (Basnet et al., 2016). Recently, transcriptome-wide association studies were used to dissect oil biosynthesis in B. napus, and 690 genes, including LPAAT, TT4, and C4H, were identified as being associated with seed oil content (Tang et al., 2021). Thus, bioinformatics analysis based on transcriptome data from multiple tissues and multiple time series at the population level may provide more insights into the coordination of seed oil biosynthesis.
In summary, identification and characterization of essential regulatory factors that play roles in improving oil yield and agricultural traits are vital missions in vegetable oil research. To reach this goal, we require a profound understanding of the regulatory mechanisms that impact critical steps in the FA biosynthetic pathway and how these regulatory mechanisms are connected to environmental and developmental signaling. The knowledge gained in such an endeavor will guide our design to enhance vegetable oil yield in crops with integrated approaches that include genome editing, protein engineering, and metabolic engineering.
Funding
This work was supported by Ministry of Education (MOE) of Singapore Tier 1 to W.M. (RG29/20), MOE of Singapore Tier 2 to W.M. (MOE-T2EP30220-0011), the National Key R&D Program of China to L.Y. (2019YFC1711100), the Hubei Hongshan Laboratory Research Fund to L.G. (2021HSZD004), and the HZAU-AGIS Cooperation Fund to L.G. (SZYJY2021004).
Author contributions
Y.Y., Q.K., A.R.Q.L., L.G., L.Y., and W.M. conceived the ideas, wrote the first draft, and prepared the figures and tables. Q.K., S.L., H.Z., L.G., L.Y., and W.M. reviewed and edited the manuscript, figures, and tables. All authors have read and approved the final version of the manuscript.
Acknowledgments
We apologize to the authors whose important works are not cited because of space limitations. The authors declare no competing interests.
Published: April 20, 2022
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Contributor Information
Liang Guo, Email: guoliang@mail.hzau.edu.cn.
Ling Yuan, Email: lyuan3@uky.edu.
Wei Ma, Email: weima@ntu.edu.sg.
References
- Adhikari N.D., Bates P.D., Browse J. WRINKLED1 rescues feedback inhibition of fatty acid synthesis in hydroxylase-expressing seeds. Plant Physiol. 2016;171:179–191. doi: 10.1104/pp.15.01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D.K. Assessing compartmentalized flux in lipid metabolism with isotopes. Biochim. Biophys. Acta. 2016;1861:1226–1242. doi: 10.1016/j.bbalip.2016.03.017. [DOI] [PubMed] [Google Scholar]
- An D., Suh M.C. Overexpression of Arabidopsis WRI1 enhanced seed mass and storage oil content in Camelina sativa. Plant Biotechnol. Rep. 2015;9:137–148. doi: 10.1007/s11816-015-0351-x. [DOI] [Google Scholar]
- Andrade F.H., Ferreiro M.A. Reproductive growth of maize, sunflower and soybean at different source levels during grain filling. Field Crops Res. 1996;48:155–165. doi: 10.1016/s0378-4290(96)01017-9. [DOI] [Google Scholar]
- Barthole G., To A., Marchive C., Brunaud V., Soubigou-Taconnat L., Berger N., Dubreucq B., Lepiniec L., Baud S. MYB118 represses endosperm maturation in seeds of Arabidopsis. Plant Cell. 2014;26:3519–3537. doi: 10.1105/tpc.114.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnet R.K., Del Carpio D.P., Xiao D., Bucher J., Jin M., Boyle K., Fobert P., Visser R.G., Maliepaard C., Bonnema G. A systems genetics approach identifies gene regulatory networks associated with fatty acid composition in Brassica rapa seed. Plant Physiol. 2016;170:568–585. doi: 10.1104/pp.15.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P.D. Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. Biochim. Biophys. Acta. 2016;1861:1214–1225. doi: 10.1016/j.bbalip.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Bates P.D., Browse J. The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J. 2011;68:387–399. doi: 10.1111/j.1365-313X.2011.04693.x. [DOI] [PubMed] [Google Scholar]
- Bates P.D., Stymne S., Ohlrogge J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013;16:358–364. doi: 10.1016/j.pbi.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Bates P.D., Johnson S.R., Cao X., Li J., Nam J.W., Jaworski J.G., Ohlrogge J.B., Browse J. Fatty acid synthesis is inhibited by inefficient utilization of unusual fatty acids for glycerolipid assembly. Proc. Natl. Acad. Sci. U S A. 2014;111:1204–1209. doi: 10.1073/pnas.1318511111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S., Mendoza M.S., To A., Harscoet E., Lepiniec L., Dubreucq B. WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J. 2007;50:825–838. doi: 10.1111/j.1365-313X.2007.03092.x. [DOI] [PubMed] [Google Scholar]
- Baud S., Feria Bourrellier A.B., Azzopardi M., Berger A., Dechorgnat J., Daniel-Vedele F., Lepiniec L., Miquel M., Rochat C., Hodges M., et al. PII is induced by WRINKLED1 and fine-tunes fatty acid composition in seeds of Arabidopsis thaliana. Plant J. 2010;64:291–303. doi: 10.1111/j.1365-313X.2010.04332.x. [DOI] [PubMed] [Google Scholar]
- Baudry A., Heim M.A., Dubreucq B., Caboche M., Weisshaar B., Lepiniec L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004;39:366–380. doi: 10.1111/j.1365-313X.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- Bemer M., van Dijk A.D.J., Immink R.G.H., Angenent G.C. Cross-family transcription factor interactions: an additional layer of gene regulation. Trends Plant Sci. 2017;22:66–80. doi: 10.1016/j.tplants.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Bogamuwa S., Jang J.C. The Arabidopsis tandem CCCH zinc finger proteins AtTZF4, 5 and 6 are involved in light-abscisic acid- and gibberellic acid-mediated regulation of seed germination. Plant Cell Environ. 2013;36:1507–1519. doi: 10.1111/pce.12084. [DOI] [PubMed] [Google Scholar]
- Bonfante P., Genre A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010;1:48. doi: 10.1038/ncomms1046. [DOI] [PubMed] [Google Scholar]
- Boulard C., Fatihi A., Lepiniec L., Dubreucq B. Regulation and evolution of the interaction of the seed B3 transcription factors with NF-Y subunits. Biochim. Biophys. Acta Gene Regul. Mech. 2017;1860:1069–1078. doi: 10.1016/j.bbagrm.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Bourgis F., Kilaru A., Cao X., Ngando-Ebongue G.F., Drira N., Ohlrogge J.B., Arondel V. Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning. Proc. Natl. Acad. Sci. U S A. 2011;108:12527–12532. doi: 10.1073/pnas.1106502108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun P. Transcription factors as tools for metabolic engineering in plants. Curr. Opin. Plant Biol. 2004;7:202–209. doi: 10.1016/j.pbi.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Canvin D.T. The effect of temperature on the oil content and fatty acidcomposition of oils from several oil seed crops. Can. J. Bot. 1965;43:63–69. doi: 10.1139/b65-008. [DOI] [Google Scholar]
- Cao D., Cheng H., Wu W., Soo H.M., Peng J. Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol. 2006;142:509–525. doi: 10.1104/pp.106.082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera C., Martínez M.J., Dardanelli J., Mónica Balzarini M. Water deficit effect on the relationship between temperature during the seed fill period and soybean seed oil and protein concentrations. Crop Sci. 2009;49:990–998. doi: 10.2135/cropsci2008.06.0361. [DOI] [Google Scholar]
- Casson S.A., Lindsey K. The turnip mutant of Arabidopsis reveals that LEAFY COTYLEDON1 expression mediates the effects of auxin and sugars to promote embryonic cell identity. Plant Physiol. 2006;142:526–541. doi: 10.1104/pp.106.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernac A., Benning C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 2004;40:575–585. doi: 10.1111/j.1365-313X.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- Cernac A., Andre C., Hoffmann-Benning S., Benning C. WRI1 is required for seed germination and seedling establishment. Plant Physiol. 2006;141:745–757. doi: 10.1104/pp.106.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.D., Ohlrogge J.B. Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 2012;287:2288–2294. doi: 10.1074/jbc.R111.290072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.D., Dyer J.M., Mullen R.T. Biogenesis and functions of lipid droplets in plants. J. Lipid Res. 2012;53:215–226. doi: 10.1194/jlr.R021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Zhang G., Li P., Yang J., Guo L., Benning C., Wang X., Zhao J. Multiple GmWRI1s are redundantly involved in seed filling and nodulation by regulating plastidic glycolysis, lipid biosynthesis and hormone signalling in soybean (Glycine max) Plant Biotechnol. J. 2020;18:155–171. doi: 10.1111/pbi.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Lee J.H., Weber H., Tohge T., Witt S., Roje S., Fernie A.R., Hellmann H. Arabidopsis BPM proteins function as substrate adaptors to a cullin3-based E3 ligase to affect fatty acid metabolism in plants. Plant Cell. 2013;25:2253–2264. doi: 10.1105/tpc.112.107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zheng Y., Dong Z., Meng F., Sun X., Fan X., Zhang Y., Wang M., Wang S. Soybean (Glycine max) WRINKLED1 transcription factor, GmWRI1a, positively regulates seed oil accumulation. Mol. Genet. Genomics. 2018;293:401–415. doi: 10.1007/s00438-017-1393-2. [DOI] [PubMed] [Google Scholar]
- Chen M., Zhang B., Li C., Kulaveerasingam H., Chew F.T., Yu H. TRANSPARENT TESTA GLABRA1 regulates the accumulation of seed storage reserves in arabidopsis. Plant Physiol. 2015;169:391–402. doi: 10.1104/pp.15.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wang Z., Zhu Y., Li Z., Hussain N., Xuan L., Guo W., Zhang G., Jiang L. The effect of transparent TESTA2 on seed fatty acid biosynthesis and tolerance to environmental stresses during young seedling establishment in Arabidopsis. Plant Physiol. 2012;160:1023–1036. doi: 10.1104/pp.112.202945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Xuan L., Wang Z., Zhou L., Li Z., Du X., Ali E., Zhang G., Jiang L. TRANSPARENT TESTA8 inhibits seed fatty acid accumulation by targeting several seed development regulators in arabidopsis. Plant Physiol. 2014;165:905–916. doi: 10.1104/pp.114.235507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Du X., Zhu Y., Wang Z., Hua S., Li Z., Guo W., Zhang G., Peng J., Jiang L. Seed Fatty Acid Reducer acts downstream of gibberellin signalling pathway to lower seed fatty acid storage in Arabidopsis. Plant Cell Environ. 2012;35:2155–2169. doi: 10.1111/j.1365-3040.2012.02546.x. [DOI] [PubMed] [Google Scholar]
- Chen N., Veerappan V., Abdelmageed H., Kang M., Allen R.D. HSI2/VAL1 silences AGL15 to regulate the developmental transition from seed maturation to vegetative growth in arabidopsis. Plant Cell. 2018;30:600–619. doi: 10.1105/tpc.17.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.F., Li L.Q., Xu Q., Kong Y.H., Wang H., Wu W.H. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell. 2009;21:3554–3566. doi: 10.1105/tpc.108.064980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Zhu W., Chen Y., Ito S., Asami T., Wang X. Brassinosteroids control root epidermal cell fate via direct regulation of a MYB-bHLH-WD40 complex by GSK3-like kinases. Elife. 2014;3:e02525. doi: 10.7554/eLife.02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio C., Ranocha P., Francoz E., Burlat V., Zheng Y., Perry S.E., Ripoll J.J., Yanofsky M., Dunand C. The class III peroxidase PRX17 is a direct target of the MADS-box transcription factor AGAMOUS-LIKE15 (AGL15) and participates in lignified tissue formation. New Phytol. 2017;213:250–263. doi: 10.1111/nph.14127. [DOI] [PubMed] [Google Scholar]
- Covas M.I., de la Torre R., Fito M. Virgin olive oil: a key food for cardiovascular risk protection. Br. J. Nutr. 2015;113(Suppl 2):S19–S28. doi: 10.1017/S0007114515000136. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A., Stahl U., Lenman M., Banas A., Lee M., Sandager L., Ronne H., Stymne S. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. U S A. 2000;97:6487–6492. doi: 10.1073/pnas.120067297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega A.J., Chapman S.C., Hall A.J. Genotype by environment interaction and indirect selection for yield in sunflower. Field Crops Res. 2001;72:17–38. doi: 10.1016/s0378-4290(01)00162-9. [DOI] [Google Scholar]
- Dean Rider S., Jr., Henderson J.T., Jerome R.E., Edenberg H.J., Romero-Severson J., Ogas J. Coordinate repression of regulators of embryonic identity by PICKLE during germination in Arabidopsis. Plant J. 2003;35:33–43. doi: 10.1046/j.1365-313x.2003.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I., Leon-Kloosterziel K.M., Koornneef M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000;122:403–414. doi: 10.1104/pp.122.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers B.J.W., Schuurmans J.A.M.J., Smeekens S.C.M. Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol. Biol. 2008;67:151–167. doi: 10.1007/s11103-008-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S., Jin C., Li D., Gao C., Qi S., Liu K., Hai J., Ma H., Chen M. MYB76 inhibits seed fatty acid accumulation in arabidopsis. Front. Plant Sci. 2017;8:226. doi: 10.3389/fpls.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P., Koláčá L., Rawsthorne S. Photosynthesis by developing embryos of oilseed rape (Brassica napus L.) J. Exp. Bot. 1996;47:1763–1769. doi: 10.1093/jxb/47.11.1763. [DOI] [Google Scholar]
- Eastmond P.J., Rawsthorne S. Comparison of the metabolic properties of plastids isolated from developing leaves or embryos of Brassica napus L. J. Exp. Bot. 1998;49:1105–1111. doi: 10.1093/jxb/49.324.1105. [DOI] [Google Scholar]
- Fatihi A., Zbierzak A.M., Dormann P. Alterations in seed development gene expression affect size and oil content of Arabidopsis seeds. Plant Physiol. 2013;163:973–985. doi: 10.1104/pp.113.226761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R.R., Gibson S.I. ABA and sugar interactions regulating development: cross-talk or voices in a crowd? Curr. Opin. Plant Biol. 2002;5:26–32. doi: 10.1016/s1369-5266(01)00225-4. [DOI] [PubMed] [Google Scholar]
- Fleet C.M., Sun T.P. A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr. Opin. Plant Biol. 2005;8:77–85. doi: 10.1016/j.pbi.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Focks N., Benning C. wrinkled1: a novel, low-seed-oil mutant of arabidopsis with a deficiency in the seed-specific regulation of carbohydrate Metabolism1. Plant Physiol. 1998;118:91–101. doi: 10.1104/pp.118.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilen K., Heilmann M., Hillmer S., Bohmer M. WRKY43 regulates polyunsaturated fatty acid content and seed germination under unfavourable growth conditions. Sci. Rep. 2017;7:14235. doi: 10.1038/s41598-017-14695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Hauge B.M., Valon C., Smalle J., Parcy F., Goodman H.M. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4:1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffman F.D., Alonso A.P., Schwender J., Shachar-Hill Y., Ohlrogge J.B. Light enables a very high efficiency of carbon storage in developing embryos of rapeseed. Plant Physiol. 2005;138:2269–2279. doi: 10.1104/pp.105.063628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg A., Carlsson A.S., Marttila S., Bhalerao R., Hofvander P. Transcriptional transitions in Nicotiana benthamiana leaves upon induction of oil synthesis by WRINKLED1 homologs from diverse species and tissues. BMC Plant Biol. 2015;15:192. doi: 10.1186/s12870-015-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E. Transcription factors for predictive plant metabolic engineering: are we there yet? Curr. Opin. Biotechnol. 2008;19:138–144. doi: 10.1016/j.copbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Guo Q., Major I.T., Howe G.A. Resolution of growth-defense conflict: mechanistic insights from jasmonate signaling. Curr. Opin. Plant Biol. 2018;44:72–81. doi: 10.1016/j.pbi.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Hafner A., Bulyk M.L., Jambhekar A., Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019;20:199–210. doi: 10.1038/s41580-019-0110-x. [DOI] [PubMed] [Google Scholar]
- Harris H.C., McWilliam J.R., Mason W.K. Influence of temperature on oil content and composition of sunflower seed. Aust. J. Agric. Res. 1978;29:1203–1212. doi: 10.1071/ar9781203. [DOI] [Google Scholar]
- Horstman A., Willemsen V., Boutilier K., Heidstra R. AINTEGUMENTA-LIKE proteins: hubs in a plethora of networks. Trends Plant Sci. 2014;19:146–157. doi: 10.1016/j.tplants.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Horstman A., Li M., Heidmann I., Weemen M., Chen B., Muino J.M., Angenent G.C., Boutilier K. The BABY BOOM transcription factor Activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis. Plant Physiol. 2017;175:848–857. doi: 10.1104/pp.17.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Liu B., Liu L., Song S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017;68:1349–1359. doi: 10.1093/jxb/erw495. [DOI] [PubMed] [Google Scholar]
- Huang R., Liu M., Gong G., Wu P., Patra B., Yuan L., Qin H., Wang X., Wang G., Liao H., et al. The Pumilio RNA-binding protein APUM24 regulates seed maturation by fine-tuning the BPM-WRI1 module in Arabidopsis. J. Integr. Plant Biol. 2021;63:1240–1259. doi: 10.1111/jipb.13092. [DOI] [PubMed] [Google Scholar]
- Hung C.Y., Lin Y., Zhang M., Pollock S., David Marks M., Schiefelbein J. A common position-dependent mechanism controls cell-type patterning and GLABRA2 regulation in the root and hypocotyl epidermis of Arabidopsis1. Plant Physiol. 1998;117:73–84. doi: 10.1104/pp.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X.J., Mao X., Hao Q.T., Liu B.L., Xue J.A., Li R.Z. Splice variants of the Castor WRI1 gene upregulate fatty acid and oil biosynthesis when expressed in tobacco leaves. Int. J. Mol. Sci. 2018;19:146. doi: 10.3390/ijms19010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Fu X. GA action: turning on de-DELLA repressing signaling. Curr. Opin. Plant Biol. 2007;10:461–465. doi: 10.1016/j.pbi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Xie Q., Wang W., Yang J., Zhang X., Yu N., Zhou Y., Wang E. Medicago AP2-domain transcription factor WRI5a is a master regulator of lipid biosynthesis and transfer during mycorrhizal symbiosis. Mol. Plant. 2018;11:1344–1359. doi: 10.1016/j.molp.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Jo L., Pelletier J.M., Harada J.J. Central role of the LEAFY COTYLEDON1 transcription factor in seed development. J. Integr. Plant Biol. 2019;61:564–580. doi: 10.1111/jipb.12806. [DOI] [PubMed] [Google Scholar]
- Jones A.L., Pruitt N.L., Lloyd D., Harwood J.L. Temperature-induced changes in the synthesis of unsaturated fatty acids by Acanthamoeba castellanii. J. Protozoology. 1991;38:532–536. doi: 10.1111/j.1550-7408.1991.tb06076.x. [DOI] [Google Scholar]
- Joshi S., Keller C., Perry S.E. The EAR motif in the arabidopsis MADS transcription factor AGAMOUS-like 15 is not necessary to promote somatic embryogenesis. Plants (Basel) 2021;10:758. doi: 10.3390/plants10040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Mano S., Kondo M., Hayashi M., Nishimura M. Extension of oil biosynthesis during the mid-phase of seed development enhances oil content in Arabidopsis seeds. Plant Biotechnol. J. 2016;14:1241–1250. doi: 10.1111/pbi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki N., Bates P.D. The effect of light conditions on interpreting oil composition engineering in Arabidopsis seeds. Plant Direct. 2018;2:e00067. doi: 10.1002/pld3.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Yamaguchi S., Lim S., Oh E., Park J., Hanada A., Kamiya Y., Choi G. SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell. 2008;20:1260–1277. doi: 10.1105/tpc.108.058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.U., Lee K.R., Jung S.J., Shin H.A., Go Y.S., Suh M.C., Kim J.B. Senescence-inducible LEC2 enhances triacylglycerol accumulation in leaves without negatively affecting plant growth. Plant Biotechnol. J. 2015;13:1346–1359. doi: 10.1111/pbi.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Jang I.C., Chua N.H. The mediator complex MED15 subunit mediates activation of downstream lipid-related genes by the WRINKLED1 transcription factor. Plant Physiol. 2016;171:1951–1964. doi: 10.1104/pp.16.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R.J., Kim H.U., Suh M.C. Development of camelina enhanced with drought stress resistance and seed oil production by co-overexpression of MYB96A and DGAT1C. Ind. Crops Prod. 2019;138:111475. doi: 10.1016/j.indcrop.2019.111475. [DOI] [Google Scholar]
- Kong Q., Ma W. WRINKLED1 as a novel 14-3-3 client: function of 14-3-3 proteins in plant lipid metabolism. Plant Signal. Behav. 2018;13:e1482176. doi: 10.1080/15592324.2018.1482176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Ma W. WRINKLED1 transcription factor: how much do we know about its regulatory mechanism? Plant Sci. 2018;272:153–156. doi: 10.1016/j.plantsci.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Kong Q., Yuan L., Ma W. WRINKLED1, a "master regulator" in transcriptional control of plant oil biosynthesis. Plants (Basel) 2019;8:238. doi: 10.3390/plants8070238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Yang Y., Guo L., Yuan L., Ma W. Molecular basis of plant oil biosynthesis: insights gained from studying the WRINKLED1 transcription factor. Front. Plant Sci. 2020;11:24. doi: 10.3389/fpls.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Ma W., Yang H., Ma G., Mantyla J.J., Benning C. The Arabidopsis WRINKLED1 transcription factor affects auxin homeostasis in roots. J. Exp. Bot. 2017;68:4627–4634. doi: 10.1093/jxb/erx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Yang Y., Low P.M., Guo L., Yuan L., Ma W. The function of the WRI1-TCP4 regulatory module in lipid biosynthesis. Plant Signal. Behav. 2020;15:1812878. doi: 10.1080/15592324.2020.1812878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Low P.M., Lim A.R.Q., Yang Y., Yuan L., Ma W. Functional antagonism of WRI1 and TCP20 modulates GH3.3 expression to maintain auxin homeostasis in roots. Plants (Basel) 2022;11:454. doi: 10.3390/plants11030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Singh S.K., Mantyla J.J., Pattanaik S., Guo L., Yuan L., Benning C., Ma W. TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR4 interacts with WRINKLED1 to mediate seed oil biosynthesis. Plant Physiol. 2020;184:658–665. doi: 10.1104/pp.20.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y., Chen S., Yang Y., An C. ABA-insensitive (ABI) 4 and ABI5 synergistically regulate DGAT1 expression in Arabidopsis seedlings under stress. FEBS Lett. 2013;587:3076–3082. doi: 10.1016/j.febslet.2013.07.045. [DOI] [PubMed] [Google Scholar]
- Kubota A., Ito S., Shim J.S., Johnson R.S., Song Y.H., Breton G., Goralogia G.S., Kwon M.S., Laboy Cintron D., Koyama T., et al. TCP4-dependent induction of CONSTANS transcription requires GIGANTEA in photoperiodic flowering in Arabidopsis. Plos Genet. 2017;13:e1006856. doi: 10.1371/journal.pgen.1006856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S.A., Jolma A., Campitelli L.F., Das P.K., Yin Y., Albu M., Chen X., Taipale J., Hughes T.R., Weirauch M.T. The human transcription factors. Cell. 2018;172:650–665. doi: 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- Lavy M., Estelle M. Mechanisms of auxin signaling. Development. 2016;143:3226–3229. doi: 10.1242/dev.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir R., Bellini C. The plant-specific dof transcription factors family: new players involved in vascular system development and functioning in Arabidopsis. Front. Plant Sci. 2013;4:164. doi: 10.3389/fpls.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.G., Park B.Y., Kim H.U., Seo P.J. MYB96 stimulates C18 fatty acid elongation in Arabidopsis seeds. Plant Biotechnol. Rep. 2015;9:161–166. doi: 10.1007/s11816-015-0352-9. [DOI] [Google Scholar]
- Lee H.G., Kim H., Suh M.C., Kim H.U., Seo P.J. The MYB96 transcription factor regulates triacylglycerol accumulation by activating DGAT1 and PDAT1 expression in arabidopsis seeds. Plant Cell Physiol. 2018;59:1432–1442. doi: 10.1093/pcp/pcy073. [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y., Shorrosh B., Beisson F., Andersson M.X., Arondel V., Bates P.D., Baud S., Bird D., Debono A., Durrett T.P., et al. Acyl-lipid metabolism. Arabidopsis Book. 2013;11:e0161. doi: 10.1199/tab.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Jin C., Duan S., Zhu Y., Qi S., Liu K., Gao C., Ma H., Zhang M., Liao Y., et al. MYB89 transcription factor represses seed oil accumulation. Plant Physiol. 2017;173:1211–1225. doi: 10.1104/pp.16.01634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Beisson F., Pollard M., Ohlrogge J. Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry. 2006;67:904–915. doi: 10.1016/j.phytochem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Lim A.R.Q., Kong Q., Singh S.K., Guo L., Yuan L., Ma W. Sunflower WRINKLED1 plays a key role in transcriptional regulation of oil biosynthesis. Int. J. Mol. Sci. 2022;23:3054. doi: 10.3390/ijms23063054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.C., Pomeranz M.C., Jikumaru Y., Kang S.G., Hah C., Fujioka S., Kamiya Y., Jang J.C. The Arabidopsis tandem zinc finger protein AtTZF1 affects ABA- and GA-mediated growth, stress and gene expression responses. Plant J. 2011;65:253–268. doi: 10.1111/j.1365-313X.2010.04419.x. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhai Z., Kuczynski K., Keereetaweep J., Schwender J., Shanklin J. WRINKLED1 regulates BIOTIN ATTACHMENT DOMAIN-CONTAINING proteins that inhibit fatty acid synthesis. Plant Physiol. 2019;181:55–62. doi: 10.1104/pp.19.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Luo X., Niu L., Xiao Y., Chen L., Liu J., Wang X., Jin M., Li W., Zhang Q., et al. Distant eQTLs and non-coding sequences play critical roles in regulating gene expression and quantitative trait variation in maize. Mol. Plant. 2017;10:414–426. doi: 10.1016/j.molp.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Liu J., Hua W., Zhan G., Wei F., Wang X., Liu G., Wang H. Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus. Plant Physiol. Biochem. 2010;48:9–15. doi: 10.1016/j.plaphy.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Liu J., Deng S., Wang H., Ye J., Wu H.W., Sun H.X., Chua N.H. CURLY LEAF regulates gene sets coordinating seed size and lipid biosynthesis. Plant Physiol. 2016;171:424–436. doi: 10.1104/pp.15.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.F., Li Q.T., Lu X., Song Q.X., Lam S.M., Zhang W.K., Ma B., Lin Q., Man W.Q., Du W.G., et al. Soybean GmMYB73 promotes lipid accumulation in transgenic plants. BMC Plant Biol. 2014;14:73. doi: 10.1186/1471-2229-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T., Ohto M.a., Yee K.M., West M.A., Lo R., Kwong R.W., Yamagishi K., Fischer R.L., Goldberg R.B., Harada J.J. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93:1195–1205. doi: 10.1016/s0092-8674(00)81463-4. [DOI] [PubMed] [Google Scholar]
- Lu C., Hills M.J. Arabidopsis mutants deficient in diacylglycerol acyltransferase display increased sensitivity to abscisic acid, sugars, and osmotic stress during germination and seedling development. Plant Physiol. 2002;129:1352–1358. doi: 10.1104/pp.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Wei W., Li Q.T., Bian X.H., Lu X., Hu Y., Cheng T., Wang Z.Y., Jin M., Tao J.J., et al. A transcriptional regulatory module controls lipid accumulation in soybean. New Phytol. 2021;231:661–678. doi: 10.1111/nph.17401. [DOI] [PubMed] [Google Scholar]
- Lu S., Sturtevant D., Aziz M., Jin C., Li Q., Chapman K.D., Guo L. Spatial analysis of lipid metabolites and expressed genes reveals tissue-specific heterogeneity of lipid metabolism in high- and low-oil Brassica napus L. seeds. Plant J. 2018;94:915–932. doi: 10.1111/tpj.13959. [DOI] [PubMed] [Google Scholar]
- Luerßen H., Kirik V., Herrmann P., Misera S. FUSCA3 encodes a protein with a conserved VP1/ABI3-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J. 1998;15:755–764. doi: 10.1046/j.1365-313x.1998.00259.x. [DOI] [PubMed] [Google Scholar]
- Lutz K.A., Martin C., Khairzada S., Maliga P. Steroid-inducible BABY BOOM system for development of fertile Arabidopsis thaliana plants after prolonged tissue culture. Plant Cell Rep. 2015;34:1849–1856. doi: 10.1007/s00299-015-1832-7. [DOI] [PubMed] [Google Scholar]
- Ma W., Kong Q., Mantyla J.J., Yang Y., Ohlrogge J.B., Benning C. 14-3-3 protein mediates plant seed oil biosynthesis through interaction with AtWRI1. Plant J. 2016;88:228–235. doi: 10.1111/tpj.13244. [DOI] [PubMed] [Google Scholar]
- Ma W., Kong Q., Grix M., Mantyla J.J., Yang Y., Benning C., Ohlrogge J.B. Deletion of a C-terminal intrinsically disordered region of WRINKLED1 affects its stability and enhances oil accumulation in Arabidopsis. Plant J. 2015;83:864–874. doi: 10.1111/tpj.12933. [DOI] [PubMed] [Google Scholar]
- Ma W., Kong Q., Arondel V., Kilaru A., Bates P.D., Thrower N.A., Benning C., Ohlrogge J.B. Wrinkled1, a ubiquitous regulator in oil accumulating tissues from Arabidopsis embryos to oil palm mesocarp. PLoS One. 2013;8:e68887. doi: 10.1371/journal.pone.0068887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeo K., Tokuda T., Ayame A., Mitsui N., Kawai T., Tsukagoshi H., Ishiguro S., Nakamura K. An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J. 2009;60:476–487. doi: 10.1111/j.1365-313X.2009.03967.x. [DOI] [PubMed] [Google Scholar]
- Manan S., Ahmad M.Z., Zhang G., Chen B., Haq B.U., Yang J., Zhao J. Soybean LEC2 regulates subsets of genes involved in controlling the biosynthesis and catabolism of seed storage substances and seed development. Front. Plant Sci. 2017;8:1604. doi: 10.3389/fpls.2017.01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano F., Aoyanagi T., Kozaki A. Atypical splicing accompanied by skipping conserved micro-exons produces unique WRINKLED1, an AP2 domain transcription factor in rice plants. Plants (Basel) 2019;8:207. doi: 10.3390/plants8070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchive C., Nikovics K., To A., Lepiniec L., Baud S. Transcriptional regulation of fatty acid production in higher plants: molecular bases and biotechnological outcomes. Eur. J. Lipid Sci. Technol. 2014;116:1332–1343. doi: 10.1002/ejlt.201400027. [DOI] [Google Scholar]
- Masaki T., Mitsui N., Tsukagoshi H., Nishii T., Morikami A., Nakamura K. ACTIVATOR of Spomin::LUC1/WRINKLED1 of Arabidopsis thaliana transactivates sugar-inducible promoters. Plant Cell Physiol. 2005;46:547–556. doi: 10.1093/pcp/pci072. [DOI] [PubMed] [Google Scholar]
- Masucci J.D., Rerie W.G., Foreman D.R., Zhang M., Galway M.E., Marks M.D., Schiefelbein J.W. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- Meinke D.W., Franzmann L.H., Nickle T.C., Yeung E.C. Leafy cotyledon mutants of arabidopsis. Plant Cell. 1994;6:1049–1064. doi: 10.1105/tpc.6.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memelink J., Gantet P. Transcription factors involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Phytochemistry Rev. 2007;6:353–362. doi: 10.1007/s11101-006-9051-z. [DOI] [Google Scholar]
- Mendes A., Kelly A.A., van Erp H., Shaw E., Powers S.J., Kurup S., Eastmond P.J. bZIP67 regulates the omega-3 fatty acid content of Arabidopsis seed oil by activating fatty acid desaturase3. Plant Cell. 2013;25:3104–3116. doi: 10.1105/tpc.113.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miray R., Kazaz S., To A., Baud S. Molecular control of oil metabolism in the endosperm of seeds. Int. J. Mol. Sci. 2021;2210:1621. doi: 10.3390/ijms22041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monke G., Seifert M., Keilwagen J., Mohr M., Grosse I., Hahnel U., Junker A., Weisshaar B., Conrad U., Baumlein H., et al. Toward the identification and regulation of the Arabidopsis thaliana ABI3 regulon. Nucleic Acids Res. 2012;40:8240–8254. doi: 10.1093/nar/gks594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L., Eichner L.J., Shaw R.J., Auwerx J. Transcriptional coregulators: fine-tuning metabolism. Cell Metab. 2014;20:26–40. doi: 10.1016/j.cmet.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J., Tan H., Zheng Q., Fu F., Liang Y., Zhang J., Yang X., Wang T., Chong K., Wang X.J., et al. LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol. 2008;148:1042–1054. doi: 10.1104/pp.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesi N., Jond C., Debeaujon I., Caboche M., Lepiniec L. The arabidopsis TT2 gene encodes and R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell. 2001;13:2099–2114. doi: 10.2307/3871430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., Wu G.Z., Ye R., Lin W.H., Shi Q.M., Xue L.J., Xu X.D., Li Y., Du Y.G., Xue H.W. Global analysis of gene expression profiles in Brassica napus developing seeds reveals a conserved lipid metabolism regulation with Arabidopsis thaliana. Mol. Plant. 2009;2:1107–1122. doi: 10.1093/mp/ssp042. [DOI] [PubMed] [Google Scholar]
- Ogas J., Cheng J.C., Sung Z.R., Somerville C. Cellular differentiation regulated by gibberellin in the Arabidopsis thaliana pickle mutant. Science. 1997;277:91–94. doi: 10.1126/science.277.5322.91. [DOI] [PubMed] [Google Scholar]
- Ogas J., Kaufmann S., Henderson J., Somerville C. PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 1999;96:13839–13844. doi: 10.1073/pnas.96.24.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F., Valon C., Kohara A., Misera S., Giraudat J. The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell. 1997;9:1265–1277. doi: 10.2307/3870380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Oh D.H., Dassanayake M., Nguyen K.T., Ogas J., Choi G., Sun T.P. Gibberellin signaling requires chromatin remodeler PICKLE to promote vegetative growth and phase transitions. Plant Physiol. 2017;173:1463–1474. doi: 10.1104/pp.16.01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J.M., Kwong R.W., Park S., Le B.H., Baden R., Cagliari A., Hashimoto M., Munoz M.D., Fischer R.L., Goldberg R.B., et al. LEC1 sequentially regulates the transcription of genes involved in diverse developmental processes during seed development. Proc. Natl. Acad. Sci. U S A. 2017;114:E6710–E6719. doi: 10.1073/pnas.1707957114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pireyre M., Burow M. Regulation of MYB and bHLH transcription factors: a glance at the protein level. Mol. Plant. 2015;8:378–388. doi: 10.1016/j.molp.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Ramsay N.A., Glover B.J. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005;10:63–70. doi: 10.1016/j.tplants.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Remy W., Taylor T.N., Hass H., Kerp H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Natl. Acad. Sci. U S A. 1994;91:11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerie W.G., Feldmann K.A., Marks M.D. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev. 1994;8:1388–1399. doi: 10.1101/gad.8.12.1388. [DOI] [PubMed] [Google Scholar]
- Rich M.K., Vigneron N., Libourel C., Keller J., Xue L., Hajheidari M., Radhakrishnan G.V., Le Ru A., Diop S.I., Potente G., et al. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science. 2021;372:864–868. doi: 10.1126/science.abg0929. [DOI] [PubMed] [Google Scholar]
- Rohde A., Kurup S., Holdsworth M. ABI3 emerges from the seed. Trends Plant Sci. 2000;5:418–419. doi: 10.1016/s1360-1385(00)01736-2. [DOI] [PubMed] [Google Scholar]
- Roscoe T.T., Guilleminot J., Bessoule J.J., Berger F., Devic M. Complementation of seed maturation phenotypes by ectopic expression of ABSCISIC ACID INSENSITIVE3, FUSCA3 and LEAFY COTYLEDON2 in arabidopsis. Plant Cell Physiol. 2015;56:1215–1228. doi: 10.1093/pcp/pcv049. [DOI] [PubMed] [Google Scholar]
- Ruta V., Longo C., Lepri A., De Angelis V., Occhigrossi S., Costantino P., Vittorioso P. The DOF transcription factors in seed and seedling development. Plants (Basel) 2020;9:218. doi: 10.3390/plants9020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter A.J., Thomas K.L., Herbert D., Henderson R.J., Lloyd D., Harwood J.L. Oxygen induction of a novel fatty acid n-6 desaturase in the soil protozoon, Acanthamoeba castellanii. Biochem. J. 2002;368:57–67. doi: 10.1042/BJ20020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska S.A., Schwender J., Ohlrogge J.B. The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol. 2004;136:2700–2709. doi: 10.1104/pp.104.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska S.A., Girke T., Benning C., Ohlrogge J.B. Contrapuntal networks of gene expression during arabidopsis seed filling[W] Plant Cell. 2002;14:1191–1206. doi: 10.1105/tpc.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S., Wang M., Dedaldechamp F., Perez-Garcia M.D., Oge L., Hamama L., Atanassova R. The sugar-signaling hub: overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018;1910:2506. doi: 10.3390/ijms19092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjaya, Durrett T.P., Weise S.E., Benning C. Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnol. J. 2011;9:874–883. doi: 10.1111/j.1467-7652.2011.00599.x. [DOI] [PubMed] [Google Scholar]
- Santos Mendoza M., Dubreucq B., Miquel M., Caboche M., Lepiniec L. LEAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Lett. 2005;579:4666–4670. doi: 10.1016/j.febslet.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Itoh H., Gomi K., Ueguchi-Tanaka M., Ishiyama K., Kobayashi M., Jeong D.H., An G., Kitano H., Ashikari M., et al. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science. 2003;299:1896–1898. doi: 10.1126/science.1081077. [DOI] [PubMed] [Google Scholar]
- Schulte L.R., Ballard T., Samarakoon T., Yao L., Vadlani P., Staggenborg S., Rezac M. Increased growing temperature reduces content of polyunsaturated fatty acids in four oilseed crops. Ind. Crops Prod. 2013;51:212–219. doi: 10.1016/j.indcrop.2013.08.075. [DOI] [Google Scholar]
- Schwender J., Goffman F., Ohlrogge J.B., Shachar-Hill Y. Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds. Nature. 2004;432:779–782. doi: 10.1038/nature03145. [DOI] [PubMed] [Google Scholar]
- Seiler G.J. Analysis of the relationships of environmental factors with seed oil and fatty acid concentrations of wild annual sunflower. Field Crops Res. 1986;15:57–72. doi: 10.1016/0378-4290(86)90101-2. [DOI] [Google Scholar]
- Serivichyaswat P., Ryu H.S., Kim W., Kim S., Chung K.S., Kim J.J., Ahn J.H. Expression of the floral repressor miRNA156 is positively regulated by the AGAMOUS-like proteins AGL15 and AGL18. Mol. Cells. 2015;38:259–266. doi: 10.14348/molcells.2015.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Sinkevicius K.W., Selinger D.A., Tarczynski M.C. The homeobox gene GLABRA2 affects seed oil content in Arabidopsis. Plant Mol. Biol. 2006;60:377–387. doi: 10.1007/s11103-005-4110-1. [DOI] [PubMed] [Google Scholar]
- Shen B., Allen W.B., Zheng P., Li C., Glassman K., Ranch J., Nubel D., Tarczynski M.C. Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol. 2010;153:980–987. doi: 10.1104/pp.110.157537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Katavic V., Yu Y., Kunst L., Haughn G. Arabidopsis glabra2 mutant seeds deficient in mucilage biosynthesis produce more oil. Plant J. 2012;69:37–46. doi: 10.1111/j.1365-313X.2011.04768.x. [DOI] [PubMed] [Google Scholar]
- Singer S.D., Zou J., Weselake R.J. Abiotic factors influence plant storage lipid accumulation and composition. Plant Sci. 2016;243:1–9. doi: 10.1016/j.plantsci.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Song G., Li X., Munir R., Khan A.R., Azhar W., Yasin M.U., Jiang Q., Bancroft I., Gan Y. The WRKY6 transcription factor affects seed oil accumulation and alters fatty acid compositions in Arabidopsis thaliana. Physiol. Plant. 2020;169:612–624. doi: 10.1111/ppl.13082. [DOI] [PubMed] [Google Scholar]
- Song J., Xie X., Chen C., Shu J., Thapa R.K., Nguyen V., Bian S., Kohalmi S.E., Marsolais F., Zou J., et al. LEAFY COTYLEDON1 expression in the endosperm enables embryo maturation in Arabidopsis. Nat. Commun. 2021;12:3963. doi: 10.1038/s41467-021-24234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.M., Zhang Y., Zhou Z.W., Lu S., Ma W., Lu C., Chen L.L., Guo L. Oil plant genomes: current state of the science. J. Exp. Bot. 2021;10:1093. doi: 10.1093/jxb/erab472. [DOI] [PubMed] [Google Scholar]
- Song Q.X., Li Q.T., Liu Y.F., Zhang F.X., Ma B., Zhang W.K., Man W.Q., Du W.G., Wang G.D., Chen S.Y., et al. Soybean GmbZIP123 gene enhances lipid content in the seeds of transgenic Arabidopsis plants. J. Exp. Bot. 2013;64:4329–4341. doi: 10.1093/jxb/ert238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S.L., Kwong L.W., Yee K.M., Pelletier J., Lepiniec L., Fischer R.L., Goldberg R.B., Harada J.J. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl. Acad. Sci. U S A. 2001;98:11806–11811. doi: 10.1073/pnas.201413498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X., Singh S.K., Patra B., Schluttenhofer C., Guo W., Pattanaik S., Yuan L. Cross-family transcription factor interaction between MYC2 and GBFs modulates terpenoid indole alkaloid biosynthesis. J. Exp. Bot. 2018;69:4267–4281. doi: 10.1093/jxb/ery229. [DOI] [PubMed] [Google Scholar]
- Sun R., Ye R., Gao L., Zhang L., Wang R., Mao T., Zheng Y., Li D., Lin Y. Characterization and ectopic expression of CoWRI1, an AP2/EREBP domain-containing transcription factor from coconut (Cocos nucifera L.) endosperm, changes the seeds oil content in transgenic Arabidopsis thaliana and rice (oryza sativa L.) Front. Plant Sci. 2017;8:63. doi: 10.3389/fpls.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S.M., Singh D.P. Tall tales from sly dwarves: novel functions of gibberellins in plant development. Trends Plant Sci. 2005;10:123–129. doi: 10.1016/j.tplants.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Taatjes D.J. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem. Sci. 2010;35:315–322. doi: 10.1016/j.tibs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H., Yang X., Zhang F., Zheng X., Qu C., Mu J., Fu F., Li J., Guan R., Zhang H., et al. Enhanced seed oil production in canola by conditional expression of Brassica napus LEAFY COTYLEDON1 and LEC1-LIKE in developing seeds. Plant Physiol. 2011;156:1577–1588. doi: 10.1104/pp.111.175000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Zhao H., Lu S., Yu L., Zhang G., Zhang Y., Yang Q.Y., Zhou Y., Wang X., Ma W., et al. Genome- and transcriptome-wide association studies provide insights into the genetic basis of natural variation of seed oil content in Brassica napus. Mol. Plant. 2021;14:470–487. doi: 10.1016/j.molp.2020.12.003. [DOI] [PubMed] [Google Scholar]
- Thakare D., Tang W., Hill K., Perry S.E. The MADS-domain transcriptional regulator AGAMOUS-LIKE15 promotes somatic embryo development in Arabidopsis and soybean. Plant Physiol. 2008;146:1663–1672. doi: 10.1104/pp.108.115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.G., Sun T.P. Update on gibberellin signaling. A tale of the tall and the short. Plant Physiol. 2004;135:668–676. doi: 10.1104/pp.104.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R., Wang F., Zheng Q., Niza V.M.A.G.E., Downie A.B., Perry S.E. Direct and indirect targets of the arabidopsis seed transcription factor ABSCISIC ACID INSENSITIVE3. Plant J. 2020;103:1679–1694. doi: 10.1111/tpj.14854. [DOI] [PubMed] [Google Scholar]
- Tiedemann J., Rutten T., Monke G., Vorwieger A., Rolletschek H., Meissner D., Milkowski C., Petereck S., Mock H.P., Zank T., et al. Dissection of a complex seed phenotype: novel insights of FUSCA3 regulated developmental processes. Dev. Biol. 2008;317:1–12. doi: 10.1016/j.ydbio.2008.01.034. [DOI] [PubMed] [Google Scholar]
- To A., Joubes J., Barthole G., Lecureuil A., Scagnelli A., Jasinski S., Lepiniec L., Baud S. WRINKLED transcription factors orchestrate tissue-specific regulation of fatty acid biosynthesis in arabidopsis. Plant Cell. 2012;24:5007–5023. doi: 10.1105/tpc.112.106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga-Wada R., Ishida T., Wada T. New insights into the mechanism of development of Arabidopsis root hairs and trichomes. Int. Rev. Cell Mol. Biol. 2011;286:67–106. doi: 10.1016/B978-0-12-385859-7.00002-1. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y., Nambara E., Naito S., McCourt P. The FUS3 transcription factor functions through the epidermal regulator TTG1 during embryogenesis in Arabidopsis. Plant J. 2004;37:73–81. doi: 10.1046/j.1365-313x.2003.01939.x. [DOI] [PubMed] [Google Scholar]
- Vanhercke T., El Tahchy A., Shrestha P., Zhou X.R., Singh S.P., Petrie J.R. Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett. 2013;587:364–369. doi: 10.1016/j.febslet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Vanhercke T., Dyer J.M., Mullen R.T., Kilaru A., Rahman M.M., Petrie J.R., Green A.G., Yurchenko O., Singh S.P. Metabolic engineering for enhanced oil in biomass. Prog. Lipid Res. 2019;74:103–129. doi: 10.1016/j.plipres.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Vanhercke T., El Tahchy A., Liu Q., Zhou X.R., Shrestha P., Divi U.K., Ral J.P., Mansour M.P., Nichols P.D., James C.N., et al. Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol. J. 2014;12:231–239. doi: 10.1111/pbi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier J., Thompson R.D. Transcriptional regulation of storage protein synthesis during dicotyledon seed filling. Plant Cell Physiol. 2008;49:1263–1271. doi: 10.1093/pcp/pcn116. [DOI] [PubMed] [Google Scholar]
- Walker A.R., Davison P.A., Bolognesi-Winfield A.C., James C.M., Srinivasan N., Blundell T.L., Esch J.J., Marks M.D., Gray J.C. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell. 1999;11:1337–1350. doi: 10.2307/3870753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Perry S.E. Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development. Plant Physiol. 2013;161:1251–1264. doi: 10.1104/pp.112.212282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.W., Zhang B., Hao Y.J., Huang J., Tian A.G., Liao Y., Zhang J.S., Chen S.Y. The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seeds of transgenic Arabidopsis plants. Plant J. 2007;52:716–729. doi: 10.1111/j.1365-313X.2007.03268.x. [DOI] [PubMed] [Google Scholar]
- Weselake R.J., Shah S., Tang M., Quant P.A., Snyder C.L., Furukawa-Stoffer T.L., Zhu W., Taylor D.C., Zou J., Kumar A., et al. Metabolic control analysis is helpful for informed genetic manipulation of oilseed rape (Brassica napus) to increase seed oil content. J. Exp. Bot. 2008;59:3543–3549. doi: 10.1093/jxb/ern206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western T.L., Burn J., Tan W.L., Skinner D.J., Martin-McCaffrey L., Moffatt B.A., Haughn G.W. Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol. 2001;127:998–1011. doi: 10.1104/pp.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R.B., Cavins J.F., Kleiman R., Black L.T. Effect of temperature on soybean seed constituents: oil, protein, moisture, fatty acids, amino acids and sugars. J. Am. Oil Chemists’ Soc. 1982;59:230–232. doi: 10.1007/bf02582182. [DOI] [Google Scholar]
- Woodward A.W., Bartel B. Auxin: regulation, action, and interaction. Ann. Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Shanklin J. Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu. Rev. Plant Biol. 2016;67:179–206. doi: 10.1146/annurev-arplant-043015-111641. [DOI] [PubMed] [Google Scholar]
- Xue L., Klinnawee L., Zhou Y., Saridis G., Vijayakumar V., Brands M., Dormann P., Gigolashvili T., Turck F., Bucher M. AP2 transcription factor CBX1 with a specific function in symbiotic exchange of nutrients in mycorrhizal Lotus japonicus. Proc. Natl. Acad. Sci. U S A. 2018;115:E9239–E9246. doi: 10.1073/pnas.1812275115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Kagaya Y., Usui H., Hobo T., Takeda S., Hattori T. Diverse roles and mechanisms of gene regulation by the Arabidopsis seed maturation master regulator FUS3 revealed by microarray analysis. Plant Cell Physiol. 2010;51:2031–2046. doi: 10.1093/pcp/pcq162. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S. The Dof family of plant transcription factors. Trends Plant Sci. 2002;7:555–560. doi: 10.1016/s1360-1385(02)02362-2. [DOI] [PubMed] [Google Scholar]
- Yang Y., Munz J., Cass C., Zienkiewicz A., Kong Q., Ma W., Sedbrook J., Benning C. Ectopic expression of WRINKLED1 affects fatty acid homeostasis in Brachypodium distachyon vegetative tissues. Plant Physiol. 2015;169:1836–1847. doi: 10.1104/pp.15.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Liu X., Wang K., Li Z., Jia Q., Zhao C., Zhang M. ABI3 with or without FUS3 highly up-regulates lipid droplet proteins and activates oil accumulation. J. Exp. Bot. 2021;73:2077–2092. doi: 10.1093/jxb/erab524. [DOI] [PubMed] [Google Scholar]
- Yang Z., Liu X., Li N., Du C., Wang K., Zhao C., Wang Z., Hu Y., Zhang M. WRINKLED1 homologs highly and functionally express in oil-rich endosperms of oat and castor. Plant Sci. 2019;287:110193. doi: 10.1016/j.plantsci.2019.110193. [DOI] [PubMed] [Google Scholar]
- Ye J., Wang C., Sun Y., Qu J., Mao H., Chua N.H. Overexpression of a transcription factor increases lipid content in a Woody perennial jatropha curcas. Front. Plant Sci. 2018;9:1479. doi: 10.3389/fpls.2018.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang H., Su T., Wu W.H., Chen Y.F. The ubiquitin E3 ligase PRU1 regulates WRKY6 degradation to modulate phosphate homeostasis in response to low-pi stress in arabidopsis. Plant Cell. 2018;30:1062–1076. doi: 10.1105/tpc.17.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Nikovics K., To A., Lepiniec L., Fedosejevs E.T., Van Doren S.R., Baud S., Thelen J.J. Docking of acetyl-CoA carboxylase to the plastid envelope membrane attenuates fatty acid production in plants. Nat. Commun. 2020;11:6191. doi: 10.1038/s41467-020-20014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap W.C., Lee F.C., Shabari Shan D.K., Musa H., Appleton D.R., Kulaveerasingam H. WRI1-1, ABI5, NF-YA3 and NF-YC2 increase oil biosynthesis in coordination with hormonal signaling during fruit development in oil palm. Plant J. 2017;91:97–113. doi: 10.1111/tpj.13549. [DOI] [PubMed] [Google Scholar]
- Zale J., Jung J.H., Kim J.Y., Pathak B., Karan R., Liu H., Chen X., Wu H., Candreva J., Zhai Z., et al. Metabolic engineering of sugarcane to accumulate energy-dense triacylglycerols in vegetative biomass. Plant Biotechnol. J. 2016;14:661–669. doi: 10.1111/pbi.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentella R., Zhang Z.L., Park M., Thomas S.G., Endo A., Murase K., Fleet C.M., Jikumaru Y., Nambara E., Kamiya Y., et al. Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell. 2007;19:3037–3057. doi: 10.1105/tpc.107.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha P., Liu S., Li Y., Ma T., Yang L., Jing Y., Lin R. The evening complex and the chromatin-remodeling factor PICKLE coordinately control seed dormancy by directly repressing DOG1 in arabidopsis. Plant Commun. 2020;1:100011. doi: 10.1016/j.xplc.2019.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z., Liu H., Shanklin J. Phosphorylation of WRINKLED1 by KIN10 results in its proteasomal degradation, providing a link between energy homeostasis and lipid biosynthesis. Plant Cell. 2017;29:871–889. doi: 10.1105/tpc.17.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z., Keereetaweep J., Liu H., Feil R., Lunn J.E., Shanklin J. Trehalose 6-phosphate positively regulates fatty acid synthesis by stabilizing WRINKLED1. Plant Cell. 2018;30:2616–2627. doi: 10.1105/tpc.18.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Bishop B., Ringenberg W., Muir W.M., Ogas J. The CHD3 remodeler PICKLE associates with genes enriched for trimethylation of histone H3 lysine 27. Plant Physiol. 2012;159:418–432. doi: 10.1104/pp.112.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Fan J., Taylor D.C., Ohlrogge J.B. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant cell. 2009;21:3885–3901. doi: 10.1105/tpc.109.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Cao X., Jia Q., Ohlrogge J. FUSCA3 activates triacylglycerol accumulation in Arabidopsis seedlings and tobacco BY2 cells. Plant J. 2016;88:95–107. doi: 10.1111/tpj.13233. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu Z., Wang X., Wang J., Fan K., Li Z., Lin W. DELLA proteins negatively regulate dark-induced senescence and chlorophyll degradation in Arabidopsis through interaction with the transcription factor WRKY6. Plant Cell Rep. 2018;37:981–992. doi: 10.1007/s00299-018-2282-9. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Ren N., Wang H., Stromberg A.J., Perry S.E. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-Like15. Plant Cell. 2009;21:2563–2577. doi: 10.1105/tpc.109.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Farnung L., Kaasinen E., Sahu B., Yin Y., Wei B., Dodonova S.O., Nitta K.R., Morgunova E., Taipale M., et al. The interaction landscape between transcription factors and the nucleosome. Nature. 2018;562:76–81. doi: 10.1038/s41586-018-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Wei Y., Jako C., Kumar A., Selvaraj G., Taylor D.C. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 1999;19:645–653. doi: 10.1046/j.1365-313x.1999.00555.x. [DOI] [PubMed] [Google Scholar]