Abstract
Sweetpotato (Ipomoea batatas (L.) Lam.) is one of the most important root crops cultivated worldwide. Because of its adaptability, high yield potential, and nutritional value, sweetpotato has become an important food crop, particularly in developing countries. To ensure adequate crop yields to meet increasing demand, it is essential to enhance the tolerance of sweetpotato to environmental stresses and other yield-limiting factors. The highly heterozygous hexaploid genome of I. batatas complicates genetic studies and limits improvement of sweetpotato through traditional breeding. However, application of next-generation sequencing and high-throughput genotyping and phenotyping technologies to sweetpotato genetics and genomics research has provided new tools and resources for crop improvement. In this review, we discuss the genomics resources that are available for sweetpotato, including the current reference genome, databases, and available bioinformatics tools. We systematically review the current state of knowledge on the polyploid genetics of sweetpotato, including studies of its origin and germplasm diversity and the associated mapping of important agricultural traits. We then outline the conventional and molecular breeding approaches that have been applied to sweetpotato. Finally, we discuss future goals for genetic studies of sweetpotato and crop improvement via breeding in combination with state-of-the-art multi-omics approaches such as genomic selection and gene editing. These approaches will advance and accelerate genetic improvement of this important root crop and facilitate its sustainable global production.
Keywords: sweetpotato, polyploid genetics, genomics, breeding
This review provides insights into recent progress in genomics resources, polyploid genetics, and conventional and molecular breeding approaches for sweetpotato. The application of genetic studies to promote crop improvement via breeding is also discussed.
Introduction
Sweetpotato (Ipomoea batatas (L.) Lam., 2n = 6x = 90) is one of the most important root crops cultivated worldwide with an annual production of ∼113 million tons (Food and Agriculture Organization of the United Nations, 2019). Because of its high yield potential and ability to adapt to a wide range of environmental conditions, sweetpotato has become an affordable source of dietary calories, protein, fiber, minerals, vitamins, and flavonoids (Woolfe, 1992; Padmaja, 2009), particularly in developing countries. Currently, orange-fleshed sweetpotato plays an important role in combating vitamin A deficiency in Africa (Kurabachew, 2015). Sweetpotato is not only cultivated for human food (storage roots and leaves) and animal feed but also as an industrial raw material to produce starch, alcohol, and natural pigments (Padmaja, 2009; Katayama et al., 2017). Development of cultivars that combine disease and pest resistance with high yields and enhanced nutritional value is essential to meet the increasing demands for quality foodstuff and industrial raw products.
Research in genetics and genomics has enabled the application of molecular breeding strategies to many crop species (Liu et al., 2020; Nawaz and Chung, 2020; Mores et al., 2021). However, genetic analysis and breeding of sweetpotato have been challenging. First, sweetpotato is a highly heterozygous hexaploid (2n = 6x = 90) and has a large, complex genome approximately 2–3 Gb in size (Ozias-Akins and Jarret, 1994). The presence of multiple homoeo-alleles across homoeologous chromosomes and the resulting complex inheritance patterns complicate estimation of allele dosages (Dufresne et al., 2014). Accurate estimation of allele dosages is important for precise mapping of genetic loci (Rosyara et al., 2016; Bourke et al., 2018; da Silva Pereira et al., 2020). Second, a large number of possible genotypes are observed in segregating populations because of the diverse combinations of parental chromosomes provided by the male and female gametes (Cervantes-Flores et al., 2008a; Yamakawa et al., 2021). The number of potential allelic combinations in F1 progeny plants is estimated to be 1.1 × 1039, as calculated by if we consider sweetpotato to be an autohexaploid. The multitude of diverse combinations poses a significant challenge to genetic mapping and also results in laborious and time-consuming efforts to screen progeny. A large number of hybrid lines are needed in the early stages of a sweetpotato breeding program, and one cultivar is typically released every 10 years after extensive field evaluations and line selections (Kumagai, 2001). Third, sweetpotato plants are almost always self-incompatible and sometimes cross-incompatible. Cross-incompatibility restricts breeding progress when parental lines with desirable traits belong to the same incompatibility group (Vimala and Hariprakash, 2011; Gurmu et al., 2013). Finally, compared with diploid crops, the availability of genomics tools and resources for sweetpotato has lagged behind, mainly because of the complexity of the genome and the relatively small number of scientists working on sweetpotato.
Because of the development of next-generation sequencing (NGS) technologies, the availability of genomics resources, and advances in breeding methodologies, significant improvements to sweetpotato have been realized over the past two decades (Figure 1). The initial genetic map, constructed in 1997 (Ukoskit and Thompson, 1997), served as the basis for subsequent genetic studies that resulted in the identification of a series of agriculturally important traits. This was accomplished using various approaches, such as mapping of quantitative trait loci (QTLs) (Mwanga et al., 2002; Li et al., 2014; da Silva Pereira et al., 2020), application of bulked segregant analysis (BSA) (Nakayama et al., 2012; Suematsu et al., 2021; Yamakawa et al., 2021), and genome-wide association studies (GWASs) (Zhang et al., 2016; Okada et al., 2019; Chen et al., 2021). A genome sequencing project was formally initiated in 2012. In 2015, the first reference genome of the wild diploid sweetpotato relative Ipomoea trifida was published (Hirakawa et al., 2015), and the sweetpotato reference genome was released in 2017 (Yang et al., 2017a). Since then, the number of genetic and genomics studies has increased, and a number of bioinformatics tools/pipelines for sweetpotato have been developed. These include Ranbow, GBSapp, ngsAssocPoly, MAPpoly, QTLpoly, OutcrossSeq, and haplotype-based phylogenetic analysis (HPA) (Wadl et al., 2018; Moeinzadeh et al., 2020; Mollinari et al., 2020; da Silva Pereira et al., 2020; Yamamoto et al., 2020; Chen et al., 2021; Yamakawa et al., 2021; Yan et al., 2021). Over the past few decades, traditional breeding and molecular breeding approaches have been widely applied to sweetpotato and have resulted in the development of many new cultivars with improved yield, nutrient content, pest resistance, and other quality factors (Newell et al., 1995; Okada et al., 2001; Otani et al., 2003; Gao et al., 2011b; Wang et al., 2019). Several advances in sweetpotato breeding have been achieved; these include a synthetic hexaploid derived from wild species (Shiotani and Kawase, 1987), the first transgenic sweetpotato plant (Otani et al., 1998), an accelerated breeding scheme (ABS) (Grüneberg et al., 2009) and somatic hybrids (Yang et al., 2009), CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9)-mediated mutagenesis via genome editing (Wang et al., 2019), and, most recently, the development of genomic selection (GS) methods (Gemenet et al., 2020a).
Figure 1.
Milestones in sweetpotato genetics and breeding
Chronological achievements in the development of genetics tools/techniques and breeding are summarized. The references (Eserman et al., 2014; Schafleitner et al., 2010; Wang et al., 2010) are given in Supplemental Table 1. QTL, quantitative trait locus; GWAS, genome-wide association study; CRISPR-Cas9, clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9.
Genomics resources in sweetpotato and its wild relatives
Sweetpotato reference genomes
A high-quality genome sequence assembly is crucial for molecular breeding in any crop species. The initial sweetpotato genome sequence was obtained using the carotenoid-rich cultivar ‘Taizhong 6’ (Yang et al., 2017a). This genome sequence was assembled using only Illumina short DNA sequencing reads (Table 1). Fifteen pseudochromosomes were ultimately generated based on gene synteny between this haplotype-improved I. batatas assembly and the Ipomoea nil genome (Hoshino et al., 2016). The novel haplotyping method of Yang et al. (2017a) enabled the first successful genomic analysis of the complex hexaploid chromosomal composition of I. batatas using polyploid DNA sequencing data. This assembly (version 2017) is available at http://public-genomes-ngs.molgen.mpg.de/SweetPotato/. To improve the sweetpotato genome assembly, it was re-sequenced using nanopore sequencing (Oxford Nanopore Technologies) and 10X Genomics techniques (Table 1). The long-read assembly was then anchored onto chromosomes using the linkage map (Rastas, 2019). This assembly (version 2019) is available at http://sweetpotao.com/.
Table 1.
Genomes and databases for the genus Ipomoea.
| Species | Ploidy | Materials | Sequencing platform | References | Total length of scaffoldsa (bp) | N50 of scaffolds (bp) | Number of scaffolds | Genome sizeb (Mb) | Number of genes | Link |
|---|---|---|---|---|---|---|---|---|---|---|
| I. batatas | hexaploid | Taizhong6 (version 2017) | Illumina, Roche 454 | Yang et al., 2017a, 2017b | 836,316,092 | 200,728 | 35,919 | 851.4 | 78,781 | Ipomoea batatas Genome Browser (http://public-genomes-ngs.molgen.mpg.de/SweetPotato/) |
| I. batatas | hexaploid | Taizhong6 (version 2019) | Nanopore, 10X Genomics | Rastas (2019) | 728,240,131 | 77,499 | 3934 | 473.8 | 64,295 | Ipomoea Genome Hub (http://sweetpotao.com/) |
| I. trifida | diploid | Mx23Hm | Illumina | Hirakawa et al. (2015) | 512,990,885 | 42,586 | 77,400 | 515.8 | 62,407 | Sweetpotato GARDEN (http://sweetpotato-garden.kazusa.or.jp/) |
| I. trifida | diploid | 0431-1 | Illumina | Hirakawa et al. (2015) | 712,155,587 | 36,283 | 181,194 | 539.9 | 109,449 | Sweetpotato GARDEN(http://sweetpotato-garden.kazusa.or.jp/) |
| I. trifida | diploid | NCNSP0306 | Illumina, PacBio, BioNano | Wu et al. (2018) | 461,997,559 | 1,237,020 | 30,394 | 526.4 | 44,158 | Sweetpotato Genomics Resource (http://sweetpotato.plantbiology.msu.edu/) |
| I. trifida | diploid | Y22 | Illumina, PacBio | Li et al. (2019) | 460,931,543 | 607,924 | 5264 | 476.4 | 30,227 | NCBI (PRJNA362521) |
| I. triloba | diploid | NCNSP0323 | Illumina, PacBio, BioNano | Wu et al. (2018) | 457,835,428 | 6,861,300 | 4008 | 495.9 | 47,008 | Sweetpotato Genomics Resource (http://sweetpotato.plantbiology.msu.edu/) |
| I. nil | diploid | Tokyo Kokei standard | Illumina, PacBio | Hoshino et al. (2016) | 734,061,355 | 4,082,476 | 3367 | 734.8 | 42,783 | Ipomoea nil (http://viewer.shigen.info/asagao/) |
| I. purpurea | diploid | field sample | Illumina, PacBio, Sequel | Gupta et al. (2021) | - | 5.77M | 402 | 602.0 | 53,973 | CoGe platform (https://genomevolution.org/coge/GenomeInfo.pl?gid=58735) |
| I. aquatica | diploid | HNUWS001 | Illumina, PacBio | Hao et al. (2021) | 513,191,976 | 2.75M | 1392 | 550.0 | 30,693 | BIGD (PRJCA002216) |
Scaffold refers to the assembly version of contigs before chromosome-scale processing.
Genome size and gene number are calculated based on the final assembly version (chromosome level, if available).
In 2012, six organizations from China, Korea, and Japan established the Trilateral Research Association of Sweetpotato genome sequencing consortium. In 2019, a draft genome of the sweetpotato cultivar ‘Xushu 18’ was assembled using PacBio and Illumina reads and chromosome-scale scaffolding using BioNano and high-throughput chromosome conformation capture (Hi-C) (Yoon, 2019). Yoon (2019) constructed a complete genome assembly of the sweetpotato cultivar ‘Xushu 18’ using DeNovoMAGIC. Their most recent assembly versions are currently unpublished.
Reference genomes of wild sweetpotato relatives
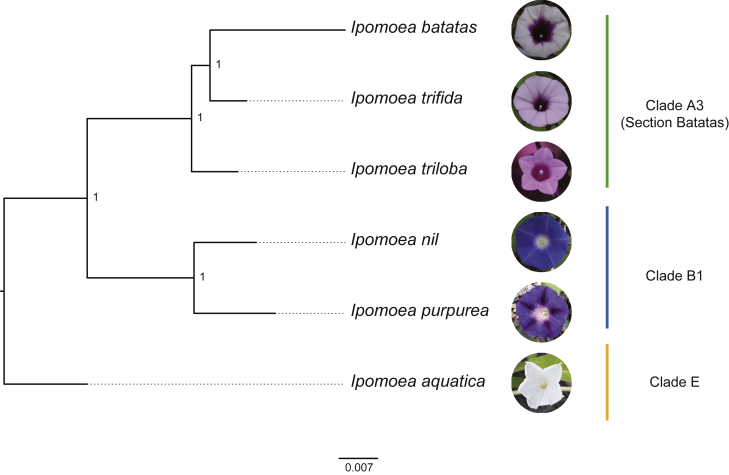
Crop wild relatives (CWRs) possess many agriculturally important traits and harbor a wealth of genetic diversity (Acosta-Gallegos et al., 2007). The CWRs of cultivated sweetpotato have the potential to contribute to breeding objectives. Over the last few decades, the CWRs of cultivated sweetpotato have been used in studies of yield, drought tolerance, and disease resistance (Khoury et al., 2015). There are approximately 800 species in the genus Ipomoea (Wood et al., 2020), and these are loosely considered to be the CWRs of cultivated sweetpotato. The New World Ipomoea species consist of five phylogenetic clades, designated clades A–E (Wood et al., 2020). The cultivated sweetpotato belongs to a subclade of clade A, clade A3, also known as Ipomoea section Batatas. Therefore, CWRs within section Batatas (such as I. trifida and Ipomoea triloba) are closely related to the sweetpotato (Diaz et al., 1996; Figure 2). The genetic relationships between sweetpotato and the CWRs in the other four clades decrease in order from clade B to clade E. Given their history of contributing to our understanding of the sweetpotato genome, reference genomes of its CWRs are important for evolutionary studies as well as improvement of the cultivated sweetpotato. At present, reference genomes of five sweetpotato CWRs are available (Table 1).
Figure 2.
The phylogeny of sweetpotato and its wild relatives
The phylogenetic relationships were inferred from a concatenated alignment matrix of 9776 single-copy ortholog sequences across six Ipomoea species with published genomes. Values at the nodes indicate bootstrap support (1 = 100%). The phylogenetic clades are based on those of Wood et al. (2020). Shown are photographs of Ipomoea flowers by Yuqin Wang (I. triloba and I. aquatica), M.Y. (I. purpurea), and Ming Li (I. batatas, I. trifida, and I. nil).
I. trifida, a wild species in section Batatas (Figure 2), is likely to be the diploid progenitor of sweetpotato (Jarret et al., 1992; Yang et al., 2017a). The assembly of the much smaller diploid I. trifida genome was used as an indirect means of viewing the much larger and more complicated genome of the hexaploid crop species I. batatas. Hirakawa et al. (2015) performed the first de novo genome assembly of two lines of diploid I. trifida (Mx23Hm and 0431-1) using Illumina DNA sequencing reads (Table 1). Although the genomes were not chromosome-level assemblies, they were the first reference genomes of any sweetpotato CWR and provided the foundation for future studies of the hexaploid sweetpotato genome (Hirakawa et al., 2015). Wu et al. (2018) published the first chromosome-level reference genome of I. trifida (Table 1). They used Illumina paired-end and mate-pair reads in the assembly. PacBio long reads and BioNano genome maps were used for gap filling and to improve the assembly, respectively. The total length of the pseudomolecules was 373.4 Mb (80.8% of the assembly in 461 scaffolds anchored to 15 pseudomolecules) (Wu et al., 2018). Li et al. (2019) also reported a chromosome-level genome assembly of an I. trifida variety (Y22) (Table 1). They integrated whole-genome shotgun reads, single-molecule long reads (PacBio RS II), and genotyping-by-sequencing (GBS) genetic maps. This I. trifida genome represents a vast improvement over previous genomes because of the high quality of the assembly (Li et al., 2019). For example, evidence of naturally occurring horizontal gene transfer was identified in this genome, and it therefore represents a vehicle for the analysis of genome evolution in the genus Ipomoea.
I. triloba also belongs to section Batatas and is a close CWR of sweetpotato (Figure 2). A chromosome-level reference genome of I. triloba has been published (Wu et al., 2018) (Table 1). This reference genome was assembled using Illumina paired-end and mate-pair reads and was improved with PacBio reads, BioNano genome maps, and a linkage map. The total length of the pseudomolecules was 443.3 Mb (96.8% of the assembly in 216 scaffolds).
I. nil, the Japanese morning glory, is a popular ornamental plant with significant commercial value that has been used as a model species for analysis of the photoperiodic flowering response (Hoshino et al., 2016). I. nil nests in phylogenetic clade B1 and is therefore relatively distantly related to sweetpotato (Figure 2). Hoshino et al. (2016) sequenced and assembled the genome of this species using PacBio long reads (Table 1). Residual errors were identified and corrected using Illumina reads. The authors constructed linkage maps for all 15 chromosomes using single-nucleotide polymorphisms (SNPs) as markers. This assembly was the first pseudochromosome-level genome assembly in the botanical family Convolvulaceae.
Ipomoea purpurea, a species that also belongs to clade B1 (Figure 2), is a common noxious field weed that is distributed worldwide. Its genome was assembled from PacBio Sequel whole-genome shotgun data (Gupta et al., 2021; Table 1). In total, the 602-Mb genome assembly consists of 402 scaffolds with a scaffold N50 of 5.77 Mb. The assembly was collapsed into the expected 15 haploid chromosomes by performing pseudomolecule scaffolding with a Phase Genomics Hi-C map. Approximately 53,973 genes were annotated from the I. purpurea genome.
Ipomoea aquatica (also known as water spinach) is consumed as a popular vegetable in East, South, and Southeast Asia (Wu et al., 2018). I. aquatica is a semi-aquatic tropical plant that has medicinal value (Malalavidhane et al., 2001, 2003). I. aquatica belongs to clade E in the phylogenetic tree (Figure 2) and is a distant CWR of sweetpotato. The I. aquatica genome was assembled de novo using PacBio sequencing and 10X Genomics reads (Hao et al. 2021). The 162 scaffolds (415.77 Mb) were anchored to 15 pseudochromosomes using a high-density genetic map (Table 1).
Databases
Ipomoea batatas Genome Browser
The Ipomoea batatas genome browser (http://public-genomes-ngs.molgen.mpg.de/SweetPotato/) is an early database created by the Max Planck Institute for Molecular Genetics that provides access to the sweetpotato genome (version 2017) published by Yang et al. (2017a). Genomic sequences and RNA sequencing (RNA-seq) data are available to download. A browser for the sweetpotato genome and BLAT (the BLAST-like alignment tool) can be used to search for required information (Supplemental Table 2).
Ipomoea Genome Hub
The phased sweetpotato genome (version 2019) is available through the Ipomoea Genome Hub (http://sweetpotao.com/), which was jointly established by the Shanghai Chenshan Botanical Garden, the Max Planck Institute for Molecular Genetics, and the Chinese Academy of Sciences. It provides optimized reference genome annotation, RNA-seq, and expressed sequence tag (EST) data for sweetpotato. The JBrowse genome browser, haplotypes of sweetpotato, and the Ipomoea Atlas are available on this platform, which has descriptions and photos of 146 species in the genus Ipomoea (Supplemental Table 2).
Sweetpotato GARDEN
This database (http://sweetpotato-garden.kazusa.or.jp/), built by the Kazusa DNA Research Institute, contains the reference genome of the wild diploid species I. trifida which was published in 2015 (Hirakawa et al., 2015). Two different lines (Mx23Hm and 0431-1) are available for Basic Local Alignment Search Tool (BLAST) searches. It also provides genetic and Kyoto Encyclopedia of Genes and Genomes (KEGG) maps for further application (Supplemental Table 2).
Sweetpotato Genomics Resource (SGR)
This database (http://sweetpotato.plantbiology.msu.edu/) contains the reference genomes and gene expression profile information for two wild diploid species, I. trifida and I. triloba (Wu et al., 2018). The SGR database was built by the Genomic Tools for Sweetpotato team. The pseudomolecules, annotation, RNA-seq data, and gene expression data for these two species can be downloaded from this database. A JBrowse genome browser and many tools, such as BLAST, Gene Ontology (GO), ID and keyword search, and pfam accession ID search, are also available (Supplemental Table 2).
Ipomoea nil
This site (http://viewer.shigen.info/asagao/) provides access to the I. nil genome assembly (Hoshino et al., 2016). This database was established by The Morning Glory Genome Consortium. The JBrowse, BLAST/BLAT, and keyword searches are available. This database enables users to download genome, gene, annotation, RNA-seq, and transposon data for I. nil (Supplemental Table 2).
Polyploid genetics of sweetpotato
The origin of sweetpotato
The origin of sweetpotato and its polyploid genome have been debated for years. Three polyploidization hypotheses have been proposed.
-
(1)
The autopolyploid hypothesis posits that sweetpotato is an autopolyploid and that I. trifida is its only wild ancestor (Figure 3A and 3B). This hypothesis is supported by polysomic inheritance based on genetic linkage analysis (Ukoskit and Thompson, 1997; Kriegner et al., 2003; Cervantes-Flores et al., 2008a; Zhao et al., 2013a; Mollinari et al., 2020), phylogenetic analysis (Roullier et al., 2013; Muñoz-Rodríguez et al., 2018), and cytogenetic analyses (Shiotani, 1988; Shiotani and Kawase, 1989). Based on a phylogenetic analysis of chloroplast genomes, a hybridization event between sweetpotato and I. trifida that occurred after speciation of sweetpotato resulted in chloroplast capture from I. trifida (Roullier et al., 2013; Muñoz-Rodríguez et al., 2018; Figure 3B).
-
(2)
The second origin hypothesis suggests that sweetpotato is a segmental allopolyploid that carries three partially differentiated subgenomes originally from I. trifida, Ipomoea tenuissima, and Ipomoea littoralis, based on an analysis of 811 conserved single-copy genes (Gao et al., 2020; Figure 3C).
-
(3)
The third allopolyploid hypothesis suggests that sweetpotato was formed by hybridization between two progenitor species but that the identities of the progenitor species are controversial. This allopolyploid hypothesis is supported by cytogenetic evidence because two of the genomes show closer homology to one another than to the third (Magoon et al., 1970). Nishiyama (1971) suggested that sweetpotato originated from I. trifida 3x, which is a hybrid between Ipomoea × leucantha and I. littoralis based on cytogenetic analysis (Figure 3D). Based on morphological data, Austin (1988) concluded that the cultivated sweetpotato is derived from a hybridization event between I. trifida and I. triloba (Figure 3E). From an analysis of nucleotide sequence variation in the Waxy gene intron, Gao et al. (2011a) suggested that sweetpotato arose via hybridization between I. tenuissima and I. littoralis (Figure 3F). Based on phylogenetic analyses of homologous haplotypes, it is likely that sweetpotato arose from a cross between I. trifida and tetraploid I. batatas 4x (Yang et al., 2017a; Yan et al., 2021; Figure 3G). Recently, Muñoz-Rodríguez et al. (2022) described an autotetraploid relative from Ecuador as a new species, Ipomoea aequatoriensis, and identified it as the tetraploid progenitor of sweetpotato based on morphological and phylogenetic analyses (Figure 3H).
Figure 3.
Hypotheses to describe the origin of cultivated sweetpotato
(A) The autopolyploid hypothesis suggests that I. trifida is the only progenitor of sweetpotato. Adapted from Shiotani (1988).
(B) Sweetpotato originated from I. trifida, and after the speciation of sweetpotato, a hybridization event occurred between sweetpotato and I. trifida. Adapted from Muñoz-Rodríguez et al. (2018).
(C) The species tree embodying the two hybridization networks demonstrates the segmental allopolyploid hypothesis. From Gao et al. (2020).
(D) The allopolyploid hypothesis of Nishiyama (1971): sweetpotato is derived from a triploid I. trifida that arose from hybridization of Ipomoea ×leucantha and I. littoralis.
(E) The allopolyploid hypothesis of Austin (1988): sweetpotato arose via hybridization between I. trifida and I. triloba.
(F) The allopolyploid hypothesis of Gao et al. (2011a, 2011b): sweetpotato arose via hybridization between I. tenuissima and I. littoralis.
(G) The allopolyploid hypothesis of Yang et al. (2017a) and Yan et al. (2021): sweetpotato is likely to be derived from a triploid that arose from a cross between I. trifida and I. batatas 4x.
(H) The allopolyploid hypothesis of Muñoz-Rodríguez et al. (2022): I. aequatoriensis arose from a whole-genome duplication in I. trifida. Sweetpotato (I. batatas) is likely to be derived from a cross between I. trifida and I. aequatoriensis. Subsequent introgression between I. trifida and sweetpotato resulted in chloroplast capture from I. trifida.
A phylogenetic analysis based on a haplotype-resolved genome showed that the six sets of sweetpotato chromosomes are divided into two clusters that, in turn, are composed of two sets and four sets (Yang et al., 2017a). This is consistent with the B1B1B2B2B2B2 genomic formula of sweetpotato proposed by Shiotani and Kawase (1987) based on cytogenetic analyses. Genomic and cytogenetic analyses suggest that sweetpotato arose from a cross between a diploid and a tetraploid progenitor (Shiotani and Kawase, 1987; Yang et al., 2017a). The diploid progenitor is most likely I. trifida, whereas the identity of the tetraploid progenitor remains under debate. An extant tetraploid relative (tentatively identified as I. batatas 4x) shows a closer relationship with sweetpotato than does I. trifida (Jarret et al., 1992; Jarret and Austin, 1994; Rajapakse et al., 2004; Srisuwan et al., 2006; Feng et al., 2018).
Kyndt et al., 2015 reported the discovery of two horizontally transferred DNA (T-DNA) sequences from Agrobacterium spp., IbT-DNA1 and/or IbT-DNA2, that are integrated into the genomes of sweetpotato and some of its wild relatives (Kyndt et al., 2015; Quispe-Huamanquispe et al., 2019). These horizontally transferred DNA sequences were suggested to be natural genetic markers that are useful for identifying the origin of the cultivated sweetpotato and its relationship to its CWRs (Quispe-Huamanquispe et al., 2019). Yan et al. (2021) analyzed sweetpotato and representative wild tetraploid relatives that contain IbT-DNA insertions from all relevant geographic locations. Their results suggest that I. batatas 4x consists of two lineages, the Ecuador lineage and the basal lineage. The Ecuador lineage is the sister group of cultivated sweetpotato, and the basal lineage is the probable tetraploid progenitor of sweetpotato, a conclusion supported by phylogenetics based on both homologous haplotypes and whole genome-wide variations. Based on morphological and phylogenetic analyses of nuclear DNA and the chloroplast genome, Muñoz-Rodríguez et al. (2022) have described the Ecuador lineage of I. batatas 4x as a new species, I. aequatoriensis, and identified this lineage as the tetraploid progenitor of sweetpotato. In addition, they consider the other tetraploid lineage related to the basal lineage of Yan et al. (2021) as being a hybrid between sweetpotato and I. trifida. Therefore, the tetraploid progenitor of sweetpotato is still controversial. The key to confirming the tetraploid progenitor will be to establish an effective method or standard to distinguish the possible tetraploid progenitor and the hybrid offspring, and it is crucial to include data from real or simulated hybrids between sweetpotato and I. trifida in future studies.
The B1 and B2 subgenomes of sweetpotato retain a high degree of similarity (Magoon et al., 1970). Therefore, polysomic inheritance in sweetpotato (with some preferential pairing) resembles that of an autohexaploid (Ukoskit and Thompson, 1997; Kriegner et al., 2003; Cervantes-Flores et al., 2008a; Zhao et al., 2013a; Mollinari et al., 2020). Thus, sweetpotato has been treated as an autohexaploid during genetic map construction and genetic mapping (Mollinari et al., 2020; da Silva Pereira et al., 2020; Yamamoto et al., 2020; Chen et al., 2021).
Genetic diversity analysis of sweetpotato germplasm
Sweetpotato germplasm collections, such as those maintained by the International Potato Center (CIP) and the United States Department of Agriculture (USDA), can acquire and conserve an increasing number of I. batatas genotypes. In this effort, knowledge about the genetic diversity maintained in gene banks facilitates the most efficient use of resources and improves the general understanding of how the genetic relationships of sweetpotato crop genetic resources can be used in support of breeding and related activities (Buteler et al., 2002; Hu et al., 2003; Campos and Caligari, 2017). Traditionally, biochemical and morphological markers have been used to characterize sweetpotato diversity (Huamán et al., 1999). However, although morphological data (descriptors) provide useful information, their use has certain limitations, and biochemical markers often have low levels of polymorphism. In both instances, the expression of morphological traits and biochemical markers tends to be influenced by the plant growth stage(s) and various environmental factors (Nadeem et al., 2018).
Beginning in the 1990s, molecular markers based on allelic polymorphisms were developed, and they have been widely utilized to estimate genetic diversity in sweetpotato. Early studies of sweetpotato used a variety of markers, including restriction fragment length polymorphisms (RFLPs), random amplified polymorphic DNA (RAPD) (Jarret and Austin, 1994; Gichuki et al., 2003), amplified fragment length polymorphisms (AFLPs) (Zhang et al., 2000, 2004), inter-simple sequence repeats (ISSRs) (Li et al., 2008; Moulin et al., 2012), ESTs (Marian et al., 2018), and simple sequence repeats (SSRs) (Veasey et al., 2008; Som et al., 2014; Yang et al., 2015; Zawedde et al., 2015; Feng et al., 2018; Lee et al., 2019; Palumbo et al., 2019; Anglin et al., 2021). These markers have been used to characterize and define the accessions in sweetpotato germplasm collections and to examine population structure and gene pools of sweetpotato originating from Latin America, South America, Central America, Oceania, Asia, and Africa (Zhang et al., 2000; Gichuki et al., 2003; Li et al., 2008; Som et al., 2014).
Over the past decade, improvements in high-throughput DNA sequencing technology have enabled the identification of huge numbers of SNPs in the genomes of crop plants. The effectiveness of SNP analysis in detecting polymorphisms is much greater than that of other classes of molecular markers, and there are many ways to analyze genome-wide SNP data (Khlestkina and Salina, 2006). SNP markers have been adopted for certification of sweetpotato cultivars and breeding lines (Supplemental Table 3). Specific length amplified fragment sequencing (SLAF-seq) has been used to identify genome-wide SNPs in sweetpotato and to assess the population structure and genetic diversity of 197 sweetpotato accessions, most of which were from China (Su et al., 2017). Restriction site-associated DNA sequencing (RAD-seq) was also used to analyze the relationships among sweetpotato accessions and different ploidy forms of I. trifida (Feng et al., 2018), as well as the genetic relationships among 81 sweetpotato accessions (Feng et al., 2020). Wadl et al. (2018) used GBSpoly, an optimized GBS protocol for highly heterozygous and polyploid genomes, to determine the population structure and genetic diversity within a subset of the USDA sweetpotato collection. Wu et al. (2018) reported the first whole-genome re-sequencing (WGRS) study in sweetpotato and revisited the phylogeny and genetic diversity of the 16 sweetpotato cultivars in the Mwanga diversity panel (MDP). Using WGRS data, Yan et al. (2021) examined the relationships among the diploid and tetraploid wild relatives of sweetpotato and hexaploid sweetpotato cultivars based on consensus genome-wide variation analysis and an HPA pipeline.
Functional genomics and genetic mapping
Functional genomics studies are essential for marker-assisted breeding and crop improvement using genome editing (Monden and Tahara, 2017; Zhang et al., 2017). In sweetpotato, forward genetics approaches, such as QTL mapping, GWAS, and BSA, have been used to map economically important genes or genomic regions. The mapped genes include those affecting yield, root quality, biotic resistance, and various morphological traits (Table 2). Storage root yield is potentially the most important agronomic trait in sweetpotato. Thus, a number of independent studies have been conducted that focused on root yield and/or the number of storage roots per plant, and these studies identified many associated QTLs (Chang et al., 2009; Li et al., 2014; Yada et al., 2017; Okada et al., 2019; da Silva da Silva Pereira et al., 2020; Chen et al., 2021). The expansin gene IbEXP4A has been suggested as a candidate gene for determining the number of roots per plant (Chen et al., 2021). Storage root thickening is another important characteristic of interest to sweetpotato breeders. Suematsu et al. (2021) detected a major QTL (qRT1) on chromosome 6 (chr06) that regulates root thickness in I. trifida. The pattern of storage root formation and bulking is important when the crop is harvested. Commercial farming can require synchronized maturity or discontinuous storage root formation and bulking (DCSRFAB) at harvest. Multiple harvesting, which is predominantly practiced in sub-Saharan Africa, requires cultivars with continuous storage root formation and bulking (CSRFAB) to ensure availability of product over a longer growing season. Bararyenya et al. (2020) identified 12 and seven candidate genes that were annotated for CSRFAB and DCSRFAB, respectively.
Table 2.
Overview of trait association studies in sweetpotato.
| Trait type | Trait | Population | Candidate loci | Markers | Method | Software | Reference |
|---|---|---|---|---|---|---|---|
| Yield | number of roots per plant (SS), weight of roots per plant (SZ), biomass in early stage (SZS) | 1807 F1 progenies derived from Yuzi7 × Xu18 | 4 QTLs (IbEXP4A) for SS; 2 QTLs for SZ; 1 QTL for SZS | 1,670,312 SNPs (WGRS) | GWAS | OutcrossSeq | Chen et al. (2021) |
| number of commercial roots per plant (NOCRs), number of noncommercial roots per plant (NONCs), total number of storage roots per plant (TNRs), commercial root yield (CYTHA), total root yield (RYTHA), foliage yield (FYTHA) | 315 F1 progenies derived from Beauregard × Tanzania | 2 QTLs for NOCR; 4 QTLs for NONC; 4 QTLs for TNR; 1 QTL for CYTHA; 1 QTL for RYTHA; 1 QTL for FYTHA | 30,684 SNPs (GBSpoly) | QTL | QTLpoly | Pereira et al. (2020) | |
| root yield | 92 F1 progenies derived from PSL × 90IDN-47 | 1 peak in 90IDN-47 and 2 peaks in PSL for yield | 17,940 SNPs | GWAS | R Package rrBLUP 4.6 | Okada et al. (2019) | |
| root yield | 287 F1 progenies derived from New Kawogo × Beauregard | 12 QTLs | 250 SSRs | GWAS | SAS 9.4 | Yada et al. (2017) | |
| root yield | 202 F1 progenies derived from Xushu 18 × Xu 781 | 4 QTLs in Xushu 18 and 5 QTLs in Xu 781 | Xushu 18: 1936 AFLPs and 141 SSRs; Xu 781: 1824 AFLPs and 130 SSRs | QTL | MapQTL 4.0 | Li et al. (2014) | |
| top weight (TW), root weight (RW), root number (RN) | NT: 119 F1 progenies derived from Nancy Hall (♀) × Tainung 27 (♂) TN: 112 F1 progenies derived from Tainung 27 (♀) × Nancy Hall (♂) | 1 QTL in population NT and 3 QTLs in population TN for TW; 2 QTLs in population NT and 1 QTL in population TN for RW; 1 QTL in population NT for RN | 100 ISSRs | QTL | QTL Cartographer | Chang et al. (2009) | |
| Root development | root thickness | I. trifida BC1F1 population | 1 major QTL on chr06 (2.94–8.71 Mb) | SNPs (WGRS) | BSA | QTLseqr 0.7.5.2 | Suematsu et al. (2021) |
| continuous storage formation and bulking (CSRFAB), discontinuous storage root formation and bulking (DCSRFAB) | 358 sweetpotato genotypes | 13 SNPs and 13 candidate genes (CSLD1, NHH, P-LNPHP, CmlP, HVA22, PLLSP, Amp-M1, KAKU4, P-RID, SAG, CLE-related, MAP3K-like, and NF-Y) for CSRFAB; 5 SNPs and 5 candidate genes (TRO-Z, DUF803, hypothetical, AGC-Kinase, and GBLD) for DCSRFAB | 33,068 SNPs (DArTseq) | GWAS | R package GAPIT 3.0 | Bararyenya et al. (2020) | |
| Quality | amylase activity in the whole meal (amylase), dry-matter content (DMC), tuber surface smoothness (PC), root surface color (PS), root flesh color (RS) | 1807 F1 progenies derived from Yuzi7 × Xu18 | 1 QTL for amylase; 2 QTLs for DMC; 2 QTLs for PC; 1 QTL for PS; 1 QTL for RS | 1,670,312 SNPs (WGRS) | GWAS | OutcrossSeq | Chen et al. (2021) |
| anthocyanin content | 94 F1 progenies derived from Konaishin × Akemurasaki | 1 major QTL (Myb-related gene) | 15,430 and 15,403 Akemurasaki-specific and Konaishin-specific simplex SNPs (WGRS) | BSA | QTL-seq 1.4.4 | Yamakawa et al. (2021) | |
| DMC, starch content (SC), β-carotene content (BC), flesh color (FC) | 315 F1 progenies derived from Beauregard × Tanzania | 5 QTLs for DM; 2 QTLs for SC; 2 QTLs for BC (phytoene synthase gene, 15-cis-phytoene desaturase violaxanthin de-epoxidase and ORANGE); 3 QTLs for FC (phytoene synthase gene, 15-cis-phytoene desaturase violaxanthin de-epoxidase and lycopene β-cyclase) | 30,684 SNPs (GBSpoly) | QTL | QTLpoly | Gemenet et al. (2020b) | |
| FC (related to anthocyanin content) | 104 sweetpotato accessions with anthocyanin variation in root flesh | chr12: 17,809,090–23,930,961 (IbMYB1-2) | 724,438 SNPs (88 transcriptomes/16 WGRS) | GWAS/eQTL | EMMAX | Zhang et al. (2020a) | |
| anthocyanin content | 94 F1 progenies derived from Konaishin × Akemurasaki | Genes on LGs 2, 5, 10–15 (IbF3H, IbDFR, IbMYB1, IbWD40, IbUF3GT, IbCHI, IbCHS, IbANS, and IbbHLH3) | 59,675 SNPs (ddRAD-Seq) | GWAS/QTL | ngsAssocPoly/ MapQTL 6.0 | Haque et al., (2020b) | |
| β-carotene content (BC), DMC, starch content (SC) | 52 F1 progenies derived from J-Red × Choshu | 3 QTLs in J-Red and 2 QTLs in Choshu for BC; 2 QTLs in J-Red for DM; 2 QTLs in J-Red and 3 QTLs in Choshu for SC | J-Red: 5952 SNPs (ddRAD-Seq), 228 retrotransposon-based markers, and 161 SSRs; Choshu: 5640 SNPs (ddRAD-Seq), 192 retrotransposon-based markers, and 176 SSRs | GWAS/QTL | TASSEL 5.2.49/MapQTL 6.0 | Haque et al., (2020a) | |
| root skin color (RSC), internode length (LI) | F1 progenies derived from Xushu18 × K123-11 (KX-F1); self-pollinated progenies of Xushu18 (X18-S1) | 1 peak in KX-F1 and 1 peak in X18-S1 for RSC; 1 peak in KX-F1 and 1 peak in X18-S1 for LI | SNPs (ddRAD-Seq) | GWAS | ngsAssocPoly | Yamamoto et al. (2020) | |
| LI, root skin thickness (RST), main RSC (MRSC), secondary RSC (SRSC) | 137 F1 progenies derived from Yeseumi × Annobeny | 3 QTLs for LI; 1 QTL for RST; 15 QTLs for MRSC; 2 QTLs for SRSC | 210 SSRs | QTL | WinQTL Cartographer 2.5 | Kim et al. (2017) | |
| BC, DMC, starch content (SC) | 287 F1 progenies derived from New Kawogo × Beauregard | 8 QTLs for BC; 4 QTLs for DM; 6 QTLs for SC | 250 SSRs | GWAS | SAS 9.4 | Yada et al. (2017) | |
| Dry-matter and starch content (DMSC), BC, starch composition (SCP) | 239 genotypes have various morphological types and geographical origins | 32 QTLs for DMSC; 16 QTLs for BC; 17 QTLs for SC | 887 SSRs | GWAS | TASSEL 5.0 | Zhang et al., (2016) | |
| starch content | 202 F1 progenies derived from Xushu 18 × Xu 781 | 2 QTLs in Xushu 18 and 6 QTLs in Xu 781 | Xushu 18: 1936 AFLPs and 141 SSRs; Xu 781: 1824 AFLPs and 130 SSRs | QTL | MapQTL 4.0 | Yu et al., (2014) | |
| DMC | 202 F1 progenies derived from Xushu 18 × Xu 781 | 5 QTLs in Xushu 18 and 22 QTLs in Xu 781 | Xushu 18: 1936 AFLPs and 141 SSRs; Xu 781: 1824 AFLPs and 130 SSRs | QTL | MapQTL 4.0 | Zhao et al., (2013a) | |
| BC, DMC, starch content (SC) | 240 F1 progenies derived from Tanzania × Beauregard | 3 positive QTLs and 1 negative QTL in Beauregard and 3 positive QTLs and 1 negative QTL in Tanzania for BC; 4 positive QTLs and 4 negative QTLs in Beauregard and 4 positive QTLs and 1 negative QTL in Tanzania for DM; 3 positive QTLs and 4 negative QTLs in Beauregard and 3 positive QTLs and 2 negative QTLs in Tanzania for SC | Beauregard: 726 AFLPs; Tanzania: 927 AFLPs | QTL | WinQTL Cartographer | Cervantes-Flores et al. (2011) | |
| BC | 55 genotypes were selected from 73 F1 genotypes developed from the maternal clones Beauregard, Excel, L94-96, L89-110, L86-33, and L96-117 | 17 markers were selected by discriminant analysis, and 9 markers were selected by logistic regression | 259 AFLPs | DA/LRG | SAS | Mwamburi Mcharo and LaBonte (2010) | |
| RSC, FC, root shape (RS) | NT: 119 F1 progenies derived from Nancy Hall (♀) × Tainung 27 (♂) TN: 112 F1 progenies derived from Tainung 27 (♀) × Nancy Hall (♂) | 3 QTLs in population NT and 3 QTLs in population TN for RSC; 4 QTLs in population NT and 2 QTLs in population TN for FC; 3 QTLs in population NT for RS | 100 ISSRs | QTL | QTL Cartographer | Chang et al. (2009) | |
| basal branching number (FZS), diameter of the stem between the fifth and sixth expanded leaves (JC), length of the stem between the fifth and sixth expanded leaves (JJC), color of the stem between the fifth and sixth expanded leaves (JS), color of the leaf-petiole conjunction part of the sixth expanded leaf (MJS), length of petiole in the sixth expanded leaf (YBC), color of veins in the sixth expanded leaf (YMS), leaf shape of the sixth expanded leaf (YX) | 1807 F1 progenies derived from Yuzi7 × Xu18 | 2 QTLs for FZS; 4 QTLs for JC; 1 QTL for JJC; 1 QTL (IbMYB1) for JS; 1 QTL (IbMYB1) for MJS; 2 QTLs for YBC; 1 QTL (IbMYB1) for YMS; 2 QTLs (IbFBW2) for YX | 1,670,312 SNPs (WGRS) | GWAS | OutcrossSeq | Chen et al. (2021) | |
| Biotic resistance | root rot (caused by Fusarium solani) | 300 F1 progenies derived from Jizishu 1 × Longshu 9 | 6 QTLs in Jizishu 1 and 2 QTLs in Longshu 9 | Jizishu 1: 484 SSRs; Longshu 9: 573 SSRs | QTL | MapQTL 5.0 | Ma et al. (2020) |
| weevil resistance (WS) | 92 F1 progenies derived from PSL × 90IDN-47 | 1 peak in 90IDN-47 and 1 peak in PSL for WS | 17,940 SNPs (ddRAD-Seq) | GWAS | R Package rrBLUP 4.6 | Okada et al. (2019) | |
| root-knot nematode resistance | 113 F1 progenies derived from J-Red × Choshu | 3 QTLs in J-Red and 1 QTL in Choshu | J-Red: 5952 SNPs (ddRAD-Seq), 228 retrotransposon-based markers, and 161 SSRs; Choshu: 5640 SNPs (ddRAD-Seq), 192 retrotransposon-based markers, and 176 SSRs | GWAS/QTL | TASSEL 5.0/ MapQTL 6.0 | Sasai et al. (2019) | |
| stem nematode resistance | 196 F1 progenies derived from Xushu 18 × Xu 781 | 4 markers displayed linkage to the Rsn1 gene | 200 SRAPs | BSA | Map Manager QTXb17 | Zhao et al., (2013b) | |
| root-knot nematode resistance | 92 F1 progenies derived from Koganesengan × Hi-starch | 9 AFLP markers that were present in resistant bulked DNAs and absent in the susceptible bulks; 1 QTL was mapped to the region around E33M53_090 | 360 AFLPs | BSA/QTL | MapQTL 5.0 | Nakayama et al. (2012) | |
| root-knot nematode resistance | 240 F1 progenies derived from Tanzania × Beauregard | 7 QTLs in Tanzania and 2 QTLs in Beauregard | 947 AFLPs | QTL | WinQTL Cartographer | Cervantes-Flores et al. (2008b) | |
| root-knot nematode resistance | 48 F1 genotypes developed in LSU and 55 F1 progenies developed in CIP | 5 and 4 AFLP markers that had a strong and significant association with respect to the resistance trait in the LSU and CIP populations | LSU population: 229 AFLPs; CIP population: 220 AFLPs | DA/LRG | SAS | Mcharo et al. (2005a) | |
| sweetpotato virus disease resistance (SPVD) | 92 genotypes had mild or no symptoms of SPVD severity | 4 were selected by discriminant analysis and logistic regression statistical methods | 350 AFLPs | DA/LRG | SAS | Miano et al. (2008) | |
| sweetpotato chlorotic stunt virus resistance (SPCSV), sweetpotato feathery mottle virus resistance (SPFMV) | 87 F1 progenies derived from Tanzania × Wagabolige | 7 QTLs for SPCSV; 4 QTLs for SPFMV | 232 AFLPs and 37 RAPD markers | QTL/DA/LRG | QGENE/SAS | Mcharo et al. (2005b) | |
| SPCSV, SPFMV, sweetpotato virus disease resistance (SPVD) | F1 progenies derived from Tanzania × Wagabolige | 9 QTLs for SPCSV; 4 QTLs for SPFMV; 7 QTLs for SPVD | Tanzania: 330 AFLPs; Wagabolige: 252 AFLPs | QTL | QGENE | Mwanga et al. (2002) |
LSU, Louisiana State University; CIP, International Potato Center; QTL, quantitative trait locus; SNP, single-nucleotide polymorphism; AFLP, amplified fragment length polymorphism; GBS, genotyping by sequencing; ddRAD-Seq, Double-digest restriction-site associated DNA; SSR, simple sequence repeat; ISSR, inter-simple sequence repeat; WGRS, whole-genome re-sequencing; DArTseq, deamination adjacent to RNA modification targets; SRAP, sequence-related amplified polymorphism; RAPD, random amplified polymorphic DNA; GWAS, genome-wide association study; BSA, bulked segregant analysis; eQTL, expression quantitative trait locus; DA, discriminant analysis; LRG, logistic regression.
Sweetpotatoes are used for a wide variety of purposes; therefore, the quality traits of sweetpotato are diverse. Dry-matter content and starch content are two related quality traits (Gemenet et al., 2020b), and both of them contribute to eating quality as well as to starch and ethanol production (Yamakawa, 1998; Kitahara et al., 2017). Many QTLs have been identified as being associated with these traits that include dry-matter content (Cervantes-Flores et al., 2011; Zhao et al., 2013a; Zhang et al., 2016; Yada et al., 2017; Gemenet et al., 2020b; Haque et al., 2020a; Chen et al., 2021), starch content (Cervantes-Flores et al., 2011; Yu et al., 2014; Zhang et al., 2016; Yada et al., 2017; Haque et al., 2020a; da Silva Pereira et al., 2020), starch composition (Zhang et al., 2016), and amylase activity (Chen et al., 2021). Sweetpotato is a significant source of vitamin A (β-carotene) and anthocyanins, and breeding for improved nutritional value has become increasingly important. Several genes or genomic regions responsible for β-carotene content (Mcharo and LaBonte, 2010; Cervantes-Flores et al., 2011; Zhang et al., 2016; Yada et al., 2017; Haque et al., 2020a; Gemenet et al., 2020b), anthocyanin content (Haque et al., 2020b; Yamakawa et al., 2021), and related root flesh color (Chang et al., 2009; Zhang et al., 2020a; Gemenet et al., 2020b; Chen et al., 2021) have been mapped. QTLs associated with additional sweetpotato root traits have been investigated. These include root skin smoothness (Chen et al., 2021), root skin thickness (Kim et al., 2017), root skin color (Chang et al., 2009; Kim et al., 2017; Yamamoto et al., 2020; Chen et al., 2021), and root shape (Chang et al., 2009). QTLs associated with vine traits have also been mapped, and these include top weight, internode length, stem diameter, stem color, leaf-petiole junction color, petiole length, vein color, and leaf shape (Chang et al., 2009; Kim et al., 2017; Yamamoto et al., 2020; Chen et al., 2021).
Sweetpotato can be affected by a number of fungal, bacterial, and virus or virus-like pathogens as well as nematodes and insect pests (Martin and Jones, 1986; Clark et al., 2013). Diseases and pest infestations can decrease the yield and quality of the sweetpotato crop (Jansson and Raman, 1991; Ma et al., 2020). Therefore, QTLs associated with resistances to economically important pests and diseases have been studied. These include resistance to sweetpotato weevil (Okada et al., 2019), root-knot nematode (Mcharo et al., 2005a; Cervantes-Flores et al., 2008b; Nakayama et al., 2012; Sasai et al., 2019), stem nematode (Zhao et al., 2013b), sweetpotato virus disease (Mwanga et al., 2002; Mcharo et al., 2005b; Miano et al., 2008), and root rot caused by the ascomycete fungus Fusarium solani (Ma et al., 2020).
New genetics tools for polyploid sweetpotato
Sweetpotato is a suitable model for studying the genetics of a complex polyploid crop species. In recent years, many bioinformatics tools and pipelines have been developed for sweetpotato (Table 3). Although these genetics tools were developed for sweetpotato, they can also be applied to other polyploid species. Haplotype phasing facilitates research on polyploid genetics, physiology, evolution, and crop breeding (Zhang et al., 2020b), and a number of related tools have been developed. For example, Ranbow, a tool for haplotype reconstruction of polyploid genomes from short-read sequencing data, has been used successfully to phase half of the hexaploid genome of sweetpotato (Yang et al., 2017a; Moeinzadeh et al., 2020). Recently, the HPA pipeline, which takes full advantage of the homologous variation in polyploids to disentangle complex relationships, was used to identify the closest tetraploid genotype related to sweetpotato (Yan et al., 2021). Bioinformatics programs have been developed for polyploid genetics and genetic mapping. These include tools for allele dosage estimation (GBSapp [Wadl et al., 2018] and ngsAssocPoly [Yamamoto et al., 2020]), genomic imputation (OutcrossSeq; Chen et al., 2021), genetic map construction (MAPpoly; Mollinari et al., 2020), QTL mapping (QTLpoly [da Silva Pereira et al., 2020] and polyploid QTL-seq [Yamakawa et al., 2021]), and GWAS using low-coverage resequencing data via ngsAssocPoly (Yamamoto et al., 2020) and OutcrossSeq (Chen et al., 2021). Using very low-coverage resequencing data (1.5×) from an F1 population, OutcrossSeq successfully identified genomic regions associated with multiple agricultural traits (Chen et al., 2021).
Table 3.
Bioinformatics tools for genetic studies in sweetpotato.
| Software/pipeline | Purpose | Object | Reference | Link |
|---|---|---|---|---|
| HPA | genetic relationship detection between polyploids using homologous haplotypes | polyploid | Yan et al. (2021) | https://github.com/YanMengxiao/HPA |
| Ranbow | polyploid haplotype reconstruction | polyploid | Moeinzadeh et al. (2020) | https://github.com/moeinzadeh/Ranbow.git |
| Polyploid QTL-seq | polyploid QTL analysis by whole-genome resequencing (BSA-based) | autopolyploid | Yamakawa et al. (2021) | |
| Autopolyploid-Plant module of OutcrossSeq | GWAS in F1 population with whole-genome low-coverage resequencing data | autopolyploid | Chen et al. (2021) | https://github.com/xhhuanglab/OutcrossSeq |
| ngsAssocPoly | GWAS with low-coverage NGS-based genotyping data | autopolyploid | Yamamoto et al. (2020) | https://github.com/yame-repos/ngsAssocPoly |
| MAPpoly | genetic map construction | autopolyploid | Mollinari et al. (2020) | https://github.com/mmollina/MAPpoly |
| QTLpoly | QTL mapping in F1 population | autopolyploid | da Silva Pereira et al. (2020) | https://github.com/guilherme-pereira/qtlpoly |
| GBSapp | SNP calling, filtering, and allele dosage calling | polyploid | Wadl et al. (2018) | https://github.com/bodeolukolu/GBSapp |
HPA, haplotype-based phylogenetic analysis; QTL, quantitative trait locus; BSA, bulked segregant analysis; GWAS, genome-wide association study; NGS, next-generation sequencing; SNP, single-nucleotide polymorphism.
Progress in sweetpotato breeding
Traditional breeding
Traditional breeding approaches that involve hybridization of selected clones, visual evaluation of progeny, and subsequent rounds of selection have been widely applied to sweetpotato. These efforts have resulted in the generation of new cultivars with improved yield, nutrient content, pest resistance, and other quality factors. This process is labor intensive and often requires evaluation of extremely large progeny populations. Because of cross-incompatibility, multiple parents are usually employed in a polycross (Jones, 1965). This prevents the identification of superior parents (Quispe-Huamanquispe et al., 2019), although molecular markers may be used to assist with their identification (Martin, 1965; Hwang et al., 2002). Currently, 16 incompatibility groups have been identified in sweetpotato (Katayama et al., 2017). Additional issues of concern include poor flowering and sterility in some environments. Sweetpotato breeding faces more challenges than normally encountered in the breeding of other crop species (Martin, 1965).
In a polycross breeding strategy, 20–30 or more parental lines are selected and used to produce 10,000–100,000 true seeds. These seeds provide the progeny that are subsequently used for gene pool separation and selection. In a typical polycross nursery, the male parent of the selected progeny is often unknown. In the past century, polycross strategies have been successfully used in sweetpotato breeding programs at Louisiana State University (LSU), the Xuzhou Sweetpotato Research Center in China, and the National Crops Resources Research Institute (NaCRRI) in Kampala, Uganda. The leading sweetpotato cultivar ‘Beauregard’ was developed using polycross selection and was released by LSU (Rolston et al., 1987). Between 1999 and 2003, 22 sweetpotato cultivars were released by the NaCRRI. These were also developed using a polycross selection process that included 24 parents (Mwanga et al., 2016).
To increase the chances of detecting/selecting desirable minor and recessive genes, polycrossing in combination with recurrent selection has become a popular breeding approach. In this method, a polycross population is generated by controlled crossing, and the progeny with desirable traits are selected for use as parents in a second round of polycrossing. The white-fleshed boniato-type cultivar ‘Arapey’ was developed by the Instituto Nacional de Investigación Agropecuaria in Uruguay using a polycross-recurrent selection approach (Lebot, 2010). To further increase breeding efficiency, an ABS can be used in sweetpotato. An ABS approach involves a multi-location field evaluation in the initial generation instead of multiple evaluations over a period of years in a single selection environment (Grüneberg et al., 2015).
CWRs often express higher levels of biotic and abiotic stress resistance than cultivated species or their commercial cultivars. Successful hybrid crosses between I. batatas and one or more of its wild relatives have been reported. This approach has been proposed as a means to integrate stress tolerance traits into commercial cultivars. Several sweetpotato cultivars that were derived from hybridization between I. batatas and I. trifida have been reported (Nishiyama, 1971; Shiotani and Kawase, 1987).
As an alternative to conventional hybridization that could overcome cross incompatibility and ploidy differences, somatic hybridization involving fusion of protoplasts of two species has been explored. A storage-root-producing hybrid was generated via somatic hybridization between the sweetpotato cultivar ‘Kokei 14’ and I. triloba (Yang et al., 2009). Although interspecific hybridization and somatic hybridization may ultimately prove useful in efforts to improve the crop, plant regeneration is still a bottleneck in the production of new cultivars.
Mutation breeding strategies have been used to improve the sweetpotato crop. Shoot apices from irradiated plants of the cultivar ‘Kokei 14’ were cultured to form embryogenic callus cultures. Regenerated plants exhibited higher yields and altered storage root flesh color compared with the controls (Wang et al., 2007). Shin et al. (2011) regenerated plants from irradiated stem segments that varied with respect to storage root size, shape, and yield.
Not all sweetpotato clones flower readily, profusely, or in synchrony. Various approaches have been used to promote flowering and enable hybridization to occur. An approach used successfully in some breeding programs is the utilization of a flower-inducing rootstock (Lam et al., 1959) such as Ipomoea carnea (Dukes et al., 2019). Other approaches to promote flowering include the use of growth regulators such as 2,4-dichlorophenoxyacetic acid (2,4-D), gibberellic acid (GA3), and 1-naphthaleneacetic acid (NAA) and the adjustment of photoperiod and/or temperature (Lam et al., 1959; Mubayiwa et al., 2016).
Molecular breeding
Molecular breeding offers the promise of a more rapid and efficient means of crop improvement and production of novel cultivars. In general, molecular breeding approaches can be classified into two types: (1) molecular marker-assisted breeding and (2) genome-modified breeding.
As described under “Functional genomics and genetic mapping,” molecular markers such as SSRs, RFLPs, AFLPs, and SNPs have been used in QTL mapping and GWAS analyses in sweetpotato. Gemenet et al. (2020c) developed 30 reliable SNP markers for quality assurance and control in a genomics-assisted breeding program. These markers were selected based on population structure and genetic distance. Six trait-specific markers were also selected for quality control. However, because of the complex hexaploid nature of the sweetpotato genome, successful application of marker-assisted selection (MAS) and GS has been limited (Grüneberg et al., 2009).
Genome-modified breeding (including breeding that involves transgenes and genome editing) relies on the technical feasibility of successful plant genetic transformation. Agrobacterium tumefaciens-mediated transformation of sweetpotato using embryogenic suspension cultures provides a powerful platform for genome-modified breeding in this crop (Gama et al., 1996; Yang et al., 2011). Transgenic sweetpotatoes have been generated that possess weevil resistance (expressing cowpea trypsin inhibitor), virus resistance (expressing the coat protein gene of sweetpotato feathery mottle virus), herbicide resistance (expressing the herbicide resistance bar gene), and stress tolerance (expressing the Low Osmotic Stress five gene) (Newell et al., 1995; Okada et al., 2001; Otani et al., 2003; Gao et al., 2011b). Transgenic sweetpotato plants have also been developed by endogenous gene overexpression or RNA interference (RNAi). Changes in the expression of the starch synthase I (SSI), starch branching enzyme II (SBEII), and granule-bound starch synthase I (GBSSI) genes have been shown to improve starch content (Otani et al., 2007; Zhou et al., 2015; Wang et al., 2017).
CRISPR-Cas9 gene editing technology, which allows precisely targeted genome modifications, has been used successfully to edit two starch biosynthetic pathway genes (GBSSI and SBEII) in sweetpotato. In GBSSI knockout plants, the amylose content was reduced, and in SBEII knockout plants, the amylose content was increased (Wang et al., 2019; Lyu et al., 2021).
Current challenges and future perspectives
The long-term efforts of breeders and plant scientists have resulted in the production and distribution of many high-quality sweetpotato cultivars. However, compared with other important crops, sweetpotato breeding has lagged behind because of its complex polyploid genome. With the recent explosive growth in genome sequencing and editing technologies, a combination of state-of-the-art techniques and molecular breeding strategies (Figure 4) will greatly facilitate genetic improvement of sweetpotato for sustainable global production.
Figure 4.
Strategies for sweetpotato genetics and breeding in the future
(1) Genome resources. Haplotype-phased genomes and pan-genome sequencing provide basic information for genetics and breeding. CWR, crop wild relative.
(2) Genetic mapping and marker-assisted selection (MAS). Genetic mapping enables association of specific genes, SNPs, or markers with agricultural traits and facilitates MAS. QTL, quantitative trait locus; GWAS, genome-wide association study; SNP, single-nucleotide polymorphism.
(3) Genomic selection (GS). GS targets multiple complex traits simultaneously and accelerates breeding in sweetpotato.
(4) High-throughput phenotyping. Automatic and high-throughput phenotyping technologies make it possible to handle a large number of progeny in breeding programs. UAV, unmanned aerial vehicle; AI, artificial intelligence.
(5) Conventional hybridization. Hybridization of selected clones, visual evaluation of progeny, and subsequent selection.
(6) Mutagenesis. Creating random mutations in the genome using chemical or radiation treatment with subsequent selection of plants with desirable traits.
(7) Genome editing. Precise editing of the target site using CRISPR-Cas9-based tools. The transgene cassette is removed by chromosomal segregation after self-pollination or outcrossing in subsequent generations.
(8) Multi-omics. An efficient means to identify the genes and gene networks involved in controlling a target trait. mGWAS, metabolite genome-wide association study.
Haplotype-phased genomes and pan-genomes
When assembling reference genomes, homoeologous chromosomes (chromosomes that are partially homologous because of a common ancestry) are sometimes collapsed together, and the resulting assembly is considered a “mosaic” reference. This type of assembly can result in false variants, imprecise gene annotation, and misleading biological interpretations (Korlach et al., 2017; Koren et al., 2018). Sweetpotato, as a highly heterozygous hexaploid, requires a haplotype-phased assembly to understand the molecular basis of trait variability and polyploid evolution. A haplotype-phased assembly captures all allelic variants and can be used to examine allele-specific expression. It reveals the functionally important haplotypes that are critical for the fixation of desirable traits (Zhang et al., 2020b). Although the current sweetpotato reference genome is half phased (Yang et al., 2017a), the haplotypes are too fragmented to infer biological functions. Therefore, alternate technologies, such as Hi-C and/or single-cell sequencing of gamete genomes, are required to achieve a fully haplotype-phased assembly of the sweetpotato genome.
A single reference genome cannot adequately represent the full range of genetic diversity of sweetpotato. Therefore, a pan-genome approach is required to investigate the entire gene repertoire of the crop (Golicz et al., 2016; Tao et al., 2019). The pan-genome enables the identification of genetic variants, particularly larger structural variations (SVs) such as presence/absence variants (PAVs) and copy number variants (CNVs), which play important roles in the genetic determination of agronomic traits (Lu et al., 2015; Zhao et al., 2018; Lye and Purugganan, 2019).
Phenotyping
In addition to genotypic information, phenotypic data are also critical for genetic advancement in a breeding program (Araus and Cairns, 2014). To deal with the large number of progeny plants in a breeding population, breeding programs can benefit greatly from automated high-throughput phenotyping technologies. Phenomics aims to analyze the relationship between genes and agronomic/horticultural traits in support of precision breeding (Zhao et al., 2019). High-throughput plant phenotyping platforms that use red green blue (RGB) imaging (https://www.qubitphenomics.com/rgb-structural-imaging/), chlorophyll fluorescence imaging, hyperspectral imaging, thermal imaging, and lidar, have been widely used to improve the efficiency and accuracy of phenotypic data collection (Zhao et al., 2019). In field environments, ground-based field phenotyping and unmanned aerial vehicles (UAVs) have been used. For example, the Eidgenössische Technische Hochschule Zürich field phenotyping platform, which contains a digital single-lens reflex (DSLR) camera, laser scanner, and thermal camera, was used to monitor canopy cover, canopy height, and other traits in winter wheat, maize, and soybean (Kirchgessner et al., 2017). A fixed-wing UAV was used for weed detection, estimation of net photosynthesis, and stress detection in maize, peach, and citrus plantings (Yang et al., 2017b).
Sweetpotato is a highly polymorphic crop with numerous genetically complex morphological traits (Jackson et al., 2018). With the help of RGB imaging, colorimetry, and other technologies, high-quality morphological characterization data can be obtained. Such data can be useful in identifying target traits (superior progeny) with greater accuracy. Despite their many advantages, automated, multifunctional, and high-throughput phenotyping technologies have yet to be widely applied to sweetpotato. Currently, phenotyping technologies typically focus on the aerial parts of plants. Information on the development of underground parts (such as storage roots) is important in sweetpotato. However, obtaining high-throughput phenotypic data of storage root development during the growing period clearly poses a significant challenge. UAV-based hyperspectral imagery and machine learning have been used to predict end-of-season tuber yield and tuber set in potatoes (Sun et al., 2020).
Other novel and emerging technologies have also found some applications in sweetpotatoes, including evaluation of harvested storage roots for size, shape, interior and exterior color, and insect damage. Villordon and Carroll (2002) used digital imaging to assess herbicide damage in sweetpotato storage roots. Villordon et al. (2020) used three-dimensional (3D) imaging technology to assess the surface area and volume of sweetpotato storage roots because these characteristics relate to post-harvest product recovery. Dangles and Heider (2015) evaluated high-resolution, UAV-acquired thermal infrared images for the detection of heat-tolerant varieties of sweetpotato. Other technologies, such as artificial intelligence (AI) (Vásquez-Villalobos et al., 2018) and machine learning (ML) (Villordon et al., 2010), are finding applications in the culture and processing of sweetpotato crops.
MAS and GS
MAS breeding programs use DNA-based markers that are known to be linked to desirable genes and/or favorable traits. Utilization of MAS has facilitated and accelerated the breeding of sweetpotato (Zhang et al., 2016). Several strategies, including identification of QTLs and association mapping, have been used to identify the linked markers (Table 2), and DNA marker loci linked to root quality and biotic resistance genes have been reported (Nakayama et al., 2012; Zhao et al., 2013b; Zhang et al., 2016; Sasai et al., 2019). However, to our knowledge, DNA markers have not yet been used in an MAS program with sweetpotato (Yada et al., 2017; Haque et al., 2020a). MAS is used mainly for selection of traits controlled by genes with relatively large effects. Therefore, this approach is not suitable for use with complex traits, such as yield, that are controlled by many genes or QTLs with relatively small individual effects (Xu et al., 2020).
GS utilizes genome-wide DNA markers, and it was developed for complex traits, such as yield, that are affected by a large number of genes, each of which typically has a small effect (Hickey et al., 2019; Xu et al., 2020). GS offers several advantages compared with traditional MAS. GS targets multiple traits simultaneously without the need to identify the QTLs related to the target traits and without the need for phenotyping during the later stages of breeding (Nakaya and Isobe, 2012; Hickey et al., 2019). Gemenet et al. (2020a) evaluated the sequencing depth, genotype quality, and predictive models required for accurate GS applications in sweetpotato. This was the first attempt to apply GS to sweetpotato. It is anticipated that sweetpotato breeding programs will routinely employ MAS and GS in the future.
Integration of multi-omics approaches in functional genomics studies
In the “omics” era of the life sciences, data are being generated at an unprecedented rate. These data represent layers of information that reflect various types of biological systems, including, but not limited to, the genome, transcriptome, epigenome, proteome, and metabolome (Li and Chen, 2014; Li and Yan, 2020). A multi-omics approach has already been shown to be an efficient means of characterizing complex biological systems, such as determining the genes and the gene networks involved in controlling a target trait (Li and Yan, 2020; Wu et al., 2021). This capability will support future breeding activities for the improvement of sweetpotato. To date, multi-omics studies in sweetpotato are limited. In two recent studies, integration of transcriptomic and metabolomic data facilitated the identification of genes and metabolites involved in anthocyanin accumulation and the mechanism of saccharification in tuberous roots of sweetpotato (He et al., 2020; Li et al., 2021a). Genome-wide analysis of expression QTLs (eQTLs) has also been applied to sweetpotato and was used to reveal the regulatory architecture of gene expression variation (Zhang et al., 2020a; Chen et al., 2021).
Metabolite GWAS (mGWAS) and the integration of metabolomics and transcriptomics are powerful tools for exploring the genetic and biochemical basis of plant metabolism (Alseekh et al., 2015; Fang and Luo, 2019). These combined approaches have been applied to the study of metabolite-related quality traits in many crops, including maize, rice, wheat, and tomato (Chen et al., 2014, 2020; Wen et al., 2014; Zhu et al., 2018). In sweetpotato, many important quality traits are also related to plant metabolites, including sugar, starch, anthocyanin, β-carotene, and proteins in storage roots and the content of vitamins, flavonoids, and phenolic compounds in leaves (Padmaja, 2009; Johnson and Pace, 2010). Identification of the genetic determinants of metabolite production has an important role in the improvement of sweetpotato. Integration of metabolomics, genomics, and transcriptomics data, such as mGWAS, provides a means to determine the mechanisms that regulate metabolites in sweetpotato.
Genome editing for crop improvement
Reports of CRISPR-Cas9-based genome editing first appeared in 2013 (Cong et al., 2013; Feng et al., 2013; Mao et al., 2013). Since then, genome editing technologies have proven to be powerful and efficient tools for the improvement of many crop species. At present, genome editing has been widely used to introduce/modify agronomically important traits, such as increased yield, improved nutritional quality, and resistance to biotic and abiotic stresses, in multiple crops, including rice, wheat, maize, tomato, and potato (Lu et al., 2017; Soyk et al., 2017; Tang et al., 2017; D’Ambrosio et al., 2018; Ye et al., 2018; Miao et al., 2019; Zhang et al., 2019; Zhong et al., 2019; Butt et al., 2020; Zhang et al., 2020c; Li et al., 2021b; Zhan et al., 2021). CRISPR-Cas-based genome editing has been extended to targeted mutagenesis, base editing, and precisely targeted gene/allele replacement or tagging in plants. mportantly, using CRISPR-Cas9 technology, transgenes present in the genomes of genome-edited plants can be removed by chromosomal segregation via a simple self-pollination or hybridization step. Gene editing technologies continue to be developed and utilized (Mao et al., 2013; Lu and Zhu, 2017; Lu et al., 2020).
CRISPR-Cas9 gene editing technology has been successfully applied to sweetpotato (Wang et al., 2019) to generate plants with improved starch content, demonstrating the feasibility of using this technology to improve root quality traits. As functional genomics and genetic studies continue to identify an increasing number of sweetpotato genes controlling agronomically important traits, those genes will become potential targets for genome editing.
Funding
This work was funded by the Ministry of Science and Technology of the People’s Republic of China (grants 2019YFD1000700, 2019YFD1000704-2, 2019YFD1000703, and 2019YFD1000701-2), the Shanghai Municipal Afforestation & City Appearance and Environmental Sanitation Administration (G182402, G192413, G192414, G222411, and G202402), the State Key Laboratory of Subtropical Silviculture (KF2019), the Youth Innovation Promotion Association CAS, and the Bureau of Science and Technology for Development CAS (KFJ-BRP-017-42).
Acknowledgments
We thank Dr. Ruiqing Lyu for assistance with editing the figures and Prof. Ling Yuan and Dr. David Zaitlin for help with revising the manuscript. The authors declare no conflict of interest.
Author contributions
M.Y., H.N., Y.W., X.W., and J.Z. wrote and edited the manuscript. M.Y. and H.N. designed the figures. M.Y., Y.W., and X.W. drew the original version of the figures. R.J. wrote and revised the manuscript. J.Y. and H.W. designed and revised the manuscript.
Published: May 5, 2022
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information can be found online at Plant Communications Online.
Contributor Information
Hongxia Wang, Email: hxwang@cemps.ac.cn.
Jun Yang, Email: jyang03@cemps.ac.cn.
Supplemental information
References
- Acosta-Gallegos J.A., Kelly J.D., Gepts P. Prebreeding in common bean and use of genetic diversity from wild germplasm. Crop Sci. 2007;47:S-44–S-59. doi: 10.2135/cropsci2007.04.0008ipbs. [DOI] [Google Scholar]
- Alseekh S., Tohge T., Wendenberg R., Scossa F., Omranian N., Li J., Kleessen S., Giavalisco P., Pleban T., Mueller-Roeber B., et al. Identification and mode of inheritance of quantitative trait loci for secondary metabolite abundance in tomato. Plant Cell. 2015;27:485–512. doi: 10.1105/tpc.114.132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin N.L., Robles R., Rossel G., Alagon R., Panta A., Jarret R.L., Manrique N., Ellis D.D. Genetic identity, diversity, and population structure of CIP's sweetpotato (I. batatas) germplasm collection. Front. Plant Sci. 2021;12:660012. doi: 10.3389/fpls.2021.660012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus J.L., Cairns J.E. Field high-throughput phenotyping: the new crop breeding frontier. Trends Plant Sci. 2014;19:52–61. doi: 10.1016/j.tplants.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Austin D.F. Exploration, Maintenance, and Utilization of Sweetpotato Genetic Resources. CIP; 1988. The taxonomy, evolution and genetic diversity of sweet potatoes and related wild species; pp. 27–60. [Google Scholar]
- Bararyenya A., Olukolu B.A., Tukamuhabwa P., Grüneberg W.J., Ekaya W., Low J., Ochwo-Ssemakula M., Odong T.L., Talwana H., Badji A., et al. Genome-wide association study identified candidate genes controlling continuous storage root formation and bulking in hexaploid sweetpotato. BMC Plant Biol. 2020;20:3–16. doi: 10.1186/s12870-019-2217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke P.M., Voorrips R.E., Visser R.G.F., Maliepaard C. Tools for genetic studies in experimental populations of polyploids. Front. Plant Sci. 2018;9:513. doi: 10.3389/fpls.2018.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteler M.I., LaBonte D.R., Jarret R.L., Macchiavelli R. Microsatellite-based paternity analysis in polyploid sweetpotato. J. Am. Soc. Hortic. Sci. 2002;127:392–396. doi: 10.21273/jashs.127.3.392. [DOI] [Google Scholar]
- Butt H., Rao G.S., Sedeek K., Aman R., Kamel R., Mahfouz M. Engineering herbicide resistance via prime editing in rice. Plant Biotechnol. J. 2020;18:2370–2372. doi: 10.1111/pbi.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos H., Caligari P.D.S. Springer International Publishing; 2017. Genetic Improvement of Tropical Crops. [Google Scholar]
- Cervantes-Flores J.C., Yencho G.C., Kriegner A., Pecota K.V., Faulk M.A., Mwanga R.O.M., Sosinski B.R. Development of a genetic linkage map and identification of homologous linkage groups in sweetpotato using multiple-dose AFLP markers. Mol. Breed. 2008;21:511–532. doi: 10.1007/s11032-007-9150-6. [DOI] [Google Scholar]
- Cervantes-Flores J.C., Yencho G.C., Pecota K.V., Sosinski B., Mwanga R.O.M. Detection of quantitative trait loci and inheritance of root-knot nematode resistance in sweetpotato. J. Am. Soc. Hortic. Sci. 2008;133:844–851. doi: 10.21273/jashs.133.6.844. [DOI] [Google Scholar]
- Cervantes-Flores J.C., Sosinski B., Pecota K.V., Mwanga R.O.M., Catignani G.L., Truong V.D., Watkins R.H., Ulmer M.R., Yencho G.C. Identification of quantitative trait loci for dry-matter, starch, and β-carotene content in sweetpotato. Mol. Breed. 2011;28:201–216. doi: 10.1007/s11032-010-9474-5. [DOI] [Google Scholar]
- Chang K.Y., Lo H.F., Lai Y.C., Yao P.J., Lin K.H., Hwang S.Y. Identification of quantitative trait loci associated with yield-related traits in sweet potato (Ipomoea batatas) Bot. Stud. 2009;50:43–55. [Google Scholar]
- Chen W., Gao Y., Xie W., Gong L., Lu K., Wang W., Li Y., Liu X., Zhang H., Dong H., et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat. Genet. 2014;46:714–721. doi: 10.1038/ng.3007. [DOI] [PubMed] [Google Scholar]
- Chen J., Hu X., Shi T., Yin H., Sun D., Hao Y., Xia X., Luo J., Fernie A.R., He Z., et al. Metabolite-based genome-wide association study enables dissection of the flavonoid decoration pathway of wheat kernels. Plant Biotechnol. J. 2020;18:1722–1735. doi: 10.1111/pbi.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Fan W., Ji F., Hua H., Liu J., Yan M., Ma Q., Fan J., Wang Q., Zhang S., et al. Genome-wide identification of agronomically important genes in outcrossing crops using OutcrossSeq. Mol. Plant. 2021;14:556–570. doi: 10.1016/j.molp.2021.01.003. [DOI] [PubMed] [Google Scholar]
- Clark C.A., Ferrin D.M., Smith T.P., Holmes G.J. 2nd ed. The American Phytopathological Society; St. Paul: 2013. Compendium of Sweetpotato Diseases, Pests, and Disorders. [Google Scholar]
- Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Wu X., Jiang W., Marraffini L., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangles O., Heider B. Detect the heat tolerance of sweet potatoes in Peru. PIX4D. 2015. https://www.pix4d.com/blog/detect-the-heat-tolerance-of-sweet-potatoes-in-peru
- Diaz J., Schmiediche P., Austin D.F. Polygon of crossability between eleven species of Ipomoea: section batatas (Convolvulaceae) Euphytica. 1996;88:189–200. doi: 10.1007/bf00023890. [DOI] [Google Scholar]
- Dufresne F., Stift M., Vergilino R., Mable B.K. Recent progress and challenges in population genetics of polyploid organisms: an overview of current state-of-the-art molecular and statistical tools. Mol. Ecol. 2014;23:40–69. doi: 10.1111/mec.12581. [DOI] [PubMed] [Google Scholar]
- Dukes P.D., Jones A., Schalk J.M. `Inducer’, a tree morning glory rootstock cultivar for use in breeding sweetpotatoes. HortScience. 2019;25:238–239. doi: 10.21273/hortsci.25.2.238. [DOI] [Google Scholar]
- D’Ambrosio C., Stigliani A.L., Giorio G. CRISPR/Cas9 editing of carotenoid genes in tomato. Transgenic Res. 2018;27:367–378. doi: 10.1007/s11248-018-0079-9. [DOI] [PubMed] [Google Scholar]
- Eserman L.A., Tiley G.P., Jarret R.L., Leebens-Mack J.H., Miller R.E. Phylogenetics and diversification of morning glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. Am. J. Bot. 2014;101:92–103. doi: 10.3732/ajb.1300207. [DOI] [PubMed] [Google Scholar]
- Fang C., Luo J. Metabolic GWAS-based dissection of genetic bases underlying the diversity of plant metabolism. Plant J. 2019;97:91–100. doi: 10.1111/tpj.14097. [DOI] [PubMed] [Google Scholar]
- Feng Z., Zhang B., Ding W., Liu X., Yang D.L., Wei P., Cao F., Zhu S., Zhang F., Mao Y., et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013;23:1229–1232. doi: 10.1038/cr.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.Y., Li M., Zhao S., Zhang C., Yang S.T., Qiao S., Tan W.F., Qu H.J., Wang D.Y., Pu Z.G. Analysis of evolution and genetic diversity of sweetpotato and its related different polyploidy wild species I. trifida using RAD-seq. BMC Plant Biol. 2018;18:181–212. doi: 10.1186/s12870-018-1399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Zhao S., Li M., Zhang C., Qu H., Li Q., Li J., Lin Y., Pu Z. Genome-wide genetic diversity detection and population structure analysis in sweetpotato (Ipomoea batatas) using RAD-seq. Genomics. 2020;112:1978–1987. doi: 10.1016/j.ygeno.2019.11.010. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations . 2019. FAOSTAT Statistics Database.http://www.fao.org/faostat/%20Advance%20Access [Google Scholar]
- Gama M.I.C.S., Leite R.P., Cordeiro A.R., Cantliffe D.J. Transgenic sweet potato plants obtained by Agrobacterium tumefaciens -mediated transformation. Plant Cell. Tissue Organ. Cult. 1996;46:237–244. doi: 10.1007/bf02307100. [DOI] [Google Scholar]
- Gao M., Ashu G.M., Stewart L., Akwe W.A., Njiti V., Barnes S. Wx intron variations support an allohexaploid origin of the sweetpotato [Ipomoea batatas (L.) Lam] Euphytica. 2011;177:111–133. doi: 10.1007/s10681-010-0275-z. [DOI] [Google Scholar]
- Gao S., Yuan L., Zhai H., Liu C., He S., Liu Q. Transgenic sweetpotato plants expressing an LOS5 gene are tolerant to salt stress. Plant Cell. Tissue Organ. Cult. 2011;107:205–213. doi: 10.1007/s11240-011-9971-1. [DOI] [Google Scholar]
- Gao M., Soriano S.F., Cao Q., Yang X., Lu G. Hexaploid sweetpotato (Ipomoea batatas (L.) Lam.) may not be a true type to either auto-or allopolyploid. PLoS One. 2020;15:e0229624. doi: 10.1371/journal.pone.0229624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemenet D.C., Lindqvist-Kreuze H., De Boeck B., da Silva Pereira G., Mollinari M., Zeng Z.B., Craig Yencho G., Campos H. Sequencing depth and genotype quality: accuracy and breeding operation considerations for genomic selection applications in autopolyploid crops. Theor. Appl. Genet. 2020;133:3345–3363. doi: 10.1007/s00122-020-03673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemenet D.C., da Silva Pereira G., De Boeck B., Wood J.C., Mollinari M., Olukolu B.A., Diaz F., Mosquera V., Ssali R.T., David M., et al. Quantitative trait loci and differential gene expression analyses reveal the genetic basis for negatively associated β-carotene and starch content in hexaploid sweetpotato [Ipomoea batatas (L.) Lam.] Theor. Appl. Genet. 2020;133:23–36. doi: 10.1007/s00122-019-03437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemenet D.C., Kitavi M.N., David M., Ndege D., Ssali R.T., Swanckaert J., Makunde G., Craig Yencho G., Gruneberg W., Carey E., et al. Development of diagnostic SNP markers for quality assurance and control in sweetpotato [Ipomoea batatas (L.) Lam.] breeding programs. PLoS One. 2020;15:e0232173–e0232219. doi: 10.1371/journal.pone.0232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gichuki S.T., Berenyi M., Zhang D., Hermann M., Schmidt J., Glössl J., Burg K. Genetic diversity in sweetpotato [Ipomoea batatas (L.) Lam.] in relationship to geographic sources as assessed with RAPD markers. Genet. Resour. Crop Evol. 2003;50:429–437. doi: 10.1023/a:1023998522845. [DOI] [Google Scholar]
- Golicz A.A., Batley J., Edwards D. Towards plant pangenomics. Plant Biotechnol. J. 2016;14:1099–1105. doi: 10.1111/pbi.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüneberg W., Mwanga R., Andrade M., Espinoza J. 2009. Selection Methods. Part 5: Breeding Clonally Propagated Crops. Plant Breed. Farmer Particip. Advance Access. [Google Scholar]
- Grüneberg W.J., Ma D., Mwanga R.O.M., Carey E.E., Huamani K., Diaz F., Eyzaguirre R., Guaf E., Jusuf M., Karuniawan A., et al. 2015. Advances in Sweetpotato Breeding from 1992 to 2012. [Google Scholar]
- Gupta S., Harkess A., Soble A., Van Etten M., Leebens-Mack J., Baucom R.S. Vol. 4. 2021. p. 6. (Inter-chromosomal Linkage Disequilibrium and Linked Fitness Cost Loci Influence the Evolution of Nontarget Site Herbicide Resistance in an Agricultural Weed). [Google Scholar]
- Gurmu F., Hussein S., Laing M. Self-and cross-incompatibilities in sweetpotato and their implications on breeding. Aust. J. Crop Sci. 2013;7:2074–2078. [Google Scholar]
- Hao Y., Bao W., Li G., Gagoshidze Z., Shu H., Yang Z., Cheng S., Zhu G., Wang Z. Sci. Hortic.; Amsterdam: 2021. The Chromosome-Based Genome Provides Insights into the Evolution in Water Spinach; p. 289. [Google Scholar]
- Haque E., Tabuchi H., Monden Y., Suematsu K., Shirasawa K., Isobe S., Tanaka M. QTL analysis and gwas of agronomic traits in sweetpotato (Ipomoea batatas l.) using genome wide SNPs. Breed. Sci. 2020;70:283–291. doi: 10.1270/jsbbs.19099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque E., Yamamoto E., Shirasawa K., Tabuchi H., Yoon U.H., Isobe S., Tanaka M. Genetic analyses of anthocyanin content using polyploid GWAS followed by QTL detection in the sweetpotato (Ipomoea batatas L.) storage root. Plant Root. 2020;14:11–21. doi: 10.3117/plantroot.14.11. [DOI] [Google Scholar]
- He L., Liu X., Liu S., Zhang J., Zhang Y., Sun Y., Tang R., Wang W., Cui H., Li R., et al. Transcriptomic and targeted metabolomic analysis identifies genes and metabolites involved in anthocyanin accumulation in tuberous roots of sweetpotato (Ipomoea batatas L.) Plant Physiol. Biochem. 2020;156:323–332. doi: 10.1016/j.plaphy.2020.09.021. [DOI] [PubMed] [Google Scholar]
- Hickey L.T., Hafeez A.N., Robinson H., Jackson S.A., Leal-Bertioli S.C.M., Tester M., Gao C., Godwin I.D., Hayes B.J., et al. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019;37:744–754. doi: 10.1038/s41587-019-0152-9. [DOI] [PubMed] [Google Scholar]
- Hirakawa H., Okada Y., Tabuchi H., Shirasawa K., Watanabe A., Tsuruoka H., Minami C., Nakayama S., Sasamoto S., Kohara M., et al. Survey of genome sequences in a wild sweet potato, Ipomoea trifida (H. B. K.) G. Don. DNA Res. 2015;22:171–179. doi: 10.1093/dnares/dsv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A., Jayakumar V., Nitasaka E., Toyoda A., Noguchi H., Itoh T., Shin-I T., Minakuchi Y., Koda Y., Nagano A.J., et al. Genome sequence and analysis of the Japanese morning glory Ipomoea nil. Nat. Commun. 2016;7:13295. doi: 10.1038/ncomms13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Nakatani M., Lalusin A.G., Kuranouchi T., Fujimura T. Genetic analysis of sweetpotato and wild relatives using inter-simple sequence repeats (ISSRs) Breed. Sci. 2003;53:297–304. doi: 10.1270/jsbbs.53.297. [DOI] [Google Scholar]
- Huamán Z., Aguilar C., Ortiz R. Selecting a Peruvian sweetpotato core collection on the basis of morphological, eco-geographical, and disease and pest reaction data. Theor. Appl. Genet. 1999;98:840–844. doi: 10.1007/s001220051142. [DOI] [Google Scholar]
- Hwang S.Y., Tseng Y.T., Lo H.F. Application of simple sequence repeats in determining the genetic relationships of cultivars used in sweet potato polycross breeding in Taiwan. Sci. Hortic. (Amsterdam). 2002;93:215–224. doi: 10.1016/s0304-4238(01)00343-0. [DOI] [Google Scholar]
- Michael Jackson D., Harrison H.F., Jarret R.L., Wadl P.A. Color analysis of storage roots from the USDA, ARS sweetpotato (Ipomoea batatas) germplasm collection. Genet. Resour. Crop Evol. 2018;65:1217–1236. doi: 10.1007/s10722-018-0609-6. [DOI] [Google Scholar]
- Jansson R.K., Raman K.V. Westview Press; Boulder: 1991. Sweet Potato Pest Management: A Global Perspective. [Google Scholar]
- Jarret R.L., Austin D.F. Genetic diversity and systematic relationships in sweetpotato (Ipomoea batatas (L.) Lam.) and related species as revealed by RAPD analysis. Genet. Resour. Crop Evol. 1994;41:165–173. doi: 10.1007/bf00051633. [DOI] [Google Scholar]
- Jarret R.L., Gawel N., Whittemore A. Phylogenetic relationships of the sweetpotato [Ipomoea batatas (L.) Lam.] J. Am. Soc. Hortic. Sci. 1992;117:633–637. doi: 10.21273/jashs.117.4.633. [DOI] [Google Scholar]
- Johnson M., Pace R.D. Sweet potato leaves: properties and synergistic interactions that promote health and prevent disease. Nutr. Rev. 2010;68:604–615. doi: 10.1111/j.1753-4887.2010.00320.x. [DOI] [PubMed] [Google Scholar]
- Jones A. A proposed breeding procedure for sweetpotato 1. Crop Sci. 1965;5:191–192. doi: 10.2135/cropsci1965.0011183x000500020033x. [DOI] [Google Scholar]
- Katayama K., Kobayashi A., Sakai T., Kuranouchi T., Kai Y. Recent progress in sweetpotato breeding and cultivars for diverse applications in Japan. Breed. Sci. 2017;67:3–14. doi: 10.1270/jsbbs.16129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlestkina E.K., Salina E.A. SNP markers: methods of analysis, ways of development, and comparison on an example of common wheat. Russ. J. Genet. 2006;42:585–594. doi: 10.1134/s1022795406060019. [DOI] [PubMed] [Google Scholar]
- Khoury C.K., Heider B., Castaneda-A N.P., Achicanoy H.A., Sosa C.C., Miller R.E., Scotland R.W., Wood J.R.I., Rossel G., Eserman L.A., et al. Distributions, ex situ conservation priorities, and genetic resource potential of crop wild relatives of sweetpotato [Ipomoea batatas (L.) lam., I. series batatas] Front. Plant Sci. 2015;6:1–14. doi: 10.3389/fpls.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Chung I.K., Kim K.M. Construction of a genetic map using EST-SSR markers and QTL analysis of major agronomic characters in hexaploid sweet potato (Ipomoea batatas (L.) Lam) PLoS One. 2017;12 doi: 10.1371/journal.pone.0185073. e0185073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner N., Liebisch F., Yu K., Pfeifer J., Friedli M., Hund A., Walter A. The ETH field phenotyping platform FIP: a cable-suspended multi-sensor system. Funct. Plant Biol. 2017;44:154–168. doi: 10.1071/fp16165. [DOI] [PubMed] [Google Scholar]
- Kitahara K., Nakamura Y., Otani M., Hamada T., Nakayachi O., Takahata Y. Carbohydrate components in sweetpotato storage roots: their diversities and genetic improvement. Breed. Sci. 2017;67:62–72. doi: 10.1270/jsbbs.16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren S., Rhie A., Walenz B.P., Dilthey A.T., Bickhart D.M., Kingan S.B., Hiendleder S., Williams J.L., Smith T.P.L., Phillippy A.M. De novo assembly of haplotype-resolved genomes with trio binning. Nat. Biotechnol. 2018;36:1174–1182. doi: 10.1038/nbt.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlach J., Gedman G., Kingan S.B., Chin C.-S., Howard J.T., Audet J.-N., Cantin L., Jarvis E.D. De novo PacBio long-read and phased avian genome assemblies correct and add to reference genes generated with intermediate and short reads. Gigascience. 2017;6:1–16. doi: 10.1093/gigascience/gix085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegner A., Cervantes J.C., Burg K., Mwanga R.O.M., Zhang D. A genetic linkage map of sweetpotato [Ipomoea batatas (L.) Lam.] based on AFLP markers. Mol. Breed. 2003;11:169–185. doi: 10.1023/a:1022870917230. [DOI] [Google Scholar]
- Kumagai T. Breeding and varieties of sweetpotato. Farming Jpn. 2001;35:11–17. [Google Scholar]
- Kurabachew H. The role of orange fleshed sweet potato (Ipomea batatas) for combating vitamin A deficiency in Ethiopia: a review. Int. J. Food Sci. Nutr. Eng. 2015;5:141–146. [Google Scholar]
- Kyndt T., Quispe D., Zhai H., Jarret R., Ghislain M., Liu Q., Gheysen G., Kreuze J.F. The genome of cultivated sweet potato contains Agrobacterium T-DNAs with expressed genes: an example of a naturally transgenic food crop. Proc. Natl. Acad. Sci. U S A. 2015;112:5844–5849. doi: 10.1073/pnas.1419685112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S.L., Thompson A.E., McCollum J.P. Induction of flowering in the sweetpotato. Proc. Amer. Soc. Hort. Sci. 1959;74:453–462. [Google Scholar]
- Lebot V. 2010. Root and tuber crops. Root tuber crop. Adv. Access published 2010. [DOI] [Google Scholar]
- Lee K.J., Lee G., Lee J., Sebastin R., Shin M., Cho G., Hyun D.Y. Agronomy; 2019. Genetic Diversity of Sweet Potato (Ipomoea Batatas L. Lam) Germplasms Collected Worldwide Using Chloroplast SSR Markers. [Google Scholar]
- Li Y., Chen L. Big biological data: challenges and opportunities. Genomics. Proteomics Bioinformatics. 2014;12:187–189. doi: 10.1016/j.gpb.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yan J. Sustainable agriculture in the era of omics: knowledge-driven crop breeding. Genome Biol. 2020;21:154. doi: 10.1186/s13059-020-02073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Liu Q.C., Zhai H., Ma D.F., Wang X., Li X.Q., Wang Y.P. Genetic diversity in main parents of sweetpotato in China as revealed by ISSR marker. Acta Agron. Sin. 2008;34:972–977. doi: 10.3724/sp.j.1006.2008.00972. [DOI] [Google Scholar]
- Li H., Zhao N., Yu X., Liu Y., Zhai H., He S., Li Q., Ma D., Liu Q. Identification of QTLs for storage root yield in sweetpotato. Sci. Hortic. (Amsterdam) 2014;170:182–188. doi: 10.1016/j.scienta.2014.03.017. [DOI] [Google Scholar]
- Li M., Yang S., Xu W., Pu Z., Feng J., Wang Z., Zhang C., Peng M., Du C., Lin F., et al. The wild sweetpotato (Ipomoea trifida) genome provides insights into storage root development. BMC Plant Biol. 2019;19:119–217. doi: 10.1186/s12870-019-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Kou M., Arisha M.H., Tang W., Ma M., Yan H., Wang X., Wang X., Zhang Y., Liu Y., et al. Transcriptomic and metabolic profiling of high-temperature treated storage roots reveals the mechanism of saccharification in sweetpotato (Ipomoea batatas (L.) Lam.) Int. J. Mol. Sci. 2021;22:6641. doi: 10.3390/ijms22136641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Jiao G., Sun Y., Chen J., Zhong Y., Yan L., Jiang D., Ma Y., Xia L. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnol. J. 2021;19:937–951. doi: 10.1111/pbi.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Fernie A.R., Yan J. The past, present, and future of maize improvement: domestication, genomics, and functional genomic routes toward crop enhancement. Plant Commun. 2020;1:100010–100019. doi: 10.1016/j.xplc.2019.100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Zhu J.K. Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol. Plant. 2017;10:523–525. doi: 10.1016/j.molp.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Lu F., Romay M.C., Glaubitz J.C., Bradbury P.J., Elshire R.J., Wang T., Li Y., Li Y., Semagn K., Zhang X., et al. High-resolution genetic mapping of maize pan-genome sequence anchors. Nat. Commun. 2015;6:6914. doi: 10.1038/ncomms7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Ye X., Guo R., Huang J., Wang W., Tang J., Tan L., Zhu J. kang, Chu C., Qian Y. Genome-wide targeted mutagenesis in rice using the CRISPR/Cas9 system. Mol. Plant. 2017;10:1242–1245. doi: 10.1016/j.molp.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Lu Y., Tian Y., Shen R., Yao Q., Wang M., Chen M., Dong J., Zhang T., Li F., Lei M., et al. Targeted, efficient sequence insertion and replacement in rice. Nat. Biotechnol. 2020;38:1402–1407. doi: 10.1038/s41587-020-0581-5. [DOI] [PubMed] [Google Scholar]
- Lye Z.N., Purugganan M.D. Copy number variation in domestication. Trends Plant Sci. 2019;24:352–365. doi: 10.1016/j.tplants.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Lyu R., Ahmed S., Fan W., Yang J., Wu X., Zhou W., Zhang P., Yuan L., Wang H. Engineering properties of sweet potato starch for industrial applications by biotechnological techniques including genome editing. Int. J. Mol. Sci. 2021;22:9533. doi: 10.3390/ijms22179533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Gao W., Liu L., Liu M., Zhao N., Han M., Wang Z., Jiao W., Gao Z., Hu Y., et al. Identification of QTL for resistance to root rot in sweetpotato (Ipomoea batatas (L.) Lam) with SSR linkage maps. BMC Genomics. 2020;21:366. doi: 10.1186/s12864-020-06775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoon M.L., Krishnan R., Vijaya Bai K. Cytological evidence on the origin of sweet potato. Theor. Appl. Genet. 1970;40:360–366. doi: 10.1007/bf00285415. [DOI] [PubMed] [Google Scholar]
- Malalavidhane S., Wickramasinghe S.M.D.N., Jansz E.R. An aqueous extract of the green leafy vegetable Ipomoea aquatica is as effective as the oral hypoglycaemic drug tolbutamide in reducing the blood sugar levels of wistar rats. Phyther. Res. 2001;15:635–637. doi: 10.1002/ptr.851. [DOI] [PubMed] [Google Scholar]
- Malalavidhane T.S., Wickramasinghe S.M.D.N., Perera M.S.A., Jansz E.R. Oral hypoglycaemic activity of Ipomoea aquatica in streptozotocin-induced, diabetic wistar rats and type II diabetics. Phyther. Res. 2003;17:1098–1100. doi: 10.1002/ptr.1345. [DOI] [PubMed] [Google Scholar]
- Mao Y., Zhang H., Xu N., Zhang B., Gou F., Zhu J.K. Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol. Plant. 2013;6:2008–2011. doi: 10.1093/mp/sst121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian D.Q., Kwadwo A., David A.-K., Ruth N.P., John A.-A., Belinda A., Harrison D. Use of expressed sequence tags-derived simple sequence repeat (SSR) markers for population studies of released and elite sweet potato. Int. J. Genet. Mol. Biol. 2018;10:14–25. doi: 10.5897/ijgmb2017.0159. [DOI] [Google Scholar]
- Martin F.W. Incompatibility in the sweet potato. A review. A. Review. Econ. Bot. 1965;19:406–415. doi: 10.1007/bf02904812. [DOI] [Google Scholar]
- Martin F.W., Jones A. Breeding sweet potatoes. Plant Breed. Rev. 1986;4:313–345. doi: 10.1002/9781118061015.ch10. [DOI] [Google Scholar]
- Mcharo M., LaBonte D.R. Multivariate selection of AFLP markers associated with β-carotene in sweetpotatoes. Euphytica. 2010;175:123–132. doi: 10.1007/s10681-010-0193-0. [DOI] [Google Scholar]
- Mcharo M., LaBonte D.R., Clark C., Hoy M., Oard J.H. Molecular marker variability for southern root-knot nematode resistance in sweetpotato. Euphytica. 2005;144:125–132. doi: 10.1007/s10681-005-5271-3. [DOI] [Google Scholar]
- Mcharo M., LaBonte D., Mwanga R.O.M., Kriegner A. Associating molecular markers with virus resistance to classify sweetpotato genotypes. J. Am. Soc. Hortic. Sci. 2005;130:355–359. doi: 10.21273/jashs.130.3.355. [DOI] [Google Scholar]
- Miano D.W., LaBonte D.R., Clark C.A. Identification of molecular markers associated with sweet potato resistance to sweet potato virus disease in Kenya. Euphytica. 2008;160:15–24. doi: 10.1007/s10681-007-9495-2. [DOI] [Google Scholar]
- Miao C., Wang Z., Zhang L., Yao J., Hua K., Liu X., Shi H., Zhu J.K. The grain yield modulator miR156 regulates seed dormancy through the gibberellin pathway in rice. Nat. Commun. 2019;10:3822. doi: 10.1038/s41467-019-11830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeinzadeh M.H., Yang J., Muzychenko E., Gallone G., Heller D., Reinert K., Haas S., Vingron M. Ranbow: a fast and accurate method for polyploid haplotype reconstruction. Plos Comput. Biol. 2020;16:e1007843. doi: 10.1371/journal.pcbi.1007843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollinari M., Olukolu B.A., Pereira G.d.S., Khan A., Gemenet D., Yencho G.C., Zeng Z.B. Unraveling the hexaploid sweetpotato inheritance using ultra-dense multilocus mapping. G3 Genes, Genomes, Genet. 2020;10:281–292. doi: 10.1534/g3.119.400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monden Y., Tahara M. Genetic linkage analysis using DNA markers in sweetpotato. Breed. Sci. 2017;67:41–51. doi: 10.1270/jsbbs.16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mores A., Borrelli G.M., Laidò G., Petruzzino G., Pecchioni N., Amoroso L.G.M., Desiderio F., Mazzucotelli E., Mastrangelo A.M., Marone D. Genomic approaches to identify molecular bases of crop resistance to diseases and to develop future breeding strategies. Int. J. Mol. Sci. 2021;22:5423. doi: 10.3390/ijms22115423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin M.M., Rodrigues R., Gonçalves L.S.A., Sudré C.P., Pereira M.G. A comparison of RAPD and ISSR markers reveals genetic diversity among sweet potato landraces (Ipomoea batatas (L.) Lam.) Acta Sci. - Agron. 2012;34:139–147. doi: 10.4025/actasciagron.v34i2.12616. [DOI] [Google Scholar]
- Mubayiwa M., Mutasa W., Gasura E., Mabasa S. Grafting and 2.4-dichlorophenoxyacetic acid induced flowering in non-flowering sweetpotato in sub-tropics. South Afr. J. Bot. 2016;106:153–157. doi: 10.1016/j.sajb.2016.07.005. [DOI] [Google Scholar]
- Muñoz-Rodríguez P., Carruthers T., Wood J.R.I., Williams B.R.M., Weitemier K., Kronmiller B., Ellis D., Anglin N.L., Longway L., Harris S.A., et al. Reconciling conflicting phylogenies in the origin of sweet potato and dispersal to Polynesia. Curr. Biol. 2018;28:1246–1256.e12. doi: 10.1016/j.cub.2018.03.020. [DOI] [PubMed] [Google Scholar]
- Muñoz-Rodríguez P., Wells T., Wood J.R.I., Carruthers T., Anglin N.L., Jarret R.L., Scotland R.W. New Phytologist; 2022. Discovery and Characterization of Sweetpotato’s Closest Tetraploid Relative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanga R.O.M., Kriegner A., Cervantes-Flores J.C., Zhang D.P., Moyer J.W., Yencho G.C. Resistance to sweetpotato chlorotic stunt virus and sweetpotato feathery mottle virus is mediated by two separate recessive genes in sweetpotato. J. Am. Soc. Hortic. Sci. 2002;127:798–806. doi: 10.21273/jashs.127.5.798. [DOI] [Google Scholar]
- Mwanga R.O.M., Kyalo G., Ssemakula G.N., Niringiye C., Yada B., Otema M.A., Namakula J., Alajo A., Kigozi B., Makumbi R.N.M., et al. ‘NASPOT 12 O’ and ‘NASPOT 13 O’ sweetpotato. HortScience. 2016;51:291–295. doi: 10.21273/hortsci.51.3.291. [DOI] [Google Scholar]
- Nadeem M.A., Nawaz M.A., Shahid M.Q., Doğan Y., Comertpay G., Yıldız M., Hatipoğlu R., Ahmad F., Alsaleh A., Labhane N., et al. DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018;32:261–285. doi: 10.1080/13102818.2017.1400401. [DOI] [Google Scholar]
- Nakaya A., Isobe S.N. Will genomic selection be a practical method for plant breeding? Ann. Bot. 2012;110:1303–1316. doi: 10.1093/aob/mcs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Tanaka M., Takahata Y., Matsui K., Iwahori H., Sano Z. ichi, Yoshinaga M. Development of AFLP-derived SCAR markers associated with resistance to two races of southern root-knot nematode in sweetpotato. Euphytica. 2012;188:175–185. doi: 10.1007/s10681-012-0678-0. [DOI] [Google Scholar]
- Nawaz M.A., Chung G. Genetic improvement of cereals and grain legumes. Genes (Basel). 2020;11:1255. doi: 10.3390/genes11111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell C.A., Lowe J.M., Merryweather A., Rooke L.M., Hamilton W.D.O. Transformation of sweet potato (Ipomoea batatas (L.) Lam.) with Agrobacterium tumefaciens and regeneration of plants expressing cowpea trypsin inhibitor and snowdrop lectin. Plant Sci. 1995;107:215–227. doi: 10.1016/0168-9452(95)04109-8. [DOI] [Google Scholar]
- Nishiyama I. Evolution and domestication of the sweet potato. Bot. Mag. 1971;84:377–387. doi: 10.15281/jplantres1887.84.377. [DOI] [Google Scholar]
- Okada Y., Saito A., Nishiguchi M., Kimura T., Mori M., Hanada K., Sakai J., Miyazaki C., Matsuda Y., Murata T. Virus resistance in transgenic sweetpotato [Ipomoea batatas L. (Lam)] expressing the coat protein gene of sweet potato feathery mottle virus. Theor. Appl. Genet. 2001;103:743–751. doi: 10.1007/s001220100654. [DOI] [Google Scholar]
- Okada Y., Monden Y., Nokihara K., Shirasawa K., Isobe S., Tahara M. Genome-wide association studies (GWAS) for yield and weevil resistance in sweet potato (Ipomoea batatas (L.) Lam) Plant Cell Rep. 2019;38:1383–1392. doi: 10.1007/s00299-019-02445-7. [DOI] [PubMed] [Google Scholar]
- Otani M., Shimada T., Kimura T., Saito A. Transgenic plant production from embryogenic callus of sweet potato (Ipomoea batatas (L.) Lam.) Using Agrobacterium Tumefaciens. Plant Biotechnol. 1998;15:11–16. [Google Scholar]
- Otani M., Wakita Y., Shimada T. Production of herbicide-resistant sweetpotato (Ipomoea batatas (L.) Lam.) plants by Agrobacterium tumefaciens-mediated transformation. Breed. Sci. 2003;53:145–148. doi: 10.1270/jsbbs.53.145. [DOI] [Google Scholar]
- Otani M., Hamada T., Katayama K., Kitahara K., Kim S.H., Takahata Y., Suganuma T., Shimada T. Inhibition of the gene expression for granule-bound starch synthase I by RNA interference in sweet potato plants. Plant Cell Rep. 2007;26:1801–1807. doi: 10.1007/s00299-007-0396-6. [DOI] [PubMed] [Google Scholar]
- Ozias-Akins P., Jarret R.L. Nuclear DNA content and ploidy levels in the genus Ipomoea. J. Am. Soc. Hortic. Sci. 1994;119:110–115. doi: 10.21273/jashs.119.1.110. [DOI] [Google Scholar]
- Padmaja G. In: The Sweetpotato. Loebenstein G., Thottappilly G., editors. Springer Netherlands; Dordrecht: 2009. Uses and nutritional data of sweetpotato; pp. 189–234. [Google Scholar]
- Palumbo F., Galvao A.C., Nicoletto C., Sambo P., Barcaccia G. Diversity analysis of sweet potato genetic resources using morphological and qualitative traits and molecular markers. Genes (Basel) 2019;10:840. doi: 10.3390/genes10110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quispe-Huamanquispe D.G., Gheysen G., Yang J., Jarret R., Rossel G., Kreuze J.F. The horizontal gene transfer of Agrobacterium T-DNAs into the series Batatas (Genus Ipomoea) genome is not confined to hexaploid sweetpotato. Sci. Rep. 2019;9:12584–12613. doi: 10.1038/s41598-019-48691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse S., Nilmalgoda S.D., Molnar M., Ballard R.E., Austin D.F., Bohac J.R. Phylogenetic relationships of the sweetpotato in Ipomoea series Batatas (Convolvulaceae) based on nuclear β-amylase gene sequences. Mol. Phylogenet. Evol. 2004;30:623–632. doi: 10.1016/s1055-7903(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Rastas P. Shanghai Chenshan Botanic Garden; Shanghai: 2019. Linkage Mapping Guided Assembly of Hexaploid Sweet Potato; p. 23. [Google Scholar]
- Rolston L.H., Clark C.A., Cannon J.M., Randle W.M., Riley E.G., Wilson P.W., Robbins M.L. Beauregard' sweet potato. HortScience. 1987;22:1338–1339. [Google Scholar]
- Rosyara U.R., De Jong W.S., Douches D.S., Endelman J.B. Software for genome-wide association studies in autopolyploids and its application to potato. Plant Genome. 2016;9 doi: 10.3835/plantgenome2015.08.0073. plantgenome2015-plantgenome2108. [DOI] [PubMed] [Google Scholar]
- Roullier C., Duputié A., Wennekes P., Benoit L., Fernández Bringas V.M., Rossel G., Tay D.B., McKey D., Lebot V. Disentangling the origins of cultivated sweet potato (Ipomoea batatas (L.) lam.) PLoS One. 2013;8:e62707. doi: 10.1371/journal.pone.0062707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai R., Tabuchi H., Shirasawa K., Kishimoto K., Sato S., Okada Y., Kuramoto A., Kobayashi A., Isobe S., Tahara M., et al. Development of molecular markers associated with resistance to Meloidogyne incognita by performing quantitative trait locus analysis and genome-wide association study in sweetpotato. DNA Res. 2019;26:399–409. doi: 10.1093/dnares/dsz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafleitner R., Tincopa L.R., Palomino O., Rossel G., Robles R.F., Alagon R., Rivera C., Quispe C., Rojas L., Pacheco J.A., et al. A sweetpotato gene index established by de novo assembly of pyrosequencing and Sanger sequences and mining for gene-based microsatellite markers. BMC Genom. 2010;11:604. doi: 10.1186/1471-2164-11-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.M., Kim B.K., Seo S.G., Jeon S.B., Kim J.S., Jun B. ki, Kang S.Y., Lee J.S., Chung M.N., Kim S.H. Mutation breeding of sweet potato by gamma-ray radiation. Afr. J. Agric. Res. 2011;6:1447–1454. [Google Scholar]
- Shiotani I. International Potato Center (CIP); Lima (Peru): 1988. Genomic Structure and the Gene Flow in Sweet Potato and Related species. Report of the First Planning Converence on Exploration, Maintenance and Utilization of Sweet Potato Genetic Resources; pp. 61–73. [Google Scholar]
- Shiotani I., Kawase T. Synthetic hexaploids derived from wild species related to sweet potato. Jpn. J. Breed. 1987;37:367–376. doi: 10.1270/jsbbs1951.37.367. [DOI] [Google Scholar]
- Shiotani I., Kawase T. Genomic structure of the sweet potato and hexaploids in Ipomoea trifida (HBK) Don. Jpn. J. Breed. 1989;39:57–66. doi: 10.1270/jsbbs1951.39.57. [DOI] [Google Scholar]
- da Silva Pereira G., Gemenet D.C., Mollinari M., Olukolu B.A., Wood J.C., Diaz F., Mosquera V., Gruneberg W.J., Khan A., Buell C.R., et al. Multiple QTL mapping in autopolyploids: a random-effect model approach with application in a hexaploid sweetpotato full-sib population. Genetics. 2020;215:579–595. doi: 10.1534/genetics.120.303080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som K., Vernon G., Isaac A., Eric Y.D., Jeremy T.O., Tignegre J.B., Belem J., Tarpaga M.V. Diversity analysis of sweet potato (Ipomoea batatas [L.] Lam) germplasm from Burkina Faso using morphological and simple sequence repeats markers. Afr. J. Biotechnol. 2014;13:729–742. doi: 10.5897/ajb2013.13234. [DOI] [Google Scholar]
- Soyk S., Müller N.A., Park S.J., Schmalenbach I., Jiang K., Hayama R., Zhang L., Van Eck J., Jiménez-Gómez J.M., Lippman Z.B. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat. Genet. 2017;49:162–168. doi: 10.1038/ng.3733. [DOI] [PubMed] [Google Scholar]
- Srisuwan S., Sihachakr D., Siljak-Yakovlev S. The origin and evolution of sweet potato (Ipomoea batatas Lam.) and its wild relatives through the cytogenetic approaches. Plant Sci. 2006;171:424–433. doi: 10.1016/j.plantsci.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Su W., Wang L., Lei J., Chai S., Liu Y., Yang Y., Yang X., Jiao C. Genome-wide assessment of population structure and genetic diversity and development of a core germplasm set for sweet potato based on specific length amplified fragment (SLAF) sequencing. PLoS One. 2017;12:e0172066–e0172114. doi: 10.1371/journal.pone.0172066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu K., Tanaka M., Isobe S. Identification of a major QTL for root thickness in diploid wild sweetpotato (Ipomoea trifida) using QTL-seq. Plant Prod. Sci. 2021;25:120–129. doi: 10.1080/1343943x.2021.1927766. [DOI] [Google Scholar]
- Sun C., Feng L., Zhang Z., Ma Y., Crosby T., Naber M., Wang Y. Prediction of end-of-season tuber yield and tuber set in potatoes using in-season uav-based hyperspectral imagery and machine learning. Sensors (Switzerland) 2020;20:5293–5313. doi: 10.3390/s20185293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Mao B., Li Y., Lv Q., Zhang L., Chen C., He H., Wang W., Zeng X., Shao Y., et al. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017;7:14438–14512. doi: 10.1038/s41598-017-14832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Zhao X., Mace E., Henry R., Jordan D. Exploring and exploiting pan-genomics for crop improvement. Mol. Plant. 2019;12:156–169. doi: 10.1016/j.molp.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Ukoskit K., Thompson P.G. Autopolyploidy versus allopolyploidy and low-density randomly amplified polymorphic DNA linkage maps of sweetpotato. J. Am. Soc. Hortic. Sci. 1997;122:822–828. doi: 10.21273/jashs.122.6.822. [DOI] [Google Scholar]
- Vásquez-Villalobos V., Hernández-Bracamonte O., Rojas-Naccha J., Ninaquispe-Zare V., Rojas-Padilla C., Vásquez-Angulo J. Moisture prediction of sweet potato-quinoa-kiwicha flakes dried by rotary drum dryer using artificial intelligence. Sci. Agropecu. 2018;9:83–91. doi: 10.17268/sci.agropecu.2018.01.09. [DOI] [Google Scholar]
- Veasey E.A., Borges A., Rosa M.S., Queiroz-Silva J.R., Bressan E.d.A., Peroni N. Genetic diversity in Brazilian sweet potato (Ipomoea batatas (L.) Lam., Solanales, Convolvulaceae) landraces assessed with microsatellite markers. Genet. Mol. Biol. 2008;31:725–733. doi: 10.1590/s1415-47572008000400020. [DOI] [Google Scholar]
- Villordon A., Carroll H. Digital image analysis of sweetpotato storage roots in herbicide trials. HortScience. 2002;37:669–670. doi: 10.21273/hortsci.37.4.669. [DOI] [Google Scholar]
- Villordon A., Clark C., Smith T., Ferrin D., LaBonte D. Combining linear regression and machine learning approaches to identify consensus variables related to optimum sweetpotato transplanting date. HortScience. 2010;45:684–686. doi: 10.21273/hortsci.45.4.684. [DOI] [Google Scholar]
- Villordon A., Gregorie J.C., LaBonte D. Direct measurement of sweetpotato surface area and volume using a low-cost 3D scanner for identification of shape features related to processing product recovery. HortScience. 2020;55:722–728. doi: 10.21273/hortsci14964-20. [DOI] [Google Scholar]
- Vimala B., Hariprakash B. Variability of morphological characters and dry matter content in the hybrid progenies of sweet potato (Ipomoea batatas (L) Lam) Gene Conserv. 2011;10:1–13. [Google Scholar]
- Wadl P.A., Olukolu B.A., Branham S.E., Jarret R.L., Yencho G.C., Jackson D.M. Genetic diversity and population structure of the USDA sweetpotato (Ipomoea batatas) germplasm collections using GBSpoly. Front. Plant Sci. 2018;9:1166. doi: 10.3389/fpls.2018.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang F., Zhai H., Liu Q. Production of a useful mutant by chronic irradiation in sweetpotato. Sci. Hortic. (Amsterdam) 2007;111:173–178. doi: 10.1016/j.scienta.2006.10.007. [DOI] [Google Scholar]
- Wang Z., Fang B., Chen J., Zhang X., Luo Z., Huang L., Chen X., Li Y. De novo assembly and characterization of root transcriptome using Illumina paired-end sequencing and development of cSSR markers in sweetpotato (Ipomoea batatas) BMC Genom. 2010;11:726. doi: 10.1186/1471-2164-11-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Y., Zhang H., Zhai H., Liu Q., He S. A soluble starch synthase i gene, IbSSI, alters the content, composition, granule size and structure of starch in transgenic sweet potato. Sci. Rep. 2017;7:2315. doi: 10.1038/s41598-017-02481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wu Y., Zhang Y., Yang J., Fan W., Zhang H., Zhao S., Yuan L., Zhang P. CRISPR/Cas9-based mutagenesis of starch biosynthetic genes in sweet potato (Ipomoea Batatas) for the improvement of starch quality. Int. J. Mol. Sci. 2019;20:4702. doi: 10.3390/ijms20194702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Li D., Li X., Gao Y., Li W., Li H., Liu J., Liu H., Chen W., Luo J., et al. Metabolome-based genome-wide association study of maize kernel leads to novel biochemical insights. Nat. Commun. 2014;5:3438. doi: 10.1038/ncomms4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J.R.I., Muñoz-Rodríguez P., Williams B.R.M., Scotland R.W. A foundation monograph of Ipomoea (Convolvulaceae) in the new World. PhytoKeys. 2020;143:1–823. doi: 10.3897/phytokeys.143.32821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfe J.A. Cambridge University Press; 1992. Sweet Potato: An Untapped Food Resource. [Google Scholar]
- Wu S., Lau K.H., Cao Q., Hamilton J.P., Sun H., Zhou C., Eserman L., Gemenet D.C., Olukolu B.A., Wang H., et al. Genome sequences of two diploid wild relatives of cultivated sweetpotato reveal targets for genetic improvement. Nat. Commun. 2018;9:4580–4612. doi: 10.1038/s41467-018-06983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Han L., Li Q., Wang G., Zhang H., Li L. Using interactome big data to crack genetic mysteries and enhance future crop breeding. Mol. Plant. 2021;14:77–94. doi: 10.1016/j.molp.2020.12.012. [DOI] [PubMed] [Google Scholar]
- Xu Y., Liu X., Fu J., Wang H., Wang J., Huang C., Prasanna B.M., Olsen M.S., Wang G., Zhang A. Enhancing genetic gain through genomic selection: from livestock to plants. Plant Commun. 2020;1:100005. doi: 10.1016/j.xplc.2019.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada B., Brown-Guedira G., Alajo A., Ssemakula G.N., Owusu-Mensah E., Carey E.E., Mwanga R.O.M., Yencho G.C. Genetic analysis and association of simple sequence repeat markers with storage root yield, dry matter, starch and β-carotene content in sweetpotato. Breed. Sci. 2017;67:140–150. doi: 10.1270/jsbbs.16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa O. Development of sweetpotato cultivars for new processing use in Japan. Trop. Agric. 1998;75:284–287. [Google Scholar]
- Yamakawa H., Haque E., Tanaka M., Takagi H., Asano K., Shimosaka E., Akai K., Okamoto S., Katayama K., Tamiya S. Polyploid QTL-seq towards rapid development of tightly linked DNA markers for potato and sweetpotato breeding through whole-genome resequencing. Plant Biotechnol. J. 2021;19:2040–2051. doi: 10.1111/pbi.13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E., Shirasawa K., Kimura T., Monden Y., Tanaka M., Isobe S. Genetic mapping in autohexaploid sweet potato with low-coverage NGS-based genotyping data. G3 Genes, Genomes, Genet. 2020;10:2661–2670. doi: 10.1534/g3.120.401433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Li M., Moeinzadeh M.-H., Quispe-Huamanquispe D.G., Fan W., Nie H., Wang Z., Heider B., Jarret R., Kreuze J. Haplotype-based phylogenetic analysis uncovers the tetraploid progenitor of sweet potato. Res. Sq. 2021 doi: 10.21203/rs.3.rs-750500/v1. [DOI] [Google Scholar]
- Yang Y., Guan S., Zhai H., He S., Liu Q. Development and evaluation of a storage root-bearing sweetpotato somatic hybrid between Ipomoea batatas (L.) Lam. and I. triloba L. Plant Cell. Tissue Organ. Cult. 2009;99:83–89. doi: 10.1007/s11240-009-9578-y. [DOI] [Google Scholar]
- Yang J., Bi H.P., Fan W.J., Zhang M., Wang H.X., Zhang P. Efficient embryogenic suspension culturing and rapid transformation of a range of elite genotypes of sweet potato (Ipomoea batatas [L.] Lam.) Plant Sci. 2011;181:701–711. doi: 10.1016/j.plantsci.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Yang J., Moeinzadeh M.H., Kuhl H., Helmuth J., Xiao P., Haas S., Liu G., Zheng J., Sun Z., Fan W., et al. Haplotype-resolved sweet potato genome traces back its hexaploidization history. Nat. Plants. 2017;3:696–703. doi: 10.1038/s41477-017-0002-z. [DOI] [PubMed] [Google Scholar]
- Yang G., Liu J., Zhao C., Li Z., Huang Y., Yu H., Xu B., Yang X., Zhu D., Zhang X., et al. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: current status and perspectives. Front. Plant Sci. 2017;8:1111. doi: 10.3389/fpls.2017.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.S., Su W.J., Wang L.J., Jian L., Chai S.S., Liu Q.C. Molecular diversity and genetic structure of 380 sweetpotato accessions as revealed by SSR markers. J. Integr. Agric. 2015;14:633–641. doi: 10.1016/s2095-3119(14)60794-2. [DOI] [Google Scholar]
- Ye M., Peng Z., Tang D., Yang Z., Li D., Xu Y., Zhang C., Huang S. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants. 2018;4:651–654. doi: 10.1038/s41477-018-0218-6. [DOI] [PubMed] [Google Scholar]
- Yoon U.H. The 1st International Convolvulaceae Meeting; 2019. Strategy and Application of Sweetpotato Genome Sequecing Project by TRAS Collaboration; p. 22. [Google Scholar]
- Yu X.X., Zhao N., Li H., Jie Q., Zhai H., He S.Z., Li Q., Liu Q.C. Identification of QTLs for starch content in sweetpotato (Ipomoea batatas (L.) Lam.) J. Integr. Agric. 2014;13:310–315. doi: 10.1016/s2095-3119(13)60357-3. [DOI] [Google Scholar]
- Zawedde B.M., Ghislain M., Magembe E., Amaro G.B., Grumet R., Hancock J. Characterization of the genetic diversity of Uganda’s sweet potato (Ipomoea batatas) germplasm using microsatellites markers. Genet. Resour. Crop Evol. 2015;62:501–513. doi: 10.1007/s10722-014-0175-5. [DOI] [Google Scholar]
- Zhan X., Lu Y., Zhu J.K., Botella J.R. Genome editing for plant research and crop improvement. J. Integr. Plant Biol. 2021;63:3–33. doi: 10.1111/jipb.13063. [DOI] [PubMed] [Google Scholar]
- Zhang D., Cervantes J., Huamán Z., Carey E., Ghislain M. Assessing genetic diversity of sweet potato (Ipomoea batatas (L.) Lam.) cultivars from tropical America using AFLP. Genet. Resour. Crop Evol. 2000;47:659–665. [Google Scholar]
- Zhang D., Rossel G., Kriegner A., Hijmans R. AFLP assessment of diversity in sweetpotato from Latin America and the Pacific region: its implications on the dispersal of the crop. Genet. Resour. Crop Evol. 2004;51:115–120. doi: 10.1023/b:gres.0000020853.04508.a0. [DOI] [Google Scholar]
- Zhang K., Wu Z., Tang D., Lv C., Luo K., Zhao Y., Liu X., Huang Y., Wang J. Development and identification of SSR markers associated with starch properties and β-carotene content in the storage root of sweet potato (Ipomoea batatas L.) Front. Plant Sci. 2016;7:1–21. doi: 10.3389/fpls.2016.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang J., Lang Z., Botella J.R., Zhu J.-K. Genome editing—principles and applications for functional genomics research and crop improvement. CRC. Crit. Rev. Plant Sci. 2017;36:291–309. doi: 10.1080/07352689.2017.1402989. [DOI] [Google Scholar]
- Zhang A., Liu Y., Wang F., Li T., Chen Z., Kong D., Bi J., Zhang F., Luo X., Wang J., et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019;39:47. doi: 10.1007/s11032-019-0954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yu Y., Shi T., Kou M., Sun J., Xu T., Li Q., Wu S., Cao Q., Hou W., et al. Genome-wide analysis of expression quantitative trait loci (eQTLs) reveals the regulatory architecture of gene expression variation in the storage roots of sweet potato. Hortic. Res. 2020;7:90. doi: 10.1038/s41438-020-0314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wu R., Wang Y., Yu J., Tang H. Unzipping haplotypes in diploid and polyploid genomes. Comput. Struct. Biotechnol. J. 2020;18:66–72. doi: 10.1016/j.csbj.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhou Z., Bai J., Tao X., Wang L., Zhang H., Zhu J.K. Disruption of MIR396e and MIR396f improves rice yield under nitrogen-deficient conditions. Natl. Sci. Rev. 2020;7:102–112. doi: 10.1093/nsr/nwz142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Yu X., Jie Q., Li H., Li H., Hu J., Zhai H., He S., Liu Q. A genetic linkage map based on AFLP and SSR markers and mapping of QTL for dry-matter content in sweetpotato. Mol. Breed. 2013;32:807–820. doi: 10.1007/s11032-013-9908-y. [DOI] [Google Scholar]
- Zhao N., Zhai H., Yu X., Liu Z.S., He S.Z., Li Q., Liu Q.C. Development of SRAP markers linked to a gene for stem nematode resistance in sweetpotato, Ipomoea batatas (L.) Lam. J. Integr. Agric. 2013;12:414–419. doi: 10.1016/s2095-3119(13)60241-5. [DOI] [Google Scholar]
- Zhao Q., Feng Q., Lu H., Li Y., Wang A., Tian Q., Zhan Q., Lu Y., Zhang L., Huang T., et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018;50:278–284. doi: 10.1038/s41588-018-0041-z. [DOI] [PubMed] [Google Scholar]
- Zhao C., Zhang Y., Du J., Guo X., Wen W., Gu S., Wang J., Fan J. Crop phenomics: current status and perspectives. Front. Plant Sci. 2019;10:714. doi: 10.3389/fpls.2019.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Liu C., Qi X., Jiao Y., Wang D., Wang Y., Liu Z., Chen C., Chen B., Tian X., et al. Mutation of ZmDMP enhances haploid induction in maize. Nat. Plants. 2019;5:575–580. doi: 10.1038/s41477-019-0443-7. [DOI] [PubMed] [Google Scholar]
- Zhou W., Yang J., Hong Y., Liu G., Zheng J., Gu Z., Zhang P. Impact of amylose content on starch physicochemical properties in transgenic sweet potato. Carbohydr. Polym. 2015;122:417–427. doi: 10.1016/j.carbpol.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Zhu G., Wang S., Huang Z., Zhang S., Liao Q., Zhang C., Lin T., Qin M., Peng M., Yang C., et al. Rewiring of the fruit metabolome in tomato breeding. Cell. 2018;172:249–261.e12. doi: 10.1016/j.cell.2017.12.019. e12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.