Abstract
The arabinose-inducible promoter PBAD is subject to all-or-none induction, in which intermediate concentrations of arabinose give rise to subpopulations of cells that are fully induced and uninduced. To construct a host-vector expression system with regulatable control in a homogeneous population of cells, the araE gene of Escherichia coli was cloned into an RSF1010-derived plasmid under control of the isopropyl-β-d-thiogalactopyranoside-inducible Ptac and Ptaclac promoters. This gene encodes the low-affinity, high-capacity arabinose transport protein and is controlled natively by an arabinose-inducible promoter. To detect the effect of arabinose-independent araE expression on population homogeneity and cell-specific expression, the gfpuv gene was placed under control of the arabinose-inducible araBAD promoter (PBAD) on the pMB1-derived plasmid pBAD24. The transporter and reporter plasmids were transformed into E. coli strains with native arabinose transport systems and strains deficient in one or both of the arabinose transport systems (araE and/or araFGH). The effects of the arabinose concentration and arabinose-independent transport control on population homogeneity were investigated in these strains using flow cytometry. The araE, and araE araFGH mutant strains harboring the transporter and reporter plasmids were uniformly induced across the population at all inducer concentrations, and the level of gene expression in individual cells varied with arabinose concentration. In contrast, the parent strain, which expressed the native araE and araFGH genes and harbored the transporter and reporter plasmids, exhibited all-or-none behavior. This work demonstrates the importance of including a transport gene that is controlled independently of the inducer to achieve regulatable and consistent induction in all cells of the culture.
In 1957, Novick and Weiner (14) studied expression of the lac operon in the presence of inducer concentrations less than that needed for maximal induction (subsaturating concentrations). This early study demonstrated that a fraction of cells in the population was fully induced while the remainder was uninduced and that the number of fully induced cells varied directly with the concentration of inducer. They referred to this mechanism as “all-or-none” or autocatalytic gene expression (14). Autocatalytic gene expression systems contain the genes encoding the transporter under the control of the transported molecule (the inducer). More recently, autocatalytic behavior was also reported for the ara operon (18).
Although the all-or-none phenomenon associated with autocatalytic expression systems was demonstrated more than 40 years ago, many of the expression systems currently available continue to be based on similar frameworks and used without regard for this phenomenon. For systems in which population heterogeneity is not important and high-level gene expression is desired, autocatalytic systems remain an ideal choice; expression can be induced to a maximal level in all cells of the population. However, for many applications, intermediate expression levels are necessary to reduce metabolic burden or to achieve specific intracellular conditions. In these cases, one would like expression to be very low in the absence of inducer and vary directly with the level of inducer (6).
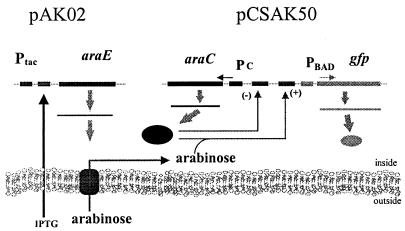
The ara operon is one of the most well-studied autocatalytic systems. In this system, genes encoding the arabinose transporters (araE and araFGH) and arabinose catabolic genes (araBAD) are under arabinose-inducible control through AraC; arabinose binds to the AraC protein, which positively regulates expression of transporters and negatively regulates its own expression (Fig. 1). Engineered plasmid vectors carrying the araC-PBAD fragment from the ara operon have been used successfully in Escherichia coli and Salmonella enterica serovar Typhimurium as recombinant gene expression systems (3). This self-regulating system provides fine control of expression, tight repression in the absence of inducer, and induction over a 1,000-fold range in the presence of inducer (3). With the development of broad-host-range plasmids containing the araC-PBAD repressor-promoter assemblage (13), this system is now also available for use in nonenteric, gram-negative bacteria.
FIG. 1.
Decoupled transporter-reporter system. AraC regulates expression from its own promoter (Pc) and from the araBAD promoter (PBAD). In the decoupled system, the araE genes, encoding the arabinose transporters, were placed under control of Ptac (pAK02).
Unfortunately, the response of the araC-PBAD system in individual cells to arabinose concentration is not linear. In a recent study, Siegele and Hu (18) demonstrated all-or-none behavior of E. coli cells containing PBAD::gfp constructs exposed to intermediate arabinose concentrations similar to that observed for the lac operon by Novick and Weiner (14). This phenomenon was attributed to the inducible l-arabinose transport systems (araE and araFGH).
Simulation experiments indicated that replacement of the native promoter for the gene encoding the transport protein with a constitutive or independently inducible promoter should effectively decouple the inducer transport-induction loop and eliminate autocatalytic responses (1). Using this strategy, it is possible to vary the average level of gene expression of the population by changing the level of gene expression in individual cells rather than the fraction of the population that is fully induced. In this article, we describe a transporter-reporter system used to examine the effect of independent expression of transport gene on reporter gene expression across the bacterial population.
MATERIALS AND METHODS
Reagents, strains, and plasmids.
Chemical reagents were purchased from Fisher Scientific and Sigma (St. Louis, Mo.). Restriction enzymes, DNA polymerase, and T4 DNA ligase were obtained from and used as recommended by Boehringer Mannheim (Indianapolis, Ind.) and New England Biolabs (Beverly, Mass.). Oligonucleotide synthesis and sequencing were done by Genemed (South San Francisco, Calif.).
All relevant strains and plasmids used in this study are listed in Table 1. E. coli was grown at 37°C in Luria-Bertani (LB) medium supplemented with ampicillin (100 μg/ml) for transformants with pMB1-derived plasmids, chloramphenicol (20 μg/ml) for pMMB-derived plasmids, or a combination of antibiotics for transformants harboring both plasmids.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and characteristicsa | Reference or source |
|---|---|---|
| E. coli strains | ||
| CW2513 | K-12 wild type | 5 |
| CW2549 | araE201 | 5 |
| CW2553 | araE201 ΔaraFGH::kan | 5 |
| CW2587 | araE201 ΔaraFGH::kan srl::Tn10 recA59 | 5 |
| DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu) 7697 galU galK1 rpsL nupG | Life Technologies Inc. (Gaithersburg, Md.) |
| Plasmids | ||
| pAK01 | pMMB206 lacIqPtaclacUV5::araE Cmr | This work |
| pAK02 | pMMB207 lacIqPtac::araE Cmr | This work |
| pCSAK50 | pTC40 PBAD::gfpuv Apr | This work |
| pGFPuv | pUC18 gfpuv Apr | Clontech |
| pKKATEB | pKK223-3 Ptac::araFGH Kanr Apr | 5 |
| pTC40 | pBAD24 PBAD::lacZ Apr | 11 |
Cmr, chloramphenicol resistance; Apr, ampicillin resistance; Kanr, kanamycin resistance.
Plasmids were transformed into E. coli by electroporation using an E. coli Pulser (Bio-Rad Inc., Hercules, Calif.) with a field strength of 18 kV/cm. Electrotransformants were selected on LB agar containing the appropriate antibiotics. Plasmids were prepared by standard plasmid purification techniques (16) or with the Qiagen spin isolation kit (Qiagen Inc., Valencia, Calif.).
All cloning was done using standard techniques (16) in commercially available E. coli DH10B. The gene encoding the low-affinity, high-capacity arabinose transporter, araE, of E. coli was amplified from the genomic DNA of E. coli W3110 (prepared by the method of Pospiech and Neumann [15]) using PCR and the primers for the 5′ end of the gene (5′-CACGAATTCGTCTTACTCTCTGCTGGCAG-3') and the 3′ end of the gene (5′-TATGTCGACAACGGCCAAGTGCCCAATCT-3′ or 5′-CGCAAGCTTAACGGCCAAGTGCCCAATCT-3′). These PCR products were digested with EcoRI and SalI or with EcoRI and HindIII and then cloned into the RSF1010-derived plasmids pMMB206 and pMMB207 (12) under control of PtaclacUV5 and Ptac promoters, forming plasmids pAK01 and pAK02, respectively. Plasmids pAK01, pAK02, and pKKATEB (carrying the araFGH genes encoding the low-capacity, high-affinity system under control of Ptac promoter) were introduced into the transport-deficient strains CW2549, CW2553, and CW2587 and the parental strain CW2513 (5).
Plasmid pCSAK50 (containing the gfp gene under control of the araBAD promoter) was constructed by placing a 0.7-kb PCR fragment containing an altered gfpuv gene into pTC40, which contained the lacZ gene under control of the PBAD promoter (2). In short, the gfpuv gene from the pGFPuv vector (Clontech, Palo Alto, Calif.) was modified by PCR and gene splicing by overlap extension to remove the XhoI and SalI restriction sites inside the coding region so that the gene could be cloned into pTC40 (2). The modifications were designed to ensure that no amino acid was changed in removing restriction sites. The gfpuv gene was inserted after PBAD immediately upstream of the lacZ gene into the BstEII and Asp718 restriction sites. Finally, the lacZ gene was deleted using the PvuII restriction endonuclease, and the resulting DNA fragment was ligated to form pCSAK50.
Induction experiments.
Induction experiments were performed in C medium supplemented with 3.4% glycerol as a carbon source (4). For all constructs with the PBAD promoter, E. coli strains were grown at 37°C under antibiotic selection with or without isopropyl-β-d-thiogalactopyranoside (IPTG) to an optical density at 600 nm (OD600) of 0.6 to 0.8. Cells were collected by centrifugation (15,000 × g), washed in C medium without a carbon source, resuspended in fresh C medium containing antibiotics, glycerol, IPTG, and/or arabinose (for the induction of gene expression) to an OD600 of 0.1 to 0.2, and incubated for 6 h. Samples were taken routinely during the subsequent growth period for analysis.
Culture fluorescence was measured on a Versafluor Fluorimeter (Bio-Rad Inc.) with 360/40-nm-wavelength excitation and 520/10-nm-wavelength emission filters. Flow cytometry was performed on a Beckman-Coulter EPICS XL flow cytometer (Beckman Instruments Inc., Palo Alto, Calif.) equipped with an argon laser (emission at a wavelength of 488 nm and 15 mW) and a 525-nm-wavelength band pass filter. Prior to the analysis, sampled cells were washed with phosphate-buffered saline that had been filtered (filter pore size, 0.22 μm), diluted to an OD600 of 0.05, and placed on ice. For each sample, 30,000 events were collected at a rate between 500 and 1,000 events/s.
l-Arabinose transport assay.
The arabinose transport assay was performed using l-[14C]arabinose (American Radiolabeled Chemicals Inc., St. Louis, Mo.) and a Beckman LS 8000 scintillation counter. All cultures were grown on C medium with antibiotics and were preinduced with IPTG for 6 h to fully induce the transport genes and were not exposed to arabinose prior to addition of radioactive arabinose. Cells were harvested by centrifugation (12,000 × g), washed in C medium without arabinose, and resuspended in fresh medium to an OD600 of 0.5. An aliquot of cells (0.5 ml) was added to 0.5 ml of medium at 37°C containing l-[14C]arabinose. The final arabinose concentration was 0.02% (1.33 mM) and had a specific activity of 55 μCi/mmol. Samples were placed at 37°C in a rotating incubator for 2 min. After incubation, the cells were filtered onto a 0.22-μm-pore-size Millipore membrane at room temperature (17) and washed with 10 ml of C medium containing 0.02% unlabeled arabinose. Filters were transferred into scintillation vials, filled with CytoScint scintillation liquid (ICN Biomedicals, Irvine, Calif.), shaken, and counted. A sample without cells was used to determine the background signal from nonspecific adsorption of labeled arabinose to the filters. Transport-deficient strains containing only control vectors (no transport genes) could not grow on minimal medium with arabinose as a carbon source and allowed no arabinose transport when induced with IPTG, arabinose, or both. Nonetheless, nonspecific adsorption of arabinose to cells was observed. It represented less than 10% of total radioactivity accumulated by catabolism-deficient and transport-deficient strains. All results presented here were corrected for such adsorption as well as for radioactivity nonspecifically bound to the filter.
RESULTS
Arabinose transport.
To determine if the cloned and overexpressed araE gene was functional, an arabinose transport assay was performed. Since in any inducible system inducer must be transported into the cell, knowledge of its transport is important for understanding the effect it may play on the promoter modulation. Under normal conditions, arabinose is transported into E. coli via arabinose permeases. (AraE and AraFGH) and any changes in transport efficiency will influence intracellular sugar pools and affect PBAD induction.
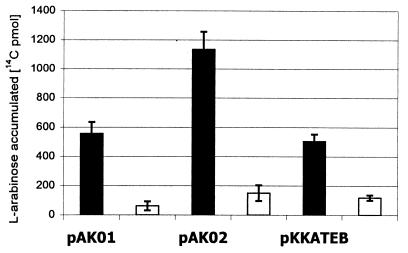
l-Arabinose accumulation experiments were performed in E. coli CW2587, which is devoid of functional chromosomal copies of both high- and low-affinity transport systems (5). IPTG-induced E. coli CW2587 carrying pAK02 (Ptac::araE) accumulated approximately twofold-more arabinose than CW2587 carrying pAK01 (PtaclacUV5::araE) and CW2587 carrying pKKATEB (Ptac::araFGH) (Fig. 2). In the presence of IPTG, the cells containing pAK01 or pAK02 accumulated eightfold-more arabinose than the uninduced controls with each plasmid, while the cells containing pKKATEB accumulated fourfold-more arabinose than the control. The arabinose accumulation data presented above clearly demonstrated that (PtaclacUV5::araE, Ptac::araE, and Ptac::araFGH are IPTG responsive. That the plasmid-encoded araE genes were necessary for arabinose transport was confirmed by testing growth of the various strains on minimal solid medium containing arabinose. In the absence of a plasmid-encoded araE (pAK01 or pAK02), CW2513 was the only strain able to grow on C medium with arabinose as a carbon source. All transport-deficient strains harboring plasmid pAK02 (Ptac::araE) grew well on agar plates containing arabinose as a carbon source with or without induction using IPTG.
FIG. 2.
l-[14C]arabinose accumulation by E. coli CW2587 with various transporter-reporter systems. Cells harbored arabinose transport genes on pAK01 (PtaclacUV5::araE), pAK02 (Ptac::araE), or pKKATEB (Ptac::araFGH) and the reporter gene on pCSAK50 (araC-PBAD::gfpuv). Data for cultures preinduced with 32 μg of IPTG per ml for 6 h prior to addition of l-[14C]arabinose (black bars) and uninduced cells (open bars) are shown. Assays were performed 2 min after radioactive arabinose was added. All numbers are the averages of triplicate experiments.
Induction experiments.
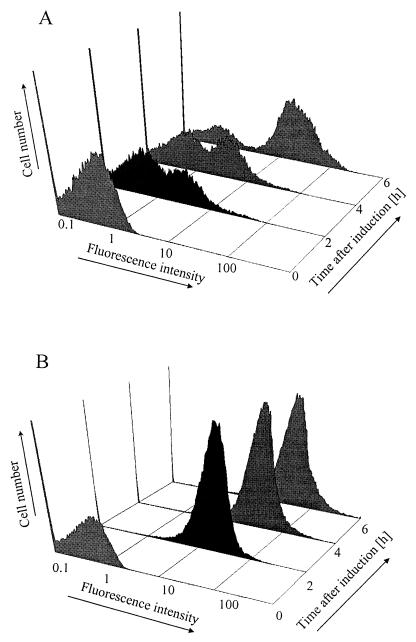
Flow cytometry experiments were conducted to examine the homogeneity of gene expression in several strains with mutations in none, one, or both of the arabinose transport systems and containing the IPTG-inducible arabinose transport gene on pAK02 (Ptac::araE) and the gfp gene under control of PBAD promoter pCSAK50 (PBAD::gfp). Flow cytometry analysis showed that the fluorescence of individual cells could be reliably detected (Fig. 3 and 4). After only 2 h of induction with arabinose, the cultures produced enough green fluorescent protein (GFP) to give a quantifiable fluorescence signal. Prior to arabinose addition (time zero), both cultures displayed background fluorescence intensities. Two hours after induction, cultures of CW2513 had two distinct subpopulations (Fig. 4A). With time, the mean fluorescence of the subpopulation that was fully induced increased, and the fraction of the population that was uninduced decreased. Six hours after induction, only 10 to 15% of the cells remained uninduced. In contrast to the results obtained with CW2513, cultures of CW2587 had a homogeneous population at all times after induction (Fig. 4B).
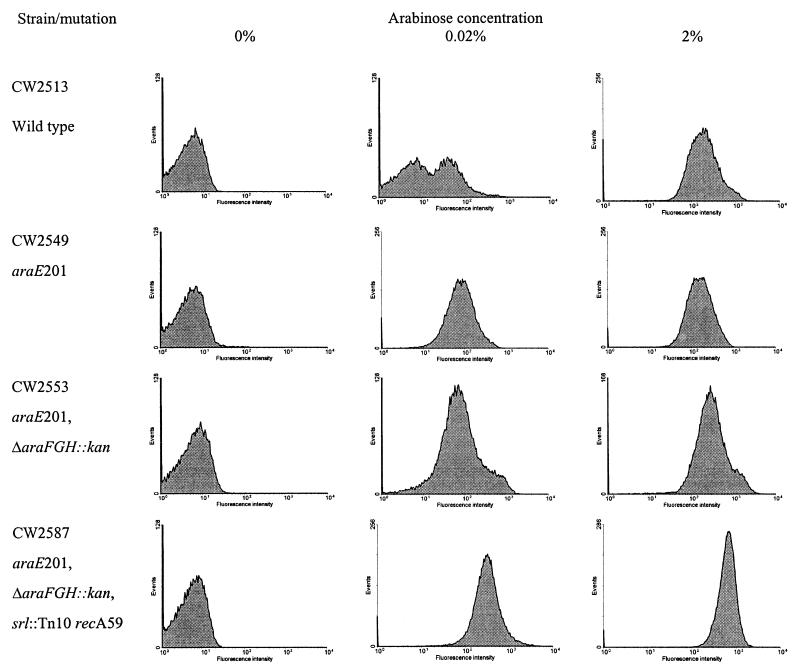
FIG. 3.
Population homogeneity of various E. coli strains harboring the transporter-reporter plasmid system. All cultures harbored the transporter gene on pAK02 (Ptac::araE) and reporter gene on pCSAK50 (araC-PBAD::gfpuv). Cells were grown in the presence of 32 μg of IPTG per ml. Flow cytometry analysis was performed 4 h after addition of arabinose. In control experiments, all strains harbored pCSAK50 and pMMB207. Experiments were performed in triplicate. Results from a single experiment are presented.
FIG. 4.
Time course of fluorescence intensity in E. coli CW2513 (A) and CW2587 (B) harboring pAK02 (Ptac::araE) and pCSAK50 (araC-PBAD::gfpuv). All cells were preinduced with 32 μg of IPTG per ml for 6 h prior to arabinose induction. Arabinose (0.02%) and IPTG (32 μg/ml) were added to the culture at time zero.
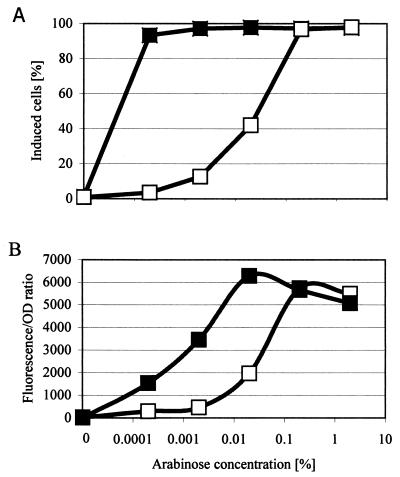
The population-averaged fluorescence and the fraction of the CW2513 population induced varied with the arabinose concentration (Fig. 5). In contrast, CW2587 harboring both transporter and reporter plasmids was induced homogeneously at all arabinose concentrations tested (0.0002% to 2%) and all times after addition of inducer. The population-averaged fluorescence increased with arabinose concentration; at subsaturating arabinose concentrations (0.0002 to 0.02%), the population-averaged fluorescence increased linearly with the log of the arabinose concentration and was about three times higher than that of CW2513 cultures. In addition, CW2587 was homogeneously induced at arabinose concentrations as low as 0.0002%, 3 orders of magnitude lower than the minimal concentration necessary for homogeneous induction of CW2513.
FIG. 5.
GFP expression as function of the arabinose concentration in cultures of E. coli CW2513 (open squares) and CW2587 (filled squares) harboring pAK02 (Ptac::araE) and pCSAK50 (araC-PBAD::gfpuv). Cells were preinduced with 32 μg of IPTG per ml. Six hours after addition of arabinose, the percentage of the population that was induced (A) and the population-averaged fluorescence (Fluorescence/OD ratio) (B) were determined.
To determine the gene locus primarily responsible for the all-or-none response, induction experiments were performed on several strains with and without chromosomal copies of arabinose transporter genes. The wild-type parental strain (CW2513), containing plasmids pAK02 and pCSAK50 and a functional chromosomal copy of araE, displayed two subpopulations at intermediate arabinose concentrations (Fig. 3) (the intermediate concentration of 0.02% is shown as representative of all intermediate concentrations). In contrast, the araE-deficient CW2549 and araE- and araFGH-deficient CW2553 and CW2587 strains displayed a single, homogeneous population when induced with the same intermediate concentrations of arabinose. At a saturating arabinose concentration (2%), all cultures displayed a single population. In a negative-control experiment, all strains were transformed with pCSAK50 and the pMMB207 cloning vector (no araE); only CW2513 was induced and showed two populations at intermediate arabinose levels (data not shown).
DISCUSSION
Inducible promoter systems have been used extensively to control and probe cellular processes and to express heterologous genes for high-level protein production. For many years, it has been known that the lacZYA operon suffers from an all-or-none expression response (11, 14). Recently, it was shown that the ara operon has similar characteristics (18). These carbohydrate-responsive systems have evolved this all-or-none method of gene expression to allow low-level expression in the absence of the substrate and rapid, high-level response when the substrate is present. These characteristics are ideal for overexpression of a heterologous gene; in the absence of inducer, the gene is not expressed, and in the presence of inducer, the gene is overexpressed. Unfortunately, these characteristics are not suitable for the subtle and regulatable control of gene expression that may be essential for probing cellular processes and for manipulating cellular metabolism. These reasons have motivated the work described above.
The all-or-none or autocatalytic phenomenon arises because the transporter for the inducer is under control of the inducer itself. Upon exposure to an inducer, the inducer is transported into the cell by some minimal number of transporters in the membrane. If a threshold level of inducer accumulates inside the cell, the transporter gene and any other associated genes are induced. The production of more transport protein and the subsequent import of more inducer rapidly cascades to maximal gene expression.
Several strategies can be used to decouple autocatalytic systems. One strategy is to place the gene for the transport system under control of a promoter that is not regulated by the inducer that the transport system transports. This can be accomplished by transforming a transport-deficient strain or a strain carrying a low-capacity transport system with a plasmid that carries an independently regulated transporter. The second strategy is to synthesize an inducer analog that can diffuse across the cell membrane without a transport system (e.g., IPTG). In the latter case, one may need to use a transport-deficient strain, as an inducer analog may be transported by the same transport proteins that act on the natural inducer and suffer the same all-or-none regulation. Hence, we chose the former strategy.
The high-capacity arabinose transporter system has been studied extensively (7–10, 17). AraE is a proton symporter, in contrast to AraFGH, which is binding protein dependent. In this work, the expression of araE was engineered to be dependent on the IPTG concentration in the growth medium (Fig. 1). The vectors carrying araE contained either a strong Ptac promoter or a moderate PtaclacUV5 promoter and the gene encoding the lac repressor (laclq) for tight control over expression. In addition, expression of araE from the low-copy-number RSF1010-based plasmids (approximately 12 copies per cell) minimized any possible toxic effects of overexpression of membrane-associated proteins.
Our results showed that plasmid encoded L-arabinose transport in the transport-deficient strains was not completely dependent on IPTG. Even in the absence of IPTG, PBAD was induced above basal expression levels in the presence of arabinose. However, the more tightly regulated PtaclacUV5 promoter system had a lower basal expression level and consequently less arabinose accumulated inside the cells. There are two potential reasons. (i) The Ptac promoter was leaky. (ii) Arabinose was transported via other transporters. In the early experiments with the Ptac::araFGH system (pKKATEB), Horazdovsky and Hogg (5) found that even in the absence of the inducer, a significant level of l-arabinose accumulated in a similar strain. Thus, the basal expression level of the transport gene from the plasmid might be high enough to allow transport of arabinose in amounts sufficient for induction of arabinose-dependent promoters. In any case, the gene encoding the transport protein would be controlled independently of arabinose and should not suffer from all-or-none regulation.
As demonstrated above, IPTG preinduction led to a significant increase in arabinose transport at intermediate arabinose levels. In this case, the cultures were exposed to arabinose for a very short time, so there was minimal effect of arabinose on the expression of genes encoding the transport proteins. However, when both IPTG and arabinose were present in the culture broth for a long period of time, there was a significant inhibition of radioactively labeled arabinose accumulation (data not shown). Most likely, the unlabeled arabinose that accumulated inside the cells inhibited further accumulation of labeled arabinose.
Introduction of an independently regulated transporter in CW2513 had no effect on population homogeneity. In contrast, expression of the independently regulated transporter in the araE mutant strains CW2549, CW2553, and CW2587 resulted in a single population in cultures preinduced with IPTG. In the case of CW2587, a homogeneous population was achieved even at the lowest arabinose level tested (0.0002%), at least 100-fold-less inducer than was needed to induce all of the other strains to comparable levels. Through the entire range of arabinose concentrations, the transport-deficient cultures demonstrated higher specific and overall GFP production compared to that of wild-type CW2513.
It should be noted that the recA strain CW2587 had a more uniform population than the recA+ strains CW2549 and CW2553. As recA is known to affect plasmid stability, this result is not surprising. Nevertheless, the recA+ strains have homogeneous populations at all arabinose concentrations.
Finally, at saturating arabinose concentrations (2%), a decline in the specific fluorescence was observed. This phenomenon might be ascribed to arabinose catabolism, resulting in a lower internal arabinose concentration and lower GFP production per cell.
The presence of a functional araE gene on the chromosome would appear to be primarily responsible for the all-or-none response. However, it is not entirely clear why the plasmid-encoded transport system did not alleviate the all-or-none response in the araE+ CW2513. It is possible that once arabinose was transported into CW2513, expression from the chromosomal copy of the arabinose transport gene was higher than the low-level, IPTG-induced expression from the plasmid.
In summary, the results presented here show that replacing the native inducer transport system with one under independent control can eliminate the all-or-none response characteristic of many inducible systems. Furthermore, by placing the transport system under independent control, the resulting inducible promoter is regulatable with significantly lower inducer concentrations. This design should prove useful in metabolic redesign of cells and should serve as a prototype for redesign of other inducible promoter systems suffering from the all-or-none phenomenon.
ACKNOWLEDGMENTS
We thank R. W. Hogg for providing E. coli strains and pKKATEB, K. L. Jones and C. D. Smolke for stimulating discussions, L. Hua for excellent technical assistance, and H. Nolla for assistance with the flow cytometry.
This research was supported in part by the ERC Program of the National Science Foundation under award number EEC-9731725, by National Science Foundation grant BES-9502495, and by a Swiss National Scientific Foundation fellowship (Bourse de Relève du FNRS) to A.K.
REFERENCES
- 1.Carrier T A, Keasling J D. Investigating autocatalytic gene expression systems through mechanistic modeling. J Theor Biol. 1999;201:25–36. doi: 10.1006/jtbi.1999.1010. [DOI] [PubMed] [Google Scholar]
- 2.Carrier T A, Keasling J D. Library of synthetic 5′ secondary structures to manipulate mRNA stability in Escherichia coli. Biotech Prog. 1999;15:58–64. doi: 10.1021/bp9801143. [DOI] [PubMed] [Google Scholar]
- 3.Guzman L M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmstetter C E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968;31:507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- 5.Horazdovsky B F, Hogg R W. Genetic reconstitution of the high-affinity l-arabinose transport system. J Bacteriol. 1989;171:3053–3059. doi: 10.1128/jb.171.6.3053-3059.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keasling J D. Gene expression tools for metabolic engineering of bacteria. Trends Biotechnol. 1999;17:452–460. doi: 10.1016/s0167-7799(99)01376-1. [DOI] [PubMed] [Google Scholar]
- 7.Kolodrubetz D, Schleif R. l-Arabinose transport systems in Escherichia coli K-12. J Bacteriol. 1981;148:472–479. doi: 10.1128/jb.148.2.472-479.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolodrubetz D, Schleif R. Regulation of the l-arabinose transport operons in Escherichia coli. J Mol Biol. 1981;151:215–227. doi: 10.1016/0022-2836(81)90512-x. [DOI] [PubMed] [Google Scholar]
- 9.MacPherson A J, Jones-Mortimer M C, Henderson P J. Identification of the AraE transport protein of Escherichia coli. Biochem J. 1981;196:269–283. doi: 10.1042/bj1960269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiden M C, Jones-Mortimer M C, Henderson P J. The cloning, DNA sequence, and overexpression of the gene araE coding for arabinose-proton symport in Escherichia coli K12. J Biol Chem. 1988;263:8003–8010. [PubMed] [Google Scholar]
- 11.Maloney P C, Rotman B. Distribution of suboptimally induced β-D-galactosidase in Escherichia coli. The enzyme content of individual cells. J Mol Biol. 1973;73:77–91. doi: 10.1016/0022-2836(73)90160-5. [DOI] [PubMed] [Google Scholar]
- 12.Morales V M, Bäckman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 13.Newman J R, Fuqua C. Broad-host-range expression vectors that carry the l-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene. 1999;227:197–203. doi: 10.1016/s0378-1119(98)00601-5. [DOI] [PubMed] [Google Scholar]
- 14.Novick A, Weiner M. Enzyme induction as an all-or-none phenomenon. Proc Natl Acad Sci USA. 1957;43:553–556. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pospiech A, Neumann B. A versatile quick-prep of genomic DNA from Gram-positive bacteria. Trends Genet. 1995;11:217–218. doi: 10.1016/s0168-9525(00)89052-6. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Maniatis T, Fritsch E F. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 17.Schleif R. An l-arabinose binding protein and arabinose permeation in Escherichia coli. J Mol Biol. 1969;46:185–196. doi: 10.1016/0022-2836(69)90065-5. [DOI] [PubMed] [Google Scholar]
- 18.Siegele D A, Hu J C. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc Natl Acad Sci USA. 1997;94:8168–8172. doi: 10.1073/pnas.94.15.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]