Abstract
Objective
Robot‐assisted surgery has been promoted worldwide in recent years. The development of a domestic orthopaedic robot and its clinical application are therefore of great significance. This study aimed to compare the early clinical and radiographic outcomes of domestic robot‐assisted total knee arthroplasty (RA‐TKA) with conventional manual total knee arthroplasty (CM‐TKA).
Methods
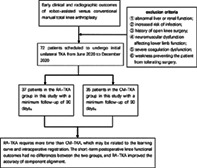
A total of 77 patients who underwent primary single‐sided TKA from June to December 2020 were prospectively enrolled; resulting in the inclusion of 72 patients. The patients were randomly divided into the RA‐TKA group (37 cases, with TKA being assisted by the Yuanhua Orthopaedic Robotic System) and the CM‐TKA group (35 cases, with TKA being performed using conventional tools). Knee function was evaluated by the knee range of motion (ROM), the American Knee Society Score (KSS), and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Postoperative radiographic results were evaluated by full‐length weight‐bearing X‐rays of the lower limb and anteroposterior and lateral X‐rays of the knee were obtained preoperatively and at 90 days postoperative. The operative duration, blood loss, postoperative knee function, radiographic outcomes, and incidence of complications were compared by Student's t‐test, Mann–Whitney U test, or chi‐square test. Serum levels of inflammatory markers before the operation and 1, 3, and 30 days after the operation were recorded and compared between the two groups.
Results
The operation was significantly longer in the RA‐TKA group than in the CM‐TKA group (154.3 vs 115.2 min, p < 0.001). There was no significant difference in blood loss (933 vs 863 ml, p = 0.519) between the two groups. The knee ROM, KSS, and WOMAC were significantly improved in both groups 90 days after the operation compared with before the operation (p < 0.05), but there were no significant differences between the two groups (p > 0.05). The incidence of postoperative deep vein thrombosis was not statistically different between the two groups. In the radiographic findings at 90 days postoperatively we found the frequency of lateral tibial component (LTC) angle outliers was significantly lower in the RA‐TKA group (3.0% vs 29.4%, p = 0.003). The neutrophil‐to‐lymphocyte ratio (NLR) was significantly lower in the RA‐TKA group than in the CM‐TKA group on day 1 after surgery (9.9 vs 12.7, p = 0.024).
Conclusions
RA‐TKA requires more time than CM‐TKA, which may be related to the learning curve and intraoperative registration. The short‐term postoperative knee functional outcomes had no differences between the two groups, and RA‐TKA improved the accuracy of tibial component alignment. Further follow‐up studies are required to investigate the long‐term outcomes.
Keywords: Component alignment, conventional Manual total knee arthroplasty, Functional outcomes, Inflammatory response, Robot‐assisted total knee arthroplasty
We compared the early clinical outcomes of the RA‐TKA and CM‐TKA groups through a prospective study. The advantages of RA‐TKA in terms of radiographic results as well as inflammatory indicators and the disadvantages of prolonging the surgery are described.
Introduction
Knee osteoarthritis (KOA) is a chronic joint disease. As the disease progresses patients often develop knee pain, stiffness, and deformity, limiting the patient's activities 1 . Total knee arthroplasty (TKA) has long been considered the definitive treatment for KOA, and successful TKA can reduce joint pain and restore function. Although there have been tremendous improvements in surgical techniques and postoperative rehabilitation, approximately 20% of patients are unsatisfied after primary TKA 2 , with anterior knee pain as one of the most common causes of dissatisfaction 3 . Other causes reported in the literature include polyethylene liner wear and knee instability 4 , which are all related to improper prosthetic component placement 5 . In previous TKA surgeries, especially in patients with severe KOA, it was difficult for even experienced surgeons to ensure precise intraoperative placement of the prosthesis due to the severe deformity of the knee. The results of lower extremity force line reconstruction with conventional manual total knee arthroplasty (CM‐TKA) depend on the operator's surgical experience and the standardization of the procedure. A large number of reports have shown that the use of robot‐assisted total knee arthroplasty (RA‐TKA) can improve knee component alignment 6 , 7 , 8 , 9 . The ultimate aim of any new technique is to achieve better clinical results, knee pain and function are the most subjective and important parts of the knee after TKA surgery, but there has been controversy about whether the robot will result in better pain and function scores. One study compared the long‐term follow‐up results of RA‐TKA with those of CM‐TKA and found no difference in joint function or pain scores between the two group 10 . Other studies, however, have suggested that RA‐TKA can help improve patient satisfaction 6 , 7 .
Opponents of this new technology argue that it will prolong the surgery and add additional tests and costs. The increased surgery time in RA‐TKA is of clear clinical relevance as increasing surgery time has been shown to increase the risk of peri‐prosthetic joint infections 11 . Proponents, on the other hand, argue that the operative time for RA‐TKA does not differ significantly from CM‐TKA as long as the learning curve is passed 12 . Some investigators have concluded from analysis of inflammatory indicators of blood that RA‐TKA will reduce injury. The measurement of serum markers of inflammation provides an objective method for assessing the relative invasiveness of a surgery 9 . However, such studies are currently scarce and do not provide a comprehensive description of the types of inflammatory markers and the timing of changes.
In recent years, a variety of different robotic systems for assisting orthopaedic surgery (with or without images, different cutting systems, and different planning methods) have been promoted worldwide. However, the purchase and promotion of these imported robots can only be implemented in some large tertiary hospitals in China due to the limitations of intellectual property rights and the difficulty of subsequent maintenance. The development of a domestic orthopaedic robot with independent intellectual property rights and its clinical application are therefore of great significance. In June 2020, we completed the first domestic RA‐TKA procedure in China, with satisfactory initial clinical results. However, there have been no validated clinical randomized controlled trials (RCTs) to verify its short‐ and long‐term clinical efficacy. The objective is: (i) to evaluate surgery‐related outcomes and early postoperative function in RA‐TKA and CM‐TKA; (ii) to verify whether RA‐TKA can obtain a more precise prosthesis position in the imaging results at 90 days postoperatively compared to CM‐TKA; (iii) to investigate whether there is a difference in inflammatory response from the two groups in the early postoperative period.
Materials and Methods
Patients
This study was registered in the Chinese Clinical Trials Registry (Registration number: ChiCTR2100042323) and approved by the ethics committee of Chinese PLA General Hospital (S2020‐005‐01). A total of 77 patients scheduled to undergo initial unilateral TKA from June 2020 to December 2020 were prospectively included; five patients voluntarily withdrew from the trial for personal reasons, resulting in 72 cases being included for analysis. Regarding patients understanding of the benefits and risks of this trial, willingness to participate, and provision of written informed consent was obtained, informed consent was obtained, and the patients were randomized into groups using an online number generator (www.random.org). There were 37 patients in the RA‐TKA group and 35 patients in the CM‐TKA group. The inclusion criteria were as follows: (i) age 18–80 years, with no gender restriction; (ii) diagnosis of end‐stage KOA unresponsive to conservative treatment; (iii) determination of the requirement for initial unilateral TKA by the investigator. The exclusion criteria were as follows: (i) abnormal liver or renal function (alanine transaminase or aspartate transaminase >1.5 times normal, blood urea nitrogen >8.3 mmol/L, serum creatinine >115 μmol/L); (ii) diabetes mellitus and poorly controlled blood glucose level leading to an increased risk of infection, as judged by the investigator; (iii) history of open knee surgery; (iv) neuromuscular dysfunction affecting lower limb function; (v) severe coagulation dysfunction; and (vi) concomitant severe medical or surgical disease or weakness preventing the patient from tolerating surgery. Dropout criteria for this study were intolerance of adverse effects of the procedure, patient desire for alternative treatment, or no reason for voluntary withdrawal from the trial.
There was no significant difference between the RA‐TKA and CM‐TKA groups in terms of age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, operative side, or preoperative deformity (p > 0.05) (Table 1).
TABLE 1.
Demographic data and preoperative radiographic aberrations in the RA‐TKA and CM‐TKA groups
| Parameter | RA‐TKA (n = 37) | CM‐TKA (n = 35) | Statistical value | p value |
|---|---|---|---|---|
| Age (years) | 64.5 (5.3) | 63.4 (7.2) | t = 0.807 | 0.422 a |
| Number of males/females (n, %) | χ2 = 0.908 | 0.341 b | ||
| Males | 11 (29.7) | 7 (20) | ||
| Females | 26 (70.3) | 28 (80) | ||
| BMI (kg/m2) | 26.2 (3.8) | 26.4 (3.1) | t = −0.286 | 0.776 a |
| ASA class (n, %) | 0.671 c | |||
| I | 1 (2.7) | 1 (2.8) | ||
| II | 35 (94.5) | 31 (88.5) | ||
| III | 1 (2.7) | 3 (8.5) | ||
| Operative side (n, %) | χ2 = 0.878 | 0.349 b | ||
| Left | 21 (56.8) | 16 (45.7) | ||
| Right | 16 (43.2) | 19 (54.3) | ||
| HKA angle (°) | 171.0 (4.0) | 171.0 (4.0) | t = 0.041 | 0.968 a |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CM‐TKA, conventional manual total knee arthroplasty; HKA, hip‐knee‐ankle; RA‐TKA, robot‐assisted total knee arthroplasty
Student's t‐test
Pearson chi‐square test
Fisher's exact test.
Surgical Techniques and Postoperative Care
All surgeries were performed by two senior joint surgeons. A tourniquet was applied intraoperatively, and all patients underwent surgery using a standard medial parapatellar approach with patellar eversion. In both groups, the joint was replaced with a unified fixed‐platform and posterior cruciate ligament sacrificing prosthesis (Unique knee, Zhengtian, Tianjin, China).
Surgical Steps of CM‐TKA
CM‐TKA was completed using the measured osteotomy method. Proximal tibial osteotomy was performed using an extramedullary positioning system with a 3°–5° posterior inclinaton in the sagittal plane and perpendicular to the mechanical axis in the coronal plane of the tibia. The distal femur was osteotomized using an intramedullary positioning system at an angle of 5°–7° valgus based on the preoperative measurement of the lower limb force line and the anatomical axis of the femur. The posterior condyles were osteotomized at an angle 3°–5° of external rotation to the posterior condylar line base on the transepicondylar axis. After completion of the osteotomy, a trial mold was fitted to assess the soft tissue balance; moderate soft tissue release was performed to provide a balanced, symmetrical flexion‐extension gap and neutral mechanical limb alignment. The prosthesis was fitted after confirmation that the flexion‐extension gap was balanced.
Surgical Steps of RA‐TKA
The RA‐TKA group used a surgical assist system (YUANHUA‐TKA, Shenzhen, China) in the following steps. Step 0, Preoperative planning: Anatomical landmark identification based on preoperative 3D CT reconstructions was performed to plan the osteotomy. Step 1, Stabilization pins implanted: Femoral stabilization pins were placed in the femoral stem 8–10 cm from the top edge of the patella, while tibial stabilization pins were inserted 6–8 cm below the tibial tuberosity on the medial surface of the tibia; bone movement monitors were attached to these pins to achieve optical motion capture tracking. Step 2, Registration: Intraoperative registration of the femur and tibia was performed using a probe after routine incision exposure (Fig. 1). After registration, the system entered the “Planning Module.” Step 3, Osteotomy: When prompted for a feasible osteotomy, the operator operated the pendulum saw and started cutting the bone with the assistance of the robotic arm. The part to be removed was shown in green in the femur and tibia model. During the osteotomy process, the system automatically adjusted the position and angle of the saw at the end of the arm to align the saw blade with the current plane of osteotomy and restrict the operator to the current plane of osteotomy. When the green part of the navigation view was cleared, the current osteotomy was completed. Step 4, Evaluation and Prosthesis Placement: After the osteotomy was completed, a trial mold was fitted to assess the mobility and stability of the knee joint, surgeons also performed soft tissue releases if necessary and the prosthesis was fitted after confirming correct mobility and stability.
Fig. 1.
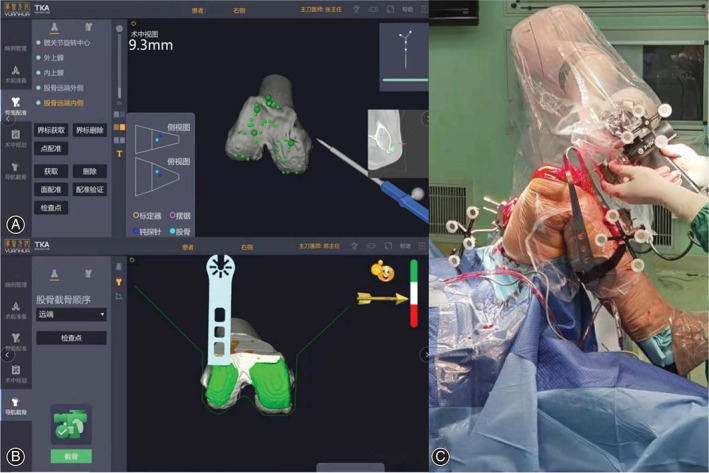
(A) Registration of the distal femur prior to osteotomy. (B) Surgical software interface, green represent the safe range of osteotomy. (C) Robotic arm‐assisted osteotomy
Postoperative Care
Postoperatively, both groups adopted the clinical pathway of Enhanced Recovery After Surgery (ERAS) 13 , patients were treated with a uniform anti‐infective, analgesic, and anticoagulant regimen. On the day after surgery, patients began weight‐bearing exercises with the aid of a floor walker as well as active and passive knee flexion and extension exercises in a non‐weight‐bearing state. Postoperative incision healing and complications, such as vascular nerve damage, were documented.
Functional Assessments
The duration of surgery and length of hospital stay were recorded in both groups, and the theoretical blood loss was calculated according to the hemoglobin balance method 14 , 15 . Knee function was evaluated by the preoperative and 90‐day postoperative knee flexion‐extension range of motion (ROM), American Knee Society Score (KSS) 16 , and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) 17 . All patients underwent color Doppler ultrasound examination of the lower limb vessels at 30 days postoperatively to assess the incidence of lower limb thrombosis.
Radiographic Assessments
Full‐length weight‐bearing X‐rays of the lower limb and anteroposterior and lateral X‐rays of the knee were obtained preoperatively and at 90 days postoperative for determination of the following angles: (i) the tangent of the medial and lateral femoral condyles is the lateral transverse axis of the femur of the knee. The angle between this axis and the mechanical axis of the femur is measured as the frontal femoral component (FFC; optimum, 90°); (ii) using the medial and lateral tibial plateau tangents as the lateral transverse tibial axis, measure the angle between this axis and the mechanical axis of the tibia frontal tibial component (FTC; optimum, 90°); (iii) the angle between the axis of the lateral femoral prosthesis and the anatomical axis of the femur as the lateral femoral component (LFC; optimum, 0°); (iv) angle between the transverse axis of the lateral tibial prosthesis and the anatomical axis of the tibia as the lateral tibial component (LTC; optimum, 87°, determined based on the characteristics of the prosthesis used and after consultation with clinicians); and (v) hip‐knee‐ankle (HKA; optimum, 180°) (Fig. 2). An outlier was defined by a difference of ≥3° from the optimum value 18 .
Fig. 2.
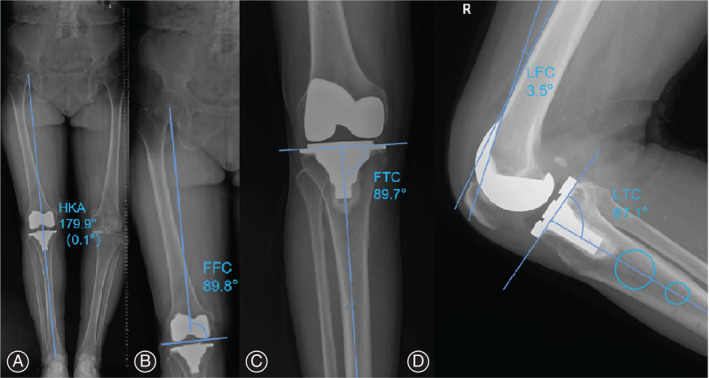
Measurement of evaluated angles. (A) Measurement of the postoperative hip‐knee‐ankle angle. (B) Measurement of the frontal femoral component angle. (C) Measurement of the frontal tibial component angle. (D) Measurement of the lateral femoral component and lateral tibial component angles
Each angle measurement was performed independently by two experienced orthopaedic surgeons not involved in the clinical trial using Digimizer (version 5.4.3, MedCalc Software, Ostend, Belgium) software, and the final results were averaged between the two. If the difference between the two values was excessive (≥0.5°), a third senior orthopaedic surgeon, who was not involved in the clinical trial, took the measurements, and the final result was the average of the two similar measurements out of the three.
Measurement of Inflammatory Indicators
Patients' venous blood indices were recorded preoperatively and 1, 3, and 30 days postoperatively. The C‐reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), and neutrophil‐to‐lymphocyte ratio (NLR) were analyzed and compared based on serum samples from both groups to assess trends in the early postoperative inflammatory response.
Statistical Analysis
SPSS 26.0 statistical software (version 26.0, IBM SPSS, NY, USA) was used to perform statistical analysis of the relevant data. Trends in inflammatory indexes were plotted using prism (version 9.3.1, GraphPad Software, San Diego, USA). The Kolmogorov–Smirnov test was used to assess whether the variables followed a normal distribution. Based on the result of the normality test, independent‐samples t tests or Mann–Whitney U tests were used to analyze continuous variables. Data that did not conform to a normal distribution are expressed as the median (Q1, Q3), and measures that conformed to a normal distribution are expressed as the mean (SD), and independent‐samples t tests were used for comparisons between groups. Chi‐square or Fisher's exact tests were used to compare categorical data. Differences were considered statistically significant at p < 0.05.
Results
General Results
All surgery were completed successfully. The operative duration was significantly longer in the RA‐TKA group than in the CM‐TKA group (154.3 vs 115.2 min, p < 0.001). There was no significant difference between the two groups in terms of the length of hospital stay (9.1 vs 8.4 days, p = 0.175) or theoretical intraoperative blood loss (933.0 vs 863.4 ml, p = 0.519).
Complications and Functional Outcomes
The postoperative incisions all showed stage I healing, with no complications, such as poor incision healing or vascular or nerve injuries. No serious complications such as acute PJI or non‐healing incision occurred in either group. Doppler color ultrasound examination of both lower limb veins at 30 days postoperatively showed lower limb venous thrombosis in 10 patients in the RA‐TKA group compared to 11 patients in the CM‐TKA group, with no significant difference in the incidence of lower limb venous thrombosis between the two groups (27.8% vs 31.4%, p = 0.736). No significant differences existed between the two groups in terms of the ROM, KSS, or WOMAC preoperatively or at 90 days postoperatively (p > 0.05) (Table 2).
TABLE 2.
Surgical data and clinical outcomes in the CM‐TKA and RA‐TKA groups
| Parameter | RA‐TKA (n = 37) | CM‐TKA (n = 35) | Statistical value | p value |
|---|---|---|---|---|
| Preoperative functional assessment | ||||
| KSS | 32 (22, 54) | 28 (18, 37) | Z = −1.793 | 0.073 b |
| WOMAC | 159.8 (42.8) | 162.9 (42.4) | t = −0.315 | 0.754 a |
| ROM (°) | 105 (90,120) | 105 (90,120) | Z = −0.361 | 0.718 b |
| Operation and LOS | ||||
| Operative duration (min) | 154.3 (20.7) | 115.2 (30.2) | t = 0.815 | 0.001 a |
| Intraoperative blood loss (ml) | 933.0 (453.7) | 863.4 (457.5) | t = 0.648 | 0.519 a |
| LOS (day) | 9.1 (2.0) | 8.4 (2.1) | t = −1.370 | 0.175 a |
| Complications | ||||
| Day 30 DVT (n, %) | 10 (27.8) | 11 (31.4) | χ2 = 0.114 | 0.736 c |
| Postoperative functional assessment | ||||
| Day 90 KSS | 67 (58.3,72) | 66(61,71) | Z = ‐0.547 | 0.584 b |
| Day 90 WOMAC | 12.5 (13.2) | 19.4 (31.4) | t = −1.206 | 0.232 a |
| Day 90 ROM (°) | 115 (110, 125) | 120 (105, 130) | Z = −0.903 | 0.366 b |
Abbreviations: CM‐TKA, conventional manual total knee arthroplasty; DVT, deep vein thrombosis; KSS, American Knee Society Score; LOS, length of stay; RA‐TKA, robot‐assisted total knee arthroplasty; ROM, range of motion; WOMAC, Western Ontario McMaster Universities Osteoarthritis Index
Student's t‐test
Mann–Whitney U‐test
Pearson chi‐square test.
Radiographic Outcomes
At 90 days postoperative, three patients were unable to attend hospital for review, so a total of 69 patients' radiographic reports were included in this outcome. There were no significant differences in the incidence of HKA (17.1% vs 35.3%, p = 0.086), FFC (5.7% vs 20.6%, p = 0.067), FTC (2.9% vs 8.8%, p = 0.289) or LFC (42.4% vs 55.9%, p = 0.271) angle outliers between the two groups postoperatively. However, the incidence of outliers for the LTC angle was significantly lower in the RA‐TKA group than in the CM‐TKA group (3.0% vs 29.4%, p = 0.001), and the mean LTC angle in the RA‐TKA group was closer to the expected value of 87° (Table 3).
TABLE 3.
Comparison of radiographic findings between the two groups 90 days postoperatively
| Parameter | RA‐TKA (n = 35) | CM‐TKA (n = 34) | Statistical value | p value |
|---|---|---|---|---|
| HKA angle (°) | 178.2(1.2) | 177.4(2.1) | t = 1.988 | 0.052 a |
| Outlier (n, %) | 6 (17.1%) | 12 (35.3%) | χ2 = 2.947 | 0.086 b |
| FFC angle (°) | 91.2 (1.1) | 91.6 (2.2) | t = −0.998 | 0.323 a |
| Outlier (n, %) | 2 (5.7%) | 7 (20.6%) | χ2 = 3.364 | 0.067 b |
| FTC angle (°) | 90.1 (1.5) | 90.2 (1.6) | t = 0.830 | 0.721 a |
| Outlier (n, %) | 1 (2.9%) | 3 (8.8%) | χ2 = 1.124 | 0.289 b |
| LFC angle (°) | 2.9 (2.5) | 3.4 (2.9) | t = −0.816 | 0.417 a |
| Outlier (n, %) | 14 (42.4%) | 19 (55.9%) | χ2 = 1.214 | 0.271 b |
| LTC angle (°) | 87.1 (1.2) | 85.5 (2.2) | t = 3.517 | 0.001 a |
| Outlier (n, %) | 1 (3.0%) | 10 (29.4%) | χ2 = 9.075 | 0.003 b |
Abbreviations: CM‐TKA, conventional manual total knee arthroplasty; FFC, frontal femoral component; FTC, frontal tibial component; HKA, hip‐knee‐ankle; LFC, lateral femoral component; LTC, lateral tibial component; RA‐TKA, robot‐assisted total knee arthroplasty; TKA, total knee arthroplasty
Student's t‐test
Pearson chi‐square test.
Inflammatory Indicators
There were no statistically significant differences (p > 0.05) in the CRP level or ESR between the two groups preoperatively or 1, 3, or 30 days postoperatively (Fig. 3A–C). However, the NLR was significantly lower in the RA‐TKA group 1 day postoperatively than in the CM‐TKA group (9.88 vs 12.65, p = 0.024), while there were no significant differences between the two groups at the remaining time points (p > 0.05).
Fig. 3.
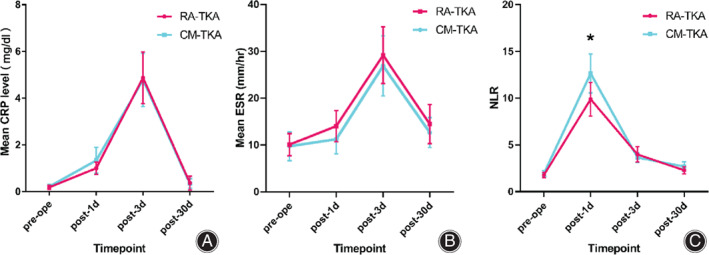
Inflammatory indicators. (A) Trend of the CRP level at four time points. (B) Trend of the ESR at four time points. (C) Trend of the NLR at four time points; The NLR was significantly lower in the RA‐TKA group than in the CM‐TKA group on the first postoperative day (p = 0.024). CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; NLR, neutrophil‐to‐lymphocyte ratio; RA‐TKA, robot‐assisted total knee arthroplasty; CM‐TKA, conventional manual total knee arthroplasty
Discussion
RA‐TKA Prolongs Surgery Time
In this study, the average operative duration in the RA‐TKA group was 42 min longer than that in the CM‐TKA group, which is similar to the results previously reported by Sang‐Woo Jeon et al. using ROBODOC (Curexo Technology Corporation, Sacramento, CA, USA) in robot‐assisted TKA 19 . In robot‐assisted TKA, most of the extra operative time is spent on setup, femoral and tibial fixation, and alignment. This seems to be a disadvantage of RA‐TKA that needs to be improved to shorten the time spent on nonsurgical tasks. In a retrospective study by Vermue et al., it was concluded that the adoption of RA‐TKA varied between operators, with a learning curve ranging between 11 and 43 stations 20 . During the surgery in this study, the major causes of the prolonged surgery time were the tedious steps of registration of important bony landmarks and the pending improvement of the registration success rate. However, this condition will no longer bother the surgeon as one becomes more proficient in the surgery procedure. Thus, differences in the learning curve of different surgeons are also an important reason for the prolonged duration of RA‐TKA.
RA‐TKA and CM‐TKA Have Similar Early Clinical Outcomes
Nikhil Agarwal et al. reviewed 22 published studies that included short‐ and long‐term follow‐up examinations; 12 of these studies found statistically better clinical outcomes for RA‐TKA than CM‐TKA, while nine studies found no difference, and one study did not assess clinical outcomes 6 . After a 2‐year follow‐up of 116 patients who underwent TKA, Yim et al. found no significant difference in knee function scores between the RA‐TKA and CM‐TKA groups. In contrast, a study by Kayani et al. concluded that RA‐TKA resulted in greater pain reduction, improved early functional recovery, and shortened the length of hospital stay 21 . There has been debate regarding whether the use of RA‐TKA results in better postoperative joint function than CM‐TKA. In this study, the ROM, KSS, and WOMAC in the two groups were compared at 90 days postoperatively, and no significant differences were found. This suggests that the clinical outcome of RA‐TKA was not significantly better than that of CM‐TKA in the early postoperative period in this study, but further follow‐up is needed to determine whether there is a difference in the long‐term outcome. The reasons for the different outcomes are unclear and may be related to the surgeon still being on the learning curve, but there have been no reports of RA‐TKA yielding significantly lower postoperative scores than CM‐TKA.
RA‐TKA Has Better Radiographic Results
Batailler et al. analyzed 26 studies on MAKO RA‐TKA and concluded that the CT‐based RA‐TKA system resulted in better prosthesis positioning than CM‐TKA, with comparable or slightly better functional outcomes at 1 year 7 . Song et al. prospectively analyzed 50 cases of RA‐TKA vs 50 cases of CM‐TKA and found that RA‐TKA reduced the probability of abnormal prosthesis alignment values and improved the ability to achieve flexion‐extension gap balance compared to CM‐TKA 22 . The postoperative radiographic findings in this study indicated a significantly lower incidence of outliers for the LTC angle in the RA‐TKA group than in the CM‐TKA group, and the mean values were markedly closer to the preoperatively planned LTC angles; both of these differences were statistically significant. Although the other angular data also suggested a trend of more precise lower limb alignment and component positioning in the RA‐TKA group, none of the differences were significant. While most studies, including this one, suggest that RA‐TKA improves prosthesis positioning, there is a lack of long‐term follow‐up evidence to support this, and further research is needed to determine whether this results in less prosthesis wear and a lower revision rate.
RA‐TKA Has a More Moderate Inflammatory Response in the Early Postoperative Period
Kayani et al. found that RA‐TKA was associated with a transient reduction in the inflammatory response in the early postoperative period (day 7) but that there was no difference in the systemic inflammatory response in the immediate (<48 h) or late postoperative period (day 28) compared with conventional TKA 9 . NLR have been demonstrated as stable biomarkers that evaluate the inflammatory response as they mediate inflammation by various biochemical mechanisms 23 . In their 2017 study, Wasko et al. concluded that while both the CRP level and NLR were significantly higher after TKA, the NLR showed a faster changing kinetic pattern in response to the surgical trauma than the CRP level 24 . Yombi et al. demonstrated that the early postoperative NLR normalized more rapidly than the CRP level in 587 patients treated with TKA. They examined the CRP level and NLR preoperatively and 2, 4, 21, and 42 days postoperatively. One‐fifth of the patients did not reach a normal CRP level within 3 weeks postoperatively. However, only 4.5% had an elevated NLR (>5) at the same time point. They concluded that based on these two trials, the NLR may be a better biomarker for tracking the inflammatory response after 25 . In our study, the NLR was lower in the RA‐TKA group than in the CM‐TKA group on the first postoperative day, and there was no difference between the two groups on the third or 30th postoperative day. Neutrophils rapidly increase in injured tissue in response to high concentrations of inflammatory mediators and damageassociated molecular patterns (DAMPs) 26 . It has also been shown that elevated NLR is a predictor of poor outcome following surgical injuries 23 . Even if no significant distinction was found for both CRP and ESR, the difference in NLR could indicate a variation in the postoperative inflammatory response between the two groups. We speculate that there may be several reasons for this, the first being that RA‐TKA does not use open marrow localization during femoral osteotomy; bone marrow and macrophages in the marrow are important for progression of the acute‐phase response to trauma and surgery and directly affect serum inflammatory marker levels 27 , 28 . Second, the robotic arm will limit the extent and angle of the osteotomy, eliminating the need for thorough intraoperative exposure of the osteotomy surface and reducing soft tissue stripping. At the same time, RA‐TKA allows for real‐time tracking of the soft tissue balance after fitting of the trial mold, reducing unnecessary loosening. A cadaveric study conducted by Khlopas et al. found that with the preservation of PCL, RA‐TKA was associated with lower rates of posterior cruciate ligament injury and tibial subluxation than CM‐TKA 29 . This demonstrates that RA‐TKA has a better protective effect on soft tissues. However, further studies are needed to verify whether these are the reasons for the reduced inflammatory response.
Limitations
This study is a rigorous randomized controlled trial that compared the early clinical outcomes of two surgical approaches. There are also some limitations to this study, the first of which is the relatively small sample size; while the sample size calculation performed prior to the study meets the requirements of an RCT, the findings still need to be validated in a large‐sample study in the future. Second, the surgeries in the study were performed by two surgeons and could have been influenced by differences in operator acceptance of RA‐TKA and experience, and there may have been interoperator bias. Third, the follow‐up period was relatively short, and the long‐term efficacy in terms of the postoperative functional scores needs to be further observed and analyzed.
Conclusions
In summary, the KSS and WOMAC after RA‐TKA are similar to those after CM‐TKA. Compared to CM‐TKA, RA‐TKA could improve the accuracy of prosthesis placement and reduce the inflammatory response in the early postoperative period, showing advantages in the above two terms but prolonging the operation. Additionally, we suggest that studies on serum inflammatory indicators should exclude patients with an inflammatory arthropathy and the long‐term efficacy of RA‐TKA requires further follow‐up studies over longer periods.
Ethics statement
This study was approved by the ethics committee of Chinese PLA General Hospital (S2020‐005‐01).
Acknowledgment
The authors would like to thank Tao Deng for his help with the clinical follow‐up and data collection.
Jiazheng Xu and Liangliang Li contributed equally to this work and are considered co‐first authors.
Funding information: This work was supported by the National Key Research and Development Program of China [Grant numbers 2020YFC2004900] and Key Research and Development Program of the Ministry of Science and Technology—clinical application and optimization of intelligent wheelchair and walker products [Grant numbers 2020YFC2007405].
Grant sources: XJZ and LLL designed the study and wrote the article. FJ, XC, and NM participated in the analysis of the data and contributed to the interpretation of results. CW and HLB provided guidance on the design of the study and revised the article. ZGQ and CJY are mainly responsible for this project. All authors read and approved the final manuscript.
Disclosure: The authors declare that they have no conflict of interest.
Contributor Information
Guoqiang Zhang, Email: gqzhang301@163.com.
Jiying Chen, Email: jiyingchen_301@163.com.
References
- 1. Zhang Y, Liu H. Safety of Total knee arthroplasty in the treatment of knee osteoarthritis and its effect on postoperative pain and quality of life of patients. Contrast Media Mol Imaging. 2021;2021:6951578–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gunaratne R, Pratt DN, Banda J, Fick DP, Khan RJK, Robertson BW. Patient dissatisfaction following Total knee arthroplasty: a systematic review of the literature. J Arthroplasty. 2017;32(12):3854–60. [DOI] [PubMed] [Google Scholar]
- 3. Laubach M, Hellmann JT, Dirrichs T, Gatz M, Quack V, Tingart M, et al. Anterior knee pain after total knee arthroplasty: a multifactorial analysis. J Orthop Surg (Hong Kong). 2020;28(2):2309499020918947. [DOI] [PubMed] [Google Scholar]
- 4. Li CY, Ng Cheong Chung KJ, Ali OME, Chung NDH, Li CH. Literature review of the causes of pain following total knee replacement surgery: prosthesis, inflammation and arthrofibrosis. EFORT Open Rev. 2020;5(9):534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manghwani J, Vaidya SV, Patel H, Vaidya CS. Does total contact of the patella with the femoral trochlea during no thumb test significantly reduce anterior knee pain? Knee. 2019;26(6):1338–47. [DOI] [PubMed] [Google Scholar]
- 6. Agarwal N, To K, McDonnell S, Khan W. Clinical and radiological outcomes in robotic‐assisted Total knee arthroplasty: a systematic review and meta‐analysis. J Arthroplasty. 2020;35(11):3393–409. [DOI] [PubMed] [Google Scholar]
- 7. Batailler C, Fernandez A, Swan J, Servien E, Haddad FS, Catani F, et al. MAKO CT‐based robotic arm‐assisted system is a reliable procedure for total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2020;29:3585–98. [DOI] [PubMed] [Google Scholar]
- 8. Blakeney WG, Carrero V, Fau KR, Wall SJ. Computer‐assisted techniques versus conventional guides for component alignment in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93(19):1377–84. [DOI] [PubMed] [Google Scholar]
- 9. Kayani B, Tahmassebi J, Ayuob A, Konan S, Oussedik S, Haddad FS. A prospective randomized controlled trial comparing the systemic inflammatory response in conventional jig‐based total knee arthroplasty versus robotic‐arm assisted total knee arthroplasty. Bone Joint J. 2021;103‐B:113–22. [DOI] [PubMed] [Google Scholar]
- 10. Yim JH, Song EK, Khan MS, Sun ZH, Seon JK. A comparison of classical and anatomical total knee alignment methods in robotic total knee arthroplasty: classical and anatomical knee alignment methods in TKA. J Arthroplasty. 2013;28(6):932–7. [DOI] [PubMed] [Google Scholar]
- 11. Blanco JF, Díaz A, Melchor FR, da Casa C, Pescador D. Risk factors for periprosthetic joint infection after total knee arthroplasty. Arch Orthop Trauma Surg. 2020;140(2):239–45. [DOI] [PubMed] [Google Scholar]
- 12. Khlopas A, Sodhi N, Sultan AA, Chughtai M, Molloy RM, Mont MA. Robotic arm‐assisted Total knee arthroplasty. J Arthroplasty. 2018;33(7):2002–6. [DOI] [PubMed] [Google Scholar]
- 13. Morrell AT, Layon DR, Scott MJ, Kates SL, Golladay GJ, Patel NK. Enhanced recovery after primary Total hip and knee arthroplasty: a systematic review. J Bone Joint Surg Am. 2021;103(20):1938–47. [DOI] [PubMed] [Google Scholar]
- 14. Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58:277–80. [DOI] [PubMed] [Google Scholar]
- 15. Li LA‐O, Fu J, Xu C, Guan H, Ni M, Chai W, et al. Factors associated with blood loss in ankylosing spondylitis patients with hip involvement undergoing primary total hip arthroplasty: a cross‐sectional retrospective study of 243 patients. J Orthop Surg Res. 2020;15:541. 10.1186/s13018-020-02064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the knee society clinical rating system. Clin Orthop Relat Res. 1989;248:13–4. [PubMed] [Google Scholar]
- 17. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 18. Ensini A, Catani F, Leardini A, Romagnoli M, Giannini S. Alignments and clinical results in conventional and navigated total knee arthroplasty. Clin Orthop Relat Res. 2007;457:156–62. [DOI] [PubMed] [Google Scholar]
- 19. Jeon SW, Kim KI, Song SJ. Robot‐assisted Total knee arthroplasty does not improve long‐term clinical and radiologic outcomes. J Arthroplasty. 2019;34(8):1656–61. [DOI] [PubMed] [Google Scholar]
- 20. Vermue H, Luyckx T, Winnock de Grave P, Ryckaert A, Cools AS, Himpe N, et al. Robot‐assisted total knee arthroplasty is associated with a learning curve for surgical time but not for component alignment, limb alignment and gap balancing. Knee Surg Sports Traumatol Arthrosc. 2020;30:593–602. 10.1007/s00167-020-06341-6. [DOI] [PubMed] [Google Scholar]
- 21. Kayani B, Konan S, Tahmassebi J, Pietrzak JRT, Haddad FS. Robotic‐arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital discharge compared with conventional jig‐based total knee arthroplasty: a prospective cohort study. Bone Joint J. 2018;100‐b(7):930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic‐assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inose H, Kobayashi Y, Yuasa M, Hirai T, Yoshii T, Okawa A. Procalcitonin and neutrophil lymphocyte ratio after spinal instrumentation surgery. Spine (Phila pa 1976). 2019;44(23):E1356–e1361. [DOI] [PubMed] [Google Scholar]
- 24. Wasko MK, Struminski M, Bobecka‐Wesolowska K, Kowalczewski J. Neutrophil‐to‐lymphocyte ratio shows faster changing kinetics than C‐reactive protein after total hip and knee arthroplasty. J Orthop. 2017;10:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yombi JC, Schwab PE, Thienpont E. Neutrophil‐to‐lymphocyte ratio (NLR) distribution shows a better kinetic pattern than C‐reactive protein distribution for the follow‐up of early inflammation after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3287–92. [DOI] [PubMed] [Google Scholar]
- 26. Fouladseresht H, Bolandparvaz S, Abbasi HR, Abdolrahimzadeh Fard H, Paydar S. The neutrophil‐to‐lymphocyte ratio at the time of admission: a new prognostic indicator for hospital mortality of trauma patients. Iran J Allergy Asthma Immunol. 2021;20(1):33–45. [DOI] [PubMed] [Google Scholar]
- 27. Tanavalee A, Honsawek S, Rojpornpradit T, Sakdinakiattikoon M, Ngarmukos S. Inflammation related to synovectomy during total knee replacement in patients with primary osteoarthritis: a prospective, randomised study. J Bone Joint Surg Br. 2011;93(8):1065–70. [DOI] [PubMed] [Google Scholar]
- 28. Tsunoda K, Sonohata M, Kugisaki H, Someya S, Honke H, Komine M, et al. The effect of air tourniquet on Interleukin‐6 levels in Total knee arthroplasty. Open Orthop J. 2017;11:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khlopas A, Chughtai M, Hampp EL, Scholl LY, Prieto M, Chang TC, et al. Robotic‐arm assisted Total knee arthroplasty demonstrated soft tissue protection. Surg Technol Int. 2017;30:441–6. [PubMed] [Google Scholar]


