Abstract
Objective
Surgical site infection is a common complication of surgery, especially in orthopedics. Povidone‐Iodine (PI) is one of the oldest and most commonly used disinfectants in surgery. However, the toxicity and antimicrobial effect of PI have not been discussed. In addition, no study has explored the optimum PI concentration for sterilization and tissue healing. This study explores the germicidal efficacy of different concentrations PI, in addition, the toxicity and antibacterial effects of PI irrigation in fracture surgery are also discussed.
Methods
Methicillin‐resistant Staphylococcus aureus and Pseudomonas aeruginosa (P. aeruginosa) were used to evaluate the germicidal efficacy of PI in vitro and in vivo. In vitro, the effects of PI on bacterial growth were analyzed. 2.5%, 1.25%, 0.5%, 0.25%, 0.05%, 0.025%, 0.005%, 0.0025% and 0% PI was added into the bacterial suspension, besides, the bacterial algebra and growth rate were tested. Meanwhile, the fluorescence intensity of viable bacteria was also tested to evaluate the effects of PI on bacterial survival. In vivo, first, femoral fracture with wound infection rat models were established. Second, thyroid gland sections, blood thyroxine, urinary iodine, wound local skin, muscle and bone tissue sections, serum creatinine and alanine aminotransferase, serum and bone local tissue interleukin‐6 (IL‐6), interleukin‐10 (IL‐10), bone morphogenetic protein (BMP‐2), vascular endothelial growth factor (VEGF) and transforming growth factor (TGF‐β1) were detected in rat femoral shaft fracture model with 5%, 2.5%, 0.5%, 0.05%, and 0% PI irrigation. Third, tissue bacteria culture was tested in rat femoral fracture with wound infection model with different concentrations PI irrigation.
Results
In vitro, 2.5%, 1.25%, 0.5% PI inhibited the growth of bacteria. 1.25%, 0.5% PI killed all the bacteria, while 0.25%, 0.05% PI had not killed bacteria after about 10 min. The iodine absorption of 5%, 2.5%, 0.5% PI irrigation did not cause thyroid injury. The 5%, 2.5%, 0.5% PI irrigation did not make serum creatinine and alanine aminotransferase abnormal and can remove bacteria from wounds. The 0.5%, 2.5% PI irrigation can promote tissue healing and increase BMP‐2, VEGF, TGF‐β1, IL‐10, in addition, decrease IL‐6. 5% PI irrigation would inhibit tissue healing, and increase IL‐6, decrease BMP‐2, VEGF, TGF‐β1, IL‐10.
Conclusions
Povidone‐Iodine was a widely used disinfectant and 2.5%, 1.25% and 0.5% PI could effectively kill bacteria. Five percent and lower concentration PI irrigation was safe and could not cause thyroid, kidney and liver damage. The 0.5% PI irrigation was beneficial for tissue healing but 5% PI irrigation was the opposite.
Keywords: Povidone‐iodine irrigation, Antibacterial properties, Toxicity of povidone‐iodine, Pseudomonas aeruginosa, MRSA
0.5% PI irrigations are safe and PI irrigation could significantly reduce the incidence of wound infection. Also, 0.5% PI irrigation could promote tissue healing and the mechanisms may be related with BMP‐2, VEGF, TGF‐β1, IL‐6, IL‐10.
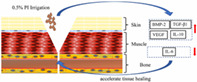
Introduction
Surgical site infection (SSI) is a common complication of surgery, especially in orthopedics. According to the literature, the incidence of SSI is 5%–15%. 1 , 2 , 3 The fracture surgery is more prone to SSI due to its particularity and poor soft tissue conditions. Once infection has occurred, debridement, renovation and even amputation may be required. However, the treatment can aggravate the lesion and have a great impact on the quality of life of the patients. At present, there are several methods to prevent infection in the clinic, but its incidence has not been significantly reduced. 3 In addition, multidrug‐resistant bacterial infections may also occur. Searching for a broad‐spectrum and effective method to prevent SSI has become a research hotspot.
Povidone‐Iodine (PI) is one of the oldest and most commonly used disinfectants in surgery. As early as in the 19th century, PI had been used to prevent SSI. 4 , 5 , 6 , 7 The concentration of PI used can vary, depending on the site of surgery (0.5%–10%). 8 PI kills bacteria by releasing free iodine, which binds with proteins. 4 , 6 , 7 , 8 , 9 Once bound, the iodine is carried across the cell membrane into the cell cytoplasm via polyvinylpyrrolidone. 7 , 8 , 9 The free iodine has a microbicidal effect within 15 s.
Bigliardi et al. 8 summarized the effects of PI in wound healing and infection. They reviewed the studies on the use of PI in surgery and the control of infection. The current studies find that PI had the broad antimicrobial spectrum, even the multidrug‐resistant bacteria. 8 Besides, PI rarely causes bacterial resistance and is also effective for biofilms formation. In addition, PI can reduce the inflammatory response. However, Bigliardi et al. 8 also summarized some current controversies, such as cytotoxicity, tissue toxicity, iodine absorption damage, suitable concentration of clinical application and so on.
Povidone‐Iodine is associated with tissue toxicity. The toxic effects have been reported on various types of tissues, such as skin, brain, lung, joint, etc. 10 , 11 , 12 , 13 Even 0.025% PI can make skin fibroblast growth progressively retarded. 14 Iodine is absorbed by the body's mucosa as PI irrigation during operation, which can cause body iodine poisoning and renal failure. 15
Li et al. 10 used PI to rinse the right cerebral cortex of rabbits and found that PI caused the micro‐structure changes of brain. They thought that PI was toxic to brain tissues. Ozkiris et al. 11 investigated the ototoxic effects of PI applied to the middle ear cavity and proposed that high concentration PI solution may cause significant ototoxic effects. Sato et al. 12 analyzed the toxicity of PI to epithelial cells. They used the PI concentrations lower than clinically used and found that 0.01 μM of PI made rat epithelial cells apoptosis, besides, the minimal concentration of PI caused apoptosis was 0.001 μM. Newton et al. 13 put their focus on fracture surgery. They examined whether PI irrigation damaged the function of human osteoblast cells. They used 0.35% PI and PI exhibited a significant inhibiting effects on cell proliferation. In addition, PI decreased the CII‐SDHB expression of osteoblasts and reduced the bone nodule mineralization formation. They concluded that PI had a rapid and detrimental effect on human osteoblasts. 13
Several studies have reported that intraoperative PI irrigation can reduce the incidence of postoperative infection. 4 , 5 , 6 , 7 , 8 , 9 , 16 Although animal studies have been performed on PI, they have all shown that PI irrigation can reduce postoperative infection; some have even found that it can accelerate tissue healing. 17 , 18 , 19
The pathway of interleukin‐6 (IL‐6), interleukin‐10 (IL‐10), bone morphogenetic protein (BMP‐2), vascular endothelial growth factor (VEGF) and transforming growth factor (TGF‐β1) during wound healing process were complex. Farahpour et al. 20 evaluated the effects of salvia officinalis essential oil (SOO) on an infected wound mice model. They found that SOO increased the tissue total antioxidant capacity level and reduced the malondialdehyde, interleukin‐1β and tumor necrosis factor‐α levels. Farahpour et al. 21 also explored the effects of Zataria multiflora Boiss essential oil (ZME) on a mice infected wounds model. They proposed that ZME increased the expression of TGF‐β, IL‐10 and VEGF and accelerated the wound healing. Besides, their team also analyzed the ointments from Mentha piperita essential oil (M. piperita) on wound healing and found M. piperita up‐regulated the expression levels of IL‐1β, TGF‐β1 and IL‐10. 22
Ruder and Springer 4 proposed that diluted PI lavage provided a safe and inexpensive method to reduce the periprosthetic joint infections. Roeckner et al. 17 summarized the PI irrigation for prevention of wound infection. They found that PI irrigation reduced the risk of postoperative wound infections and fever. Kanno et al. 5 analyzed the effects of PI irrigation on wound P. aeruginosa infection. They found that 1% PI irrigation reduced bacterial count of wound surface. Besides, they proposed that 1% PI irrigation promoted wound re‐epithelialization. Panchmatia et al. 7 demonstrated the effectiveness and safety of PI sinonasal rinses as an adjunctive therapy. They used a prospective cohort study and found 0.08% PI sinonasal rinse reducing infection. Matsuura et al. 9 also found that PI irrigation decreasing the infection incidence of surgery. However, the toxicity and antimicrobial effect of PI have not been discussed. In addition, no study has explored the optimum PI concentration for sterilization and tissue healing.
The aim of the present study was: first, the effects of different concentrations of PI on bacterial growth and bacterial survival were explored in vitro. Second, the iodine absorption of PI irrigation was assessed, and the effects of PI irrigation on skin, muscle and bone tissue healing were discussed. Also, the effects of PI irrigation on inflammatory reaction, kidney and liver function, and wound bacteria survival were analyzed in vivo. Methicillin‐resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (P. aeruginosa) were selected for this study, as they are the most common drug‐resistant bacteria in SSI.
Materials and Methods
Experimental Material
Five percent PI (effective iodine concentration, 5000 mg/L; Sihuan Medical Device Factory Co. Ltd., Beijing, China) was used. Luria Broth (LB) powder (containing 10 g peptone, 5 g yeast powder and 10 g NaCl) was purchased from Solarbio Science & Technology Co., Ltd. (Beijing). Phosphate buffer saline (PBS) was purchased from Merck KGaA (Darmstadt, Germany), microbial activity test kit Bac Titer‐GloTM from Promega Corporation (Madison, WI; USA) and sterile physiological saline from Baxter Medical Products Co. Ltd. (Deerfield, IL, USA) Sodium pentobarbital was provided by the Medical Research Center of Beijing Chaoyang Hospital affiliated to Capital Medical University. RatIL‐6 (CSB‐E04640r), IL‐10 (CSB‐E04595r), BMP‐2 (CSB‐E04508r), VEGF (CSB‐E04757r) and TGF‐β1 (CSB‐E04727r) ELISA kits were purchased from CUSABIO Bioengineering Co. Ltd. (Houston, TX, USA)
MRSA (ATCC6538) standard strain and P. aeruginosa (ATCC9027) were provided by the Guangdong Microbial Species Preservation Center (Beijing, China).
Female adult Sprague‐Dawley (SD) rats (Charles River Laboratories, Beijing), aged 8 weeks and weighing 190––210 g, were kept in separate cages under a 12 h light/dark cycle at 23.6°C and 35% humidity, and fed a sterilized chow diet with free water intake. All procedures complied with the ARRIVE guidelines and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The present study was approved by the Capital Medical University Ethics Committee on the use of animals in research and education (approval no. AEEI‐2020‐096). The humane endpoints of this study were as follows: extremely difficult to move to get the food or water, limb necrosis, self‐eating behavior and screaming by touching lightly. At the end of the experiments or reached the humane endpoints, rats were euthanized by using an excessive dose of sodium pentobarbital (100 mg/kg, intraperitoneal injection). Animal death was confirmed as no breath and heart beating.
Experimental Process
The bactericidal effects of different concentrations of PI were studied in vitro, with the aim of identifying the suitable effective concentration of PI for sterilization. Experiments had two parts. For the first part, MRSA and P. aeruginosa were used. Different concentrations of PI were added to the bacterial suspension, and the bacterial growth rate and total amount of bacteria were examined. For the second part, MRSA and P. aeruginosa were used with different concentrations of PI added to the bacterial suspension, and Bac Titer‐Glo™ (Promega Corporation) were used to detect the number of viable bacteria.
In the in vivo experiments, the optimum concentration of PI for sterilization and tissue healing was analyzed. Experiments had three parts and 126 rats were used. The rat femoral shaft fracture model was using different concentrations of PI irrigation when modeling. Seventy rats were divided into seven groups. Iodine uptake was evaluated by thyroid gland, blood thyroxine and urinary iodine detection. Skin, muscle, bone and tissue sections were obtained from around the wound to observe tissue damage and healing to evaluate the toxicity or enhancement of PI. Serum creatinine and alanine aminotransferase detection were performed to evaluate whether PI caused kidney and liver injury. Meanwhile, IL‐6, IL‐10, BMP‐2, VEGF and TGF‐β1 were examined in the serum and tissue near the bone fracture to evaluate the inflammatory reaction. For the third part, the wound infection model of rats with femoral shaft fracture was using with different concentrations of PI irrigation when modeling. Fifty‐six rats were divided into 14 groups. A tissue bacteria culture was used to examine the sterilization of the PI. The number of rats was calculated by Stata 12.0 as preliminary experimental results.
Luria Broth medium was made using LB, powder (containing 10 g peptone, 5 g yeast powder and 10 g NaCl) dissolved in 1 L sterile PBS. It was then filtered by 0.22 μm and Chilled to 4°C. Bacterial strain powder was dissolved by LB medium and gently shaken and mixed. Bacteria was cultured at 37°C, at 200 rpm/min overnight. Next, at 10,000 rpm/min for 3 min, precipitate was acquired and resuspended in LB medium. Bacterial passage was performed three times. According to the method provided by Morales‐Fernandez et al, 23 a double concentration of the culture medium was used to dilute the bacterial solution to the optical density (OD) value of 0.03 at 650 nm, which comprised bacterial concentration A. Another dose of bacterial liquid was diluted by PBS to an approximate OD value of 1 at 650 nm, which comprised bacterial concentration B.
Phosphate buffer saline was used to dilute 5% PI solution at a concentration of 2.5%, 1.25%, 1%, 0.5%, 0.25%, 0.1%, 0.05%, 0.025%, 0.01%, 0.005% and 0.0025%.
The animal experiments were in two parts. The first part, the rat femoral shaft fracture model was used. Seventy rats were divided into seven groups: 5% PI, 2.5% PI, 0.5% PI, 0.05% PI, 0% PI, sham‐operated (F) and control (C) groups. In this part, rats were without infection. The femoral shaft fracture rat model received 5%, 2.5%, 0.5%, 0.05% and 0% PI irrigation in the PI group. The F group comprised just the fracture model, without infection or irrigation. The C group was normally feed. In the second part, the wound infection rat model with femoral shaft fracture was used. According to relevant literature reports and preliminary experimental results, five concentrations of PI irrigation were selected for the animal experiments. Twenty‐eight rats were randomly divided into seven groups: The 5% PI + infection (5% PI + I), 2.5% PI + infection (2.5% PI + I), 0.5% PI + infection group (0.5% PI + I), 0.05% PI + infection (0.05% PI + I), 0% PI + infection (0% PI + I), sham‐operated (F‐I) and control (C‐I) groups. These are MRSA infection part and P. aeruginosa infection part was also used 28 rats divided into seven groups. The femoral shaft fracture with wound infection rat model received 5%, 2.5%, 0.5%, 0.05% and 0% PI irrigation in the PI + infection group. The F‐I group comprised just the fracture model, without infection or irrigation. The C‐I group was normally feed.
Wound irrigation was simulated in a clinical surgery. PI (5%, 2.5%, 0.5%, 0.05% or 0%) was used after the bone fracture was made, before the muscle was sutured. The F group comprised just the fracture model, without infection or irrigation. The C group was normally fed. The wound was slowly irrigated with PI for 5 min, followed by physiological saline for 5 min, which was repeated three times as referred to previous studies. 1 , 4 , 6 The muscle and incision were then sutured (details in Supplementary Materials 1).
Evaluation Indicators
Different concentrations of PI (2.5%, 1.25%, 0.5%, 0.25%, 0.05%, 0.025%, 0.005%, 0.0025% and 0%) were used in the bacteriostatic experiment. Next, 125 μl of different PI concentrations and 125 μl bacterial suspension (bacterial concentration A) per well were added to a 96‐well plate. The OD value was recorded at 650 nm following the addition of bacterial suspension and marked as A0. Next, bacteria were cultivated at 37°C and shaken at 200 rpm/min. The OD value was recorded at 650 nm Every hour for nine hand marked as A t (t = 1, 2, 3, 4, 5, 6, 7, 8, 9). Bacterial algebra (g) and growth rate (μ) were calculated by absorbance: 23
Different concentrations of PI (1.25%, 0.5%, 0.25%, 0.05%, 0.005% and 0%) were used in the sterilization experiment. Next, 80 μl of different PI concentrations and 20 μl bacterial suspension (bacterial concentration B) per well were added to an opaque 96‐well plate. Bacteria were cultivated in 37°C and shaken at 200 rpm/min. Next, 100 μl Bac Titer‐Glo™ was added at 1, 2, 5 or 10 min. The fluorescence intensity was recorded using a microplate reader after 5 min; it was defined as 0 when the luminous value was 0 or negative.
Iodine absorption was evaluated by thyroid gland, plasma thyroxine and urinary iodine detection in all animal groups.
The thyroid gland was assessed by examining its weight and a tissue section. The thyroid gland weight and section were obtained at post‐irrigation days 1, 3, 5, 7, 14 and 28. Rats were sacrificed, and intact thyroid glands (containing two lateral lobes and isthmus) were harvested under microscopy. Following tissue weighing, thyroid gland was fixed in 4% polyformaldehyde, dehydrated in gradient ethanol and diphenylamine, embedded, sliced and HE‐stained to observe whether the thyroid tissue structure was destroyed.
Plasma thyroxine and urinary iodine were detected by ELISA on post‐irrigation days 1, 3, 5, 7, 14 and 28. All procedures were performed following the manufacturer's instructions.
Wound skin, muscle and bone tissue from the fracture site were obtained on post‐irrigation days 1, 3, 5, 7, 14 and 28 to evaluate the effects of PI on skin, muscle and bone healing.
Rats were sacrificed and right thighs were harvested. Soft and bone tissue were separated. Soft tissue was fixed in 4% polyformaldehyde, dehydrated in gradient ethanol and diphenylamine, embedded, sliced and HE‐stained to observe, while the bone tissue was fixed in 4% polyformaldehyde, decalcified in 20% EDTA, dehydrated in gradient ethanol and diphenylamine, embedded, sliced and HE‐stained to observe.
An X‐ray of the right thigh was performed on post‐irrigation days 1, 3, 5, 7, 14 and 28 to evaluate the effects of PI on bone healing.
Rats were anesthetized by an intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight). An image station system (In‐Vivo FX PRO; Bruker BioSpin) was used to evaluate fracture healing by the illumination source adjusted to the X‐ray, with a gamma value of 1.7. Exposure time was set at 1 min and posterior–anterior and lateral views were captured to evaluate the formation of callus.
Anteroposterior position was set at the supine position and the limbs were fixed in the right position. Lateral position was set at left lateral position and the right lower limb was straight.
Adobe photoshop CS3 was used to select the region of interest (ROI), which was 5 mm above and below the fracture line. In addition, Image‐Pro Plus 6.0 was used to analyze the grayscale value of the bone and callus, and modify the color. Green represented the callus and red the bone. The ratio of the area of the callus to that of the bone tissue was calculated using Image‐Pro Plus 6.0.
The Tarlov score was used to assess the recovery of the lower limb function in rats. The modified Tarlov score of rats was observed before modeling, and 1, 2 and 4 weeks after modeling.
The rectal temperature of the rats was measured before modeling, and 1, 2 and 4 weeks after modeling.
A digital thermometer (Lifetech Scientific Co., Ltd., Shenzhen, China) was used to measure the rectal temperature, which was swapped for a rectal probe to minimize the stress response 2 days before the experiment. The rats were gently handled and removed from their cages. The probe was inserted 1 cm into the rectum and the temperature was measured at 09:00 a.m. 20
Blood was harvested on post‐irrigation days 1, 3, 5, 7, 14 and 28. Blood was centrifuged at 3000 rpm/min for 25 min, to separate the serum. Rat BMP‐2, VEGF, TGF‐β1, IL‐6 and IL‐10 ELISA kits were used to measure the serum cytokine levels. All procedures were performed following the manufacturer's instructions.
Tissue cytokines were tested by immunohistochemistry. Rats were sacrificed on post‐irrigation days 1, 3, 5, 7, 14 and 28. The wound soft tissues were sent to Wuhan Servicebio Co. Ltd. for Immunohistochemical detection. Image‐pro plus 6.0 was used to analysis the positive area ratio (analysis methods was in Supplementary Materials 2).
Blood was harvested on post‐irrigation days 1, 3, 5, 7, 14 and 28. Blood was centrifuged at 3000 rpm/min for 25 min to separate the serum. Serum creatinine and alanine aminotransferase were tested using an automatic biochemical analyzer (Beckman Coulter, Inc., USA).
Rats were sacrificed on post‐irrigation days 1, 3, 5, 7, 14 and 28. Wound skin and muscle sutures were removed. Pharyngeal swab was used to obtain the skin and muscle secretions. The secretions were inoculated on different LB agar media at 37°C for 72 h. Colony formation counts were measured to evaluate the effects of PI on wound bacteria survival.
Statistical Analysis
SPSS 23.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Measurement data were reported as the mean ± deviation, and enumeration data were as a percentage. One‐way ANOVA was used to compare the differences in measurement data, and χ 2 those in enumeration data, among the groups. Least significant difference tests were used for multiple comparisons in case of homogeneity of variance, otherwise a Dunnett T3 test was used. P < 0.05 was considered to indicate a statistically significant difference.
Results
Evaluation of Antibacterial Properties of PI at Different Concentrations
The 0.5%, 1.25% and 2.5% PI inhibits the growth of bacteria, while 0.25%, 0.05%, 0.025%, 0.005%, 0.0025% and 0% PI does not. The growth curves of MRSA and P. aeruginosa were observed in the PBS group, which served as the control group. The growth curves of MRSA and P. aeruginosa showed a similar exponential trend at 4–9 h. The growth rates of MRSA and P. aeruginosa remained high at 6, 7, 8 and 9 h.
The growth of MRSA and P. aeruginosa was inhibited by 0.5%, 1.25% and 2.5% PI, with no proliferation and the growth rate at ~0.
The effect of 0.05%, 0.025%, 0.005% and 0.0025% PI on the proliferation of MRSA was weak, and the curve was nearly parallel to that of the PBS group (P > 0.05; Supplementary Materials 3 for details).
The 1.25% and 0.5% PI kills all bacteria within 1 min, while 0.25%, 0.05% and 0.005% PI does not completely kill them at ~10 min. The fluorescence intensity of the 1.25°C and 0.5% PI groups was ~0 within 1 min, which indicated that 1.25% and 0.5% PI can completely kill MRSA and P. aeruginosa in 1 min. The fluorescence intensity of 0.25%, 0.05% and 0.005% PI had a downward trend, but the luminosity was still >200 at 10 min, irrespective of whether it was MRSA or P. aeruginosa. The results indicated that 0.25%, 0.05% and 0.005% PI could not kill all the bacteria, with a lot of them surviving (Supplementary Materials 3 for details).
The wound bacterial culture was examined on post‐irrigation days 1, 3, 5, 7, 14 and 28. The 5% PI + I, 2.5% PI + I, 0.5% PI + I and F‐I groups had a negative wound bacterial culture, while the 0.05% PI + I and 0% PI + F groups had a positive wound bacterial culture on days 1, 3, 5, 7, 14 and 28.
Evaluation of the Toxicity of PI Irrigation at Different Concentrations
Povidone‐Iodine irrigation‐induced iodine absorption does not cause thyroid injury, and the urine iodine of rats is normal after irrigation for 24 h. Iodine absorption was evaluated by thyroid gland, plasma thyroxine and urinary iodine detection.
The thyroid gland weight and section were examined on post‐irrigation days 1, 3, 5, 7, 14 and 28. No statistical difference in thyroid weight was observed among groups, and no structure destruction was found in the thyroid sections among groups. In addition, there were no significant differences in the serum thyroxine levels between groups. Urinary iodine was detected on post‐irrigation days 1, 3, 5, 7, 14 and 28 and there was no significant difference in urinary iodine among all groups.
Serum creatinine and alanine aminotransferase was tested on post‐irrigation days 1, 3, 5, 7, 14 and 28 and were found to be within the normal range, with no statistical differences observed (Fig. 1).
Fig. 1.
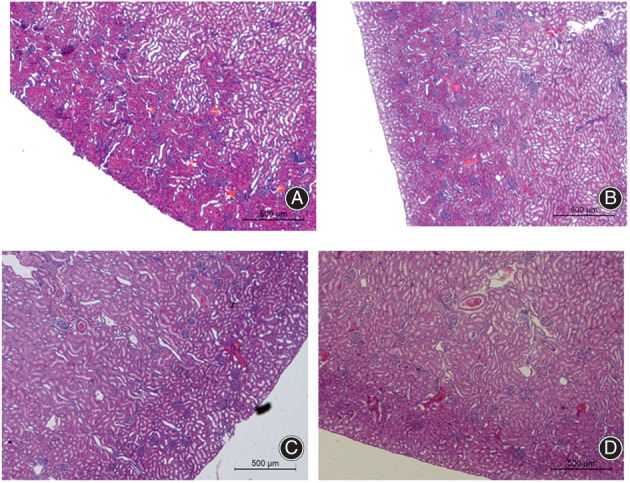
Kidney section in some groups, which shows that the kidney was not damaged. (A) 5% PI group at 7 days; (B) 0.5% PI group at 7 days; (C) 5% PI group at 28 days; (D) 0.5% PI group at 28 days.
Evaluation of the Physiological and Biochemical Effect of PI Irrigation at Different Concentrations
In the 0% PI, F, 0.05% PI, 2.5% PI and 0.5% PI groups, the incision exudation disappeared 3–5 days after irrigation and the incision healed in most rats 14 days after irrigation. In addition, at 14 days, the suture was removed and no rats showed incision dehiscence.
In the 0% PI, F and 0.05% PI groups, the incision healed well, but some of the surface squamous epithelium of the skin had not yet formed. In the 2.5% PI and 0.5% PI groups, the inflammatory reaction in the skin section was weaker than that in the 0% PI, F and 0.05% PI groups. In the 2.5% PI and 0.5% PI groups, a large number of fibroblasts was observed, and the proliferation of the squamous epithelium and fibrous tissue was clear under the microscope at 7 days. Most of the squamous epithelium of the skin surface had completely covered the wound at 14 days. In the 5% PI group, the incision exudation was higher than that in the other groups, and the incision exhibited obvious redness at 7 days. The incision still exhibited redness and swelling at 14 days. The suture was removed and the wound cracked at 14 days, which indicated poor skin healing. A large number of inflammatory cells were found to have infiltrated the skin section at 7 and 14 days (Fig. 2).
Fig. 2.
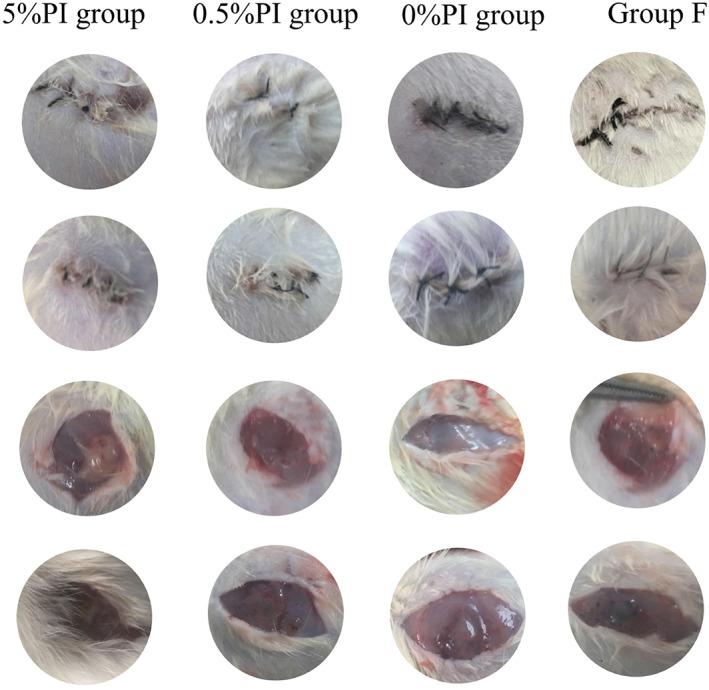
Seven days after femoral fracture irrigated by PI. The healing of skin and muscle tissue are shown. The wound in 5% PI group is red and swollen with necrotic tissue in the muscle tissue. The skin of 0.5% PI group, 0% PI group and group F healed well without redness and swelling and no necrosis of muscle tissue is observed.
In the 0% PI, F, 0.05% PI, 2.5% PI and 0.5% PI groups, no obvious necrosis in the muscle tissue of the wound could be observed by the naked eye. Muscle sections revealed normal inflammatory reaction at 7 days, and fibrous tissue formation at 14 days. In the 5% PI group, muscle necrosis was observed in some rats at 7 days. In the muscle sections, muscle cells were found to be broken with a large number of infiltrating inflammatory cells at 7 days. A higher inflammatory cell infiltration was observed in the 5% PI than in the other groups (Fig. 2).
An X‐ray of the right thigh was performed on post‐irrigation days 1, 3, 5, 7, 14 and 28 to evaluate the effects of PI on bone healing. In the 0% PI, F and 0.05% PI groups, callus formation appeared at 7 days and increased at 14 days. At 28 days, the fracture line was blurred and half of the rats had exhibited medullary recanalization. Half of the rats in the 0.5% and 2.5% PI groups had more calluses than the 0% PI, F and C groups at 14 days. In the 5% PI irrigation group, there was no obvious callus formation in any of the rats at 7 days, with mild callus formation at 14 days. In addition, the fracture line was still clearly visible at 28 days.
The 0.5% and 2.5% PI irrigation could increase the callus area ratio of the ROI, as compared with the 0% PI, F and C groups at 14 and 28 days. However, 5% PI irrigation had the opposite effect.
Rats were sacrificed 1, 3, 5, 7, 14 and 28 days after irrigation to evaluate the effects of PI on bone healing. In the 0% PI, F and 0.05% PI groups, fibrous and cartilaginous calluses were formed in the bone sections at 14 days. Fibroblasts aggregated and chondrocytes proliferated actively. Intrachondral osteogenesis, new bone trabeculae and angiogenesis were observed. At 28 days, the calluses in the cartilage decreased and chondroid calcification and lamellar bone formation were observed in the bone section. 0.5°C and 2.5% PI irrigation enhanced callus formation, as compared with the 0% PI, F and 0.05% PI groups at 14 days, while 5% PI irrigation had the opposite effects.
In the 0% PI, F, 0.05% PI groups, the Tarlov function score gradually recovered at 7, 14 and 28 days. The Tarlov function score of the 5% PI irrigation group was lower than that in the 0% PI, F and 0.05% PI groups, and the 0.5% and 2.5% PI irrigation groups at 7, 14 and 28 days. The 0.5% and 2.5% PI irrigation improved their score at 14 days, as compared with the 0% PI, F and 0.05% PI groups, but there were no statistical differences among the 0% PI, F and 0.05% PI, and 0. 5% and 2.5% PI irrigation groups at 28 days (Figs 3 and 4).
Fig. 3.
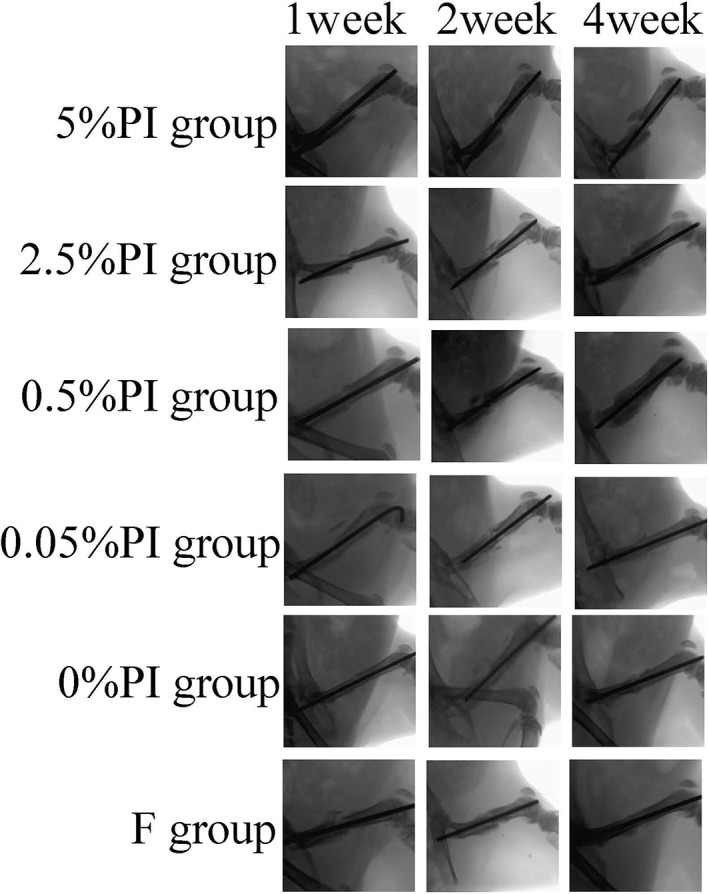
X‐ray lateral position of fracture in each group. It can be seen that the fracture line of 5% PI group is still clear at 4 weeks, while the callus amount of 2.5% PI group and 0.5% PI group at 2 weeks is significantly more than other groups.
Fig. 4.
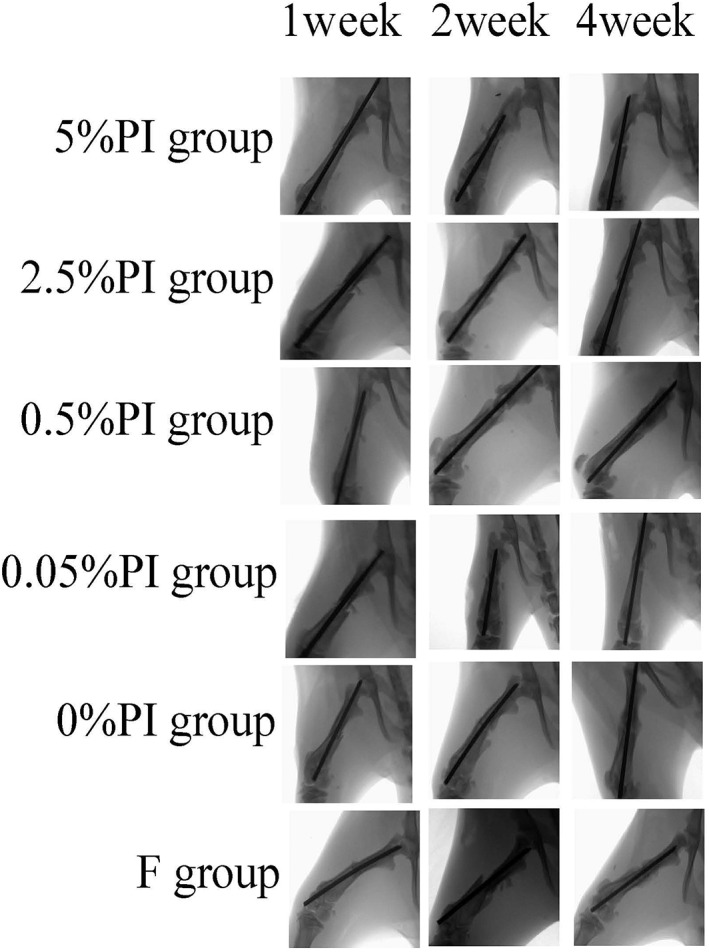
X‐ray anteroposterior position of fracture in each group. The results are the same as in the lateral position.
The rectal temperature of the rats was measured prior to modeling, and 7, 14 and 28 days after modeling. There was no significant difference in body temperature among the groups. The temperature peaked on post‐irrigation day 2 and returned to normal on post‐irrigation day 3.
Fitting degree R of the ELISA standard curve for BMP‐2, VEGF, TGF‐β1, IL‐6 and IL‐10 was above 0.99. Serum BMP‐2, VEGF, TGF‐β1 peaked at 7 days and decreased at 14 and 28 days in all groups. The 0.5% and 2.5% PI irrigation increased BMP‐2, VEGF and TGF‐β1 levels, as compared with the 0% PI, F and 0.05% PI group at 7 and 14 days. However, 5% PI irrigation had the opposite effects. No significant difference in serum BMP‐2, VEGF and TGF‐β1 were observed in any of the groups at 28 days. Serum IL‐6 and IL‐10 peaked at 7 days and decreased at 14 and 28 days in all groups. The 0.5% and 2.5% PI irrigation decreased the level of IL‐6 and increased that of IL‐10 at 7 and 14 days. The 5% PI irrigation had the opposite effects to those of the 0% PI, F and 0.05% PI groups. No statistical difference in serum IL‐6 or IL‐10 was observed in any of the groups at 28 days.
Cytokines from tissue were tested on post‐irrigation days 1, 3, 5, 7, 14 and 28. The local BMP‐2, VEGF and TGF‐β1 levels peaked at 14 days and decreased at 28 days in all groups. The 0.5% and 2.5% PI irrigation increased BMP‐2, VEGF and TGF‐β1 expression, as compared with the 0% PI, F and 0.05% PI groups at 7 and 14 days. However, 5% PI irrigation had the opposite effects. No significant differences in BMP‐2, VEGF or TGF‐β1 were observed at 28 days. The IL‐6 and IL‐10 expression in the tissue local to the bone fracture peaked at 14 days and decreased at 28 days in all groups. The 0.5% and 2.5% PI irrigation decreased the level of IL‐6 and increased that of IL‐10 at 7 and 14 days. 5% PI irrigation had the opposite effects to those of the 0% PI, F and 0.05% PI groups. No statistical difference in IL‐6 or IL‐10 was observed in any of the groups at 28 days. (Figs 5 and 6).
Fig. 5.
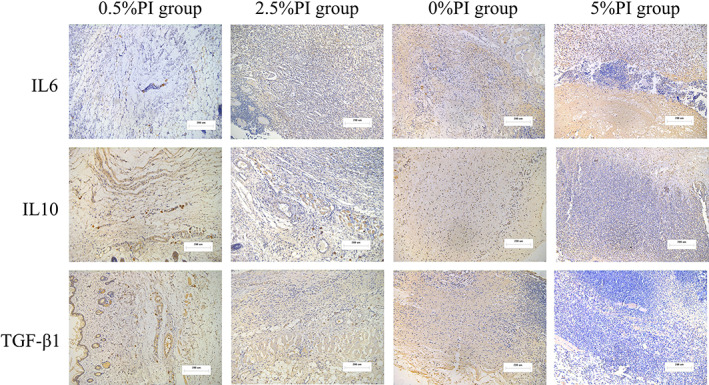
Immunohistochemical staining images. The 0.5% and 2.5% PI irrigation increased TGF‐β1, IL10 expression and decreased IL6 expression, as compared with the 0% PI group at 14 days. The 5% PI irrigation had the opposite effects. Images ×100; original scale bar, 200 μm.
Fig. 6.
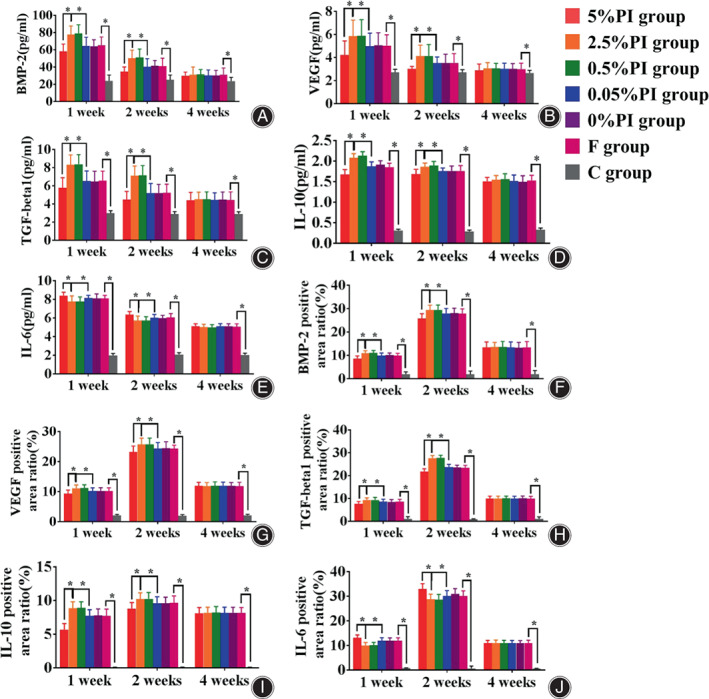
Cytokines in serum and bone tissue of rats in each group. BMP‐2, VEGF, TGF‐β1 and IL‐10 of 5% PI group are lower than other groups, while IL‐6 is higher than other groups at 1 and 2 weeks. BMP‐2, VEGF, TGF‐β1 and IL‐10 of 2.5% PI group and 0.5 PI group are higher than other groups, while IL‐6 is lower than other groups at 1 and 2 weeks. *p < 0.05. (A) Serum BMP‐2; (B) serum VEGF; (C) serum TGF‐β1; (D) serum IL‐10; (E) serum IL‐6; (F) bone tissue BMP‐2; (G) bone tissue VEGF; (H) bone tissue TGF‐β1; (I) bone tissue IL‐10; (J) bone tissue IL‐6.
Discussion
Our study showed that showed that 0.5%, 1.25% and 2.5% PI could inhibit bacterial growth, but 0.25%, 0.05%, 0.025%, 0.005%, 0.0025% and 0% PI did not significantly inhibit it. An in vivo experiment showed that 107 CFU/ml MRSA or 106 CFU/ml P. aeruginosa could establish a stable wound infection rat model. 5%, 2.5%, 0.5%, 0.05% or 0% PI irrigation is relatively safe for the body and PI irrigation could significantly reduce the incidence of wound infection in rats. 2.5% and 0.5% PI irrigation could promote skin, muscle and bone healing, and the mechanism may involve BMP‐2, VEGF, TGF‐β 1, IL‐6, IL‐10.
Surgical Infection and PI Irrigation
Incision infection is the most common postoperative complication in surgery, 1 , 2 , 3 , 6 , 7 and the identification of ways to prevent it has been a hot research topic. With the progress of society, aseptic conditions in surgery have become more and more strict, with an increasing number of antibiotics developed; however, the incidence of incision infection has not decreased significantly. 4 , 5 , 6 Studying the drugs that can reduce the incision infection and not cause the emergence of drug‐resistant bacteria is key to its prevention.
The chemical properties of PI were reported as early as in 1957. 7 , 8 , 9 PI is a polyvinylpyrrolidone‐iodine complex. This clathrate is more soluble in water than iodine. When PI is dissolved in water, it does not exist in a single form. It is not a compound but a complex and there are >10 forms in its aqueous solution. 8 PVP‐I is composed of polyvinylpyrrolidone and iodine. Polyvinylpyrrolidone has no bactericidal effect. However, PVP‐I has a lasting bactericidal effect by slowly releasing free iodine ions in water. It can oxidize microbial protoplasm proteins and cause bacterial denaturation and death.
Bactericidal Effect of PI
The bactericidal effect of PI is not concentration‐dependent. An inversion phenomenon was observed in the relationship between bactericidal efficacy and PI concentration. The bactericidal efficacy of the diluent at the appropriate concentration was better than that of the original solution. This inversion phenomenon was mainly manifested in the killing ability of S. aureus. This may be associated with the decrease in the attractiveness of povidone to the iodine ion, and the increase in the release of ion following PI dilution. In 1982, Berkelman et al. 21 found that 0.1%–1% PI could completely kill S. aureus in 15 s, while 5% iodophor solution and 0.01% PI needed 1 min to get the same results. Zafer et al 22 used 20 ml 10% PI on enema‐administered rabbits for three consecutive days. It was found that only serum iodine increased after day 1 and lasted to day 7, with no effect on thyroxine T3, thyroxine T4 and TSH. Gozeneli et al. 23 showed that abdomens washed with 2% PI did not cause significant changes in TSH, thyroxine T3 and T4. Other studies have shown that pleural cavity irrigation, mediastinal irrigation, and iodine‐coated internal fixators do not cause thyroid dysfunction. 24 , 25 In addition, PI is considered to be a weak allergen, with its allergy incidence rate at only 0.4%. 26
In orthopedic clinical work, PI solution or PI diluted in normal saline are often used to irrigate the incision, which has been shown to have a good effect on preventing infection. 7 , 27 De Luna et al. 27 used the 3% PI low pressure pulse method to wash wounds during spinal surgery, which effectively prevented incision infection. Ulivieri et al. 7 used 6.15% PI to soak for 1 min before suturing the incision during spinal surgery, and then rinsed with normal saline, which effectively prevented the infection of the incision. Brown et al. 28 used 0.35% PI solution to wash for 3 min during hip and knee replacement surgery to prevent deep periprosthetic infection.
Toxic Effect of PI
However, PI has toxic effects on fibroblasts, osteoblasts, myoblasts and chondrocytes, and its toxicity is significantly correlated with time and concentration. 10 , 11 , 12 , 13 , 14 Fibroblasts play an important role in all stages of fracture healing. Hirsch et al. 29 found that PI was significantly toxic in fibroblasts at a concentration of >0.3% in vitro. Osteoblasts are the most important cells in fracture healing. In an in vitro experiment, Liu et al. 30 used 0%, 0.001%, 0.01%, 0.1%, 0.35% and 1% PI to treat human osteoblasts, fibroblasts and myoblasts for 3 min. The results showed that 0.1%, 0.35% and 1% PI could significantly inhibit cell migration. Furthermore, as suggested by the cell survival rate 0.1%, 0.35% and 1% PI were significantly toxic to human osteoblasts, fibroblasts and myoblasts in vitro.
Philp et al. 31 also found that 0.35% PI solution had a strong inhibitory effect on the proliferation and mineralization of human osteoblasts in vitro, with the inhibition lasting 7 days, and the proliferation of osteoblasts decreasing by 40%–50%, as compared with that of normal cells. Chondrocytes are also important cells in fracture healing. It has been found that chondrocytes have the ability to transform into osteoblasts during fracture healing. Von Keudell et al. 32 used 0.35%, 1.4%, 3.5% and 5% PI to immerse the cartilage for 1, 3 and 6 min. When the cartilage surface was exposed to 5% PI solution for 6 min, >70% chondrocytes from the 300‐um thickness cartilage died, even in low concentrations of PI solution (0.35%). Even following short‐time (1‐min) exposure, PI was found to cause damage on the surface of articular cartilage. Kataoka et al. 33 injected 0.1 ml PI solution into the knee joint of rats for seven consecutive days. At a concentration exceeding and including 6.25%, cartilage damage occurred. Husodo et al. 17 found that the infiltration of 10% PI for 2 min after irrigation had adverse effects on fracture healing in rats, resulting in an increase in the percentage of cartilage and fibrous tissue. Mesenchymal stem cells (MSC) are a type of primordial cell with a key role in fracture healing. MSC are mainly derived from the bone marrow and can differentiate into osteocytes and chondrocytes during fracture healing. When injury occurs, the local inflammatory reaction is intense, and a large number of cytokines and bone growth‐related factors are increased, including IL‐6, VEGF and BMP‐2. The MSC are activated and transported to the injured site for repair. Jiang et al. 34 found that the use of low‐concentration (~0.036%) PI infiltrating protein scaffolds can promote the subchondral osteogenesis of MSC.
Suitable Germicidal Concentration of PI
It was found that 0.25%, 0.05%, 0.025%, 0.005%, 0.0025% and 0% PI did not significantly inhibit bacterial growth, while 0.5%, 1.25% and 2.5% PI could inhibit it. The results showed that when the PI concentration was <0.5%, there was no bacteriostatic effect, but when it was >0.5%, there was a bacteriostatic effect. It was found that 1.25% and 0.5% PI could kill bacteria in 1 min, but 0.25, 0.05 and 0.005 PI could not kill bacteria in 10 min. Through an in vitro experiment, it was found that 0.5% PI can effectively inhibit bacterial growth and kill bacteria.
In vivo, a stable incision infection model was first established. It was found that 1 × 107 CFU/ml MRSA or 1 × 106 CFU/ml P. aeruginosa could establish a stable wound infection rat model. Next, 5%, 2.5%, 0.5%, 0.05% or 0% PI was used to wash the wound. Thyroid weight, serum thyroxine and urinary iodine were measured at 1, 3, 5, 7, 14 and 28 days after the model was established. No obvious abnormality was found. At the same time, it was found that 5%, 2.5%, 0.5%, 0.05% or 0% PI could not damage the thyroid structure of rats and 5%, 2.5%, 0.5%, 0.05% or 0% PI washing could not cause abnormalities in serum creatine and alanine aminotransferase. Therefore, we concluded that 5%, 2.5%, 0.5%, 0.05% or 0% PI flushing is relatively safe for the body.
Furthermore, bacteria were cultured in the washed wounds of rats. The results showed that the wounds washed with 5%, 2.5% and 0.5%, 0.05% or 0% PI had no bacterial growth on days 1, 3, 5, 7, 14 and 28 after the model was established. The results showed that PI irrigation could significantly reduce the incidence of wound infection in rats.
Effects of PI on Tissue Healing
In order to determine the local toxicity of iodophor, local skin, muscle and bone healing was evaluated in rats. 35 , 36 It was found that 2.5% and 0.5% PI could promote skin, muscle and bone healing. Washing with 2.5% and 0.5% PI could promote the repair of skin and muscle fiber tissue, regeneration of bone tissue and formation of calluses. However, 5%, 0.05% and 0% PI did not promote skin, muscle and bone healing. On the contrary, washing with 5% PI could slow down the healing of skin, muscle and bone tissue, exhibiting PI toxicity. The inflammatory response and promotion of tissue repair was also discussed. It was found that 2.5% and 0.5% PI could reduce IL‐6 and increase BMP‐2, VEGF, TGF‐β 1 and IL‐10 in the blood of rats 7 and 14 days after model establishment, while 5% PI could increase the levels of IL‐6 and decrease those ofBMP‐2, VEGF, TGF‐β1 and IL‐10 in the blood of rats 7 and 14 days after model establishment. The results showed that 2.5% and 0.5% PI could reduce the inflammatory response in the body, and that the promotion of tissue healing might be associated with BMP‐2, VEGF and TGF‐β1. However, 5% PI can promote the inflammatory response and reduce the levels of BMP‐2, VEGF and TGF‐β1, which may be the reason of inhibiting tissue healing. The mechanisms of 2.5% and 0.5% PI irrigation promoting tissue healing need to be further explored.
Limitations
Although we discovered a lot, this study also has many shortcomings. First of all, we have not carried out an in‐depth study on the mechanism, why different iodine‐volt irrigation has different effects on cytokines, and why the regulation of tissue healing is different. Second, we plan to develop a low‐concentration sustained release system of iodine volts, which can not only kill bacteria but also promote tissue healing, but the relevant carriers need further research.
Conclusion
Povidone‐Iodine was a widely used disinfectant and 2.5%, 1.25% and 0.5% PI could effectively kill bacteria. 5% and lower concentration PI irrigation was safe and could not cause thyroid, kidney and liver damage.0.5% PI irrigation was benefit for tissue healing but 5% PI irrigation was playing an against role.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors. The present study was approved by the Capital Medical University Ethics Committee on the use of animals in research and education (approval no. AEEI‐2020‐096).
Author Contributions
Junlin Zhou designed this study. Wenrui Lv and Dong Wang did the experiments; Dong Wang analyzed the data; Dong Wang wrote the article. Xinli Huang and Junlin Zhou revised the article. All the authors read and approved the final manuscript.
Supporting information
Supplementary Materials 1.
Supplementary Materials 2.
Supplementary Materials 3.
Reference
- 1. Ulivieri S, Toninelli S, Petrini C, Giorgio A, Oliveri G. Prevention of post‐operative infections in spine surgery by wound irrigation with a solution of povidone‐iodine and hydrogen peroxide. Arch Orthop Trauma Surg. 2011;131(9):1203–6. [DOI] [PubMed] [Google Scholar]
- 2. Lakomkin N, Sathiyakumar V, Wick B, Shen MS, Jahangir AA, Mir H, Obremskey WT, Dodd AC, Sethi MK et al Incidence and predictive risk factors of postoperative sepsis in orthopedic trauma patients. J OrthopTraumatol 2017;18(2):151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cook GE, Markel DC, Ren W, Webb LX, McKee MD, Schemitsch EH et al Infection in orthopaedics. J Orthop Trauma 2015;29 Suppl 12:S19‐23. [DOI] [PubMed] [Google Scholar]
- 4. Ruder JA, Springer BD. Treatment of periprosthetic joint infection using antimicrobials: dilute povidone‐iodine lavage. J Bone Jt Infect. 2017;2(1):10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kanno E, Tanno H, Suzuki A, Kamimatsuno R, Tachi M. Reconsideration of iodine in wound irrigation: the effects on Pseudomonas aeruginosa biofilm formation. J Wound Care. 2016;25(6):335–9. [DOI] [PubMed] [Google Scholar]
- 6. Edmiston CE Jr, Spencer M, Leaper D. Antiseptic irrigation as an effective interventional strategy for reducing the risk of surgical site infections. Surg Infect (Larchmt). 2018;19(8):774–80. [DOI] [PubMed] [Google Scholar]
- 7. Payandeh J, Al‐Salman R, Kakande E, et al. The efficacy of diluted topical povidone‐iodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study. Eur Arch Otorhinolaryngol. 2019;276(12):3373–81. [DOI] [PubMed] [Google Scholar]
- 8. Bigliardi PL, Alsagoff SAL, El‐Kafrawi HY, et al. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260–8. [DOI] [PubMed] [Google Scholar]
- 9. Matsuura K, Miyazaki D, Sasaki SI, Yakura K, Inoue Y, Sakamoto M et al Effectiveness of timely intraoperative iodine irrigation during cataract surgery. Jpn J Ophthalmol 2016;60(6):433–8. [DOI] [PubMed] [Google Scholar]
- 10. Li SH, Wang Y, Gao HB, et al. Experimental study on the toxicity of povidone‐iodine solution in brain tissues of rabbits. Int J Clin Exp Med. 2015;8(9):14863–70. [PMC free article] [PubMed] [Google Scholar]
- 11. Ozkiris M, Kapusuz Z, Saydam L. Ototoxicity of different concentrations povidone‐iodine solution applied to the middle ear cavity of rats. Indian J Otolaryngol Head Neck Surg. 2013;65(2):168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sato S, Miyake M, Hazama A, Omori K. Povidone‐iodine‐induced cell death in cultured human epithelial HeLa cells and rat oral mucosal tissue. Drug Chem Toxicol. 2014;37(3):268–75. [DOI] [PubMed] [Google Scholar]
- 13. Newton Ede MP, Philp AM, Philp A, Richardson SM, Mohammad S, Jones SW et al Povidone‐iodine has a profound effect on in vitro osteoblast proliferation and metabolic function and inhibits their ability to mineralize and form bone. Spine (Phila Pa 1976). 2016;41(9):729–34. [DOI] [PubMed] [Google Scholar]
- 14. Balin AK, Pratt L. Dilute povidone‐iodine solutions inhibit human skin fibroblast growth. Dermatol Surg. 2002;28(3):210–4. [DOI] [PubMed] [Google Scholar]
- 15. Lakhal K, Faidherbe J, Choukhi R, Boissier E, Capdevila X. Povidone iodine: features of critical systemic absorption. Ann Fr Anesth Reanim. 2011;30(7–8):e1–3. [DOI] [PubMed] [Google Scholar]
- 16. Sindhura H, Harsha RH, Shilpa RH. Efficacy of subgingival irrigation with 10% povidone‐iodine as an adjunct to scaling and root planing: a clinical and microbiological study. Indian J Dent Res. 2017;28(5):514–8. [DOI] [PubMed] [Google Scholar]
- 17. Roeckner JT, Sanchez‐Ramos L, Mitta M, Kovacs A, Kaunitz AM. Povidone‐iodine 1% is the most effective vaginal antiseptic for preventing post‐cesarean endometritis: a systematic review and network meta‐analysis. Am J Obstet Gynecol. 2019;221(3):261.e1–261.e20. [DOI] [PubMed] [Google Scholar]
- 18. Wang L, Qin W, Zhou Y, Chen B, Zhao X, Zhao H, Mi E, Mi E, Wang Q, Ning J et al Transforming growth factor beta plays an important role in enhancing wound healing by topical application of povidone‐iodine. Sci Rep 2017;7(1):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCrory P. To wash or not to wash? That is the question. Br J Sports Med. 2003;37(3):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang D, Liu Y, Zhao Y, Zhou J. Low dose of protein a pretreatment can alleviate the inflammatory reaction and the bio‐safety was evaluated in vivo. J Chin Med Assoc. 2016;79(7):400–8. [DOI] [PubMed] [Google Scholar]
- 21. Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone‐iodine solutions. J Clin Microbiol. 1982;15(4):635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zafer N, Dulger M, Oguz M, Unal A, Cengiz S. The alteration on serum thyroid hormones and iodine levels when the 10% povidone iodine solution was topically performed on ano‐rectal mucosa. Mater Med Pol. 1992;24(4):249–51. [PubMed] [Google Scholar]
- 23. Morales‐Fernandez L, Fernandez‐Crehuet M, Espigares M, Moreno E, Espigares E. Study of the hormetic effect of disinfectants chlorhexidine, povidone iodine and benzalkonium chloride. Eur J Clin Microbiol Infect Dis. 2014;33(1):103–9. [DOI] [PubMed] [Google Scholar]
- 24. Findik G, Gezer S, Aydogdu K, Öz G, Kucukbayrak A, Tastepe I, Karaoglanoglu N, Kaya S et al Effect of intrapleural povidone‐iodine lavage on thyroid hormones in thoracic surgery. Thorac Cardiovasc Surg 2010;58(4):225–8. [DOI] [PubMed] [Google Scholar]
- 25. Lachapelle JM. Allergic contact dermatitis from povidone‐iodine: a re‐evaluation study. Contact Dermatitis. 2005;52(1):9–10. [DOI] [PubMed] [Google Scholar]
- 26. Norman G, Atkinson RA, Smith TA, Rowlands C, Rithalia AD, Crosbie EJ, Dumville JC, Cochrane Wounds Group et al Intracavity lavage and wound irrigation for prevention of surgical site infection. Cochrane Database Syst Rev 2017;10:CD012234, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Luna V, Mancini F, De Maio F, et al. Intraoperative disinfection by pulse irrigation with povidone‐iodine solution in spine surgery. Adv Orthop. 2017;2017:7218918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27–30. [DOI] [PubMed] [Google Scholar]
- 29. Hirsch T, Koerber A, Jacobsen F, Dissemond J., Steinau H.U., Gatermann S., al‐Benna S., Kesting M., Seipp H.M., Steinstraesser L. et al Evaluation of toxic side effects of clinically used skin antiseptics in vitro. J Surg Res 2010;164(2):344–50. [DOI] [PubMed] [Google Scholar]
- 30. Liu JX, Werner JA, Buza JIII, et al. Povidone‐iodine solutions inhibit cell migration and survival of osteoblasts, fibroblasts, and myoblasts. Spine (Phila Pa 1976). 2017;42(23):1757–62. [DOI] [PubMed] [Google Scholar]
- 31. Philp AM, Raja S, Philp A, Newton Ede MP, Jones SW. The effect of vancomycin and gentamicin antibiotics on human osteoblast proliferation, metabolic function, and bone mineralization. Spine (Phila Pa 1976). 2017;42(3):202–7. [DOI] [PubMed] [Google Scholar]
- 32. von Keudell A, Canseco JA, Gomoll AH. Deleterious effects of diluted povidone‐iodine on articular cartilage. J Arthroplasty. 2013;28(6):918–21. [DOI] [PubMed] [Google Scholar]
- 33. Kataoka M, Tsumura H, Kaku N, Torisu T. Toxic effects of povidone‐iodine on synovial cell and articular cartilage. Clin Rheumatol. 2006;25(5):632–8. [DOI] [PubMed] [Google Scholar]
- 34. Jiang Y, Chen L, Zhang S, Tong T., Zhang W., Liu W., Xu G., Tuan R.S., Heng B.C., Crawford R., Xiao Y., Ouyang H.W. et al Incorporation of bioactive polyvinylpyrrolidone‐iodine within bilayered collagen scaffolds enhances the differentiation and subchondral osteogenesis of mesenchymal stem cells. Acta Biomater 2013;9(9):8089–98. [DOI] [PubMed] [Google Scholar]
- 35. Lin J, Ding J, Dai Y, Wang X, Wei J, Chen Y et al Antibacterial zinc oxide hybrid with gelatin coating. Mater Sci Eng C Mater Biol Appl 2017;81:321–6. [DOI] [PubMed] [Google Scholar]
- 36. Li S, Dong S, Xu W, Tu S, Yan L, Zhao C, Ding J, Chen X et al Antibacterial hydrogels. Adv Sci (Weinh) 2018;5(5):1700527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials 1.
Supplementary Materials 2.
Supplementary Materials 3.


