Abstract
The expression of the moa locus, which encodes enzymes required for molybdopterin biosynthesis, is enhanced under anaerobiosis but repressed when the bacterium is able to synthesize active molybdenum cofactor. In addition, moa expression exhibits a strong requirement for molybdate. The molybdate enhancement of moa transcription is fully dependent upon the molybdate-binding protein, ModE, which also mediates molybdate repression of the mod operon encoding the high-affinity molybdate uptake system. Due to the repression of moa in molybdenum cofactor-sufficient strains, the positive molybdate regulation of moa is revealed only in strains unable to make the active cofactor. Transcription of moa is controlled at two sigma-70-type promoters immediately upstream of the moaA gene. Deletion mutations covering the region upstream of moaA have allowed each of the promoters to be studied in isolation. The distal promoter is the site of the anaerobic enhancement which is Fnr-dependent. The molybdate induction of moa is exerted at the proximal promoter. Molybdate-ModE binds adjacent to the −35 region of this promoter, acting as a direct positive regulator of moa. The molybdenum cofactor repression also appears to act at the proximal transcriptional start site, but the mechanism remains to be established. Tungstate in the growth medium affects moa expression in two ways. Firstly, it can act as a functional molybdate analogue for the ModE-mediated regulation. Secondly, tungstate brings about the loss of the molybdenum cofactor repression of moa. It is proposed that the tungsten derivative of the molybdenum cofactor, which is known to be formed under such conditions, is ineffective in bringing about repression of moa. The complex control of moa is discussed in relation to the synthesis of molybdoenzymes in the bacterium.
Molybdenum is an essential trace element required for the activity of several enzymes in the form of a molybdenum cofactor which is found in bacteria, plants, and animals. All oxo-molybdoenzymes described to date contain a molybdenum cofactor which consists in its most simple form of a pterin, molybdopterin, complexed to molybdenum. Molybdopterin has a terminally phosphorylated, four-carbon alkyl side chain with a dithiolene group, the two sulfur atoms of which are ligands to the molybdenum (28). Crystallographic structures of several molybdoenzymes are now available (4, 18, 19, 33).
In Escherichia coli the mo- loci are responsible for molybdenum transport and the synthesis of the molybdenum cofactor, and all of these loci have been characterized (12, 13, 15, 26, 29, 30). The moa and moe loci are required for molybdopterin biosynthesis. The first three genes (moaABC) of the five genes at the moa locus (30) are required for the first committed step of molybdopterin biosynthesis, namely the formation of precursor Z from a guanosine nucleotide or closely related compound (39).
The modABCD operon of E. coli encodes the high-affinity molybdate uptake system of the bacterium. Strains defective at this locus are pleiotropically unable to synthesize active molybdoenzymes, such as nitrate reductase. The introduction of high levels of molybdate into the growth medium, however, restores molybdoenzyme activities to mod strains, indicating that molybdate can enter the bacterium by another route under such conditions (6, 27, 31). Transcription of modABCD is repressed under conditions of high molybdate availability (23, 29, 31). The repression is mediated by the ModE protein, which is encoded by a second operon expressed divergently from modABCD (7, 20, 25, 38).
In vitro studies have demonstrated that dimeric ModE directly binds two molybdate ions with a Kd of about 0.8 μM and that the molybdate-ModE complex binds to mod DNA at a site which spans the modA transcription start site. This is consistent with its role as a transcriptional repressor of modA (2, 8, 22). The ModE protein has a wider role in mediating molybdate regulation of other operons which include dmsA, the structural operon for the molybdoenzyme dimethyl sulfoxide reductase (21), hyc, the hydrogenase 3 structural operon (34), and narG, which encodes the molybdoenzyme nitrate reductase A (34). Hall and colleagues recently reported the crystallographic structure of ModE, which appears to possess distinct DNA- and molybdate-binding domains (9).
Genetic analysis of the regulation of moa has revealed that its expression is enhanced under anaerobic growth conditions but is repressed in strains able to synthesize active molybdenum cofactor (3). McNicholas et al. (22) reported that molybdate-ModE is able to bind at the moa promoter region and that moa expression displayed a twofold ModE dependency. However, a dependency of moa expression on molybdate has not been demonstrated. We report here that internal molybdate availability exerts a major influence on moa expression via a mechanism in which ModE acts directly as a positive regulator at one of the two moa promoters. This effect is distinct from and in addition to the anaerobic molybdenum-cofactor regulation of moa. This work exploits our previous finding that ModE is able to bind tungstate, a molybdate analogue, in a manner that is indistinguishable from that of molybdate (2).
MATERIALS AND METHODS
Bacterial strains, bacteriophage, and plasmids.
All strains (derivatives of E. coli strain K-12), bacteriophage, and plasmids used in this study are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) medium at 37°C, but strains carrying a Mucts prophage were grown at 30°C. Ampicillin was added, where required, to final concentrations of 125 μg/ml in liquid media and 50 μg/ml in solid media. Tetracycline and gentamicin were added, where required, to final concentrations of 25 and 10 μg/ml, respectively. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was added to media at 125 μg/ml when necessary. Isopropyl-β-d-thiogalactopyranoside (IPTG) was used as an inducer for the lac operon at a concentration of 0.3 mM. Aerobic growth conditions were achieved by placing 5 to 10 ml of culture in 50-ml conical flasks and incubating with vigorous shaking on an orbital shaker. Anaerobic growth was performed in an anaerobic chamber under an atmosphere containing carbon dioxide and hydrogen (GasPak; BBL Microbiology).
TABLE 1.
Bacterial strains, bacteriophage, and plasmids
| Strain, bacteriophage, or plasmida | Relevant genotype or characteristics | Source or reference |
|---|---|---|
| Strains | ||
| RK4353 | F′ araD139 Δ(argF-lac)U169 deoC1 flbB5201 gyrA 219 relA1 rpsL150 non-9 ptsF25 | 37 |
| DB1004 | As RK4353; φ(moaB::lacZ)4 | 3 |
| DB1060 | As RK4353; [λpφ(moaB::lacZ)4] | 3 |
| SE1597 | Δ(lacU)169 rpsL; modC118 | K. T. Shanmugan |
| RK4921 | As RK4353; zbh-623::Tn10 (near mod) | V. Stewart |
| LA27 | As DB1004; modC118 zbh-623::Tn10 | This work |
| LA29 | As LA27; modE::Gm | This work |
| TL28 | As DB1060; modC118 zbh-623::Tn10 | This work |
| LA30 | As TL28; modE::Gm | This work |
| MC4100 | F′ araD139 Δ(argF-lac)U169 deoC1 flbB5201 relA1 rpsL150 ptsF25 rbsR | Lab stocks |
| RM102 | As MC4100; fnr Δ(srl-recA)306::Tn10 | A. Bock |
| MC1000 | F′ araD139 Δ(araABC-leu) 7679 galU galK Δ(lac)X74 rpsL thi | Lab stocks |
| HORF55 | As MC1000; modE::Gm | 38 |
| NS3 | As MC4100; moeB | Lab stocks |
| Bacteriophage | ||
| λpφ(moa-lacZ)4 | λpφ(moaB::lacZ)4 | This work |
| Plasmids | ||
| pMLB524 | Ampr [Lac+] | 35 |
| pAA182 | Ampr ′lacZ | 14 |
| pEM101 | 1.16-kb PvuII fragment of moa DNA in pAA182 | This work |
| pEM114* | As pEM101, but 0.74-kb insert | This work |
| pEM117* | As pEM101, but 0.80-kb insert | This work |
| pEM118* | As pEM101, but 0.54-kb insert | This work |
| pEM220† | As pEM101, but 0.37-kb insert | This work |
*, insert constructed by deleting from the pAA182 multiple cloning site an EcoRI site upstream of the insert in plasmid pEM101; †, constructed by deleting from the vector's multiple cloning (BamHI) site downstream of the insert in pEM101.
Strain construction.
Generalized transduction between E. coli strains was carried out using a derivative of bacteriophage P1 essentially as described previously (35). A P1vir lysate was prepared with strain RK4921 (zbh-623::Tn10 [near mod]) and used as the donor in a cross with strain SE1597 (modC118). Recombinants selected as Tetr colonies were screened for loss of molybdenum-dependent formate hydrogenlyase activity by their inability to produce gas during glucose fermentation and for the nitrate reductase-negative phenotype by the nitrite overlay procedure (6). A P1vir lysate prepared with the resultant strain (as SE1597; zbh-623::Tn10) was used as the donor with acceptor strains DB1060 [as RK4353; λpφ(moa::lacZ)4] and DB1004 [φ(moa::lacZ)4] to give strains LA27 (as DB1004; modC118) and TL28 (as DB1060; modC118), respectively. Strain HORF55 was used as the donor in P1-mediated crosses with strains LA27 and TL28 to construct strains LA29 (as LA27; modE::Gm) and LA30 (as TL28; modE::Gm), respectively. Drug resistance was used for initial selection in all crosses.
Enzymes, reagents, and recombinant DNA techniques.
Restriction endonucleases, T4 DNA ligase, Klenow fragment of DNA polymerase I, and nuclease Bal 31 were purchased from Northumbria Biologicals. Sequenase was obtained from U.S. Biochemicals Corp. through Cambridge Bioscience (Cambridge, Mass.). Avian myeloblastosis virus reverse transcriptase was purchased from International Biotechnologies Inc. Human placental ribonuclease inhibitor and all radionucleotides were obtained from Amersham PLC. The isolation of plasmid DNA, restriction digestion, ligation, E. coli transformation, and agarose gel electrophoresis were essentially as described by Maniatis et al. (17).
The assay for β-galactosidase activity was performed essentially as described by Miller (24). Cells were assayed during early to mid-log phase following permeabilization by the addition of sodium dodecyl sulfate and chloroform. All assays were performed in triplicate and the mean values were reported in Miller units (24). Individual assays rarely differed from the mean by more than 10%.
Isolation of λpφ(moa-lacZ) phage and construction of lysogens.
Strain DB1004 [φ(moaB::lacZ)4] was grown to mid-log phase, and the cells were harvested and resuspended in a half-volume of 10 mM magnesium sulfate for lysate preparation. The suspension was exposed (2 min) to short-wave (260-nm) irradiation, diluted 10 times in LB medium, and incubated with shaking at 37°C in foil-covered tubes until lysis occurred (approximately 4 h). Cell debris was removed by centrifugation and the supernatants were used as low-titer lysates for production of Lac+ plaques when grown in the presence of X-Gal and IPTG. A high-titer lysate was used to construct moa::lacZ reporter lysogens by transduction into appropriate acceptor strains.
To prepare recombinant λ phage DNA, solid polyethylene glycol (PEG 8000) was dissolved at room temperature in a large-scale lysate to a final concentration of 10% (wt/vol). The phage was pelleted and then dispersed in a minimum volume (about 5 ml per liter of lysate) of buffer (50 mM Tris HCl [pH 7.5], 10 mM MgSO4). Following a phenol-chloroform extraction, the aqueous solution was layered over a glycerol step-gradient (5 to 40% [wt/vol]) and centrifuged at 70,000 × g for 90 min to pellet the phage. Residual host-derived DNA and RNA were removed using DNase and RNase. The phage coat was liberated by suspension in 0.5% (wt/vol) sodium dodecyl sulfate, 50 mM Tris HCl (pH 7.5), 400 mM EDTA, and 1 mg of proteinase K/ml. DNA was ethanol precipitated and resuspended in a suitable volume of sterile water or 10 mM Tris HCl (pH 8.0), and 1 mM EDTA.
Subcloning the moa promoter region and generation of deletion mutations.
A plasmid carrying the moa promoter region was isolated by shotgun cloning of EcoRI-digested phage λpφ(moaB::lacZ) DNA into the EcoRI-digested promoter-cloning vector pMLB524 and by selecting for Lac+ transformants of MC4100 (35).
Further subcloning of the moa promoter region gave rise to pEM101, which contained a 1.16-kb fragment of moa DNA in the SmaI site of pAA182, a transcriptional reporter plasmid carrying a promoterless lacZ gene. Deletion mutations in the moa promoter region were isolated as described below.
For 5′ deletions, plasmid pEM101 was digested with EcoRI and treated with nuclease Bal 31, and overhangs were filled in with Klenow fragment. Following digestion with BamHI, the blunt-end BamHI fragments were ligated to SmaI-BamHI-cleaved pAA182. The plasmids were characterized by agarose gel electrophoresis following EcoRI-BamHI digestion or by direct DNA sequence determination.
For 3′ deletions, plasmid pEM101 was digested with BamHI and treated with nuclease Bal 31, and overhangs were filled in with Klenow fragment and religated. The deleted plasmids were characterized by sequencing through the insert DNA region.
The extent of moa DNA remaining in the deletion constructs pEM114 and pEM117 was determined by DNA sequencing using the oligonucleotide primer L9 (5′-GCCCTGGTGGCAATCT-3′), which hybridizes to moa DNA between nucleotides +87 and +72, located between S1 and the translational start of moaA. The extent of the DNA insert remaining in plasmid pEM118 could not be determined in this manner, presumably due to loss of the primer binding site. The size of this insert was estimated from its electrophoretic mobility. The deletion end point in plasmid pEM220 was determined by DNA sequencing using an oligonucleotide primer which hybridizes to a region upstream of the distal transcriptional start site.
Double-stranded DNA sequencing reactions were performed as described previously (32).
The insert in plasmid pEM101 comprises 1,164 bp of chromosomal DNA, of which 587 nucleotides lie upstream of the translational start of moaA.
Analysis of transcription initiation sites.
Cultures of strain RK4353 carrying plasmid pEM101 were grown to an optical density at 600 nm of 0.6, and total RNA was recovered according to the method of Figueroa et al. (5). The dried RNA was resuspended in 30 μl of sterile H2O and hybridized with 0.1 pmol of 32P-labeled oligonucleotide primer. Primer extension was performed using the method of Inoue and Cech (11). The oligonucleotide primer L9 (see Fig. 2) was used in all the experiments shown. The same oligonucleotide primer was used in DNA sequencing reactions with plasmid pEM101 DNA as the template to produce a reference sequencing ladder, which was electrophoresed adjacent to the transcript-analysis samples in a 6% (wt/vol) denaturing polyacrylamide gel containing 6 M urea.
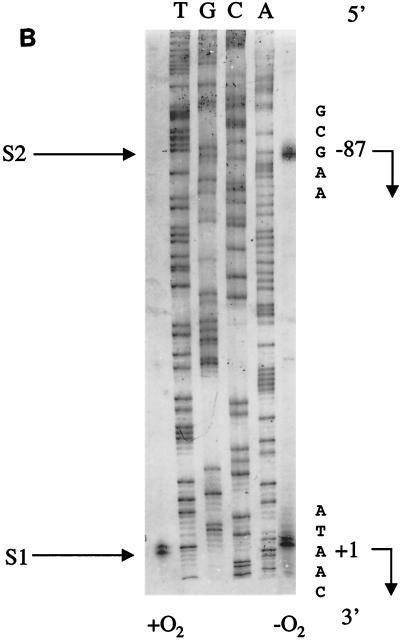
FIG. 2.
(A) Nucleotide sequence of the moa promoter region. Part of the moa upstream sequence is shown. Indicated are the different plasmid-borne 5′ (pEM114 and pEM117) and 3′ (pEM220) deletion end points. The position of binding of the primer (L9) used in the primer extension reactions is indicated by underlining and the L9 label. Transcriptional start sites, termed S1 (proximal) and S2 (distal), are shown in bold type. The Fnr recognition half-sites are underlined. The start codon of moaA is boxed and is in lowercase type, as is the portion of the moaA sequence shown. (B) Primer extension analysis of moa transcription. RNA was recovered from parental (RK4353) cells carrying plasmid pEM101, grown both aerobically (+O2) and anaerobically (−O2) in LB medium. The autoradiograph was reversed prior to photography to show the sequence of the coding strand. The DNA sequence shown was obtained using primer L9 (see Materials and Methods). The nucleotide sequence around each initiation site is shown on the right. The transcript labeled as S1 begins at nucleotide +1, and transcript S2 begins at position −87.
RESULTS
Molybdate activates moa expression under anaerobic growth conditions in a molybdenum cofactor-deficient background.
To assess the possible molybdate regulation of moa, moa-lacZ reporter strains defective in modC, which encodes part of the high-affinity molybdate transporter, were constructed in order to obtain molybdate starvation conditions in normal growth media.
In strains unable to synthesize molybdenum cofactor, reduction of the internal availability of molybdenum (modC) resulted in a much-reduced expression of moa compared to that found for the parental strain, DB1004 (Fig. 1A). Because strain LA27 (modC) is molybdenum cofactor-deficient, the reduced expression cannot arise from the previously described molybdenum cofactor-mediated repression. This suggests that intracellular molybdenum availability acts positively on moa via a mechanism that is independent of the molybdenum cofactor.
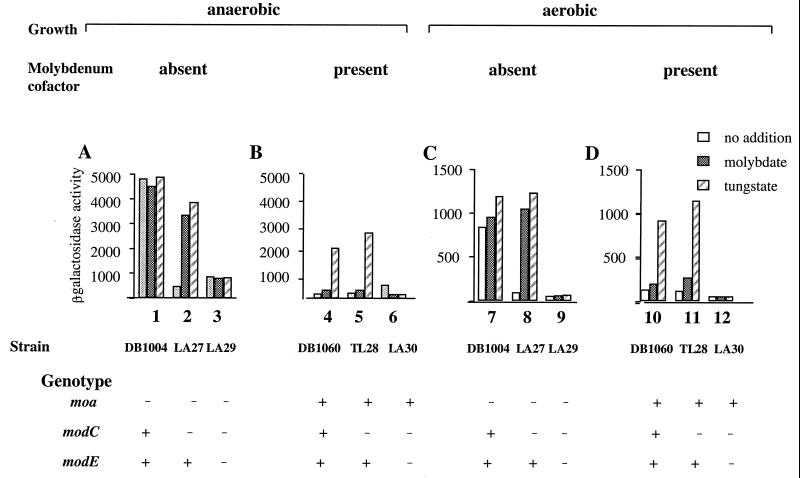
FIG. 1.
Expression of moa: ModE-dependent molybdate and tungstate regulation. All growths were performed at 37°C in LB medium. Additions to the growth media were sodium molybdate and sodium tungstate to a final concentration of 10 mM, as indicated. β-Galactosidase activity is expressed in Miller units (24). (A) Strains defective in molybdenum cofactor (moa), under anaerobic growth. (B) Strains which were molybdenum cofactor sufficient, under anaerobic growth. (C) Strains defective in molybdenum cofactor (moa), under aerobic growth. (D) Strains which were molybdenum cofactor sufficient, under aerobic growth. The strains used were as follows: 1 and 7, DB1004 [φ(moaB::lacZ)4]; 2 and 8, LA27 (as DB1004; modC118); 3 and 9, LA29 (as LA27; modE::Gm); 4 and 10, DB1060 [λpφ(moaB::lacZ)4]; 5 and 11, TL28 (as DB1060; modC118); 6 and 12, LA30 (as TL28; modE::Gm).
The internal molybdate deficiency brought about by mutation of modC (strain LA27) can be overcome by growth in the presence of excess molybdate. Under such conditions, expression of moa returned to levels similar to those found for the parental reporter strain (DB1004). The suppression of the modC phenotype with respect to moa expression by molybdate addition to the growth medium clearly points to a role for molybdenum in the activation of moa.
To establish whether ModE may be responsible for mediating the molybdenum-dependent activation, strain LA29 (modC, modE) which was additionally defective in modE was constructed. Expression of moa was much reduced in this strain but the addition of molybdate to the growth medium was without effect (Fig. 1A). The ModE protein is, therefore, required for the molybdenum-dependent activation of moa. Since it is established that ModE binds molybdate, it appears that the internal availability of molybdate, rather than any other form of molybdenum, is the signal for moa activation. The ModE-mediated, molybdate-dependent regulation of moa can result in a fivefold increase in activation.
Molybdate cannot activate moa in a molybdenum cofactor-sufficient background.
Baker and Boxer (3) reported that moa is repressed in molybdenum cofactor-sufficient strains. As anticipated, in the presence of functional molybdenum cofactor, moa was repressed {strain DB1060 [moa+/φ(moaB-lacZ)]}, and the addition of molybdate to the growth medium was without effect (Fig. 1B). Strain TL28 [moa+/φ(moaB-lacZ), modC], in the presence or absence of added molybdate, displayed levels of moa expression similar to the repressed levels in strain DB1060. Furthermore, loss of ModE function was without effect, since expression in strain LA30 [moa+/φ(moaB-lacZ) modC modE] remained low and uninfluenced by the addition of molybdate. The clear ModE-dependent molybdate activation of moa observed in molybdenum cofactor-deficient backgrounds is not found in strains possessing functional molybdenum cofactor. It is evident that molybdenum cofactor-dependent repression is dominant over the ModE-requiring molybdate activation. However, high expression of moa clearly requires ModE.
Aerobic expression of moa displays similar control characteristics to those present during anaerobic growth.
The aerobic expression of moa was monitored in a manner similar to that described above. The relative patterns of expression were closely similar to those found for anaerobically grown cultures (Fig. 1, compare C and D). In the molybdenum cofactor-deficient strain DB1004, the anaerobic enhancement was four- to fivefold higher, whereas in the molybdenum cofactor-sufficient strain (DB1060), the anaerobic enhancement was much lower. Molybdate availability, therefore, activates moa during both anaerobic and aerobic growth in a ModE-dependent manner and is a major positive control on moa.
Tungstate activates moa expression in a ModE-dependent manner.
Tungstate is a close analogue of molybdate in biological systems. Tungstate interacts with isolated ModE indistinguishably from molybdate, and the tungstate-ModE complex also interacts with DNA in a manner similar to that of the molybdate-ModE complex (2). The effects of tungstate on moa regulation were investigated.
The addition of tungstate to the growth media of molybdenum cofactor-deficient strains LA27 (modC) and LA29 (modC modE) influenced moa expression in a way similar to that found for molybdate (Fig. 1A and C). The expression of moa in strain LA27 was enhanced to a comparable level to that found for the parental strain, DB1004, when grown in tungstate. The addition of tungstate to the growth medium for strain LA29 (modE) could not restore the tungstate activation seen in strain LA27. A similar result was also obtained following tungstate addition to the growth medium for strain LA30 (modC modE). We conclude that for a molybdenum cofactor-deficient strain, tungstate, like molybdate, is able to activate moa expression in a ModE-dependent manner.
In contrast to molybdate, tungstate relieves molybdenum cofactor-dependent repression of moa.
The low moa expression in the (moa+) merodiploid strain DB1060 can be explained by repression brought about by the molybdenum cofactor sufficiency of the strain. This is revealed by the enhanced expression of moa observed when mutations in other (mo−) genes required for molybdenum cofactor biosynthesis are introduced into such merodiploids. This repression could be mediated by the cofactor itself, a derivative of the cofactor, or a process which is dependent on the active cofactor (3).
Growth in the presence of tungstate gives rise to a biologically inactive tungsten form of the molybdenum cofactor, which leads to the formation of inactive forms of molybdoenzymes (16). The expression of moa is low in DB1060, a molybdenum cofactor-sufficient strain, but on the addition of tungstate to the growth medium, expression was increased some 10-fold (Fig. 1B and D). This was in contrast to the lack of effect of molybdate addition to this strain. Similarly, the addition of tungstate to the growth medium for strain TL28 (modC) gave rise to enhanced moa expression; however, molybdate addition was without effect. The presence or absence of functional ModE did not influence this tungstate-specific effect, which was observed only in molybdenum cofactor-sufficient strains.
These observations cannot be explained by the ModE-dependent tungstate enhancement of moa demonstrated earlier for the molybdenum cofactor-deficient strains (Fig. 1A and C). This tungstate effect is dependent upon the ability of the strain to synthesize functional molybdenum cofactor under normal growth conditions. The most likely explanation is that the tungstate leads to the formation of a nonfunctional tungsten form of the cofactor, which is unable to effect moa repression via the molybdenum cofactor-dependent mechanism.
Identification of two transcriptional start sites at moa.
Plasmid pEM101 consists of approximately 0.6 kb of DNA upstream of the moaA translational start inserted into the multiple cloning site of the transcriptional (LacZ) reporter plasmid vector, pAA182. The moa DNA in pEM101 was subcloned from a Lac+ bacteriophage λ [φ(moaB-lacZ)], a transducing phage induced from strain DB1004. That the moa promoter region was present in pEM101 was confirmed by the retention of the moa expression characteristics by the plasmid (data not shown). This retention also confirmed that moa regulation is exerted at the level of transcription.
The insert in plasmid pEM101 comprised 1,164 bp of chromosomal DNA, and part of the DNA sequence which lies upstream of the moaA translational start is shown in Fig. 2A. Primer extension analysis revealed two transcriptional start sites (Fig. 2B). The proximal transcriptional start site, S1, lies 130 bp upstream of the translational start of moaA; the distal start site, S2, lies a further 87 bp upstream of S1 (Fig. 2A).
Transcripts originated from S2 only when mRNA was isolated from cells grown anaerobically. Transcripts from S1, however, were seen following growth under both aerobic and anaerobic conditions regardless of whether expression from the distal transcriptional start was active.
Analysis of expression from deletion clones.
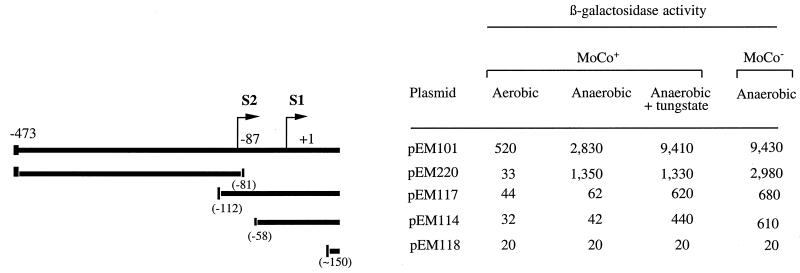
We determined, by using deletion analysis, the expression characteristics of the independent moa promoters in order to assess their contribution to the overall control of the locus. Three 5′-deleted plasmids were obtained following Bal 31 digestion from the EcoRI site in pEM101. These constructs are pEM114 (∼0.74 kb insert; carrying only S1), pEM117 (∼0.80 kb insert; carrying S1 and S2 with 25 bp upstream of S2), and pEM118 (∼0.54 kb; whole promoter region absent). A further deletion, in plasmid pEM220 (insert 0.37 kb), was obtained by Bal 31 digestion originating from the BamHI site at the 3′ limit of the insert in pEM101. The structures of the deletion clones are summarized in Fig. 3.
FIG. 3.
Properties of promoter-deletion clones. The direction of transcription is indicated by arrows and the position of the first nucleotide in each transcript is given. The DNA present in each of the deleted clones is shown by the broad lines, with the deletion end point given in parentheses. The expression from each of the constructs is given as β-galactosidase activity (Miller units). MoCo+ indicates that the strain (RK4353) is able to synthesize functional molybdenum cofactor. MoCo− indicates that the strain (NS3; moeB) is unable to synthesize functional molybdenum cofactor. Each plasmid was examined in both a MoCo+ strain (RK4353) and a MoCo− strain (NS3; moeB). The bacteria were grown in LB medium at 37°C. Growth conditions and additions were as described in Materials and Methods.
Since strains carrying mutations in either moa or moe cannot synthesize molybdopterin (28), plasmids pEM101, pEM220, pEM117, pEM114, and pEM118 were transformed into the molybdenum cofactor-deficient strain NS3 (moeB) in order to assess the capacity of the deletion constructs to respond to moa effectors. The results were compared to those for the intact promoter plasmid, pEM101 (Fig. 3).
In the molybdenum cofactor-sufficient background, expression from pEM101 was anaerobically enhanced fivefold. This anaerobic expression was still partially repressed, since tungstate addition led to a further threefold enhancement. Such repression was absent from the corresponding moeB strain. Expression in this strain was, as anticipated, similar to that of the parental strain when grown with tungstate.
Plasmid pEM220 obtained from the 3′ deletion of pEM101 retains only 8 nucleotides of moa DNA downstream of S2. This construct showed little or no promoter activity under aerobic conditions but exhibited a 40-fold enhancement during anaerobic growth. This is consistent with expression from S2 occuring only in anaerobic cultures (Fig. 2B). The addition of tungstate to the anaerobic growth medium was without effect on expression from pEM220, suggesting that expression from S2 is not influenced by molybdate or the molybdenum cofactor. Placing this plasmid into a strain genetically lacking the functional cofactor, however, did lead to a further small increase in anaerobic expression, the significance of which is presently unclear. Plasmid pEM101, however, displayed high levels of anaerobic expression in the moeB strain similar to those obtained following the addition of tungstate to the molybdenum cofactor-sufficient strain (RK4353). We conclude that the distal promoter, as indicated from the transcript analysis, is responsible for the anaerobic enhancement of moa expression.
The deletion clone pEM114 has lost the distal (S2) start and an additional 28 nucleotides downstream. This plasmid retains the −35 and −10 regions of the proximal promoter. Under all conditions examined, expression from this clone was lower than that for the parental plasmid, pEM101. For this clone in the absence of tungstate, expression was not increased during anaerobic growth. During anaerobic growth the addition of tungstate to the molybdenum cofactor-sufficient parental strain carrying pEM114 raised expression. A similar derepression was also seen when the plasmid was present in the molybdenum cofactor-deficient (moeB) strain. Overall, these results suggest that the loss of S2 has two effects. Firstly, the level of expression is lower. Secondly, the anaerobic enhancement is lost. The results for pEM117, which has only 25 bp of DNA upstream of S2, were similar to those found for pEM114.
Transcription-start analysis on pEM114 and pEM117 confirmed that transcription was from S1 in both plasmids. As would be expected from the loss of the −35 region of S2, transcription from S2 in pEM117 could not be detected even following anaerobic growth (data not shown). Expression from these two 5′-deletion clones is much lower than that from the parental plasmid, which may reflect a requirement for sequences 5′ to S2 being required for full expression from S1. As anticipated, expression from plasmid pEM118 (both S1 and S2 absent) was negligible under all conditions tested.
In the absence of S2 (pEM117 and pEM114), there is both a tungstate enhancement in the molybdenum cofactor-sufficient parental strain background and a derepression to a similar level in the molybdenum cofactor-deficient strain. We conclude that the promoter associated with S1 is subject to repression in molybdenum cofactor-sufficient strains.
If the two moa promoters worked independently in situ, the expression observed when both promoters are functional should equal the sum of their individual expressions. This is not the case. The proximal promoter clearly overlaps part of the distal promoter.
Expression from the distal promoter is insensitive to molybdate.
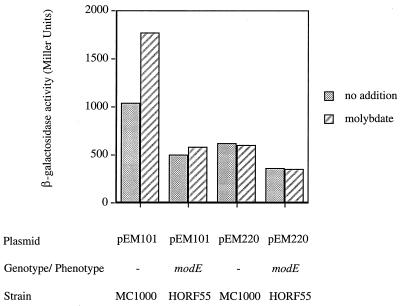
The plasmid pEM101 possesses both promoter regions and retains the overall characteristics of moa regulation. Plasmid pEM220 contains only the distal promoter. In order to establish whether the distal promoter is regulated by ModE, the plasmids were each transformed into a modE strain (HORF55) and expression was monitored following anaerobic growth (Fig. 4). In the modE strain, expression from pEM101 was reduced approximately twofold from the level for the parental strain background. Expression from pEM101 was enhanced some 75% by molybdate addition to the growth medium in the modE+ strain, but in the modE-deficient strain the addition of molybdate was without effect. This is consistent with the results obtained for the chromosomal moa-lacZ strains. When only S2 is present (pEM220), neither the presence of molybdate in the growth medium nor the lack of modE influences expression. This strongly indicates that the ModE-dependent molybdate control of moa is effected through the proximal promoter.
FIG. 4.
ModE does not regulate moa expression from the distal promoter. The plasmids were present in the strains as indicated. Cells were grown under anaerobic conditions at 37°C in LB medium with 125 μg of ampicillin/ml. Where indicated, sodium molybdate was added at a final concentration of 10 mM. β-Galactosidase activity is expressed in Miller units (24).
Fnr is required for the anaerobic regulation of moa at the distal promoter.
The fnr gene encodes a positive transcriptional regulator required for the expression of a number of anaerobically expressed genes (36). A putative Fnr recognition sequence (GATGAT-N4-ATCAAA), centered at −39.5 nucleotides upstream of S2, was found (Fig. 2A). This site differs from the consensus sequence in only one base. Baker and Boxer previously reported that the fnr gene is not required for the anaerobic expression of moa (3). In our earlier studies, single-copy moaB-lacZ chromosomal translational gene fusions which contained the intact promoter region upstream of the moa operon were used. Since the previous results reflected the combined effects of both promoters and the anaerobic enhancement of moa can be studied directly using pEM220, we decided to reinvestigate the possible involvement of fnr in moa regulation.
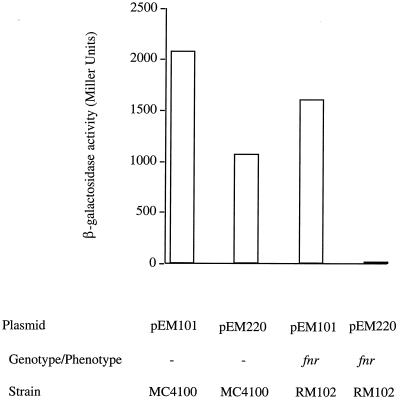
During anaerobic growth high levels of expression were observed from plasmid pEM101 (both promoters) when present in the parental strain. Plasmid pEM220 (distal promoter only) also displayed a high level of expression, although slightly lower than that for pEM101. When expression from pEM101 was examined in the fnr strain, a small reduction to about 70% of the parental strain level was obtained (Fig. 5). This is comparable to the results reported previously by Baker and Boxer (3) for the chromosomal fusions, which were not considered significant at the time. However, when expression of pEM220 was examined in the fnr strain, a large reduction (60-fold or more) was observed. It is clear, therefore, that full anaerobic expression of moa requires the Fnr protein. This effect is mediated through the distal promoter. The full fnr dependency is apparent only when expression from the distal promoter (pEM220) is examined in isolation.
FIG. 5.
Fnr controls the anaerobic expression of moa at the distal promoter. The plasmids were present in the strains as indicated. All growths were performed under anaerobic conditions at 37°C in LB medium with 125 μg of ampicillin/ml. β-Galactosidase activity is expressed in Miller units (24).
DISCUSSION
Molybdate addition to the growth medium brings about an activation of moa expression during both anaerobic and aerobic growth of some four- to fivefold over that found in its absence. Molybdate acts as a major positive regulator of moa and its action requires the ModE protein. The molybdate activation of moa, however, is revealed only in a molybdenum cofactor-deficient background, since in molybdenum cofactor-sufficient strains, moa is effectively repressed. ModE is a major regulator of moa, since in modE strains moa expression levels remain low regardless of growth conditions or molybdate or molybdenum-cofactor availability. A summary of our findings regarding the regulation of moa is shown in Fig. 6.
FIG. 6.
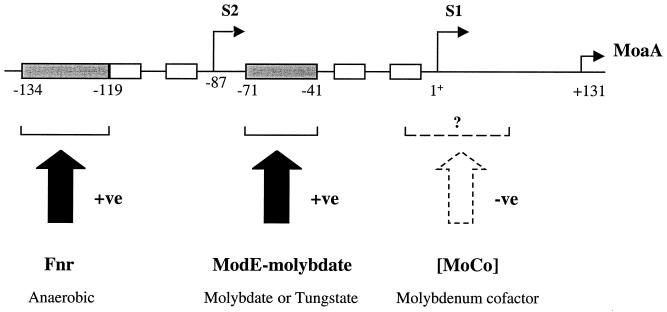
Regulation of moa expression. The diagram is a schematic representation of the DNA sequence upstream of moaA indicating the two transcriptional start sites, S1 and S2, along with their ς70 −10 and −35 regions, which are shown as open boxes. Numbering of the DNA sequence is with reference to the S1 transcription start site as +1. Anaerobic regulation is effected by the positive global regulator Fnr, the putative binding site for which is shown as the filled box (−134 to −119) upstream of S2. The positive regulation by molybdate is effected by the ModE-molybdate complex, which acts at the filled boxed region (−71 to −41) upstream of S1. Tungstate is also capable of effecting this regulation. Molybdenum cofactor (MoCo)-mediated negative regulation appears to be exerted at the S1 transcription start site. The mechanism for this regulation is presently unknown. Tungstate in the growth medium is able to alleviate the molybdenum cofactor repression.
ModE has been best characterized as a transcriptional repressor for the modABCD operon (8, 38). Although molybdate-ModE has been shown to mediate enhanced expression of dmsA under certain conditions, its mode of action is thought to be indirect, namely that of a transcriptional repressor of a direct repressor of dmsA (21). Recently, molybdate-ModE has been proposed as a secondary transcriptional activator of both hyc and narG (34). In the case of hyc, the ModE-molybdate complex is thought to bind about 95 bp upstream of the main formate-dependent positive transcriptional regulator, FhlA. In the regulation of narG, ModE-molybdate does not bind directly to the narG promoter region but binds to the narK-narXL intragenic region. This suggests that its effect on narG is mediated via the narXL operon (34). McNicholas et al. (22), by employing DNaseI-protection experiments, defined the ModE binding site at moa and we have confirmed this by gel shift analysis (data not shown). The ModE-molybdate binding site at moa is centered at −57.5 with respect to the proximal moa transcription start site and, therefore, is adjacent to the −35 region of this promoter. The magnitude of the ModE-dependent molybdate activation of moa, the direct binding of ModE to the proximal moa promoter region, and the persistent low levels of moa expression in modE strains point to molybdate-ModE being a major direct, positive transcriptional regulator of this operon. Previously, McNicholas et al. (22) reported, from analysis of a molybdenum cofactor-sufficient strain, a twofold, ModE-dependent but molybdate-independent enhancement of moa expression.
The molybdate regulation of both the hyc and narG operons also displays a ModE-independent, MoeA-dependent component (10). There is no evidence for such regulation of moa, since all the molybdate effects appear to be totally dependent upon ModE.
Enhanced expression of moa under anaerobic growth conditions has been reported previously (3). This effect, like the molybdate effect, is only fully apparent in strains that lack functional molybdenum cofactor. Only a modest twofold enhancement of moa to a very low level can be seen in molybdenum cofactor-sufficient strains. In molybdenum cofactor-deficient strains also lacking ModE activity, anaerobiosis elevates moa expression about eightfold over the aerobic levels but only to a relatively modest level (∼800 Miller units). However, anaerobic growth of such strains with a functional ModE results in a further fourfold increase in moa expression (Fig. 1). Under aerobic growth conditions, the ModE-dependent molybdate activation effects a 10-fold or so enhancement to an intermediate (1,100 units) level of expression. ModE-molybdate, therefore, is required for full moa expression.
The molybdate- and anaerobic-dependent enhancements of moa appear independent and distinct. This interpretation is consistent with our finding of two promoters at moa. Analysis of expression from the individual moa promoters clearly showed that the global anaerobic transcriptional regulator, Fnr, acts positively at the highly active, distal moa promoter. Full expression of moa, during anaerobic growth with high molybdate availability, requires the action of the two positive regulators, ModE and Fnr. ModE and Fnr mediate their effects specifically and distinctly at the proximal and distal promoters, respectively. The promoter subclones containing only the proximal promoter generally displayed poor expression. Given the close proximity of the transcriptional start sites, it is likely that the proximal promoter region overlaps the distal transcription start. Further work is required to illuminate how expression from each of the two promoters is related to that of the other.
The molybdate and anaerobic control of moa described above is apparent only in strains that are molybdenum cofactor-deficient. That molybdenum cofactor sufficiency leads to tight moa repression was identified by Baker and Boxer (3). It is abundantly clear from the present work that the molybdenum cofactor-dependent repression of moa is dominant over both the molybdate and anaerobic effects. The mechanism of this repression has not been established but it appears from the analysis of the promoter subclones that the repression is mediated at the proximal promoter region. Experiments designed to isolate a molybdenum cofactor-dependent repressor are currently under way.
A large enhancement of moa transcription is observed when tungstate is present in the growth medium. ModE binds tungstate in a virtually identical manner to molybdate, and ModE-tungstate can bind to the modA promoter (2). In strains that are deficient in molybdenum cofactor it is clear that tungstate can also bring about a ModE-dependent activation of moa. Under these conditions, tungstate and molybdate are equivalent with respect to their positive effect on moa expression.
In strains genetically able to synthesize molybdenum cofactor, the effect of tungstate in the growth medium is more complicated. Under these conditions tungstate leads to a full derepression of moa via a mechanism that is independent of ModE. This demonstrates a second ModE-independent tungstate effect on moa which is dependent upon the capacity of the bacterium to synthesize active molybdenum cofactor in the absence of tungstate. It is known that tungsten can substitute for molybdenum in the molybdenum cofactor to form a biologically inactive derivative (16). We propose that the tungsten cofactor derivative cannot mediate moa repression. This gives a plausible explanation for this tungstate effect on moa. Either the tungsten-cofactor derivative is unable to bind to the putative molybdenum cofactor-repressor, or the tungsten cofactor-repressor complex cannot productively bind to the proximal transcriptional start region.
The moa operon encodes the enzymes required for the first dedicated step of molybdopterin synthesis. It is appropriate, therefore, that this locus should be subject to transcriptional control, and the expression of moa is indeed highly regulated. The regulation is generally consistent with what is known concerning molybdoenzyme biosynthesis in E. coli. The positive molybdate control should ensure that molybdopterin biosynthesis is down regulated under conditions of limited molybdate availability. When there is sufficient molybdenum cofactor, further molybdopterin synthesis would not be required. Almost all of the E. coli molybdoenzymes are present predominantly during anaerobic growth. The anaerobic molybdoenzymes nitrate reductases and DMSO reductase under fully induced conditions are of high abundance in the bacterium. This points to a relatively high requirement for the molybdenum cofactor during anaerobiosis. The anaerobic regulation of moa, therefore, acts to ensure appropriate molybdopterin availability for the synthesis of these metabolically important enzymes.
ACKNOWLEDGMENTS
This work was supported by a Biotechnology and Biological Sciences Research Council grant to D. H. Boxer and a studentship to L. A. Anderson.
A. Bock, S. Busby, R. Eichenlaub, M. Berman, K. T. Shanmugam, and V. Stewart are thanked for gifts of strains or plasmids.
REFERENCES
- 1.Amy N K, Rajagopalan K V. Characterization of molybdenum cofactor from Escherichia coli. J Bacteriol. 1979;140:114–124. doi: 10.1128/jb.140.1.114-124.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson L A, Palmer T, Price N C, Bornemann S, Boxer D H, Pau R N. Characterisation of the molybdenum-responsive ModE regulatory protein and its binding to the promoter region of the modABCD (molybdenum transport) operon of Escherichia coli. Eur J Biochem. 1997;246:119–126. doi: 10.1111/j.1432-1033.1997.00119.x. [DOI] [PubMed] [Google Scholar]
- 3.Baker K P, Boxer D H. Regulation of the chlA locus of Escherichia coli K12: involvement of molybdenum cofactor. Mol Microbiol. 1991;5:901–907. doi: 10.1111/j.1365-2958.1991.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 4.Dias J M, Than M E, Humm A, Huber R, Bourenkov G P, Bartunik H D, Bursakov S, Calvete J, Caldeira J, Carneiro C, Moura J J, Romao M J. Crystal structure of the first dissimilatory nitrate reductase at 1.9 Å solved by MAD methods. Struct Fold Des. 1999;7:65–79. doi: 10.1016/s0969-2126(99)80010-0. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa N, Wills N, Bossi L. Common sequence determinants of the response of a prokaryotic promoter to DNA bending and supercoiling. EMBO J. 1991;10:941–949. doi: 10.1002/j.1460-2075.1991.tb08028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaser J H, DeMoss J A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971;108:854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunden A M, Ray R M, Rosentel J K, Healy F G, Shanmugam K T. Repression of the Escherichia coli modABCD (molybdate transport) operon by ModE. J Bacteriol. 1996;178:735–744. doi: 10.1128/jb.178.3.735-744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunden A M, Self W T, Villain M, Blalock J E, Shanmugan K T. An analysis of the binding of repressor protein ModE to modABCD (molybdate transport) operator/promoter DNA of Escherichia coli. J Biol Chem. 1999;274:24308–24315. doi: 10.1074/jbc.274.34.24308. [DOI] [PubMed] [Google Scholar]
- 9.Hall D R, Gourley D G, Leonard G A, Duke E M H, Anderson L A, Boxer D H, Hunter W N. The high-resolution crystal structure of the molybdate-dependent transcriptional regulator (ModE) from Escherichia coli: a novel combination of domain folds. EMBO J. 1999;18:1435–1446. doi: 10.1093/emboj/18.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasona A, Self W T, Ramesh M R, Shanmugam K T. Molybdate-dependent transcription of hyc and nar operons of Escherichia coli requires MoeA protein and ModE-molybdate. FEMS Microbiol Lett. 1998;169:111–116. doi: 10.1111/j.1574-6968.1998.tb13306.x. [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Cech T R. Secondary structure of the circular form of the Tetrahymena rRNA intervening sequence: a technique for RNA structure analysis using chemical probes and reverse transcriptase. Proc Natl Acad Sci USA. 1985;82:648–652. doi: 10.1073/pnas.82.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iobbi-Nivol C, Palmer T, Whitty P W, McNairm E, Boxer D H. The mob locus of Escherichia coli K12 required for molybdenum cofactor biosynthesis is expressed at very low levels. Microbiology. 1995;141:1663–1671. doi: 10.1099/13500872-141-7-1663. [DOI] [PubMed] [Google Scholar]
- 13.James R, Dean D, Debbage J. Five open reading frames upstream of the dnaK gene of E. coli. DNA Sequence. 1993;3:327–332. doi: 10.3109/10425179309020832. [DOI] [PubMed] [Google Scholar]
- 14.Jayaraman P S, Peakman T C, Busby S W J, Quincey R V, Cole J A. Location and sequence of the promoter of the gene for the NADH-dependent nitrite reductase of Escherichia coli and its regulation by oxygen, the FNR protein and nitrite. J Mol Biol. 1987;196:781–788. doi: 10.1016/0022-2836(87)90404-9. [DOI] [PubMed] [Google Scholar]
- 15.Kamdar K P, Shelton M E, Finnerty V. The Drosophila molybdenum cofactor gene cinnamon is homologous to three Escherichia coli cofactor proteins and the rat protein gephyrin. Genetics. 1994;137:791–801. doi: 10.1093/genetics/137.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kletzin A, Adams M W W. Tungsten in biological systems. FEMS Microbiol Rev. 1996;18:5–63. doi: 10.1016/0168-6445(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 17.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 18.McAlpine A S, McEwan A G, Bailey S. The high resolution crystal structure of DMSO reductase in complex with DMSO. J Mol Biol. 1998;275:613–623. doi: 10.1006/jmbi.1997.1513. [DOI] [PubMed] [Google Scholar]
- 19.McMaster J, Enemark J H. The active sites of molybdenum- and tungsten-containing enzymes. Curr Opin Chem Biol. 1998;2:201–207. doi: 10.1016/s1367-5931(98)80061-6. [DOI] [PubMed] [Google Scholar]
- 20.McNicholas P M, Chiang R C, Gunsalus R P. The Escherichia coli modE gene: effects of mutations on molybdate dependent modA expression. FEMS Microbiol Lett. 1996;145:117–123. doi: 10.1016/0378-1097(96)00398-9. [DOI] [PubMed] [Google Scholar]
- 21.McNicholas P M, Chiang R C, Gunsalus R P. Anaerobic regulation of the Escherichia coli dmsABC operon requires the molybdate-responsive regulator ModE. Mol Microbiol. 1998;27:197–208. doi: 10.1046/j.1365-2958.1998.00675.x. [DOI] [PubMed] [Google Scholar]
- 22.McNicholas P M, Rech S A, Gunsalus R P. Characterization of the ModE DNA-binding sites in the control regions of modABCD and moaABCDE of Escherichia coli. Mol Microbiol. 1997;23:515–524. doi: 10.1046/j.1365-2958.1997.d01-1864.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller J, Scott D, Amy N. Molybdenum-sensitive transcriptional regulation of the chlD locus of Escherichia coli. J Bacteriol. 1987;169:1853–1860. doi: 10.1128/jb.169.5.1853-1860.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 25.Mouncey N J, Mitchenall L A, Pau R N. The modE gene product mediates molybdenum-dependent expression of genes for the high-affinity molybdate transporter and modG in Azotobacter vinelandii. Microbiology. 1996;142:1997–2004. doi: 10.1099/13500872-142-8-1997. [DOI] [PubMed] [Google Scholar]
- 26.Nohno A, Kasai Y, Saito T. Cloning and complete nucleotide sequence of the Escherichia coli chlEN operon involved in molybdopterin biosynthesis. J Bacteriol. 1988;170:4097–4102. doi: 10.1128/jb.170.9.4097-4102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pau R N, Klipp W, Leimkuhler S. Molybdenum transport, processing and gene regulation. In: Winkelmann G, Carrano C J, editors. Transition metals in microbial metabolism. Newark, N.J: Harwood Academic Publishers; 1997. pp. 217–234. [Google Scholar]
- 28.Rajagopalan K V, Johnson J L. The pterin molybdenum cofactors. J Biol Chem. 1992;267:10199–10202. [PubMed] [Google Scholar]
- 29.Rech S, Deppenmeier U, Gunsalus R P. Regulation of the molybdate transport operon, modABCD, of Escherichia coli in response to molybdate availability. J Bacteriol. 1995;177:1023–1029. doi: 10.1128/jb.177.4.1023-1029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivers S L, McNairn E, Blasco F, Giordano G, Boxer D H. Molecular genetic analysis of the moa operon of Escherichia coli K-12 required for molybdenum cofactor biosynthesis. Mol Microbiol. 1993;8:1071–1081. doi: 10.1111/j.1365-2958.1993.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 31.Rosentel J K, Healy F, Maupin-Furlow J A, Lee J H, Shanmugam K T. Molybdate and regulation of mod (molybdate transport), fdhF, and hyc (formate hydrogenlyase) operons in Escherichia coli. J Bacteriol. 1995;177:4857–4864. doi: 10.1128/jb.177.17.4857-4864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1997;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider F L, Huber R, Schindelin H, Kisker C, Knablein J. Crystal structure of dimethyl sulfoxide reductase from Rhodobacter capsulatus at 1.88 Å resolution. J Mol Biol. 1996;263:53–69. doi: 10.1006/jmbi.1996.0555. [DOI] [PubMed] [Google Scholar]
- 34.Self W T, Grunden A M, Hasona A, Shanmugam K T. Transcriptional regulation of molybdoenzyme synthesis in Escherichia coli in response to molybdenum: ModE-molybdate, a repressor of the modABCD (molybdate transport) operon is a secondary transcriptional activator for the hyc and nar operons. Microbiology. 1999;145:41–55. doi: 10.1099/13500872-145-1-41. [DOI] [PubMed] [Google Scholar]
- 35.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 36.Spiro S, Guest J R. FNR and its role in oxygen-related gene expression in Escherichia coli. FEMS Microbiol Rev. 1990;6:399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- 37.Stewart V, MacGregor C H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982;151:788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walkenhorst H M, Hemschemeier S K, Eichenlaub R. Molecular analysis of the molybdate uptake operon, modABCD, of Escherichia coli and modR, a regulatory gene. Microbiol Res. 1995;150:347–361. doi: 10.1016/S0944-5013(11)80016-9. [DOI] [PubMed] [Google Scholar]
- 39.Wuebbens M W, Rajagopalan K V. Investigation of the early steps of molybdopterin biosynthesis in Escherichia coli through the use of in vivo labeling studies. J Biol Chem. 1995;270:1082–1087. doi: 10.1074/jbc.270.3.1082. [DOI] [PubMed] [Google Scholar]