Abstract
Mutants of the bacterium Acinetobacter sp. strain ADP1 were selected to grow on benzoate without the BenM transcriptional activator. In the wild type, BenM responds to benzoate and cis,cis-muconate to activate expression of the benABCDE operon, which is involved in benzoate catabolism. This operon encodes enzymes that convert benzoate to catechol, a compound subsequently degraded by cat gene-encoded enzymes. In this report, four spontaneous mutants were found to carry catB mutations that enabled BenM-independent growth on benzoate. catB encodes muconate cycloisomerase, an enzyme required for benzoate catabolism. Its substrate, cis,cis-muconate, is enzymatically produced from catechol by the catA-encoded catechol 1,2-dioxygenase. Muconate cycloisomerase was purified to homogeneity from the wild type and the catB mutants. Each purified enzyme was active, although there were differences in the catalytic properties of the wild type and variant muconate cycloisomerases. Strains with a chromosomal benA::lacZ transcriptional fusion were constructed and used to investigate how catB mutations affect growth on benzoate. All of the catB mutations increased cis,cis-muconate-activated ben gene expression in strains lacking BenM. A model is presented in which the catB mutations reduce muconate cycloisomerase activity during growth on benzoate, thereby increasing intracellular cis, cis-muconate concentrations. This, in turn, may allow CatM, an activator similar to BenM in sequence and function, to activate ben gene transcription. CatM normally responds to cis,cis-muconate to activate cat gene expression. Consistent with this model, muconate cylcoisomerase specific activities in cell extracts of benzoate-grown catB mutants were low relative to that of the wild type. Moreover, the catechol 1,2-dioxygenase activities of the mutants were elevated, which may result from CatM responding to the altered intracellular levels of cis,cis-muconate and increasing catA expression. Collectively, these results support the important role of metabolite concentrations in controlling benzoate degradation via a complex transcriptional regulatory circuit.
Acinetobacter sp. strain ADP1 can catabolize benzoate as the sole source of carbon and energy via the β-ketoadipate pathway, a well-studied pathway for the microbial dissimilation of aromatic compounds (Fig. 1) (8). Growth on benzoate involves the coordinated expression of at least 15 ben and cat genes clustered on the ADP1 chromosome (Fig. 2). Among these genes are benM and catM, which encode homologous LysR-type transcriptional regulators (5, 18, 22). Chromosomal disruption of benM prevents growth on benzoate, although mutants that acquire the ability to grow with benzoate as the sole carbon source arise at a frequency of approximately 10−5 (5). To improve our understanding of the complex regulatory circuit governing benzoate degradation in ADP1, we characterized spontaneous mutants that grow on benzoate in the absence of BenM.
FIG. 1.
Catechol branch of the β-ketoadipate pathway of Acinetobacter sp. strain ADP1. The compounds and genes relevant to these studies are in boldface and italicized, respectively. Rectangles indicate the catA- and catB-encoded enzymes that produce and consume cis,cis-muconate, respectively. CoA, coenzyme A.
FIG. 2.
Restriction map of the chromosomal ben-cat region. Genes and their transcriptional directions are shown relative to some of the KpnI (K), ClaI (C), HindIII (H), SphI (Sp), EcoRI (E), SalI (S), and HincII (Hc) restriction endonuclease sites. Numerical subscripts distinguish sites described in Table 1. Triangles mark the insertion sites of the ΩS (21) and lacZ::Kmr (13) cassettes of strains in Table 1. An enlargement of the catM-catB region illustrates the approximate sites of mutations (asterisks) found in the strains indicated in the attached boxes. Within each box, the nucleotide (nt) position and base change of the mutation are shown, followed by the corresponding amino acid change in the CatB protein. Below the enlarged map, striped lines represent 1.4-kbp SphI-to-SalI (A), 1.0-kbp HindIII-to-SalI (B), 1.3-kbp EcoRI-to-SalI (C), 0.3-kbp EcoRI-to-HindIII (D), and 0.9-kbp HindIII-to-EcoRI (E) DNA fragments. These fragments from different mutants were ligated to appropriately digested cloning vector pUC19 (27). Whether (+) or not (−) the resultant plasmids (pBAC258-307 and pBAC439-446; Table 1) could transform benM mutant strain ISA36 to a strain able to grow on benzoate as the sole carbon source is indicated on the right.
BenM normally responds to benzoate and cis,cis-muconate to activate transcription of the benABCDE operon, whose gene products convert benzoate to catechol. CatM, in turn, regulates catechol degradation by activating transcription of the cat genes in response to cis,cis-muconate. CatM and BenM are 59% identical in protein sequence. In addition, the operator-promoter regions to which the two regulatory proteins bind have similar DNA sequences (5, 22). The CatM-regulated genes include catA, which encodes a dioxygenase that produces cis,cis-muconate from catechol, and the catBCIJFD genes, which are needed to generate tricarboxylic acid cycle intermediates from cis,cis-muconate (Fig. 1 and 2). The catA gene is regulated by BenM, as well as by CatM. CatM, unlike BenM, does not respond to benzoate as an inducer, nor does CatM normally substitute for BenM in activating ben gene expression (5).
The inability of benM-disrupted mutants to express the benABCDE operon can be overcome by at least two types of mutations upstream of the benA coding sequence. One mutation in the benA promoter results in constitutive ben gene expression (5). A different point mutation in this region enables cis,cis-muconate, but not benzoate, to induce benABCDE expression in the absence of BenM (5). This latter mutation may allow CatM to activate ben gene expression in vivo.
As described here, benM-disrupted mutants able to grow on benzoate that had wild-type benA promoter sequences were chosen for further investigation. Since catM was considered a possible site for mutations that would allow growth on benzoate, this genetic region was characterized in four independently isolated strains. In each case, mutations were identified not in catM but rather in the adjacent catB structural gene. This latter gene encodes muconate cycloisomerase (also known as muconate-lactonizing enzyme), an enzyme that converts cis,cis-muconate to muconolactone (Fig. 1) (15). Since muconate cycloisomerase is required for growth on benzoate, the variant CatB enzymes were predicted to be functional. This prediction was tested by investigating the enzymatic activities of cell extracts and purified proteins. In addition, the effects of the catB mutations on ben gene expression were studied. A model is presented in which muconate cycloisomerase helps to control the internal cellular concentration of cis,cis-muconate, thereby affecting transcriptional regulation by the CatM activator protein.
MATERIALS AND METHODS
Chemicals.
Q-Sepharose, phenyl-Sepharose, and S75 gel filtration media were purchased from Amersham Pharmacia Biotech. cis,cis-Muconate was obtained from Celgene.
Bacterial strains, growth conditions, and DNA manipulations.
All of the Acinetobacter strains used were derived from BD413 (11), also designated ADP1 (Table 1). Escherichia coli DH5α (GIBCO BRL) was used as a plasmid host. Bacteria were cultured in Luria-Bertani (LB) broth or minimal medium at 37°C as previously described (23, 25). Succinate, benzoate, or anthranilate was added as a carbon source to minimal medium at a final concentration of 10, 3, or 2.5 mM, respectively. Antibiotics were added as needed at the following final concentrations: tetracycline, 6 μg/ml; kanamycin, 25 μg/ml; streptomycin, 12.5 μg/ml; spectinomycin, 12.5 μg/ml; ampicillin, 150 μg/ml. Generation times were determined by monitoring the optical density at 600 nm of culture samples during the course of growth. Standard methods were used for chromosomal and plasmid DNA purifications, restriction enzyme digestions, electrophoresis, ligations, and E. coli transformations (23).
TABLE 1.
Acinetobacter strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Acinetobacter strains | ||
| ADP1 | Wild type (BD413) | 11 |
| ISA36 | benM::ΩS4036 | 5 |
| ACN32 | benA::lacZ-Kmr5032 | 5 |
| ACN42 | benM::ΩS4036 benA::lacZ-Kmr5032 Δ(catM-catB)3205 | 5 |
| ACN47 | benM::ΩS4036 benA::lacZ-Kmr5032 | 5 |
| ACN148 | benM::ΩS4036 catB5148(CatB R198L) | This study |
| ACN151 | benM::ΩS4036 catB5151 (CatB R99C) | This study |
| ACN152 | benM::ΩS4036 catB5152 (CatB 210QQL) | This study |
| ACN155 | benM::ΩS4036 catB5155 (CatB P328S) | This study |
| ACN159 | benM::ΩS4036 benA::lacZ-Kmr5032 catB5148 (CatB R198L) | This study |
| ACN162 | benM::ΩS4036 benA::lacZ-Kmr5032 catB5151 (CatB R99C) | This study |
| ACN163 | benM::ΩS4036 benA::lacZ-Kmr5032 catB5152 (CatB 210QQL) | This study |
| ACN166 | benM::ΩS4036 benA::lacZ-Kmr5032 catB5155 (CatB P328S) | This study |
| Plasmids | ||
| pUC19 | Apr; cloning vector | 27 |
| pRK415 | Tcr; broad-host-range cloning vector | 12 |
| pET21b | Apr; T7 expression vector | Novagen |
| pKOK6 | Source of promoterless lacZ-Kmr cassette | 13 |
| pHP45 | Source of ΩS | 21 |
| pIGG16 | Tcr; used to isolate benA region by gap repair methodc | 5 |
| pBAC14 | benM in pRK415 | 5 |
| pBAC54 | benA::lacZ-Kmr in pUC19 Apr | 5 |
| pBAC200 | Tcr; part of ORF1 and -2, part of catJ and catFD (K1 to C1 and C3 to E2; Fig. 2), used to isolate catM-B region by gap repair methodc | This study |
| pBAC213 | benA region of ACN152 obtained with pIGG16 | This study |
| pBAC215 | benA region of ACN148 obtained with pIGG16 | This study |
| pBAC217 | benA region of ACN151 obtained with pIGG16 | This study |
| pBAC220 | benA region of ACN155 obtained with pIGG16 | This study |
| pBAC230 | cat region (C1 to C3; Fig. 2) from ACN148 obtained with pBAC200 | This study |
| pBAC232 | cat region (C1 to C3; Fig. 2) from ACN151 obtained with pBAC200 | This study |
| pBAC233 | cat region (C1 to C3; Fig. 2) from ACN152 obtained with pBAC200 | This study |
| pBAC236 | cat region (C1 to C3; Fig. 2) from ACN155 obtained with pBAC200 | This study |
| pBAC238 | cat region (C1 to C3; Fig. 2) from ISA36 obtained with pBAC200 | This study |
| pBAC253 | 5.3-kbp fragment (E1 to E2; Fig. 2) from pBAC230 in pUC19 | This study |
| pBAC254 | 4.6-kbp fragment (E1 to K2; Fig. 2) from pBAC233 in pUC19 | This study |
| pBAC258 | Fragment C (Fig. 2) from pBAC230 (ACN148) in pUC19 | This study |
| pBAC259 | 5.3-kbp fragment (E1 to E2; Fig. 2) from pBAC232 in pUC19 | This study |
| pBAC260 | Fragment C (Fig. 2) from pBAC233 (ACN152) in pUC19 | This study |
| pBAC267 | 1.5-kbp fragment (Sp1 to Sp2; Fig. 2) from pBAC236 in pUC19 | This study |
| pBAC269 | Fragment A (Fig. 2) from pBAC236 (ACN155) in pUC19 | This study |
| pBAC271 | Fragment B (Fig. 2) from pBAC236 (ACN155) in pUC19 | This study |
| pBAC307 | Fragment C (Fig. 2) from pBAC232 (ACN151) in pUC19 | This study |
| pBAC351 | NdeIb-to-XhoIb wild-type catB fragment in pET21b | This study |
| pBAC352 | NdeIb-to-XhoIbcatB5148 (CatB R198L) fragment in pET21b | This study |
| pBAC353 | NdeIb-to-XhoIbcatB5152 (CatB 210QQL) fragment in pET21b | This study |
| pBAC354 | NdeIb-to-XhoIb catB5151 (CatB R99C) fragment in pET21b | This study |
| pBAC355 | NdeIb-to-XhoIb catB5155 (CatB P328S) fragment in pET21b | This study |
| pBAC439 | Fragment E (Fig. 2) from pBAC230 (ACN148) in pUC19 | This study |
| pBAC440 | Fragment D (Fig. 2) from pBAC230 (ACN148) in pUC19 | This study |
| pBAC441 | Fragment E (Fig. 2) from pBAC232 (ACN151) in pUC19 | This study |
| pBAC442 | Fragment D (Fig. 2) from pBAC232 (ACN151) in pUC19 | This study |
| pBAC443 | Fragment E (Fig. 2) from pBAC233 (ACN152) in pUC19 | This study |
| pBAC444 | Fragment D (Fig. 2) from pBAC233 (ACN152) in pUC19 | This study |
| pBAC445 | Fragment E (Fig. 2) from pBAC236 (ACN155) in pUC19 | This study |
| pBAC446 | Fragment D (Fig. 2) from pBAC236 (ACN155) in pUC19 | This study |
Apr, ampicillin resistant; Kmr, kanamycin resistant; Tcr, tetracycline resistant.
Restriction site added by PCR.
Method of Gregg-Jolly and Ornston (7).
Isolation of plasmids with the benA and catB regions of mutant strains and DNA sequencing.
The gap repair method of Gregg-Jolly and Ornston (7) was used as previously described to isolate the benA region from the chromosome of a mutant and transfer it to a plasmid (5). Acinetobacter mutants were transformed with pIGG16 (Table 1), which was linearized at the sole KpnI site and was unable to replicate in Acinetobacter. Flanking this KpnI site, the plasmid contains DNA identical in sequence to the adjacent upstream and downstream regions of the chromosomal segment to be isolated. Homologous recombination in vivo generates a circular plasmid carrying the chromosomal region of interest and a drug resistance marker, which allows selection, from the original vector.
The cat region mutations were similarly isolated by the gap repair method with ClaI-linearized pBAC200, which contains DNA upstream and downstream of the catM-catB region. Plasmid pBAC200 was constructed by ligating an 830-bp KpnI-to-ClaI fragment containing part of open reading frames 1 (ORF1) and ORF2 (K1 to C1 in Fig. 2) and a 2.3-kbp ClaI-to-EcoRI fragment (C3 to E2 in Fig. 2) containing part of catJ and catFD into the KpnI- and EcoRI-digested pRK415 vector (12). Recipient strains were transformed with pBAC200, linearized at the sole ClaI site, and homologous recombination in vivo generated plasmids which carried the chromosomal region of the mutants extending from ORF2 (C1 in Fig. 2) to catJ (C3 in Fig. 2). As described later, subclones were constructed with the pUC19 vector (27).
The catB genes were sequenced from plasmid templates using forward and reverse pUC/M13 sequencing primers (Promega) that recognize pUC19 sequences and using two oligonucleotide primers that hybridize to catB sequences, 5′-GTCGATCATGTAATTGCC-3′ and 5′-ATGCACGGTTCACATCTA-3′, purchased from Genosys Biotechnologies. DNA sequencing was carried out with an automated sequencer (ABI373A; Applied Biosystems Inc.), at the University of Georgia Molecular Genetics Instrumentation Facility.
Construction and selection of Acinetobacter mutants.
Spontaneous mutants of ISA36 were isolated after 2 to 3 days of incubation on solid minimal medium with benzoate as the sole carbon source as previously described (5). Strains with chromosomal benA::lacZ fusions were generated using plasmid pBAC54 (5), which contains a promoterless lacZ cassette under the transcriptional control of benA. As previously described (5), pBAC54 was linearized by digestion with the restriction endonuclease Asp718 and used to transform recipient strains. Since the lacZ cassette is followed by a marker that confers resistance to kanamycin (13), transformants were selected with this antibiotic. Sensitivity of the transformants to ampicillin indicated that the vector portion of pBAC54 was not retained in the Acinetobacter strains and that the benA::lacZ-Kmr5032 allele (Table 1; Fig. 2) had been chromosomally incorporated by allelic replacement.
Transformation assay to localize mutations.
The natural transformability of the Acinetobacter strains was exploited to test whether DNA fragments containing mutations could confer the ability to grow on benzoate to strain ISA36, as described in previous studies (19). A 5-ml culture of recipient strain ISA36, which had been grown overnight with succinate as the sole carbon source with appropriate antibiotics, was diluted (1:25) into 5 ml of the same medium. The diluted culture was incubated with aeration for approximately 3 h, and 100 μl was then spread onto solid medium containing benzoate as the sole carbon source with no antibiotics. Approximately 0.1 to 5 μg of donor plasmid DNA in a volume of 1 to 10 μl was added in small spots to the plate containing the recipient cells. Plasmids containing wild-type benM (pBAC14; Table 1) or the wild-type catM-catB region (pBAC238; Table 1) were used as positive and negative controls, respectively. Plates were incubated for up to several days until colonies corresponding to the positive control were visible. This assay allows DNA with a mutation to replace the corresponding region of the chromosome by homologous recombination. The pUC19-based plasmids are not maintained in Acinetobacter bacteria.
β-Galactosidase assays.
Cultures were grown overnight in 5 ml of LB with or without 3 mM benzoate or 3 mM cis,cis-muconate. Alternatively, cultures were grown in minimal medium with anthranilate as the sole carbon source. Cells were lysed with sodium dodecyl sulfate and chloroform, and β-galactosidase activities were determined as described by Miller (17).
Preparation of Acinetobacter cell extracts and measurement of catechol 1,2-dioxygenase (CatA) and muconate cycloisomerase (CatB) activities.
Acinetobacter cultures were grown with benzoate as the sole carbon source. Cells were harvested and disrupted by sonication, and the cell extract was removed as previously described (25). Catechol 1,2-dioxygenase and muconate cycloisomerase activities were assayed spectrophotometrically by the increase or decrease in cis,cis-muconate concentration, respectively, as indicated by A260 (15, 20). Protein concentrations were determined by the method of Bradford (2) with bovine serum albumin as the standard.
Metabolite monitoring by high-performance liquid chromatography.
Samples from cultures growing on benzoate were taken at timed intervals, and whole cells were removed from the medium as previously described (5). A 10-μl aliquot from each sample of cell-free culture medium was separated on a C18 reverse-phase column from Bio-Rad Laboratories. The metabolites benzoate, catechol, and cis,cis-muconate were identified and quantified as previously described (5).
Expression of Acinetobacter catB genes in E. coli and purification of wild-type and variant muconate cyloisomerases.
Plasmids for the expression of Acinetobacter catB genes from the T7 promoter of vector pET21b (Novagen) were constructed with a PCR-based method. The forward oligonucleotide primer, 5′-GCGAATTCCATATGTATAAATCAG-3′, annealed to catB sequences beginning with the translational start codon (in italics), and added an NdeI recognition site (in bold). The reverse primer, 5′-ATACTCGAGTTAATGACGGCGTAA-3′, annealed to sequences in the last 15 nucleotides of catB and added an XhoI recognition site (in bold) after the TAA ochre codon (opposite strand, in italics). In individual reactions, these primers amplified DNAs from pBAC238 (wild-type catB), pBAC253 (catB5148), pBAC259 (catB5151), pBAC254 (catB5152), and pBAC267 (catB5155). The resulting PCR fragments were digested with NdeI and XhoI and ligated to similarly digested pET21b, generating pBAC351, pBAC352, pBAC354, pBAC353, and pBAC355, respectively (Table 1). The Acinetobacter sequences of these plasmids were confirmed with the T7 promoter and T7 terminator primers (from Novagen) in sequencing reactions.
LB-grown cultures of E. coli host strain BL21(DE3) (Stratagene) carrying each of the catB expression plasmids pBAC351 to -355 were induced with isopropyl-β-d-thiogalactopyranoside (100 μg/ml). After induction, cultures were maintained at 37°C for 3 to 5 h and harvested by centrifugation (6,000 × g). Crude extracts (50 ml per 4-liter culture) were prepared by sonication of resuspended cell pellets. The crude extract was filtered and applied to Q-Sepharose and phenyl-Sepharose columns as described previously (26). An additional step was added to obtain pure enzyme. Fractions eluting from the phenyl-Sepharose column were applied to a Superdex HiLoad 26/60 gel filtration column (Pharmacia) in 50 mM Tris (pH 8.0) buffer, and fractions with activity were pooled. In some cases, it was necessary to purify these fractions further by separation on a Mono Q column with a linear gradient of 0 to 400 mM NaCl in 50 mM Tris (pH 8.0) buffer. Enzyme purity was estimated by sodium dodecyl sulfate-polyacrylamide gels stained with Coomassie brilliant blue R-250 (Bio-Rad). Analyses of muconate cycloisomerase activities were carried out as described previously (26), using a range of 10 to 100 μM cis,cis-muconate in a solution containing 1 mM MnCl2, 0.1 mM cis,cis-muconate, and 33 mM Tris-HCl (pH 8.0) in a total volume of 1 ml. Three-dimensional pictures of the active site were made using Molscript (14) and Raster 3D (16).
RESULTS
Isolation of benM mutants that can grow on benzoate.
Extended incubation using benzoate as the sole carbon source selected spontaneous mutants of ISA36, a strain that has its benM gene disrupted by a cassette conferring resistance to streptomycin and spectinomycin (ΩS; Table 1; Fig. 2) (5). Four such mutants were independently isolated and designated ACN148, ACN151, ACN152, and ACN155 (Table 1). Each strain retained the drug resistance of the chromosomal benM disruption. To assess whether there were benA operator-promoter mutations, the benA chromosomal region of each mutant was isolated to yield plasmids pBAC215, pBAC217, pBAC213, and pBAC220 (Table 1). Each plasmid was tested for the ability to transform ISA36 to grow on benzoate as described in Materials and Methods. This procedure does not rely on complementation with the DNA in trans but rather provides an opportunity for the transforming DNA to replace the homologous chromosomal region. Although this approach was previously successful in identifying three benA region mutations (5), in no case tested here did the benA region of a mutant confer on ISA36 the ability to grow on benzoate. The mutations responsible for this phenotype, therefore, were likely to be in different chromosomal regions.
The catM region was considered a possible site for mutations that could enable growth on benzoate in the absence of BenM. To isolate this chromosomal region from each of the mutants, plasmid pBAC200 was constructed and used in the gap repair method (7). The 5.3-kbp chromosomal regions between ClaI sites in ORF2 and catJ (C1 to C3; Fig. 2) were isolated from ACN148, ACN151, ACN152, and ACN155 to yield plasmids pBAC230, pBAC232, pBAC233, and pBAC236, respectively (Table 1). Using the transformation assay described above, each of these plasmids was able to confer on ISA36 the ability to grow on benzoate. We next attempted to localize possible mutations that were in the cat gene regions of the plasmids.
Identification of mutations in catB that were responsible for growth on benzoate.
Fragments of DNA from the mutants were ligated to vectors, and the resulting plasmids were tested for the ability to transform the benM mutant ISA36 to grow on benzoate (Fig. 2). In each case, the DNA region conferring this ability could be localized to a 1.3-kbp region between an EcoRI site in catM and a SalI site in catB. The entire Acinetobacter DNA insert was sequenced for the smallest plasmid from each mutant that was capable of transforming ISA36 into a strain able to grow on benzoate (pBAC258, pBAC307, pBAC260, and pBAC271; Table 1).
Each plasmid contained a mutation in the catB structural gene. The mutant allele from ACN148, designated catB5148, had a point mutation that changed codon 198 from CGT to CTT, causing a change from arginine to leucine (R198L) in the gene product. A point mutation was also found in the allele from ACN151, designated catB5151, changing wild-type CGC codon 99 to TGC. The protein encoded by this allele would have a cysteine rather than an arginine at the 99th amino acid residue (R99C). Strain ACN152 had a 9-bp insertion (TTCAACAGC) at position 626 in the nucleotide sequence of the catB gene that appeared to be a duplication of the neighboring sequences. The catB5152 allele encodes two extra glutamine residues and one leucine residue (210QQL) between isoleucine and glutamine residues that correspond to residues 209 and 210 in wild-type CatB. The final mutant, ACN155, had a point mutation changing the wild-type 328 codon CCT of catB to TCT, encoding a change from proline to serine (P328S) in the protein. None of the mutations caused premature termination or a change in the reading frame of the catB gene, consistent with the prediction that each of the mutants selected for growth on benzoate needs a functional muconate cycloisomerase.
Expression of a benA::lacZ transcriptional fusion in strains carrying the catB mutations.
The ability of mutations in catB to enable growth on benzoate without BenM suggested that these mutations increased expression of the benABCDE genes. To assess ben gene expression, a benA::lacZ fusion was introduced into the chromosome of ACN148, ACN151, ACN152, and ACN155 to generate strains ACN159, ACN162, ACN163, and ACN166, respectively (Table 1). In each case, the fusion replaced wild-type benA, thereby preventing benzoate degradation, as shown previously (5). Since benzoate could not be used as a sole carbon source, strains were grown in rich medium (LB) to which either benzoate or cis,cis-muconate was added as a possible inducer. Expression of lacZ under the control of the benA promoter was assessed as β-galactosidase (LacZ) activity and reported as a percentage of that of cis,cis-muconate-induced strain ACN32, which has wild-type benM (Fig. 3A).
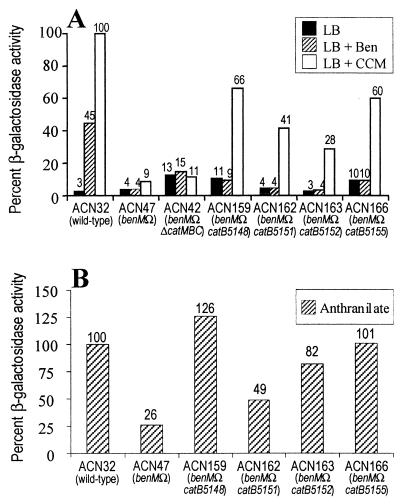
FIG. 3.
β-Galactosidase (LacZ) activity resulting from expression of a chromosomal benA::lacZ fusion expressed as a percentage of that for induced strain ACN32. Values are shown above the bars. (A) Cells were grown in rich medium (LB) with or without benzoate (Ben) or cis,cis-muconate (CCM) as an inducer. A 100% activity level corresponded to 5,459 Miller units. (8) Cells were grown in mimimal medium with anthranilate as the sole carbon source. A 100% activity level corresponded to 1,001 Miller units. The activities shown are averages of at least three experiments, and the corresponding standard deviations were <20% of the average values.
The ben gene expression in each of the catB mutants was inducible by cis,cis-muconate but not by benzoate (Fig. 3A). The addition of cis,cis-muconate, relative to the corresponding uninduced culture, increased β-galactosidase levels between 6- and 10-fold. In the aforementioned strain ACN32, β-galactosidase was inducible by either benzoate or cis,cis-muconate, as previously demonstrated (5). The pattern of inducibility in the catB mutants suggested a possible role for CatM in regulating ben gene expression. Consistent with this possibility, β-galactosidase levels did not increase in response to cis,cis-muconate or benzoate in strain ACN42, which lacks both CatM and BenM (5).
Strain ACN47, in which benM is disrupted, differs from ACN159, ACN162, ACN163, and ACN166 by having a wild-type catB allele. In ACN47, cis,cis-muconate caused an approximately twofold induction of benA expression that resulted in low levels of β-galactosidase activity (Fig. 3A). This may indicate that relatively low internal concentrations of cis,cis-muconate are sufficient for BenM but not CatM to induce high levels of benA expression (5). The β-galactosidase levels in ACN159, ACN162, ACN163, and ACN166 were significantly higher than in ACN47 but still lower than in a strain with wild-type benM (ACN32).
Expression of the benA::lacZ fusion was also tested under conditions that allowed cis,cis-muconate to be generated internally during metabolism rather than from exogenous addition to the cultures. Strains were grown on anthranilate as the sole carbon source, and β-galactosidase levels were measured (Fig. 3B). Anthranilate, like benzoate, is converted to catechol and further degraded by the cat-encoded enzymes with cis,cis-muconate produced transiently (Fig. 1) (3). When grown on anthranilate, the catB mutants had β-galactosidase levels that were more similar to those of ACN32 than were those of cells grown in the presence of exogenous cis,cis-muconate (Fig. 3B compared to Fig. 3A). When cis,cis-muconate was generated during anthranilate catabolism, strains ACN159 and ACN166 had β-galactosidase activities that were comparable to those of ACN32. Moreover, in strains lacking BenM, the catB mutations resulted in β-galactosidase levels that were approximately two- to fivefold higher than those of ACN47, which has wild-type catB. During growth on anthranilate, mutations in catB might cause cis,cis-muconate to be degraded more slowly than in strains having wild-type muconate cycloisomerase, and this in turn might alter CatM-dependent cis,cis-muconate induction of gene expression. To test the predictions of this hypothesis, the catB mutants were characterized further.
Enzyme activities, growth rates, and cis,cis-muconate accumulation in catB mutants.
The effects of the catB mutations were assessed by measuring the specific activity of muconate cycloisomerase (CatB) in cell extracts of benzoate-grown cultures (Table 2). As predicted, all of the strains had detectable muconate cycloisomerase activity. The catB mutations appeared to reduce muconate cycloisomerase activity in vivo in the mutants without preventing growth on substrates dissimilated via the catechol branch of the β-ketoadipate pathway (Fig. 1).
TABLE 2.
Induction of CatA and CatB during growth on benzoate
| Strain | Relevant characteristic(s) | Avg sp act (nmol/min/mg of protein)a ± SD
|
CatA/CatB ratio sp act | Generation time (min)b for growth on benzoate | |
|---|---|---|---|---|---|
| CatB | CatA | ||||
| ADP1 | Wild type | 192 ± 93 | 353 ± 129 | 1.8 | 72 |
| ACN148 | benM::ΩS4036 catB5148 (CatB R198L) | 69 ± 26 | 607 ± 98 | 8.8 | 119 |
| ACN151 | benM::ΩS4036 catB5151 (CatB R99C) | 179 ± 39 | 710 ± 173 | 4.0 | 99 |
| ACN152 | benM::ΩS4036 catB5152 (CatB 210 QQL) | 56 ± 31 | 584 ± 179 | 10.4 | 115 |
| ACN155 | benM::ΩS4036 catB5155 (CatB P328S) | 70 ± 25 | 480 ± 11 | 6.9 | 77 |
The values shown for CatA (catechol 1,2-dioxygenase) and CatB (muconate cycloisomerase) are averages of 4 to 12 experiments.
The values shown are averages of at least three experiments. Standard deviations were no more than 15% of the values shown.
Most of the catB mutants, which were selected for growth on benzoate, grew somewhat more slowly on this carbon source than the wild type (Table 2). In contrast, when anthranilate was used as the sole carbon source, none of the mutants grew more slowly than the wild type (data not shown). Since anthranilate and benzoate are each degraded to catechol (Fig. 1), the growth rate on benzoate may be limited by the rate of benzoate conversion to catechol rather than by the defect in the catB-encoded enzyme. Thus, the most significant effect of the catB mutations on benzoate degradation in the absence of BenM appears to be the increase in expression of the benABCDE operon in response to cis,cis-muconate.
The catB mutations may alter the cellular levels of cis,cis-muconate that are available to activate CatM. To assess whether the expression of catA, which is also regulated by CatM, was affected in the catB mutants, the specific activities of catechol 1,2-dioxygenase (CatA) were determined in cell extracts of benzoate-grown cultures (Table 2). In all of the catB mutants, catechol 1,2-dioxygenase levels were higher than those in the wild-type strain. The relatively high standard deviations make it difficult to conclude the significance of the increase in CatA activity in ACN152 and ACN155. Nevertheless, both of these strains had significantly reduced CatB activities, which indicates that the CatA/CatB activity ratios in both strains were higher than in ADP1. Similarly, the significance in the reduced specific activity of CatB in ACN151 relative to ADP1 was not clear. However, the use of a saturating concentration of substrate in the assay may be higher than the substrate concentration in vivo. This could mask the catalytic effects that occur during growth on benzoate due to an increased Km for cis,cis-muconate of the variant muconate cycloisomerase (see below). The two-fold increase in the specific activity of CatA in ACN151 relative to ADP1 supports the hypothesis that during growth on benzoate, the catB mutation in ACN151 causes lowered muconate cycloisomerase activity and increased internal concentrations of cis,cis-muconate.
In each case, the results were consistent with the ability of catB mutations to increase CatM-activated gene expression. Higher levels of catA expression could also increase the rate of cis,cis-muconate formation, since the catA-encoded enzyme cleaves catechol to form this compound. The higher-than-normal ratio of CatA/CatB activity in the mutants (Table 2) may cause an increase in the steady-state intracellular levels of cis,cis-muconate during growth on benzoate. Samples of culture medium isolated during growth on benzoate were tested by high-performance liquid chromatography for the presence of metabolites. In no case was a higher-than-normal level of cis,cis-muconate detected in the medium external to the cells (data not shown).
Catalytic properties of purified wild-type and variant muconate cycloisomerases.
To understand how the catB mutations affected muconate cycloisomerase activity, the mutant catB alleles were expressed in E. coli and the variant enzymes were purified. The wild-type enzyme and three of the variants were purified to homogeneity. Despite numerous efforts, it was not possible to purify the protein encoded by the catB5152 allele, which is predicted to encode a protein with three additional amino acids (210 QQL). Invariably, activity was lost during the chromatographic procedures.
Spectrophotometric assays of enzyme activity and standard Lineweaver-Burk analyses were used to determine kcat and Km values for the purified proteins (Table 3). These values had previously been reported for muconate cycloisomerase from Acinetobacter sp. strain ADP1 (Km, 130 μM; kcat, 3,700 min−1) (26) and Pseudomonas putida (Km, 40 μM; kcat, 12,600 min−1) (26). In our studies, the kcat values for two of the variants of muconate cycloisomerase (R99C and P328S) were similar to that measured here for the ADP1 wild-type enzyme (kcat, 3,810 min−1; Table 3). The enzyme encoded by the catB5148 allele (R198L muconate cycloisomerase) had an almost twofold increase in kcat. Tempering the increase in kcat for R198L muconate cycloisomerase was a fourfold increase in Km, which yielded a two-fold overall decrease in catalytic efficiency (kcat/ Km). Likewise, R99L muconate cycloisomerase also had a significantly higher Km than the wild-type enzyme. The third variant investigated, P328S muconate cycloisomerase, did not have Km and kcat values that differed significantly from those of the wild type despite the lower-than-normal specific activity for this enzyme in cell extracts of the mutant carrying the corresponding allele, catB5155 (Table 2).
TABLE 3.
Kinetic parameters for variants of muconate cycloisomerase
| Enzyme | Km (μM)a | kcat (min−1)a | kcat/Km (min−1 μM−1) |
|---|---|---|---|
| Wild type | 53 | 3,810 | 72 |
| R198L | 216 | 7,300 | 34 |
| R99C | 145 | 3,120 | 21 |
| P328S | 49 | 3,700 | 76 |
Deviations in Km and kcat were estimated to be less than 10% of the reported values.
DISCUSSION
Activation of ben gene expression by mutations in catB
Strains lacking BenM do not express the benABCDE genes at levels that support growth on benzoate (5). As reported here, four DNA fragments with different catB mutations each transformed a strain without BenM to grow on benzoate. The transformation assays indicated that mutations in the catB gene were sufficient for the restoration of growth. The ability of the catB mutations to alter ben gene expression was confirmed using a benA::lacZ transcriptional fusion (Fig. 3). The BenM-independent activation of benA expression by exogenous cis,cis-muconate was significantly increased in strains carrying the selected catB mutations. In these mutants, cis,cis-muconate induced the expression of benA to levels 28 to 66% of that of an identically grown strain with wild-type benM (ACN32; Fig. 3A). Nevertheless, full induction of the wild type requires benzoate (5). In the catB mutants, unlike the wild type, benzoate did not increase benA expression when added alone (Fig. 3A) or together with cis,cis-muconate (L. S. Collier, unpublished data). Therefore, the BenM-independent ben gene expression might be mediated by a transcriptional regulator that responds to cis,cis-muconate but not benzoate.
Several lines of evidence suggested that CatM was responsible for activating ben gene expression in the catB mutants. Not only is CatM known to respond to cis,cis-muconate and not benzoate, but DNA sequences upstream of benA are very similar to those involved in CatM regulation (5, 22). There is 56% sequence identity in an alignment of two 73-nucleotide regions, one normally used for BenM-mediated expression of benA and the other upstream of catB that is regulated by CatM. DNase I footprinting experiments (4) and transcription assays with purified proteins (B. M. Bundy, unpublished data) demonstrated that CatM can activate benA expression in vitro in response to cis,cis-muconate. In vivo, catM appears to control whether cis,cis-muconate will induce benA expression in a strain lacking BenM. In the presence of catM, but not in its absence, expression is inducible, albeit at low levels (strain ACN47 versus strain ACN42; Fig. 3). Finally, consistent with the possibility that the catB mutations increase CatM-regulated gene expression, strains with these mutations had higher-than-normal levels of catechol 1,2-dioxygenase, an enzyme encoded by the CatM-regulated catA gene (Table 2).
Importance of intracellular cis, cis-muconate concentrations for regulation.
The altered levels of catechol 1,2-dioxygenase (CatA) raised the possibility that the effect on benA expression depends on the combination of higher CatA and lower CatB activity in the catB mutants. This combination of altered enzyme levels could increase internal cis,cis-muconate accumulation and increase CatM-activated gene expression during growth on benzoate. However, problems with correlating the level of BenM-independent benA expression with the intracellular concentration of cis,cis-muconate lie in the difficulty of measuring this concentration accurately. Although no differences were detected in the amounts of cis,cis-muconate excreted by different strains into the culture medium during growth on benzoate, the results were inconclusive concerning internal metabolite accumulations. Whether cis,cis-muconate diffuses freely out of the cell or whether export is regulated remains unknown. Moreover, the high-performance liquid chromatography procedures used for metabolite monitoring may not detect small yet physiologically significant concentration differences.
During growth on anthranilate, compared to growth on LB with added cis,cis-muconate, BenM-independent expression of benA increased relative to expression in a strain containing BenM (ACN32). When provided with exogenous cis,cis-muconate, the catB mutants do not need CatA to produce this metabolite. In contrast, during growth on anthranilate, internal cis,cis-muconate accumulation should depend on the rate of its formation from catechol, catalyzed by CatA, and the rate of its conversion to muconolactone, catalyzed by muconate cycloisomerase (CatB). During growth on anthranilate, the catB mutants induced expression of a benA::lacZ fusion to levels that were 49 to 126% of that of strain ACN32, which carries wild-type benM (Fig. 3B). Therefore, expression of benA in the catB mutants relative to that in ACN32 was higher during growth on anthranilate than with cis,cis-muconate provided exogenously. It is possible that the former growth conditions yield higher internal levels of cis,cis-muconate than the latter and that these levels are elevated by the high CatA/CatB ratios in the mutants. Exogenous cis, cis-muconate may be taken into the cell concomitant with its degradation such that high internal levels of this compound do not accumulate (6).
With anthranilate as the carbon source, the absence of benM should not affect the initial conversion of anthranilate to catechol, from which cis,cis-muconate can be readily generated by induction of CatA. However, during growth on benzoate, cis,cis-muconate formation requires the initial expression of the benABCDE operon without the presence of cis,cis,-muconate. In wild-type cells, benzoate can interact with BenM to activate the initial expression of the ben genes. Previous studies of metabolite flow using nuclear magnetic resonance techniques have demonstrated that in strains lacking BenM, the background level of ben and cat gene expression is sufficient for the slow metabolism of benzoate, even if this degradation does not support growth (5). Investigations of strains with the benA::lacZ fusion also indicate that under noninducing growth conditions benA can be expressed at low levels without BenM (Fig. 3). In the benM mutants, this background level of ben gene expression could account for the initial formation of cis,cis-muconate that allows activated ben gene expression in response to this metabolite.
The level of BenM-independent ben gene expression normally induced by cis,cis-muconate appears to be below a threshold needed to support growth on benzoate. However, it is difficult to estimate the value of this threshold. The cis,cis-muconate-induced benA::lacZ expression in the benM+ ACN32 strain was approximately 30% of that of the same strain induced with both cis,cis-muconate and benzoate (not shown in Fig. 3) (5). In the catB mutants, benA::lacZ expression never greatly exceeded that of comparably grown ACN32 without benzoate (i.e., conditions of submaximal benA expression). Therefore, the benA expression levels in the catB mutants were well below that of the fully induced wild-type strain. This suggests that the threshold level of ben gene expression for growth is no more than several fold higher than the normal levels that can be attained in the absence of BenM.
The important regulatory role played by metabolite concentrations during the catabolism of benzoate is suggested by the frequency with which the catB mutations were selected. To date, 10 independently isolated strains have been characterized that allow growth on benzoate in the absence of BenM (4). Whereas two of the strains growing on benzoate had promoter mutations upstream of benA that increased constitutive levels of ben gene expression (5), the four strains described here had catB mutations that allowed ben gene expression to be regulated. Although the remaining mutations are still under investigation, they too allowed regulated ben gene expression (4). It may be that some mutations that increase constitutive ben gene expression in an unregulated fashion produce lethal levels of cis,cis-muconate, since high levels of this compound generated from benzoate catabolism are known to be toxic to bacterial cells (6). In the wild-type strain, a complex regulatory circuit has evolved to balance the expression of multiple operons involved in benzoate degradation. Increased concentrations of cis,cis-muconate would normally increase the CatM-regulated induction of muconate cycloisomerase. Strains lacking catB that cannot relieve the buildup of this toxic metabolite will not grow in the presence of benzoate, even if other carbon sources are provided (3). It therefore seems reasonable that lowered, as well as elevated, levels of muconate cycloisomerase activity should be able to play a regulatory role in catabolism.
Comparisons of variant and wild-type muconate cycloisomerases.
Since the wild-type muconate cycloisomerase of ADP1 is 52% identical and 69% similar in sequence to that of P. putida PRS2000, it seemed likely that these enzymes would have analogous structures (1). Crystallography of the P. putida enzyme reveals an active site that contains an octahedrally coordinated Mn atom at the C-terminal end of the barrel β-strands in the αβ-barrel domain (Fig. 4A) (9, 10, 24). The Mn atom is essential for activity and is coordinated by three carboxylate ligands from protein and three solvent water molecules (Fig. 4B) (10). Docking studies suggest that the substrate does not coordinate the metal but rather that the metal ion serves as an electrophile through one of the water molecules. The substrate appears to interact with Glu327 and Lys169, with steric constraints placed by Phe329 and Ile54.
FIG. 4.
Monomeric structure of muconate cycloisomerase from P. putida (10; PDB code, 1 muc) (A) and expanded view of the active site (B). Helices in red mark the region from panel A that is enlarged in panel B. Amino acid changes in the variant ADP1 muconate cycloisomerases described in this report are indicated parenthetically below residues that correspond in a pairwise alignment of the homologous Acinetobacter and P. putida sequences. Superscript numbers associated with P. putida residues were derived from the crystallographic coordinates. Asp198, Glu224, and Asp249 are conserved in both enzymes and are ligands to the Mn atom, as are three water molecules (not shown). Lys169, Arg196, Glu327, and Phe329 are also conserved between the enzymes and are important for positioning of the substrate and its activation for catalysis.
The proximity of Gln201 (and, by analogy, Arg198 in the Acinetobacter enzyme) to the catalytic Mn atom (Fig. 4B) raises the possibility that the R198L muconate cycloisomerase has altered metal-binding properties that affect the overall efficiency of the enzyme. In contrast, the position of the P328S substitution, which corresponds to Pro331 in the P. putida enzyme (Fig. 4B), suggests an effect on substrate binding rather than metal binding. The P328S variant had Km and kcat values similar to those of the wild-type enzyme, whereas the strain encoding the variant had a reduced specific activity of muconate cycloisomerase, relative to that of the wild type in cell extracts of benzoate-grown cultures. Therefore, the primary effect of the mutation in vivo may be on protein stability.
Protein stability may also be a factor in the inability to purify 210QQL muconate cycloisomerase. The three amino acid insertion predicted to occur in the alpha helix (G) that links β-strands 6 and 7, may destabilize the enzyme in vivo and in vitro. Strand 6 contains Asp198, and strand 7 contains Glu224, both of which are ligands to the central Mn atom (Fig. 4B) (10). The implications of the substitution in the fourth variant of these investigations, the R99C muconate cycloisomerase, are not readily apparent. The position of this substitution corresponds to Thr102 of the P. putida enzyme (10). Thr102 is located in the helix C of the N-terminal domain, which is distant from the active-site Mn and substrate-binding pocket (Fig. 4A).
Taken together, these results suggest that an increase in the intracellular cis,cis-muconate concentration can activate BenM-independent benA expression. The disruption of benM appears to create a selective pressure for decreased muconate cycloisomerase activity. In this way, it was possible to isolate functional enzymes with activities only slightly altered relative to those of the wild-type enzyme. Such a selection is usually difficult to design intentionally. Here, the selection for growth on benzoate provided an opportunity to investigate structure-function relationships of muconate cycloisomerase, an interesting and well-characterized enzyme.
ACKNOWLEDGMENTS
This research was supported by National Science Foundation grant MCB-9808784 (to E.L.N.) and research training grant BIR9413235 (support for N.J.C).
We thank Matthew Wisdom and Jennifer Knight for investigating the identity of the catB mutations and Timothy Hoover for useful suggestions.
REFERENCES
- 1.Aldrich T L, Frantz B, Gill B, Kilbane J F, Chakrabarty A M. Cloning and complete nucleotide sequence determination of the catB gene encoding cis,cis-muconate lactonizing enzyme. Gene. 1987;52:185–195. doi: 10.1016/0378-1119(87)90045-x. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Bundy B M, Campbell A L, Neidle E L. Similarities between the antABC-encoded anthranilate dioxygenase and the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADP1. J Bacteriol. 1998;180:4466–4474. doi: 10.1128/jb.180.17.4466-4474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier L S. Ph.D. thesis. University of Georgia, Athens. Transcriptional regulation of benzoate degradation by BenM and CatM in Acinetobacter sp. strain ADP1. 2000. [Google Scholar]
- 5.Collier L S, Gaines III G L, Neidle E L. Regulation of benzoate degradation in Acinetobacter sp. strain ADP1 by BenM, a LysR-type transcriptional activator. J Bacteriol. 1998;180:2493–2501. doi: 10.1128/jb.180.9.2493-2501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaines G L, III, Smith L, Neidle E L. Novel nuclear magnetic resonance spectroscopy methods demonstrate preferential carbon source utilization by Acinetobacter calcoaceticus. J Bacteriol. 1996;178:6833–6841. doi: 10.1128/jb.178.23.6833-6841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregg-Jolly L A, Ornston L N. Recovery of DNA from the Acinetobacter calcoaceticus chromosome by gap repair. J Bacteriol. 1990;172:6169–6172. doi: 10.1128/jb.172.10.6169-6172.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harwood C S, Parales R E. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 9.Hasson M S, Schlichting I, Moulai J, Taylor K, Barrett W, Kenyon G L, Babbit P C, Gerlt J A, Petsko G A, Ringe D. Evolution of an active site: the structure of a new crystal form of muconate lactonizing enzyme compared with mandelate racemase and enolase. Proc Natl Acad Sci USA. 1998;95:10396–10401. doi: 10.1073/pnas.95.18.10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helin S, Kahn P C, Guha B L, Mallows D G, Goldman A. The refined X-ray structure of muconate lactonizing enzyme from Pseudomonas putida PRS2000 at 1.85 Å resolution. J Mol Biol. 1995;254:918–941. doi: 10.1006/jmbi.1995.0666. [DOI] [PubMed] [Google Scholar]
- 11.Juni E, Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keen T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 13.Kokotek W, Lotz W. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene. 1989;84:467–471. doi: 10.1016/0378-1119(89)90522-2. [DOI] [PubMed] [Google Scholar]
- 14.Kraulis P J. Molscript—a program to produce both detailed and schematic plots of protein structures. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- 15.Meagher R B, Ngai K-L, Ornston L N. Muconate cycloisomerase. Methods Enzymol. 1990;188:126–130. doi: 10.1016/0076-6879(90)88023-4. [DOI] [PubMed] [Google Scholar]
- 16.Merritt E A, Bacon D J. Raster 3D: photorealistic molecular graphics. Macromol Crystallogr. 1997;277:505–524. doi: 10.1016/s0076-6879(97)77028-9. [DOI] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 18.Neidle E L, Hartnett C, Ornston L N. Characterization of Acinetobacter calcoaceticus catM, a repressor gene homologous in sequence to transcriptional activator genes. J Bacteriol. 1989;171:5410–5421. doi: 10.1128/jb.171.10.5410-5421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neidle E L, Ornston L N. Cloning and expression of the Acinetobacter calcoaceticus catechol 1,2-dioxygenase structural gene catA in Escherichia coli. J Bacteriol. 1986;165:557–563. doi: 10.1128/jb.168.2.815-820.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngai K-L, Neidle E L, Ornston L N. Catechol and chlorocatechol 1,2-dioxygenases. Methods Enzymol. 1990;188:122–126. doi: 10.1016/0076-6879(90)88022-3. [DOI] [PubMed] [Google Scholar]
- 21.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Arroyo C E, Schell M A, Gaines III G L, Neidle E L. catM encodes a LysR-type transcriptional activator regulating catechol degradation in Acinetobacter calcoaceticus. J Bacteriol. 1995;177:5891–5898. doi: 10.1128/jb.177.20.5891-5898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Schell U, Helin S, Kajander T, Schlömann M, Goldman A. Structural basis for the activity of two muconate cycloisomerase variants towards substituted muconates. Proteins. 1999;34:125–136. [PubMed] [Google Scholar]
- 25.Shanley M S, Neidle E L, Parales R E, Ornston L N. Cloning and expression of Acinetobacter calcoaceticus catBCDE genes in Pseudomonas putida and Escherichia coli. J Bacteriol. 1986;165:557–563. doi: 10.1128/jb.165.2.557-563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vollmer M D, Hoier H, Hecht H-J, Schell U, Gröning J, Goldman A, Schlömann M. Substrate specificity of and product formation by muconate cycloisomerases: an analysis of wild-type enzymes and engineered variants. Appl Envrion Microbiol. 1998;64:3290–3299. doi: 10.1128/aem.64.9.3290-3299.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]