Abstract
Purpose
To assess the longitudinal changes in crystalline lens in persistent non-myopic and myopic children.
Methods
Four cohorts of children were recruited from Guangzhou, China, from first year of kindergarten (G0, n = 1129), first year of primary school (G1, n = 1324), fourth year of primary school (G4, n = 1854), and first year of junior high school (G7, n = 867) in 2018 and followed up annually for 2 years. All children received cycloplegic autorefraction and ocular biometry measurement. Children were classified into categories of persistent non-myopia (PNM; spherical equivalent refraction [SER] ≥−0.5 diopter [D] at baseline and during follow-up), persistent myopia (PM; SER <−0.5 D at baseline and during follow-up), or newly developed myopia (NDM: SER ≥−0.5 D at baseline and <−0.5 D during follow-up).
Results
The mean (SD) age was 3.69 (0.34) years for children in G0, 6.79 (0.35) years in G1, 9.52 (0.42) years in G4, and 12.56 (0.38) years in G7. A LOWESS plot showed a three-stage pattern of change in lens thickness (LT) in PNM children including a rapid decrease from 3 to 7 years of age and a slower decrease from 7 to 11 years, followed by an increase thereafter. Similar trends were observed in the PM and NDM groups, although there was less change in LT. In contrast, lens power (LP) decreased consistently in all cohorts during the follow-up. No significant changes in LT or LP were observed around myopia onset.
Conclusions
The lens showed a three-stage pattern of change in LT, whereas there was continuous loss of LP in children ages 3 to 15 years.
Keywords: lens thickness, lens power, myopia, children, longitudinal
With a global myopia boom heralding an ever-increasing prevalence, it has been predicted that 49.8% of the global population will be myopic by 2050.1,2 Previous investigations regarding biometric changes during myopia development have largely focused on the axial length (AL) and corneal curvature (CR).3,4 The AL/CR ratio is the biometric parameter that most correlates with mean spherical equivalent refraction (SER).5,6
Unlike corneal power, which stabilizes after 2 years of age,7 AL increases for the first two decades of life and into the third before it stabilizes.8 The lens also undergoes substantial optical and structural changes that continue throughout life.9,10 However, the role of the lens and its interplay with AL changes during myopia development has not been investigated sufficiently. Several previous studies demonstrated decreasing lens thickness (LT) and lens power (LP) with age in childhood,5,11–13 but there is insufficient detailed longitudinal data to provide an overall picture of how the lens changes from preschool years to adolescence,14–16 and it is unclear whether the lens is actively modulated in relation to axial elongation during the onset of myopia,15
A better understanding of the age-dependent properties of the crystalline lenses and its association with AL growth in children is fundamental to studying the mechanism of myopic development and progression and could be facilitated by long-term longitudinal data from both emmetropic and myopic children. Therefore, we conducted a cohort study in two outer-suburban areas in China, where the prevalence and incidence of myopia are substantially lower than in more urban areas, to investigate the longitudinal changes in AL, LT, and LP among children with different refractive development patterns from 3 to 15 years of age.
Methods
Study Population
This study was a prospective longitudinal study with 7050 children from four different grades recruited from the Zengcheng District (20 primary schools and 11 kindergartens) and Huadu District (five primary schools and three junior high schools) of Guangzhou, China. The study population was divided into four subpopulations: (1) 1433 children at the first-year kindergarten level (grade 0, G0); (2) 1561 participants at the first year of primary school (grade 1, G1); (3) 2671 participants at the fourth year of primary school (grade 4, G4); and (4) 1385 participants at the first year of junior high school (grade 7, G7). The baseline data were collected in 2018, and follow-up examinations were performed annually afterward. Children in these grades were chosen to maximize the follow-up period within the same school. Written informed consent was obtained from the study participants’ parents or legal guardians. This study adhered to the tenets of the Declaration of Helsinki and has been registered on clinicaltrial.gov (NCT03589937). This study was approved by the Institutional Review Board of Zhongshan Ophthalmic Center, Guangzhou, China (2018KYPJ079).
Examinations and Measurement
Participant demographics, including age and gender, were recorded at baseline. Baseline examinations included height and weight measurement, visual acuity test, slit-lamp ocular examinations, ophthalmoscopy, cycloplegic autorefraction, and ocular biometry examinations. Height (measured to the nearest 0.1 cm) and weight (measured to the nearest 0.1 kg) were measured using a height and weight monitor (RGZ-120-RT, Wuxi Weigher Factory Co., Ltd; Suhong, China) with children standing in light clothing and without shoes. Before cycloplegia, ocular biometry was measured using non-contact, partial-coherence laser interferometry (IOLMaster 700; Carl Zeiss Meditec, Wetzlar, Germany). The average of five measurements giving the lowest standard deviation (not exceeding 0.1 mm) was recorded for each eye. Cycloplegia was achieved with two drops of 1% cyclopentolate administered 5 minutes apart, with an additional drop administered after 20 minutes. Cycloplegia was considered complete if the pupillary light reflex was absent and the pupil was dilated to at least 6 mm. Otherwise, a third drop of cyclopentolate was administered. Children who still failed for successful cycloplegia were excluded from the analysis. Cycloplegic autorefraction (KR8800; Topcon, Tokyo, Japan) was performed for all children, and three successive readings with standard error less than 5% were obtained for each eye. Slit-lamp examination and indirect ophthalmoscopy were performed by an ophthalmologist to assess for any ocular abnormality. The same equipment was used and same protocol followed throughout the study.
Statistical Analysis
Baseline and 2 years of follow-up data were included in the current analysis (2018–2020). The study population was divided into three refractive status groups: persistent non-myopia group (PNM; spherical equivalent refraction [SER] ≥−0.5 diopter [D] at baseline and during the follow-up), persistent myopia group (PM; SER <−0.5 D at baseline and during the follow-up), and newly developed myopia group (NDM; SER ≥−0.5 D at baseline and SER <−0.5 D during the follow-up). Children fulfilling any of the following criteria were excluded from the analysis: (1) unavailable data on SER or ocular biometry measurement, (2) history of orthokeratology treatment or myopia corrective surgery, (3) history of ocular diseases (e.g., strabismus, retinal disease) or ocular trauma, (4) severe astigmatism (cylinder power ≤−5 D), (5) severe hyperopia (SER >5 D), (6) high myopia at first grade (G1, SER <−5 D), (7) insufficient follow-up data to determine the myopia development status mentioned above, or (8) unable to satisfy cycloplegia requirements. Because correlations were high (≥93%) between the right and left eye data in the same individual, only right eye data were used in the current analysis. The SER was calculated as the spherical power (D) plus half of the cylinder power (D). Because the LP cannot be directly measured, it was calculated according to the widely used Bennett's equation (Supplementary Material).15,17
The normality in the distribution of continuous variables was checked using the Kolmogorov–Smirnov normality test and histogram. Distributions of baseline age, SER, LT, LP, and AL were expressed as mean (SD) for children in different refractive status groups and in different grades. Gender distribution was expressed as absolute and relative frequencies. The baseline distributions among refractive status groups were compared by χ2 test for sex, and ANOVA was used for all other characteristics. The annual change in LT, LP, and AL during the follow-up for each subgroup of study population is expressed as mean (SD), and P for trend across refractive status groups was calculated by a linear regression model. A locally weighted scatterplot smoothing (LOWESS) plot with a smoothing value of 0.50 was drawn to show the changes in LT and LP for the study participants with an age range of 3 to 15 years. Linear regression models were fitted to assess the associations between changes in LT/LP ratio and AL for PM and PNM children, as well as the associations between LT/LP ratio change and AL before and after myopia onset. Bivariate single-factor linear regression was used to assess the difference in association between the LT/LP ratio and AL before and after myopia onset. A line chart was used to demonstrate the mean 1-year change in SE, LP, LT, and AL both before and after myopia onset. The year children first met the myopia criteria was defined as year 0 (onset year), the first year prior to onset year was −1, and two years prior was −2. The year following the onset year was designated as +1. A two-sided P value of 0.05 or less was considered statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Of the 7050 children enrolled, 1876 (26.61%) were further excluded, including 94 (1.33%) without available SER or ocular biometry data, 258 (3.66%) with history of orthokeratology treatment or myopia corrective surgery, 310 (4.40%) with history of ocular diseases, 8 (0.11%) with severe astigmatism, 14 (0.20%) with severe hyperopia, 8 (0.11%) with high myopia at first grade, 1146 (16.26%) with insufficient follow-up data to determine the myopia development status, and 38 (0.54%) without successful cycloplegia. Thus, data from 5174 (73.39%) children were included in the final analysis.
Baseline characteristics of the study participants are shown in Table 1. The G0 cohort included 1129 children with a mean (SD) age of 3.68 (0.34) years (52.35% boys), the G1 cohort included 1324 children with a mean (SD) age of 6.80 (0.35) years (55.66% boys), the G4 cohort included 1854 children with a mean (SD) age of 9.53 (0.42) years (54.21% boys), and the G7 cohort included 867 children with a mean (SD) age of 12.56 (0.38) years (53.40% boys). Of the included 5174 children, 3797 (73.38%) were PNM, 680 (13.14%) were PM, and 697 (13.47%) were NDM. The distributions of LT, LP, and AL were significantly different among the PNM, PM, and NDM group in G1 (all P < 0.05), G4 (all P < 0.001), and G7 (all P < 0.001).
Table 1.
Baseline Characteristics of the Study Participants
| Total (N = 5174) | Persistent Non-Myopia (N = 3797) | Persistent Myopia (N = 680) | Newly Developed Myopia (N = 697) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | P * |
| G0† | 1129 | 1126 | 1 | 2 | |||||
| Age (y) | 1049 | 3.68 (0.34) | 1047 | 3.68 (0.34) | 1 | 3.78 (−) | 1 | 4.09 (−) | — |
| Male | 1129 | 591 (52.35) | 1080 | 564 (52.22) | 1 | 0 (0.00) | 2 | 0 (0.00) | — |
| LT (mm) | 239 | 3.59 (0.12) | 237 | 3.59 (0.12) | 1 | 3.38 (/) | 1 | 3.73 (/) | — |
| LP (D) | 239 | 26.58 (1.41) | 237 | 26.59 (1.41) | 1 | 24.95 (/) | 1 | 26.81 (/) | — |
| AL (mm) | 243 | 22.10 (0.60) | 241 | 22.09 (0.60) | 1 | 22.89 (/) | 1 | 22.19 (/) | — |
| G1 | 1324 | 1239 | 10 | 75 | |||||
| Age (y) | 1173 | 6.80 (0.35) | 1096 | 6.79 (0.35) | 8 | 6.91 (0.27) | 69 | 6.83 (0.34) | 0.426 |
| Male | 1324 | 737 (55.66) | 1136 | 631 (55.55) | 10 | 5 (50.00) | 75 | 37(49.33) | 0.464 |
| LT (mm) | 1108 | 3.44 (0.14) | 1030 | 3.45 (0.14) | 8 | 3.40 (0.16) | 70 | 3.40 (0.12) | 0.030 |
| LP (D) | 1101 | 24.24 (1.38) | 1023 | 24.27 (1.38) | 8 | 23.60 (1.17) | 70 | 23.79 (1.40) | 0.007 |
| AL (mm) | 1120 | 22.63 (0.67) | 1042 | 22.59 (0.66) | 8 | 23.52 (0.75) | 70 | 23.02 (0.61) | <0.001 |
| G4 | 1854 | 1107 | 248 | 499 | |||||
| Age | 1744 | 9.53 (0.42) | 1026 | 9.50 (0.43) | 241 | 9.59 (0.35) | 477 | 9.54 (0.41) | 0.008 |
| Male | 1854 | 1005 (54.21) | 1047 | 611 (58.36) | 248 | 123 (49.60) | 499 | 235 (47.09) | <0.001 |
| LT (mm) | 1672 | 3.38 (0.15) | 956 | 3.40 (0.15) | 241 | 3.33 (0.15) | 475 | 3.38 (0.15) | <0.001 |
| LP (D) | 1637 | 23.01 (1.48) | 935 | 23.25 (1.45) | 235 | 22.29 (1.41) | 467 | 22.89 (1.44) | <0.001 |
| AL (mm) | 1654 | 23.25 (0.80) | 950 | 23.03 (0.71) | 236 | 24.08 (0.77) | 468 | 23.28 (0.68) | <0.001 |
| G7 | 867 | 325 | 415 | 121 | |||||
| Age (y) | 846 | 12.56 (0.38) | 312 | 12.56 (0.40) | 415 | 12.56 (0.37) | 119 | 12.55 (0.36) | 0.922 |
| Male | 867 | 463 (53.40) | 314 | 200 (63.69) | 421 | 186 (44.18) | 121 | 69 (57.02) | <0.001 |
| LT (mm) | 781 | 3.35 (0.17) | 256 | 3.39 (0.17) | 408 | 3.32 (0.16) | 117 | 3.34 (0.15) | <0.001 |
| LP (D) | 796 | 21.87 (1.42) | 263 | 22.22 (1.48) | 414 | 21.62 (1.36) | 119 | 21.94 (1.31) | <0.001 |
| AL (mm) | 801 | 24.08 (0.99) | 267 | 23.41 (0.70) | 415 | 24.62 (0.93) | 119 | 23.72 (0.57) | <0.001 |
The P values were calculated by χ2 test for sex and by ANOVA for all other variables.
Among 1129 G0 children, 886 children were enrolled in 2019 and did not have baseline ophthalmic data.
Table 2 shows the mean annual changes in LT, LP, and AL for children in different myopia development groups in the four cohorts. LT reduced from G0 to G4 and started to increase in G7. Even myopic children with the fastest progression rate (first quartile) demonstrated an increase in LT in the G7 cohort (n = 207; LT change, 0.007 mm) (Supplementary Table S1). This changing pattern of LT was consistently observed among the PNM, PM, and NDM groups, although the degree of lens thinning was significantly greater among myopic children in comparison with PNM children. It should also be noted that the number of participants was insufficient for a meaningful estimation among PM and NDM in the G0 cohort. Consistently decreasing LP was observed among G0 to G7, but with a slower rate in the older age cohorts. Children in the PM and NDM group demonstrated larger LP decreases than the PNM group. AL increased in all four cohorts and three refractive status groups, but the extent of AL increases was significantly smaller in the PNM group and older cohorts. Separate analyses for annual changes in LT, LP, and AL from the year 2018 to 2019, as well as from 2019 to 2020, produced similar findings (Supplementary Tables S2, S3).
Table 2.
Mean Annual Changes in LT, LP and AL During the Follow-Up
| Persistent Non-Myopia | Persistent Myopia | Newly Developed Myopia | |||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | P for Trend* | |
| G0 | 1126 | 1 | 2 | ||||
| LT (mm) | 908 | −0.046 (0.039) | 1 | −0.060 (/) | 2 | −0.078 (0.025) | — |
| LP (D) | 880 | −0.858 (0.530) | 1 | −1.232 (/) | 2 | −1.066 (0.523) | — |
| AL (mm) | 943 | 0.178 (0.091) | 1 | 0.515 (/) | 2 | 0.617 (0.206) | — |
| G1 | 1239 | 10 | 75 | ||||
| LT (mm) | 1137 | −0.027 (0.021) | 10 | −0.026 (0.014) | 73 | −0.037 (0.024) | <0.001 |
| LP (D) | 1114 | −0.500 (0.295) | 10 | −0.547 (0.282) | 73 | −0.595 (0.522) | <0.001 |
| AL (mm) | 1148 | 0.163 (0.076) | 10 | 0.388 (0.236) | 73 | 0.403 (0.169) | <0.001 |
| G4 | 1107 | 248 | 499 | ||||
| LT (mm) | 1020 | −0.005 (0.025) | 244 | −0.010 (0.017) | 471 | −0.020 (0.027) | <0.001 |
| LP (D) | 1019 | −0.352 (0.336) | 243 | −0.405 (0.298) | 470 | −0.536 (0.339) | <0.001 |
| AL (mm) | 1027 | 0.172 (0.097) | 244 | 0.403 (0.163) | 472 | 0.419 (0.144) | <0.001 |
| G7 | 325 | 421 | 121 | ||||
| LT (mm) | 297 | 0.013 (0.019) | 413 | 0.015 (0.015) | 119 | 0.002 (0.017) | <0.001 |
| LP (D) | 296 | −0.264 (0.371) | 411 | −0.195 (0.200) | 120 | −0.302 (0.263) | 0.940 |
| AL (mm) | 303 | 0.107 (0.083) | 413 | 0.217 (0.110) | 120 | 0.290 (0.131) | <0.001 |
P for trend was calculated by the linear regression model.
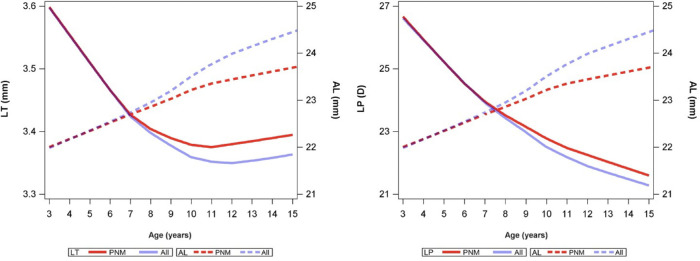
Figure 1 simulates the changes in LT and LP for children in all four cohorts, covering an age range of 3 to 15 years. For PNM children, we observed a three-stage LT change pattern as follows: LT decreased quickly from 3 to 7 years (S1, rapidly decreasing stage), further decreased but at a slower rate from 7 to 11 years (S2, slowly decreasing stage), and increased after 11 years (S3, increasing stage). Similar growth pattern was observed for boys and girls (Supplementary Figure S1). After further including NDM and PM children, the LT decrease was more significant after 7 years of age, followed by an increasing trend after around 12 years of age. We can also see that AL increased faster after including myopic children and slowed down at 11 or 12 years of age (Fig. 1). Regarding LP, a consistent decreasing trend was observed, and a slower decreasing rate occurred at around 10 to 11 years (Fig. 1, Supplementary Fig. S1).
Figure 1.
Change in LT and AL with increasing age among persistent myopia and non-myopia children during follow-up. The curves were estimated with LOWESS plots with a smoothing value of 0.50.
Interaction analysis showed that in the G4 and G7 cohorts, the association between changes in LT and AL and the association between changes in LP and AL were significantly different between PM and PNM children (Table 3, Supplementary Fig. S2). Using data from 697 NDM children during the follow-up, we found significant associations between LT/LP ratio and AL changes both before and after myopia onset (all P < 0.001) (Table 4), but the association was significantly smaller after myopia onset than before myopia onset (P < 0.001 for LT, P = 0.003 for LP). In addition, the SE and AL change peaked at 1 year before myopia onset, but no significant changes in LT or LP were observed around the time of myopia onset (Fig. 2).
Table 3.
Associations Among Changes in Lens Thickness, Lens Power, and Axial Length for Children with Persistent Myopia and Non-Myopia During the Follow-Up
| Linear Regression Model* | ||||
|---|---|---|---|---|
| LT Change | LP Change | |||
| β (95% CI) | P | β (95% CI) | P | |
| G0 | ||||
| AL growth | — | — | — | — |
| PM (vs. PNM) | — | — | — | — |
| AL growth × PM† | — | — | — | — |
| G1 | — | — | — | |
| AL growth | −0.11 (−0.12, −0.10) | <0.001 | −1.66 (−1.87, −1.46) | <0.001 |
| PM (vs. PNM) | 0.0001 (−0.01, 0.01) | 0.987 | −0.05 (−0.22, 0.11) | 0.535 |
| AL growth × PM† | 0.08 (0.03, 0.13) | 0.002 | 1.61 (0.85, 2.37) | <0.001 |
| G4 | ||||
| AL growth | −0.11 (−0.12, −0.09) | <0.001 | −1.65 (−.1.83, −1.46) | <0.001 |
| PM (vs. PNM) | −0.004 (−0.008, −0.0008) | 0.015 | −0.05 (−0.09, −0.009) | 0.02 |
| AL growth × PM† | 0.07 (0.04, 0.10) | <0.001 | 0.85 (0.45, 1.25) | <0.001 |
| G7 | ||||
| AL growth | −0.09 (−0.12, −0.07) | <0.001 | −1.49 (−1.84, −1.14) | <0.001 |
| PM (vs. PNM) | 0.003 (0.0005, 0.005) | 0.020 | 0.05 (0.02, 0.08) | 0.004 |
| AL growth × PM† | 0.05 (0.02, 0.08) | <0.001 | 0.79 (0.38, 1.20) | <0.001 |
For each of subsamples, the regression co-efficient (β) was estimated by a separate model with LT growth and LP change as the dependent variables and AL growth and refractive status (PM vs. PNM) as the independent variables, with an interaction term included, adjusting for sex and height as covariates that are not presented in the table. AL growth and height were centered by subtracting the mean value.
Interaction terms with a positive β value indicate that one unit of AL growth in the PM group would cause more change in outcome than that in the PNM group.
Table 4.
Linear Regression for Associations Among Changes in Lens Thickness, Lens Power, and Axial Length Before and After Myopia Onset
| LT Change | LP Change | |||||||
|---|---|---|---|---|---|---|---|---|
| Before Myopia Onset | After Myopia Onset | Before Myopia Onset | After Myopia Onset | |||||
| Grade | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| AL growth, unstandardized estimation* | −0.10 (−0.12, −0.09) | <0.001 | −0.03 (−0.05, −0.01) | 0.001 | −1.58 (−1.76, −1.39) | <0.001 | −0.53 (−0.79, −0.26) | <0.001 |
| Difference (95% CI) | P | Difference (95% CI) | P | |||||
| Before vs. after† | −0.05 (−0.06, −0.04) | <0.001 | −0.22 (−0.37, −0.07) | 0.003 | ||||
The estimation was derived from a separate regression model with LT growth and LP change as the dependent variable and AL growth as independent variable, adjusting for sex and height as covariates that are not presented in the table.
Bivariate single-factor linear regression for estimating the difference of the effect of AL growth on the outcome before and after myopia onset, adjusting for sex and height as covariates that are not presented in the table.
Figure 2.
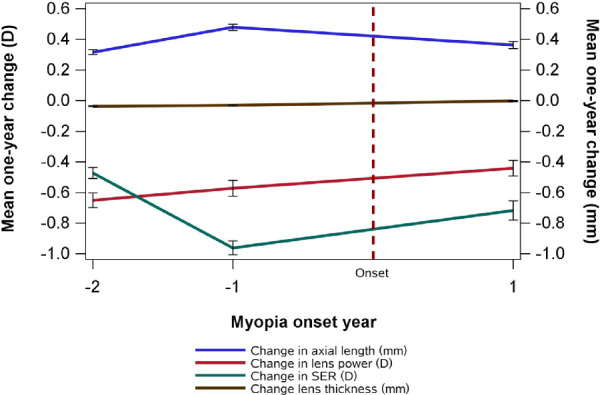
Change in LT, LP, AL, and SE before and after myopia onset. The year children first met the myopia criteria was defined as year 0 (onset year), the first year prior to onset year was −1, and two years prior was −2. The year following the onset year was designated as +1.
Discussion
Based on the longitudinal observation of crystalline lens and AL changes in a large sample of Chinese children, our data show that, in the physiological development of the eye globe, AL continues to grow and LP continues to decrease from 3 to 15 years of age. The measured LT is substantially reduced over time until the child reaches 11 years old. The reductions in LT and LP would partially offset the myopia shift caused by axial elongation, and more substantial decreases in LT and LP were observed among myopic children. After 11 years of age, LT started to increase, presumably as a result of increased synthesis of cortex fibers outpacing the compaction of lens fibers.
Most previous studies investigating the lens change in children and adolescents have been cross-sectional in design and focused on the LP change in children 6 years of age or older. The LT change could better reflect morphological changes in the lens but has been less studied. In a cross-sectional study including 596 children from Qinghai, China, Lu et al.11 reported that LP decreased from 6 to 13 years of age followed by a slight increase from 13 to 16 years of age. Hashemi et al.18 included 6624 Iranian children in their study and found decreasing LP from 6 to 12 years of age. Xiong et al.10 included 1992 Chinese participants 6 to 18 years old and found a three-stage change in LP characterized by a significant change before 10 years of age, which was then reduced between 10 and 14 years of age, and plateaued after 14 years of age. Regarding LT change, Shih et al.19 used data from a nationwide survey of 11,656 students from 7 to 18 years of age and found that the crystalline LT decreased from 7 until 11 or 12 years of age, followed by a subsequent increase, similar to our findings. These studies clearly demonstrate different lens growth patterns at different ages, but the cross-sectional design limits the quality of evidence due to potential cohort effects.
Limited longitudinal studies exist regarding crystalline lens growth in childhood. Based on the Orinda Longitudinal Study of Myopia (OLSM), Zadnik et al.,20 Mutti et al.,13 and Jones et al.14 provided among the first longitudinal evidence demonstrating a change in lens development patterns when children reach approximately 10 years of age. Later, Iribarren et al.21 studied results on 1747 children (6–12 years) from the Singapore Cohort Study of the Risk Factors for Myopia (SCORM), identifying a continued but flattened curve of LP loss at the age of 10 years; a similar trend was observed in our data (Fig. 1B). The study by Wong et al.22 included 1775 Asian children (6–10 years) with at least three visits, and they identified a U-shape growth curve for LT with a trough at nearly 9 years in non-myopic children and 10 years for myopic children. We also identified a reversal in LT change (from decreasing to increasing) at around 11 years of age among PNM children, whereas this turning point was delayed at an older age when further including myopic children (12 years). This is consistent with data from the Correction of Myopia Evaluation Trial (COMET) study, in which 469 myopic children (6–12 years; −1.25 to −4.5 D) were prospectively followed for up to 14 years and found that, between 6 and 18 years of age, the lenses thinned and then thickened with a turning point at a mean age of 11.56 (2.04) years.23 To our knowledge, only two longitudinal studies have reported the lens changes during the preschool stage. Using keratophakometry and ultrasonography, Mutti et al.24 identified continued lens thinning and LP loss for children between 3 months and 6.5 years of age. The reductions in LT (−0.06 mm/y) and LP (−1.23 D/y) in children 3 to 6.5 years old in their study were larger than those observed in our G0 cohort (−0.046 mm/y and −0.858 D/y, respectively). Ma et al.25 reported a mean LP change of −0.93 D/year based on one-year follow-up of 458 children 3 to 5 years old from Shanghai, China. They also found larger LP loss (compared to axial elongation) for eyes with baseline SER <+1.00 D and smaller LP loss for those with SER above this range, and they suggested that the lens may compensate for increasing AL to maintain the refractions in a mildly hyperopic ranges in the preschool stage.
By including children between 3 and 15 years of age, we have revealed a three-stage LT growth pattern with two stage-changing points at 7 and 11 years of age during physiologic ocular development among PNM children. This three-stage pattern also applied to myopic children, although there was a difference in the inflection points. We speculate that the extent of delay in lens thickening is related to the prevalence and severity of myopia in the given population, with more delay in populations with higher myopia prevalence and individuals with higher degrees of myopia.
The effective decrease in LT and LP associated with increasing AL in early childhood appears to represent an attempt by the lens to actively compensate for axial elongation in order to achieve emmetropization. The LT significantly reduced its rate of change after 7 years, when most hyperopic eyes become emmetropic. But, in myopic eyes, more rapid eye growth was accompanied by larger lens thinning after 7 years of age. However, whether causal relationships exist between axial elongation and lens change could not be directly proved in this study. We observed that LT began to increase for all children in the G7 cohort, regardless of myopia status or myopia progression rate, suggesting that the crystalline lens ceases to thin and flatten even as the eye continues to grow during this stage, a physiologically intrinsic process. LP, on the other hand, continued to decrease during the study period in all four cohorts, suggesting that the lens starts to thicken while continuing to slowly lose power at this stage.
It is unknown whether the cessation of lens thinning is due to the lens reaching a physiologic limit below which it is unable to lose power efficiently or a certain age at which point it is programmed to increase. Mutti et al.13,26 previously suggested that lens thinning in early years was largely attributed to zonular traction with eye growth, and the slowing rate at 10 years could be explained by reaching a limit with equatorial growth; however, this mechanical tension theory was not supported by other studies.27,28 As suggested in a review by Iribarren,16 compaction might better explain the lens thinning in the first decade of life, and the lens ceases to thin when the rate of compaction in the nucleus equals the growth rate of newly developed cortex. The LP results from a co-effect of changes in the shape and the gradient refractive index of the lens. With lens thinning, the lens loses surface power with reduced anterior and posterior curvature, and the internal power also slowly decreases as the gradient of the refractive index increases. In the study by Twelker et al.,29 the equivalent refractive index was nearly 1.45 in early infancy but dropped to 1.42 by age 10 in Asian children. After 10 years of age, despite no further lens thinning, the LP continued to decrease due to a further reduced internal refractive index, but at a much slower rate.
In accordance with previous studies,10,26,30 we identified more significant associations between the LT/LP ratio and AL in PNM children than in PM children. We also observed a weaker association between the LT/LP ratio and AL after myopia onset in the NDM children in our cohorts, which suggests that the crystalline lens change in relation to axial elongation is different between non-myopic and myopic children. A peak SER decreasing rate and corresponding peak AL increasing rate at 1 year before myopia onset has been consistently identified in many previous studies.12,22 However, the changes to the crystalline lens before and after myopia onset are unclear. Xiang et al.15 examined Chinese twins 7 to 15 years old and did not find any significant changes at onset, a finding that was supported by Xiong et al.,30 who included 1465 Chinese children (6–8 years old) and observed similar LP loss between those who remain emmetropic and those who became myopic during the 2-year follow-up. Mutti et al.26 compared 732 children (6–14 years old) who became myopic and 596 age- and gender-matched emmetropic children in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study (CLEERE). They followed these children from 5 years before myopia onset to 5 years after myopia onset and found that the lens stopped compensatory thinning within ±1 year of myopia onset in myopic children compared to emmetropes. In the Singapore Cohort Study of the Risk Factors for Myopia (SCORM), Rozema et al.12 compared 480 emmetropic children, 303 newly onset myopic children, and 509 persistent myopic children (age range, 6–9 years) during a 3- to 6-year follow-up. They found significantly higher LP loss (−0.71 D/y) up to 1 year before myopia onset, which slowed down rapidly in the following period.12 Our findings that LT and LP did not exhibit significant changes around the time of myopia onset were consistent with the two above-mentioned studies in Chinese populations.15,30 Further studies are needed to understand the association between lens changes and myopia onset and whether there is ethnic heterogeneity in this association.
Strengths of the current study include a large study population with a wide age range and a consistent study methodology with regular use of cycloplegic refraction. There were several limitations. First, this study included a 2-year follow-up for four age cohorts of children with different baseline ages, which is not as ideal as following the same cohort for an extended period of time, but nonetheless this approach still provides sufficient evidence regarding longitudinal changes, given that the year-to-year cohort effects are likely to be small. Second, the sample sizes of PM and NDM children in the G0 and G1 cohorts were relatively small due to a low prevalence and incidence of myopia in this age group. Third, there is currently no method for directly measuring LP in vivo, as determining LP from phakometric data requires assumptions about the internal gradient index. The LP was calculated in our study using Bennett's formula, which is a validated and widely used method but has been reported to possibly produce errors in 5% of the cases.17 Fourth, one should note that the observed mean annual changes of LT in several subgroups, despite being statistically significant, were smaller than the repeatability and reproducibility limits of IOLMaster. Efforts were made to minimize the risk of bias, including requiring the same experienced personnel to perform the measurement and recording the average of five measurements giving the lowest standard deviation as the final value for LT at each study visit. In addition, the LT changes in each subgroup were statistically significant despite being small in extent, and we also found significant associations between the longitudinal LT changes with both baseline age and baseline SER (Supplementary Table S4, Supplementary Fig. S3), suggesting that the observed values were real changes instead of random or systematic measurement error. Finally, the follow-up time before or after myopia onset was limited, hindering us from providing a more thorough analysis regarding the role of crystalline lens in myopia onset.
In conclusion, our study has allowed us to describe a three-stage LT growth pattern accompanied by continuously decreasing LP in childhood, as demonstrated in both persistent myopic and non-myopic Chinese children. The crystalline lens could actively compensate for extra axial elongation in myopic children, but this process is significantly diminished after myopia onset. The lens is programmed to eventually cease thinning, although later in myopic children.
Supplementary Material
Acknowledgments
Supported by the Science and Technology Planning Project of Guangzhou (202002030405) and Medical Scientific Research Foundation of Guangdong Province (A2020146).
Disclosure: X. Han, None; R. Xiong, None; L. Jin, None; Q. Chen, None; D. Wang, None; S. Chen, None; X. Chen, None; J. Ha, None; Y. Li, None; Y. Qu, None; R. Lin, None; M. He, None; I.G. Morgan, None; Y. Zeng, None; Y. Liu, None
References
- 1. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123(5): 1036–1042. [DOI] [PubMed] [Google Scholar]
- 2. Baird PN, Saw SM, Lanca C, et al.. Myopia. Nat Rev Dis Primers. 2020; 6(1): 99. [DOI] [PubMed] [Google Scholar]
- 3. Morgan IG, Wu PC, Ostrin LA, et al.. IMI risk factors for myopia. Invest Ophthalmol Vis Sci. 2021; 62(5): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li FF, Lu SY, Tang SM, et al.. Genetic associations of myopia severities and endophenotypes in children. Br J Ophthalmol. 2021; 105(8): 1178–1183. [DOI] [PubMed] [Google Scholar]
- 5. Guo X, Fu M, Ding X, Morgan IG, Zeng Y, He M.. Significant axial elongation with minimal change in refraction in 3- to 6-year-old Chinese preschoolers: the Shenzhen Kindergarten Eye Study. Ophthalmology. 2017; 124(12): 1826–1838. [DOI] [PubMed] [Google Scholar]
- 6. He X, Sankaridurg P, Naduvilath T, et al.. Normative data and percentile curves for axial length and axial length/corneal curvature in Chinese children and adolescents aged 4–18 years [published online ahead of print September 16, 2021]. Br J Ophthalmol, 10.1136/bjophthalmol-2021-319431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mutti DO, Mitchell GL, Jones LA, et al.. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005; 46(9): 3074–3080. [DOI] [PubMed] [Google Scholar]
- 8. Truckenbrod C, Meigen C, Brandt M, et al.. Longitudinal analysis of axial length growth in a German cohort of healthy children and adolescents. Ophthalmic Physiol Opt. 2021; 41(3): 532–540. [DOI] [PubMed] [Google Scholar]
- 9. Han X, Guo X, Lee PY, Morgan IG, He M.. Six-year changes in refraction and related ocular biometric factors in an adult Chinese population. PLoS One. 2017; 12(8): e0183364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiong S, Zhang B, Hong Y, et al.. The associations of lens power with age and axial length in healthy Chinese children and adolescents aged 6 to 18 years. Invest Ophthalmol Vis Sci. 2017; 58(13): 5849–5855. [DOI] [PubMed] [Google Scholar]
- 11. Lu T, Song J, Wu Q, et al.. Refractive lens power and lens thickness in children (6–16 years old). Sci Rep. 2021; 11(1): 19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rozema J, Dankert S, Iribarren R, Lanca C, Saw SM.. Axial growth and lens power loss at myopia onset in Singaporean children. Invest Ophthalmol Vis Sci. 2019; 60(8): 3091–3099. [DOI] [PubMed] [Google Scholar]
- 13. Mutti DO, Zadnik K, Fusaro RE, Friedman NE, Sholtz RI, Adams AJ.. Optical and structural development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci. 1998; 39(1): 120–133. [PubMed] [Google Scholar]
- 14. Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K.. Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci. 2005; 46(7): 2317–2327. [DOI] [PubMed] [Google Scholar]
- 15. Xiang F, He M, Morgan IG.. Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology. 2012; 119(7): 1478–1484. [DOI] [PubMed] [Google Scholar]
- 16. Iribarren R. Crystalline lens and refractive development. Prog Retin Eye Res. 2015; 47: 86–106. [DOI] [PubMed] [Google Scholar]
- 17. Rozema JJ, Atchison DA, Tassignon MJ.. Comparing methods to estimate the human lens power. Invest Ophthalmol Vis Sci. 2011; 52(11): 7937–7942. [DOI] [PubMed] [Google Scholar]
- 18. Hashemi H, Pakzad R, Iribarren R, Khabazkhoob M, Emamian MH, Fotouhi A.. Lens power in Iranian schoolchildren: a population-based study. Br J Ophthalmol. 2018; 102(6): 779–783. [DOI] [PubMed] [Google Scholar]
- 19. Shih YF, Chiang TH, Lin LL.. Lens thickness changes among schoolchildren in Taiwan. Invest Ophthalmol Vis Sci. 2009; 50(6): 2637–2644. [DOI] [PubMed] [Google Scholar]
- 20. Zadnik K, Mutti DO, Fusaro RE, Adams AJ.. Longitudinal evidence of crystalline lens thinning in children. Invest Ophthalmol Vis Sci. 1995; 36(8): 1581–1587. [PubMed] [Google Scholar]
- 21. Iribarren R, Morgan IG, Chan YH, Lin X, Saw SM.. Changes in lens power in Singapore Chinese children during refractive development. Invest Ophthalmol Vis Sci. 2012; 53(9): 5124–5130. [DOI] [PubMed] [Google Scholar]
- 22. Wong HB, Machin D, Tan SB, Wong TY, Saw SM.. Ocular component growth curves among Singaporean children with different refractive error status. Invest Ophthalmol Vis Sci. 2010; 51(3): 1341–1347. [DOI] [PubMed] [Google Scholar]
- 23. Gwiazda J, Norton TT, Hou W, Hyman L, Manny R, COMET Group. longitudinal changes in lens thickness in myopic children enrolled in the Correction of Myopia Evaluation Trial (COMET). Curr Eye Res. 2016; 41(4): 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mutti DO, Sinnott LT, Lynn Mitchell G, et al.. Ocular component development during infancy and early childhood. Optom Vis Sci. 2018; 95(11): 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma Y, Lin S, Morgan IG, et al.. Eyes grow towards mild hyperopia rather than emmetropia in Chinese preschool children. Acta Ophthalmol. 2021; 99(8): e1274–e1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mutti DO, Mitchell GL, Sinnott LT, et al.. Corneal and crystalline lens dimensions before and after myopia onset. Optom Vis Sci. 2012; 89(3): 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berntsen DA, Sinnott LT, Mutti DO, Zadnik K.. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci. 2012; 53(2): 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phelps Brown N, Bron A. Lens Disorders: A Clinical Manual of Cataract Diagnosis. 3rd ed. Oxford, UK: Butterworth-Heinemann; 1996: 17–24. [Google Scholar]
- 29. Twelker JD, Mitchell GL, Messer DH, et al.. Children's ocular components and age, gender, and ethnicity. Optom Vis Sci. 2009; 86(8): 918–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiong S, He X, Sankaridurg P, et al.. Accelerated loss of crystalline lens power initiating from emmetropia among young school children: a 2-year longitudinal study. Acta Ophthalmol. 2022; 100(4): e968–e976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.