Abstract
Purpose
We aimed to investigate the possible effect of SARS-CoV-2 vaccination on sperm quality by evaluating semen analyses of men prior to vaccination and 6–14 months after vaccination.
Methods
This was a retrospective cohort study, conducted in a university-affiliated in vitro fertilization center between October 2021 and March 2022, including men not previously infected with the SARS-CoV-2 virus who received at least 2 doses of the Pfizer-BioNTech (BNT162b2) SARS-CoV-2 vaccine. Semen analyses of samples given pre-vaccination and 6–14 months post-vaccination were analyzed for the parameters of volume, concentration, motility, morphology, and total motile count (TMC) and compared. These parameters were also compared separately for men who received a third (booster) dose and for men with pre-vaccination normal and abnormal sperm. Correlations between time from vaccination and post-vaccination sperm parameters were also assessed.
Results
Fifty-eight men were included in the final analysis. Semen volume (2.9 ± 1.4 vs. 2.9 ± 1.6 ml), sperm concentration (42.9 ± 37.9 vs. 51.5 ± 46.2 million/ml), motility (42.5 ± 23.1 vs. 44.3 ± 23.4 percent), morphology (8.8 ± .16.6 vs. 6.6 ± 8.8 percent), and TMC (55.7 ± 57.9 vs. 71.1 ± 77.1 million) were comparable between the pre- and post-vaccination samples. This was true for the entire study cohort, for the subgroup of men who received a third dose and for the subgroups of men with a pre-vaccination normal and abnormal semen samples. No correlation was found between time from vaccination and post-vaccination sperm parameters.
Conclusions
The Pfizer-BioNTech (BNT162b2) SARS-CoV-2 vaccine does not impair any of the sperm parameters over a relatively long-time interval of 6 to 14 months from vaccination.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02621-x.
Keywords: Fertility, Sperm parameters, COVID-19, SARS-CoV-2 vaccine, BNT162b2 vaccine
Introduction
The COVID-19 pandemic was declared in March 2020, spreading rapidly throughout the world with devastating consequences, including short- and long-term morbidity and mortality [1]. One of the mechanisms in which the SARS-CoV-2 virus causes infection is binding to the angiotensin-converting enzyme 2 (ACE-2) through the spike glycoprotein [2]. As ACE-2 receptors are located on the blood-testis barrier, the male reproductive system is a potential target for the virus. This has promoted research on the effects of the SARS-CoV-2 virus on the male reproductive system. While majority of studies demonstrated the absence of SARS-CoV-2 RNA in the semen of infected men [3, 4], few studies [5, 6] showed a minor negative effect on sperm parameters within the first 2–3 months after infection.
The mRNA SARS-CoV-2 vaccine, introduced in late 2020, dramatically reduced COVID-19 infection and transmission with consequent decrease in hospitalization and death rates within the general population [7, 8]. Following the implementation of the mRNA SARS-CoV-2 vaccine, several studies were conducted to explore possible adverse outcomes of the vaccine on male fertility, confirming its safety on short-term follow-up [9–11].
Previous data focused on relatively short-term (under 6 months, mostly up to 3 months) outcomes [9, 11], while data on longer intervals between vaccination and semen evaluation remains scarce. Vaccine hesitancy is driven, among other factors, by unfounded fear of possible long-term adverse effects on fertility [12]. Moreover, safety results of long-term follow-up are essential to reassure men that have already been vaccinated. Therefore, long-term outcomes are necessary to encourage vaccine uptake and decrease hesitancy.
The aim of this study was to evaluate sperm parameters of non-infected men who received at least two Pfizer-BioNTech (BNT162b2) SARS-CoV-2 vaccines and had a sperm analysis prior to vaccination and 6–14 months post-vaccination.
Materials and methods
Study population
We performed a retrospective cohort study at a tertiary university teaching hospital between October 2021 and March 2022. Included were men, in the process of IVF treatment with a female partner, who received at least two Pfizer-BioNTech (BNT162b2) SARS-CoV-2 vaccines and had submitted a sperm specimen prior and after vaccination. Both men with normal and abnormal semen analysis were included in the study, to evaluate possible effect in both groups. Excluded were men with known azoospermia (absence of sperm cells in the semen), men who were tested positive for SARS-CoV-2 (rapid antigen or PCR testing) at any point and those for whom date of vaccination was unavailable.
Specimen collection and analysis
Sperm specimens were collected as part of our standard protocol of care for couples evaluated for infertility. Following abstinence for 2–3 days, each man provided a semen sample either at home or in our laboratory in a sterile cup by means of masturbation and delivered it within 60 min to our laboratory for evaluation by a single embryologist. For all patients, the second sperm specimen was collected at least 6 months after the first vaccine dose. Patients for which a third (booster) dose was administered were recorded as well.
Semen analysis included evaluation of the semen sample, including the use of the Mackler chamber (Sefi Medical Instruments, Israel) for the assessment of sperm motility and concentration. The following semen parameters were evaluated—sample volume (milliliter (ml)), concentration (million/ml), motility (%), normal morphology (%), and total motile count (million). The classification of normal sperm parameters was according to the widely accepted WHO semen analysis reference range guidelines [13]. We analyzed two specimens from each sample to determine the average value of all sperm parameters: concentration, motility, and morphology.
Sperm morphology evaluation was done using Quick Stain (Biological Industries, Sartorius AG, Germany). The stained slides were prepared by the laboratory staff according to the manufacturer’s instructions. The evaluation was performed at a final magnification of X400 (PLAN CN 40x/0.65 Ph2 OLYMPUS) by counting 100 cells. Spermatozoa classification as normal or abnormal morphologically was according to WHO criteria (13). A smear of 20 ul 1:1 mix (stain and semen) was evaluated at a X400 magnification (PLAN CN 40x/0.65 Ph2 OLYMPUS) by counting at least 100 cells (unstained and red-stained cells).
Data collection and analysis
Information was extracted from the electronic medical record software used to manage all data within the IVF unit at our medical center. Data retrieved included demographic, past medical history, indication for IVF treatment, and semen analysis-related data—semen volume, sperm concentration (million/ milliliter), motility, morphology, and total motile count pre- and post-vaccination. For patients with several sperm specimens, we used the latest specimen collected after vaccination.
These sperm parameters were compared pre-vaccination and post-vaccination in the entire cohort. A subgroup analysis for men who received a third dose (booster dose) was also performed. Additionally, a subgroup analysis for men with pre-vaccination normal and abnormal sperm was done, to test for possible differences in the vaccination effect according to the initial sperm quality. Linear correlation between the parameter of time from vaccination (days) and post-vaccination sperm parameters was also performed to assess possible gradual continuous effects.
Ethical approval
This study was approved by the Human Investigation Review Board of Hadassah Hebrew University Medical center (IRB 0216–22-HMO) and conforms to the provisions of the declaration of Helsinki.
Power analysis
The sample size power calculation for a paired t test was directed to detect an 8 million/ml difference (represents a 20% difference for an average concentration of 40 million/ml) in sperm concentration pre- and post-vaccination and achieve a significance level of 5% (two tailed) and power of 80%, with a SD of 20 million/ml. We calculated that a total of 52 patients would provide sufficient power to detect this difference with statistical significance.
Statistical analysis
For quantitative variables, the comparison between independent variables of the study groups was performed using Student’s t test. Semen parameters pre- and post-vaccination were compared by Student’s t test for paired samples (as each patient had a semen analysis pre- and post-vaccination).
Testing the association between two categorical variables was carried out using either the Chi-square test or the Fisher’s exact test, as indicated. The Fisher’s exact test was applied in analyses of small samples, when more than 20% of cells had expected frequencies of less than five. Cross-sectional association of the days from first vaccination to sperm parameters—volume, concentration, motility, morphology, and total motile count—were tested using the Pearson correlation coefficient (“r”) in linear regression models. For all comparisons, a p value of < 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS software (version 23; IBM Corp).
Results
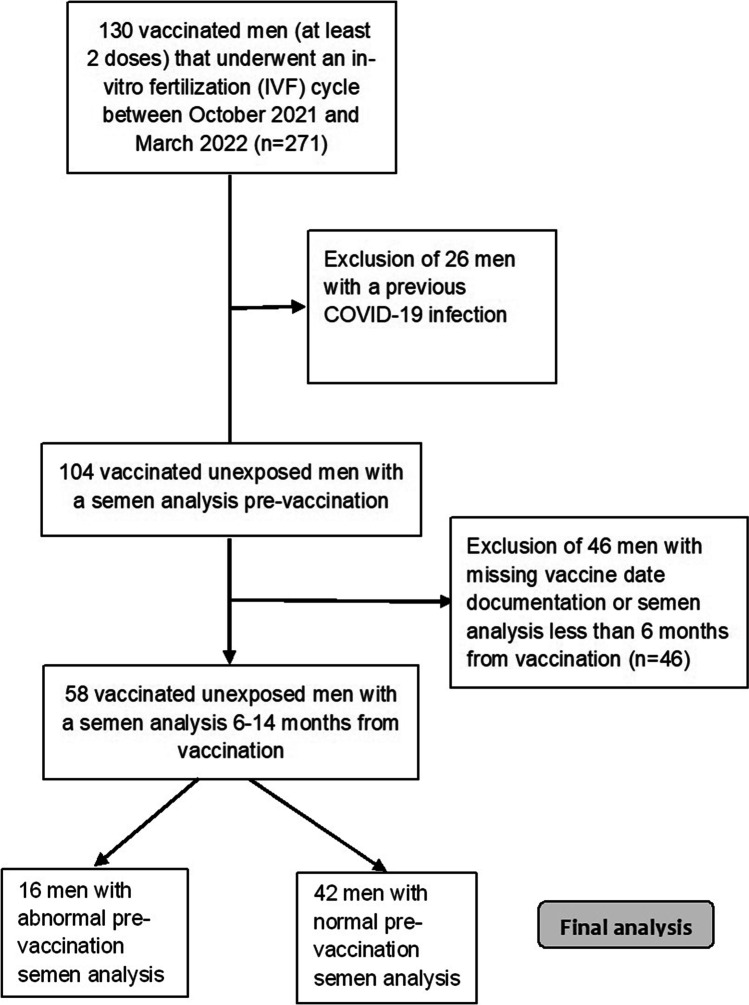
In our IVF unit, 130 men underwent an Assisted Reproduction Technology (ART) cycle between October 2021 and March 2022. Out of these men, 104 were uninfected with the SARS-CoV-2 virus, and 85 of them had received at least 2 vaccination doses (3 weeks apart) with documented vaccination dates. Finally, 58 men who had pre- and post-vaccination semen analyses performed at least 6 months from the first vaccination were included in the study (Fig. 1).
Fig. 1.
Flow chart of study population
The average age at pre-vaccination semen analysis was 38.0 ± 6.1 years (median of 38 years). Sixteen men (27.6%) were diagnosed with male factor infertility, thirteen of them had two abnormal parameters or oligoasthenoteratozoospermia. The average number of days from vaccination to post-vaccination semen analysis was 319.0 ± 61.4 days (median of 317 days). Thirteen men (22.4%) had post-vaccination semen analysis 6–9 months after vaccination, while for 45 men (77.6%), it was performed 9–14 months after vaccination. The basic characteristics and reproductive background of the study population are presented in Table 1. In the entire study population, the post-vaccination TMC parameter was higher in 27 men and lower in 28 men (due to missing data regarding motility in one of the semen analyses, TMC was not calculated for the remaining three men).
Table 1.
Pre-vaccination characteristics of the study population: men vaccinated with at least two doses of SARS-CoV-2 mRNA vaccine without a previous exposure to the SARS-CoV-2 virus
| Number of men | 58 |
| Age (years) pre vaccination | 38.0 ± 6.1 (38.0) |
| Smoker | 3/58 (5.2%) |
| Father to children | 10/58 (17.2%) |
| Chronic disease or medication taken | 4/58 (%) |
| Testicular trauma or infection | 2/58 (3.4%) |
| Varicocele | 8/58 (13.8%) |
| Testicular (varicocele/hydrocele/undescended testicle) or inguinal hernia surgeries | 7/58 (12.1%) |
| Third vaccination given | 47/58 (81.0%) |
| Testosterone levels (mIU/ml)a | 16.3 ± 13.8 (15.1) |
| FSH levelsa (mIU/ml) a | 5.7 ± 2.9 (4.3) |
| LH levelsa (mIU/ml)a | 4.2 ± 1.5 (2.9) |
| Infertility diagnosis | |
| Male factor infertility | 16/58 (27.6%) |
| Ovulation disorder | 2/58 (3.4%) |
| Endometriosis | 4/58 (6.9%) |
| Ovarian insufficiency/ age related infertility | 11/58 (19.0%) |
| PGT | 6/58 (10.3%) |
| RPL | 2/58 (3.5%) |
| Female fertility preservation | 2/58 (3.5%) |
| Mechanical factor | 3/58 (5.2%) |
| Unexplained infertility | 12/58 (20.7%) |
| Days from first vaccination to semen analysis | 319.0 ± 61.4 (317.5) |
| Months from first vaccination to semen analysis | |
| 6–9 months | 13/58 (22.4%) |
| 9–14 months | 45 (77.6%) |
Data presented as n/N(%) or mean ± SD (median)
FSH, follicular stimulating hormone; LH, luteinizing hormone; PGT, preimplantation genetic testing; RPL, recurrent pregnancy loss
aHormonal profile pre-vaccination was available for 18 patients
When comparing pre- and post-vaccination semen analyses in the entire cohort (Table 2), we found no difference in semen volume (2.9 ± 1.4.0 vs. 2.9 ± 1.6 ml, respectively; NS), concentration (42.9 ± 37.9 vs. 51.5 ± 46.2 million/ml, respectively; NS), motility (43.2 ± 22.6 vs. 44.1 ± 23.6 percent, respectively; NS), normal morphology (1.5 ± 2.0 vs. 3.8 ± 3.5 percent, respectively; NS), and total motile count (56.7 ± 58.0 vs. 71.1 ± 77.8 million, respectively; NS).
Table 2.
Comparison of semen analysis results of men vaccinated with at least two doses of SARS-CoV-2 mRNA with semen samples pre-vaccination and post-vaccination
| Parameter | Pre-vaccination | Post-vaccination* | P value |
|---|---|---|---|
| Entire cohort (n = 58) | |||
| Semen parameters | |||
| Volume (ml) | 2.9 ± 1.4 (3.0) | 2.9 ± 1.6 (3.0) | 0.956 |
| Concentration (million/ml) | 42.9 ± 37.9 (33.5) | 51.5 ± 46.2 (48.0) | 0.060 |
| Motility (%) | 42.5 ± 23.1 (44.5) | 44.3 ± 23.4 (43.0) | 0.790 |
| Normal morphologya (%) | 8.8 ± .16.6 (4.0) | 6.6 ± 8.8 (3.5) | 0.132 |
| Total motile count (million) | 55.7 ± 57.9 (30.3) | 71.1 ± 77.1 (39.5) | 0.187 |
| Men who received 3 vaccinations (n = 47) | |||
| Semen parameters | |||
| Volume (ml) | 2.9 ± 1.5 (3.0) | 2.9 ± 1.7 (3.0) | 0.870 |
| Concentration (million/ml) | 42.0 ± 38.7 (30.0) | 54.7 ± 47.5 (50.0) | 0.017 |
| Motility (%) | 41.4 ± 21.9 (44.0) | 44.0 ± 25.0 (43.7) | 0.706 |
| Normal morphologya (%) | 6.2 ± 11.9 (3.0) | 7.1 ± 9.2 (4.0) | 0.054 |
| Total motile count (million) | 49.0 ± 53.1 (29.8) | 71.0 ± 73.2 (44.3) | 0.064 |
Data presented as mean ± SD (median)
aMorphology data was available for 8 patients in the entire cohort and for 7 patients in the three vaccination group
Similar results were demonstrated in a subgroup analysis including men who received three vaccine doses (n = 47) (Table 2), except for the concentration parameter, which was higher post-vaccination (54.7 ± 47.5 vs. 42.0 ± 38.7 million/ml, p = 0.017).
Between the pre- and post-vaccination semen analyses, one man had stopped receiving Copaxone treatment for multiple sclerosis (diagnosis was ruled out), one man received antibiotic treatment for possible genitourinary tract infection, and another started taking multivitamin. For all three, sperm parameters were slightly improved in the post-vaccination analysis.
An additional analysis was performed separately for men with normal sperm parameters (n = 42) and for men (n = 16) with abnormal sperm with similar results showing comparable semen parameters pre- and post-vaccination (Table 3).
Table 3.
Comparison of semen analysis results of vaccinated men with normal (n = 42) and abnormal (n = 16) semen parameters pre-vaccination and post-vaccination
| Parameter | Pre-vaccination | Post-vaccination* | P value |
|---|---|---|---|
| Men with normal sperm | |||
| Semen parameters | |||
| Volume (ml) | 2.9 ± 1.3 (2.9) | 2.9 ± 1.5 (3.0) | 0.743 |
| Concentration (million/ml) | 56.0 ± 36.6 (50.0) | 66.8 ± 44.6 (65.0) | 0.084 |
| Motility (%) | 51.1 ± 18.5(52.0) | 47.2 ± 22.7 (45.0) | 0.274 |
| Normal morphologya (%) | 10.5 ± 17.8 (5.0) | 7.9 ± 10.0 (4.0) | 0.296 |
| Total motile count (million) | 74.2 ± 57.4 (49.5) | 89.8 ± 79.6 (75.0) | 0.275 |
| Men with abnormal semen analysis | |||
| Semen parameters | |||
| Volume (ml) | 2.8 ± 1.7 (3.3) | 3.0 ± 1.8 (2.8) | 0.630 |
| Concentration (million/ml) | 8.5 ± 8.1 (5.3) | 11.5 ± 17.6 (4.4) | 0.368 |
| Motility (%) | 18.8 ± 17.3 (16.0) | 35.6 ± 24.3 (35.0) | 0.053 |
| Total motile count (million) | 5.1 ± 5.8 (1.7) | 15.6 ± 27.3 (3.8) | 0.146 |
Data presented as mean ± SD (median)
aMorphology data was available for 5 patients in the normal sperm group and for 3 in the abnormal sperm group (therefore not evaluated and not presented)
When evaluating the linear correlation (Pearson correlation coefficient) between time from vaccination (days) and the parameters of semen volume, concentration, motility, and total motile count, we found no correlation with any of these parameters (Suppl. Table 1).
Discussion
In this study, we investigated semen analyses of vaccinated men 6–14 months after vaccination. We found no difference in sperm parameters pre- and post-vaccination in IVF patients treated with the Pfizer-BioNTech (BNT162b2) SARS-CoV-2 vaccine. Similar results were found for men who received a third vaccine dose (booster dose). All post-vaccination sperm specimens were collected at least 6 months after vaccination (median 317 days) enabling relatively long-term follow-up for the main outcome measures.
Our findings are in accordance with previous studies. Lifshitz et al. presented data from 75 fertile men 1–2 months after receiving their second vaccine dose showing that sperm parameters were within the WHO normal range, with less than 5% of abnormal parameters [9], though pre-vaccination semen samples were not available for comparison, as the authors mentioned. Similar results were noted in a study of 45 healthy volunteers scheduled for mRNA COVID-19 vaccination who provided semen samples prior to vaccination and 75 days after their second vaccine dose [10]. In a more recent study, semen parameters and fertilization rates were compared pre- and post-vaccination in patients undergoing ART. At a median follow-up of 75 days, no differences were noted between groups [11].
The process of spermatogenesis is a complex one which has been estimated to take approximately 74 days [14]. Theoretically, a vaccine administered during this time-period could instigate an immune response affecting spermatozoa at different stages of maturity. However, the same response may target germ cells which have not yet entered spermatogenesis. In this study, we intentionally excluded patients who received their vaccine less than 6 months prior to semen sample collection. We believe this enabled us to better assess the true effect of vaccination on sperm parameters at all stages.
Our results are unique in that they focus on longer follow-up than previously reported. While most studies reported on outcomes up to 3 months post-vaccination, most of the men included in this study were vaccinated 9–14 months prior to the last semen sample collection. While future studies will surely assess vaccine safety for longer follow-up periods, these results are encouraging for both providers and patients.
In the subgroup of men who received three vaccines, an increase in sperm concentration post-vaccination was detected. This finding might be explained, at least partially, by the three men in this group that recieved multivitamins or antibiotics or discontinued pharmacological treatment.
This study has several limitations. Firstly, all men participating in this study were part of couples undergoing IVF treatment and included men with male factor infertility, as opposed to studies that evaluated sperm donors. Secondly, data on sperm morphology, progressive motility assessment and DNA integrity and fragmentation were unavailable for part of our cohort. All subjects were vaccinated with the Pfizer BioNTech mRNA vaccine, limiting our conclusions to patients who received this specific formulation. Finally, we included patients with male factor infertility, and though each sperm analysis was paired, this may hamper our ability to draw conclusions to the general population.
Conclusions
Our study shows that the Pfizer-BioNTech (BNT162b2) SARS-CoV-2 vaccine does not negatively affect semen parameters, even in men with male factor infertility. We further found this to be true in patients who received a booster dose. The long-term follow-up presented here is key to establish vaccine safety and address patients’ hesitancy towards vaccination and provide reassurance for vaccinated men.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- COVID-19
Coronavirus disease
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- mRNA
Messenger ribonucleic acid
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Gilad Karavani and Henry H. Chill have equal contribution as first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of SARS-CoV-2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamyan L, Elagin V, Vechorko V, Stepanian A, Dashko A, Doroshenko, et al. A review of recent studies on the effects of SARS-CoV-2 infection and SARS-CoV-2 vaccines on male reproductive health. Med Sci Monit. 2022;28:e935879. doi: 10.12659/MSM.935879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donders GGG, Bosmans E, Reumers J, Donders F, Jonckheere J, Salembier G, et al. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril. 2022;117:287–296. doi: 10.1016/j.fertnstert.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26:2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harder T, Koch J, Vygen-Bonnet S, Külper-Schiek W, Pilic A, Reda S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. 2021;26:2100563. doi: 10.2807/1560-7917.ES.2021.26.28.2100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lifshitz D, Haas J, Lebovitz O, Raviv G, Orvieto R, Aizer A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod Biomed Online. 2022;44:145–149. doi: 10.1016/j.rbmo.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, Ory J, et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA. 2021;326:273–274. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reschini M, Pagliardini L, Boeri L, Piazzini F, Bandini V, Fornelli G, et al. COVID-19 vaccination does not affect reproductive health parameters in men. Front Public Health. 2022;10:839967. doi: 10.3389/fpubh.2022.839967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz P, Zizzo J, Balaji NC, Reddy R, Khodamoradi K, Ory J, et al. Fear about adverse effect on fertility is a major cause of COVID-19 vaccine hesitancy in the United States. Andrologia. 2022;54:e14361. doi: 10.1111/and.14361. [DOI] [PubMed] [Google Scholar]
- 13.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 14.Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science. 1963;140:184–186. doi: 10.1126/science.140.3563.184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.