Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection leads to the development of coronavirus disease 2019 (COVID-19), which causes endothelial dysfunction (ED), oxidative stress (OS), and inflammatory disorders. These changes cause hypoxia and cytokine storm with the development of cardio-pulmonary complications. Bioactive lipids and other polyunsaturated fatty acids participate in a vital role in the SARS-CoV-2 infection process. One of these mediators is the anti-inflammatory compound, lipoxin (LX). LXs are produced from arachidonic acid (AA) by collaboration between 5-lipoxygenase (5-LO) and 12–15 LO during cell interactions. Thus, our goal was to review the probable role of LXs in COVID-19 regarding the effects of LXs on the inflammatory signaling pathways that are linked with COVID-19 pathogenesis and complications.
Keywords: Arachidonic acid, COVID-19, Inflammation, Lipoxins, SARS-CoV-2
Background
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) was primarily documented as the probable cause of acute respiratory infection in Wuhan, Hubei province of China in late December 2019 (Al-Kuraishy et al. 2021). The World Health Organization (WHO) designated this infection as coronavirus disease 2019 (COVID-19) (Attallah et al. 2021). SARS-CoV-2 utilizes angiotensin-converting enzyme type 2 (ACE2) as an entry point (Al-Kuraishy et al. 2020). Interaction of SARS-CoV-2 with ACE2 provokes a series of inflammatory changes causing cell injury and hyperinflammation (Al-Kuraishy et al. 2021). ACE2 is broadly distributed in a diverse cellular system including enterocytes, cardiomyocytes, pulmonary alveolar cells, neurons, and testes (Al-Kuraishy et al. 2021). The clinical presentation of COVID-19 is frequently asymptomatic in 85% of the cases, though 15% of cases are presented with moderate to severe forms of the disease owing to the progress of acute lung injury (ALI). Also, 5% of COVID-19 patients may be seriously affected and need ventilation due to the progression of acute respiratory distress syndrome (ARDS) (Al-Kuraishy et al. 2021).
ACE2 is a peptidase that metabolizes the vasoconstrictor angiotensin II (Ang II) to the vasodilator Ang1-7 and Ang1-9. Thus, reduction of ACE2 during SARS-CoV-2 infection persuades vasoconstriction and development of endothelial dysfunction (ED), oxidative stress (OS), and inflammatory disorders (Al-Kuraishy et al. 2021). These changes cause hypoxia and cytokine storm with the development of cardio-pulmonary complications (Al-Kuraishy et al. 2021). In reaction to diverse viral infections, pro-inflammatory, anti-inflammatory cytokines, and bioactive lipids are released from the immune cells (Onohuean et al. 2021). Bioactive lipids and other polyunsaturated fatty acids participate in a vital role in the process of viral infections including SARS-CoV-2 infection (Das 2020). One of these mediators is the anti-inflammatory lipoxin (LX).
As LXs are potent anti-inflammatory mediators, we aimed at reviewing the potential role of LXs in COVID-19 regarding the effects of LXs on the inflammatory signaling pathways which are linked with COVID-19 pathogenesis.
Characteristic of lipoxin
LX is a bioactive autocoid from arachidonic acid (AA) made by different cell types. Two types of LX are identified, LXA4 and LXB4, in addition, two epimers are recognized and they are 15-epi-LXA4 and 15-epi-LXB4 (Bäck et al. 2014). LXs were first described in 1984 by Laurate Samuelsson and his colleagues (Serhan et al. 1984). Succeeding studies confirmed that LXs had anti-inflammatory effects by resolving inflammation in different infectious and non-infectious inflammatory disorders (Hughes et al. 2017). LXs and epimers inhibit chemotaxis of neutrophils, macrophage activations, the release of pro-inflammatory cytokines, and antibody productions from B cells. As well, LXs block various ligands involved in the release of pro-inflammatory cytokines such as formyl peptide receptor (FPR) (Bai et al. 2019). LXs are generated by collaboration between 5-lipoxygenase (5-LO) and 12–15 LO during cell interactions (Fig. 1). The 15-epi-LXs are formed and designated by aspirin acetylated cyclooxygenase 2 (COX-2) in collaboration with 5-LO. The 15-epi-LXA4 is known as aspirin-triggered LX (ATL). LXA4 receptors were initially named FPR, which was renamed ALX receptors (Romano et al. 2007).
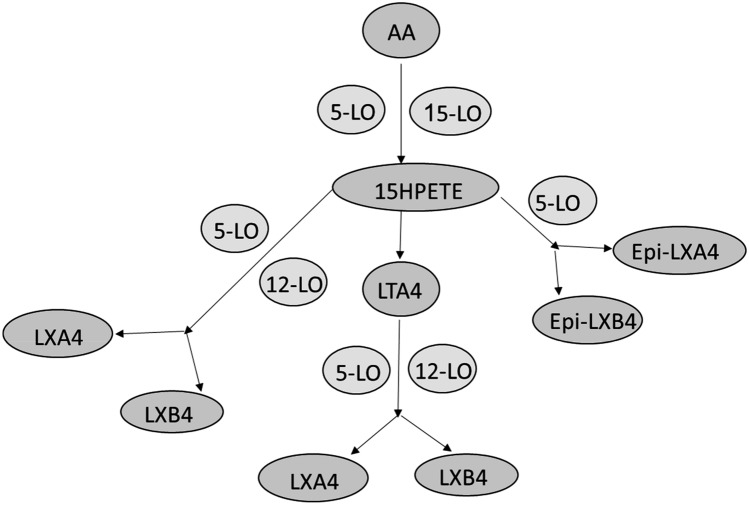
Fig. 1.
Biosynthesis and pathway of lipoxins (LXs) from arachidonic acid (AA) through lipoxygenase (LO) 5 and 15. AA is converted to 15-hydroxy peroxy eicosatetraenoic acid (15HPETE), which form LTA4 that directly forms LXA4 and LXB5. The 15HPETE can form LXA4 and LXB5 through 5/12-LO, or can form Epi-LXB4 and Epi-LXA4 through 5-LO
LXs are generated from AA by 5-LO via transcellular and unicellular biosynthetic pathways. Specifically, in the trans-cellular pathway, LXs are formed by 12-LO derived from platelet-neutrophil interaction, while in the unicellular pathway LXs are formed by 5-LO (Prieto et al. 2010). In addition, two different pathways for biosynthesis of LXs are triggered that are aspirin-triggered (AT) and statins triggered (ST) LXs. Aspirin induces acetylation of COX-2 that increases AA substrate for 5-LO, while statins increase conversion of AA to 15-LXs (Petri et al. 2017; Planagumà et al. 2010).
LXs are metabolized by macrophages 15-hydroxyprostaglandin dehydrogenase to inactive metabolites. However, 15-epi-LXA4, 15-epi-LXB4, and LX synthetic analogs are resistant to being metabolized. LXs enhance brain endocannabinoids and exert similar effects to the endogenous endocannabinoid system. LXA4 exerts a protective effect against memory impairment in animals through the activation of cannabinoid receptor 1 (CB1) (Pertwee 2012).
Moreover, specialized pro-resolving lipid mediators (SPMs) which are formed from polyunsaturated fatty acids (PUFAs) during acute and chronic inflammations, are involved in the resolution process (Bannenberg and Serhan 2010). The SPMs play a role in the reduction of neutrophil infiltration, induction of neutrophil apoptosis and/or efferocytosis, and counter-regulation of cytokines and chemokines with induction of macrophage polarization toward anti-inflammatory M2 type (Bannenberg and Serhan 2010).
Taken together, the main functions of LXs are inhibition of pro-inflammatory cytokines, activation of anti-inflammatory cytokines, activation of phagocytosis by monocytes/macrophages, and removing apoptotic neutrophils (efferocytosis), and suppression of the release of reactive oxygen species (ROS). This is in addition to increasing the production of the anti-platelet prostaglandin (prostacyclin I2) with a vasodilator effect by releasing nitric oxide (NO) (Luo et al. 2013; Miao et al. 2015). Besides, LXs promote the release of anti-inflammatory cytokines by increasing the entry of nuclear factor erythroid 2 related factors (Nrf2) which increases the production of heme oxygenase 1 (HMOX-1) (Wu et al. 2013). Further, HMOX-1 improves the synthesis of antioxidant glutathione which neutralizes ROS/peroxynitrite and prevents the oxidative stress (OS) (Wu et al. 2013).
It has been reported that LXB4 and other analogs attenuate inflammatory signaling pathways such as nuclear factor kappa B (NF-κB) and activated protein 1 (AP-1) (Liu et al. 2018). As well, LXB4 stimulates the expression of the suppressors of the cytokine signaling (SOCS) proteins. This inhibits the activation of the signal transducer and activator transcription (STAT), leading to an increase in the expression of the pro-inflammatory cytokines (Machado et al. 2006). Indeed, LXA4 can bind aryl hydrocarbon receptor (AHR) and activate nuclear xenobiotic response elements causing activation of the expression of the anti-inflammatory cytokines (Abma et al. 2020). LXs are effective against various inflammatory disorders including bacterial sepsis-induced ALI/ARDS, respiratory syncytial virus (RSV) induced-ALI, and different parasitic infections (Lee et al. 2017). As well, LXs reduce allergic reactions and inflammation in asthma by reducing leukotriene C (LTC) (Liu et al. 2021).
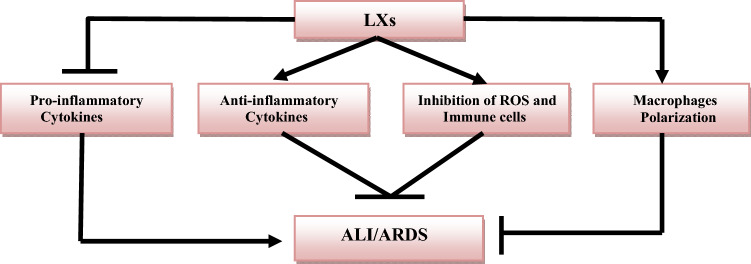
Taken together, LXs have anti-inflammatory and antioxidant effects by reducing the release of the pro-inflammatory cytokines and the generation of ROS. Therefore, LXs or LX receptor agonists could be effective against different inflammatory disorders (Fig. 2).
Fig. 2.
Role of lipoxins (LXs) in the prevention of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
Role of lipoxin in respiratory disorders
It has been reported that LXA4 can reduce pulmonary inflammation and bronchial hyper-responsiveness, so LXA4 agonists and analogs could be a new therapeutic strategy in the management of asthma (Kong et al. 2017). A pilot study involved 50 asthmatic patients treated with LXA4 methyl ester compared to inhaled bronchodilators showed that LXA4 methyl ester was more effective with fewer adverse effects compared to the standard anti-asthmatic therapy (Kong et al. 2017). Ricklefs et al. found that expression of ALX receptors is correlated with lung inflammation and asthma severity (Ricklefs et al. 2017). Besides, a case–control study comprised 22 asthmatic children compared with 22 healthy persons found that LXA4 serum level is correlated with asthmatic severity (Mohamed et al. 2020). Furthermore, LXs which reduce neutrophil recruitments are decreased in patients with cystic fibrosis compared to the controls (Karp et al. 2004). Administration of LXs analogs inhibits neutrophil-mediated inflammation, and decreases bacterial inflammation and the severity of cystic fibrosis (Karp et al. 2004). Thornton et al. illustrated that LXA4 promotes antibiotic efficacy against Pseudomonas aeruginosa by reducing the expression of virulence genes in patients with cystic fibrosis (Thornton et al. 2021).
LXs are also effective against the development of ALI and ARDS, an experimental study showed that mesenchymal cells attenuate the development of ALI in mice by activating the release of LXA4 (Fang et al. 2015). Repair of the lung alveolar structures and resolution of ALI required a balance between inflammatory molecular signals and inflammatory reactions that need an active metabolic and biochemical process (Fang et al. 2015). LXs have dual pro-resolution and anti-inflammatory activities, thereby reducing the pulmonary epithelial and endothelial permeability (Fang et al. 2015; Levy et al. 2001). LXs through interaction with ALX/FPR2 promote the alveolar fluid clearance and resolution of the inflammations in ALI (Fang et al. 2015). El-Kebir et al. revealed that 15-Epi-LXA4 enhances the resolution of ALI by inhibiting myeloperoxidase (MPO)-induced stimulation of extracellular signal-regulated kinase-mediated phosphorylation (Kebir et al. 2009). MPO is released from neutrophils and acts as a paracrine and autocrine mediator increasing MPO release and delaying the intrinsic apoptosis of neutrophils (Mayadas and Cullere 2005).
LXA4 through its anti-inflammatory action inhibits the expression of neutrophils Mac-1 and other adhesion molecules (Chiang et al. 2006). Ye et al. observed that LXA4 inhibits acute pancreatitis-induced ALI through modulation of Nrf2/HO-1 and suppression of generation of ROS-induced inflammation and development of ALI (Ye et al. 2019). Moreover, Wang et al. review study found that various anti-inflammatory mediators including LXA4 play a critical role in the enhancement of the alveolar fluid clearance in ARDS (Wang et al. 2018). A systematic review regarding the role of the inflammatory mediators in the progression of ARDS revealed that aspirin through inhibition of COX-2 increases the production of ATL, which inhibits NF-κB and production of IL-8. ATL promotes phagocytosis of apoptotic neutrophils and reduces the severity of ARDS (Toner et al. 2015).
Concerning the role of LXs in respiratory viral infections, Ciloniz et al. an experimental study observed that dysregulation of the anti-inflammatory LXA4 is associated with lethal H5N1 influenza infection in mice due to the reduction of the protective effect of LXA4 (Cilloniz et al. 2010). As well, H5N1 influenza infection induces a reduction in the expression of SOCS2, which is needed in the action of LXA4 (Russell and Schwarze 2014). Therefore, the administration of LXA4 analogs may reduce the severity of H5N1 infection. It has been shown that respiratory syncytial virus (RSV) infection in 5-LO deficient mice is exaggerated causing severe pulmonary inflammation compared with wild-type mice (Shirey et al. 2014). Treatment with LXA4 analogs in 5-LO deficient mice with RSV infection restored the body's anti-inflammatory capacity against respiratory viral infections (Shirey et al. 2014). Moreover, alveolar macrophages subjected to different viral infections produce a large amount of LXA4 (Kim 1990). Thus, a higher LXA4 concentration is linked with various respiratory viral infections and could be a surrogate biomarker in this regard. Therefore, LXA4 receptor antagonists may reduce the resolution of viral and bacterial-induced ALI (Gotts et al. 2018).
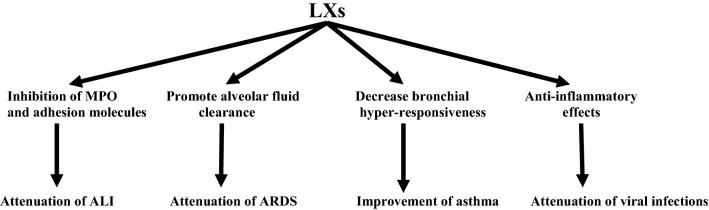
These observations support the potential role of LXs in attenuating respiratory viral infections through the modulation of anti-inflammatory response. Thus, LXA4 receptor agonists or LXA4 analogs could be novel therapeutic modalities in attenuating respiratory viral infections and their associated complications like ALI and ARDS (Fig. 3).
Fig. 3.
Protective effects of lipoxins (LXs) in respiratory disorders: LXs attenuate acute lung injury (ALI) through inhibition of neutrophil myeloperoxidase (MPO). They also attenuate acute respiratory distress syndrome (ARDS) by improving the alveolar fluid clearance and improve asthma by reducing bronchial hyper-responsiveness. Moreover, they attenuate respiratory viral infections by their anti-inflammatory effects
Role of lipoxin in SARS-CoV-2 infection
Bioactive lipids including the pro-inflammatory mediators like prostaglandins (PGs), leukotrienes (LTs), and thromboxane A2 (TXA2), and the anti-inflammatory mediators like LXs, protectin, maresins, and resolvins are formed from AA during acute inflammations and infections. These bioactive lipid mediators augment macrophage phagocytic activity and at the same time resolve the inflammatory process, and enhance microbial clearance (Norris et al. 2018). It has been shown that the bioactive lipid, LXs, and AA, can modulate SARS-CoV-2 infection through inhibition of viral entry, inhibition of viral replication, downregulation of the expression of ACE2, and suppression of the pro-inflammatory cytokines (Das 2021). Therefore, deficiency of AA and bioactive lipid mediators may augment viral infections due to interruption of the anti-inflammatory process. Pal et al. reported that the paucity of SPMs in obese patients increases the risk of SARS-CoV-2 infection (Pal et al. 2020). Thus, oral or intravenous administrations of AA and LXs could be effective in COVID-19 by increasing the resistance and recovery from SARS-CoV-2 infection (Das 2021). Lee observed that SPMs mainly LXs are regarded as a potential therapy against SARS-CoV-2 infection through modulation of the viral-inflammation circuits (Lee 2021). Regidor et al. proposed that SPMs can reduce the severity of SARS-CoV-2 infection and its associated complications including ALI, immunothrombosis, and cytokine storm via attenuating the release of the pro-inflammatory cytokines (Regidor et al. 2020). Likewise, Hammock hypothesized that SARS-CoV-2 infection is linked with endoplasmic stress and the development of eicosanoids storm due to the generation of pro-inflammatory cytokines (Hammock 2020). Therefore, anti-inflammatory drugs, via increasing the anti-inflammatory LXs, may counteract eicosanoids storm in COVID-19.
LXs and LXs receptor agonist, BML-111, can induce autophagy in lung alveolar macrophages and protect from ALI through modulation of the mitogen-activated protein kinase (MAPK) signaling pathway (Liu et al. 2018). Besides, different studies revealed a potential role of LXs in preventing ALI and ARDS (Fang et al. 2015; Levy et al. 2001; Kebir et al. 2009; Mayadas and Cullere 2005). So, LXs and LXs receptor agonists might be effective in the reduction of SARS-CoV-2 infection-induced ALI/ARDS by modulation of the hyperinflammation and inflammatory signaling pathways.
Of note, SARS-CoV-2 infection is associated with activation of various inflammatory signaling molecules including NF-κB, STAT3, MAPK, mammalian target of rapamycin (mTOR), and nod-like receptor pyrin 3 (NLPR3) inflammasome. Activation of these inflammatory molecules is linked with the development of hyperinflammation, cytokine storm, ED, OS, immunothrombosis, pulmonary thromboembolic disorders, and ALI/ARDS (Rex et al. 2021; Shah et al. 2020). Inhibition of these inflammatory signaling molecules may reduce COVID-19 severity and the associated complications. Interestingly, LXs inhibit NF-κB, AP-1, and STAT3 in different experimental studies and clinical trials (Liu et al. 2018; Abma et al. 2020). Besides, LXs block the activation of human MAPK, (Chen et al. 2014) and they can reduce inflammation by inhibiting mTOR in Kaposi sarcoma-associated herpes viral infection (Chandrasekharan and Sharma-Walia 2015). Furthermore, they can suppress the activation of NLPR3 inflammasome in chronic obstructive pulmonary disease (Cao et al. 2018). Thus, LXs may reduce the inflammatory changes induced by activation of MAPK, mTOR, and NLPR3 inflammasome in COVID-19.
In addition, SARS-CoV-2 induces a paradoxical activation of SOCS and abnormal immune response (Johnson et al. 2020). Johnson and his colleagues suggested that SOCS antagonists might be beneficial in this regard. However, using of these antagonists led to severe exacerbation of COVID-19 due to the uncontrolled release of pro-inflammatory cytokines (Johnson et al. 2020). Of note, LXB4 stimulates SOCS signaling with subsequent inhibition of STAT3-dependent release of the pro-inflammatory cytokines (Machado et al. 2006). Therefore, LXs is regarded as an endogenous inhibitor that prevents the progression of inflammatory disorders and may reduce SARS-CoV-2-induced hyperinflammation and complications.
In COVID-19, Nrf2 which inhibits the production of ROS and induces the release of the anti-inflammatory cytokines is inhibited by SARS-CoV-2 leading to OS and inflammatory disorders (Cuadrado et al. 2020). For this reason, activation of Nrf2 can decrease the inflammation, OS, and restore tissue repair in both ALI and ARDS (Cuadrado et al. 2020). Besides, SARS-CoV-2 infection, old age, and metabolic disorders are associated with low levels of stress protein in particular HMOX-1 protein. Although, exaggerated immune response and hyperinflammation induce the expression of HMOX-1 to counterbalance the inflammatory burden (Hooper 2020). Activators of HMOX-1 such as melatonin, resveratrol, statins, and curcumin can improve COVID-19 outcomes (Hooper 2020). An observational cohort study illustrated that HMOX-1 serum level was increased in patients with severe COVID-19 and correlated with low oxygen saturation (Hooper 2020). Herein, LXs, which encourage the release of the anti-inflammatory cytokines via increasing the entry of Nrf2 and inducing HMOX-1, (Wu et al. 2013) could be beneficial in the mitigation of hyperinflammation and OS in COVID-19.
Furthermore, AHR which is activated by LXs has a protective effect against lung hyper-responsiveness and injury through modulation of the cell recruitments and inflammation (Michaudel et al. 2020). Bock revealed that AHR has anti-inflammatory and pro-inflammatory roles by releasing LXs and pro-inflammatory cytokines, respectively (Bock 2020). AHR is mainly involved in the resolution of the inflammatory process through the LXs-dependent pathway (Bock 2020). However, Anderson et al., proposed that AHR is implicated in the pathogenesis of SARS-CoV-2 infection and it is linked with COVID-19 severity (Anderson et al. 2020). Therefore, AHR antagonists could be prophylactic and therapeutic agents in COVID-19 severity. This observation raises a clue about the controversy concerning the relation of AHR with LXs in COVID-19.
Indeed, LXs have a potent anti-inflammatory effect through inhibition of the expression of toll-like receptor 4 (TLR-4) and myeloid differentiation gene 88 (MyD88), which are involved in NF-κB activation and release of the pro-inflammatory cytokines (Ali et al. 2020). In COVID-19, the TLR-4/MyD88 axis is activated and associated with the release of pro-inflammatory cytokines and the development of cytokine storm (Cuevas et al. 2021). Thus, LXs through inhibition of TLR-4/MyD88, may reduce SARS-CoV-2-induced hyperinflammation and cytokine storm in COVID-19.
Also, immunothrombosis and pulmonary thromboembolic disorders are common hallmarks of COVID-19 due to ED, OS, and hyperinflammation (Loo et al. 2021). Yeung et al. illustrated that 15-LO metabolites, mainly LXs, have anti-platelet effects thereby reducing clot formation and maintaining normal body homeostasis (Yeung and Holinstat 2011). Similarly, LXs activate the release of tissue factors which inhibit the induction of clotting factors and the development of thrombosis (Maderna et al. 2000). With these findings, LXs and LXs analogs and/or receptor agonists may mitigate thrombotic events in COVID-19 and reduce the risk of pulmonary micro-thrombosis.
Remarkably, leukotrienes (LTs) are triggered during SARS-CoV-2 infection leading to pulmonary and extra-pulmonary inflammatory changes with the risk of development of cytokine storm (Al-Kuraishy et al. 2021). Al-kuraishy et al. found that LT antagonists can alleviate pulmonary and extra-pulmonary manifestations of COVID-19 (Al-Kuraishy et al. 2021). It has been reported that LXs reduce the expression of LTs and attenuate the associated inflammatory disorders (Papayianni et al. 1996). In this sense, LXs and LXs analogs can mitigate COVID-19 manifestations through suppression of the LT pathway.
Additionally, SARS-CoV-2 infection induces down-regulation of ACE2 with subsequent elevation of vasoconstrictor and pro-inflammatory AngII with reduction of the vasodilator and protective Ang1-7 and Ang1-9 (Elekhnawy and Negm 2022). High circulating AngII in COVID-19 patients triggers cardio-pulmonary complications including pulmonary thrombosis and ALI/ARDS (Elekhnawy and Negm 2022). Recently, it has been shown that LXA4 inhibits the excitatory auto-antibodies against the angiotensin 1 receptor (AT1R) of AngII and prevents the development of preeclampsia (Liu et al. 2020). Chen et al. study revealed that LX agonist BML-111 protects and prevents the occurrence of ALI and hepatic injury by reduction of ACE activity and increasing the activity of ACE2 and Ang1-7 (Chen et al. 2019). Therefore, LXs and LXs agonists through regulation of the renin-angiotensin system (RAS) can attenuate AngII-induced cardiopulmonary complications in COVID-19.
Moreover, expression of the anti-inflammatory FPRs is reduced in COVID-19 due to an unbalanced immune-inflammatory response leading to a delay in the resolution of inflammatory changes (Perretti and Godson 2020). FPRs are activated by LXs leading to anti-inflammatory effects (Ge et al. 2020). Therefore, LXs/FPRs axis may reduce SARS-CoV-2 infection-induced hyperinflammation. Besides, the anti-inflammatory effects of LXs are mediated by increasing the release of NO through activation of nitric oxide synthase (NOs) (Paul-Clark et al. 2004). In SARS-CoV-2 infection, there is severe ED and a reduction in the release of NO (Green 2020). Restoration of NO by dietary nitrate improves the endothelial function and prevents platelet-endothelial interaction and the development of thrombosis (Green 2020). In this sense, LXs analogs could be potential agents in the attenuation of ED caused by NO deficiency.
Without a doubt, LXs increase the polarization of macrophages from classical inflammatory (M1) to the alternative anti-inflammatory (M2), thereby reducing the inflammatory effect of macrophages (Li et al. 2021). In SARS-CoV-2 infection, M1 macrophages are predominant leading to an increase in the release of the pro-inflammatory cytokines and hyperinflammation (Ferraro et al. 2021).
Finally, LXs are regarded as endogenous activators of the CB1 receptor; promoting the activity of the endocannabinoid system (ECS) (Pertwee 2012). It has been shown that ECS is involved in the regulation of the immune response by inhibiting the release of the pro-inflammatory cytokines and improving the release of the anti-inflammatory cytokines (Briand-Mésange et al. 2020). In COVID-19, activation of ECS is associated with a reduction in the severity of SARS-CoV-2 infection through inhibition of the development of cytokine storm (Onaivi and Sharma 2020).
Taken together, LXs have immunomodulating effects on SARS-CoV-2 infection in COVID-19 through modulation of the immune cells and response of monocytes/macrophages phagocytic activity with inhibition of inflammatory signaling pathways. Therefore, LXs and LXs analogs could be effective in the management of COVID-19.
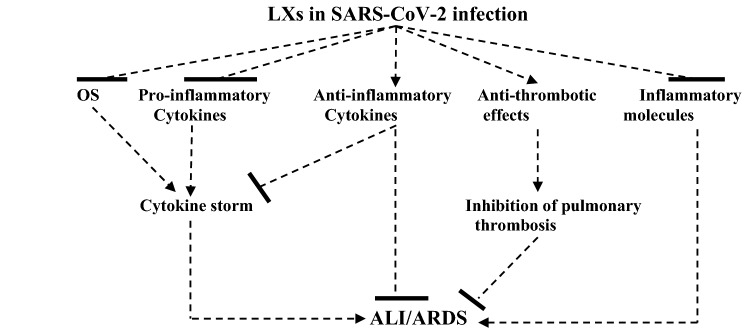
The present study had several limitations including a paucity of preclinical trials and prospective clinical studies regarding the role of LXs in COVID-19. However, this narrative review shed light on the anti-inflammatory role of LXs in COVID-19 and gave suggested mechanisms for the beneficial effect of LXs in COVID-19 (Fig. 4).
Fig. 4.
Role of lipoxin (LXs) in SARS-CoV-2 infection: LXs inhibit oxidative stress (OS), pro-inflammatory cytokines, and inflammatory molecules with anti-inflammatory and anti-thrombotic effects. These effects inhibit the development of cytokine storm and pulmonary thrombosis with subsequent suppression of the development of ALI and ARDS
Conclusions
LXs have anti-inflammatory effects by inhibiting the inflammatory signaling pathways and releasing the pro-inflammatory cytokines with augmentation of the anti-inflammatory cytokines. Taken together, LXs, LXs analogs, and LXs agonists could be effective therapeutic modalities in the management of COVID-19. These observations need to be confirmed by experimental, preclinical, and clinical studies.
Acknowledgements
The authors would like to thank Tanta university for support.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis, and writing the first draft was performed by all authors. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
All data are available in the manuscript.
Declarations
Confict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
Ali I. Al-Gareeb, Email: dr.alialgareeb78@yahoo.com
Engy Elekhnawy, Email: engy.ali@pharm.tanta.edu.eg.
Hayder M. Al-kuraishy, Email: Hayderm36@yahoo.com
References
- Abma W, Noreby M, Wheelock CE, Dahlen SE, Adner M, Säfholm J. Lipoxin A4 reduces house dust mite and TNFα-induced hyperreactivity in the mouse trachea. Prostaglandins Other Lipid Mediat. 2020;1(149):106428. doi: 10.1016/j.prostaglandins.2020.106428. [DOI] [PubMed] [Google Scholar]
- Ali M, Yang F, Jansen JA, Walboomers XF. Lipoxin suppresses inflammation via the TLR4/MyD88/NF-κB pathway in periodontal ligament cells. Oral Dis. 2020;26(2):429–438. doi: 10.1111/odi.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Al-Buhadily AK, Al-Harchan NA, Lugnier C. COVID-19 and phosphodiesterase enzyme type 5 inhibitors. J Microsc Ultrastruct. 2020;8(4):141. doi: 10.4103/JMAU.JMAU_63_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Qusty N, Cruz-Martins N, Batiha GE. Sequential doxycycline and colchicine combination therapy in COVID-19: the salutary effects. Pulm Pharmacol Ther. 2021;1(67):102008. doi: 10.1016/j.pupt.2021.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Alqarni M, Cruz-Martins N, El-Saber BG. Pleiotropic effects of tetracyclines in the management of COVID-19: emerging perspectives. Front Pharmacol. 2021;23(12):136. doi: 10.3389/fphar.2021.642822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Almulaiky YQ, Cruz-Martins N, Batiha GE. Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of Covid-19: the enigmatic entity. Eur J Pharmacol. 2021;15:174196. doi: 10.1016/j.ejphar.2021.174196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Alexiou A, Batiha GE. Niclosamide for COVID-19: bridging the gap. Mol Biol Rep. 2021;18:1–8. doi: 10.1007/s11033-021-06770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Cruz-Martins N, Batiha GE. The potential role of neopterin in COVID-19: a new perspective. Mol Cell Biochem. 2021;476(11):4161–4166. doi: 10.1007/s11010-021-04232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Carbone A, Mazzoccoli G. Aryl hydrocarbon receptor role in co-ordinating SARS-CoV-2 entry and symptomatology: linking cytotoxicity changes in COVID-19 and cancers; modulation by racial discrimination stress. Biology. 2020;9(9):249. doi: 10.3390/biology9090249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attallah NG, El-Kadem AH, Negm WA, Elekhnawy E, El-Masry TA, Elmongy EI, Altwaijry N, Alanazi AS, Al-Hamoud GA, Ragab AE. Promising antiviral activity of agrimonia pilosa phytochemicals against severe acute respiratory syndrome coronavirus 2 supported with in vivo mice study. Pharmaceuticals. 2021;14(12):1313. doi: 10.3390/ph14121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck M, Powell WS, Dahlén SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR review 7. Br J Pharmacol. 2014;171(15):3551–3574. doi: 10.1111/bph.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Wang J, He Z, Yang M, Li L, Jiang H. Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin A4 by targeting transforming growth factor β (TGF-β)/smad pathway and pro-inflammatory cytokines. Med Sci Monit. 2019;25:3069. doi: 10.12659/MSM.914860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta. 2010;1801(12):1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW. Aryl hydrocarbon receptor (AHR)-mediated inflammation and resolution: non-genomic and genomic signaling. Biochem Pharmacol. 2020;15:114220. doi: 10.1016/j.bcp.2020.114220. [DOI] [PubMed] [Google Scholar]
- Briand-Mésange F, Trudel S, Salles J, Ausseil J, Salles JP, Chap H. Possible role of adipose tissue and the endocannabinoid system in coronavirus disease 2019 pathogenesis: can rimonabant return? Obesity. 2020;28(9):1580–1581. doi: 10.1002/oby.22916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhou X, Yin Z, Yu X, Yang Q, Guo Q, Tian D, Xiong X, Xu G, Kuang X. The anti-inflammatory effect of BML-111 on COPD may be mediated by regulating NLRP3 inflammasome activation and ROS production. Prostaglandins Other Lipid Mediat. 2018;1(138):23–30. doi: 10.1016/j.prostaglandins.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan JA, Sharma-Walia N. Lipoxins: nature’s way to resolve inflammation. J Inflamm Res. 2015;8:181. doi: 10.2147/JIR.S90380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wu RF, Su L, Zhou WD, Zhu MB, Chen QH. Lipoxin A4 regulates expression of the estrogen receptor and inhibits 17β-estradiol induced p38 mitogen-activated protein kinase phosphorylation in human endometriotic stromal cells. Fertil Steril. 2014;102(1):264–271. doi: 10.1016/j.fertnstert.2014.03.029. [DOI] [PubMed] [Google Scholar]
- Chen QF, Hao H, Kuang XD, Hu QD, Huang YH, Zhou XY. BML-111, a lipoxin receptor agonist, protects against acute injury via regulating the renin angiotensin-aldosterone system. Prostaglandins Other Lipid Mediat. 2019;1(140):9–17. doi: 10.1016/j.prostaglandins.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58(3):463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Cilloniz C, Pantin-Jackwood MJ, Ni C, Goodman AG, Peng X, Proll SC, Carter VS, Rosenzweig ER, Szretter KJ, Katz JM, Korth MJ. Lethal dissemination of H5N1 influenza virus is associated with dysregulation of inflammation and lipoxin signaling in a mouse model of infection. J Virol. 2010;84(15):7613–7624. doi: 10.1128/JVI.00553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A, Pajares M, Benito C, Jiménez-Villegas J, Escoll M, Fernández-Ginés R, Yagüe AJ, Lastra D, Manda G, Rojo AI, Dinkova-Kostova AT. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol Sci. 2020 doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas AM, Clark JM, Potter JJ. Increased TLR/MyD88 signaling in patients with obesity: is there a link to COVID-19 disease severity? Int J Obes. 2021;45(5):1152–1154. doi: 10.1038/s41366-021-00768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das UN. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch Med Res. 2020;51(3):282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das UN. Bioactive lipids in COVID-19-further evidence. Arch Med Res. 2021;52(1):107–120. doi: 10.1016/j.arcmed.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, József L, Pan W, Wang L, Petasis NA, Serhan CN, Filep JG. 15-Epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180(4):311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elekhnawy E, Negm WA. The potential application of probiotics for the prevention and treatment of COVID-19. Egyptian Journal of Medical Human Genetics. 2022;23(1):1–9. doi: 10.1186/s43042-022-00252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, Matthay MA. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol. 2015;195(3):875–881. doi: 10.4049/jimmunol.1500244. [DOI] [PubMed] [Google Scholar]
- Ferraro E, Germanò M, Mollace R, Mollace V, Malara N. HIF-1, the Warburg effect, and macrophage/microglia polarization potential role in COVID-19 pathogenesis. Oxid Med Cell Longev. 2021;12:2021. doi: 10.1155/2021/8841911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Zhang S, Wang J, Xia F, Wan JB, Lu J, Ye RD. Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity. FASEB J. 2020;34(5):6920–6933. doi: 10.1096/fj.201903206R. [DOI] [PubMed] [Google Scholar]
- Gotts JE, Wu X, Croze R, Gronert K, Fang X, Chun L, Matthay MA. 2018 Antagonism of the Lipoxin A4 receptor impairs the resolution of both bacterial and viral lung injury in mice. Ind105. critical care: ventilator induced lung injury and ards-from mice to biomarkers in ards. American Thoracic Society
- Green SJ. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22(4):149. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock BD. The overlooked storm in coronavirus disease 2019 (COVID-19)? Eicosanoids. 2020;30(3):Q1. doi: 10.1016/j.ajpath.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL. COVID-19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones. 2020;25:707–710. doi: 10.1007/s12192-020-01126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EL, Becker F, Flower RJ, Buckingham JC, Gavins FN. Mast cells mediate early neutrophil recruitment and exhibit anti-inflammatory properties via the formyl peptide receptor 2/lipoxin A4 receptor. Br J Pharmacol. 2017;174(14):2393–2408. doi: 10.1111/bph.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HM, Lewin AS, Ahmed CM. SOCS, Intrinsic Virulence Factors, and Treatment of COVID-19. Front Immunol. 2020;23(11):2803. doi: 10.3389/fimmu.2020.582102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5(4):388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- Kim SJ. Elevated formation of lipoxins in viral antibody-positive rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990;3(2):113–118. doi: 10.1165/ajrcmb/3.2.113. [DOI] [PubMed] [Google Scholar]
- Kong X, Wu SH, Zhang L, Chen XQ. Pilot application of lipoxin A4 analog and lipoxin A4 receptor agonist in asthmatic children with acute episodes. Exp Ther Med. 2017;14(3):2284–2290. doi: 10.3892/etm.2017.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH. Role of specialized pro-resolving lipid mediators and their receptors in virus infection: a promising therapeutic strategy for SARS-CoV-2 cytokine storm. Arch Pharmacal Res. 2021;4:1–5. doi: 10.1007/s12272-020-01299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Nakahira K, Dalli J, Siempos II, Norris PC, Colas RA, Moon JS, Shinohara M, Hisata S, Howrylak JA, Suh GY. NLRP3 inflammasome deficiency protects against microbial sepsis via increased lipoxin B4 synthesis. Am J Respir Crit Care Med. 2017;196(6):713–726. doi: 10.1164/rccm.201604-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Li QQ, Ding DH, Wang XY, Sun YY, Wu J. Lipoxin A4 regulates microglial M1/M2 polarization after cerebral ischemia-reperfusion injury via the notch signaling pathway. Exp Neurol. 2021;1(339):113645. doi: 10.1016/j.expneurol.2021.113645. [DOI] [PubMed] [Google Scholar]
- Liu M, Chen S, Shang Y, Yao S. Aspirin-triggered lipoxin A4 attenuates lipopolysaccharide-induced acute lung injury by inhibiting activation of mitogen-activated protein kinases and NF-κB in mice. Int J Clin Exp Pathol. 2018;11(5):2570. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhou K, Liao L, Zhang T, Yang M, Sun C. Lipoxin A4 receptor agonist BML-111 induces autophagy in alveolar macrophages and protects from acute lung injury by activating MAPK signaling. Respir Res. 2018;19(1):1–1. doi: 10.1186/s12931-018-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Cheng F, Xu Q, Huang W, Wang S, Sun R, Ye D, Zhang D. Lipoxin A 4 suppresses angiotensin II type 1 receptor autoantibody in preeclampsia via modulating caspase-1. Cell Death Dis. 2020;11(1):1–7. doi: 10.1038/s41419-020-2281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wei L, He C, Chen R, Meng L. Lipoxin A4 inhibits ovalbumin-induced airway inflammation and airway remodeling in a mouse model of asthma. Chem Biol Interact. 2021;1(349):109660. doi: 10.1016/j.cbi.2021.109660. [DOI] [PubMed] [Google Scholar]
- Loo J, Spittle DA, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76(4):412–420. doi: 10.1136/thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- Luo CL, Li QQ, Chen XP, Zhang XM, Li LL, Li BX, Zhao ZQ, Tao LY. Lipoxin A4 attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013;28(1502):1. doi: 10.1016/j.brainres.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. Anti-inflammatory actions of lipoxin A 4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12(3):330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- Maderna P, Godson C, Hannify G, Murphy M, Brady HR. Influence of lipoxin A4 and other lipoxygenase-derived eicosanoids on tissue factor expression. Am J Physiol Cell Physiol. 2000;279(4):C945–C953. doi: 10.1152/ajpcell.2000.279.4.C945. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, Cullere X. Neutrophil β2 integrins: moderators of life or death decisions. Trends Immunol. 2005;26(7):388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Miao GS, Liu ZH, Wei SX, Luo JG, Fu ZJ, Sun T. Lipoxin A4 attenuates radicular pain possibly by inhibiting spinal ERK, JNK and NF-κB/p65 and cytokine signals, but not p38, in a rat model of non-compressive lumbar disc herniation. Neuroscience. 2015;6(300):10–18. doi: 10.1016/j.neuroscience.2015.04.060. [DOI] [PubMed] [Google Scholar]
- Michaudel C, Bataille F, Maillet I, Fauconnier L, Colas C, Sokol H, Straube M, Couturier-Maillard A, Dumoutier L, Van Snick J, Quesniaux VF. Ozone-induced aryl hydrocarbon receptor activation controls lung inflammation via interleukin-22 modulation. Front Immunol. 2020;25(11):144. doi: 10.3389/fimmu.2020.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed KU, Mansour SA, Abdelsalam SM, Zaki SM. Evaluation of Lipoxin A4 as a marker of severity of bronchial asthma in pediatrics in Zagazig University Hospital. Egypt J Hosp Med. 2020;81(1):1269–1274. doi: 10.21608/ejhm.2020.112317. [DOI] [Google Scholar]
- Norris PC, Arnardottir H, Sanger JM, Fichtner D, Keyes GS, Serhan CN. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot Essent Fatty Acids. 2018;1(138):81–89. doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Sharma V. Cannabis for COVID-19: can cannabinoids quell the cytokine storm? Future Sci OA. 2020 doi: 10.2144/fsoa-2020-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onohuean H, Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Batiha GE. COVID-19 and development of heart failure: mystery and truth. Naunyn Schmiedebergs Arch Pharmacol. 2021;4:1–9. doi: 10.1007/s00210-021-02147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Gowdy KM, Oestreich KJ, Beck M, Shaikh SR. Obesitydriven deficiencies of specialized pro resolving mediators may drive adverse outcomes during SARS CoV 2 infection. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol. 1996;156(6):2264–2272. [PubMed] [Google Scholar]
- Paul-Clark MJ, van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-Epi-lipoxin A4–mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200(1):69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Godson C. Formyl peptide receptor type 2 agonists to kick-start resolution pharmacology. Br J Pharmacol. 2020;177(20):4595–4600. doi: 10.1111/bph.15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Lipoxin A4 is an allosteric endocannabinoid that strengthens anandamide-induced CB1 receptor activation. Proc Natl Acad Sci. 2012;109(51):20781–20782. doi: 10.1073/pnas.1218529110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri MH, Laguna-Fernandez A, Arnardottir H, Wheelock CE, Perretti M, Hansson GK, Bäck M. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E−/− mice. Br J Pharmacol. 2017;174(22):4043–4054. doi: 10.1111/bph.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planagumà A, Pfeffer MA, Rubin G, Croze R, Uddin M, Serhan CN, Levy BD. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A 4. Mucosal Immunol. 2010;3(3):270–279. doi: 10.1038/mi.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P, Cuenca J, Través PG, Fernández-Velasco M, Martín-Sanz P, Boscá L. Lipoxin A 4 impairment of apoptotic signaling in macrophages: implication of the PI3K/Akt and the ERK/Nrf-2 defense pathways. Cell Death Differ. 2010;17(7):1179–1188. doi: 10.1038/cdd.2009.220. [DOI] [PubMed] [Google Scholar]
- Regidor PA, Santos FG, Rizo JM, Egea FM. Pro resolving inflammatory effects of the lipid mediators of omega 3 fatty acids and its implication in SARS COVID-19. Med Hypotheses. 2020;1(145):110340. doi: 10.1016/j.mehy.2020.110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex DA, Dagamajalu S, Kandasamy RK, Raju R, Prasad TS. SARS-CoV-2 signaling pathway map: a functional landscape of molecular mechanisms in COVID-19. J Cell Commun Signal. 2021;28:1–8. doi: 10.1007/s12079-021-00632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs I, Barkas I, Duvall MG, Cernadas M, Grossman NL, Israel E, Bleecker ER, Castro M, Erzurum SC, Fahy JV, Gaston BM. ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight. 2017;2:14. doi: 10.1172/jci.insight.93534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M, Recchia I, Recchiuti A. Lipoxin receptors. Sci World J. 2007;1(7):1393–1412. doi: 10.1100/tsw.2007.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CD, Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2014;141(2):166–173. doi: 10.1111/imm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun. 1984;118(3):943–949. doi: 10.1016/0006-291X(84)91486-4. [DOI] [PubMed] [Google Scholar]
- Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. Overview of immune response during SARS-CoV-2 infection: lessons from the past. Front Immunol. 2020;7(11):1949. doi: 10.3389/fimmu.2020.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey KA, Lai W, Pletneva LM, Karp CL, Divanovic S, Blanco JC, Vogel SN. Role of the lipoxygenase pathway in RSV-induced alternatively activated macrophages leading to resolution of lung pathology. Mucosal Immunol. 2014;7(3):549–557. doi: 10.1038/mi.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JM, Walker JM, Sundarasivarao PK, Spur BW, Rodriguez A, Yin K. Lipoxin A4 promotes reduction and antibiotic efficacy against pseudomonas aeruginosa biofilm. Prostaglandins Other Lipid Mediat. 2021;1(152):106505. doi: 10.1016/j.prostaglandins.2020.106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care. 2015;19(1):1–9. doi: 10.1186/s13054-015-1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yan SF, Hao Y, Jin SW. Specialized pro-resolving mediators regulate alveolar fluid clearance during acute respiratory distress syndrome. Chin Med J. 2018;131(8):982. doi: 10.4103/0366-6999.229890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Liu ZJ, Miao S, Zou LB, Cai L, Wu P, Ye DY, Wu Q, Li HH. Lipoxin A4 ameliorates cerebral ischaemia/reperfusion injury through upregulation of nuclear factor erythroid 2-related factor 2. Neurol Res. 2013;35(9):968–975. doi: 10.1179/1743132813Y.0000000242. [DOI] [PubMed] [Google Scholar]
- Ye W, Zheng C, Yu D, Zhang F, Pan R, Ni X, Shi Z, Zhang Z, Xiang Y, Sun H, Shi K. Lipoxin A4 ameliorates acute pancreatitis-associated acute lung injury through the antioxidative and anti-inflammatory effects of the Nrf2 pathway. Oxid Med Cell Longev. 2019;6:2019. doi: 10.1155/2019/2197017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J, Holinstat M. 12-Lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovasc Hematol Agents Med Chem. 2011;9(3):154–164. doi: 10.2174/187152511797037619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the manuscript.