Abstract
In clinical settings, oxygen therapy is administered to preterm neonates and to adults with acute and chronic conditions such as COVID-19, pulmonary fibrosis, sepsis, cardiac arrest, carbon monoxide poisoning, and acute heart failure. In non-clinical settings, divers and astronauts may also receive supplemental oxygen. In addition, under current standard cell culture practices, cells are maintained in atmospheric oxygen, which is several times higher than what most cells experience in vivo. In all the above scenarios, the elevated oxygen levels (hyperoxia) can lead to increased production of reactive oxygen species from mitochondria, NADPH oxidases, and other sources. This can cause cell dysfunction or death. Acute hyperoxia injury impairs various cellular functions, manifesting ultimately as physiological deficits. Chronic hyperoxia, particularly in the neonate, can disrupt development, leading to permanent deficiencies. In this review, we discuss the cellular activities and pathways affected by hyperoxia, as well as strategies that have been developed to ameliorate injury.
Graphical abstract
• Hyperoxia promotes overproduction of reactive oxygen species (ROS).
• Hyperoxia dysregulates a variety of signaling pathways, such as the Nrf2, NF-κB and MAPK pathways.
• Hyperoxia causes cell death by multiple pathways.
• Antioxidants, particularly, mitochondria-targeted antioxidants, have shown promising results as therapeutic agents against oxygen toxicity in animal models.
Keywords: Hyperoxia, Reactive oxygen species, Oxygen toxicity, Mitochondria, Cell death, Antioxidants
Introduction
Oxygen toxicity was first systematically studied in animal species in the late nineteenth century. Paul Bert demonstrated oxygen toxicity in the central nervous system (CNS), manifesting as loss of consciousness, seizures, and even death in animals exposed to hyperbaric hyperoxia (100% O2 at total pressures above 1 atm) (Bert 1878). James Lorrain Smith described pulmonary toxicity in animals exposed to normobaric hyperoxia (above 21% or 160 mm Hg, at 1 atm). Although mice, rats, and birds could tolerate moderately elevated O2 levels (~ 40%) for over a week, higher levels (~ 80%) were shown to be lethal within several days. Inspection of deceased animals revealed injury and inflammation in lungs and other tissues (Smith 1899).
In humans, exposure to hyperoxic conditions is routinely encountered in supplemental oxygen therapy administered to patients to address blood hypoxemia and tissue hypoxia in a variety of pathological conditions. In the 1950s, for example, oxygen therapy became a common practice to treat underdeveloped and underweight premature newborns (Tin and Gupta 2007). Many neonates who received oxygen therapy in the following decades suffered serious complications as a result of chronic hyperoxia exposure, including blindness and abnormal brain and lung development (Tin and Gupta 2007). More recently, oxygen therapy has been widely employed to treat patients with severe COVID-19 who have sustained significant lung injury that compromises gas exchange (Ospina-Tascón et al. 2021). Although this is acutely necessary and successfully addresses hypoxemia and tissue hypoxia, the detrimental effects of oxygen toxicity necessitate that it be administered with caution (Perrone et al. 2017). In non-clinical settings, examples of operational exposure to hyperoxia include military and recreational divers (van Ooij et al. 2016; Wingelaar et al. 2017) and astronauts (Thirsk et al. 2009). In these situations as well, the inherent toxicity of oxygen necessitates that exposure to hyperoxia be limited.
A very different and broad context in which hyperoxia is routinely encountered is mammalian cell culture (Abbas et al. 2021; Al-Ani et al. 2018). In mammals breathing atmosphere of 20–21% O2, the O2 levels in the alveolar airspace are only ~ 14% due to the dynamics of incomplete lung volume exchange and constant diffusion of O2 into the pulmonary circulation. In mammalian tissues where cells are utilizing O2 diffusing into the cell from extracellular fluid to support oxidative phosphorylation and other oxygen-requiring reactions, O2 levels are even lower, ranging from 2 to 6% (Keeley and Mann 2019). Despite this, routine cell culture is performed in incubators that regulate CO2 but not O2, conditions in which O2 equilibrates to 18–19%. Thus, cells cultured under standard conditions are in fact experiencing hyperoxia. Many cellular processes measured in “physioxia” (2–6% O2) are different compared to 18–19% O2, such as ROS production (Maddalena et al. 2017), proliferation and senescence (Packer and Fuehr 1977; Busuttil et al. 2003; Parrinello et al. 2003), mitochondrial function (Moradi et al. 2021b), and response to drugs (Yan et al. 2010; Fonseca et al. 2018; Otto-Ślusarczyk et al. 2021) and hormones (Moradi et al. 2021a). It is thus imperative to implement physiological O2 conditions in cell culture, in order to improve the quality and validity of in vitro studies and avoid artificial outcomes.
In this review, we examine the cellular pathways and mechanisms of cellular damage and physiological dysregulation associated with normobaric oxygen toxicity. The effects of hyperbaric oxygen therapy and its associated toxicity have been reviewed elsewhere (Ciarlone et al. 2019; Doolette and Mitchell 2010).
Hyperoxia drives reactive oxygen species formation
The acute toxicity associated with hyperoxia arises due to increased rates of production of ROS from molecular oxygen, which results in macromolecular damage and dysregulated signaling pathways. The production and roles of ROS in biology have been extensively reviewed elsewhere (Brand 2010; Alfadda and Sallam 2012; Zorov et al. 2014); thus here, we provide only a brief summary and then focus on the sources of excess ROS production in hyperoxia specifically. Superoxide anion (O2•–) and hydrogen peroxide (H2O2) are produced in mitochondria, endoplasmic reticulum (ER), peroxisomes, and the cytosol by enzymes including respiratory complexes (Turrens 2003), NADPH oxidases (NOX) (Bedard and Krause 2007), uncoupled nitric oxide synthase (Montezano and Touyz 2012), monoamine oxidase (Pizzinat et al. 1999), and xanthine oxidase (Battelli et al. 2016). In most instances, O2• – is the progenitor ROS, produced by the single electron reduction of molecular O2. However, it is rapidly dismuted into H2O2, either spontaneously or via enzymatic catalysis by superoxide dismutases (SOD) (McCord and Fridovich 1969). Three isoforms have been described: SOD1 in the cytosol (Crapo et al. 1992), SOD2 in the mitochondria (Weisiger and Fridovich 1973), and SOD3 in the extracellular space (Marklund 1984). H2O2 is relatively long-lived in cells and is also electrically neutral so it can diffuse across membranes. It has well-characterized roles as a signaling molecule (Sies 2017) and in pathological processes (Gough and Cotter 2011). In the presence of iron ions, H2O2 is further reduced through the Haber–Weiss and Fenton reactions to form the highly reactive and toxic hydroxyl radical (HO•) (Lipinski 2011). While O2•– and H2O2 preferentially react with Fe-S clusters and cysteine residues in proteins, (HO•) indiscriminately oxidizes lipids, proteins, and DNA (D’Autréaux and Toledano 2007). These chemical modifications can alter the composition, structure, and function of the affected molecules. H2O2 is neutralized to water by catalase, glutathione peroxidases (GPX), and thioredoxin (Kurutas 2016). Moreover, ROS can also react with nitric oxide to form reactive nitrogen species (RNS) like peroxynitrite, which further contribute to oxidative stress and disrupt nitric oxide-mediated signaling (Brown and Borutaite 2006).
Several decades ago, hyperoxia was shown to increase cyanide-resistant respiration—a proxy of ROS production—in lung slices from rats (Freeman and Crapo 1981). This has been reproduced in an isolated perfused rat lung preparation exposed to 95% O2, using an Amplex Red-based assay to detect H2O2 (Audi et al. 2018). Yusa et al. observed a similar increase in H2O2 production in brain tissue of rats exposed to 99% O2 (Yusa et al. 1987). Increased formation of ROS (O2• –, HO•, and alkyl radicals) was also observed in sheep microvascular endothelial cell suspensions exposed to 100% O2 (Sanders et al. 1993). In MCF-7 cells exposed to 95% O2 for 44 h, mitochondrial O2• – levels, reported by the mitochondrial matrix-targeted fluorescent probe MitoSOX™, were elevated relative to 20% O2 (Pinterić et al. 2018). In isolated porcine lung mitochondria, higher rates of ROS production (measured as H2O2) were observed with increasing O2 levels above 20% (Turrens et al. 1982). Thus, there is evidence from multiple orthologous approaches that hyperoxia increases rates of ROS production.
NOX enzymes are also important sources of cellular ROS in hyperoxia. Genetic ablation/silencing or pharmacological inhibition of NOX1, NOX2, or NOX4 significantly decreases ROS production and prevents injury in a wide range of hyperoxia exposure models (Parinandi et al. 2003; Zhang et al. 2003; Brueckl et al. 2006; Usatyuk et al. 2007; Pendyala et al. 2009; Carnesecchi et al. 2009; Auten et al. 2009; Chan et al. 2013; Audi et al. 2018). Maddalena et al. showed a similar effect in cell culture, where increased H2O2 production at 18% O2 versus 5% O2 could be largely abolished by pharmacological inhibition of NOX activities (Maddalena et al. 2017). Thus, there is strong evidence for the participation of NOX enzymes in ROS production and oxidative damage in hyperoxia. The relative importance of mitochondrial versus NOX-derived ROS production may depend on cell type and associated differences in mitochondrial abundance and NOX expression. In addition, other ROS-producing enzymes likely also contribute, but have been less extensively studied and may be quantitatively less important.
Hyperoxia-induced ROS oxidize lipids, DNA, and proteins
Polyunsaturated fatty acids, such as arachidonic acid and linoleic acid, are particularly vulnerable to peroxidation by ROS, which can lead to the alteration of membrane structure and function (Catalá 2009). Lipid peroxidation involves a free radical mechanism that ends in the formation of lipid hydroperoxides and smaller end-products such as 4-hydroxynoneal (4-HNE), malonyldialdehyde (MDA), and 8-isoprostane, among others (Niki 2008). Some of these subproducts damage biomolecules and harm organelle function. For example, 4-HNE and MDA can form adducts with DNA and proteins (Guéraud et al. 2010). In vivo and in vitro studies have reported increased lipid peroxidation caused by hyperoxia, as measured by the formation of MDA, 4-HNE, and 8-isoprostane (Wispe et al. 1986; Block 1988; Vacchiano and Tempel 1994; Bandali et al. 2004; D’Agostino et al. 2009). Some of these have observed morphological and structural alterations to the plasma membrane, such as blebbing, and altered fluidity (Vacchiano and Tempel 1994; Wispe et al. 1986). More comprehensive lipidomic approaches reveal extensive phospholipid species changes associated with hyperoxia. For example, in lung tissue from mice exposed to 100% O2 for 72 h and then allowed to recover for 4 days, extensive remodeling was observed in virtually all phospholipid species (Peterson et al. 2020). The enzyme peroxiredoxin 6 (Prdx6) seems to have an important role in the detoxification of lipid hydroperoxides and repair of phospholipid membranes in hyperoxia. Overexpression of Prdx6 decreased MDA production and prolonged survival of mice exposed to hyperoxia (Wang et al. 2004). In turn, Prdx6-deficient mice show a delayed recovery from lipid peroxidation following hyperoxia (Li et al. 2015a). Moreover, lungs from mice with a mutation that renders Prdx6 unable to bind phospholipids show no recovery post-hyperoxic exposure, unlike control animals (Fisher et al. 2018). Phospholipid peroxidation is a cause of cell death, mainly via ferroptosis (Sharma and Flora 2021) and apoptosis (Nakagawa 2004).
Oxidative DNA damage can occur as direct base modifications or as strand breaks. One of the main products of the former type is 8-oxo-2′-deoxyguanosine (8-oxo-dG). Elevated levels of DNA strand breaks were observed in mouse HyHEL-10 cells exposed to hyperbaric hyperoxia (Cacciuttolo et al. 1993). Agarwal and Sohal observed increased 8-oxo-dG levels in houseflies exposed to 100% O2 for 3 days (Agarwal, and Sohal 1994). Exposure of mice to 60% O2 for longer than 2 h results in a significant increase of 8-oxo-dG in lung tissue and urine samples (Kundumani-Sridharan et al. 2019). Fluorescence microscopy of lung tissue revealed that both nuclear and mitochondrial DNAs were affected by hyperoxia.
Proteins are also important targets of oxidation, with important repercussions in both physiological signaling and pathological processes. Due to its chemical reactivity and the ability of sulfur to adopt a variety of oxidation states, cysteine residues are a primary target of oxidation in hyperoxia (Paulsen and Carroll 2013). H2O2-mediated oxidation of cysteine results in the formation of distinct chemotypes such as sulfenic acids and disulfides, both of which are reversible (Poole and Nelson 2008). Reversible oxidation of cysteine residues of proteins can act as a molecular switch to upregulate or downregulate a variety of signaling pathways (Finkel 2011). However, further oxidation of sulfenic acid by ROS leads to the largely irreversible formation of sulfinic and sulfonic acids, which is associated with loss of function and degradation (Tomin et al. 2019). A thorough discussion of the mechanisms involved in redox signaling can be found elsewhere (Sies and Jones 2020; Go and Jones 2013).
Protein carbonylation is another irreversible form of oxidation that occurs mainly at aliphatic amino acid residues and has been associated with a variety of diseases (Davies 2005; Suzuki et al. 2010). Protein carbonyls are measured by their reaction with 2,4-dinitrophenylhydrazine (DNPH), producing the colored compounds hydrazones (Suzuki et al. 2010). A study by Sohal et al. was one of the first to observe protein carbonylation caused by hyperoxia (Sohal et al. 1993). They found that houseflies exposed to 100% O2 for 3 days have an increased content of protein carbonyls that persists even after recovery at room air. Mori et al. detected increased levels of protein carbonyls in astrocytes treated with hyperbaric hyperoxia (Mori et al. 2007). Interestingly, they also showed that treating healthy astrocytes with either the supernatant of astrocytes cultured on hyperbaric hyperoxia or with the protein extract from this supernatant induces neuronal cell death. Another report showed that hyperoxia increases oxidation of thiol groups, formation of lipid peroxidation products and protein adducts, and formation of bityrosines, another marker of protein oxidation (Tatarkova et al. 2011). In summary, through the redox modification of biomolecules, hyperoxia induces a variety of adaptive and pathological changes in cellular macromolecules.
Hyperoxia injury in tissues and organs
Acute toxicity of hyperoxia was observed in the earliest recorded experiments (Bert 1878; Smith 1899). O2 levels above 80% were fatal to mice within 3 days. Lung inflammation and edema were evident post-mortem. Hyperoxic acute lung injury (HALI) has now been well characterized, and pathological characteristics include damaged pulmonary capillary endothelium, alveolar type I epithelial cell death, type II epithelial cell hypertrophy, interstitial edema, neutrophil accumulation, altered surfactant production, and decreased lung compliance (Kallet and Matthay 2013; Amarelle et al. 2021).
In addition to acute injury, long-term exposure to supplemental oxygen has been well characterized. In neonates, hyperoxia interferes with lung development, leading to developmental abnormalities that persist into adulthood. Animal models, particularly murine models, have been widely used to investigate the effects of hyperoxia in the lungs of newborns. This is due to the fact that lung development in rodents during postnatal days 1–5 is similar to that of preterm newborn infants (born before the 37th week) (Berger and Bhandari 2014; O’Reilly and Thébaud 2014). During the final 6 weeks of pregnancy, antioxidant enzymes are upregulated within the developing lung in order to prepare the fetus for respiration at extrauterine O2 levels (Frank and Groseclose 1984). As such, preterm neonates lack sufficient antioxidant capacity and are more vulnerable to oxygen toxicity; this acute damage leads to long-term pathologies like bronchopulmonary dysplasia (BPD), which is characterized by inflammation, fibrosis, decreased alveolarization, disruption of the alveolar-capillary membrane, impaired surfactant production, and pulmonary microvascular dysplasia (Bhandari 2010; Wang and Dong 2018). Indeed, exposure of preterm newborn animals to hyperoxia impairs type II alveolar epithelial cell proliferation and differentiation, causes interstitial thickening, delays alveolar development, and increases immune cell reactivity (Bucher and Roberts 1981; Yee et al. 2006; Bouch et al. 2015). Many of these pathological changes persist even in adulthood.
More recent findings have revealed an important link between hyperoxia and COVID-19. The expression of the SARS-CoV-2 co-receptor TMPRSS11D, a transmembrane protease required for efficient viral entry into host cells, was found to be higher in lung tissue from experimental mice BPD models, human BPD patients, and human and mouse epithelial cells exposed to hyperoxia (Myti et al. 2020). Another study found that neonatal hyperoxia upregulates the expression of angiotensin converting enzyme 2 (ACE2) and TMPRSS2—the receptor and co-receptor of the virus, respectively—in type II alveolar epithelial cells from mice by 2 months of age and that these protein levels remained higher than in control animals even at 12 months (Yee et al. 2020). These results suggest that, although necessary to treat respiratory failure and hypoxemia, treatment with supplemental oxygen may facilitate and accelerate the SARS-CoV-2 infection cycle by upregulating the receptor and co-receptors of the virus. In addition, patients who were born preterm may be at greater risk for COVID-19.
Elevated pO2 in the alveoli drive an increase in dissolved O2 concentrations in blood, and this is rapidly communicated to all internal organs where it can cause oxidative damage and disrupt cell signaling processes. From the earliest studies of hyperoxia in animals (Bert 1878), it has been clear that the brain is highly susceptible to hyperoxia injury. This is particularly true in neonates progressing through critical developmental milestones. During embryonic brain development, neuronal migration is near complete by the 24th week of gestation; however, glial maturation continues to occur postnatally (Reich et al. 2017). In preterm animal models, hyperoxia causes neuronal cell death and disturbs glial maturation and neural connectivity in the cortex, basal ganglia, hypothalamus, striatum, hippocampus, and white matter (Felderhoff-Mueser et al. 2004, 2005; Dean et al. 2014). A proteomic study by Kaindl et al. revealed that treatment of newborn mice with hyperoxia alters the expression of proteins involved in vesicle trafficking, cell growth and differentiation, neuronal migration, and axonal arborization (Kaindl et al. 2008). Behavioral tests have revealed that postnatal exposure to hyperoxia leads to impaired motor coordination, spatiotemporal learning, and memory in rodents in their adolescence and young adulthood (Schmitz et al. 2012; Ramani et al. 2013; Serdar et al. 2016). In humans, clinical studies have reported detrimental effects of hyperoxia in patients with stroke and traumatic brain injury (Davis et al. 2009; Rincon et al. 2014).
Hyperoxia injury of the retina has been widely studied. Treatment of premature infants with supplemental oxygen leads to visual impairment and even blindness in adulthood (Saugstad 2006), a pathology termed retinopathy of prematurity (ROP). As such, experimental oxygen-induced retinopathy models have been used to investigate the mechanisms of oxygen toxicity in the retina. ROP is characterized by hyperoxia-mediated arrest of retinal vascularization, followed by hypoxia due to poor vascularization, which in turn induces vasoproliferation and subsequent retinal detachment (Hellström et al. 2013).
Heart function is also impaired in hyperoxia. In neonatal mice, sustained hyperoxia causes left ventricular dysfunction (Ramani et al. 2015). Similarly, right ventricular dysfunction has been observed in adult mice that were exposed to neonatal hyperoxia (Menon et al. 2018). Hyperoxia has been shown to alter the redox and metabolic state of mouse cardiomyocytes, impairing action potential generation, dysregulating the expression of several potassium and sodium channels, and ultimately leading to left-ventricular hypertrophy and decreased cardiac output (Panguluri et al. 2013; Chapalamadugu et al. 2015; Vysotskaya et al. 2018). In human cardiomyocytes, hyperoxia induces cell death, upregulates proinflammatory cytokines, and alters the expression of genes involved in cell-cycle regulation, metabolism, and signaling ex vivo (Hafner et al. 2017). Further, a meta-analysis revealed that heart failure patients are more susceptible to the hemodynamic effects of hyperoxia, such as cardiac output decline (Smit et al. 2018).
Virtually all organs and tissues have been shown to be targets of oxygen toxicity, though some are less well studied. Endocrine glands (Bean and Johnson 1954), the liver (Wong et al. 2001; Rogers et al. 2010; Zangl et al. 2014), the kidney (Hess and Menzel 1970), the gastrointestinal tract (Chen and Chou 2016; Liu et al. 2020; Li and Liu 2022), and adipose tissue (Soares et al. 2016) are all adversely affected by hyperoxia.
Hyperoxia directly and indirectly modulates diverse signaling pathways
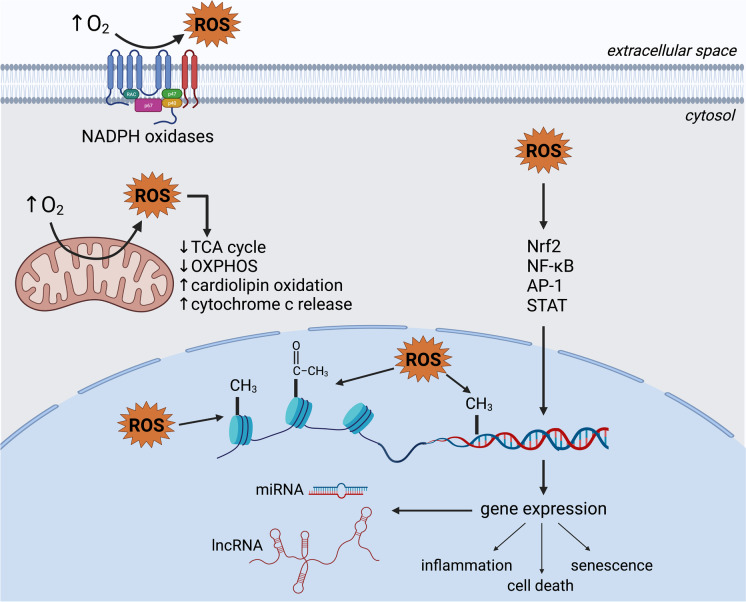
Hyperoxia compromises cellular functions via macromolecular oxidative damage and by dysregulating cellular signaling processes. A wide range of signaling pathways is altered by ROS in hyperoxia (Fig. 1). In many instances, these are regulated by the oxidation of specific cysteine residues in key proteins, which can either activate or inhibit protein activities, in either case modulating signaling (Zhang et al. 2016). A central pathway that is regulated in this way involves the nuclear factor erythroid 2-related factor 2 (Nrf2) (Bellezza et al. 2018). When activated, Nrf2 modulates the intracellular oxidative stress response by binding the antioxidant response element (5′-TGACXXXGC-3′) of genes encoding antioxidant enzymes, enzymes involved in glutathione metabolism, and enzymes involved in the generation of reducing equivalents (Tonelli et al. 2018). The transcriptional activity of Nrf2 is regulated by its physical interaction with the cytosolic Kelch-like ECH-associated protein 1 (Keap1), which facilitates Nrf2’s proteasomal degradation under normal oxidative conditions. Keap1 binding of Nrf2 is inhibited when excessive cytosolic ROS modify key sensor cysteines in Keap1 (Baird and Yamamoto 2020), leading to Nrf2’s escape of degradation, translocation to the nucleus, and promotion of transcriptional activity on target genes.
Fig. 1.
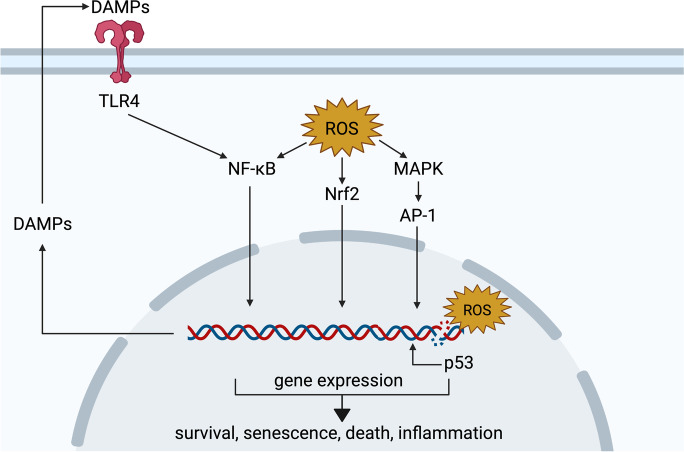
Signaling pathways affected in hyperoxia. Excessive ROS modulate intracellular signaling, including via nuclear factor erythroid 2-related factor 2 (Nrf2), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and mitogen-activated protein kinase (MAPK) pathways. In parallel, oxidative damage to DNA activates p53, which induces the transcription of target genes. In turn, damage-associated molecular patterns (DAMPs) upregulated by these pathways are released into the extracellular space, where they can bind receptors such as the toll-like receptor 4 (TLR4) and further activate the NF-κB pathway. Signaling events orchestrated by these and other pathways determine the outcome of hyperoxia-mediated oxidative stress and may include cell survival, senescence, death, and inflammation. Created with BioRender.com
The Nrf2-Keap1 system appears to play an important role in hyperoxia. As early as 2002, genome-wide genetic linkage analysis had identified Nrf2 as a critical gene in the response to hyperoxia in two strains of laboratory mice (Cho et al. 2002b). Exposure of mice to hyperoxia (95–98% O2) significantly increased Nrf2 mRNA levels and DNA-binding activity, with concomitant increases in mRNA levels of target genes (Cho et al. 2002a). Supporting this finding, Nrf2−/− mice were subsequently shown to be susceptible to lung injury resulting from exposure to 95–98% O2. Similar results have been reported by Reddy et al. and Cho et al. (Reddy et al. 2009; Cho et al. 2012). In both studies, Nrf2 knockout mice showed increased lung injury and inflammation, while failing to recover normally upon return to normoxia. In contrast, increasing Nrf2 activity by reducing Keap1 protein levels offers protection against the injurious effects of hyperoxia (Tamatam et al. 2020). Taken together, these studies indicate that the Nrf2-Keap1 pathway plays a key role in the response to oxygen toxicity, reducing tissue injury and improving recovery.
ROS also promotes the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a transcription factor broadly involved in immune and inflammatory responses, via direct oxidation of regulatory cysteine residues (Lingappan 2018). However, the relationship between NF-κB and ROS is complex and bidirectional. Similar to Nrf2, under normal conditions, NF-κB remains bound by regulatory IκB proteins in the cytosol, preventing its translocation, DNA binding, and transcription regulation. Oxidative modifications to specific amino acids in IKB kinase (IKK) and IκBα can lead to NF-κB activation. NF-κB can induce the transcription of both antioxidant and pro-oxidant genes, suggesting a possible role in cell survival under oxidative stress (Morgan and Liu 2011); however, there is conflicting evidence regarding whether hyperoxia-mediated NF-κB activation is deleterious (Wright et al. 2010; Zara et al. 2013; Chou and Chen 2020; Li et al. 2020) or beneficial (Barazzone-Argiroffo et al. 2003; Franek et al. 2004; Yang et al. 2004; McKenna et al. 2014; Michaelis et al. 2014). This is likely due to the numerous genes regulated by NF-κB and to the multiplicity of NF-κB protein subunits and regulators that can be activated or inactivated through distinct pathways depending on the tissue and cell type.
Hyperoxia also appears to impact the Janus kinase-signal transducer and activator of transcription (Jak-STAT) pathway. STAT-induced gene expression regulates biological processes like proliferation, survival, and inflammation (Hu et al. 2021). Some evidence suggests a beneficial role of STAT proteins in hyperoxia through the induction of the protective enzyme heme oxygenase-1 (HO-1) and the inhibition of matrix metalloproteinase (MMP) upregulation (Lee et al. 2000; Lian et al. 2005). Likewise, deletion of STAT3 increased lung injury and alveolar capillary leak in mice exposed to hyperoxia (Hokuto et al. 2004). However, STAT3 activation in hyperoxic rats has also been linked to the pathogenesis of retinopathy of prematurity (Byfield et al. 2009; Ren et al. 2021).
Oxidative DNA damage driven by hyperoxia triggers activation and upregulation of p53, which orchestrates cell cycle arrest, decreased proliferation, senescence, or cell death (O’Reilly et al. 1998; Shenberger and Dixon 1999; Das et al. 2004; Das and Dashnamoorthy 2004; Maniscalco et al. 2005; Klimova et al. 2009; Parikh et al. 2019; You et al. 2019; Scaffa et al. 2021). Ataxia telangiectasia-mutated (ATM) and ATM-and-Rad3 related (ATR) are two kinases that are activated by damaged DNA. Both have also been shown to activate p53 in hyperoxia (Das and Dashnamoorthy 2004; Das et al. 2004; Kulkarni and Das 2008; Resseguie et al. 2015). Upregulation of p21 in hyperoxia and subsequent cell cycle arrest and induction of senescence has been demonstrated in multiple studies (Mcgrath 1998; Shenberger and Dixon 1999; Rancourt et al. 2001; Nyunoya et al. 2003; Das et al. 2004; Das and Dashnamoorthy 2004; Londhe et al. 2011; You et al. 2019; Parikh et al. 2019).
Receptors involved in proinflammatory pathways, such as toll-like receptor-4 (TLR4), are implicated in the inflammatory phase of hyperoxia injury. TLR4 can be activated by cytokines produced in a paracrine or autocrine fashion, and by damage-associated molecular patterns (DAMPs) like high mobility group box 1 (HMGB1) released by damaged cells. Indeed, TLR4 activation in hyperoxia promotes cell death and inflammation through the NF-κB-mediated induction of proinflammatory cytokines IL-6, IL-8, and TNF-α (Ogawa et al. 2007; Liu et al. 2015; Huang et al. 2016a). Contrastingly, TLR4 activation has also been associated with survival and protection against hyperoxia. Zhang et al. reported that TLR4-deficient mice are more susceptible to apoptosis induced by hyperoxia (Zhang et al. 2005). In another study with TLR4-deficient mice, reconstitution of endothelial TLR4 prolonged the survival of TLR4-KO animals post-hyperoxia (Takyar et al. 2016).
DAMPs are often upregulated by oxidative stress-mediated transcription factors (e.g., NF-κB) and are subsequently released into the extracellular space where they can activate pattern recognition receptors in immune cells and in the same cell, resulting in a positive feedback loop that further exacerbates damage (Roh and Sohn 2018). Accumulation of extracellular HMGB1 has been observed in hyperoxic mice, where treatment with neutralizing HMGB1 antibodies attenuated pulmonary edema, structural alterations, and inflammation (Entezari et al. 2014; Yu et al. 2016).
The mitogen-activated protein kinase (MAPK) pathway leading to the transcriptional response mediated by activator protein-1 (AP-1) has also been implicated in hyperoxia-induced signaling. It has been demonstrated that apoptosis signal-regulating kinase 1 (ASK1), upstream activator of Jun N-terminal kinase (JNK) and p38, is activated upon oxidation of specific cysteine residues (Nadeau et al. 2009, 2007). In hyperoxia, extracellular signal-regulated kinase 1/2 (ERK1/2) has been mostly associated with survival and protective effects, while p38 and JNK activation has been mainly linked to cell death and injury (reviewed by Porzionato et al. 2015).
In conclusion, hyperoxia affects cellular signaling via a wide range of pathways resulting in numerous changes in cell behavior. This arises due to the preponderance of individual interactions of ROS with these signaling pathways and their interconnectedness. These wide-ranging effects likely underlie the observed impacts of hyperoxia on growth and development.
Hyperoxia drives cellular senescence
Replicative senescence is caused by DNA damage and/or telomere shortening leading to the induction of cell cycle arrest pathways and permanent exit from the cell cycle. Its initiation is regulated by the transcription factors p53, p21, and retinoblastoma protein (pRb) (Kumari and Jat 2021). Hyperoxia has been characterized as an initiator of cell cycle arrest and cellular senescence in mammalian cell lines. Early studies performed by Balin et al. showed that elevated O2 levels shortened the replicative lifespan of WI-38 human fibroblasts (Balin et al. 1977). It was later observed that cell culture in 40% O2 causes telomere shortening in these cells, halting their proliferation at the G1 phase (von Zglinicki et al. 1995). Saretzki et al. demonstrated the elevation of gene expression markers of senescence in BJ human neonatal foreskin fibroblasts exposed to 40% O2 for 4–6 weeks (Saretzki et al. 1998).
The activity of senescence associated (SA)-β-galactosidase, a well-recognized downstream senescence marker (Lee et al. 2006), is elevated in retinal pigment epithelial (RPE) cells cultured at 40% O2 (Honda et al. 2002). Parikh et al. also observed elevated SA-β-galactosidase activity, accompanied by increased p21, pRb, phosphorylated p53 levels, and DNA damage markers, in human fetal airway smooth muscle cells exposed to 40% O2 for 7 days (Parikh et al. 2019). Similarly, primary human fetal lung fibroblasts cultured at 40% O2 for 7 days showed increased SA-β-galactosidase activity, DNA damage markers, and G2/M phase arrest, along with upregulation of p21 and p53. Additionally, hyperoxic fibroblasts had elevated expression of proinflammatory and profibrotic factors, as measured by RT-PCR (You et al. 2019). As mentioned above, standard cell culture conditions of 18–19% O2 are hyperoxic relative to in vivo physioxia (2–6%). Parrinello et al. demonstrated that the onset of premature replicative senescence in mouse embryo fibroblasts cultured at 18% O2 occurred much earlier than in 3% O2 (Parrinello et al. 2003). This was associated with accelerated accumulation of DNA damage and mutations (Busuttil et al. 2003) and further exacerbated by knockout of DNA repair genes. Thus, hyperoxia drives replicative senescence in various cell types, apparently via the accumulation of DNA damage and mutations.
Mitochondria are key targets of oxygen toxicity
Mitochondrial respiration is both an important source of ROS in hyperoxia and a main target. Consistently, cells exposed to elevated O2 levels exhibit reduced rates of respiration (Das 2013; Hals et al. 2017; Pinterić et al. 2018; Schoonen et al. 1990). Respiration-deficient (ρ°) HeLa cells tolerate hyperoxic conditions that are otherwise toxic to their wild-type counterparts. Moreover, the toxicity of hyperoxia is restored when these cells are repopulated with respiratory-competent mitochondria (Li et al. 2004). Similarly, uncoupling HeLa cell respiration with carbonyl cyanide m-chlorophenyl hydrazone (CCCP), thus lowering membrane potential, reduces mitochondrial ROS production rates in hyperoxia and confers tolerance to such conditions (Li et al. 2004).
Inhibition of oxidative phosphorylation in hyperoxia is associated with inhibition of several key enzymes at high O2 levels. For example, pyruvate dehydrogenase (PDH) complex activity is reduced in lungs of mice and rats exposed to 95–100% O2 (Kimura et al. 1983; Tanaka et al. 2020). Inactivation of PDH complex limits pyruvate oxidation in the tricarboxylic acid (TCA) cycle and is in many cases partially compensated for by increased glycolytic rates (Tanaka et al. 2020). α-Ketoglutarate dehydrogenase (α-KGDH) is similarly inhibited by hyperoxia. In HeLa cells exposed to 98% O2 for 4 days, α-KGDH activity was almost completely abolished, and this was accompanied by a fall in glutamine/glutamate utilization (Schoonen et al. 1990). The TCA cycle enzyme aconitase, which catalyzes the isomerization of citrate to isocitrate, is particularly sensitive to inhibition by O2• –. Inactivation of mitochondrial aconitase in hyperoxia has been reported both in vivo and in vitro (Gardner et al. 1994; Morton et al. 1998). Additionally, hyperoxia inhibits the activities of mitochondrial respiratory complexes I and II (Das 2013; Schoonen et al. 1990). Proteomic analysis of hippocampal tissue of neonatal mice that were exposed to 85% O2 until postnatal day 12 showed altered expression of subunits of several respiratory complexes, along with decreased ATP-linked oxygen consumption, even at 14 weeks of age—approximately 12 weeks after mice were returned to normoxia (Ramani et al. 2019). Thus, short-term exposure to hyperoxia both acutely and chronically inhibits mitochondrial bioenergetic function. The inhibition of mitochondrial bioenergetic function by hyperoxia results in mitochondrial dysfunction, evidenced by a reduced membrane potential (Audi et al. 2022) and increased fission of mitochondrial networks (Ma et al. 2018).
Mitochondrial DNA (mtDNA) is localized to the matrix (Richter 1995), which makes it proximal to several sources of excess ROS production during hyperoxia. Perhaps not surprisingly, increased levels of mtDNA oxidative damage are observed with hyperoxia exposure. Roper et al. reported elevated mtDNA oxidative damage in lung epithelial cells of mice breathing a 100% O2 atmosphere for up to 72 h (Roper et al. 2004). In experiments with isolated mouse lung epithelial cells in culture, exposure to 60% O2 for 24 h increased 8-oxo-dG by over fivefold (Kundumani-Sridharan et al. 2019). Treatment of rat lungs with a mitochondria-targeted DNA repair enzyme, endonuclease III, protects against hyperoxia-induced mtDNA damage ex vivo (Gebb et al. 2013). Similarly, Kim et al. reported that overexpression of another DNA repair enzyme, mitochondrial 8-oxoguanine DNA glycosylase (mt-OGG1), reduces oxidant-induced mtDNA lesions and apoptosis in alveolar epithelial cells exposed to hyperoxia in vitro (Kim et al. 2014). Effects on non-lung tissues have also been reported. For example, rats housed in 60% O2 for 21 days developed cataracts coincidentally with elevated mtDNA damage in lens tissue (Zhang et al. 2010). It is unclear the extent to which mtDNA damage and mutations can explain the phenotypic response to hyperoxia observed in vivo, since cells have a relatively high tolerance to mtDNA damage (Chomyn et al. 1992; Miyabayashi et al. 1992; Carelli and Chan 2014).
Cardiolipin (CL) is another mitochondrial target of hyperoxia. CL is a unique phospholipid with two phosphate groups and four acyl chains that in eukaryotic organisms is exclusively located in the mitochondria, mainly in the inner mitochondrial membrane (IMM). CL has an essential role in the stability of respiratory chain supercomplexes (often termed as respirosomes) (Pfeiffer et al. 2003) and dimerization of ATP synthase (Acehan et al. 2011). CL also interacts with cytochrome c (cyt c) on the outer surface of the IMM. ROS-mediated oxidation of CL causes its dissociation from cyt c, which then leads to the release of cyt c into the cytosol (Kagan et al. 2005; Shidoji et al. 1999; Polyak et al. 1997; Ott et al. 2002), a hallmark of apoptosis. Treatment of human lens epithelial B-3 (HLE B-3) cells with 80% O2 for 48 h causes a reduction in CL content (Huang et al. 2006). An oxidative lipidomics study by mass spectrometry revealed CL peroxidation in mouse endothelial lung cells subjected to 72-h hyperoxia (95–100% O2) both in vivo and in vitro. Lipid peroxidation was accompanied by apoptosis, measured by caspases 3 and 7 activity and TUNEL assay (Tyurina et al. 2010). It is evident that hyperoxia-mediated CL oxidation is a major trigger of mitochondrial toxicity and cell death. Further mechanistic details will be explored in the next section.
The uncoupling proteins UCP2 and UCP3 have been studied in the context of hyperoxia. These IMM proteins produce a relatively minor loss of membrane potential associated with decreased rates of ROS production in some experimental models. UCP3 overexpression in mouse C2C12 myotubes exposed to hyperoxia ameliorates protein carbonylation levels (Barreiro et al. 2009). Similarly, reduced UCP2 expression in MLE-12 cells and lungs from mice exposed to hyperoxia is accompanied by enhanced O2• – production and alveolar epithelial apoptosis. These effects were abrogated by thioredoxin overexpression, which upregulated UCP2 via PGC-1α. This latter study also showed increased hyperoxic lung injury in UCP2-deficient mice (Raghavan et al. 2022). Together, these findings support the notion that UCP2/3 might decrease mROS production rates, providing additional targets for possible therapeutic intervention.
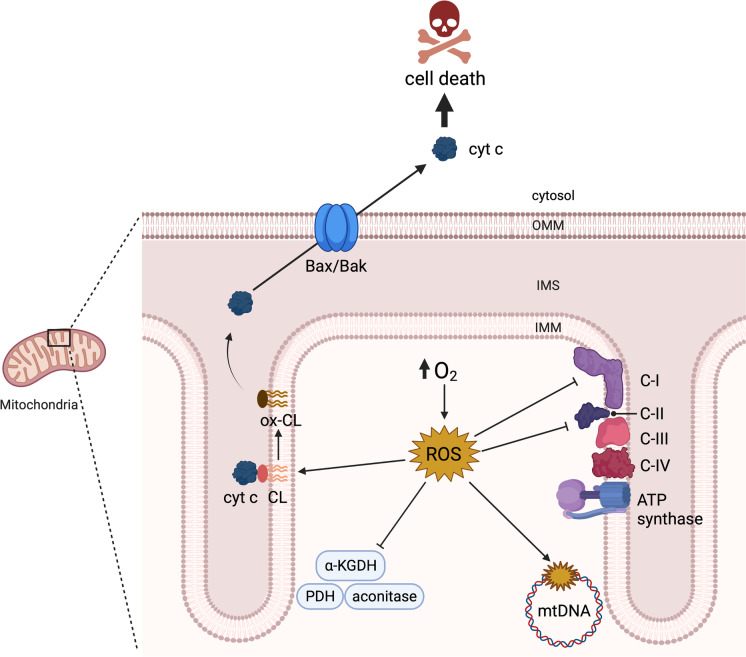
In summary, hyperoxia targets mitochondrial bioenergetics and function, mediates CL and mtDNA damage, and ultimately promotes the mitochondrial pathway of apoptotic cell death (Fig. 2).
Fig. 2.
Mitochondrial targets of hyperoxia-mediated injury. Hyperoxia drives the over production of mitochondrial reactive oxygen species (ROS), which inhibit metabolic enzymes such as aconitase, α-ketoglutarate dehydrogenase (α-KGDH), and pyruvate dehydrogenase (PDH), and respiratory complexes I and II, leading to bioenergetic failure. mROS oxidize mitochondrial DNA (mtDNA) and cardiolipin (CL), further promoting dysfunction and leading to the release of cytochrome c (cyt c) into the cytosol through Bcl-2-associated X protein/Bcl-2 homologous antagonist killer (Bax/Bak) oligomers to instigate apoptosis. Created with BioRender.com
Hyperoxia causes cell death via multiple pathways
Cell death is associated with oxygen toxicity. It occurs via multiple pathways, including apoptosis, necrosis, necroptosis, pyroptosis, and ferroptosis. Due to the complexity of cell injury caused by hyperoxia, there is no consensus regarding the relative contributions of each in hyperoxia, and they likely vary according to the experimental model used. A key event in the intrinsic pathway of apoptosis is the release of cyt c and other proapoptotic factors from the mitochondrial inter-membrane space into the cytosol, which occurs through two main mechanisms (reviewed by Garrido et al. 2006).
One mechanism is the permeabilization of the outer mitochondrial membrane (OMM) through the oligomerization of the proteins Bax and Bak. Apoptotic stimuli cause the cytosolic protein Bax to localize to the OMM, where it forms oligomers with itself and with the constitutively present Bak. Bax and Bak oligomers are then responsible for the release of proapoptotic factors into the cytosol. Activation and oligomerization of Bax/Bak is influenced by oxidative stress, seemingly via Cys-62 oxidation of Bax promoting its translocation to mitochondria (D’Alessio et al. 2005; Nie et al. 2008). Oxidation of Bax/Bak has not yet been demonstrated in a hyperoxic model, but the relationship between these proteins and excessive ROS production is clear. Treatment of rat alveolar epithelial cells with SOD/catalase mimetic EUK-134 prevented Bax activation, cyt c release, and apoptosis after hyperoxic exposure, indicating that ROS production occurs upstream of Bax activation in this model (Buccellato et al. 2004). Budinger et al. demonstrated that deficiency of Bax and Bak can protect against hyperoxia-induced apoptosis. There is thus a clear role of Bax/Bak in hyperoxia-mediated apoptotic cell death (Budinger et al. 2011).
A second mechanism underlying the intrinsic apoptosis pathway is the opening of the mitochondrial permeability transition pore (mPTP), which leads to mitochondrial swelling and rupture to release pro-apoptotic factors including cyt c (reviewed by Bernardi and di Lisa 2015; Bernardi et al. 2021). Oxidative stress is a key factor inducing mitochondrial permeability transition. mPTP opening is triggered by thiol oxidants and can be prevented by thiol reductants (Fagian et al. 1990; Lenartowicz et al. 1991; Valle et al. 1993; Bernardes et al. 1994; Kowaltowski et al. 1996). Addition of catalase, lipid peroxide inhibitors, iron chelators, and dietary and synthetic antioxidants similarly protect against mPTP opening (Castilho et al. 1995; Kowaltowski et al. 1996; Singh et al. 2013; Daniel et al. 2018; Teixeira et al. 2018; Baburina et al. 2019). Moreover, oxidative modifications to specific cysteine residues of the adenine nucleotide translocator (ANT) (Costantini et al. 2000; McStay et al. 2002), cyclophilin D (CypD) (Nguyen et al. 2011), and the oligomycin sensitivity conferral protein (OSCP, a subunit of ATP synthase complex) (Carraro et al. 2020) seem to play a crucial role in mPTP assembly and opening. mPTP-mediated cell death has been linked to hyperoxic injury (Pagano et al. 2004). Cyt c release and mitochondrial swelling were observed in epithelial alveolar cells from mice exposed to 100% O2 for 72 h in vivo. These changes were prevented by treatment with cyclosporine A (CsA), an inhibitor of mPTP opening. Thus, there is strong evidence hyperoxia causes cell death via the intrinsic apoptotic pathway, involving a variety of mechanisms.
Hyperoxia can also cause cell death through the extrinsic apoptosis pathway mediated through interaction of extracellular signaling molecules with cell death receptors such as Fas and CD40. Increased lung tissue Fas expression is seen in murine models of oxygen toxicity in vivo and in vitro (Barazzone et al. 1998; de Paepe et al. 2005). Similarly, protein and mRNA levels of Fas, FasL, and Fas-associated death domain (FADD) are increased in brain tissues from rats exposed to 80% O2. In this study, trafficking of the Fas receptor to the plasma membrane was found to be increased in hyperoxia in vitro. In turn, lack of functional Fas receptors in mice provided neuroprotection against hyperoxic injury (Dzietko et al. 2008). Conversely, there is also evidence indicating that the extrinsic pathway is not required for hyperoxia-induced cell death, as genetic ablation of Fas and CD40 do not confer protection against lung injury in murine models (Barazzone et al. 1998; Barazzone Argiroffo et al. 2002).
A recent study by Tong et al. found that hyperoxia induces a non-canonical type of apoptotic cell death via ER stress (Tong et al. 2021). ER stress induces the unfolded protein response (UPR). Inositol-requiring enzyme 1α (IRE1α), one of the most conserved UPR signaling proteins, induces activation of the transcription factor X-box binding protein 1 (XBP1), which in turn induces the expression of ER chaperones and protein degradation components (Chen and Brandizzi 2013; Gong et al. 2017). However, overactivation of the UPR results in IRE1α-mediated JNK activation, which can promote both intrinsic and extrinsic apoptotic pathways (Verma and Datta 2012). Tong et al. showed that prolonged exposure (7–14 days) of newborn rats to hyperoxia caused phosphorylation of IRE1α and JNK in lung tissues, indicating an involvement of the ER stress-associated apoptosis. There is also evidence of the activation of the ER stress-related caspase-12 (Lamkanfi et al. 2004) in murine hyperoxic models (Zhang et al. 2013; Huang et al. 2016b). Furthermore, treatment with ER-stress inhibitor 4-phenyl butyric acid protects against hyperoxic-cell death both in vivo and in vitro (Pao et al. 2021). Recent evidence showed role of the mitochondrial protein A-kinase anchoring protein 1 (Akap1) and ER stress in protection against hyperoxia; deletion of Akap1 resulted in increased ER stress-associated cell death (Sidramagowda Patil et al. 2022).
In many studies of hyperoxia, cell death resembles necrosis. Kazzaz et al. did not observe markers of apoptosis in A549 human lung adenocarcinoma cells cultured at 95% O2 for 7 days (Kazzaz et al. 1996). Rather, morphological features of these cells were typical of necrotic cell death, including swelling and enlarged nuclei and mitochondria. However, this study did show apoptotic cell death in lungs from mice exposed to 100% O2 for 48 h in vivo. Although contrasting, these results are not necessarily surprising. A direct exposure of cultured cells to 95% O2 represents a more severe hyperoxia (perhaps 20 × physioxia) than would be experienced by alveolar cells of mice breathing 100% O2 (approximately 5 × greater than in normoxia). Further, in this study, A549 cells were exposed to hyperoxia for 7 days, while animals were exposed for only 48 h.
Necroptosis is a form of programmed cell death that shares characteristics of both apoptosis and necrosis. In hyperoxia, the expression of receptor-interacting proteins (RIP) 1 and 3—key proteins in this pathway—was increased in the bronchoalveolar lavage fluid of rats exposed to hyperbaric hyperoxia (100% O2 at 1875 mmHg) for 6 h. Inhibition of necroptosis by the RIP1 inhibitor necrostatin-1 and the ROS scavenger edaravone protected the animals against lung pathology (Han et al. 2018). Pyroptosis is a form of programmed necrotic and inflammatory cell death that appears to participate in hyperoxic injury. There is an increasing amount of recent evidence showing that inhibition of the pyroptosis pathway, either by pharmacologically or genetically targeting the inflammasome formation, or other elements of the pathway, protects against hyperoxic injury (Fukumoto et al. 2013; Galam et al. 2016; Zhang et al. 2017b; Dapaah-Siakwan et al. 2019; Mendha et al. 2021; Wang et al. 2022a). A more recently described form of cell death is ferroptosis which, like pyroptosis and necroptosis, is a regulated cell death with necrotic phenotype. It is characterized by, among other things, lipid peroxidation (Cao and Dixon 2016), leading to loss of membrane integrity and rupture of the cell (Jiang et al. 2021). Two murine models have reported the involvement of ferroptosis in lung injury induced by hyperoxia (Jia et al. 2021; Chou and Chen 2022).
Taken together, the data indicate that multiple modes of cell death are involved in hyperoxic injury. The relative importance of any single pathway is likely dependent, in part, on the severity and duration of hyperoxia exposure. More research is needed to better understand these relationships.
Epigenetic responses to hyperoxia
Effects of hyperoxia exposure during the neonatal period can last into adulthood, suggesting that long-lasting epigenetic alterations might be involved. Epigenetic regulation of gene expression occurs via DNA methylation, covalent histone modifications, and the expression of non-coding RNAs (Aguilera et al. 2010). All these mechanisms are known to be affected by oxidative stress (reviewed by García-Guede et al. 2020). Unsurprisingly, both aberrant DNA methylation (Panayiotidis et al. 2004; Zhu et al. 2015; Chen et al. 2017; Bik-Multanowski et al. 2018) and histone modification have been identified in experiments with hyperoxia (Londhe et al. 2011; Zhu et al. 2015; Coarfa et al. 2020).
Noncoding RNA molecules, including miRNAs (19–25 nucleotides) and long non-coding RNAs (lncRNAs; > 200 nucleotides) are also affected by hyperoxia. miRNA molecules regulate gene expression by silencing specific mRNAs. On the other hand, lncRNAs are a highly heterogenous class of RNAs that act through a wide variety of mechanisms. For instance, lncRNA can bind specific miRNAs and inhibit their function by “sponging” them (reviewed by Panni et al. 2020). Using miRNA microarray, Zhang et al. identified 21 miRNAs that are differentially expressed in lungs from neonatal mice exposed to hyperoxia versus control mice (Zhang et al. 2011). Similarly, Bao et al. identified approximately 2000 differentially expressed lncRNAs in newborn mice exposed to hyperoxia compared to wild-type mice (Bao et al. 2016). Many subsequent studies have shown the roles of different lncRNA and miRNA molecules in hyperoxia (Table 1).
Table 1.
Evidence of the roles of noncoding RNA molecules in hyperoxia
| RNA molecule | Type of RNA | Hyperoxic model | Outcome | Reference |
|---|---|---|---|---|
| miR-150 | miRNA | Lung injury/newborn mice/95% O2/3–10 days |
• Downregulated in hyperoxia • KO decreased lung injury |
(Narasaraju et al. 2015) |
| Lung injury/primary mouse lung epithelial cells, BEAS-2B and A549 cells/95% O2/12–72 h | • Cytoprotective effect | (Zhang et al. 2017a) | ||
| miR-876-3p | miRNA |
Lung injury/newborn mice/85–100% O2/4–14 days Normal human bronchial epithelial/85% O2/24 h |
• Downregulated in hyperoxia • Predicted protective effect against injury |
(Lal et al. 2018) |
| miR-16 | miRNA | Lung injury/isolated T2AECs/60% O2 /24 h |
• Downregulated in hyperoxia • miR-16 mimics inhibited apoptosis and the TGF‐β/Smad2 and Jak/STAT3 pathways |
(Li et al. 2018) |
| miR-34a | miRNA |
Lung injury/newborn mice/100% O2/4 or 7 days Isolated T2AECs and MLE-12 cells/40–95% O2/4–48 h |
• Silencing ameliorated apoptosis in vitro and in vivo • Overexpression aggravated injury |
(Syed et al. 2017) |
|
Lung injury/newborn mice/85% O2/14 days MLg cells/85% O2/unspecified duration |
• Upregulated in hyperoxia • Deletion protected against injury |
(Ruiz-Camp et al. 2019) | ||
| miR-17 | miRNA | Lung injury/newborn mice/70% O2/4–14 days |
• Downregulated in hyperoxia • Downregulated STAT3 • Upregulation relieved pulmonary injury |
(Zhang et al. 2020) |
| Lung injury/newborn mice/85% O2/14 days |
• Downregulated in hyperoxia • Downregulation was associated with lung injury |
(Wang et al. 2020) | ||
| miR-185-5p | miRNA |
Lung injury/mice/95% O2/24–72 h MLE-15 cells/95% O2/24–48 h |
• Upregulated in hyperoxia • Upregulated RIP1 and RIP3 • Induced necroptosis and apoptosis |
(Carnino et al. 2020) |
| miR-96 | miRNA |
OIR/newborn rats/cycling 10–50% O2 every 24 h/14 days Retinal vaso-obliteration/newborn rats/80% O2/5 days HRMECs/80% O2/1–48 h |
• Downregulated in hyperoxia • Overexpression promoted vascular repair in vivo and protected against endothelial dysfunction in vitro |
(Desjarlais et al. 2020) |
| miR-101-3p | miRNA | Lung injury/newborn mice/65% O2/7–14 days |
• Overexpression mitigated injury • Downregulated HMGB3 and TGF-ß1/Smad3 axis |
(Yuan et al. 2020) |
| miR-18a | miRNA |
Lung injury/mouse/95% O2/7 days MLE-12 cells/95% O2/12–48 h |
• Downregulated in hyperoxia • Overexpression prevented pyroptosis and relieved lung injury |
(Zou et al. 2020) |
| miR-29b | miRNA | Plasma from preterm infants lung injury/newborn mice/85% O2/14 days |
• Downregulated in hyperoxia • Improved alveolarization and decreased expression of ECM proteins |
(Durrani-Kolarik et al. 2017) |
| miR-29a | miRNA | Lung injury/newborn mice/ > 90% O2/4 days |
• Upregulated in hyperoxia • Inhibition alleviated injury |
(Hu et al. 2020) |
| miR-199a-5p | miRNA |
Lung injury/newborn mice/100% O2/7 days Mouse MLE-12 cells and RAW264.70 cells/95% O2/4–24 h |
• Upregulated in hyperoxia • Mimic treatment worsened injury |
(Alam et al. 2019) |
| miR-20b | miRNA | Lung injury/rats/95% O2/48 h |
• Downregulated in hyperoxia • Overexpression downregulated Mfn1/2 and reduced apoptosis |
(Mu et al. 2021) |
| miR-214 | miRNA |
Lung injury/newborn rats/95% O2/7 days Alveolar epithelial cells/85% O2/24 h |
• Downregulated in hyperoxia • Overexpression restored alveolarization in vivo and decreased apoptosis in vitro |
(Zhang et al. 2021b) |
| miR-421 | miRNA |
Lung injury/newborn mice/85% O2/7 days MLE-12 cells/85% O2/6 h |
• Upregulated in hyperoxia • Downregulation was associated with alleviated injury • Mimic treatment abrogated Rian-mediated protection |
(Tao et al. 2021) |
| miR-194-5p | miRNA |
Lung injury/newborn mice/ > 90% O2/4 days BEAS-2B cells/95% O2/48 h |
• Mediated hyperoxic injury • Upregulation blocked CASC2-mediated protection |
(Ji et al. 2021) |
| miR‐181c‐5p | miRNA | HLMECs/80% O2/12–24 h |
• Upregulated in hyperoxia • miR‐181c‐5p mimic downregulated NCAPG and enhanced apoptosis |
(Wu et al. 2021) |
| miR-342-5p | miRNA |
Lung injury/newborn mice/100% O2/4–7 days T2AECs and MLE-12 cells/95% O2/2–48 h |
• Downregulated in hyperoxia • Overexpression and mimic treatment ameliorated injury |
(Wen et al. 2021) |
| miR-299-3p | miRNA | OIR/newborn mice/75% O2/5 days |
• Downregulated in hyperoxia • Overexpression reduced apoptosis |
(Wang et al. 2022b) |
| FOXD3-AS1 | lncRNA | Lung injury/primary mouse lung epithelial cells, BEAS-2B and A549 cells/95% O2/12–72 h |
• Upregulated in hyperoxia in vivo and in vitro • Deletion is cytoprotective in vivo and in vitro |
(Zhang et al. 2017a) |
| Xist | lncRNA | Lung injury/newborn mice/65% O2/7–14 days |
• Upregulated in hyperoxia • Silencing protects against injury |
(Yuan et al. 2020) |
| H19 | lncRNA | Lung injury/newborn mice/70% O2/4–14 days |
• Upregulated in hyperoxia • Silencing upregulated miR-17, downregulated STAT3, and relieved injury |
(Zhang et al. 2020) |
| MEG3 | lncRNA |
Lung injury/mice/95% O2/7 days MLE-12 cells/95% O2/12–48 h |
• Upregulated in hyperoxia • Knockdown inhibited NLRP3 inflammasome, caspase-1, and pyroptosis |
(Zou et al. 2020) |
| MALAT1 | lncRNA |
BPD patients A549 cells 92% O2/48 h |
• Upregulated in BPD • Downregulated in hyperoxia in vitro • Silencing promoted apoptosis |
(Zhang et al. 2021a) |
| Rian | lncRNA |
Lung injury/newborn mice/85% O2/7 days MLE-12 cells/85% O2/6 h |
• Downregulated in vivo and in vitro • Overexpression downregulated miR-421 and alleviated injury |
(Tao et al. 2021) |
| CASC2 | lncRNA |
Lung injury/newborn mice/ > 90% O2/4 days/10 days recovery in normoxia BEAS-2B cells/95% O2/48 h |
• Poorly expressed in hyperoxic mice • Overexpression ameliorated lung injury in vivo • Inhibited apoptosis of epithelial cells in vitro |
(Ji et al. 2021) |
| DLEU2 | lncRNA | HLMECs/80% O2/12–24 h |
• Downregulated in hyperoxia • Overexpression inhibited miR‐181c‐5p and hyperoxic damage |
(Wu et al. 2021) |
| TUG1 | lncRNA | OIR/newborn mice/75% O2/5 days |
• Upregulated in hyperoxia • Knockdown reduced pathological alterations, apoptosis, inflammation, and miR-299-3p expression |
(Wang et al. 2022b) |
Abbreviations: CASC2, cancer susceptibility candidate 2; DLEU2, deleted in lymphocytic leukemia 2; ECM, extracellular matrix; FOXD3-AS1, FOXD3 antisense RNA 1; HMGB3, high mobility group box 3; HLMECs, human lung microvascular endothelial cells; HPMECs, human pulmonary microvascular endothelial cells; HRMECs, human retinal microvascular endothelial cells; Jak, Janus kinase; KO, knockout; MALAT1, metastasis-associated lung adenocarcinoma transcript; MEG3, maternally expressed 3; Mfn1/2, mitofusin 1/2; NCAPG, non-SMC condensin I complex subunit G; NLRP3, NLR family pyrin domain containing 3; OIR, oxygen-induced retinopathy; RIP1/3, receptor-interacting protein 1/3; Smad2, small mothers against decapentaplegic 2; STAT3, signal transducer and activator of transcription 3; TGF-β, transforming growth factor-β; TUG1, taurine up-regulated 1; T2AECs, type II alveolar epithelial cells; Xist, X-inactive specific transcript
Therapeutic approaches to ameliorating hyperoxia injury
Preventing or mitigating oxidative damage caused by increased ROS levels produced by cells during supplemental oxygen therapy (i.e., hyperoxia) can allow hypoxemia and tissue hypoxia to be corrected while avoiding or reducing oxygen toxicity. Strategies to reduce hyperoxic injury have been investigated in humans and rodent models and address key aspects including ROS production/neutralization, apoptotic cell death, and the inflammatory response, among others (Table 2).
Table 2.
Therapeutic candidates studied to treat hyperoxic injury
| Candidate | Mechanism of action | Models | Outcomes | References |
|---|---|---|---|---|
| Antioxidants | ||||
| MitoTEMPO/MitoTEMPOL | Mitochondrial O2•– scavenger | Murine BPD, various in vitro models |
↑Alveolarization ↓Right ventricular hypertrophy ↓ACE2 and TMPRSS2 expression ↓Proinflammatory cytokines ↓Cell death ↓Mitochondrial fragmentation |
(Datta et al. 2015; Forred et al. 2017; Ma et al. 2018; Yee et al. 2020) |
| Ascorbate (vitamin C) | Alkyl hydroperoxide scavenger, regenerates reduced tocopherol |
Healthy patients, congestive heart failure patients, Murine HALI, various in vitro models |
↑Left ventricular function ↓Hyperventilation ↓Oxidative biomarkers in blood ↓HMGB1 levels ↓Leukocyte infiltration ↓Lipid and protein oxidation ↓Vasoconstriction |
(Mak et al. 2002; Al-Shmgani et al. 2012; Gao et al. 2012; Patel et al. 2020; Fernandes et al. 2021) |
| Tocopherol (vitamin E) | Alkyl hydroperoxide scavenger | In vitro OIR, neonatal rabbits, other in vitro models |
↓Lipid and protein oxidation ↓Vascular cell injury ↓Surfactant system impairment |
(Tripathi and Tripathi 1984; Ward and Roberts 1984; Wispe et al. 1986; Al-Shmgani et al. 2012) |
| Retinol/retinoic acid (vitamin A) | ROS scavenger, retinoid X receptor agonist | Murine BPD |
↑Surfactant protein levels ↑Alveolar maturation ↓Lung damage ↓Growth retardation ↓MIP-2 expression |
(Zimová-Herknerová et al. 2008; James et al. 2010; Gelfand et al. 2020) |
| Coenzyme Q10 | ROS scavenger | Murine neonatal organ injury |
↑Antioxidant enzyme activity in heart, kidney, and brain ↓Oxidative stress in liver |
(Lee et al. 2022) |
| N-acetylcysteine | L-cysteine prodrug, replenishes GSH | Murine HALI, in vitro models |
↑Mitochondrial membrane potential ↓Lung damage ↓Cell death ↓Cyt c release ↓HGMB1 and RAGE expression ↓TLR2/4 and NF-κB activity ↓Proinflammatory cytokine secretion |
(Huang et al. 2016a; Qiao et al. 2019; Zou et al. 2019) |
| Curcumin and analogs | ROS scavenger, multiple molecular targets | Murine BPD |
↑Relaxation of tracheal smooth muscle ↑Lung maturation ↑Alveolarization ↑PPAR-γ activation ↑Catalase activity ↓Apoptosis ↓ERK1/2 activation ↓TNF-α expression ↓TGF-β signaling |
(Sakurai et al. 2011, 2013; Stamenkovska et al. 2020) |
| Sulforaphane | ROS scavenger, Nrf2 inducer, NF-κB inhibitor, other targets | Murine BPD and HALI |
↑Nrf2-mediated transcriptional response ↑Macrophage function ↓Inflammatory cell infiltration ↓LDH levels ↓Mucous hypersecretion |
(McGrath-Morrow et al. 2014; Cho et al. 2019; Patel et al. 2020) |
| Resveratrol | ROS scavenger, SIRT1, multiple molecular targets | Murine brain injury, murine HALI and BPD |
↑SIRT1/PGC-1α signaling ↑PGC-1α, NRF1, and TFAM expression ↑Mitochondrial biogenesis ↑SOD and GSH ↓Alveolar simplification ↓Lung fibrosis ↓Apoptosis ↓Mitochondrial dysfunction ↓p53 expression ↓Proinflammatory cytokine release ↓Wnt/β-catenin signaling |
(Özdemir et al. 2014; Xu et al. 2015; Zhu et al. 2020, 2021; Kang et al. 2021; Yang et al. 2022) |
| Quercetin | ROS scavenger, multiple molecular targets | Murine BPD, fetal airway smooth muscle cells |
↑Alveolarization ↓Inflammation ↓NF-κB levels ↓Lipid peroxidation ↓Senescence |
(Maturu et al. 2018; Parikh et al. 2019) |
| Anthocyanins | ROS scavenger | Murine OIR, HUVECs |
↑Nrf2 gene targets ↑Cell viability ↓Mitochondrial dysmorphology ↓Endothelial cell proliferation |
(Cimino et al. 2013; Ercan et al. 2019) |
| Caffeine | ROS scavenger, A2AR antagonist, multiple molecular targets | Murine BPD, neonatal murine brain injury |
↑Alveolar development ↑Weight gain ↓DNA damage ↓A2AR expression ↓Proinflammatory cytokines ↓Inflammatory infiltration ↓Apoptosis ↓ER stress ↓NLRP3 inflammasome expression ↓NF-κB activation ↓MMP2 levels |
(Endesfelder et al. 2017, 2019; Teng et al. 2017; Chen et al. 2020c) |
| Indole-3-carbinol | ROS scavenger, AhR agonist/inducer, other targets | Murine BPD |
↑AhR gene targets ↑Alveolarization ↑NF-κB target genes ↓Fibrosis |
(Guzmán-Navarro et al. 2021) |
| Tetrandrine | ROS scavenger, multiple molecular targets | Murine BPD |
↑Antioxidant enzymes ↓Apoptosis ↓Inflammation ↓Fibrotic markers ↓NF-κB and ERK1/2 signaling |
(Jiao et al. 2020) |
| Antiapoptotic | ||||
| Cyclosporin A | Cyclophilin D inhibitor; delays mPTP opening | Murine |
↓Cyt c release ↓Mitochondrial swelling ↓Lung damage |
(Pagano et al. 2004) |
| TRP601 | Caspase inhibitor | Murine brain injury |
↓Apoptosis ↓Neurodegeneration |
(Sifringer et al. 2012) |
| Anti-inflammatory | ||||
| Interleukin-10 | Anti-inflammatory cytokine | Murine HALI, fetal alveolar cells (in vitro) |
↑Survival ↑VEGF release ↑Proliferation ↑Jak1 and TYK2 phosphorylation ↓Lung injury ↓Cell death ↓NF-κB activation ↓Proinflammatory cytokines ↓iNOS and NO levels ↓MMP2 and MMP9 activities |
(Lee and Kim 2011; Li et al. 2015b; Lee and Lee 2015) |
| Interleukin-1 receptor antagonist | Anti-inflammatory cytokine | Murine BPD, murine BPD-pulmonary hypertension |
↑Pulmonary small vessels ↑Immune cell viability ↓Pulmonary vascular resistance ↓Lung structural disintegration ↓Cardiac fibrosis ↓Immune cell activation ↓Proinflammatory cytokines |
(Nold et al. 2013; Bui et al. 2019) |
| Acetylsalicylic acid | COX inhibitor | Murine HALI |
↓NF-κB activation ↓ROS ↓Proinflammatory cytokines ↓Macrophages ↓Neutrophil infiltration ↓Lung edema |
(Chen et al. 2020b; Tung et al. 2022) |
| Ibuprofen | COX inhibitor | Murine OIR |
↓Retinopathy score ↓Extra-retinal nuclei count per section |
(Sharma et al. 2003) |
| HIF-1 upregulators | ||||
| FG-4095 | PHD inhibitor | Fetal baboon lung explants, primate BPD, distinct cell lines |
↑HIF-1/2α target genes ↑Angiogenesis ↑Alveolar surface area ↑Lung compliance |
(Asikainen et al. 2006, 2005) |
| Dimethyloxalylglycine | PHD inhibitor | Murine OIR |
↑Peripheral vascularity ↓Neovascularization ↓Ischemia |
(Sears et al. 2008; Trichonas et al. 2013) |
| Roxadustat | PHD inhibitor | Murine BPD |
↑Survival ↑Alveolarization ↑eNOS expression ↑VEGF expression |
(Huang et al. 2021) |
| Others | ||||
| Memantine | NMDA receptor antagonist | Murine brain injury |
↑Neuron viability ↓Apoptosis |
(Polat et al. 2020) |
| Lacosamide | Enhances slow Na+ channel inactivation | Murine brain injury |
↑Neuron viability ↓Apoptosis |
(Polat et al. 2020) |
| Vitamin D | Vitamin D receptor agonist | Murine BPD |
↑Alveolarization ↑VEGF and VEGFR2 expression ↑HIF-1α expression ↓Alveolar simplification ↓Apoptosis ↓TLR4 expression ↓IFN‐γ and IL‐1β expression ↓Neutrophil extracellular traps ↓Proinflammatory cytokines |
(Kose et al. 2017; Yao et al. 2017; Chen et al. 2020a; Wang and Jiang 2021) |
| Metformin | AMPK activator | Murine BPD, HUVECs |
↑Radial alveolar count ↑Vascular proliferation ↑ATP levels ↑Lung capillary number ↓Mortality ↓Inflammation ↓Fibrosis |
(Chen et al. 2015; Yadav et al. 2020) |
| Rosiglitazone | PPAR-γ agonist | Murine BPD and HALI, preterm rabbits |
↑Radial alveolar count ↑Alveolar sacculation ↑Lung maturation ↑Surfactant proteins ↑VEGF expression ↓Wnt and TGF-β signaling ↓Neutrophil influx |
(Richter et al. 2016; Rehan et al. 2010; Dasgupta et al. 2009) |
| Alda-1 | ALDH2 activator | Murine HALI, HMVECs |
↑Mitochondrial membrane potential ↑Akt/mTOR signaling ↓Alveolar damage ↓Inflammation ↓Immune cell infiltration ↓Bax and cyt c levels ↓4-HNE levels |
(Sidramagowda Patil et al. 2019, 2021) |
Abbreviations: ACE2, angiotensin converting enzyme 2; AhR, aryl hydrocarbon receptor; ALDH2, aldehyde dehydrogenase 2; Alda-1, ALDH2 activator 1; AMPK, adenosine monophosphate-activated kinase; ATP, adenosine triphosphate; A2AR, A2A adenosine receptor; BPD, bronchopulmonary dysplasia; COX, cyclooxygenase; cyt c, cytochrome c; ER, endoplasmic reticulum; ERK1/2, extracellular signal-regulated kinase 1/2; GSH, reduced glutathione; HALI, hyperoxic acute lung injury; HIF-1/2, hypoxia-inducible factor 1/2; HMGB1, high mobility group box 1; HUVECs, human umbilical vein endothelial cells; Jak, Janus kinase; IFN-γ, interferon γ; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; MIP-2, macrophage inflammatory protein 2; MMP2, matrix metalloproteinase 2; mPTP, mitochondrial permeability transition pore; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing 3; NMDA, N-methyl-D-aspartate; NRF1, nuclear respiratory factor 1; Nrf2, nuclear factor erythroid 2-related factor 2; NO, nitric oxide; eNOS, endothelial NO synthase; iNOS, inducible NO synthase; OIR, oxygen-induced retinopathy; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator 1 α; PHD, prolyl hydroxylase domain; PPAR-γ, peroxisome proliferator-activated receptor γ; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; SIRT1, sirtuin 1; SOD, superoxide dismutase; TMPRSS2, transmembrane protease, serine 2; TGF-β, transforming growth factor β; TFAM, transcription factor a, mitochondrial; TLR2/4, toll-like receptor 2/4: TNF-α, tumor necrosis factor α; TYK2, tyrosine kinase 2; VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; 4-HNE, 4-Hydroxynoneal
Mitochondrial oxygen toxicity in hyperoxia has been targeted using synthetic O2• – scavengers like Mito-TEMPO and Mito-TEMPOL. Both molecules have a triphenylphosphonium (TPP) moiety with a single, shielded, positive charge that drives accumulation in the matrix in proportion to membrane potential. Mito-TEMPO has been shown to ameliorate lung damage and injury in several models of hyperoxia injury (see Alva et al. in press).
Non-mitochondria-targeted antioxidants can also reduce the extent of hyperoxia injury. Ascorbic acid is effective in reducing the levels of the oxidative damage biomarker 8-isoprostane in young men breathing 100% O2 (Fernandes et al. 2021). The antioxidant N-aceylcysteine reduces lung injury in rats exposed to 90% O2 (Qiao et al. 2019). Similarly, tocopherol, retinol, and coenzyme Q have all shown efficacy in various models of hyperoxia injury.
Therapeutic strategies targeting the response to oxidant injury have also been investigated. As outlined above, elevated cytosolic ROS levels generate a compensatory response via the Nrf2-Keap1 system. A variety of phytochemicals have been shown to ameliorate hyperoxia-mediated injury via this signaling pathway. For example, the plant polyphenolic compound curcumin activates Nrf2 indirectly (Park et al. 2021). Both curcumin (Sakurai et al. 2013) and its synthetic analogues (Stamenkovska et al. 2020) reduce lung damage due to hyperoxia in neonatal rats. Similar results have been obtained with resveratrol (Yang et al. 2022). Another phytochemical, sulforaphane, ameliorates hyperoxia-induced lung injury in Nrf2+/+ but not Nrf2−/− mice (Cho et al. 2019). Taken together, these studies suggest that administration of select phytochemicals might be beneficial in treating hyperoxia injury.
Strategies for avoiding apoptotic cell death have also been investigated. The mPTP inhibitor cyclosporin A has successfully reduced hyperoxic injury in a murine model (Pagano et al. 2004). Similarly, the caspase inhibitor TRP601 reduces hyperoxia injury in mice (Sifringer et al. 2012).
Although the overproduction of ROS appears to be responsible for acute hyperoxia injury, the disruption of O2-dependent cellular signaling is clearly important in the long-term manifestations of oxygen toxicity. Prolyl hydroxylase inhibitors that stabilize HIF-1 ameliorate hyperoxia injury in a wide range of experimental models. This relates to the fact that HIF-1α degradation is not complete under physioxic conditions in vivo (i.e., 2–6% O2) (Yan et al. 2010; Bracken et al. 2006; reviewed by Stuart et al. 2019), and it can therefore play a role in growth and development. In contrast, hyperoxia that increases tissue pO2 will be associated with reduced HIF-1α levels and concomitant loss of HIF-1 signaling activity. Further research is needed to determine how all of these studies can be translated in human patients in order to evaluate their potential efficacy in clinical practice.
Conclusions and future directions
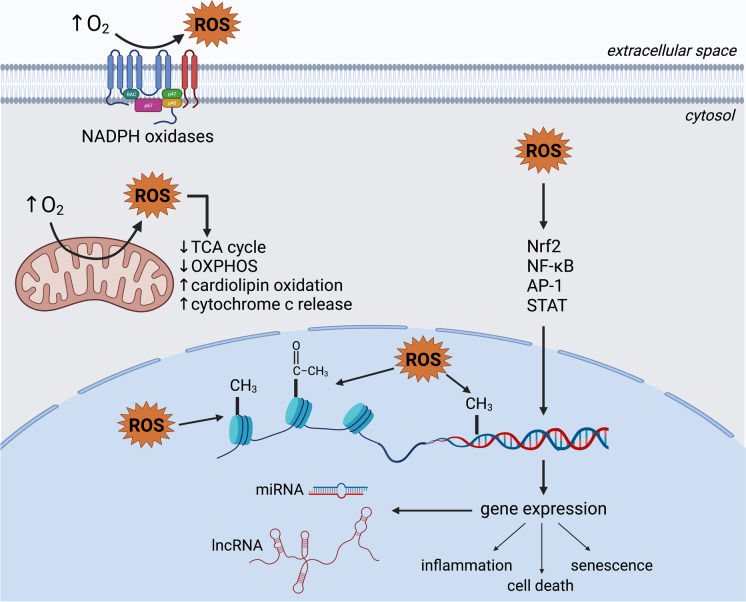
Hundreds of studies with isolated proteins and organelles, cultured cells, animal models, and humans indicate widespread oxidative injury and dysregulation of O2-dependent signaling processes in hyperoxia (Fig. 3), leading to serious and sometimes permanent pathologies. Virtually all experiments have been of relatively short duration (< 1 week); however, oxygen supplementation in severe COVID-19 and COPD can be needed for longer periods. Furthermore, the vast majority of studies have focused on the developmental effects of oxygen toxicity in the neonate. Future studies should focus on improving our understanding of long-term effects of exposure in adults.
Fig. 3.
Molecular mechanisms and cellular pathways of hyperoxia. Through an increased production of reactive oxygen species, hyperoxia dysregulates signaling pathways and promotes epigenetic modifications, resulting in altered gene expression, and ultimately leading to senescence, inflammation, and death. In the mitochondria, hyperoxia inhibits respiration and promotes cardiolipin oxidation and cytochrome c release, further contributing to the induction of cell death pathways. Created with BioRender.com
Since there is no apparent alternative to addressing the tissue hypoxemia associated with oxygen supplementation in patients experiencing reduced lung function, effective strategies must be aimed at ameliorating the negative effects of hyperoxia. A wide variety of approaches has been studied in pre-clinical models, and there is no shortage of candidate molecules showing some efficacy. However, given the vast range of oxygen effects on cells, this is a complex problem. Nonetheless, establishing these strategies is of immediate importance, given the ongoing need for oxygen therapy related to the COVID-19 pandemic and beyond.
Equally important, in terms of scientific data quality, is addressing the issue of oxygen toxicity in mammalian cell culture. Virtually all cell culture is performed under substantially hyperoxic conditions, with important effects on cellular activities. It would be a reasonable assumption that cells grown in standard conditions (~ 18% O2) may be somewhat preconditioned and thus less sensitive to the toxicity of severe hyperoxic environments (> 60% O2) compared to cells grown at physioxia. This underlines the need for revisiting previous results obtained from cell culture-based research, including studies using in vitro hypoxia and hyperoxia models. It is thus imperative that cell culturists be aware of how the hyperoxia of cell culture affects their experiments and make adjustments to avoid this problem. While the elevated cost of commercially available O2-regulating incubators may seem like a barrier to implement physioxia in cell culture workflows, we have recently developed an inexpensive cell culture incubator capable of maintaining physioxia (Samokhin et al. 2022) that can be employed for this purpose. Given the pervasive nature of oxygen’s effects on cellular function, it is a key parameter to regulate in vitro.
Acknowledgements
We thank Georgina Gardner for proofreading and helpful comments.
Author contribution
RA and JAS wrote the manuscript. MM, AB, LL, LS, AB, CC, AJ, DO, and TAM contributed research and notes.
Funding
JAS is funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant RGPIN 2020–05274. RA is supported by a Mitacs Globalink Graduate Fellowship.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approvals
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consent to publish this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas M, Moradi F, Hu W, Regudo KL, Osborne M, Pettipas J, et al. Vertebrate cell culture as an experimental approach – limitations and solutions. Comp Biochem Physiol B Biochem Mol Biol. 2021;254:110570. doi: 10.1016/j.cbpb.2021.110570. [DOI] [PubMed] [Google Scholar]
- Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J. 2011;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sohal RS. DNA oxidative damage and life expectancy in houseflies. Proc Natl Acad Sci U S A. 1994;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera O, Fernández AF, Muñoz A, Fraga MF. epigenetics and environment: a complex relationship. J Appl Physiol. 2010;109:243–251. doi: 10.1152/japplphysiol.00068.2010. [DOI] [PubMed] [Google Scholar]
- Alam MA, Betal SGnee, Aghai ZH, Bhandari V. Hyperoxia causes miR199a-5p-mediated injury in the developing lung. Pediatr Res. 2019;86:579–588. doi: 10.1038/s41390-019-0524-3. [DOI] [PubMed] [Google Scholar]
- Al-Ani A, Toms D, Kondro D, Thundathil J, Yu Y, Ungrin M. Oxygenation in cell culture: critical parameters for reproducibility are routinely not reported. PLoS One. 2018;13. 10.1371/journal.pone.0204269. [DOI] [PMC free article] [PubMed]
- Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shmgani HS, Moate RM, Sneyd JR, Macnaughton PD, Moody AJ. Hyperoxia-induced ciliary loss and oxidative damage in an in vitro bovine model: the protective role of antioxidant vitamins E and C. Biochem Biophys Res Commun. 2012;429:191–196. doi: 10.1016/j.bbrc.2012.10.113. [DOI] [PubMed] [Google Scholar]
- Alva R, Abbas M, Bagshaw OR, Moffatt C, Gardner G, Stuart JA. Mitochondrial oxygen toxicity; In de Oliveria MR (ed): Mitochondrial Intoxication, edn 1. Academic Press, in press.
- Amarelle L, Quintela L, Hurtado J, Malacrida L. Hyperoxia and lungs: what we have learned from animal models. Front Med (Lausanne) 2021;8:606678. doi: 10.3389/fmed.2021.606678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho W-B, Flippin LA, et al. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci U S A. 2005;102:10212–10217. doi: 10.1073/pnas.0504520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asikainen TM, Chang L-Y, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J. 2006;20:1698–1700. doi: 10.1096/fj.06-5887fje. [DOI] [PubMed] [Google Scholar]
- Audi SH, Friedly N, Dash RK, Beyer AM, Clough Av, Jacobs ER. Detection of hydrogen peroxide production in the isolated rat lung using Amplex red. Free Radic Res. 2018;52:1052–1062. doi: 10.1080/10715762.2018.1511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audi SH, Ganesh S, Taheri P, Zhang X, Dash RK, Clough Av, et al. Depolarized mitochondrial membrane potential and protection with duroquinone in isolated perfused lungs from rats exposed to hyperoxia. J Appl Physiol. 2022;132:346–356. doi: 10.1152/japplphysiol.00565.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auten RL, Mason SN, Auten KM, Brahmajothi M. Hyperoxia impairs postnatal alveolar epithelial development via NADPH oxidase in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2009;297:L134–L142. doi: 10.1152/ajplung.00112.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baburina Y, Krestinin R, Odinokova I, Sotnikova L, Kruglov A, Krestinina O. Astaxanthin inhibits mitochondrial permeability transition pore opening in rat heart mitochondria. Antioxidants. 2019;8:576. doi: 10.3390/antiox8120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird L, Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 2020;40:e0009920. doi: 10.1128/MCB.00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balin AK, Goodman DBP, Rasmussen H, Cristofalo VJ. The effect of oxygen and vitamin E on the lifespan of human diploid cells in vitro. J Cell Biol. 1977;74:58–67. doi: 10.1083/jcb.74.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandali KS, Belanger MP, Wittnich C, Wittnich Hyperoxia C. Hyperoxia causes oxygen free radical-mediated membrane injury and alters myocardial function and hemodynamics in the newborn. Am J Physiol Heart Circ Physiol. 2004;287:H553–H559. doi: 10.1152/ajpheart.00657.2003. [DOI] [PubMed] [Google Scholar]
- Bao TP, Wu R, Cheng HP, Cui XW, Tian ZF. Differential expression of long non-coding RNAs in hyperoxia-induced bronchopulmonary dysplasia. Cell Biochem Funct. 2016;34:299–309. doi: 10.1002/cbf.3190. [DOI] [PubMed] [Google Scholar]
- Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet P-F. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 1998;19:573–581. doi: 10.1165/ajrcmb.19.4.3173. [DOI] [PubMed] [Google Scholar]
- Barazzone Argiroffo C, Donati YR, Boccard J, Rochat AF, Vesin C, Kan C-D, et al. CD40-CD40 ligand disruption does not prevent hyperoxia-induced injury. Am J Pathol. 2002;160:67–71. doi: 10.1016/S0002-9440(10)64350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzone-Argiroffo C, Pagano A, Juge C, Trailler IM, Rochat A, Vesin C, et al. Glucocorticoids aggravate hyperoxia-induced lung injury through decreased nuclear factor-kB activity. Am J Physiol Lung Cell Mol Physiol. 2003;284:L197–L204. doi: 10.1152/ajplung.00239.2002. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Garcia-Martínez C, Mas S, Ametller E, Gea J, Argilés JM, et al. UCP3 overexpression neutralizes oxidative stress rather than nitrosative stress in mouse myotubes. FEBS Lett. 2009;583:350–356. doi: 10.1016/j.febslet.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid Med Cell Longev. 2016;2016. 10.1155/2016/3527579. [DOI] [PMC free article] [PubMed]
- Bean JW, Johnson PC. Adrenocortical response to single and repeated exposure to oxygen at high pressure. Am J Physiol. 1954;179:410–414. doi: 10.1152/ajplegacy.1954.179.3.410. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol. 2014;307:L936–L947. doi: 10.1152/ajplung.00159.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes CF, Meyer-Fernandes JR, Basseres DS, Castilho RF, Vercesi AE. Ca2+-dependent permeabilization of the inner mitochondrial membrane by 4,4’-diisothiocyanatostilbene-2,2’-disulfonic acid (DIDS) Biochim Biophys Acta. 1994;1188:93–100. doi: 10.1016/0005-2728(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Bernardi P, di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Carraro M, Lippe G. The mitochondrial permeability transition: recent progress and open questions. FEBS J. 2021 doi: 10.1111/febs.16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert P. La pression barométrique: Recherches de physiologie expérimentale, ed 1. Paris, 1878.
- Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med. 2010;15:223–229. doi: 10.1016/j.siny.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik-Multanowski M, Revhaug C, Grabowska A, Dobosz A, Madetko-Talowska A, Zasada M, et al. Hyperoxia induces epigenetic changes in newborn mice lungs. Free Radic Biol Med. 2018;121:51–56. doi: 10.1016/j.freeradbiomed.2018.04.566. [DOI] [PubMed] [Google Scholar]
- Block ER. Interaction between oxygen and cell membranes: modification of membrane lipids to enhance pulmonary artery endothelial cell tolerance to hypoxia. Exp Lung Res. 1988;14:937–958. doi: 10.3109/01902148809064185. [DOI] [PubMed] [Google Scholar]
- Bouch S, O’reilly M, Harding R, Sozo F. Neonatal exposure to mild hyperoxia causes persistent increases in oxidative stress and immune cells in the lungs of mice without altering lung structure. Am J Physiol Lung Cell Mol Physiol. 2015;309:L488–L496. doi: 10.1152/ajplung.00359.2014. [DOI] [PubMed] [Google Scholar]
- Bracken CP, Fedele AO, Linke S, Balrak W, Lisy K, Whitelaw ML, et al. Cell-specific regulation of hypoxia-inducible factor (HIF)-1α and HIF-2α stabilization and transactivation in a graded oxygen environment. J Biol Chem. 2006;281:22575–22585. doi: 10.1074/jbc.M600288200. [DOI] [PubMed] [Google Scholar]
- Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Interactions between nitric oxide, oxygen, reactive oxygen species and reactive nitrogen species. Biochem Soc Trans. 2006;34:953–956. doi: 10.1042/BST0340953. [DOI] [PubMed] [Google Scholar]
- Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, et al. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. 2006;34:453–463. doi: 10.1165/rcmb.2005-0223OC. [DOI] [PubMed] [Google Scholar]
- Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GRS. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem. 2004;279:6753–6760. doi: 10.1074/jbc.M310145200. [DOI] [PubMed] [Google Scholar]
- Bucher JR, Roberts RJ. The development of the newborn rat lung in hyperoxia: a dose-response study of lung growth, maturation, and changes in antioxidant enzyme activities. Pediatr Res. 1981;15:999–1008. doi: 10.1203/00006450-198107000-00005. [DOI] [PubMed] [Google Scholar]
- Budinger GRS, Mutlu GM, Urich D, Soberanes S, Buccellato LJ, Hawkins K, et al. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am J Respir Crit Care Med. 2011;183:1043–1054. doi: 10.1164/rccm.201002-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui CB, Kolodziej M, Lamanna E, Elgass K, Sehgal A, Rudloff I, et al. Interleukin-1 receptor antagonist protects newborn mice against pulmonary hypertension. Front Immunol. 2019;10:1480. doi: 10.3389/fimmu.2019.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busuttil RA, Rubio M, Dollé MET, Campisi J, Vijg J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell. 2003;2:287–294. doi: 10.1046/j.1474-9728.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Byfield G, Budd S, Elizabeth Hartnett M. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Invest Ophthalmol vis Sci. 2009;50:3360–3365. doi: 10.1167/iovs.08-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciuttolo MA, Trinh L, Lumpkin JA, Rao G. Hyperoxia induces DNA damage in mammalian cells. Free Radic Biol Med. 1993;14:267–276. doi: 10.1016/0891-5849(93)90023-N. [DOI] [PubMed] [Google Scholar]
- Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli V, Chan DC. Mitochondrial DNA: impacting central and peripheral nervous systems. Neuron. 2014;84:1126–1142. doi: 10.1016/j.neuron.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Métrailler-Ruchonnet I, Schäppi M, et al. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med. 2009;180:972–981. doi: 10.1164/rccm.200902-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnino JM, Lee H, He X, Groot M, Jin Y. Extracellular vesicle-cargo miR-185-5p reflects type II alveolar cell death after oxidative stress. Cell Death Discov. 2020;6:82. doi: 10.1038/s41420-020-00317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro M, Jones K, Sartori G, Schiavone M, Antonucci S, Kucharczyk R, et al. The unique cysteine of F-ATP synthase OSCP subunit participates in modulation of the permeability transition pore. Cell Rep. 2020;32:108095. doi: 10.1016/j.celrep.2020.108095. [DOI] [PubMed] [Google Scholar]
- Castilho RF, Kowaltowski AJ, Meinicke AR, Bechara EJH, Vercesi AE. Permeabilization of the inner mitochondrial membrane by Ca2+ ions is stimulated by t-butyl hydroperoxide and mediated by reactive oxygen species generated by mitochondria. Free Radic Biol Med. 1995;18:479–486. doi: 10.1016/0891-5849(94)00166-H. [DOI] [PubMed] [Google Scholar]
- Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Chan EC, van Wijngaarden P, Liu GS, Jiang F, Peshavariya H, Dusting GJ. Involvement of Nox2 NADPH oxidase in retinal neovascularization. Invest Ophthalmol Vis Sci. 2013;54:7061–7067. doi: 10.1167/iovs.13-12883. [DOI] [PubMed] [Google Scholar]
- Chapalamadugu KC, Panguluri SK, Bennett ES, Kolliputi N, Tipparaju SM. High level of oxygen treatment causes cardiotoxicity with arrhythmias and redox modulation. Toxicol Appl Pharmacol. 2015;282:100–107. doi: 10.1016/j.taap.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brandizzi F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013;23:547–555. doi: 10.1016/j.tcb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Chou HC. Hyperoxia disrupts the intestinal barrier in newborn rats. Exp Mol Pathol. 2016;101:44–49. doi: 10.1016/j.yexmp.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Chen X, Walther FJ, Sengers RMA, Laghmani EH, Salam A, Folkerts G, et al. Metformin attenuates hyperoxia-induced lung injury in neonatal rats by reducing the inflammatory response. Am J Physiol Lung Cell Mol Physiol. 2015;309:L262–L270. doi: 10.1152/ajplung.00389.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Liu YC, Chen YJ, Chou HC. Genome-wide analysis of DNA methylation in hyperoxia-exposed newborn rat lung. Lung. 2017;195:661–669. doi: 10.1007/s00408-017-0036-z. [DOI] [PubMed] [Google Scholar]
- Chen C, Weng H, Zhang X, Wang S, Lu C, Jin H, et al. Low-dose vitamin D protects hyperoxia-induced bronchopulmonary dysplasia by inhibiting neutrophil extracellular traps. Front Pediatr. 2020;8:335. doi: 10.3389/fped.2020.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Tung YT, Wei CH, Lee PY, Chen W. Anti-inflammatory and reactive oxygen species suppression through aspirin pretreatment to treat hyperoxia-induced acute lung injury in nf-κb–luciferase inducible transgenic mice. Antioxidants. 2020b;9. 10.3390/antiox9050429 [DOI] [PMC free article] [PubMed]
- Chen S, Chen S, Wu Q, Wu Q, Zhong D, Zhong D, et al. Caffeine prevents hyperoxia-induced lung injury in neonatal mice through NLRP3 inflammasome and NF-κB pathway. Respir Res. 2020;21:140. doi: 10.1186/s12931-020-01403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H-Y, Jedlicka AE, Reddy SPM, Kensler TW, Yamamoto M, Zhang L-Y, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Cho H-Y, Jedlicka AE, Reddy SPM, Zhang L-Y, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia Nrf2 is a candidate gene. Am J Respir Cell Mol Biol. 2002;26:42–51. doi: 10.1165/ajrcmb.26.1.4536. [DOI] [PubMed] [Google Scholar]
- Cho HY, van Houten B, Wang X, Miller-Degraff L, Fostel J, Gladwell W, et al. Targeted deletion of Nrf2 impairs lung development and oxidant injury in neonatal mice. Antioxid Redox Signal. 2012;17:1066–1082. doi: 10.1089/ars.2011.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Miller-DeGraff L, Blankenship-Paris T, Wang X, Bell DA, Lih F, et al. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol Appl Pharmacol. 2019;364:29–44. doi: 10.1016/j.taap.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A, Martinuzzi A, Yoneda M, Daga A, Johns D, Lai ST, et al. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci U S A. 1992;89:4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HC, Chen CM. Cathelicidin attenuates hyperoxia-induced intestinal injury through inhibition of NF-κB activity in newborn rats. Exp Mol Pathol. 2020;113:104269. doi: 10.1016/j.yexmp.2019.104269. [DOI] [PubMed] [Google Scholar]
- Chou HC, Chen CM. Hyperoxia induces ferroptosis and impairs lung development in neonatal mice. Antioxidants. 2022;11:641. doi: 10.3390/antiox11040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlone GE, Hinojo CM, Stavitzski NM, Dean JB: CNS function and dysfunction during exposure to hyperbaric oxygen in operational and clinical settings. Redox Biol. 2019;27. 10.1016/j.redox.2019.101159 [DOI] [PMC free article] [PubMed]
- Cimino F, Speciale A, Anwar S, Canali R, Ricciardi E, Virgili F, et al. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8:391–399. doi: 10.1007/s12263-012-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coarfa C, Grimm SL, Katz T, Zhang Y, Jangid RK, Walker CL, et al. Epigenetic response to hyperoxia in the neonatal lung is sexually dimorphic. Redox Biol. 2020;37:101718. doi: 10.1016/j.redox.2020.101718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini P, Belzacq A-S, la Vieira H, Larochette N, de Pablo MA, Zamzami N, et al. Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene. 2000;19:307–314. doi: 10.1038/sj.onc.1203299. [DOI] [PubMed] [Google Scholar]
- Crapo JD, Ouryf T, Rabouille C, Slot JW, Chang L-Y. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino DP, Olson JE, Dean JB. Acute hyperoxia increases lipid peroxidation and induces plasma membrane blebbing in human U87 glioblastoma cells. Neuroscience. 2009;159:1011–1022. doi: 10.1016/j.neuroscience.2009.01.062. [DOI] [PubMed] [Google Scholar]
- D’Alessio M, de Nicola M, Coppola S, Gualandi G, Pugliese L, Cerella C, et al. Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J. 2005;19:1504–1506. doi: 10.1096/fj.04-3329fje. [DOI] [PubMed] [Google Scholar]
- D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Daniel OO, Adeoye AO, Ojowu J, Olorunsogo OO. Inhibition of liver mitochondrial membrane permeability transition pore opening by quercetin and vitamin E in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 2018;504:460–469. doi: 10.1016/j.bbrc.2018.08.114. [DOI] [PubMed] [Google Scholar]
- Dapaah-Siakwan F, Zambrano R, Luo S, Duncan MR, Kerr N, Donda K, et al. Caspase-1 inhibition attenuates hyperoxia-induced lung and brain injury in neonatal mice. Am J Respir Cell Mol Biol. 2019;61:341–354. doi: 10.1165/rcmb.2018-0192OC. [DOI] [PubMed] [Google Scholar]
- Das KC. Hyperoxia decreases glycolytic capacity, glycolytic reserve and oxidative phosphorylation in MLE-12 cells and inhibits complex I and II function, but not complex IV in isolated mouse lung mitochondria. PLoS One. 2013;8:e73358. [DOI] [PMC free article] [PubMed]
- Das KC, Dashnamoorthy R. Hyperoxia activates the ATR-Chk1 pathway and phosphorylates p53 at multiple sites. Am J Physiol Lung Cell Mol Physiol. 2004;286:L87–L97. doi: 10.1152/ajplung.00203.2002. [DOI] [PubMed] [Google Scholar]
- Das KC, Ravi D, Holland W. Increased apoptosis and expression of p21 and p53 in premature infant baboon model of bronchopulmonary dysplasia. Antioxid Redox Signal. 2004;6:109–116. doi: 10.1089/152308604771978417. [DOI] [PubMed] [Google Scholar]
- Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, et al. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-b and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1031–L1041. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Kim GA, Taylor JM, Gugino SF, Kathryn Farrow XN, Schumacker PT, et al. Mouse lung development and NOX1 induction during hyperoxia are developmentally regulated and mitochondrial ROS dependent. Am J Physiol Lung Cell Mol Physiol. 2015;309:L369–L377. doi: 10.1152/ajplung.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Davis DP, Meade W, Sise MJ, Kennedy F, Simon F, Tominaga G, et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrama. 2009;26:2217–2223. doi: 10.1089/neu.2009.0940. [DOI] [PubMed] [Google Scholar]
- de Paepe ME, Mao Q, Chao Y, Powell JL, Rubin LP, Sharma S. Hyperoxia-induced apoptosis and Fas/FasL expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L647–L659. doi: 10.1152/ajplung.00445.2004. [DOI] [PubMed] [Google Scholar]
- Dean JM, Bennet L, Back SA, McClendon E, Riddle A, Gunn AJ. What brakes the preterm brain? An arresting story. Pediatr Res. 2014;75:227–233. doi: 10.1038/pr.2013.189. [DOI] [PubMed] [Google Scholar]
- Desjarlais M, Wirth M, Rivera JC, Lahaie I, Dabouz R, Omri S, et al. MicroRNA-96 promotes vascular repair in oxygen-induced retinopathy - a novel uncovered vasoprotective function. Front Pharmacol. 2020;11. 10.3389/fphar.2020.00013. [DOI] [PMC free article] [PubMed]
- Doolette DJ, Mitchell SJ. Hyperbaric conditions. Compr Physiol. 2010;163–201. [DOI] [PubMed]
- Durrani-Kolarik S, Pool CA, Gray A, Heyob KM, Cismowski MJ, Pryhuber G, et al. miR-29b supplementation decreases expression of matrix proteins and improves alveolarization in mice exposed to maternal inflammation and neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2017;313:L339–L349. doi: 10.1152/ajplung.00273.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzietko M, Boos V, Sifringer M, Polley O, Gerstner B, Genz K, et al. A critical role for Fas/CD-95 dependent signaling pathways in the pathogenesis of hyperoxia-induced brain injury. Ann Neurol. 2008;64:664–673. doi: 10.1002/ana.21516. [DOI] [PubMed] [Google Scholar]
- Endesfelder S, Weichelt U, Strauß E, Schlör A, Sifringer M, Scheuer T, et al. Neuroprotection by caffeine in hyperoxia-induced neonatal brain injury. Int J Mol Sci. 2017;18:187. doi: 10.3390/ijms18010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endesfelder S, Strauß E, Scheuer T, Schmitz T, Bührer C. Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respir Res. 2019;20:88. doi: 10.1186/s12931-019-1063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezari M, Javdan M, Antoine DJ, Morrow DMP, Sitapara RA, Patel V, et al. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2014;2:314–322. doi: 10.1016/j.redox.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan Z, Haberal N, Helvacioglu F, Daǧdeviren A, Yilmaz G. Effect of intravitreal and intraperitoneal cyanidin-3-glucoside injection in oxygen-induced retinopathy mouse model. Indian J Ophthalmol. 2019;67:801–805. doi: 10.4103/ijo.IJO_166_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagian MM, Pereira-da-Silva L, Martins IS, Vercesi AE. Membrane protein thiol cross-linking associated with the permeabilization of the inner mitochondrial membrane by Ca2+ plus prooxidants. J Biol Chem. 1990;265:19955–19960. doi: 10.1016/S0021-9258(17)45467-6. [DOI] [PubMed] [Google Scholar]
- Felderhoff-Mueser U, Bittigau P, Sifringer M, Jarosz B, Korobowicz E, Mahler L, et al. Oxygen causes cell death in the developing brain. Neurobiol Dis. 2004;17:273–282. doi: 10.1016/j.nbd.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Felderhoff-Mueser U, Sifringer M, Polley O, Dzietko M, Leineweber B, Mahler L, et al. Caspase-1-processed interleukins in hyperoxia-induced cell death in the developing brain. Ann Neurol. 2005;57:50–59. doi: 10.1002/ana.20322. [DOI] [PubMed] [Google Scholar]
- Fernandes IA, Mattos JD, Campos MO, Rocha MP, Mansur DE, Rocha HM, et al. Reactive oxygen species play a modulatory role in the hyperventilatory response to poikilocapnic hyperoxia in humans. J Physiol. 2021;599:3993–4007. doi: 10.1113/JP281635. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AB, Vasquez-Medina JP, Dodia C, Sorokina EM, Tao JQ, Feinstein SI. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018;14:41–46. doi: 10.1016/j.redox.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca J, Moradi F, Valente AJF, Stuart JA. Oxygen and glucose levels in cell culture media determine resveratrol’s effects on growth, hydrogen peroxide production, and mitochondrial dynamics. Antioxidants. 2018;7:157. doi: 10.3390/antiox7110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forred BJ, Daugaard DR, Titus BK, Wood RR, Floen MJ, Booze ML, et al. Detoxification of mitochondrial oxidants and apoptotic signaling are facilitated by thioredoxin-2 and peroxiredoxin-3 during hyperoxic injury. PLoS ONE. 2017;12:e0168777. doi: 10.1371/journal.pone.0168777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franek WR, Morrow DMP, Zhu H, Vancurova I, Miskolci V, Darley-Usmar K, et al. NF-κB protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic Biol Med. 2004;37:1670–1679. doi: 10.1016/j.freeradbiomed.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Frank L, Groseclose EE. Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res. 1984;18:240–244. doi: 10.1203/00006450-198403000-00004. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. doi: 10.1016/S0021-9258(19)68544-3. [DOI] [PubMed] [Google Scholar]
- Fukumoto J, Fukumoto I, Tamarapu Parthasarathy P, Cox R, Huynh B, Kollongod Ramanathan G, et al. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol. 2013;305:C182–C189. doi: 10.1152/ajpcell.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galam L, Rajan A, Failla A, Soundararajan R, Lockey RF, Kolliputi N. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. Am J Physiol Lung Cell Mol Physiol. 2016;310:L572–L581. doi: 10.1152/ajplung.00417.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol. 2012;112:483–492. doi: 10.1007/s00421-011-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Guede Á, Vera O, Ibáñez-de-Caceres I. When oxidative stress meets epigenetics: implications in cancer development. Antioxidants. 2020;9:1–26. doi: 10.3390/antiox9060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner PR, Nguyen D-DH, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci U S A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- Gebb SA, Decoux A, Waggoner A, Wilson GL, Gillespie MN. Mitochondrial DNA damage mediates hyperoxic dysmorphogenesis in rat fetal lung explants. Neonatology. 2013;103:91–97. doi: 10.1159/000342632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand CA, Sakurai R, Wang Y, Liu Y, Segal R, Rehan VK. Inhaled vitamin A is more effective than intramuscular dosing in mitigating hyperoxia-induced lung injury in a neonatal rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2020;319:L576–L584. doi: 10.1152/ajplung.00266.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Jones DP. The redox proteome. J Biol Chem. 2013;288:26512–26520. doi: 10.1074/jbc.R113.464131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Wang Xz, Wang T, Chen Jj, Xie Xy, Hu H, et al. Molecular signal networks and regulating mechanisms of the unfolded protein response. J Zhejiang Univ Sci B. 2017;18:1–14. doi: 10.1631/jzus.B1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DR, Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis. 2011;2:e213. doi: 10.1038/cddis.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res. 2010;44:1098–1124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- Guzmán-Navarro G, de León MB, Martín-Estal I, Durán RCD, Villarreal-Alvarado L, Vaquera-Vázquez A, et al. Prenatal indole-3-carbinol administration activates aryl hydrocarbon receptor-responsive genes and attenuates lung injury in a bronchopulmonary dysplasia model. Exp Biol Med. 2021;246:695–706. doi: 10.1177/1535370220963789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner C, Wu J, Tiboldi A, Hess M, Mitulovic G, Kaun C, et al. Hyperoxia induces inflammation and cytotoxicity in human adult cardiac myocytes. Shock. 2017;47:436–444. doi: 10.1097/SHK.0000000000000740. [DOI] [PubMed] [Google Scholar]
- Hals I, Ohki T, Singh R, Ma Z, Björklund A, Balasuriya C, et al. Hyperoxia reduces insulin release and induces mitochondrial dysfunction with possible implications for hyperoxic treatment of neonates. Physiol Rep. 2017;5:e13447. [DOI] [PMC free article] [PubMed]
- Han CH, Guan ZB, Zhang PX, Fang HL, Li L, Zhang HM, et al. Oxidative stress induced necroptosis activation is involved in the pathogenesis of hyperoxic acute lung injury. Biochem Biophys Res Commun. 2018;495:2178–2183. doi: 10.1016/j.bbrc.2017.12.100. [DOI] [PubMed] [Google Scholar]
- Hellström A, Smith LEH, Dammann O. Retinopathy of prematurity. The Lancet. 2013;382:1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R, Menzel D. The effect of short term exposures to 100 per cent oxygen on the fine structure of proximal convoluted tubules. Experientia. 1970;26:1124–1125. doi: 10.1007/BF02112713. [DOI] [PubMed] [Google Scholar]
- Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl A-KT, et al. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Investig. 2004;113:28–37. doi: 10.1172/JCI19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Hjelmeland LM, Handa JT. Senescence associated β galactosidase activity in human retinal pigment epithelial cells exposed to mild hyperoxia in vitro. Br J Ophthalmol. 2002;86:159–162. doi: 10.1136/bjo.86.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Xie L, Yu J, Fu H, Zhou D, Liu H. Inhibition of microRNA-29a alleviates hyperoxia-induced bronchopulmonary dysplasia in neonatal mice via upregulation of GAB1. Mol Med. 2020;26:3. doi: 10.1186/s10020-019-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Estrada R, Yappert MC, Borchman D. Oxidation-induced changes in human lens epithelial cells. 1. Phospholipids. Free Radic Biol Med. 2006;41:1425–1432. doi: 10.1016/j.freeradbiomed.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Huang D, Fang F, Xu F. Hyperoxia induces inflammation and regulates cytokine production in alveolar epithelium through TLR2/4-NF-kB-dependent mechanism. Eur Rev Med Pharmacol Sci. 2016;20:1399–1410. [PubMed] [Google Scholar]
- Huang J, Han Y, Cui C, Chen M, Hou X. Caspase-12 expression in hyperoxia-induced corpus callosum damage in newborn mice. Chin J Neonatol. 2016;6:379–384. [Google Scholar]
- Huang LT, Chou HC, Chen CM. Roxadustat attenuates hyperoxia-induced lung injury by upregulating proangiogenic factors in newborn mice. Pediatr Neonatol. 2021;62:369–378. doi: 10.1016/j.pedneo.2021.03.012. [DOI] [PubMed] [Google Scholar]
- James ML, Ross AC, Bulger A, Philips JB, Ambalavanan N. Vitamin A and retinoic acid act synergistically to increase lung retinyl esters during normoxia and reduce hyperoxic lung injury in newborn mice. Pediatr Res. 2010;67:591–597. doi: 10.1203/PDR.0b013e3181dbac3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Liu Z, Dong C, Wu D, Yang S, Wu L. LncRNA CASC2 targets CAV1 by competitively binding with microRNA-194-5p to inhibit neonatal lung injury. Exp Mol Pathol. 2021;118:104575. doi: 10.1016/j.yexmp.2020.104575. [DOI] [PubMed] [Google Scholar]
- Jia D, Zheng J, Zhou Y, Jia J, Ye X, Zhou B, et al. Ferroptosis is involved in hyperoxic lung injury in neonatal rats. J Inflamm Res. 2021;14:5393–5401. doi: 10.2147/JIR.S335061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao B, Tang Y, Liu S, Guo C. Tetrandrine attenuates hyperoxia-induced lung injury in newborn rats via NF-κB p65 and ERK1/2 pathway inhibition. Ann Transl Med. 2020;8:1018. doi: 10.21037/atm-20-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, et al. Cytochrome C acts as a cardiolipin oxygenase required for release of proapoptotic Factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Kaindl AM, Sifringer M, Koppelstaetter A, Genz K, Loeber R, Boerner C, et al. Erythropoietin protects the developing brain from hyperoxia-induced cell death and proteome changes. Ann Neurol. 2008;64:523–534. doi: 10.1002/ana.21471. [DOI] [PubMed] [Google Scholar]
- Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Dong W, Li X, Ruan Y, Zhang R. Resveratrol relieves hyperoxia-induced brain injury in neonatal rats by activating Sirt1. Am J Perinatol. 2021;38:e351–e358. doi: 10.1055/s-0040-1710352. [DOI] [PubMed] [Google Scholar]
- Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S. Cellular oxygen toxicity: oxidant injury without apoptosis. J Biol Chem. 1996;271:15182–15186. doi: 10.1074/jbc.271.25.15182. [DOI] [PubMed] [Google Scholar]
- Keeley TP, Mann GE. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol Rev. 2019;99:161–234. doi: 10.1152/physrev.00041.2017. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Cheresh P, Williams D, Cheng Y, Ridge K, Schumacker PT, et al. Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J Biol Chem. 2014;289:6165–6176. doi: 10.1074/jbc.M113.515130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura RE, Thulin GE, Wender D, Warshaw JB. Decreased oxidative metabolism in neonatal rat lung exposed to hyperoxia. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1501–1505. doi: 10.1152/jappl.1983.55.5.1501. [DOI] [PubMed] [Google Scholar]
- Klimova TA, Bell EL, Shroff EH, Weinberg FD, Snyder CM, Dimri GP, et al. Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J. 2009;23:783–794. doi: 10.1096/fj.08-114256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose M, Bastug O, Sonmez MF, Per S, Ozdemir A, Kaymak E, et al. Protective effect of vitamin D against hyperoxia-induced lung injury in newborn rats. Pediatr Pulmonol. 2017;52:69–76. doi: 10.1002/ppul.23500. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Castilho RF, Grijalba MT, Bechara EJH, Vercesi AE. Effect of inorganic phosphate concentration on the nature of inner mitochondrial membrane alterations mediated by Ca2+ ions: a proposed model for phosphate-stimulated lipid peroxidation. J Biol Chem. 1996;271:2929–2934. doi: 10.1074/jbc.271.6.2929. [DOI] [PubMed] [Google Scholar]
- Kulkarni A, Das KC. Differential roles of ATR and ATM in p53, Chk1, and histone H2AX phosphorylation in response to hyperoxia: ATR-dependent ATM activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L998–L1006. doi: 10.1152/ajplung.00004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9:645593. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundumani-Sridharan V, Subramani J, Raghavan S, Maiti GP, Owens C, Walker T, et al. Short-duration hyperoxia causes genotoxicity in mouse lungs: protection by volatile anesthetic isoflurane. Am J Physiol Lung Cell Mol Physiol. 2019;316:L903–L917. doi: 10.1152/ajplung.00142.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal CV, Olave N, Travers C, Rezonzew G, Dolma K, Simpson A, et al. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight. 2018;3:e93994. doi: 10.1172/jci.insight.93994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Kalai M, Vandenabeele P. Caspase-12: an overview. Cell Death Differ. 2004;11:365–368. doi: 10.1038/sj.cdd.4401364. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim CK. Effect of recombinant IL-10 on cultured fetal rat alveolar type II cells exposed to 65%-hyperoxia. Respir Res. 2011;12:68. doi: 10.1186/1465-9921-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lee DG. rIL-10 enhances IL-10 signalling proteins in foetal alveolar type II cells exposed to hyperoxia. J Cell Mol Med. 2015;19:1538–1547. doi: 10.1111/jcmm.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Camhi SL, Yoke Chin B, Alam J, Choi AMK. AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:L175–L182. doi: 10.1152/ajplung.2000.279.1.L175. [DOI] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Lee CH, Lee MS, Yang RC, Hsu CS, Su TC, Chang PS, et al. Using a neonatal rat model to explore the therapeutic potential of coenzyme Q10 in prematurity under hyperoxia. Environ Toxicol. 2022;37:1472–1482. doi: 10.1002/tox.23499. [DOI] [PubMed] [Google Scholar]
- Lenartowicz E, Bernardi P, Felice Azzone G. Phenylarsine oxide induces the cyclosporin A-sensitive membrane permeability transition in rat liver mitochondria. J Bioenerg Biomembr. 1991;23:679–688. doi: 10.1007/BF00785817. [DOI] [PubMed] [Google Scholar]
- Li TM, Liu DY. Mechanism of neonatal intestinal injury induced by hyperoxia therapy. J Immunol Res. 2022;2022:2316368. doi: 10.1155/2022/2316368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gao X, Qian M, Eaton JW. Mitochondrial metabolism underlies hyperoxic cell damage. Free Radic Biol Med. 2004;36:1460–1470. doi: 10.1016/j.freeradbiomed.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Li H, Benipal B, Zhou S, Dodia C, Chatterjee S, Tao JQ, et al. Critical role of peroxiredoxin 6 in the repair of peroxidized cell membranes following oxidative stress. Free Radic Biol Med. 2015;87:356–365. doi: 10.1016/j.freeradbiomed.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HD, Zhang QX, Mao Z, Xu XJ, Li NY, Zhang H. Exogenous interleukin-10 attenuates hyperoxia-induced acute lung injury in mice. Exp Physiol. 2015;100:331–340. doi: 10.1113/expphysiol.2014.083337. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang W, Wu G, Ju X, Wang Y, Liu W. miR-16 inhibits hyperoxia-induced cell apoptosis in human alveolar epithelial cells. Mol Med Rep. 2018;17:5950–5957. doi: 10.3892/mmr.2018.8636. [DOI] [PubMed] [Google Scholar]
- Li K, Zhang F, Wei L, Han Z, Liu X, Pan Y, et al. Recombinant human elafin ameliorates chronic hyperoxia-induced lung injury by inhibiting nuclear factor-kappa B signaling in neonatal mice. J Interferon Cytokine Res. 2020;40:320–330. doi: 10.1089/jir.2019.0241. [DOI] [PubMed] [Google Scholar]
- Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, et al. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol. 2005;174:7250–7256. doi: 10.4049/jimmunol.174.11.7250. [DOI] [PubMed] [Google Scholar]
- Lingappan K. NF-κB in oxidative stress. Curr Opin Toxicol. 2018;7:81–86. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev. 2011;2011:809696. doi: 10.1155/2011/809696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jiang P, Du M, Chen K, Chen A, Wang Y, et al. Hyperoxia-induced immature brain injury through the TLR4 signaling pathway in newborn mice. Brain Res. 2015;1610:51–60. doi: 10.1016/j.brainres.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Liu DY, Lou WJ, Zhang DY, Sun SY. ROS plays a role in the neonatal rat intestinal barrier damages induced by hyperoxia. Biomed Res Int. 2020;2020. 10.1155/2020/8819195. [DOI] [PMC free article] [PubMed]
- Londhe VA, Sundar IK, Lopez B, Maisonet TM, Yu Y, Aghai ZH. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr Res. 2011;69:371–377. doi: 10.1203/PDR.0b013e318211c917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Beyer AM, Durand M, Clough Av, Zhu D, Toro LN, et al. Hyperoxia causes mitochondrial fragmentation in pulmonary endothelial cells by increasing expression of pro-fission proteins. Arterioscler Thromb Vasc Biol. 2018;38:622–635. doi: 10.1161/ATVBAHA.117.310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena LA, Selim SM, Fonseca J, Messner H, McGowan S, Stuart JA. Hydrogen peroxide production is affected by oxygen levels in mammalian cell culture. Biochem Biophys Res Commun. 2017;493:246–251. doi: 10.1016/j.bbrc.2017.09.037. [DOI] [PubMed] [Google Scholar]
- Mak S, Egri Z, Tanna G, Colman R, Newton GE. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol. 2002;282:H2414–H2421. doi: 10.1152/ajpheart.00947.2001. [DOI] [PubMed] [Google Scholar]
- Maniscalco WM, Watkins RH, Roper JM, Staversky R, O’Reilly MA. Hyperoxic ventilated premature baboons have increased p53, oxidant DNA damage and decreased VEGF expression. Pediatr Res. 2005;58:549–556. doi: 10.1203/01.pdr.0000176923.79584.f7. [DOI] [PubMed] [Google Scholar]
- Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturu P, Wei-Liang Y, Androutsopoulos VP, Jiang W, Wang L, Tsatsakis AM, et al. Quercetin attenuates the hyperoxic lung injury in neonatal mice: implications for bronchopulmonary dysplasia (BPD) Food Chem Toxicol. 2018;114:23–33. doi: 10.1016/j.fct.2018.02.026. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. doi: 10.1016/S0021-9258(18)63504-5. [DOI] [PubMed] [Google Scholar]
- Mcgrath SA. Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol. 1998;18:179–187. doi: 10.1165/ajrcmb.18.2.2964m. [DOI] [PubMed] [Google Scholar]
- McGrath-Morrow SA, Lauer T, Collaco JM, Lopez A, Malhotra D, Alekseyev YO, et al. Transcriptional responses of neonatal mouse lung to hyperoxia by Nrf2 status. Cytokine. 2014;65:4–9. doi: 10.1016/j.cyto.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna S, Michaelis KA, Agboke F, Liu T, Han K, Yang G, et al. Sustained hyperoxia-induced NF-κB activation improves survival and preserves lung development in neonatal mice. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1078–L1089. doi: 10.1152/ajplung.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J. 2002;367:541–548. doi: 10.1042/bj20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendha AA, Duncan M, Zambrano R, Benny M, Scmidt A, Young K, et al. Targeting pyroptosis to prevent hyperoxia-induced lung injury in neonatal rats. Pediatrics. 2021;147:734–735. doi: 10.1542/peds.147.3MA8.734b. [DOI] [Google Scholar]
- Menon RT, Shrestha AK, Reynolds CL, Barrios R, Shivanna B. Long-term pulmonary and cardiovascular morbidities of neonatal hyperoxia exposure in mice. Int J Biochem Cell Biol. 2018;94:119–124. doi: 10.1016/j.biocel.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis KA, Agboke F, Liu T, Han K, Muthu M, Galambos C, et al. IκBβ-mediated NF-κB activation confers protection against hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014;50:429–438. doi: 10.1165/rcmb.2013-0303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabayashi S, Hanamizu H, Nakamura R, Endo H, Tada K. Defects of mitochondrial respiratory enzymes in cloned cells from MELAS fibroblasts. J Inherit Metab Dis. 1992;15:797–802. doi: 10.1007/BF01800024. [DOI] [PubMed] [Google Scholar]
- Montezano AC, Touyz RM. Reactive oxygen species and endothelial function - role of nitric oxide synthase uncoupling and nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol. 2012;110:87–94. doi: 10.1111/j.1742-7843.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- Moradi F, Fiocchetti M, Marino M, Moffatt C, Stuart JA. Media composition and O2 levels determine effects of 17b-estradiol and selective estrogen receptor modulators on mitochondrial bioenergetics and cellular reactive oxygen species. Am J Physiol Cell Physiol. 2021;321:C72–C81. doi: 10.1152/ajpcell.00080.2021. [DOI] [PubMed] [Google Scholar]
- Moradi F, Moffatt C, Stuart JA. The effect of oxygen and micronutrient composition of cell growth media on cancer cell bioenergetics and mitochondrial networks. Biomolecules. 2021;11:1177. doi: 10.3390/biom11081177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Oikawa M, Tamagami T, Kumaki H, Nakaune R, Amano J, et al. Oxidized proteins in astrocytes generated in a hyperbaric atmosphere induce neuronal apoptosis. J Alzheimer’s Dis. 2007;11:165–174. doi: 10.3233/JAD-2007-11204. [DOI] [PubMed] [Google Scholar]
- Morton RL, Iklé D, White CW. Loss of lung mitochondrial aconitase activity due to hyperoxia in bronchopulmonary dysplasia in primates Loss of lung mitochondrial aconitase activity due to hyper-oxia in bronchopulmonary dysplasia in primates. Am J Physiol. 1998;274:L127–L133. doi: 10.1152/ajplung.1998.274.1.L127. [DOI] [PubMed] [Google Scholar]
- Mu G, Deng Y, Lu Z, Li X, Chen Y. MiR-20b suppresses mitochondrial dysfunction-mediated apoptosis to alleviate hyperoxia-induced acute lung injury by directly targeting MFN1 and MFN2. Acta Biochim Biophys Sin (Shanghai) 2021;53:220–228. doi: 10.1093/abbs/gmaa161. [DOI] [PubMed] [Google Scholar]
- Myti D, Gunjak M, Francisco Casado X, Khaghani Raziabad S, Claudio Nardiello X, Vadász I, et al. Elevated FiO2 increases SARS-CoV-2 co-receptor expression in respiratory tract epithelium. Am J Physiol Lung Cell Mol Physiol. 2020;319:L670–L674. doi: 10.1152/ajplung.00345.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau PJ, Charette SJ, Toledano MB, Landry J. Disulfide bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H2O2-induced c-Jun NH 2-terminal kinase activation and apoptosis. Mol Biol Cell. 2007;18:3903–3913. doi: 10.1091/mbc.e07-05-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau PJ, Charette SJ, Landry J. REDOX Reaction at ASK1-Cys250 is essential for activation of JNK and induction of apoptosis. Mol Biol Cell. 2009;20:3628–3637. doi: 10.1091/mbc.e09-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y. Initiation of apoptotic signal by the peroxidation of cardiolipin of mitochondria. Ann N Y Acad Sci. 2004;1011:177–184. doi: 10.1196/annals.1293.018. [DOI] [PubMed] [Google Scholar]
- Narasaraju T, Shukla D, More S, Huang C, Zhang L, Xiao X, et al. Role of microRNA-150 and glycoprotein nonmetastatic melanoma protein B in angiogenesis during hyperoxia-induced neonatal lung injury. Am J Respir Cell Mol Biol. 2015;52:253–261. doi: 10.1165/rcmb.2013-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Stevens Mv, Kohr M, Steenbergen C, Sack MN, Murphy E. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem. 2011;286:40184–40192. doi: 10.1074/jbc.M111.243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie C, Tian C, Zhao L, Petit PX, Mehrpour M, Chen Q. Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J Biol Chem. 2008;283:15359–15369. doi: 10.1074/jbc.M800847200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E. Lipid peroxidation products as oxidative stress biomarkers. BioFactors. 2008;34:171–180. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- Nold MF, Mangan NE, Rudloff I, Cho SX, Shariatian N, Samarasinghe TD, et al. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc Natl Acad Sci U S A. 2013;110:14384–14389. doi: 10.1073/pnas.1306859110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyunoya T, Powers LS, Yarovinsky TO, Butler NS, Monick MM, Hunninghake GW. Hyperoxia induces macrophage cell cycle arrest by adhesion-dependent induction of p21Cip1 and activation of the retinoblastoma protein. J Biol Chem. 2003;278:36099–36106. doi: 10.1074/jbc.M304370200. [DOI] [PubMed] [Google Scholar]
- O’Reilly M, Thébaud B. Animal models of bronchopulmonary dysplasia. The term rat models. Am J Physiol Lung Cell Mol Physiol. 2014;307:L948–L958. doi: 10.1152/ajplung.00160.2014. [DOI] [PubMed] [Google Scholar]
- O’Reilly MA, Staversky RJ, Stripp BR, Finkelstein JN. Exposure to hyperoxia induces p53 expression in mouse lung epithelium. Am J Respir Cell Mol Biol. 1998;18:43–50. doi: 10.1165/ajrcmb.18.1.2950m. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Tasaka S, Yamada W, Saito F, Hasegawa N, Miyasho T, et al. Role of Toll-like receptor 4 in hyperoxia-induced lung inflammation in mice. Inflamm Res. 2007;56:334–338. doi: 10.1007/s00011-007-7052-z. [DOI] [PubMed] [Google Scholar]
- Ospina-Tascón GA, Calderón-Tapia LE, García AF, Zarama V, Gómez-Álvarez F, Álvarez-Saa T, et al. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. J Am Med Assoc. 2021;326:2161–2171. doi: 10.1001/jama.2021.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto-Ślusarczyk D, Graboń W, Mielczarek-Puta M, Chrzanowska A: Teriflunomide – The common drug with underestimated oxygen - dependent anticancer potential. Biochem Biophys Rep. 2021;28. 10.1016/j.bbrep.2021.101141. [DOI] [PMC free article] [PubMed]
- Özdemir ÖMA, Gözkeser E, Bir F, Yenisey Ç. The effects of resveratrol on hyperoxia-induced lung injury in neonatal rats. Pediatr Neonatol. 2014;55:352–357. doi: 10.1016/j.pedneo.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267:423–425. doi: 10.1038/267423a0. [DOI] [PubMed] [Google Scholar]
- Pagano A, Donati Y, Métrailler I, Argiroffo CB. Mitochondrial cytochrome c release is a key event in hyperoxia-induced lung injury: protection by cyclosporin A. Am J Physiol Lung Cell Mol Physiol. 2004;286:L275–L283. doi: 10.1152/ajplung.00181.2003. [DOI] [PubMed] [Google Scholar]
- Panayiotidis MI, Rancourt RC, Allen CB, Riddle SR, Schneider BK, Ahmad S, et al. Hyperoxia-induced DNA damage causes decreased DNA methylation in human lung epithelial-like A549 cells. Antioxid Redox Signal. 2004;6:129–136. doi: 10.1089/152308604771978435. [DOI] [PubMed] [Google Scholar]
- Panguluri SK, Tur J, Fukumoto J, Deng W, Sneed KB, Kolliputi N, et al. Hyperoxia-induced hypertrophy and ion channel remodeling in left ventricle. Am J Physiol Heart Circ Physiol. 2013;304:H1651–H1661. doi: 10.1152/ajpheart.00474.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panni S, Lovering RC, Porras P, Orchard S. Non-coding RNA regulatory networks. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194417. doi: 10.1016/j.bbagrm.2019.194417. [DOI] [PubMed] [Google Scholar]
- Pao HP, Liao WI, Tang SE, Wu SY, Huang KL, Chu SJ. Suppression of endoplasmic reticulum stress by 4-PBA protects against hyperoxia-induced acute lung injury via up-regulating claudin-4 expression. Front Immunol. 2021;12:674316. doi: 10.3389/fimmu.2021.674316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P, Britt RD, Manlove LJ, Wicher SA, Roesler A, Ravix J, et al. Hyperoxia-induced cellular senescence in fetal airway smooth muscle cells. Am J Respir Cell Mol Biol. 2019;61:51–60. doi: 10.1165/rcmb.2018-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinandi NL, Kleinberg MA, Usatyuk Pv, Cummings RJ, Pennathur A, Cardounel AJ, et al. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L26–L38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- Park JY, Sohn HY, Koh YH, Jo C. Curcumin activates Nrf2 through PKCδ-mediated p62 phosphorylation at Ser351. Sci Rep. 2021;11:8430. doi: 10.1038/s41598-021-87225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Dial K, Wu J, Gauthier AG, Wu W, Lin M, et al. Dietary antioxidants significantly attenuate hyperoxia-induced acute inflammatory lung injury by enhancing macrophage function via reducing the accumulation of airway HMGB1. Int J Mol Sci. 2020;21:977. doi: 10.3390/ijms21030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen CE, Carroll KS. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev. 2013;113:4633–4679. doi: 10.1021/cr300163e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala S, Gorshkova IA, Usatyuk Pv, He D, Pennathur A, Lambeth JD, et al. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2009;11:747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone S, Bracciali C, di Virgilio N, Buonocore G. Oxygen use in neonatal care: a two-edged sword. Front Pediatr. 2017;4:143. doi: 10.3389/fped.2016.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AL, Carr JF, Ji X, Dennery PA, Yao H. Hyperoxic exposure caused lung lipid compositional changes in neonatal mice. Metabolites. 2020;10:340. doi: 10.3390/metabo10090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, et al. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- Pinterić M, Podgorski II, Sobočanec S, Popović Hadžija M, Paradžik M, Dekanić A, et al. De novo expression of transfected sirtuin 3 enhances susceptibility of human MCF-7 breast cancer cells to hyperoxia treatment. Free Radic Res. 2018;52:672–684. doi: 10.1080/10715762.2018.1462495. [DOI] [PubMed] [Google Scholar]
- Pizzinat N, Copin N, Vindis C, Parini A, Cambon C. Reactive oxygen species production by monoamine oxidases in intact cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:428–431. doi: 10.1007/PL00005371. [DOI] [PubMed] [Google Scholar]
- Polat İ, Cilaker Mıcılı S, Çalışır M, Bayram E, Yiş U, Ayanoğlu M, et al. Neuroprotective effects of lacosamide and memantine on hyperoxic brain injury in rats. Neurochem Res. 2020;45:1920–1929. doi: 10.1007/s11064-020-03056-5. [DOI] [PubMed] [Google Scholar]
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. a model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzionato A, Sfriso MM, Mazzatenta A, Macchi V, de Caro R, di Giulio C. Effects of hyperoxic exposure on signal transduction pathways in the lung. Respir Physiol Neurobiol. 2015;209:106–114. doi: 10.1016/j.resp.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Qiao J, Chen L, Huang X, Guo F. Effects of nebulized N-acetylcystein on the expression of HMGB1 and RAGE in rats with hyperoxia-induced lung injury. J Cell Physiol. 2019;234:10547–10553. doi: 10.1002/jcp.27724. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Kundumani-Sridharan V, Kumar S, White CW, Das KC. Thioredoxin prevents loss of UCP2 in hyperoxia via MKK4-p38 MAPK-PGC1a signaling and limits oxygen toxicity. Am J Respir Cell Mol Biol. 2022;66:323–336. doi: 10.1165/rcmb.2021-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani M, van Groen T, Kadish I, Bulger A, Ambalavanan N. Neurodevelopmental impairment following neonatal hyperoxia in the mouse. Neurobiol Dis. 2013;50:69–75. doi: 10.1016/j.nbd.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani M, Bradley WE, Dell’Italia LJ, Ambalavanan N. Early exposure to hyperoxia or hypoxia adversely impacts cardiopulmonary development. Am J Respir Cell Mol Biol. 2015;52:594–602. doi: 10.1165/rcmb.2013-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani M, Miller K, Brown J, Kumar R, Kadasamy J, McMahon L, et al. Early life supraphysiological levels of oxygen exposure permanently impairs hippocampal mitochondrial function. Sci Rep. 2019;9:13364. doi: 10.1038/s41598-019-49532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancourt RC, Keng PC, Helt CE. O MA: The role of p21CIP1/WAF1 in growth of epithelial cells exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2001;280:L617–L626. doi: 10.1152/ajplung.2001.280.4.L617. [DOI] [PubMed] [Google Scholar]
- Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol. 2009;182:7264–71. [DOI] [PMC free article] [PubMed]
- Rehan VK, Sakurai R, Corral J, Krebs M, Ibe B, Ihida-Stansbury K, et al. Antenatally administered PPAR-gamma agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L672–L680. doi: 10.1152/ajplung.00240.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich B, Hoeber D, Bendix I, Felderhoff-Mueser U. Hyperoxia and the immature brain. Dev Neurosci. 2017;38:311–330. doi: 10.1159/000454917. [DOI] [PubMed] [Google Scholar]
- Ren J, Jiang J, Ou W, Luo X, Xiang J, Liu G, et al. The effect of STAT3 signal pathway activation on retinopathy of prematurity. Front Pediatr. 2021;9:638432. doi: 10.3389/fped.2021.638432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resseguie EA, Staversky RJ, Brookes PS, O’Reilly MA. Hyperoxia activates ATM independent from mitochondrial ROS and dysfunction. Redox Biol. 2015;5:176–185. doi: 10.1016/j.redox.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995;27:647–653. doi: 10.1016/1357-2725(95)00025-K. [DOI] [PubMed] [Google Scholar]
- Richter J, Toelen J, Nagatomo T, Jimenez J, Vanoirbeek J, Deprest J. Transplacental administration of rosiglitazone attenuates hyperoxic lung injury in a preterm rabbit model. Fetal Diagn Ther. 2016;39:297–305. doi: 10.1159/000439199. [DOI] [PubMed] [Google Scholar]
- Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42:387–396. doi: 10.1097/CCM.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- Rogers LK, Tipple TE, Britt RD, Welty SE. Hyperoxia exposure alters hepatic eicosanoid metabolism in newborn mice. Pediatr Res. 2010;67:144–149. doi: 10.1203/PDR.0b013e3181c2df4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper JM, Mazzatti DJ, Watkins RH, Maniscalco WM, Keng PC. O MA: In vivo exposure to hyperoxia induces DNA damage in a population of alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1045–L1054. doi: 10.1152/ajplung.00376.2003. [DOI] [PubMed] [Google Scholar]
- Ruiz-Camp J, Quantius J, Lignelli E, Arndt PF, Palumbo F, Nardiello C, et al. Targeting miR-34a/ Pdgfra interactions partially corrects alveologenesis in experimental bronchopulmonary dysplasia. EMBO Mol Med. 2019;11:e9448. doi: 10.15252/emmm.201809448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai R, Li Y, Torday JS, Rehan VK. Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-beta signaling. Am J Physiol Lung Cell Mol Physiol. 2011;301:L721–L730. doi: 10.1152/ajplung.00076.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai R, Villarreal P, Husain S, Liu J, Sakurai T, Tou E, et al. Curcumin protects the developing lung against long-term hyperoxic injury in vivo hyperoxia exposure system and animal protocol. Am J Physiol Lung Cell Mol Physiol. 2013;305:L301–L311. doi: 10.1152/ajplung.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhin P, Gardner GL, Moffatt C, Stuart JA. An inexpensive incubator for mammalian cell culture capable of regulating O2, CO2, and temperature. Oxygen. 2022;2:22–30. doi: 10.3390/oxygen2010003. [DOI] [Google Scholar]
- Sanders SP, Zweier JL, Kuppusamy P, Harrison SJ, Bessert DJP, Gabrielson EW, et al. Hyperoxic sheep pulmonary microvascular endothelial cells generate free radicals via mitochondrial electron transport. J Clin Investig. 1993;91:46–52. doi: 10.1172/JCI116198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saretzki G, Feng J, von Zglinicki T, Villeponteau B. Similar gene expression pattern in senescent and hyperoxic-treated fibroblasts. J Gerontol Biol Sci. 1998;53:438–442. doi: 10.1093/gerona/53A.6.B438. [DOI] [PubMed] [Google Scholar]
- Saugstad OD. Oxygen and retinopathy of prematurity. J Perinatol. 2006;26:S46–S50. doi: 10.1038/sj.jp.7211475. [DOI] [PubMed] [Google Scholar]
- Scaffa AM, Peterson AL, Carr JF, Garcia D, Yao H, Dennery PA. Hyperoxia causes senescence and increases glycolysis in cultured lung epithelial cells. Physiol Rep. 2021;9:e14839. doi: 10.14814/phy2.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz T, Endesfelder S, Reinert MC, Klinker F, Müller S, Bührer C, et al. Adolescent hyperactivity and impaired coordination after neonatal hyperoxia. Exp Neurol. 2012;235:374–379. doi: 10.1016/j.expneurol.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Schoonen WGEJ, Wanamarta AH, van der Klei-Van Moorsel JM, Jakobs C, Joenje H. Hyperoxia-induced clonogenic killing of HeLa cells associated with respiratory failure and selective inactivation of Krebs cycle enzymes. Mutat Res/DNAging. 1990;237:173–181. doi: 10.1016/0921-8734(90)90023-K. [DOI] [PubMed] [Google Scholar]
- Sears JE, Hoppe G, Ebrahem Q, Anand-Apte B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy. Proc Natl Acad Sci U S A. 2008;105:19898–19903. doi: 10.1073/pnas.0805817105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdar M, Herz J, Kempe K, Lumpe K, Reinboth BS, Sizonenko Sv, et al. Fingolimod protects against neonatal white matter damage and long-term cognitive deficits caused by hyperoxia. Brain Behav Immun. 2016;52:106–119. doi: 10.1016/j.bbi.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Flora SJS. Positive and negative regulation of ferroptosis and its role in maintaining metabolic and redox homeostasis. Oxid Med Cell Longev. 2021;2021:9074206. doi: 10.1155/2021/9074206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J, Barr SM, Geng Y, Yun Y, Higgins RD. Ibuprofen improves oxygen-induced retinopathy in a mouse model. Curr Eye Res. 2003;27:309–314. doi: 10.1076/ceyr.27.5.309.17222. [DOI] [PubMed] [Google Scholar]
- Shenberger JS, Dixon PS. Oxygen induces S-phase growth arrest and increases p53 and p21 WAF1/CIP1 expression in human bronchial smooth-muscle cells. Am J Respir Cell Mol Biol. 1999;21:395–402. doi: 10.1165/ajrcmb.21.3.3604. [DOI] [PubMed] [Google Scholar]
- Shidoji Y, Hayashi K, Komura S, Ohishi N, Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem Biophys Res Commun. 1999;264:343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- Sidramagowda Patil S, Hernández-Cuervo H, Fukumoto J, Narala VR, Saji S, Borra M, et al. Alda-1 attenuates hyperoxia-induced mitochondrial dysfunction in lung vascular endothelial cells. Aging. 2019;11:3909–3918. doi: 10.18632/aging.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidramagowda Patil S, Hernández-Cuervo H, Fukumoto J, Krishnamurthy S, Lin M, Alleyn M, et al. Alda-1 attenuates hyperoxia-induced acute lung injury in mice. Front Pharmacol. 2021;11:597942. doi: 10.3389/fphar.2020.597942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidramagowda Patil S, Soundararajan R, Fukumoto J, Breitzig M, Hernández-Cuervo H, Alleyn M, et al. Mitochondrial protein Akap1 deletion exacerbates endoplasmic reticulum stress in mice exposed to hyperoxia. Front Pharmacol. 2022;13:762840. doi: 10.3389/fphar.2022.762840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- Sifringer M, Bendix I, Börner C, Endesfelder S, von Haefen C, Kalb A, et al. Prevention of neonatal oxygen-induced brain damage by reduction of intrinsic apoptosis. Cell Death Dis. 2012;3:e250. doi: 10.1038/cddis.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Mhatre V, Bhori M, Marar T. Vitamins E and C reduce oxidative stress and mitochondrial permeability transition caused by camptothecin - an in vitro study. Toxicol Environ Chem. 2013;95:646–657. doi: 10.1080/02772248.2013.805013. [DOI] [Google Scholar]
- Smit B, Smulders YM, van der Wouden JC, Oudemans-van Straaten HM, Spoelstra-de Man AME: Hemodynamic effects of acute hyperoxia: systematic review and meta-analysis. Crit Care. 2018;22. 10.1186/s13054-018-1968-2. [DOI] [PMC free article] [PubMed]
- Smith JL. The pathological effects due to increase of oxygen tension in the air breathed. J Physiol. 1899;24:19–35. doi: 10.1113/jphysiol.1899.sp000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares NP, Campos KKD, Pena KB, Bandeira ACB, Talvani A, Silva ME, et al. The effects of the combination of a refined carbohydrate diet and exposure to hyperoxia in mice. Oxid Med Cell Longev. 2016;2016:1014928. doi: 10.1155/2016/1014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci U S A. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovska M, Thaçi Q, Hadzi-Petrushev N, Angelovski M, Bogdanov J, Reçica S, et al. Curcumin analogs (B2BrBC and C66) supplementation attenuates airway hyperreactivity and promote airway relaxation in neonatal rats exposed to hyperoxia. Physiol Rep. 2020;8:e14555. doi: 10.14814/phy2.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JA, Aibueku O, Bagshaw O, Moradi F: Hypoxia inducible factors as mediators of reactive oxygen/nitrogen species homeostasis in physiological normoxia. Med Hypotheses. 2019;129. 10.1016/j.mehy.2019.109249. [DOI] [PubMed]
- Suzuki YJ, Carini M, Butterfield DA. Protein Carbonylation. Antioxid Redox Signal. 2010;12:323–325. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed M, Das P, Pawar A, Aghai ZH, Kaskinen A, Zhuang ZW, et al. Hyperoxia causes MIR-34a-mediated injury via angiopoietin-1 in neonatal lungs. Nat Commun. 2017;8:1173. doi: 10.1038/s41467-017-01349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takyar S, Zhang Y, Haslip M, Jin L, Shan P, Zhang X, et al. An endothelial TLR4-VEGFR2 pathway mediates lung protection against oxidant-induced injury. FASEB J. 2016;30:1317–1327. doi: 10.1096/fj.15-275024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamatam CM, Reddy NM, Potteti HR, Ankireddy A, Noone PM, Yamamoto M, et al. Preconditioning the immature lung with enhanced Nrf2 activity protects against oxidant-induced hypoalveolarization in mice. Sci Rep. 2020;10:19034. doi: 10.1038/s41598-020-75834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watanabe T, Ozawa J, Ito M, Nagano N, Arai Y, et al. Difference in pyruvic acid metabolism between neonatal and adult mouse lungs exposed to hyperoxia. PLoS ONE. 2020;15:e0238604. doi: 10.1371/journal.pone.0238604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Fang Y, Huo C. Long non-coding RNA Rian protects against experimental bronchopulmonary dysplasia by sponging miR-421. Exp Ther Med. 2021;22:781. doi: 10.3892/etm.2021.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarkova Z, Engler I, Calkovska A, Mokra D, Drgova A, Hodas P, et al. Effect of long-term normobaric hyperoxia on oxidative stress in mitochondria of the guinea pig brain. Neurochem Res. 2011;36:1475–1481. doi: 10.1007/s11064-011-0473-7. [DOI] [PubMed] [Google Scholar]
- Teixeira J, Oliveira C, Cagide F, Amorim R, Garrido J, Borges F, et al. Discovery of a new mitochondria permeability transition pore (mPTP) inhibitor based on gallic acid. J Enzyme Inhib Med Chem. 2018;33:567–576. doi: 10.1080/14756366.2018.1442831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng R-J, Jing X, Michalkiewicz T, Afolayan AJ, Wu T-J, Konduri GG. Attenuation of endoplasmic reticulum stress by caffeine ameliorates hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312:L586–L598. doi: 10.1152/ajplung.00405.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirsk R, Kuipers A, Mukai C, Williams D. The space-flight environment: the international space station and beyond. CMAJ. 2009;180:1216–1220. doi: 10.1503/cmaj.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tin W, Gupta S. Optimum oxygen therapy in preterm babies. Arch Dis Child Fetal Neonatal Ed. 2007;92:F143–F147. doi: 10.1136/adc.2005.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomin T, Schittmayer M, Honeder S, Heininger C, Birner-Gruenberger R. Irreversible oxidative post-translational modifications in heart disease. Expert Rev Proteomics. 2019;16:681–693. doi: 10.1080/14789450.2019.1645602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Li M, Liu N, Huang W, Xue X, Fu J. Hyperoxia induces endoplasmic reticulum stress-associated apoptosis via the IRE1α pathway in rats with bronchopulmonary dysplasia. Mol Med Rep. 2021;23:33. doi: 10.3892/mmr.2020.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichonas G, Lee TJ, Hoppe G, Au J, Sears JE. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy in the rat 50/10 model. Invest Ophthalmol Vis Sci. 2013;54:4919–4926. doi: 10.1167/iovs.13-12171. [DOI] [PubMed] [Google Scholar]
- Tripathi BJ, Tripathi RC. Cellular and subcellular events in retinopathy of oxygen toxicity with a preliminary report on the preventive role of vitamin E and gamma-aminobutyric acid: a study in vitro. Curr Res Eye. 1984;3:193–208. doi: 10.3109/02713688408997201. [DOI] [PubMed] [Google Scholar]
- Tung YT, Wei CH, Yen CC, Lee PY, Ware LB, Huang HE, et al. Aspirin attenuates hyperoxia-induced acute respiratory distress syndrome (ARDS) by suppressing pulmonary inflammation via the NF-κB signaling pathway. Front Pharmacol. 2022;12:793107. doi: 10.3389/fphar.2021.793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Freeman BA, Crapo JD. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982;217:411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Tyurin VA, Murat Kaynar A, Kapralova VI, Wasserloos K, Li J, et al. Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L73–L85. doi: 10.1152/ajplung.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usatyuk Pv, Romer LH, He D, Parinandi NL, Kleinberg ME, Zhan S, et al. Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem. 2007;282:23284–23295. doi: 10.1074/jbc.M700535200. [DOI] [PubMed] [Google Scholar]
- Vacchiano CA, Tempel GE. Role of nonenzymatically generated prostanoid, 8-iso-PGF, in pulmonary oxygen toxicity. J Appl Physiol. 1994;77:2912–2917. doi: 10.1152/jappl.1994.77.6.2912. [DOI] [PubMed] [Google Scholar]
- Valle VG, Fagian MM, Parentoni LS, Meinicke AR, Vercesi AE. The participation of reactive oxygen species and protein thiols in the mechanism of mitochondrial inner membrane permeabilization by calcium plus prooxidants. Arch Biochem Biophys. 1993;307:1–7. doi: 10.1006/abbi.1993.1551. [DOI] [PubMed] [Google Scholar]
- van Ooij PJAM, Sterk PJ, van Hulst RA. Oxygen, the lung and the diver: friends and foes? Eur Respir Rev. 2016;25:496–505. doi: 10.1183/16000617.0049-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma G, Datta M. The critical role of JNK in the ER-mitochondrial crosstalk during apoptotic cell death. J Cell Physiol. 2012;227:1791–1795. doi: 10.1002/jcp.22903. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Döcke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- Vysotskaya Z, Chidipi B, Rodgers JL, Tang X, Samal E, Kolliputi N, et al. Elevated potassium outward currents in hyperoxia treated atrial cardiomyocytes. J Cell Physiol. 2018;233:4317–4326. doi: 10.1002/jcp.26263. [DOI] [PubMed] [Google Scholar]
- Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene. 2018;678:177–183. doi: 10.1016/j.gene.2018.08.031. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang L. Role of vitamin D–vitamin D receptor signaling on hyperoxia-induced bronchopulmonary dysplasia in neonatal rats. Pediatr Pulmonol. 2021;56:2335–2344. doi: 10.1002/ppul.25418. [DOI] [PubMed] [Google Scholar]
- Wang Y, Manevich Y, Feinstein SI, Fisher AB. Adenovirus-mediated transfer of the 1-cys peroxiredoxin gene to mouse lung protects against hyperoxic injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1188–L1193. doi: 10.1152/ajplung.00288.2003. [DOI] [PubMed] [Google Scholar]
- Wang X, Huo R, Liang Z, Xu C, Chen T, Lin J, et al. Simvastatin inhibits NLRP3 inflammasome activation and ameliorates lung injury in hyperoxia-induced bronchopulmonary dysplasia via the KLF2-mediated mechanism. Oxid Med Cell Longev. 2022;2022:8336070. doi: 10.1155/2022/8336070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Wang YX, Ma Y, Di Y. The long-noncoding RNA TUG1 regulates oxygen-induced retinal neovascularization in mice via MiR-299. Invest Ophthalmol vis Sci. 2022;63:37. doi: 10.1167/iovs.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hong H, Li XX, Li J, Zhang ZQ: Involvement of Hdac3-mediated inhibition of microRNA cluster 17–92 in bronchopulmonary dysplasia development. Mol Med. 2020;26. 10.1186/s10020-020-00237-4. [DOI] [PMC free article] [PubMed]
- Ward JA, Roberts RJ. Vitamin E inhibition of the effects of hyperoxia on the pulmonary surfactant system of the newborn rabbit. Pediatr Res. 1984;18:329–334. doi: 10.1203/00006450-198404000-00005. [DOI] [PubMed] [Google Scholar]
- Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–4796. doi: 10.1016/S0021-9258(19)43735-6. [DOI] [PubMed] [Google Scholar]
- Wen X, Zhang H, Xiang B, Zhang W, Gong F, Li S, et al. Hyperoxia-induced miR-342-5p down-regulation exacerbates neonatal bronchopulmonary dysplasia via the Raf1 regulator Spred3. Br J Pharmacol. 2021;178:2266–2283. doi: 10.1111/bph.15371. [DOI] [PubMed] [Google Scholar]
- Wingelaar TT, van Ooij PJAM, van Hulst RA. Oxygen toxicity and special operations forces diving: hidden and dangerous. Front Physiol. 2017;8:1263. doi: 10.3389/fpsyg.2017.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wispe JR, Knight M, Roberts RJ. Lipid peroxidation in newborn rabbits: effects of oxygen, lipid emulsion, and vitamin E. Pediatr Res. 1986;20:505–510. doi: 10.1203/00006450-198606000-00004. [DOI] [PubMed] [Google Scholar]
- Wong YL, Smith Cv, McMicken HW, Rogers LK, Welty SE. Mitochondrial thiol status in the liver is altered by exposure to hyperoxia. Toxicol Lett. 2001;123:179–193. doi: 10.1016/S0378-4274(01)00397-6. [DOI] [PubMed] [Google Scholar]
- Wright CJ, Agboke F, Chen F, La P, Yang G, Dennery PA. No inhibits hyperoxia-induced NF-B activation in neonatal pulmonary microvascular endothelial cells. Pediatr Res. 2010;68:484–489. doi: 10.1203/PDR.0b013e3181f917b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhang G, Xiong H, Zhang Y, Ding G, Ge J: miR-181c-5p mediates apoptosis of vascular endothelial cells induced by hyperoxemia via ceRNA crosstalk. Sci Rep. 2021;11. 10.1038/s41598-021-95712-1. [DOI] [PMC free article] [PubMed]
- Xu W, Zhao Y, Zhang B, Xu B, Yang Y, Wang Y, et al. Resveratrol attenuates hyperoxia-induced oxidative stress, inflammation and fibrosis and suppresses Wnt/β-catenin signalling in lungs of neonatal rats. Clin Exp Pharmacol Physiol. 2015;42:1075–1083. doi: 10.1111/1440-1681.12459. [DOI] [PubMed] [Google Scholar]
- Yadav A, Rana U, Michalkiewicz T, Teng RJ, Konduri GG. Decreased AMP-activated protein kinase (AMPK) function and protective effect of metformin in neonatal rat pups exposed to hyperoxia lung injury. Physiol Rep. 2020;8:e14587. doi: 10.14814/phy2.14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, Jaeschke H. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci. 2010;117:515–523. doi: 10.1093/toxsci/kfq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Abate A, George AG, Weng Y-H, Dennery PA. Maturational differences in lung NF-κB activation and their role in tolerance to hyperoxia. J Clin Investig. 2004;114:669–678. doi: 10.1172/JCI200419300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Yang M, Shen Y, Kang L, Zhu X, Dong W, et al. Resveratrol attenuates hyperoxia lung injury in neonatal rats by activating SIRT1/PGC-1α signaling pathway. Am J Perinatol. 2022 doi: 10.1055/a-1787-3396. [DOI] [PubMed] [Google Scholar]
- Yao L, Shi Y, Zhao X, Hou A, Xing Y, Fu J, et al. Vitamin D attenuates hyperoxia-induced lung injury through downregulation of Toll-like receptor 4. Int J Mol Med. 2017;39:1403–1408. doi: 10.3892/ijmm.2017.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, et al. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1101–L1111. doi: 10.1152/ajplung.00126.2006. [DOI] [PubMed] [Google Scholar]
- Yee M, David Cohen E, Haak J, Dylag AM, O’Reilly MA. Neonatal hyperoxia enhances age-dependent expression of SARS-CoV-2 receptors in mice. Sci Rep. 2020;10:22401. doi: 10.1038/s41598-020-79595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You K, Parikh P, Khandalavala K, Wicher SA, Manlove L, Yang B, et al. Moderate hyperoxia induces senescence in developing human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2019;317:L525–L536. doi: 10.1152/ajplung.00067.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Li X, Wan Q, Han W, Deng C, Guo C. High-mobility group box-1 protein disrupts alveolar elastogenesis of hyperoxia-injured newborn lungs. J Interferon Cytokine Res. 2016;36:159–168. doi: 10.1089/jir.2015.0080. [DOI] [PubMed] [Google Scholar]
- Yuan W, Liu X, Zeng L, Liu H, Cai B, Huang Y, et al. Silencing of long non-coding RNA X inactive specific transcript (Xist) contributes to suppression of bronchopulmonary dysplasia induced by hyperoxia in newborn mice via microRNA-101-3p and the transforming growth factor-beta 1 (TGF-b1)/Smad3 Axis. Med Sci Monit. 2020;26:e922424. doi: 10.12659/MSM.922424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa T, Beckman JS, Crapo JD, Freeman BA, Hyperoxia BAF. hyperoxia increases H2O2 production by brain in vivo. J Appl Physiol. 1987;63:353–358. doi: 10.1152/jappl.1987.63.1.353. [DOI] [PubMed] [Google Scholar]
- Zangl Q, Martignoni A, Jackson SH, Ohta A, Klaunberg B, Kaufmann I, et al. Postoperative hyperoxia (60%) worsens hepatic injury in mice. Anesthesiology. 2014;121:1217–1225. doi: 10.1097/ALN.0000000000000447. [DOI] [PubMed] [Google Scholar]
- Zara S, de Colli M, Rapino M, di Valerio V, Marconi GD, Cataldi A, et al. NF-κB involvement in hyperoxia-induced myocardial damage in newborn rat hearts. Histochem Cell Biol. 2013;140:575–583. doi: 10.1007/s00418-013-1092-y. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Sasidhar M, Chupp GL, Flavell RA, Choi AMK, et al. Reactive oxygen species and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase mediate hyperoxia-induced cell death in lung epithelium. Am J Respir Cell Mol Biol. 2003;28:305–315. doi: 10.1165/rcmb.2002-0156OC. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, et al. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175:4834–4838. doi: 10.4049/jimmunol.175.8.4834. [DOI] [PubMed] [Google Scholar]
- Zhang Y, OuYang S, Zhang L, Tang XL, Song Z, Liu P. Oxygen-induced changes in mitochondrial DNA and DNA repair enzymes in aging rat lens. Mech Ageing Dev. 2010;131:666–673. doi: 10.1016/j.mad.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Peng W, Zhang S, Wang C, He X, Zhang Z, et al. MicroRNA expression profile in hyperoxia-exposed newborn mice during the development of bronchopulmonary dysplasia. Respir Care. 2011;56:1009–1015. doi: 10.4187/respcare.01032. [DOI] [PubMed] [Google Scholar]
- Zhang T, Lu H, Wang Q, Gao C. Expressions and significance of GRP78 and caspase-12 in the lungs of rats with bronchopulmonary dysplasis. Basic Clin Med. 2013;33:1460–1465. [Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Lee H, Haspel JA, Jin Y. Long noncoding RNA FOXD3-AS1 regulates oxidative stress-induced apoptosis via sponging microRNA-150. FASEB J. 2017;31:4472–4481. doi: 10.1096/fj.201700091R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wu D, Yang Y, Liu T, Liu H. Dexmedetomidine alleviates hyperoxia-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Cell Physiol Biochem. 2017;42:1907–1919. doi: 10.1159/000479609. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang P, Shen Y, Huang T, Hu X, Yu W. Mechanism of lncRNA H19 in regulating pulmonary injury in hyperoxia-induced bronchopulmonary dysplasia newborn mice. Am J Perinatol. 2020;39:1089–1096. doi: 10.1055/s-0040-1721498. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhang X, Chu X, Cheng L, Cai C. Long non-coding RNA MALAT1 plays a protective role in bronchopulmonary dysplasia via the inhibition of apoptosis and interaction with the Keap1/Nrf2 signal pathway. Transl Pediatr. 2021;10:265–275. doi: 10.21037/tp-20-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZQ, Hong H, Li J, Li XX, Huang XM. MicroRNA-214 promotes alveolarization in neonatal rat models of bronchopulmonary dysplasia via the PlGF-dependent STAT3 pathway. Mol Med. 2021;27:109. doi: 10.1186/s10020-021-00374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Fu J, Yang H, Pan Y, Yao L, Xue X. Hyperoxia-induced methylation decreases RUNX3 in a newborn rat model of bronchopulmonary dysplasia. Respir Res. 2015;16:75. doi: 10.1186/s12931-015-0239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Lei X, Wang J, Dong W. Protective effects of resveratrol on hyperoxia-induced lung injury in neonatal rats by alleviating apoptosis and ROS production. J Matern Fetal Neonatal Med. 2020;33:4150–4158. doi: 10.1080/14767058.2019.1597846. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang F, Lei X, Dong W. Resveratrol alleviates alveolar epithelial cell injury induced by hyperoxia by reducing apoptosis and mitochondrial dysfunction. Exp Biol Med. 2021;246:596–606. doi: 10.1177/1535370220975106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimová-Herknerová M, Mysliveček J, Potměšil P. Retinoic acid attenuates the mild hyperoxic lung injury in newborn mice. Physiol Res. 2008;57:33–40. doi: 10.33549/physiolres.930794. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Li J, Fan Q, Zheng X, Deng J, Wang S. Reactive oxygen and nitrogen species induce cell apoptosis via a mitochondria-dependent pathway in hyperoxia lung injury. J Cell Biochem. 2019;120:4837–4850. doi: 10.1002/jcb.27382. [DOI] [PubMed] [Google Scholar]
- Zou DM, Zhou SM, Li LH, Zhou JL, Tang ZM, Wang SH. Knockdown of long noncoding RNAs of maternally expressed 3 alleviates hyperoxia-induced lung injury via inhibiting thioredoxin-interacting protein–mediated pyroptosis by binding to miR-18a. Am J Pathol. 2020;190:994–1005. doi: 10.1016/j.ajpath.2019.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.