Abstract
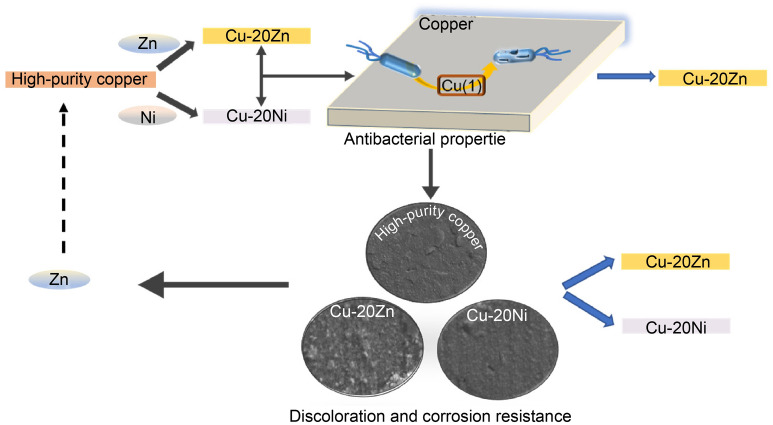
This study focused on the effects of Zn and Ni addition on the antibacterial properties and corrosion resistance of copper alloys. The antimicrobial properties of copper and copper alloys were evaluated using Escherichia coli ATCC 8739 bacterial strain by employing the overlay and plate counting methods. X-ray photoelectron spectroscopy (XPS) was used to analyze the surface composition of the alloy after contact with bacteria. A salt spray method was used to simulate an artificial sweat contact environment to test the discoloration and corrosion resistance of the alloy, and scanning electron microscopy (SEM) was used to analyze the film layer and surface material composition of the corroded samples. The addition of Ni reduced the antibacterial performance of pure copper; however, the antibacterial performance of the alloy remained fast and efficient after the addition of Zn. Moreover, the addition of Zn and Ni significantly improved the corrosion resistance and surface discoloration of copper alloys in artificial sweat environments. This study provided support for the future application of copper alloys as antimicrobial surface-contact materials with safer public and medical environments in the face of diseases spread by large populations.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12598-022-02098-8.
Abstract
本研究重点关注Zn/Ni元素的添加对铜合金抗菌性能和耐腐蚀性的影响。利用大肠杆菌(ATCC 8739),采用覆膜法和平板计数法对铜及铜合金的抗菌性能进行评估。利用X射线光电子能谱(XPS)分析与细菌接触后的合金表面成分。采用盐雾法模拟人工汗液接触环境, 测试合金的变色和耐蚀性能, 并采用扫描电子显微镜(SEM)分析腐蚀后样品的膜层和表面形貌。Ni的加入降低了铜的抗菌性能; 而Zn的加入可使铜的抗菌性能仍然快速高效。此外, 加入Zn和Ni后,明显改善了铜合金在人工汗液环境中的耐蚀性能和表面变色的情况。这项研究为未来铜合金作为抗菌表面接触材料的应用提供了支持,以便在面对大量人口传播的疾病时提供更安全的公共和医疗环境。
Several recent public health events worldwide, such as the COVID-19 pandemic, have caused millions of deaths and been detrimental to the safety of human life and the development of the world economy. These events are caused by close contact with pathogens. To provide a healthy and safe environment for human beings, it is critical to develop surface contact materials that yield good antibacterial effects and can control the spread of pathogens [1, 2]. Copper and copper alloys have attracted the attention of researchers due to their natural and efficient bactericidal ability [3–5]. The survival rates of food-borne pathogens and coronaviruses on the surfaces of copper and copper alloys are lower than those of current commonly used contact materials [6–9]. The copper content is generally considered to be the primary determinant of the antimicrobial properties of copper alloys [10–14], but the influence of the composition on the antibacterial properties remains unclear. Recent studies have shown that the antibacterial effects of copper-containing materials are dependent on the release of soluble copper or cupric ions [13–16]. It is widely accepted that free cupric ions generated from redox cycles between different states of copper (i.e., Cu(0), Cu(I), and Cu(II)) play a major role in bacterial inactivation [17–20], further research is required to determine which valence state of copper plays a major role in the deactivation of copper alloys after contact with bacteria for a short period. Additionally, as a surface contact material, copper alloy is used in hospitals and other crowded environments where there is frequent contact with human skin. Artificial sweat tends to damage the surface of the alloy; this results in corrosion and discoloration at the damaged location that affects the aesthetics and service life of the alloy [21–23]. Therefore, in addition to good antibacterial properties, superior corrosion and discoloration resistance are required to sustain these materials in an artificial sweat environment. Zn and Ni are commonly added elements to copper alloys. Zn is low-cost and exhibits good antibacterial activity [24]; alternatively, Ni can improve the corrosion resistance of copper alloys and is mostly used in the field of marine antifouling.
Therefore, in this study, high-purity copper (HPC), Cu-20Zn (wt%), and Cu-20Ni (wt%) samples were prepared to investigate the effects of Zn and Ni addition on the antibacterial properties of pure copper, as well as its corrosion and discoloration resistance to artificial sweat. This study can provide some data support and a theoretical basis for the composition optimization of antibacterial copper alloys for future applications. Photograph of microstructure of experimental alloy is shown in Fig. S1.
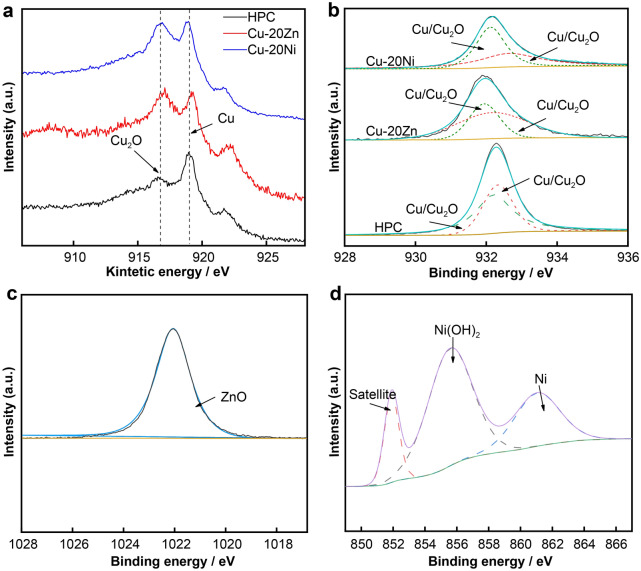
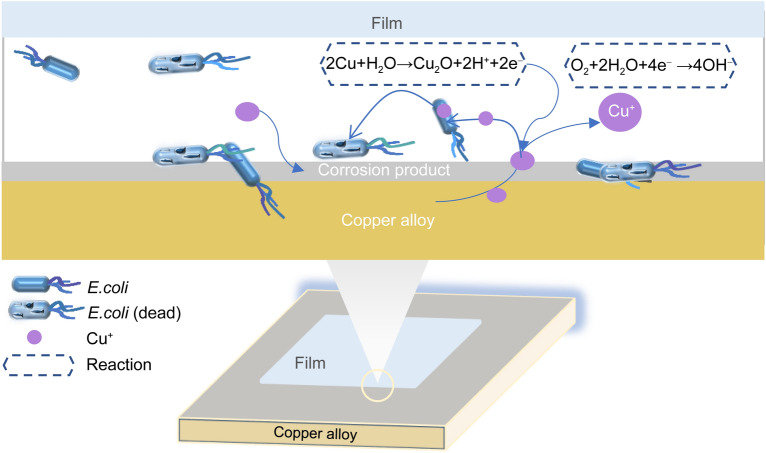
The antibacterial properties were evaluated in accordance with JIS/Z 2801–2000 using the Escherichia coli ATCC 8739 (E. coli) strain by employing the overlay and plate counting methods [25]. Figure 1 shows the colony growth diagram and antibacterial rate results for the experimental material after contact with E. coli for 15 min. Several bacterial colonies were observed on the blank control, indicating good physiological activity of E. coli. No colony growth was detected on the surface of the HPC after the experiment, whereas a small number of colonies were gathered on the surface of Cu-20Zn and Cu-20Ni alloys. The antimicrobial activity of copper was reduced by the addition of Zn and Ni, but the antibacterial rate of the Cu-20Zn alloy was still able to reach 99% in 15 min, indicating strong and rapid bactericidal activity. However, the antibacterial rate of the Cu-20Ni alloy was only 76%. The strong antibacterial properties of the copper metal and Cu-bearing alloys have been widely confirmed; the antibacterial effects are related to the content of the ions released by the copper or the elements added during the process of electrochemical corrosion on the metal surface [26–28]. X-ray photoelectron spectroscopy (XPS) was used to analyze the surface composition of the alloys after contact with the bacteria. As shown in Fig. 2, there was no Cu(II) on the surface of the alloy as indicated by the Cu 2p orbital results; however, Cu(I) and Cu(0) in the Cu 2p orbitals had similar peak shapes, and their existences could not be distinguished from the 2p orbitals alone; thus, we measured the Cu LM2 orbitals. Two peaks with kinetic energies of 916.7–918.8 eV appeared in the auger electron spectrum; this could prove the existence of Cu(I) and Cu(0) on the surfaces of the alloys. The Cu 2p3/2 orbital fitting results revealed that the binding energies of the materials were ~ 932.60–932.30 eV, respectively, indicating that Cu(I) existed in Cu2O. Zn existed in the form of ZnO on the surface of Cu-20Zn, and Ni existed as Ni(OH)2 on the surface of Cu-20Ni. XPS and antibacterial test results revealed that Cu(I) was the main solution released during the period of contact between the copper and bacteria and that the presence of Ni(OH)2 or Ni largely reduced the bacteriostatic effect of copper compared with the presence of ZnO. Utilizing the results of this study and the studies on microorganisms, the dynamic changes of the antibacterial mechanism on the surface of materials were clarified; the reaction process is shown in Fig. 3. The copper alloy underwent an electrochemical corrosion process, releasing positively charged Cu(I) to destroy the bacterial cell wall and enter the bacteria to induce bacterial death [29–31].
Fig. 1.
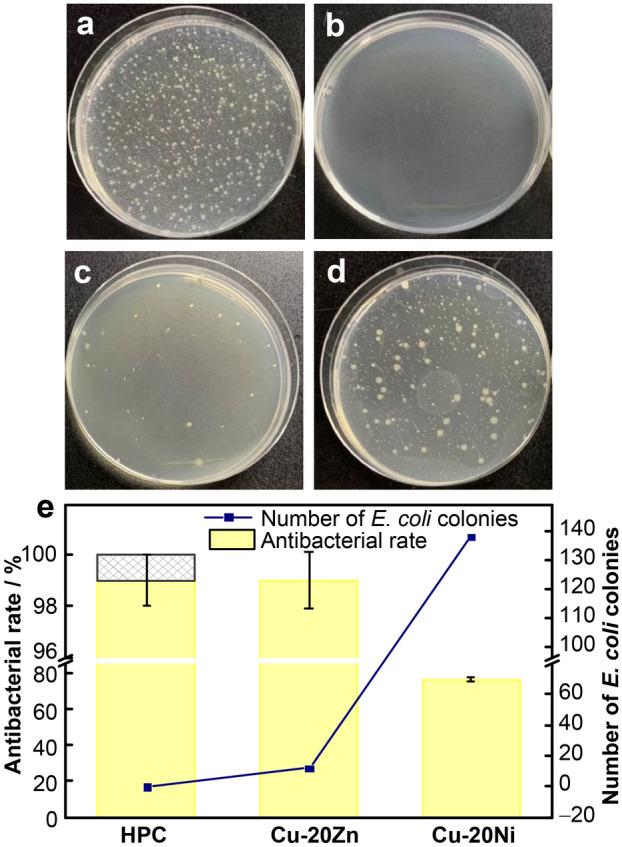
Typical photographs of colonization by E. coli for 15 min of a blank group, b HPC, c Cu-20Zn, d Cu-20Ni, e antibacterial rate in 15 min (***p < 0.001)
Fig. 2.
High-resolution XPS spectra of a Cu LMM, b Cu 2p3/2, c Zn 2p3/2, and d Ni 2p3/2
Fig. 3.
Simulation of reaction process on surface of alloy during antibacterial action
The salt spray method was used to simulate an artificial sweat contact environment to test the discoloration and corrosion resistance of the alloy. As shown in Fig. 4, the corrosion rates of the alloys decreased with corrosion time increasing. After 240 h, the corrosion rate of the HPC was the highest, reaching 0.030774 mm·year−1. The addition of Zn and Ni reduced the weight loss rate and improved the corrosion resistance of the HPC. The surface color of copper was found to be related to the state of the matrix; additionally, with the addition of these elements, the oxide formed after corrosion. Under the conditions of artificial sweat-induced accelerated atmospheric corrosion, the macroscopic surface discoloration results for the alloy after 1, 12 and 24 h were obtained, where ∆E is the change value of color difference before and after corrosion, as shown in Fig. 5. After 1 h corrosion, the surface of the HPC was uniformly corroded and discolored. A small part of the Cu-20Zn alloy was pitted and faded in color. The Cu-20Ni alloy was corroded along the grain boundary, and the corroded areas of all the experimental alloys were darker than the non-corroded areas. As the corrosion process progressed, the number of corrosion products increased, and the color change became more evident. Adding Zn and Ni changed the structure of the samples after corrosion, improving the discoloration resistance of the alloy. Cu2O was first generated during the copper oxidation process, and other compounds continued to react as the process progressed, leading to a surface color change [32].
Fig. 4.
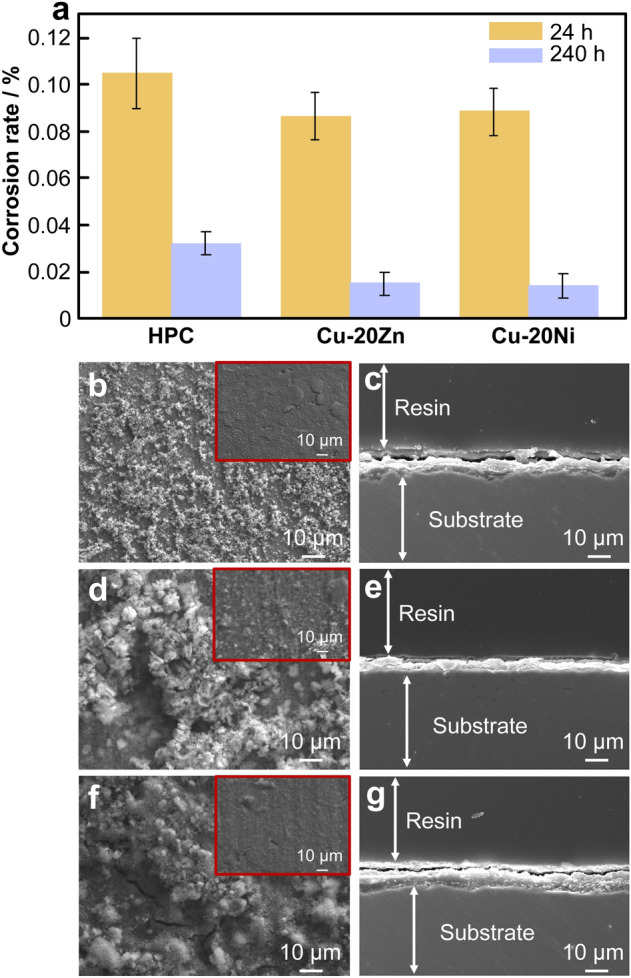
a Corrosion rate; surface SEM images of b HPC, d Cu-20Zn, and f Cu-20Ni; cross-sectional SEM images of c HPC, e Cu-20Zn, and g Cu-20Ni
Fig. 5.
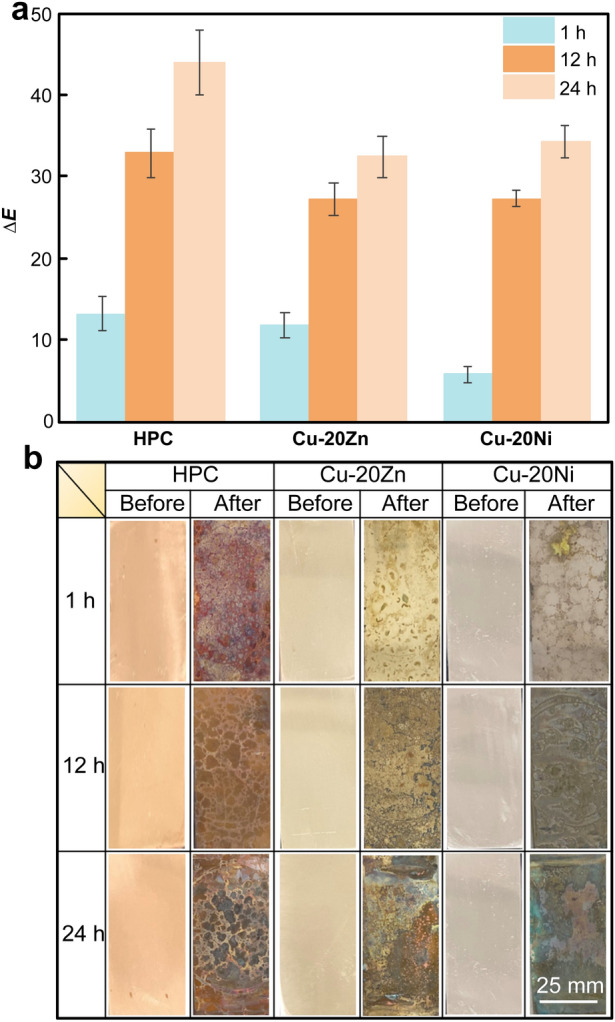
a Color difference value and b discoloration of sample surface
Scanning electron microscopy (SEM) was used to analyze the surface morphologies of the corroded samples. As shown in Fig. 4, the sample surfaces exhibited two morphologies. The top surface was a loose and rough layer, and the lower layer was relatively smooth and flat. However, the HPC surface film cracked and warped, and the corrosion product film was thick and rough. The bottom layers of Cu-20Zn and Cu-20Ni alloys were solid and firm without cracks. Analysis of the cross section of the membrane layer also revealed that the bottom layers of Cu-20Zn and Cu-20Ni were smoother and better integrated into the alloy matrix than HPC. The bottom layer of Cu-20Zn alloy had a honeycomb-like morphology. Cu-20Ni alloy had a dense, uniform film layer with a smooth cross-sectional morphology. The cross sections of the films of the two alloys were relatively flat, indicating that the two alloys had better corrosion resistance than HPC. During the acceleration of the corrosion process, the surface color changed rapidly. However, the addition of Zn and Ni promoted the formation of dense insoluble oxide products on the surface during the reaction; this protected the surface and improved the corrosion and discoloration resistance.
In summary, we found that copper and copper alloys mainly released Cu(I) during the antibacterial process, significantly contributing to the antibacterial properties. The addition of different elements had varying effects on the antibacterial performance of copper. The antibacterial performance of the alloys remained fast and efficient after the addition of Zn; particularly, the antibacterial rate was maintained at 99% after 15 min contact with E. coli. However, the addition of Ni significantly reduced the antibacterial activity of copper, as the antibacterial rate of Cu-20Ni decreased by ~ 24%. The addition of Zn and Ni was found to improve the discoloration and corrosion resistance of copper. The addition of Zn concurrently ensured strong and rapid antibacterial activity and improved the corrosion resistance of copper. Therefore, Zn can be used as the main additive element for antibacterial copper. This study may provide guidance for future composition designs and applications of copper alloys as antibacterial contact surface materials in public spaces.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financially supported by the National Key Research and Development Program of China (No. 2021YFB3700700).
Declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Footnotes
Yun Jiang and Wen-Jing Zhang contributed equally to this work.
Contributor Information
Wen-Jing Zhang, Email: wenjingzhang1987@163.com.
Xu-Jun Mi, Email: sklcopper1976@163.com.
References
- [1].Xiao MF, Zeng C, Li SH, Yuan FL. Applications of nanomaterials in COVID-19 pandemic. Rare Met. 2022;41(1):1. doi: 10.1007/s12598-021-01789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li Z, Qiao D, Xu Y. Cu-bearing high-entropy alloys with excellent antiviral properties. J Mater Sci Technol. 2021;25(1):6. doi: 10.1016/j.jmst.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Inkinen J, MäKinen R, KeinäNen-Toivola M. Copper as an antibacterial material in different facilities. Lett Appl Microbiol. 2016;64:19. doi: 10.1111/lam.12680. [DOI] [PubMed] [Google Scholar]
- [4].Michels HT, Noyce JO, Keevil CW. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett Appl Microbiol. 2009;45:191. doi: 10.1111/j.1472-765X.2009.02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marin V, Philippe H, Marc ED. Antimicrobial applications of copper. Int J Hyg Environ Health. 2016;219(07):585. doi: 10.1016/j.ijheh.2016.06.003. [DOI] [PubMed] [Google Scholar]
- [6].Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2010;77(5):1541. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mikolay A, Huggett S, Tikana L. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl Microbiol Biotechnol. 2010;87:1875. doi: 10.1007/s00253-010-2640-1. [DOI] [PubMed] [Google Scholar]
- [8].Schabrun S, Chipchase L. Healthcare equipment as a source of nosocomial infection: a systematic review. J Hosp Infect. 2006;63(3):239. doi: 10.1016/j.jhin.2005.10.013. [DOI] [PubMed] [Google Scholar]
- [9].Zhang Z, Zhang XR, Jin T, Yang CG, Sun YP, Li Q, Yang K. Antibacterial mechanism of Cu-bearing 430 ferritic stainless steel. Rare Met. 2022;41(2):559. doi: 10.1007/s12598-021-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhu L, Elguindi J, Rensing C, Ravishankar S. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol. 2012;30(1):303. doi: 10.1016/j.fm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- [11].Dinni N. The effect of nickel addition on antimicrobial, physical, and mechanical properties of copper-nickel alloy against suspensions of Escherichia coli. AIP Conf Proc. 2015;1677(070023):1. doi: 10.1063/1.4930727. [DOI] [Google Scholar]
- [12].Konieczny J, Rdzawski Z. Antibacterial properties of copper and its alloys. Arch Mater Sci Eng. 2012;56(2):53. doi: 10.1016/j.fm.2011.12.001. [DOI] [Google Scholar]
- [13].Ruparelia JP, Chatterjee AK, Duttagupta SP. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4(3):707. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [14].Ibrahim M, Wang F, Lou MM, Xie GL, Li B, Bo Z, Zhang GQ, Liu H, Wareth A. Copper as an antibacterial agent for human pathogenic multidrug resistant Burkholderia cepacia complex bacteria. J Biosci Bioeng. 2011;112(06):570. doi: 10.1016/j.jbiosc.2011.08.017. [DOI] [PubMed] [Google Scholar]
- [15].Zhou E, Qiao D, Yang Y, Xu D, Lu Y, Wang J. A novel Cu-bearing high-entropy alloy with significant antibacterial behavior against corrosive marine biofilms. J Mater Sci Technol. 2020;46:201. doi: 10.1016/j.jmst.2020.01.039. [DOI] [Google Scholar]
- [16].Jzab C, Sz C, Ksa B, Zwa B, Zh C, Kza B, Zf C, Zd X, Qza B. 3D reduced graphene oxide hybrid nano-copper scaffolds with a high antibacterial performance. Mater Lett. 2020;267:127527. doi: 10.1016/j.matlet.2020.127527. [DOI] [Google Scholar]
- [17].Rui L, Yan QL, ZhaoY L. Flower-like CuS/graphene oxide with photothermal and enhanced photocatalytic effect for rapid bacteria-killing using visible light. Rare Met. 2022;41(2):639. doi: 10.1007/s12598-021-01759-4. [DOI] [Google Scholar]
- [18].Dnp A, Nd B, Ys C, Mqk D, Au C, Xb C, Gt B, Isk C. Antibacterial mechanisms of various copper species incorporated in polymeric nanofibers against bacteria - sciencedirect. Mater Today Commun. 2020;25:101377. doi: 10.1016/j.mtcomm.2020.101377. [DOI] [Google Scholar]
- [19].Mathews S, Hans M, Mücklich F. Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl. Environ. Microbiol. 2013;79(8):2605. doi: 10.1128/AEM.03608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Horton DJ, Ha H, Foster LL. Tarnishing and Cu ion release in selected copper-base alloys: implications towards antimicrobial functionality. Electrochim Acta. 2015;169:351. doi: 10.1016/j.electacta.2015.04.001. [DOI] [Google Scholar]
- [21].Walkowicz M, Osuch P, Smyrak B, Knych T, Rudnik E. Impact of oxidation of copper and its alloys in laboratory-simulated conditions on their antimicrobial efficiency. Corros Sci. 2018;140(8):321. doi: 10.1016/j.corsci.2018.05.033. [DOI] [Google Scholar]
- [22].Yeh AC, Huang CC, Hsiao CC. Some aspects on the discoloration and antimicrobial property of a thermally passivated copper surface in a highly humid environment. Mater Trans. 2011;52(2):265. doi: 10.2320/matertrans.M2010339. [DOI] [Google Scholar]
- [23].Fredj N, Kolar JS, Prichard DM. Study of relative color stability and corrosion resistance of commercial copper alloys exposed to hand contact and synthetic hand sweat. Corros Sci. 2013;76(10):415. doi: 10.1016/j.corsci.2013.07.015. [DOI] [Google Scholar]
- [24].Zhe Z, Lichen Z, Yuting S. Mechanical properties and in vitro biodegradation of newly developed porous Zn scaffolds for biomedical applications. Mater. Des. 2016;108:136. doi: 10.1016/j.matdes.2016.06.080. [DOI] [Google Scholar]
- [25].Noyce JO, Michels H, Keevil CW. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J Hosp Infect. 2006;63(3):289. doi: 10.1016/j.jhin.2005.12.008. [DOI] [PubMed] [Google Scholar]
- [26].Zhu L, Elguindi J, Rensing C. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol. 2012;30(1):303. doi: 10.1016/j.fm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- [27].Cong P, Yang L, Shuyuan Z. Optimization of annealing treatment and comprehensive properties of Cu-containing Ti6Al4V-xCu alloys. J. Mater. Sci. Technol. 2019;35:11. doi: 10.1016/j.jmst.2019.05.020. [DOI] [Google Scholar]
- [28].Zhang E, Cong L. A new antibacterial Co-Cr-Mo-Cu alloy: preparation, biocorrosion, mechanical and antibacterial property. Mater. Sci. Eng. C. 2016;69:134. doi: 10.1016/j.msec.2016.05.028. [DOI] [PubMed] [Google Scholar]
- [29].Zhang E, Li F, Wang H, Liu J, Wang C, Li M, Yang K. A new antibacterial titanium-copper sintered alloy: preparation and antibacterial property. Mater Sci Eng C Mater Biol Appl. 2013;33(07):4280. doi: 10.1016/j.msec.2013.06.016. [DOI] [PubMed] [Google Scholar]
- [30].Yang HL, Zhu MZ, Wang JY, Ma CX, Zhou XW, Xing HX, Zhang EL, Ji SX. Optimization of mechanical and antibacterial properties of Ti-3wt%Cu alloy through cold rolling and annealing. Rare Met. 2022;41(2):610. doi: 10.1007/s12598-021-01841-x. [DOI] [Google Scholar]
- [31].Merilin R, Heiki V, Anne K, William CK, Angela I. Rapid in situ assessment of Cu-ion mediated effects and antibacterial efficacy of copper surfaces. Sci. Rep. 2018;08(01):8172. doi: 10.1038/s41598-018-26391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Leygraf C, Chang T, Herting G, Wallinder IO. The origin and evolution of copper patina colour. Corros Sci. 2019;157(8):337. doi: 10.1016/j.corsci.2019.05.025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.