Study registration: NCT04348396, registered on 10th April 2020.
Dear Sirs,
The severity of coronavirus disease 19 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection, can range from very mild to severe symptoms, depending on the multi-organ damage. Even though direct SARS-CoV-2 infection of the brain parenchyma remains a matter of debate, a wide range of neurological manifestations, including delirium, anosmia, encephalopathy, encephalitis, and cerebrovascular manifestations was reported in COVID-19 patients [9, 13]. Recent data raised the possibility that patients affected by COVID-19-associated neurological syndromes exhibit impaired amyloid processing linked to neuronal injury and neuroinflammation [22]. Inflammatory biomarkers were frequently detected at high concentrations in cerebrospinal fluid (CSF) of patients with COVID-19 neurological syndromes, suggesting that neuronal damage occurs, probably with long-term consequences that are still unknown [3]. Among the CSF biomarkers associated with neuronal injury, the neurofilament light chain (NfL) is attracting the attention of researchers and clinicians. NfL is an essential cytoskeleton protein of the axons, not specific to the central nervous system (CNS) impairment but also associated with peripheral neuropathy [4]. With a low molecular weight of 68 kDa, NfL can easily diffuse to CSF and blood. The recent development of ultrasensitive assays, such as single molecule array (SIMOA) and next-generation ELISA, allows reproducible measurement of NfL concentrations in serum or plasma [11]. It was demonstrated that NfL in both CSF and serum reflects the temporal pattern of the post-ischemic axonal injury [17]. Determining the extent of neuronal injury in COVID-19 patients is essential to better understand disease pathophysiology and to evaluate potential therapies. In adult patients affected by COVID-19, increased circulating levels of NfL were associated with acute disease severity [2, 16], also in the absence of major neurological manifestations [21]. However, several questions remain unanswered, especially in the oldest patients affected by multiple comorbidities, including neurodegenerative diseases and renal dysfunction, that could affect NfL circulating levels [15, 18].
Here, we assessed admission levels of serum NfL (sNfL) in geriatric patients with COVID-19 with the aim to evaluate the ability of circulating NfL to improve COVID‐19 mortality prediction alone and/or in association with other demographic, biochemical, and molecular circulating biomarkers.
The present study was performed on a subset of samples from the already published Report-Age COVID observational study, which was conducted at the Italian National Center on Aging (IRCCS INRCA, Ancona, Italy) to understand the predictors of adverse outcomes in older patients hospitalized and diagnosed with COVID-19 [12]. Samples were collected between 1st March 2020 and 24th June 2021. The study was conducted in adherence to the Declaration of Helsinki and has been approved by the Ethics Committee of the IRCCS INRCA (reference number CE-INRCA-20008; ClinicalTrials.gov, NCT04348396). All the included patients had been confirmed to be infected with SARS-CoV-2 by real-time reverse transcriptase-polymerase chain reaction assay regardless of the clinical symptoms. Informed consent was obtained from all individual participants included in the study. Demographic and anamnestic data, biochemical and hematological variables, information on treatments, comorbidities, and survival were collected in a retrospective manner and anonymized before release, as previously described [12]. The Clinical Frailty Scale (CFS), an ordinal 9-point scale in which the assessor evaluates the degree of frailty from clinical data, was employed to assess frailty [19].
sNfL levels were determined using commercially available kits for the Simple Plex™ Ella microfluidic platform (Protein Simple, Bio-Techne, Minneapolis, USA). Samples were prepared following the manufacturer’s instructions. Briefly, samples were diluted by mixing 25 µL of the sample with 25 µL of the sample diluent provided. All samples were run in triplicate and the mean value was considered for the analysis. Levels of NfL were measured within the linear range of each assay. Samples with an intra-assay coefficient of variation below 10.0% were included in this study. The lower limit of quantification is 2.7 pg/mL, and the upper limit is 10290 pg/mL.
Continuous variables were reported as either mean and standard deviation or median and interquartile range based on their normal or non-normal distribution (assessed using the Shapiro–Wilk test), respectively. The clinical and laboratory characteristics of the study cohort upon admission were compared according to categorized sNfL using the chi-squared test. Optimal sNfL cutoffs for predicting survival were computed using the Evaluate Cutpoints R package. Comparison of variables between groups was performed by one-way analysis of variance or Kruskal–Wallis equality-of-populations rank test as appropriate. The association between sNfL and mortality was initially investigated by Kaplan–Meier curves and statistical significance was assessed by the log-rank test for equality of survivor functions. Crude and adjusted Cox proportional hazards models were built to derive hazard ratios (HR) and 95% confidence intervals (95% CI) of the association between all independent variables and the study outcome. The length of hospital stay was used as the time to failure variable for the model. Sequential imputation using chained equations with linear regression method was applied in case of missing values in covariates. The imputation model included age, gender, and comorbidities. A two-tailed p-value < 0.05 was considered significant. Data were analyzed using STATA version15.1 Statistical Software Package for Windows (Stata Corp, College Station, TX).
A total of 205 consecutive geriatric patients, median age (IQR) 86 (82–91) years, who were hospitalized at the INRCA hospital (Ancona, Italy) due to COVID-19 were included in the analysis. 30.2% (62/205) of the enrolled patients deceased during their in-hospital stay. The mean time from hospital admission to discharge was 20.1 ± 11.4 days for survivor patients and 14.6 ± 12.3 days for deceased patients. The minimum number of days for which patients in the recovered group remained hospitalized was one day, while the maximum hospital stay was 53 days for survived patients and 56 days for deceased patients.
With respect to medication, most of the patients received corticosteroids during their hospital stay (n = 142; 69.3%); with no significant difference (p = 0.986) between deceased (n = 43; 69.4%) and survived (n = 99; 69.2%). First, two cutoffs, i.e., 85 and 258 pg/mL, were computed to maximize the ability of sNfL to predict survival in our cohort. Patients were then categorized into three groups with sNfL < 85 pg/mL (low levels), 85–258 pg/mL (intermediate levels), and > 258 pg/mL (high levels). The clinical and laboratory characteristics of the study cohort at admission according to the NfL categories are reported in Table 1.
Table 1.
Sample description
| Total | sNfL < 85 pg/mL | sNfL: 85–258 pg/mL | sNfL > 258 pg/mL | p | |
|---|---|---|---|---|---|
| N = 205 | N = 58 | N = 101 | N = 46 | ||
| Female gender, n (%) | 122 (59.5%) | 34 (58.6%) | 57 (56.4%) | 31 (67.4%) | 0.449 |
| Age (years) | 86 (82–91) | 83 (80–87) | 87 (83–91) | 87.5 (84–93) | < 0.001 |
| Length of stay (days) | 16 (9–26) | 17 (10–27) | 16 (11–25) | 13.5 (7–25) | 0.422 |
| Dead, n (%) | 62 (30.2%) | 7 (12.1%) | 32 (31.7%) | 23 (50%) | < 0.001 |
| CFS, n (%) | < 0.001 | ||||
| 1–3 | 40 (19.5%) | 22 (37.9%) | 15 (14.9%) | 3 (6.5%) | |
| 4–7 | 101 (49.3%) | 26 (44.8%) | 52 (51.5%) | 23 (50%) | |
| 8–9 | 64 (31.2%) | 10 (17.2%) | 34 (33.7%) | 20 (43.5%) | |
| Comorbidities | |||||
| History of myocardial infarction, n (%) | 23 (11.2%) | 8 (13.8%) | 12 (11.9%) | 3 (6.5%) | 0.485 |
| Dementia, n (%) | 91 (44.4%) | 13 (22.4%) | 45 (44.6%) | 33 (71.7%) | < 0.001 |
| CKD, n (%) | 64 (31.2%) | 5 (8.6%) | 38 (37.6%) | 21 (45.7%) | < 0.001 |
| Hypertension, n (%) | 154 (75.1%) | 45 (77.6%) | 71 (70.3%) | 38 (82.6%) | 0.243 |
| Stroke, n (%) | 23 (11.2%) | 4 (6.9%) | 15 (14.9%) | 4 (8.7%) | 0.257 |
| COPD, n (%) | 38 (18.5%) | 8 (13.8%) | 19 (18.8%) | 11 (23.9%) | 0.417 |
| Atrial Fibrillation, n (%) | 56 (27.3%) | 17 (29.3%) | 24 (23.8%) | 15 (32.6%) | 0.495 |
| Cancer, n (%) | 41 (20%) | 11 (19%) | 22 (21.8%) | 8 (17.4%) | 0.805 |
| CHF, n (%) | 53 (25.9%) | 11 (19%) | 28 (27.7%) | 14 (30.4%) | 0.346 |
| Diabetes, n (%) | 50 (24.4%) | 13 (22.4%) | 26 (25.7%) | 11 (23.9%) | 0.892 |
| Laboratory parameters | |||||
| Hemoglobin (g/dL) | 11.4 (10.0–12.6) | 11.9 (10.4–13.1) | 11.3 (10.0–12.4) | 10.9 (9.6–12.5) | 0.083 |
| Neutrophil % | 76.5 (65.5–85.8) | 74.1 (66.7–83.6) | 76.3 (64.9–85.9) | 79.0 (66.4–87.7) | 0.535 |
| Lymphocyte % | 15.6 (9.3–22.7) | 15.6 (9.9–24.2) | 16.4 (9.3–23.4) | 13.9 (8.1–20.1) | 0.396 |
| Eosinophil % | 0.2 (0.0–1.0) | 0.2 (0.0–1.2) | 0.3 (0.0–1.0) | 0.3 (0.0–1.0) | 0.857 |
| Basophil % | 0.1 (0.1–0.2) | 0.2 (0.1–0.3) | 0.1 (0.1–0.2) | 0.1 (0.0–0.2) | 0.275 |
| Neutrophils (× 103/ μL) | 5.5 (3.9–8.0) | 5.1 (4.0–7.5) | 5.5 (3.8–8.0) | 6.3 (4.3–10.8) | 0.183 |
| Lymphocytes (× 103/ μL) | 1.2 (0.8–1.6) | 1.1 (0.8–1.6) | 1.2 (0.8–1.7) | 1.2 (0.8–1.6) | 0.930 |
| Eosinophils (× 103/ μL) | 0.03 (0.00–0.09) | 0.03 (0.00–0.10) | 0.01 (0.00–0.10) | 0.03 (0.00–0.09) | 0.648 |
| Basophils (× 103/ μL) | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) | 0.990 |
| D-dimer (ng/mL) | 1260 (750–2320) | 930 (675–1620) | 1305 (740–2510) | 1720 (980–3360) | 0.006 |
| eGFR (mL/min) | 64.5 (45.0–83.0) | 80.0 (59.0–89.0) | 60.0 (40.0–79.0) | 47.5 (30.5–81.0) | < 0.001 |
| Fasting glucose (mg/dL) | 105.0 (82.0–132.0) | 114.0 (98.0–130.0) | 103.0 (80.0–138.0) | 98.0 (71.0–128.0) | 0.221 |
| Sodium (mmol/L) | 139.0 (136.0–143.0) | 139.0 (137.0–142.0) | 139.0 (136.0–143.0) | 140.0 (135.5–147.5) | 0.491 |
| Potassium (mmol/L) | 4.1 (3.6–4.6) | 4.2 (3.9–4.7) | 4.2 (3.7–4.6) | 3.9 (3.4–4.3) | 0.013 |
| NT-proBNP (pg/mL) | 1551 (670–4174) | 830 (340–2085) | 1598.5 (696.5–3948.5) | 2305 (1400–7777) | 0.001 |
| CRP (mg/dL) | 2.9 (1.2–8.1) | 1.9 (0.6–7.4) | 3.2 (1.5–8.1) | 3.7 (1.6–11.1) | 0.097 |
| Procalcitonin (ng/mL) | 0.080 (0.050–0.220) | 0.050 (0.050–0.145) | 0.080 (0.050–0.175) | 0.145 (0.070–0.860) | < 0.001 |
| IL-6 (pg/mL) | 34.6 (12.6–73.1) | 25.8 (8.2–65.8) | 32.6 (13.3–69.5) | 61.3 (29.7–166.0) | 0.011 |
| Ferritin (ng/mL) | 548.0 (310.0–902.0) | 452.0 (278.0–805.0) | 583.0 (310.0–907.0) | 574.5 (427.0–982.0) | 0.284 |
| NLR | 4.9 (3.0–8.9) | 4.3 (2.7–7.8) | 4.4 (2.9–9.3) | 5.7 (3.5–11.0) | 0.242 |
| dNLR | 1.9 (0.8–4.0) | 1.3 (0.4–3.2) | 2.0 (0.9–4.0) | 2.1 (0.6–4.3) | 0.249 |
| PLR | 202.3 (134.1–301.3) | 228.9 (146.4–301.3) | 196.0 (130.9–298.9) | 242.7 (115.3–304.3) | 0.580 |
| LMR | 2.4 (1.6–3.6) | 2.3 (1.6–3.5) | 2.4 (1.6–3.4) | 2.5 (1.7–4.3) | 0.390 |
Data are median (IQR) for continuous variables or number (%) for categorical variables. p-values for Chi-squared tests. In bold significant associations. CFS clinical frailty scale, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, eGFR estimated glomerular filtration rate, NLR neutrophil-to-lymphocyte ratio, dNLR derived NLR, PLR platelet-to-lymphocyte ratio, LMR lymphocyte-to-monocyte ratio, sNfL serum neurofilament light chain
The proportion of deceased COVID-19 patients was higher among patients with high levels of sNfL (50%), and this group of patients was characterized by higher median age, prevalence of dementia and chronic kidney diseases (CKD), higher levels of D-dimer, NT-proBNP, procalcitonin, interleukin-6 (IL-6), and lower estimated glomerular filtration rate (CKD-EPI eGFR) and potassium (Table 1). Spearman’s correlations with Bonferroni correction revealed that NfL serum levels were positively related to age and NT-proBNP and negatively associated with eGFR (Table 2).
Table 2.
Spearman’s rank correlation coefficients with Bonferroni correction for multiple comparisons
| sNfL | CFS | |
|---|---|---|
| sNfL | 1 | |
| CFS | 0.4529* | 1 |
| Age | 0.3995* | 0.4148* |
| Hemoglobin | − 0.1890 | 0.0003 |
| Neutrophil % | 0.1227 | 0.1315 |
| Lymphocyte % | − 0.1773 | − 0.2001 |
| Eosinophil % | − 0.0257 | − 0.0443 |
| Basophil % | − 0.1395 | − 0.0434 |
| Neutrophils (× 103 /μL) | 0.1776 | 0.2301 |
| Lymphocytes (× 103/μL) | − 0.0761 | − 0.0234 |
| Eosinophils (× 103/μL) | 0.0274 | − 0.1134 |
| Basophils (× 103 /μL) | 0.0123 | − 0.0538 |
| NLR | 0.1662 | 0.2097 |
| dNLR | − 0.0288 | 0.1309 |
| PLR | − 0.0247 | 0.0058 |
| LMR | − 0.0064 | 0.0951 |
| D-dimer | 0.2451 | 0.1215 |
| eGFR | − 0.4117* | − 0.2509 |
| Fasting glucose | − 0.1935 | − 0.0467 |
| Serum sodium | 0.1039 | 0.1781 |
| Serum potassium | − 0.1677 | − 0.0421 |
| NT-proBNP | 0.3266* | 0.2364 |
| C-reactive protein | 0.2178 | 0.2351 |
| Procalcitonin | 0.2927 | 0.1544 |
| IL-6 | 0.2648 | 0.1306 |
| Ferritin | 0.1655 | 0.1160 |
*p < 0.005. In bold significant associations. CFS clinical frailty scale, NLR neutrophil-to-lymphocyte ratio, dNLR derived NLR, PLR platelet-to-lymphocyte ratio, LMR lymphocyte-to-monocyte ratio
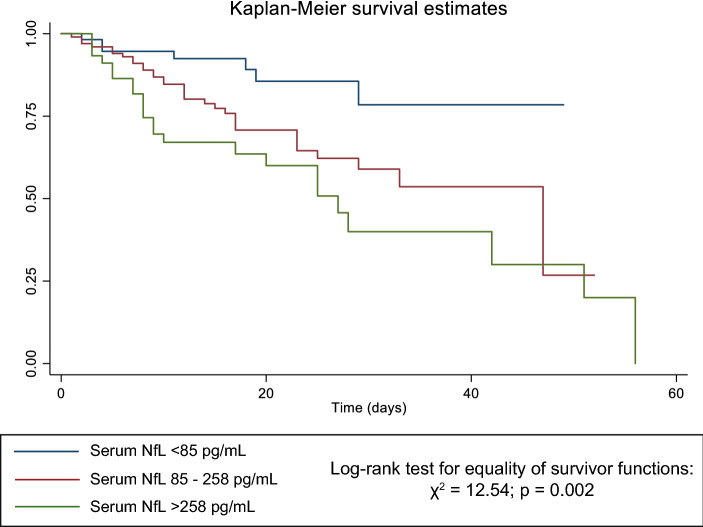
The Kaplan–Meier survival estimates and log-rank tests for comparison for COVID-19 patients grouped based on serum NfL, reported in Fig. 1, showed that patients with admission sNfL higher than 85 pg/ml (intermediate and high levels) had increased mortality compared to those with sNfL lower than 85 pg/ml. Finally, adjusted Cox regressions models evaluating the predictive value of sNfL, age, and gender (model 1) and model 1 plus CFS (model 2), showed that old age, high sNfL levels, and high CFS (≥ 8) were the best independent predictors of in-hospital mortality in geriatric patients affected by COVID-19 (Table 3). When model 2 was adjusted also for the routine laboratory parameters that differed significantly among the three groups of patients (Table 1) i.e., eGFR, D-dimer, potassium, NT-proBNP, procalcitonin and IL-6 (model 3), old age and low eGFR, but not sNfL, were independent predictors of in-hospital mortality (Table 3).
Fig. 1.
Kaplan–Meier survival estimates for categorized serum NfL
Table 3.
Cox proportional hazards models (n = 205)
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| Serum NfL (pg/mL), ref. < 85 | |||
| 85–258 | 1.99 (0.87–4.55) | 1.76 (0.76–4.06) | 1.35 (0.56–3.23) |
| > 258 | 3.38 (1.42–8.04) | 2.88 (1.20–6.90) | 1.92 (0.73–5.05) |
| Female gender | 0.86 (0.51–1.47) | 0.75 (0.43–1.28) | 0.83 (0.47–1.48) |
| Age (years) | 1.11 (1.05–1.16) | 1.09 (1.03–1.15) | 1.10 (1.04–1.16) |
| CFS, ref. 1–3 | |||
| 4–7 | 2.49 (0.94–6.61) | 2.31 (0.83–6.44) | |
| 8–9 | 3.17 (1.15–8.76) | 2.40 (0.79–7.25) | |
| D-dimer (ng/mL) | 1.00 (0.99–1.00) | ||
| eGFR (mL/min) | 0.98 (0.97–0.99) | ||
| Potassium (mmol/L) | 1.04 (0.67–1.61) | ||
| NT-proBNP (mg/L) | 1.00 (0.99–1.00) | ||
| Procalcitonin (ng/mL) | 1.05 (0.99–1.11) | ||
| IL-6 (pg/mL) | 1.00 (0.99–1.00) | ||
CFS clinical frailty scale, CKD chronic kidney disease, CHF congestive heart failure, eGFR estimated glomerular filtration rate, NfL neurofilament light chain
Based on previous findings that established NfL as a marker of axonal injury [6], we investigated whether sNfL measured in hospitalized geriatric patients affected by COVID-19 could provide clinical guidance to assess the risk of in-hospital mortality. We observed that a significantly increased number of deceased COVID-19 geriatric patients showed high levels of sNfL, and this group of patients was characterized by the oldest age, the highest prevalence of dementia, CKD and frailty, the highest levels of D-dimer, NT-proBNP, procalcitonin, IL-6 and the lowest levels of potassium. However, in the fully adjusted Cox regression model, only age and eGFR showed a significant association with an increased risk of in-hospital mortality. Our results are in line with recent data suggesting that renal function should be regarded as a potential confounder when assessing circulating NfL levels since impaired filtration can affect NfL concentration [15, 18]. Previous reports showed that circulating NfL levels are negatively related to eGFR in healthy subjects and patients with type 2 diabetes, suggesting that the effect of age on circulating NfL could be partially mediated by eGFR [1, 7]. On the other hand, Hermansson et al. reported that plasma NfL did not correlate with serum creatinine among patients infected with HIV with a mean age of 40 years, suggesting that the relationship between blood NfL and renal function becomes more evident among older adults [5]. As previously demonstrated in Parkinson’s Disease [10], sNfL showed a direct correlation with NT-proBNP. While the assessment of this cardiac biomarker in patients with neurodegenerative disorders proved useful in the assessment of subclinical cardiac damage, this close interrelationship confirms that NfL accumulates with advancing age and declining renal function, in a way similar to NT-proBNP. Notably, it has been previously shown that sNfL enhances the predictive value of NT-proBNP as a biomarker of long-term cardiovascular outcome after ischemic stroke [20] and that sNfL was associated with worse cognitive performance in patients with heart failure [14]. In this regard, the direct correlation between sNfL and NT-proBNP observed in our cohort could reflect post-ischemic neuronal damage due to impaired cerebral perfusion in patients with reduced cardiac output and renal function. Additionally, in previous investigations, the association between sNfL and eGFR was driven by the subjects with obesity whereas no such correlation was found in lean individuals [1, 15]. Unfortunately, no data were available regarding the BMI of geriatric COVID-19 patients enrolled in our study, and we were unable to test this association.
Notably, sNfL levels were not correlated with the most common routinary analyzed biomarkers that we identified as associated with increased short-term mortality risk in this setting of patients in our previous report, i.e. neutrophil, lymphocyte, and eosinophil count, blood glucose, C-reactive protein, procalcitonin, and ferritin [12].
Our study has some limitations that need to be addressed, most notably its retrospective nature, single-center design, and limited sample size. Moreover, no CSF specimens were available for NfL analysis, which could have provided a more accurate assessment of the cerebral milieu. Indeed, it is still much debated whether SARS-CoV-2 can invade the CNS. Nevertheless, we have found a strong correlation between the highest sNfL levels and the presence of dementia. This is in line with our previous report addressing the association between cerebrospinal fluid NfL levels and neurodegenerative disorders in the geriatric population [8].
In conclusion, serum NfL could be associated with severe outcomes in geriatric patients affected by COVID-19, even if caution should be used when interpreting these findings in patients with impaired renal function. Future studies are warranted to confirm its independent prognostic value in hospitalized geriatric patients.
Funding
This study was funded by the Italian Ministry of Health—Ricerca Corrente to IRCCS INRCA and by Università Politecnica delle Marche (RSA grant to FO).
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was conducted in adherence to the Declaration of Helsinki and has been approved by the Ethics Committee of the IRCCS INRCA (reference number CE-INRCA-20008; ClinicalTrials.gov, NCT04348396).
Contributor Information
Jacopo Sabbatinelli, Email: j.sabbatinelli@staff.univpm.it.
Giulia Matacchione, Email: g.matacchione@staff.univpm.it.
References
- 1.Akamine S, Marutani N, Kanayama D, Gotoh S, Maruyama R, Yanagida K, Sakagami Y, Mori K, Adachi H, Kozawa J, Maeda N, Otsuki M, Matsuoka T, Iwahashi H, Shimomura I, Ikeda M, Kudo T. Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep. 2020;10:20350. doi: 10.1038/s41598-020-76990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ameres M, Brandstetter S, Toncheva AA, Kabesch M, Leppert D, Kuhle J, Wellmann S. Association of neuronal injury blood marker neurofilament light chain with mild-to-moderate COVID-19. J Neurol. 2020;267:3476–3478. doi: 10.1007/s00415-020-10050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domingues RB, Leite F, Senne C. Cerebrospinal fluid analysis in patients with COVID-19-associated central nervous system manifestations: a systematic review. Arq Neuropsiquiatr. 2022;80:296–305. doi: 10.1590/0004-282x-anp-2021-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi T, Nukui T, Piao JL, Sugimoto T, Anada R, Matsuda N, Yamamoto M, Konishi H, Dougu N, Nakatsuji Y. Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. Brain Behav. 2021;11:e02084. doi: 10.1002/brb3.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermansson L, Yilmaz A, Price RW, Nilsson S, McCallister S, Makadzange T, Das M, Zetterberg H, Blennow K, Gisslen M. Plasma concentration of neurofilament light chain protein decreases after switching from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate. PLoS ONE. 2019;14:e0226276. doi: 10.1371/journal.pone.0226276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 7.Ladang A, Kovacs S, Lengele L, Locquet M, Reginster JY, Bruyere O, Cavalier E. Neurofilament light chain concentration in an aging population. Aging Clin Exp Res. 2022;34:331–339. doi: 10.1007/s40520-021-02054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchegiani F, Matacchione G, Ramini D, Marcheselli F, Recchioni R, Casoli T, Mercuri E, Lazzarini M, Giorgetti B, Cameriere V, Paolini S, Paciaroni L, Rossi T, Galeazzi R, Lisa R, Bonfigli AR, Procopio AD, De Luca M, Pelliccioni G, Olivieri F. Diagnostic performance of new and classic CSF biomarkers in age-related dementias. Aging (Albany NY) 2019;11:2420–2429. doi: 10.18632/aging.101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meppiel, E., Peiffer-Smadja, N., Maury, A., Bekri, I., Delorme, C., Desestret, V., Gorza, L., Hautecloque-Raysz, G., Landre, S., Lannuzel, A., Moulin, S., Perrin, P., Petitgas, P., Sella, I.F., Wang, A., Tattevin, P., de Broucker, T. and contributors to the Neuro, C.r., 2021. Neurologic manifestations associated with COVID-19: a multicentre registry. Clin Microbiol Infect. 27, 458-466 [DOI] [PMC free article] [PubMed]
- 10.Niemann L, Lezius S, Maceski A, Leppert D, Englisch C, Schwedhelm E, Zeller T, Gerloff C, Kuhle J, Choe CU. Serum neurofilament is associated with motor function, cognitive decline and subclinical cardiac damage in advanced Parkinson's disease (MARK-PD) Parkinsonism Relat Disord. 2021;90:44–48. doi: 10.1016/j.parkreldis.2021.07.028. [DOI] [PubMed] [Google Scholar]
- 11.O'Connell GC, Alder ML, Webel AR, Moore SM. Neuro biomarker levels measured with high-sensitivity digital ELISA differ between serum and plasma. Bioanalysis. 2019;11:2087–2094. doi: 10.4155/bio-2019-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivieri F, Sabbatinelli J, Bonfigli AR, Sarzani R, Giordano P, Cherubini A, Antonicelli R, Rosati Y, Del Prete S, Di Rosa M, Corsonello A, Galeazzi R, Procopio AD, Lattanzio F. Routine laboratory parameters, including complete blood count, predict COVID-19 in-hospital mortality in geriatric patients. Mech Ageing Dev. 2022;204:111674. doi: 10.1016/j.mad.2022.111674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polymeris, A.A., Coslovksy, M., Aeschbacher, S., Sinnecker, T., Benkert, P., Kobza, R., Beer, J., Rodondi, N., Fischer, U., Moschovitis, G., Monsch, A.U., Springer, A., Schwenkglenks, M., Wuerfel, J., De Marchis, G.M., Lyrer, P.A., Kuhne, M., Osswald, S., Conen, D., Kuhle, J., Bonati, L.H. and and for the Swiss, A.F.I., 2020. Serum neurofilament light in atrial fibrillation: clinical, neuroimaging and cognitive correlates. Brain Commun. 2, fcaa166. [DOI] [PMC free article] [PubMed]
- 15.Polymeris AA, Helfenstein F, Benkert P, Aeschbacher S, Leppert D, Coslovsky M, Willemse E, Schaedelin S, Blum MR, Rodondi N, Reichlin T, Moschovitis G, Wuerfel J, De Marchis GM, Engelter ST, Lyrer PA, Conen D, Kuhne M, Osswald S, Bonati LH, Kuhle J, Swiss AFI. Renal Function and Body Mass Index Contribute to Serum Neurofilament Light Chain Levels in Elderly Patients With Atrial Fibrillation. Front Neurosci. 2022;16:819010. doi: 10.3389/fnins.2022.819010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prudencio, M., Erben, Y., Marquez, C.P., Jansen-West, K.R., Franco-Mesa, C., Heckman, M.G., White, L.J., Dunmore, J.A., Cook, C.N., Lilley, M.T., Song, Y., Harlow, C.F., Oskarsson, B., Nicholson, K.A., Wszolek, Z.K., Hickson, L.J., O'Horo, J.C., Hoyne, J.B., Gendron, T.F., Meschia, J.F. and Petrucelli, L., 2021. Serum neurofilament light protein correlates with unfavorable clinical outcomes in hospitalized patients with COVID-19. Sci Transl Med. 13. [DOI] [PMC free article] [PubMed]
- 17.Pujol-Calderon F, Portelius E, Zetterberg H, Blennow K, Rosengren LE, Hoglund K. Neurofilament changes in serum and cerebrospinal fluid after acute ischemic stroke. Neurosci Lett. 2019;698:58–63. doi: 10.1016/j.neulet.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 18.Rebelos E, Rissanen E, Bucci M, Jaaskelainen O, Honka MJ, Nummenmaa L, Moriconi D, Laurila S, Salminen P, Herukka SK, Singhal T, Nuutila P. Circulating neurofilament is linked with morbid obesity, renal function, and brain density. Sci Rep. 2022;12:7841. doi: 10.1038/s41598-022-11557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockwood K, Theou O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can Geriatr J. 2020;23:210–215. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uphaus T, Bittner S, Groschel S, Steffen F, Muthuraman M, Wasser K, Weber-Kruger M, Zipp F, Wachter R, Groschel K. NfL (Neurofilament Light Chain) Levels as a Predictive Marker for Long-Term Outcome After Ischemic Stroke. Stroke. 2019;50:3077–3084. doi: 10.1161/STROKEAHA.119.026410. [DOI] [PubMed] [Google Scholar]
- 21.Verde, F., Milone, I., Bulgarelli, I., Peverelli, S., Colombrita, C., Maranzano, A., Calcagno, N., Ticozzi, N., Perego, G.B., Parati, G., Torresani, E., Ratti, A. and Silani, V., 2022. Serum neurofilament light chain levels in Covid-19 patients without major neurological manifestations. J Neurol. [DOI] [PMC free article] [PubMed]
- 22.Ziff OJ, Ashton NJ, Mehta PR, Brown R, Athauda D, Heaney J, Heslegrave AJ, Benedet AL, Blennow K, Checkley AM, Houlihan CF, Gauthier S, Rosa-Neto P, Fox NC, Schott JM, Zetterberg H, Benjamin LA, Paterson RW. Amyloid processing in COVID-19-associated neurological syndromes. J Neurochem. 2022;161:146–157. doi: 10.1111/jnc.15585. [DOI] [PMC free article] [PubMed] [Google Scholar]