Abstract
Cryptococcal meningitis is a devastating brain infection cause by encapsulated yeasts of the Cryptococcus genus. Exposure, through inhalation, is likely universal by adulthood, but symptomatic infection only occurs in a minority, in most cases, months or years after exposure. Disease has been described in almost all tissues, but it is the organism’s tropism for the central nervous system that results in the most devastating illness. While invasive disease can occur in the immunocompetent, the greatest burden by far is in immunocompromised individuals, particularly people living with human immunodeficiency virus (HIV), organ transplant recipients and those on glucocorticoid therapy or other immunosuppressive drugs. Clinical presentation is variable, but diagnosis is usually straightforward, with cerebrospinal fluid microscopy, culture, and antigen testing proving significantly more sensitive than diagnostic tests for other brain infections. Although disease incidence has reduced since the advent of effective HIV therapy, mortality when disease occurs remains extremely high, and has changed little in recent decades. This Therapy in Practice review is an update of a talk first given by JND at the European Congress on Clinical Microbiology and Infectious Diseases in 2019 in the Netherlands. The review contextualizes the most recently published World Health Organization (WHO) guidelines for the treatment of HIV-associated cryptococcal meningitis in terms of the data from large, randomized, controlled trials published between 1997 and 2022. We discuss the rationale for induction and maintenance therapy and the efficacy and undesirable effects of the current therapeutic armamentarium of amphotericin, flucytosine and fluconazole. We address recent research into repurposed drugs such as sertraline and tamoxifen, and potential future treatment options, including the novel antifungals fosmanogepix, efungumab and oteseconazole, and non-pharmaceutical solutions such as neurapheresis cerebrospinal fluid filtration.
Key points
| Significant numbers of patients have been enrolled into clinical trials of treatment for human immunodeficiency virus (HIV)-associated cryptococcal meningitis over the past 3 decades, delivering a robust foundation of data on which to base treatment guidelines. However, mortality with optimized current treatment remains high—there is a pressing need to develop novel drugs. |
| The optimal induction therapy is a single high-dose of liposomal amphotericin B (10 mg/kg) plus flucytosine (100 mg/kg/day) and high-dose fluconazole (1200 mg/day) each for 14 days. This is followed by consolidation with fluconazole (800 mg/day) for 8 weeks and then long-term maintenance. However, the availability of both liposomal amphotericin B and flucytosine is limited in many high-burden settings, meaning alternative inferior regimens have to be used. |
| Alternative, currently available antifungals and attempts at drug repurposing have so far shown disappointing efficacy, but novel antifungal agents are in development and show promise. |
Background
The human immunodeficiency virus (HIV) epidemic has raised the profile of Cryptococcus neoformans from obscure yeast to a leading cause of brain infection globally. In 2009, it was estimated there were approximately 1 million cases of cryptococcal meningitis per year [1]. Since then, due to the great strides made in access to antiretroviral therapy (ART), the global incidence has fallen to an estimated 220,000 cases per year. Of these, around 160,000 occurred in sub-Saharan Africa and 43,000 in Asia [2]. However, despite the fall in incidence, there has been little change in disease mortality, with an estimated 674,000 deaths in 2009 and 180,000 in 2014. This translates into death rates in the order of 60–80% by 6 months after diagnosis, and compares unfavorably with higher profile diseases such as tuberculosis [3], viral hemorrhagic fevers [4] and primary brain tumors [5]. However, on best available therapy, even in low-income countries, the mortality rate can be reduced to around 35% 6 months after diagnosis [6, 7]. It is striking to note that the foundation of best available therapy continues to be off-patent drugs that are more than 60 years old, reflecting the neglected status of this devastating disease. Here, we will review the data from a number of larger clinical trials published in the last 10 years that guide current treatment guidelines for HIV-associated disease, review the success of recent alternative treatment strategies and describe on-going clinical studies and therapies in development.
Epidemiology
C. neoformans was first isolated from peach juice in 1894, and the first case of human disease described in the same year by Busse [8]. However, it was the use of immunosuppressive therapy from the 1970s and the emergence of the HIV pandemic in the 1980s that saw Cryptococcus rise to prominence as one of the world’s most deadly human fungal pathogens. It has been proposed (but not accepted by the entire cryptococcal research community) that there are seven species of Cryptococcus that are responsible for the vast majority of human cases (C. neoformans, C. deneoformans, C. gattii, C. bacillisporus, C. deuterogattii, C. tetragattii and C. decagattii) [9, 10]. Clinically relevant differences between molecular genotypes are unproven, and most experts accept that the most useful clinical distinction is between two species complexes, C. neoformans and C. gattii. However, in resource-poor settings, distinguishing species complex may not be possible.
Disease most frequently occurs in immunosuppressed patients, notably people living with HIV, where the epidemic has been driven by the clonal expansion of a small number of well-defined lineages of C. neoformans [11]. The C. gattii species complex, found in tropical and sub-tropical areas as well as temperate locations such as the Pacific Northwest of the USA and Southern Australia, is less commonly implicated, but is associated with disease in immunocompetent individuals [12]. The Cryptococcus species are remarkable for their impressive capsules, which are important determinants of virulence (see Fig. 1).
Fig. 1.

India ink stain of Cryptococcus neoformans cells showing extensive capsule development and characteristic budding of daughter cells. Cell diameters excluding capsule are 5–10 um across
Exposure to C. neoformans is likely universal by adulthood, with the vast majority of adults having antibodies. Infection is not transmitted from person to person, but results from the inhalation of infectious propagules (presumed to be either spores or desiccated yeasts) from the environment, and subsequent hematogenous spread. Most disease in HIV-infected patients is considered the result of recrudescence of latent infection, occurring months to years after the initial exposure event [13]. However, outbreaks of disease illustrate symptomatic primary infection of shorter incubation is possible [14–16].
Although the vast majority of cryptococcal meningitis occurs in people with acquired immunodeficiency syndrome (AIDS) and CD4 counts < 100 cells/µL, it is also seen in other forms of immunocompromise, including solid-organ transplant recipients, hematological malignancies (particularly lymphoma), liver failure, idiopathic CD4 lymphopenia and after prolonged use of glucocorticoids or other immunosuppressive drugs. Disease in immunocompetent hosts is rare overall, but a multicenter, retrospective study of 306 cases of cryptococcal disease in HIV-seronegative individuals (predominantly caused by C. neoformans) found 22% had no underlying medical condition [17]. C. gattii is well recognized to cause meningitis in immunocompetent individuals, although infection with this species frequently manifests as pulmonary disease [16]. C. neoformans can also cause disease in apparently immunocompetent individuals, with cases of meningitis particularly being reported from Southeast Asia and China [18–21]. Table 1 summarizes the epidemiology of cryptococcosis due to C. neoformans and C. gattii.
Table 1.
Epidemiology of cryptococcosis according to infecting species
| Species | Cryptococcus neoformans | Cryptococcus gattii species complex |
|---|---|---|
| Geographic distribution | Worldwide temperate and tropical distribution | Less abundant globally; associated with tropical and subtropical regions, generally uncommon in temperate zones |
| Ecology | Avian guano, bark, tree trunk hollows | Tropical and temperate rainforest, especially eucalyptus trees, rotting wood, soil |
| Host range | Predominantly immunocompromised patients, especially HIV infection, also in immunocompetent patients | Immunocompetent individuals, sporadically in immunosuppressed, including HIV-infected patients |
| Appearance | Tan mucoid colonies; microscopically spherical to ovoid encapsulated cells | Tan mucoid colonies; microscopically mixture of globose and oblong to elliptical cells |
| Target organs | CNS infections | Pulmonary infection is more common |
CNS central nervous system, HIV human immunodeficiency virus
Clinical Manifestations
Cryptococcosis can affect any body site or structure, but meningoencephalitis is by far the most frequent syndrome, particularly in HIV-infected patients. The cause of this tropism for the central nervous system is poorly understood, but plausible explanations include fungal evasion of the host innate immune response, allowing penetration of the blood–brain barrier [22], a lack of complement defense in cerebrospinal fluid (CSF) providing a favorable growth medium for Cryptococcus [23], and dopamine-enhanced fungal virulence in the brain [24].
The commonest disease presentation is subacute or chronic, with progression of fever, malaise and headache over a period of 1–2 weeks. This is variably accompanied by neck stiffness, photophobia, vomiting, confusion and depressed level of consciousness. Visual symptoms, including reduced visual acuity, blurred [25] or double vision [26], and hearing loss [27] are also commonly reported. Untreated, the disease is universally fatal within days to weeks.
Clinical presentation varies according to the immune status of the host and infecting species of Cryptococcus. HIV-uninfected patients may experience a more insidious onset of symptoms over several months, resulting in delayed diagnosis and treatment [17]. Raised intracranial pressure, present at diagnosis in two-thirds of HIV-infected patients, appears less common in this population. C. gattii infections have been associated with an increased risk of inflammatory sequelae compared with C. neoformans, possibly as a result of stimulating more robust secretion of pro-inflammatory cytokines [12]. This may explain why C. gattii infection more commonly manifests with pulmonary disease (promoting an inflammatory response at its initial site of infection).
Pulmonary cryptococcosis is the most common non-meningeal presentation. It can range from an asymptomatic pneumonia to acute respiratory failure, with disease severity and risk of spread to other systems increasing with the level of immunocompromise. Disseminated disease can affect multiple organs simultaneously. Lesions have been described in the liver [28], lymph nodes [29], peritoneum [30], urogenital tract [31], adrenal glands [32] and eyes [33].
Diagnosis
The definitive diagnosis of cryptococcal meningoencephalitis depends upon microbiological examination of CSF obtained by lumbar puncture. When lumbar puncture is performed, it is important to measure the opening CSF pressure, because raised intracranial pressure is frequent and should be managed with therapeutic CSF drainage. The inflammatory response seen in CSF is generally milder than that seen in bacterial or tuberculous meningitis, particularly in immunosuppressed patients [34], and is typified by a mildly elevated white cell count (< 50 cells/µL) with a predominantly mononuclear cell infiltrate, modestly elevated protein and low or normal glucose. The CSF lactate is usually within the normal range or only modestly raised. Approximately 25–30% of cases of culture-proven cryptococcal meningoencephalitis have a normal CSF profile [35, 36].
Microbiological confirmation of cryptococcal disease is relatively straightforward. Microscopy, culture and antigen testing are significantly more sensitive than diagnostic tests for other brain infections. Individuals with AIDS usually have a high fungal burden, and the characteristic encapsulated oval, budding yeasts are easily visualized using an India ink preparation of CSF in > 60% of cases [37]. In recent years, simple lateral flow assays for cryptococcal antigen (CrAg) have been developed. These are inexpensive and provide rapid results when performed on CSF, blood, serum or even urine. They require minimal laboratory or technical expertise and can even be performed at the bedside. The IMMY lateral flow test has a sensitivity of > 99% for detecting Cryptococcus in the CSF of HIV-infected patients [38] and has become the mainstay of diagnosis in both low- and high-resource settings. Due to the low cost and simplicity, the World Health Organization (WHO) recommends CrAg testing of CSF as the mainstay for diagnosis [39]. Non-meningeal cryptococcosis is diagnosed through a combination of radiography, serum CrAg, histology and culture from the affected organs, e.g., sputum, bronchoalveolar lavage or lymph node biopsy. Since 2018, the WHO has recommended routine screening for cryptococcal infection in all HIV-infected patients with CD4 counts less than 100 cells/µL prior to initiating ART [39]. This is because antigenemia is associated with increased mortality in the ensuing 12 months in patients starting ART. Screening should be through CrAg testing of blood, and where positive, the patient should be carefully evaluated for signs and symptoms of meningitis and undergo a lumbar puncture, if feasible, with CSF examination and India ink or CSF CrAg assay to exclude meningitis. Patients with evidence of meningitis should receive standard treatment. Centers for Disease Control and Prevention (CDC) guidelines also recommend individuals with a blood CrAg titer ≥ 1:640 by IMMY CrAg lateral flow assay (regardless of the lumbar puncture result) should also be treated for meningitis, based on evidence that such high titers are predictive of disseminated disease and subclinical cryptococcal meningitis among HIV-infected individuals [40, 41]. If the titer is ≤ 1:320, and there is no evidence of meningitis, fluconazole can be used as preventative therapy until the patient is established on antiretrovirals and the CD4 count is > 200 cells/µL [39].
Complications
Raised intracranial pressure is an important cause of poor outcomes from cryptococcal meningoencephalitis. The pathophysiology of raised intracranial pressure is poorly understood, but may include an osmotic effect of the capsular constituents, physical blockage of drainage of CSF by yeast and shed capsule, inflammation and mass effect from cryptococcomas. Signs of rising intracranial pressure include worsening headaches, vomiting, cranial nerve palsies, seizures, blindness, a reduced level of consciousness and ultimately death from cerebral herniation. In the context of these symptoms and persistent pressure ≥ 25 cm of CSF, pressure control becomes the principal determinant of survival. Therapeutic lumbar drainage must be repeated daily, and in some instances, ventriculostomy or temporary percutaneous lumbar drains may be necessary.
Visual impairment, including total visual loss, is well described in cryptococcal disease in HIV-infected and uninfected patients and may have a prevalence of up to 20% in survivors. Again, the pathophysiology is unclear, but likely includes raised intracranial pressure, cerebral ischemia and stroke-like infarcts, optic nerve infarction and direct optic nerve invasion by fungi [21].
Complications also arise in cryptococcal disease as a result of adverse reactions to the antifungal drugs (discussed in more detail below) and antiretroviral drugs required for treatment. Amphotericin, the mainstay of treatment, is associated with anemia (which can be profound in HIV-infected patients) and renal impairment, which usually recovers when treatment is completed [6, 42]. Flucytosine causes bone marrow suppression. In individuals with AIDS, cryptococcal immune reconstitution inflammatory syndrome (IRIS), following the instigation of ART, is an important consideration. While immune recovery is essential for the successful management of cryptococcal meningoencephalitis, trials comparing early versus delayed initiation of ART in patients undergoing treatment for cryptococcal meningoencephalitis have consistently shown superior outcomes with delayed initiation of HIV treatment [37, 43, 44]. This effect was most clearly seen in the COAT study [37], which assessed survival at 26 weeks in 177 ART-naïve HIV-infected patients with cryptococcal meningitis in Uganda and South Africa, randomized to receive either early (1–2 weeks after diagnosis) or deferred (5 weeks after diagnosis) ART. Deferring ART for 5 weeks after diagnosis was associated with significantly improved survival, especially among patients with a few white cells in their CSF (< 5 per cubic millimeter). International guidelines now recommend ART should be delayed until 4–6 weeks after initiation of anti-cryptococcal therapy [39].
Treatment
Pharmacological management of cryptococcal meningitis is challenging because of the limited drug armamentarium and the toxic nature of the therapies. Only three antifungal drugs have reliable efficacy in vivo: intravenous amphotericin B, oral flucytosine and oral fluconazole.
Amphotericin B is a potent broad-spectrum fungicidal, which was first isolated from the bacterium Streptomyces nodosus in 1955 [45], and started to be used clinically in the same decade. It binds ergosterol in fungal cell membranes, inducing pore formation, electrolyte imbalance and cell death. It is effective at penetrating the blood–brain barrier and is key in sterilizing the CSF, but is associated with serious adverse effects, including anemia, electrolyte disturbances, renal impairment and infusion site reactions. Amphotericin B is available in the deoxycholate form (recommended dosage 0.7 mg/kg daily). Generic formulations are available in Asia and Southeast Asia, but only ~ 60% of sub-Saharan African countries are estimated to have stock available (see https://gaffi.org/cryptococcal-meningitis-dashboard-for-sub-saharan-africa/). In higher resource settings, the liposomal formulation (dose 4 mg/kg) is available, which has similar efficacy, but is associated with less toxicity [46, 47].
Flucytosine was also identified in the 1950s. It inhibits fungal DNA synthesis and is fungistatic in mechanism, making it less potent than amphotericin, although its effect combined with amphotericin is probably synergistic [48, 49]. It has a much lower barrier to resistance, so is only suitable for use in combination with other antifungal agents. The main side effects are gastrointestinal intolerance, presenting as abdominal pain, laboratory abnormalities including raised aminotransferases, and marrow suppression, manifesting as anemia, leukopenia and thrombocytopenia. Renal impairment caused by the concomitant administration of nephrotoxic agents, such as amphotericin B, can lead to accumulation of flucytosine and further contribute to dose-related toxicity. While flucytosine is off patent, it has extremely limited availability as a consequence of its limited disease indications, meaning that there are very few manufacturers. The price of this drug has increased significantly in recent years [50]. This results in high costs in resource-poor settings where disease burden is greatest. The chronic shortage of global manufacturers is improving thanks to recent progress on the part of Unitaid and the Clinton Health Access Initiative [51], raising hopes that flucytosine will be produced at scale to meet demand in coming years.
Fluconazole is also a broad-spectrum antifungal. It was patented in 1981 and is now cheaply available throughout the world. It acts by inhibiting the cytochrome P450 (CYP) enzyme 14α-demethylase, to which fungal cells are far more sensitive than mammalian cells. It is generally well tolerated, but may cause rash and liver enzyme abnormalities, especially at the high doses required to treat cryptococcal meningitis. Because it is an inhibitor of CYP isoenzymes, drug interactions are common and frequently complicate treatment. While prolongation of the QT interval of the cardiac cycle is listed as a potential complication, QT prolongation is generally modest in practice and does not usually warrant discontinuation of treatment [52].
These three drugs have been used for variable durations in different combinations to treat cryptococcal disease for the last 3 decades. In recent years, international guidelines have been shaped by a series of large clinical trials in HIV-infected populations, which have attempted to strike a balance between the need for rapid fungal clearance while avoiding serious drug toxicity, with practical regimens that can be implemented in low-resource settings.
Current Antifungal Recommendations and Evidence Underlying Them
In 2022, the WHO updated its 2018 guidelines for the treatment of cryptococcal meningitis in HIV-infected patients based upon the results of the recently published AMBITION trial [39, 53]. The treatment guidelines are summarized in Table 2. They recommend that treatment should be given in three distinct phases: ‘induction’ therapy, with combination high-dose antifungals in the first 2 weeks of treatment, to rapidly sterilize the CSF; ‘consolidation’ with high-dose fluconazole monotherapy for the next 8–10 weeks; and then low-dose fluconazole ‘maintenance’ therapy to prevent relapse until immune recovery occurs as a consequence of ART.
Table 2.
WHO guideline for cryptococcosis in HIV-infected patients, 2022
| Phase | Induction | Consolidation | Maintenance | |
|---|---|---|---|---|
| Option | Week 1 | Week 2 | Weeks 8–10 | Onwards |
| 1—Preferred | ||||
| Single-dose liposomal amphotericin Ba plus 14 days of flucytosineb combined with fluconazole | Fluconazole 800 mg/day | Fluconazole 200 mg/day | ||
| 2—If liposomal amphotericin is unavailable | ||||
| Amphotericin B deoxycholatec plus flucytosine | Fluconazole 1200 mg/day | Fluconazole 800 mg/day | Fluconazole 200 mg/day | |
| 3—If no amphotericin formulation is available | ||||
| Fluconazole 1200 mg/day plus flucytosineb | Fluconazole 800 mg/day | Fluconazole 200 mg/day | ||
| 4—If flucytosine is unavailable | ||||
| Liposomal amphotericin Bd plus fluconazole 1200 mg/day | Fluconazole 800 mg/day | Fluconazole 200 mg/day | ||
| 5—If liposomal amphotericin B and flucytosine are unavailable | ||||
| Amphotericin B deoxycholatec plus fluconazole 1200 mg/day | Fluconazole 800 mg/day | Fluconazole 200 mg/day | ||
All doses refer to adults; for children, refer to the WHO guidelines
WHO World Health Organization
a10 mg/kg
b100 mg/kg/day in 4 divided doses
c1 mg/kg/day
d3–4 mg/kg/day
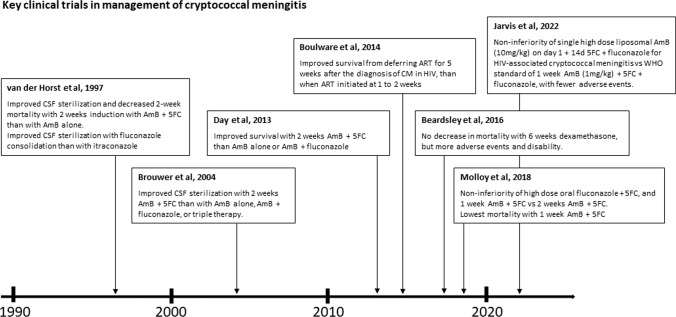
These current treatment guidelines are built on a foundation of evidence delivered from a number of large, randomized, controlled trials from the USA, Southeast Asia and Africa. The first of these was a study from 1997 conducted in the USA, which evaluated the effectiveness of 2 weeks of induction therapy with amphotericin B with or without the flucytosine in 381 patients with HIV and cryptococcal meningoencephalitis, followed by 8 weeks of consolidation therapy with an azole (Fig. 2) [54]. CSF sterilization at 2 weeks and 10 weeks was more frequent among patients receiving combination amphotericin B and flucytosine therapy compared with those receiving amphotericin B alone during induction, without increased toxicity. The study lacked the power to demonstrate a mortality benefit of the combination therapy, but a number of smaller studies subsequently confirmed improved rates of yeast clearance from CSF with this treatment combination [55, 56].
Fig. 2.
Key clinical trials in the management of cryptococcal meningitis. AmB amphotericin B, 5FC flucytosine, CM cryptococcal meningitis, CSF cerebrospinal fluid, 14d 14 days
In 2013, a randomized, three-group, open-label trial in Vietnam evaluated induction therapy in 299 HIV-infected patients with cryptococcal meningitis. Patients were randomly assigned to one of three different induction regimens: standard of care (amphotericin 1 mg/kg/day for 4 weeks), amphotericin combined with flucytosine (100 mg/kg/day) for 2 weeks, or amphotericin combined with high-dose fluconazole (800 mg/day) for 2 weeks. After the induction phase, all patients received fluconazole 400 mg/day until 10 weeks after randomization. The investigators found survival was significantly improved by 10 weeks after randomization in the patients treated with the amphotericin and flucytosine combination compared with those receiving amphotericin alone or in combination with fluconazole [6]. Lending biological plausibility to the results, the rate of clearance of yeast from CSF was significantly faster in patients receiving amphotericin and flucytosine compared with the other two arms. A survival benefit of amphotericin B plus fluconazole compared with amphotericin monotherapy was found.
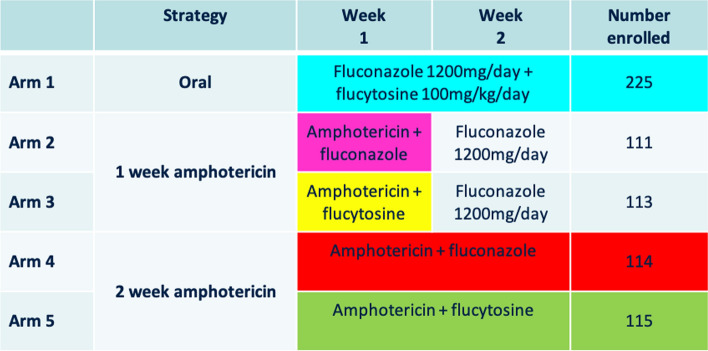
The favorable survival outcome seen from induction therapy with flucytosine in combination with amphotericin B has since been confirmed. The ACTA study, published in 2018, was a pragmatic randomized trial conducted across nine treatment sites in Africa, which evaluated alternative practical induction regimens in HIV-infected patients with cryptococcal meningitis in resource-poor settings [7]. The study investigated two main strategies. First, they wanted to test the utility of an oral-based regimen compared with 2-week intravenous amphotericin-based induction regimens. Secondly, they wanted to compare giving 2-week versus 1-week amphotericin-based induction regimens.
The investigators randomized 721 patients to an all-oral regimen of very high-dose fluconazole (1200 mg per day) plus flucytosine for 2 weeks, or 1 week of amphotericin B, or 2 weeks of amphotericin B, randomly assigning those receiving amphotericin to fluconazole or flucytosine as a partner drug. All patients received fluconazole consolidation therapy and were followed to 10 weeks. The treatment arms are illustrated in Fig. 3.
Fig. 3.
Treatment arms of the ACTA trial. Principle comparisons were between arm 1 versus arms 4 and 5 (the oral vs intravenous induction treatment strategy), and arms 2 and 3 versus arms 4 and 5 (1 week vs 2 weeks of amphotericin strategy). After induction therapy (the first 2 weeks), all patients received fluconazole 800 mg/day consolidation therapy until 10 weeks after randomization
Regarding the primary endpoints, the all-oral combination and the 1-week amphotericin B regimen both met predefined criteria for non-inferiority compared to the 2-week amphotericin induction arms (standard of care). In secondary analyses, they found that survival was better in patients on amphotericin who received flucytosine than those who received amphotericin partnered with fluconazole (71 deaths [31.1%] vs 101 deaths [45.0%]). Notably, the induction treatment regimen associated with the best survival was the one consisting of 1 week of amphotericin B plus flucytosine. This was associated with the lowest 10-week mortality (24.2%; 95% confidence interval 16.2–32.1) and lower rates of side effects compared with the other regimens. The investigators postulate that it is toxicity associated with prolonged courses of amphotericin that may result in poorer survival. WHO guidelines changed following these data, with a single week of amphotericin B combined with flucytosine recommended as first-line induction therapy in resource-poor settings since 2018. In high-resource settings benefiting from the availability of liposomal amphotericin B and optimal clinical monitoring, 2 weeks of induction therapy with amphotericin B and flucytosine was still preferred.
However, the administration of intravenous amphotericin, even for just a week, can still be a considerable logistical and financial barrier to treatment in low-income settings. Liposomal amphotericin B has a long tissue half-life and concentrates well in brain tissue. Pharmacokinetic modelling studies have suggested that single or intermittent administration of higher doses of this formulation could deliver efficient antifungal effect [57, 58]. Short intermittent high-dose liposomal amphotericin B (10 mg on day 1, 5 mg on days 3 and 6) was previously found to be as safe and effective as a standard therapy for the empirical treatment of persistent febrile neutropenia, and a small trial in cryptococcal meningitis suggested such an approach could be effective [59, 60]. This led to the landmark AMBITION trial, published in March 2022. AMBITION took place in five countries in Africa. It trialed induction therapy consisting of a single high-dose of liposomal amphotericin (10 mg/kg), combined with 14 days of flucytosine (100 mg/kg/day in divided doses) and fluconazole (1200 mg/day). This was compared with the 7-day amphotericin B deoxycholate and flucytosine combination, as recommended by WHO since 2018. Following induction therapy, all patients continue with fluconazole 800 mg/day for a further 8 weeks. The trial found that the novel induction strategy was non-inferior to the WHO guideline in terms of survival. The number of deaths amongst patients receiving the novel intervention (24.8%) was similar to that amongst patients on standard therapy (29.3%). Moreover, there were significantly fewer grade 3, grade 4 and serious adverse events in patients on the novel treatment regimen (50% vs 62.3%). An adjusted analysis suggested the novel strategy may be associated with improved survival. The novel strategy was also preferred by health care providers involved in the study because it took less time to prepare, required less monitoring and had the potential to shorten the patient’s duration of hospital stay. The WHO released Rapid Advice on line on 20 April 2022 recommending this treatment approach be used in people living with HIV, and full amended guidelines were published in June 2022 (available at https://www.who.int/publications/i/item/9789240052178). Successful global roll-out of this innovative and effective treatment approach will be critically dependent upon managing the costs and availability of liposomal amphotericin and flucytosine.
Where flucytosine is unavailable, induction therapy with amphotericin B and fluconazole is recommended on the basis that this combination has fewer side effects than a 4-week course of amphotericin B deoxycholate [6]. Dosing recommendations for fluconazole have been updated as a consequence of the ACTA study, which has shown that, when fluconazole must be used during induction, higher doses (1200 mg/day) are well tolerated and trend towards better outcomes [61]. The use of higher doses of fluconazole is further justified by the wide variability in the concentrations of fluconazole achieved in blood and CSF with standard doses, and it is clear that significant numbers of patients do not reach the notional therapeutic targets [62]. While there seems to be some variability in the susceptibility of Cryptococcus isolates to azole drugs, this does not seem to explain patient outcomes, and routine testing of drug susceptibilities is not recommended for first presentations of disease [63].
Consolidation and Maintenance
Following induction therapy, patients should receive consolidation therapy with fluconazole 800 mg/day for a minimum of 8 weeks. While it is not clear that this results in better outcomes than 400 mg/day, fluconazole is cheap and safe and, as mentioned previously, there is good evidence of wide variability in its pharmacokinetics, with significant numbers of patients being essentially underdosed at 400 mg/day. After the induction and consolidation phases of therapy, typically lasting 10–12 weeks in total, HIV-infected patients should receive ‘maintenance’ therapy with fluconazole 200 mg/day until their CD4 counts have risen consistently above 200 cells/µL and the HIV viral load is undetectable on ART [39].
Antifungal Treatment in HIV-Uninfected Patients with Meningitis
The relative abundance of evidence to guide treatment decisions for HIV-associated cryptococcal meningitis is in marked contrast with the paucity of evidence related to disease in HIV-uninfected patients. The optimal duration of therapy at all stages (induction, consolidation and maintenance) for HIV-uninfected patients is unclear—randomized controlled trials in these groups of patients are a major area of need. Trials have been hampered by the relative rarity of cases and the consequent difficulty in adequately powering studies. Given this, it would seem reasonable to enroll HIV-negative patients into ongoing trials in HIV-infected patients, where the intervention is likely to be relevant. Randomization can be stratified by HIV serostatus. In the absence of high-quality evidence, induction therapy is usually based upon combinations of amphotericin with other antifungal drugs, followed by fluconazole maintenance therapy. A particular challenge is determining when antifungal therapy can be stopped. A detailed review of treatment for this patient group is beyond the scope of this review, but a number of national bodies publish treatment guidelines based upon expert consensus [64, 65].
Adjunctive Therapies
Unlike in tuberculous or streptococcal meningitis, there is no role for the universal administration of glucocorticoids during induction therapy for HIV-associated cryptococcal meningitis. The use of dexamethasone was evaluated in a placebo-controlled, randomized trial that recruited patients across Asia and Africa [66]. Patients received amphotericin plus fluconazole with or without dexamethasone (starting at 0.3 mg/kg/day and then tapered over 6 weeks). The trial was stopped early after recruitment of 451 patients when a planned interim analysis found an increased risk of adverse events in the dexamethasone group. There was no difference seen in mortality or rate of IRIS at 10 weeks. Dexamethasone did reduce the CSF opening pressure over the first 2 weeks of treatment, but rates of fungal clearance from the CSF, rates of adverse events, and neurological outcomes at 6 months were all worse in the steroid group.
Alternative Antifungal Drugs
Itraconazole, posaconazole, voriconazole and isavuconazole are all azole drugs active against Cryptococcus and may be suitable for non-meningeal disease, although randomized controlled trial data are lacking. With the exception of voriconazole, and unlike fluconazole, these drugs have poor blood–brain–barrier penetration, which limits their use in central nervous system disease. Itraconazole has been used in consolidation therapy for cryptococcal meningitis [54]; however, CSF sterilization appears to be inferior to fluconazole, and significant differences in bioavailability between individuals mean that drug level monitoring is required. Itraconazole has a number of drug interactions and high rates of gastrointestinal toxicity. Voriconazole, in combination with flucytosine, is currently being evaluated as an option for induction therapy for cryptococcal meningitis in HIV-infected individuals in a clinical trial in China [67]. It is associated with hepatotoxicity and visual and auditory disturbance at high doses. Photosensitivity reactions may be problematic with prolonged use in sunny climates. Terbinafine has shown promise in vitro [68] and in animals [69], but is unlikely to be a practical candidate in humans due to its pharmacokinetic properties. Echinocandins have no significant activity against Cryptococcus. Of note, the reliance on azole drugs for consolidation and maintenance therapy is a particular problem in areas with high tuberculosis prevalence, where the two infections may co-exist, because of significant induction of their metabolism by rifampicin. Development of alternatives to the azoles would be a major advance.
Future Treatment Strategies for Cryptococcal Meningitis
Disappointing outcomes from conventional induction therapy, combined with the unavailability of flucytosine and liposomal amphotericin B in high-burden, resource-poor settings, makes the need for novel treatment strategies urgent. In addition to modifying current therapy as described in the ACTA and AMBITION trials, other options include repurposing existing drugs, developing novel agents and use of non-pharmaceutical interventions (Fig. 4).
Fig. 4.
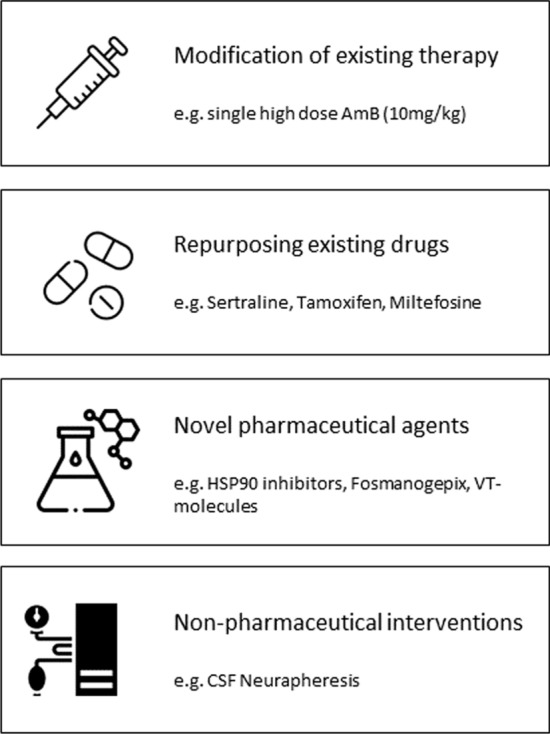
Strategies to improve treatment of cryptococcal meningitis. AmB amphotericin, CSF cerebrospinal fluid, HSP90 heat shock protein 90
Repurposed Drugs
An alternative strategy to developing novel antifungals involves repurposing existing drugs, which are safe, off-patent and commercially viable based on their ongoing use in treating other diseases. Sertraline, an inexpensive, widely prescribed selective serotonin reuptake inhibitor antidepressant, has been shown to have anti-cryptococcal activity in vitro and in mice through inhibition of intracellular vesicle transport [70, 71]. It has also demonstrated synergism with fluconazole [71]. The ASTRO-CM pilot study evaluated optimal dosing of sertraline for cryptococcal meningitis in an open-label study in Uganda, testing four different doses during induction therapy, with a primary outcome of fungal clearance from CSF at 2 weeks [72] compared to historical controls. Investigators noted a faster cryptococcal CSF clearance and a lower incidence of IRIS and relapse than occurred in historical controls. This prompted a phase III, multicenter, double-blind, placebo-controlled trial of amphotericin B and fluconazole-based induction therapy with and without sertraline 400 mg daily for 2 weeks to treat cryptococcal meningitis in HIV-infected adults [73]. Disappointingly, there was no reduction in mortality or CSF fungal clearance, and the trial was stopped for futility after 460 of a planned 550 participants had been randomized. This corroborated results from a 12-person, placebo-controlled trial in Mexico, which used a 200-mg dose [74]. The reasons for sertraline’s ineffectiveness are likely multifactorial, but include inadequate drug concentrations, underappreciated interactions with ART and possible immunomodulatory effects of sertraline. The ASTRO-CM investigators conceded the limitations of using retrospective comparisons in advancing clinical trials, particularly for combination antifungal therapy.
Another low-cost oral drug that has shown promise for repurposing is tamoxifen, a selective estrogen receptor modulator used to treat breast cancer and osteoporosis. It has anti-yeast [75] and anti-cryptococcal effects [76] and has been shown to be synergistic with fluconazole [77] and amphotericin in vitro [78]. In a mouse model of disseminated cryptococcosis, tamoxifen was synergistic with fluconazole, achieved high concentrations in the brain and inhibited growth of Cryptococcus within macrophages [79]. However, a phase II, randomized, open-label study from Vietnam, which evaluated the addition of tamoxifen 300 mg to standard induction therapy, found no difference in the rate of fungal clearance from CSF after 2 weeks of treatment [52]. A significantly higher rate of QTc interval prolongation events was noted in the tamoxifen group, making higher dosing than the 300 mg used an unattractive proposition.
Miltefosine is another potential therapeutic candidate. It is primarily used to treat leishmaniasis, in combination with amphotericin, as well as amoebic infections, where it interacts with cellular lipids and cytochrome c oxidase to induce apoptosis-like cell death. It is effective against Cryptococcus in vitro [80] and in some murine models of disseminated cryptococcosis [81], but has not yet been evaluated in a clinical trial.
Alternative candidates are continuously being evaluated in pre-clinical studies. In vitro screening of a wide selection of off-patent drugs found 43 drugs capable of inhibiting the growth of C. neoformans [82], such as ciclopirox and auranofin, although it is unclear if they will ever be tested in a clinical trial. Antiprotozoal drugs like benzimidazoles and flubendazole have also been shown to reduce fungal burden in infected mice [83].
Novel Therapeutics
Novel therapeutics that have shown promise in vitro or in mouse models include fosmanogepix, Mycograb, and the VT-molecules. Fosmanogepix is a first-in-class antifungal drug candidate that inhibits the fungal enzyme Gwt1 (a highly conserved inositol acylase in Cryptococcus spp.) in the glycosylphosphatidylinositol (GPI) biosynthesis pathway [84]. This prevents the appropriate localization of cell wall mannoproteins, compromising cell wall integrity, biofilm and germ tube formation, and fungal growth. Fosmanogepix has in vitro activity against a wide spectrum of pathogenic fungi including C. neoformans, C. gattii, Candida albicans, Candida auris and Aspergillus fumigatus and is synergistic with fluconazole [85] and liposomal amphotericin B [86]. In a murine model, it reduced cryptococcal burden in lungs and brain [87], and appears to have a favorable side effect profile in humans [88]. Fosmanogepix has been given fast track status by the US Food and Drug Administration (FDA) for several fungal infections, including Cryptococcus, and multiple ongoing studies will define its role in treating invasive disease.
Mycograb is a recombinant human antibody against heat shock protein 90 (Hsp90), which was developed as a cancer drug. Hsp90 is required for fungal cellular homeostasis, and this drug has shown good in vitro activity versus Cryptococcus [89, 90]. It appears synergistic in combination with amphotericin [91]. Mycograb initially generated considerable interest, and phase II studies were planned [92], but unfortunately, all Hsp90 inhibitors developed thus far have proved too immunosuppressive to be used as antifungals. However, recent research suggests that intrinsic differences in protein flexibility can confer selective inhibition of fungal versus human Hsp90 isoforms, raising the possibility this drug class may yet have a role [93].
New agents with greater specificity for fungal (as opposed to mammalian) CYP51 enzyme offer more hope. VT-1161 (oteseconazole), VT-1129 (quilseconazole) and VT-1598 prevent the biosynthesis of ergosterol within fungal cell membranes through inhibition of CYP51 and are highly potent in vitro against Candida spp. and Cryptococcus [94]. In a murine model of cryptococcal meningitis, this agent improved survival and the clearance of tissue fungal burden, and concentrated in brain tissue long after dosing was stopped due to its long half-life [95]. Human safety and efficacy studies are required.
Non-Pharmaceutical Options
Neurapheresis CSF filtration therapy is an emerging technology designed to treat patients with hemorrhagic stroke [96]. It has undergone initial safety and feasibility evaluation in aneurysmal subarachnoid hemorrhage, where it demonstrated the potential to safely filter CSF and remove blood and blood byproducts [97]. The device extracts contaminated CSF from the lumbar subarachnoid space via a custom dual-lumen catheter and passes it through a filtration system tailored to pathogen removal. Filtered CSF is reintroduced into the midthoracic region through the same catheter. The machine can perform around 20 filtration cycles in a 24-h period via an automated pump. The filtration technology offers a promising, one-time means of rapidly sterilizing CSF in cryptococcal meningitis, potentially reducing the need for prolonged antifungal therapy, and offering a chance to help patients presenting with high fungal loads who currently have high, early mortality rates. In vitro, filtration of CSF through this system yielded a 5-log reduction in yeast and a 1-log reduction in its polysaccharide antigen over 24 h. An analogous closed-loop system in a rabbit model achieved 97% clearance of yeasts from the subarachnoid space over 4–6 h [98]. In cryptococcal meningitis, the technology offers additional benefits beyond rapid removal of fungus from CSF. It potentially allows continuous monitoring and control of CSF pressures and may reduce the need for repeated therapeutic lumbar puncture. It may also remove other contributors to pathogenesis including cytokines and shed capsule, and even allow the direct delivery of antifungal drugs into CSF. The costs of the technology are likely to fall if it proves effective in cerebrovascular disease, and it has the potential to become affordable in centers with significant numbers of cases of cryptococcal meningitis even in low-income settings.
Conclusions
There has been something of a renaissance in the number of large, randomized, controlled trials in cryptococcal meningitis over the past 10 years, and the evidence base for prescribing has never been stronger. However, they have primarily been focused on the best use of current therapy, particularly in low-incomes settings, or drug repurposing. There has been little impact on the headline survival rates, which have changed little over the past 30 years, and there is a pressing need to develop new, more effective drugs. Moreover, HIV-uninfected patients with cryptococcal disease have been a particularly neglected group. Given that the vast majority of cases of cryptococcal disease are in low-income settings, which are unlikely to be lucrative and offer significant financial returns, we need new models for funding innovation to encourage industry and academia to work together to identify and develop novel anticryptococcal drugs. It is unsatisfactory to hope that new antifungal drugs developed for other diseases in high-income countries may also have effects that can then be applied in cryptococcal disease—we need specific efforts. While recent experience with repurposing for cryptococcal meningitis has been disappointing, such small, randomized trials, looking at the effect on the rate of clearance of yeast from CSF, should continue with the remaining candidates. As the coronavirus disease 2019 (COVID-19) pandemic has demonstrated, there is no substitute for randomized, controlled trials when trying to identify best treatments.
Declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
Drs. Day, Flower and Thuy declare that they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to the design of the review based upon a talk given by JND at the European Congress on Clinical Microbiology and Infectious Diseases in Amsterdam in 2019. NTTN and BF updated the paper, NTTN wrote the first draft; BF and JND edited the paper.
References
- 1.Park BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Rajasingham R, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soria J, et al. Mortality in hospitalized patients with tuberculous meningitis. BMC Infect Dis. 2019;19(1):9. doi: 10.1186/s12879-018-3633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakır M, et al. Validation of a severity grading score (SGS) system for predicting the course of disease and mortality in patients with Crimean-Congo hemorrhagic fever (CCHF) Eur J Clin Microbiol Infect Dis. 2015;34(2):325–330. doi: 10.1007/s10096-014-2238-0. [DOI] [PubMed] [Google Scholar]
- 5.Ostrom QT, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl 5):v1–v100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day JN, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368(14):1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molloy SF, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. 2018;378(11):1004–1017. doi: 10.1056/NEJMoa1710922. [DOI] [PubMed] [Google Scholar]
- 8.Busse O. Uber parasitare zelleinschlusse und ihre zuchtung. Zentralbl Bakteriol. 1894;16:175–180. [Google Scholar]
- 9.Kwon-Chung KJ, et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med. 2014;4(7):a019760. doi: 10.1101/cshperspect.a019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon-Chung KJ, et al. The case for adopting the "species complex" nomenclature for the etiologic agents of Cryptococcosis. mSphere. 2017;2(1):e357-16. doi: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashton PM, et al. Three phylogenetic groups have driven the recent population expansion of Cryptococcus neoformans. Nat Commun. 2019;10(1):2035. doi: 10.1038/s41467-019-10092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoffelen T, et al. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PLoS ONE. 2013;8(1):e55579. doi: 10.1371/journal.pone.0055579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirofski LA, Casadevall A. The state of latency in microbial pathogenesis. J Clin Invest. 2020;130(9):4525–4531. doi: 10.1172/JCI136221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidd SE, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc Natl Acad Sci U S A. 2004;101(49):17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrnes EJ, Marr KA. The outbreak of Cryptococcus gattii in Western North America: epidemiology and clinical issues. Curr Infect Dis Rep. 2011;13(3):256–261. doi: 10.1007/s11908-011-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi V, Chaturvedi S. Cryptococcus gattii: a resurgent fungal pathogen. Trends Microbiol. 2011;19(11):564–571. doi: 10.1016/j.tim.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappas PG, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33(5):690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 18.Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30(1):179–206. doi: 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day JN, et al. Comparative genomics of Cryptococcus neoformans var. grubii associated with meningitis in HIV infected and uninfected patients in Vietnam. PLoS Negl Trop Dis. 2017;11(6):e0005628. doi: 10.1371/journal.pntd.0005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day JN, et al. Most cases of cryptococcal meningitis in HIV-uninfected patients in Vietnam are due to a distinct amplified fragment length polymorphism-defined cluster of Cryptococcus neoformans var. grubii VN1. J Clin Microbiol. 2011;49(2):658–664. doi: 10.1128/JCM.01985-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chau TT, et al. A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam—high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis. 2010;10:199. doi: 10.1186/1471-2334-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang CL, Wang J, Zou LL. Innate immune evasion strategies against cryptococcal meningitis caused by Cryptococcus neoformans. Exp Ther Med. 2017;14(6):5243–5250. doi: 10.3892/etm.2017.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond RD, et al. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974;112(6):2260–2270. [PubMed] [Google Scholar]
- 24.Kwon-Chung KJ, Rhodes JC. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51(1):218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moodley A, et al. Early clinical and subclinical visual evoked potential and Humphrey's visual field defects in cryptococcal meningitis. PLoS ONE. 2012;7(12):e52895. doi: 10.1371/journal.pone.0052895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnamoorthy A, Joel A, Abhilash K. Cryptococcal meningitis with multiple cranial nerves palsies: a review of literature. J Glob Infect Dis. 2015;7(3):123–124. doi: 10.4103/0974-777X.161739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graybill JR, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis: the NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30(1):47–54. doi: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 28.Liu PY, Yang Y, Shi ZY. Cryptococcal liver abscess: a case report of successful treatment with amphotericin-B and literature review. Jpn J Infect Dis. 2009;62(1):59–60. [PubMed] [Google Scholar]
- 29.Philip KJ, et al. Disseminated cryptococcosis presenting with generalized lymphadenopathy. J Cytol. 2012;29(3):200–202. doi: 10.4103/0970-9371.101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JW, Arnold WC. Cryptococcal peritonitis in patients on peritoneal dialysis. Am J Kidney Dis. 1988;11(5):430–433. doi: 10.1016/S0272-6386(88)80057-X. [DOI] [PubMed] [Google Scholar]
- 31.Gopal M, et al. Cryptococcosis of the upper genital tract. AIDS Patient Care STDS. 2009;23(2):71–73. doi: 10.1089/apc.2008.0108. [DOI] [PubMed] [Google Scholar]
- 32.Ito M, et al. Disseminated Cryptococcosis with adrenal insufficiency and meningitis in an immunocompetent individual. Internal medicine (Tokyo, Japan) 2017;56(10):1259–1264. doi: 10.2169/internalmedicine.56.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai SM, Teoh SC. Ocular cryptococcosis as a presenting manifestation of cryptococcal meningitis in a patient with HIV. Int J STD AIDS. 2012;23(5):377–378. doi: 10.1258/ijsa.2009.009132. [DOI] [PubMed] [Google Scholar]
- 34.Qu J, et al. Comparison of clinical features and prognostic factors in HIV-negative adults with cryptococcal meningitis and tuberculous meningitis: a retrospective study. BMC Infect Dis. 2017;17(1):51. doi: 10.1186/s12879-016-2126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darras-Joly C, et al. Cryptococcus neoformans infection in France: epidemiologic features of and early prognostic parameters for 76 patients who were infected with human immunodeficiency virus. Clin Infect Dis. 1996;23(2):369–376. doi: 10.1093/clinids/23.2.369. [DOI] [PubMed] [Google Scholar]
- 36.Garlipp CR, Rossi CL, Bottini PV. Cerebrospinal fluid profiles in acquired immunodeficiency syndrome with and without neurocryptococcosis. Rev Inst Med Trop Sao Paulo. 1997;39(6):323–325. doi: 10.1590/S0036-46651997000600003. [DOI] [PubMed] [Google Scholar]
- 37.Boulware DR, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vijayan T, Chiller T, Klausner JD. Sensitivity and specificity of a new cryptococcal antigen lateral flow assay in serum and cerebrospinal fluid. MLO Med Lab Obs. 2013;45(3):16–20. [PMC free article] [PubMed] [Google Scholar]
- 39.Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2018. Geneva: World Health Organization. [PubMed]
- 40.Beyene T, et al. Inadequacy of high-dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)-positive human immunodeficiency virus-infected persons in an Ethiopian CrAg screening program. Clin Infect Dis. 2017;65(12):2126–2129. doi: 10.1093/cid/cix613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wake RM, et al. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among human immunodeficiency virus-infected patients. Clin Infect Dis. 2018;66(5):686–692. doi: 10.1093/cid/cix872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khoo SH, Bond J, Denning DW. Administering amphotericin B—a practical approach. J Antimicrob Chemother. 1994;33(2):203–213. doi: 10.1093/jac/33.2.203. [DOI] [PubMed] [Google Scholar]
- 43.Bisson GP, et al. Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clin Infect Dis. 2013;56(8):1165–1173. doi: 10.1093/cid/cit019. [DOI] [PubMed] [Google Scholar]
- 44.Makadzange AT, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis. 2010;50(11):1532–1538. doi: 10.1086/652652. [DOI] [PubMed] [Google Scholar]
- 45.Dutcher JD. The discovery and development of amphotericin B. Dis Chest. 1968;54(suppl 1):296–298. doi: 10.1378/chest.54.Supplement_1.296. [DOI] [PubMed] [Google Scholar]
- 46.Hamill RJ, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis. 2010;51(2):225–232. doi: 10.1086/653606. [DOI] [PubMed] [Google Scholar]
- 47.Sharkey PK, et al. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22(2):315–321. doi: 10.1093/clinids/22.2.315. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz P, et al. In vitro interaction of flucytosine with conventional and new antifungals against Cryptococcus neoformans clinical isolates. Antimicrob Agents Chemother. 2003;47(10):3361–3364. doi: 10.1128/AAC.47.10.3361-3364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pound MW, et al. Overview of treatment options for invasive fungal infections. Med Mycol. 2011;49(6):561–580. doi: 10.3109/13693786.2011.560197. [DOI] [PubMed] [Google Scholar]
- 50.Merry M, Boulware DR. Cryptococcal meningitis treatment strategies affected by the explosive cost of flucytosine in the United States: a cost-effectiveness analysis. Clin Infect Dis. 2016;62(12):1564–1568. doi: 10.1093/cid/ciw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Initiative, C.H.A. HIV market report—the state of HIV treatment, testing, and prevention in low- and middle-income countries. 2020.
- 52.Ngan NTT, et al. An open label randomized controlled trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis. Elife. 2021;10:68929. doi: 10.7554/eLife.68929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarvis JN, et al. Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med. 2022;386(12):1109–1120. doi: 10.1056/NEJMoa2111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Horst CM, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337(1):15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 55.Brouwer AE, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363(9423):1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 56.Dromer F, et al. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS ONE. 2008;3(8):e2870. doi: 10.1371/journal.pone.0002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hope WW, et al. Population pharmacokinetics of conventional and intermittent dosing of liposomal amphotericin B in adults: a first critical step for rational design of innovative regimens. Antimicrob Agents Chemother. 2012;56(10):5303–5308. doi: 10.1128/AAC.00933-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Connor L, et al. Pharmacodynamics of liposomal amphotericin B and flucytosine for cryptococcal meningoencephalitis: safe and effective regimens for immunocompromised patients. J Infect Dis. 2013;208(2):351–361. doi: 10.1093/infdis/jit164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellis M, et al. A safety and feasibility study comparing an intermittent high dose with a daily standard dose of liposomal amphotericin B for persistent neutropenic fever. J Med Microbiol. 2009;58(Pt 11):1474–1485. doi: 10.1099/jmm.0.012401-0. [DOI] [PubMed] [Google Scholar]
- 60.Jarvis JN, et al. Short-course high-dose liposomal amphotericin B for human immunodeficiency virus-associated cryptococcal meningitis: a phase 2 randomized controlled trial. Clin Infect Dis. 2019;68(3):393–401. doi: 10.1093/cid/ciy515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loyse A, et al. Comparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis. 2012;54(1):121–128. doi: 10.1093/cid/cir745. [DOI] [PubMed] [Google Scholar]
- 62.Stott KE, et al. Population pharmacokinetics and cerebrospinal fluid penetration of fluconazole in adults with cryptococcal meningitis. Antimicrob Agents Chemother. 2018;62:9. doi: 10.1128/AAC.00885-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Connor L, et al. Antifungal susceptibility does not correlate with fungal clearance or survival in AIDS-associated cryptococcal meningitis. Clin Infect Dis. 2021;73(7):e2338–e2341. doi: 10.1093/cid/ciaa1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang CC, et al. Consensus guidelines for the diagnosis and management of cryptococcosis and rare yeast infections in the haematology/oncology setting, 2021. Intern Med J. 2021;51(Suppl 7):118–142. doi: 10.1111/imj.15590. [DOI] [PubMed] [Google Scholar]
- 65.Perfect JR, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beardsley J, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374(6):542–554. doi: 10.1056/NEJMoa1509024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.clinicaltrials.gov. Three induction treatments on cryptococcal meningitis (TITOC). 2019.
- 68.Guerra CR, et al. Terbinafine inhibits Cryptococcus neoformans growth and modulates fungal morphology. Mem Inst Oswaldo Cruz. 2012;107(5):582–590. doi: 10.1590/S0074-02762012000500003. [DOI] [PubMed] [Google Scholar]
- 69.Olsen G, et al. Use of terbinafine in the treatment protocol of intestinal Cryptococcus neoformans in a dog. J Am Anim Hosp Assoc. 2012;48:216–220. doi: 10.5326/JAAHA-MS-5813. [DOI] [PubMed] [Google Scholar]
- 70.Treviño-Rangel Rde J, et al. Activity of sertraline against Cryptococcus neoformans: in vitro and in vivo assays. Med Mycol. 2016;54(3):280–286. doi: 10.1093/mmy/myv109. [DOI] [PubMed] [Google Scholar]
- 71.Zhai B, et al. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother. 2012;56(7):3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhein J, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis. 2016;16(7):809–818. doi: 10.1016/S1473-3099(16)00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rhein J, et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis. 2019;19(8):843–851. doi: 10.1016/S1473-3099(19)30127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villanueva-Lozano H, et al. Clinical evaluation of the antifungal effect of sertraline in the treatment of cryptococcal meningitis in HIV patients: a single Mexican center experience. Infection. 2018;46(1):25–30. doi: 10.1007/s15010-017-1059-3. [DOI] [PubMed] [Google Scholar]
- 75.Wiseman H, Cannon M, Arnstein HRV. Observation and significance of growth inhibition of Saccharomyces cerevisiae (A224A) by the antioestrogen drug tamoxifen. Biochem Soc Trans. 1989;17(6):1038–1039. doi: 10.1042/bst0171038. [DOI] [PubMed] [Google Scholar]
- 76.Dolan K, et al. Antifungal activity of tamoxifen: in vitro and in vivo activities and mechanistic characterization. Antimicrob Agents Chemother. 2009;53(8):3337–3346. doi: 10.1128/AAC.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spitzer M, et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol. 2011;7:499. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hai TP, et al. The combination of tamoxifen with amphotericin B, but not with fluconazole, has synergistic activity against the majority of clinical isolates of Cryptococcus neoformans. Mycoses. 2019;62(9):818–825. doi: 10.1111/myc.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butts A, et al. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. MBio. 2014;5(1):e00765-13. doi: 10.1128/mBio.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spadari CC, et al. Miltefosine has a postantifungal effect and induces apoptosis in cryptococcus yeasts. Antimicrob Agents Chemother. 2018;62(8):00312-18. doi: 10.1128/AAC.00312-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Widmer F, et al. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob Agents Chemother. 2006;50(2):414–421. doi: 10.1128/AAC.50.2.414-421.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossi SA, et al. Identification of off-patent drugs that show synergism with amphotericin B or that present antifungal action against Cryptococcus neoformans and Candida spp. Antimicrob Agents Chemother. 2020;64(4):1921-19. doi: 10.1128/AAC.01921-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nixon GL, et al. Repurposing and reformulation of the antiparasitic agent flubendazole for treatment of cryptococcal meningoencephalitis, a neglected fungal disease. Antimicrob Agents Chemother. 2018;62(4):1909-17. doi: 10.1128/AAC.01909-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao M, et al. APX001 pharmacokinetic/pharmacodynamic target determination against Aspergillus fumigatus in an in vivo model of invasive pulmonary Aspergillosis. Antimicrob Agents Chemother. 2019;63(4):2372-18. doi: 10.1128/AAC.02372-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shaw KJ, et al. In vitro and in vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against Cryptococcus. Antimicrob Agents Chemother. 2018;62(8):e00523–e618. doi: 10.1128/AAC.00523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoenigl M, et al. The antifungal pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs. 2021;81(15):1703–1729. doi: 10.1007/s40265-021-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shaw KJ, et al. In vitro and in vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against Cryptococcus. Antimicrob Agents Chemother. 2018;62(8):52318. doi: 10.1128/AAC.00523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.clinicaltrials.gov. Safety, pharmacokinetics, bioavailability, food effect, drug–drug interaction study of APX001 administered orally. 2016. Amplyx Pharmaceuticals.
- 89.Cordeiro RA, et al. Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiology (Reading) 2016;162(2):309–317. doi: 10.1099/mic.0.000222. [DOI] [PubMed] [Google Scholar]
- 90.Matthews RC, et al. Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob Agents Chemother. 2003;47(7):2208–2216. doi: 10.1128/AAC.47.7.2208-2216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nooney L, Matthews RC, Burnie JP. Evaluation of mycograb, amphotericin B, caspofungin, and fluconazole in combination against Cryptococcus neoformans by checkerboard and time-kill methodologies. Diagn Microbiol Infect Dis. 2005;51(1):19–29. doi: 10.1016/j.diagmicrobio.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 92.NCT, efficacy and safety of mycograb as adjunctive therapy for cryptococcal meningitis in patients with AIDS. https://clinicaltrials.gov/show/NCT00324025. 2006.
- 93.Marcyk PT, et al. Fungal-selective resorcylate aminopyrazole Hsp90 inhibitors: optimization of whole-cell anticryptococcal activity and insights into the structural origins of cryptococcal selectivity. J Med Chem. 2021;64(2):1139–1169. doi: 10.1021/acs.jmedchem.0c01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wiederhold NP, et al. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species, endemic fungi, including Coccidioides species, Aspergillus species and Rhizopus arrhizus. J Antimicrob Chemother. 2018;73(2):404–408. doi: 10.1093/jac/dkx410. [DOI] [PubMed] [Google Scholar]
- 95.Warrilow AGS, et al. The investigational drug VT-1129 is a highly potent inhibitor of Cryptococcus species CYP51 but only weakly inhibits the human enzyme. Antimicrob Agents Chemother. 2016;60(8):4530–4538. doi: 10.1128/AAC.00349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.https://neurapheresis.org/. Available from: https://neurapheresis.org/.
- 97.Blackburn SL, et al. Prospective trial of cerebrospinal fluid filtration after aneurysmal subarachnoid hemorrhage via lumbar catheter (PILLAR) Stroke. 2019;50(9):2558–2561. doi: 10.1161/STROKEAHA.119.025399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smilnak GJ, et al. Novel treatment of cryptococcal meningitis via neurapheresis therapy. J Infect Dis. 2018;218(7):1147–1154. doi: 10.1093/infdis/jiy286. [DOI] [PMC free article] [PubMed] [Google Scholar]