Highlights
-
•
Cinnamaldehyde microcapsules enhanced its bioavailability in mice.
-
•
Cinnamaldehyde microcapsules increased its and its metabolites level in plasma.
-
•
Cinnamaldehyde microcapsules altered intestinal flora compared with non-microcapsules.
-
•
Cinnamaldehyde microcapsules promoted the contents of butyric acid in feces.
Keywords: Cinnamaldehyde, Microcapsules, Bioavailability, Antioxidant, 16s rRNA high-throughput sequencing, Short-chain fatty acids
Abstract
The effects of cinnamaldehyde microcapsules on the concentration of cinnamaldehyde and its metabolites in plasma, urine, and feces, the antioxidant capacity, and the intestinal flora in male C57/BL6 mice were evaluated by oral administration for 7 weeks. Microencapsulation significantly increased the contents of cinnamaldehyde, cinnamyl alcohol, and methyl cinnamate in plasma and decreased those in urine and feces excretion (p < 0.05). In addition, microencapsulated cinnamaldehyde improved antioxidant capacity in liver, duodenum, and colon. Furthermore, 16S rRNA gene sequencing data suggested that microencapsulated cinnamaldehyde significantly improved the gut microbial richness and diversity, increased the abundance of Bacteroides, Bacteroidetes/Firmicutes, unclassified_f_Lachnospiraceae, Lactobacillus, and Blautia genera, and decreased in Ruminococcaceae_UCG-014, Faecalibaculum, norank_f_Muribaculaceae, and Gordonibacter genera, which was accompanied by the increased contents of butyric acid in feces. Therefore, microencapsulated cinnamaldehyde may increase its bioavailability and regulate the balance of intestinal flora.
1. Introduction
Cinnamaldehyde is a natural and plant-derived material obtained from the cinnamon and has been used in food flavors and additive ingredients for a long time (Pragyanshu et al., 2016). Cinnamaldehyde has strong antimicrobial, antioxidant, antidiabetic, and anticancer activities (Chang et al., 2021, Mohammadzamani et al., 2020). Accumulating evidence revealed that cinnamaldehyde possesses in vitro antibacterial property such as Escherichia coli, Salmonella, Staphylococcus aureus, and Enterococcus (Dunn et al., 2016, Mostafa et al., 2017). However, Lactobacillus sp. abundance was not detected and the abundance of potential beneficial bacteria, including Bifidobacteria, Roseburia sp., and Akkermansia muciniphila, were not increased in mice fed with cinnamaldehyde (Pratap et al., 2017). According to gut microbiota's central role in gut microecology, food, and stress may have an impact on the microbiota's ability to affect health and disease (Yang et al., 2020). In addition, disorders of gut microbiota may disrupt physiological homeostasis, which could result in a series of disorders, including allergy, metabolic syndrome, irritable bowel syndrome, diabetes, obesity, inflammatory bowel disease, cardiovascular disease, and cancer (Andre and Buret, 2016, Soumen and Giorgio, 2017, Hou et al., 2021). Therefore, although cinnamaldehyde has strong biological activities, its potential adverse regulation of intestinal flora may affect health negatively.
On the other hand, when exposed to oxygen, cinnamaldehyde was easily oxidised to cinnamic acid by the presence of light, temperature, and other environmental variables (Suryanti, Wibowo, Khotijah, & Andalucki, 2018). It was highly insoluble in water owing to its lipophilicity (Hill et al., 2013, Benavides et al., 2016). Therefore, these characteristics may limit the application of cinnamaldehyde as a bioactive agent. Fortunately, improved stability and efficacy as well as protection from environmental stress might be achieved by using micro-encapsulation technology (Azzi, Jraij, Auezova, Fourmentin, & Greige-Gerges, 2018). Encapsulations of cinnamaldehyde into β-cyclodextrins (β-CDs) has been found to increase their stability and in vitro antimicrobial properties and was mainly used for food preservation (Zou et al., 2021). This may be related to the unique hydrophobic inner surface of β-CDs, which have the ability to form inclusion complexes with cinnamaldehyde via the formation of noncovalent bond (Shelley and Babu, 2018, Yang et al., 2019). Moreover, microcapsules had the ability of controlling the release of the capsule material under certain circumstances (Sanbhal et al., 2018).
Therefore, we presumed that microencapsulation might improve the bioavailability of cinnamaldehyde and regulate the structure of intestinal flora by changing the phase solubility and controlling the release of cinnamaldehyde. However, microencapsulated cinnamaldehyde has yet to be studied in terms of its influence on gut flora. Cinnamaldehyde was encapsulated using β-CDs in this study. The contents of cinnamaldehyde and its metabolites were assessed in mice administered orally with microencapsulated cinnamaldehyde. Moreover, gene sequencing 16S rRNA was utilized for the impact analysis of microencapsulation of cinnamaldehyde on intestinal flora in mice. This study would provide a reference for the better application of cinnamaldehyde in functional foods.
2. Materials and methods
2.1. Reagents and chemicals
China's Shanghai Titan Scientific Co. ltd (Shanghai, China) provided the cinnamaldehyde (around 95 %). Trophic Animal Feed High-Tech Co. ltd. (Nantong, China) was entrusted with the feeds. Nanjing Jiancheng Bioengineering Institute (Nanjing, China) provided the commercially diagnostic assay kits including antioxidant biomarker and inflammatory cytokine mice test kits. Beta-cyclodextrin (purity > 97 %, MW = 1135 Da) was purchased from Seebio Biotech, Inc. (Shanghai, China). Propiophenone was used as the internal standard (IS). Tween-80 and the other chemicals were of guaranteed reagent grade and purchased from Sinopharm Chemical ReagentCo,. ltd (Shanghai, China).
2.2. Preparation of cinnamaldehyde microcapsules
Based on Yang et al. (2019) with some modified technique of preparation, the 8.0 g β-CDs was added to 100 mL deionized water at 100 °C and stirred at the speed of 500 rpm until it was completely dissolved. Meanwhile, the 5.0 g cinnamaldehyde was added to 16.0 g propanol and stirred until it was completely dissolved. When the β-CDs solution dropped to 80 °C, the β-CDs solution was agitated while the cinnamaldehyde dilution was added drop by drop stirred at the speed of 500 rpm for 2 h to form the cinnamaldehyde microencapsulation emulsion. For 24 h, this emulsion was kept at 4 °C. In order to create cinnamaldehyde microcapsules, precipitated microencapsulation was collected by suction filtering and dried in a convection oven to obtain a consistent weight. The loading capacity of cinnamaldehyde was 30.2 %. The powder was sealed in a dry and dark environment for further application.
2.3. Experimental design
Shanghai Lingchang Biotechnology Co. ltd. (Shanghai, China) provided 24 male C57/BL6 mice (6 weeks old, 19 ± 1 g each). The mice were kept in a controlled environment, unrestricted water and food access, and a specified pathogen-free habitat at 60 % relative humidity and 22 °C with a 12 h light/dark cycle. The mice were split into three groups (n = 8 per group) after a one-week period of acclimatization: the control group (the CON group, fed the AIN-93G food), the CIN group (fed the AIN-93G diet and given cinnamaldehyde by oral administration), and the MIC group (fed with AIN-93G diet and administered with cinnamaldehyde microcapsules by oral administration). Selected doses of cinnamaldehyde were based on our previous work (Supplementary data). Every of mice was given 0.2 mL of a cinnamaldehyde solution in CIN group by oral administration, which included 150 mg/kg of cinnamaldehyde, 350 mg/kg of CDs, and 0.5 % (w/v) of Tween-80. Every of MIC group mice received a 0.2 mL suspension of cinnamaldehyde microcapsules via oral administration, which included an equal quantity of cinnamaldehyde, β-CDs, and 0.5 % (w/v) Tween-80. Every of mice in the CON group was administered with 0.2 mL blank suspension (containing the equivalent amount of β-CDs and Tween-80) by oral administration. Throughout the tests, all of the mice were grown with unlimited access to food and water. For seven weeks, their body weights were measured once each week. The protocol for animal studies was approved by Shanghai Institute of Technology's Institutional Animal Care and Use Committee. Every effort was taken to minimize animal suffering and the number of animals used in this work, and the National Institutes of Health's Care and Use of Laboratory Animals was strictly adhered to for all animal procedures and experiments.
2.4. The content analysis of methyl cinnamate in plasma, cinnamyl alcohol, and cinnamaldehyde urine and feces
Fresh urine and feces were collected every 4, 8, 12, 18, and 24 h for a continuous twenty-four hours following oral administration after the mice were housed in metabolic cages for three days of pre-rearing. Blood was collected after 2 h-oral administration of cinnamaldehyde or its microcapsules at the end of experiment. When combined with physiological saline solution (1:2, w/v), feces were added and homogenized. To dilute urine samples, physiological saline solution (1:5, w/v) was used. Then, 2500 ng/ml internal standard solution (50 μL) and acetonitrile (500 μL) were added to urine samples (200 μL). A 12000 g centrifuge was used for 10 min to separate the solution. GC–MS was used to examine the 1 μL of supernatant. In the same way that urine samples are processed, plasma samples were as well. For the preparation of the reserve solutions, acetonitrile was used to dilute each of the three compounds in the ratio of 100 μg/mL to 0.3 to 30 μg/mL (0.3, 0.6, 0.9, 1.2, 1.5, 2, 5, 10, 20, 30 μg/mL) as working solutions for linear experiments. The samples were stored at −80 °C to avoid light. The content of each substance was detected by GC–MS. ARTX-WAX column (Shimadzu, 30 m × 0.25 mm × 0.25 μm film thickness, Japan) was used. The temperature increased by 10 °C/min from 50 °C to 160 °C in 1 min, and then by 20 °C/min to 280 °C in 1 min. Carrier gas helium was employed and flow rate was 1.0 mL/min at all times. The ion source and transmission cable were at both 250 °C. 70 eV electron collision ionization mode was used for ionization, with chosen ion monitoring for detection. In order to investigate all three at the same time, 92 m/Z, 105 m/Z, and 131 m/Z ions were selected. The spectra obtained from the NIST library (Shimadzu, Kyoto, Japan) were used for analysis.
2.5. Sample preparation of physiological
Cinnamaldehyde or its microcapsules were administered orally to all mice fasted overnight and they were sacrificed with ether as anesthesia two hours later. Eyeballs were removed, and the blood samples were collected in heparinized tubes. Heparinized blood samples and plasma samples were separated in a centrifuge running at 1500g for 10 min. Plasma samples were stored in the refrigerator at a temperature of −40 °C for the purposes of the subsequent investigation.
Removing and homogenizing 100 mg of the right lobe of liver, together with the duodenum, and the distal colon, was completed immediately. The supernatant was collected in a 2 mL tube after centrifugation at 4200g for 15 min at 4 °C separated the homogenate. Until further research, they were maintained at −40 °C.
2.6. Oxidative stress and biochemical assays biomarkers
Inflammatory cytokines and biochemical assays for biomarkers in the plasma including the tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). In order to determine the antioxidant capacityies in tissue samples, the activities of total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), the reduced glutathione (GSH), and catalase (CAT) and MDA concentration were measured. All the samples were measured according to the manufacturer’s instructions of commercial diagnostic kits.
2.7. Illumina MiSeq sequencing for gut microbiota of fecal sample and the contents of short-chain fatty acids (SCFAs) in the samples
Feces samples were stored at −80 °C until further analysis. The Qiagen QIAamp Fast DNA Stool Mini Kit was used in accordance with the manufacturer's instructions to extract DNA samples from the frozen feces and detect them using 1 % agarose. The V3-V4 region of the 16S rRNA gene was amplified using Polymease Chain Reaction (PCR), and its sequence was determined using Illumina high-flux technology by Shanghai Majorboi Bio-pharm Technology Co. Ltd. in Shanghai, China. Prior to sequencing on an Illumina Miseq PE300 platform, the amplicons were purified using an AxyPrep DNA gel extraction kit (Union City, USA), and quantified using a Promega Quantifluor ST fluorometer (Madison, USA).
Feces samples were tested for the presence of SCFAs in accordance with a modified technique (Nakajima et al., 2017). The ether layers containing SCFA were collected, combined, and evaluated using a GC/MS-TQ8040 in this study (Shimadzu, Kyoto, Japan). SCFAs concentrations in fecal samples were measured by using the standard external method.
2.8. Statistical analysis
SPSS 22.0 (IBM SPSS, USA) with significant criteria was used to evaluate the data of physiological, biochemical indicators (ANOVA), and Duncan's multiple range tests. At p < 0.05, differences were judged significant, while p < 0.01 was regarded highly significant. Mean ± standard deviation (SD) was used to represent all data.
The Majorbio Cloud Platform's free online platform (www.majorbio.com.) was used for the microbiological analysis. For the operational taxonomic units (OTUs) analysis, sequences having a similarity to sample sequences of at least 97 % were chosen using Usearch (version 7.0, available at https://drive5.com/uparse/). Using UCHIME, chimeric sequences were discovered and removed. The diversity analysis, principal coordinates analysis (PCoA), and the examination of the bacterial taxonomic compositions were then carried out using the acquired OTUs data. By using one-way ANOVA, the microbiota substantially difference analysis was obtained. Linear discriminant analysis (LDA) and non-parametric factorial Kruskal-Wallis sum-rank test was used to find the LEfSe multistage species difference discriminant analysis (LEfSe).
3. Results and discussions
3.1. Body weight, plasma biochemical parameters, and oxidative stress biomarkers
During the experimental period, the average body weight was increased in all groups, while in Table 1, p > 0.05, there was no statistically significant difference between the three groups. The AST and ALT activity and TNF-α concentrations in the CIN and MIC groups differed slightly from those in the CON group (p > 0.05). Additionally, the CIN group's IL-6 concentration was significantly higher than that of the CON group (p < 0.05), according to the study's findings. It was shown that patients in the MIC group had significantly lower levels of aberrant IL-6 in their blood (p < 0.05). The changes in inflammatory cytokines cloud be associated with regulation of intestinal flora.
Table 1.
The body weight and the plasma AST, ALT, TBA and inflammatory cytokines levels including TNF-α and IL-6 in mice among CON, CIN and MIC groups.
| Group | Weight (g) |
AST (U/mgprot) | ALT (U/mgprot) | IL-6 (ng/L) | TNF-α (ng/L) | |
|---|---|---|---|---|---|---|
| Initial | Final | |||||
| CON | 22.4 ± 1.0a | 25.3 ± 1.2a | 40.69 ± 9.58a | 22.83 ± 4.60a | 215.42 ± 69.38a | 51.08 ± 14.21a |
| CIN | 22.2 ± 1.2a | 24.2 ± 1.7a | 41.04 ± 4.92a | 21.30 ± 5.61a | 391.44 ± 125.28b | 53.61 ± 10.10a |
| MIC | 22.2 ± 0.9a | 24.4 ± 1.2a | 43.37 ± 8.44a | 21.55 ± 4.81a | 157.01 ± 51.57a | 55.61 ± 12.08a |
Note: AST: aspartate aminotransferase; ALT: alanine aminotransferase; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α. Data are expressed as mean ± SD (n = 8). Values with different superscript letters (a and b) were significantly differ at p < 0.05.
In the CIN group, CAT activity and GSH content were considerably higher (p < 0.05), whereas T-SOD activity was lower (p < 0.05), and MDA content in the liver was significantly lower (p < 0.05), as seen in the tissue oxidative stress data in Table 2. Compared to those in the CIN group, MIC patients showed a small rise in the activity of CAT in the liver, a considerable increase in the activity of T-AOC in the liver (p < 0.05), and a significant increase in the GSH content in the liver and colon (p < 0.05). Consistenting with previous research, cinnamaldehyde significantly increased the GSH level and the CAT and SOD activities in plasma and joints of arthritic rats (Mateen et al., 2018). The aqueous extract of cinnamon decreased MDA level in plasma, indicating that cinnamon acted in protecting lipids against oxidation (Anderson, 2008).
Table 2.
The antioxidant status in mice among CON, CIN and MIC groups.
| Group | Liver | Duodenum | Colon | |
|---|---|---|---|---|
| CAT (U/mg prot) | CON | 9.56 ± 2.69a | 571.12 ± 46.91c | 264.14 ± 22.71a |
| CIN | 14.48 ± 4.33ab | 309.30 ± 65.76b | 304.92 ± 42.22b | |
| MIC | 14.82 ± 4.81b | 164.21 ± 48.73a | 327.87 ± 17.70b | |
| T-SOD (U/mg prot) | CON | 938.13 ± 53.65a | 520.33 ± 53.34c | 107.57 ± 33.96b |
| CIN | 915.56 ± 74.71a | 320.38 ± 50.99b | 44.13 ± 18.46a | |
| MIC | 887.15 ± 72.05a | 192.47 ± 19.31a | 32.87 ± 6.75a | |
| T-AOC (U/mg prot) | CON | 0.93 ± 0.11a | 5.15 ± 0.74c | 1.20 ± 0.49a |
| CIN | 0.94 ± 0.08a | 3.43 ± 0.33b | 1.31 ± 0.18a | |
| MIC | 1.26 ± 0.22b | 2.04 ± 0.35a | 1.37 ± 0.42a | |
| GSH (nmol/mg prot) | CON | 16.51 ± 4.77a | 226.97 ± 43.96c | 66.91 ± 21.68a |
| CIN | 41.80 ± 8.10b | 161.46 ± 15.79b | 92.07 ± 15.51b | |
| MIC | 50.82 ± 6.38c | 82.75 ± 10.32a | 128.83 ± 20.67c | |
| MDA (nmol/mg prot) | CON | 1.77 ± 0.27b | 2.46 ± 0.48c | 1.31 ± 0.30a |
| CIN | 1.26 ± 0.30a | 1.44 ± 0.24b | 1.28 ± 0.40a | |
| MIC | 1.21 ± 0.53a | 0.50 ± 0.16a | 1.22 ± 0.25a |
Note: CAT: catalase; T-SOD: total superoxide dismutase; T-AOC: total antioxidant capacity; GSH: reduced glutathione; MDA: malondialdehyde. Data are expressed as mean ± SD (n = 8). Values with different superscript letters (a, b and c) were significantly differ at p < 0.05.
Intriguingly, in the duodenum, the CIN group had substantially lower CAT, T-SOD, and T-AOC activity and GSH and MDA concentration than the CON group (p < 0.05). The MDA levels were also considerably lower in the MIC group's duodenal samples than in the CIN group (p < 0.05) in spite of low antioxidant ablity, possibly causing from the increased bioavailability of cinnamaldehyde to improve exogenous antioxidant capacity and to inhibit endogenous antioxidant system. In addtion, MDA was decreased in MIC group's liver and colon without significance compared with CIN group, but GSH was significantly increased (p < 0.05). This is because redox homeostasis maintained by a complex antioxidants system involves in various mechanisms in the body (Simicic, Cudalbu, & Pierzchala, 2022). Further, the sudden decrease in the concentration of MDA (Table 2) suggested that duodenum may be the target to participate in the redox homeostasis due to one main position to absorpt nutrients.
3.2. Contents of cinnamaldehyde and its metabolites in blood, feces, and urine
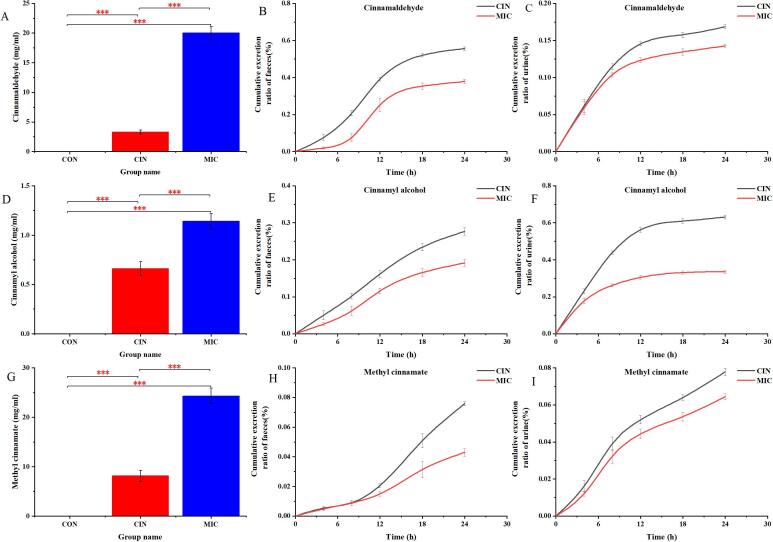
The plasma concentration of cinnamaldehyde after oral administration of cinnamaldehyde for 2 h and the cumulative excretion of cinnamaldehyde in feces and urine is shown in Fig. 1A-C. The MIC group had substantially greater plasma concentrations of cinnamaldehyde than the CIN group (p < 0.05). In fact, the CIN group's cinnamaldehyde excretion in feces and urine was greater than the MIC groups.
Fig. 1.
The concentration of cinnamaldehyde and its metabolites in plasma, urine and feces. Plasma concentrations of cinnamaldehyde (A), cinnamyl alcohol (D) and methyl cinnamate (G) in mice orally administered with cinnamaldehyde and cinnamaldehyde microcapsules for 2 h. Fecal cumulative excretion of cinnamaldehyde (B) and its metabolites cinnamyl alcohol (E) and methyl cinnamate (H) in mice. Urinary cumulative excretion of cinnamaldehyde (C) and its metabolites cinnamyl alcohol (F) and methyl cinnamate (I) in mice. Data are expressed as mean ± SD (n = 8). *p < 0.05, **p < 0.01 and ***p < 0.001. respectively.
Previous study demonstrated that the cinnamaldehyde and its metabolites, including cinnamyl alcohol and methyl cinnamate, was determined in the plasma in rats with the oral dose of 125–500 mg/kg cinnamaldehyde, but the bioavailability was relatively low (Zhao et al., 2014). As shown in Fig. 1D-I, our data showed that the levels of cinnamyl alcohol and methyl cinnamate, as metabolites of cinnamaldehyde, in plasma and fecal and urinary cumulative excretion were determined. It was shown that in plasma, the metabolite concentrations of cinnamaldehyde were greater in the MIC group than the CIN group (p < 0.05). Cinnamaldehyde and its metabolites, such as cinnamyl alcohol and methyl cinnamate, were found to be considerably higher in the MIC group's plasma than in the CIN group. Cinnamaldehyde, cinnamyl alcohol, and methyl cinnamate excretion ratios in feces and urine were lower in the MIC group than in the CIN group throughout time. These data suggested that microencapsulation improved the absorption of cinnamaldehyde, which may improve the bioavailability of cinnamaldehyde. Several previous studies demonstrated that microcapsules increased the solubility, stability and in vitro bioaccessibility of encapsulated materials and showed a significant sustained release effect (Bachir et al., 2018, Qian et al., 2021). Therefore, we presumed that microencapsulation changed the phase solubility of cinnamaldehyde and the contact between cinnamaldehyde and intestinal tract, which may be attributed to high absorption.
3.3. Gut microbiota diversity
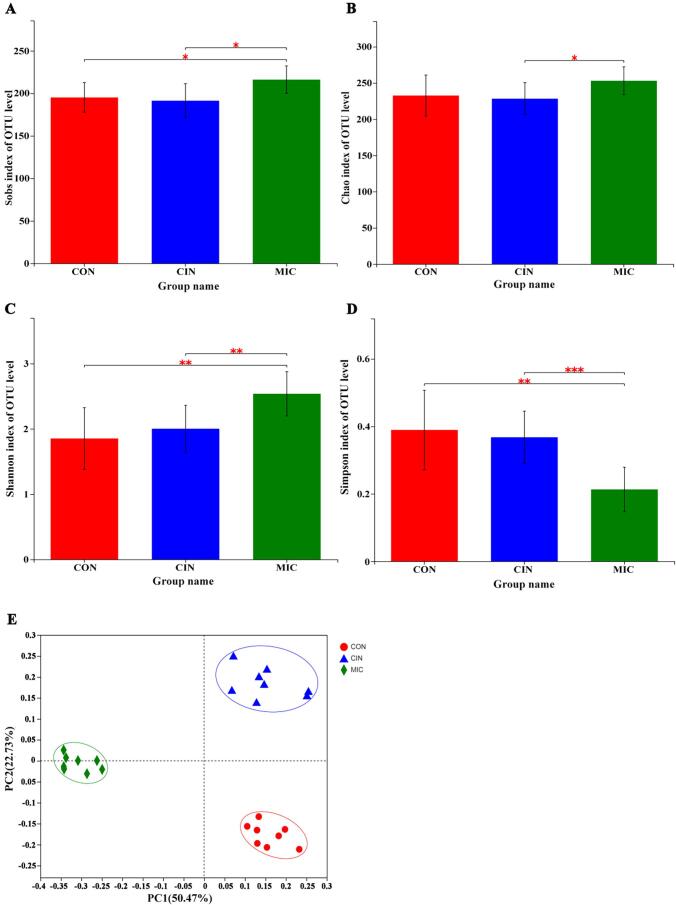
The microbial community's richness and diversity were revealed using the Alpha diversity study. There was a modest difference in sob and chao index values between the CIN group and the CON group; nonetheless, the difference was statistically significant (p < 0.05). (Fig. 2A, B). Shannon index increased significantly in the MIC group compared to CON and CIN groups, and Simpson index decreased significantly (p < 0.05) (Fig. 2C, D). Simpon index and Shannon index are commonly used in ecology to quantitatively describe the biodiversity of an area. The larger the Simpson index value, the lower the community diversity. On the contrary, the larger the Shannon value, the higher the community diversity. Our data indicated that cinnamaldehyde microcapsules increased the gut microbial richness and diversity. The gut microbial richness and diversity were negatively correlated in the occurrence of intestinal diseases (Wen et al., 2022).
Fig. 2.
The analysis of the microbial community's alpha index diversity using the Student's t-test for the Sobs, Chao, Shannon, and Simpson indices (D). *p < 0.05, **p < 0.01 and ***p < 0.001. respectively. the OTU level PCoA analysis of the gut microbiota in groups (E) (n = 8).
As shown in Fig. 2E, the PCoA analysis according to the spearman_approx distance confirmed that the intake of cinnamaldehyde altered the community structure of the gut microbiota in mice at PC2 (22.37 %). Significant dissimilarities in the structures of fecal microorganisms were observed between the MIC group and the other two groups at PC1 (50.47 %) and PC2, indicating that cinnamaldehyde microcapsules had a more prevailing regulatory impact on the gut microbiota than cinnamaldehyde. Overall, these results revealed that cinnamaldehyde microcapsules could affect the structure of gut microbiota.
3.4. Gut microbiota composition
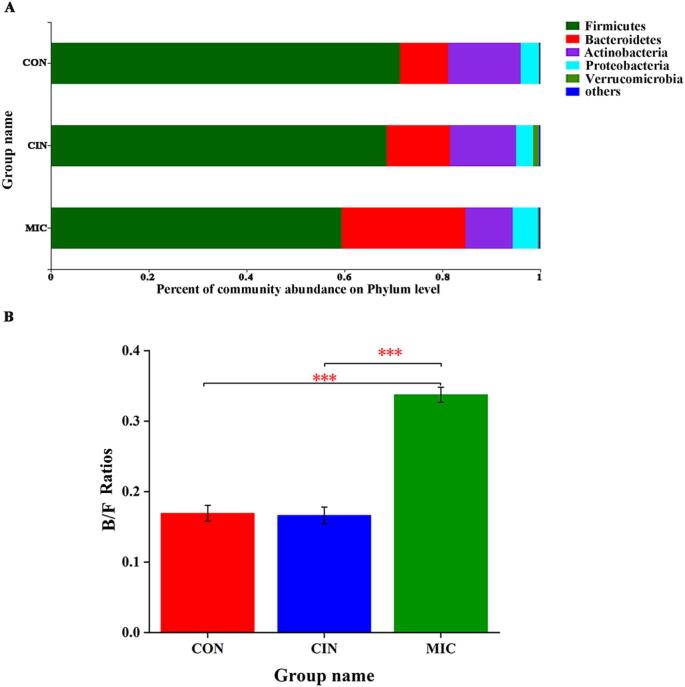
As shown in Fig. 3A, the main intestinal dominant bacteria in each group were composed of Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia, and Firmicutes and Bacteroides had the greatest relative abundance (account for 80 %). Although the species did not change, the abundance of the flora was regulated. The relative abundance of the phyla Firmicutes and Actinobacteria decreased while the phylum Bacteroidetes increased in the CIN and MIC group as compared to the CON group. The MIC group had the most profound changes in Bacteroidetes/Firmicutes (B/F) ratios, with a greater B/F ratios in the MIC group than in the CIN or CON groups (Fig. 3B). Bacteroides are regarded as the primary contributors to the complex homeostasis maintained by gut microbiota in a healthy condition (Gibiino, Lopetuso, Scaldaferri, Rizzatti, Binda, & Gasbarrini, 2018) and the increase in the abundance of Firmicutes was thought to be closely associated with an unhealthy state (Mitesh, Ansarullah, Ilian, & Helen, 2017). The decline of Firmicutes and ascend of Bacteroides played a decisive role on the change of B/F ratio. Therefore, the increased ratio of B/F might represent a potential benefit to the health (Sun et al., 2022). In addition, the CIN group had the largest concentration of Verrucomicrobia, whereas the MIC group had the highest concentration of Proteobacteria.
Fig. 3.
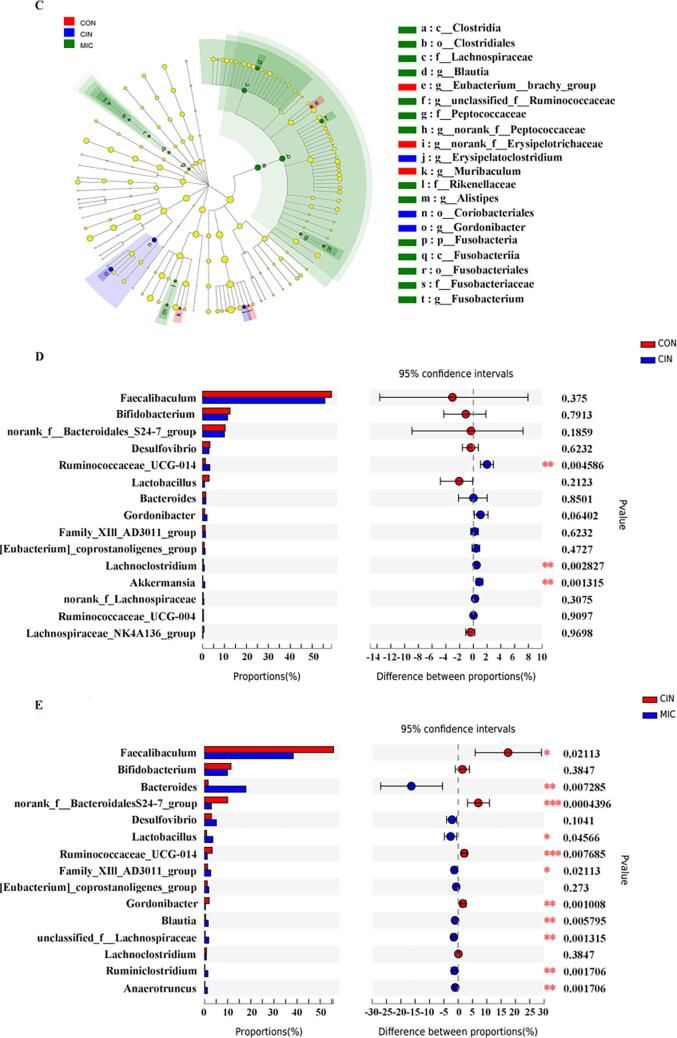
Wilcoxon rank-sum test bar plot and analysis of the gut microbiota composition. The phylum-level distributions of the gut microbiota community components (A). The bar plot displays the variations in relative abundance of the various phyla of gut microbiota in different colors. Using the LEfSe analysis, a taxonomic cladogram from family to genus was created. (B) The bar plot displays the changes in Bacteroidetes/Firmicutes (B/F) ratios in the three groups.(C)Different colored nodes reflect species with considerably high relative abundance in the relevant groups, whereas yellow nodes show species with no significantly varied relative abundance across groups (p > 0.05). The different colors represent various groups. The letters designate the names of taxons that vary significantly across groupings. The top 15 gut microbiota with noticeably varied genus-level abundances of CON and CIN (D)and CIN and MIC (E) *p < 0.05, **p < 0.01 and ***p < 0.001.
It was shown that supplementation with cinnamaldehyde microcapsules affected the gut microbiota at both the family and genus level using the LEfSe technique, which analyzed 4 families and 10 genera. There were 20 intestinal markers in total, with LDA score greater than 2.0 (Fig. 3C). The results showed that Eubacterium_brachy_group, norank_f_Erysipelotrichaceae, and Muribaculum genera were enriched in the CON group. Erysipelatoclostridium and Gordonibacter genera were more abundant in the CIN group. These families comprised of Lachnospiraceae, Peptococcaceae, Rikenellaceae, and Fusobacteriaceae and genera including Blautia, unclassified_f_Ruminococcaceae, norank_f_Peptococcaceae, Alistipes, and Fusobacterium were dominant in the MIC group.
Furthermore, we used the Wilcoxon rank-sum test to identify significant changes in the gut microbiota across groups at the species level (Fig. 3D, E). According to the findings, the CIN group had considerably more Ruminoccaceae UCG-014, Lachnoclostridium, and Akkermansia, whereas Faecalibaculum, Bifidobacterium, and Lactobacillus had a somewhat lower abundance (p > 0.05) than the CON group. Comparing the MIC group to that of the CIN group, we found that the abundance of Faecalibaculum, norank-f-Bacteroidales-S24-7-group, Ruminococcaceae UCG-014, and Gordonibacter was significantly reduced (p < 0.05), while Bacteroides, Lactobacillus, Family XIII-AD3011-group, Blautia, unclassified-f-Lachnospiraceae, Ruminiclostridium, and Anaerotruncus were significantly increased (p < 0.05).
Our data showed that non-microencapsulated cinnamaldehyde reduced the abundances of certain intestinal probiotics, such as Lactobacillus, suggesting that cinnamaldehyde may disturb the balance of intestinal flora which may involve inflammatory mechanism. Accounting for the addition of cinnamaldehyde increased the IL-6 concentration compared to CON. On the contrary, microencapsulated cinnamaldehyde increased the abundances of Lactobacillus accompanying with the reduced IL-6 levle. As a consequence of the findings, it was concluded that cinnamaldehyde microcapsules might be used in a broader range of applications to regulate intestinal flora more effectively.
Our findings also revealed that, as compared to the CIN group, mice exposed to cinnamaldehyde microcapsules exhibited significantly higher abundances of the taxa Bacteroides, Lactobacillus, unclassified_f_Lachnospiraceae, and Blautia. Bacteroides have been shown to have a variety of beneficial functions, including the ability to intervene in intestinal inflammation. These functions included maintaining intestinal balance by preserving the diversity of the gut microbiota and preventing inflammation by regulating cytokine production balance (Tan, Zhao, Zhang, Zhai, & Chen, 2019). Lactobacillus has the benefits of protecting the integrity of intestinal mucosa and avoiding inflammatory bowel disease through activation of signaling pathway induced by accelerating immunocyte secretion (Hou et al., 2018, Milad et al., 2021). The significant difference of inflammatory factor IL-6 by cinnamaldehyde and cinnamaldehyde microcapsules may be related to the regulation of these intestinal flora in this study.
3.5. Content of short chain fatty acids (SCFAs)
As a result of gut microbiota, SCFAs are one of the most significant metabolic products. Butyric acid and total SCFAs in feces were considerably lower in the CIN group compared to the CON group (p < 0.05), whereas the concentrations of acetic acid and propionic acid were somewhat lower with no significant difference (p > 0.05) compared to the CON group (Table 3). Propionic acid, butyric acid, and total acid content in feces in the MIC group were substantially higher than in the CIN group (p < 0.05). MIC patients had greater levels of butyric acid (p < 0.05) compared to those in the control group. No significant difference was seen in the concentrations of acetic acid and propionic acid between MIC and CON groups (p > 0.05).
Table 3.
The contents of short chain fatty acids (SCFAs) including propionic acid, butyric acid, acetic acid and total acid in feces among CON, CIN and MIC groups.
| Group | SCFAs (μmol/g) |
|||
|---|---|---|---|---|
| Acetic acid | Propionic acid | Butyric acid | Total acid | |
| CON | 16.00 ± 1.14a | 6.00 ± 0.41ab | 7.01 ± 0.64b | 29.13 ± 1.47b |
| CIN | 15.41 ± 0.61a | 5.78 ± 0.21a | 6.23 ± 0.57a | 27.47 ± 0.83a |
| MIC | 16.49 ± 1.05a | 6.50 ± 0.66b | 7.84 ± 0.52c | 32.18 ± 1.42b |
Note: Data are expressed as mean ± SD (n = 8). Values with different superscript letters (a, b and c) were significantly differ at p < 0.05.
Through the synthesis of SCFAs and their vast regulatory roles in the interaction between bacteria and hosts, the gut microbiota may be able to affect host homeostasis (Blacher, Levy, Tatirovsky, & Elinav, 2017). The present study showed that cinnamaldehyde microcapsules significantly increased the contents of butyric acid reduced by cinnamaldehyde, which was the probable reason that encapsulation of cinnamaldehyde mainly promoted the concentration of instead of propionic acid compared to the control. This difference was consistent with the result that cinnamaldehyde microcapsules increased butyric-producing bacteria. A similar finding was found in the study of Atractylodeskoreana (Nakai) Kitam, which improved the intestinal butyric acid level in rats with rheumatoid arthritis by regulating the intestinal flora that is positively or negatively correlated with butyric acid production (Pang, Ma, Xu, Zhang, & Cai, 2021).
Butyric acid is the preferred metabolic substrate for colonocytes and provides the majority of the energy necessary for their physiological activity. Several studies had indicated that butyric acid participated in adipose differentiation and could hydrolyze triglyceride-rich lipoproteins, thus, the increase of butyric acid is beneficial to the lipid homeostasis across biological system (Cardona et al., 2018; He & Moreau, 2019). Studies had shown a link between reduced butyric acid content and gastrointestinal diseases (Zhang, Wang, Zhao, Liu, Wang, & Zhou, 2021). In this study, increased abundances of butyrate-producing bacteria, such as Bacteroides, Lactobacillus, unclassified f Lachnospiraceae, and Blautia, demonstrate that cinnamaldehyde microcapsules may influence the makeup of the gut microbiota to affect SCFA synthesis. Therefore, it is important to focus on any possible cinnamaldehyde microcapsule prevention in conditions brought on by low butyric acid.
Unclassified_f_Lachnospiraceae and Blautia had been illustrated to be instrumental in producing butyric acid and were associated with the inhibition of intestinal disorders such as IBS (Zhang et al., 2019). Furthermore, the abundances of Faecalibaculum, norank_f_Muribaculaceae, Ruminococcaceae_UCG-014, and Gordonibacter genera were clearly lower in the MIC group than in the CIN group. It has been shown that lowering these microbiotas prevents gut dysbiosis, obesity-related metabolic problems, and hypertension, enteritis, and other ailments (Bai et al., 2019, Pei et al., 2019). Therefore, microencapsulation of cinnamaldehyde may compensate for the potential deficiency of cinnamaldehyde by altering the regulatory effect of cinnamaldehyde on intestinal flora. However, further work exploring its related mechanism is necessary.
4. Conclusions
In summary, the sustained release of cinnamaldehyde microcapsules could increase the contents of cinnamaldehyde and its metabolites in plasma and reduce their cumulative excretion ratio. This may be beneficial to increase the bioavailability of cinnamaldehyde. Further, microencapsulation of cinnamaldehyde increased the ratio of B/F and the abundance of Lactobacillus and Bacteroides and the contents of butyric acid in feces. Thus, the supplementation of microencapsulated cinnamaldehyde could improve the bioavailability of cinnamaldehyde and regulate the balance of intestinal flora. The results of this research may help to improve how cinnamaldehyde is used in functional foods.
Ethical statement
The authors declare that the animal experiment protocol was passed by the Institutional Animal Care and Use Committee of Shanghai Institute of Technology in the present study. All animal procedures and tests were performed experiment was strictly conducted according the Care and Use of Laboratory Animals of the National Institutes of Health, and all possible efforts were made to minimize the suffering and number of animals utilized in this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Shanghai Engineering Research Center of Dairy Biotechnology [19DZ2281400]. The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100441.
Contributor Information
Ying Xiao, Email: y-xiaomn@163.com.
Junli Miao, Email: miaojunli@brightdairy.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anderson R.A. Chromium and polyphenols from cinnamon improve insulin sensitivity. Proceedings of the Nutrition Society. 2008;67(1):48–53. doi: 10.1017/S0029665108006010. [DOI] [PubMed] [Google Scholar]
- Azzi J., Jraij A., Auezova L., Fourmentin S., Greige-Gerges H. Novel findings for quercetin encapsulation and preservation with cyclodextrins, liposomes, and drug-in-cyclodextrin-in-liposomes. Food Hydrocolloids. 2018;81:328–340. doi: 10.1016/j.foodhyd.2018.03.006. [DOI] [Google Scholar]
- Andre, G, Buret. (2016). Good Bugs, Bad Bugs in the Gut: The Role of Microbiota Dysbiosis in Chronic Gastrointestinal Consequences of Infection. The American Journal of Gastroenterology Supplements, 3(2), 25-32. 10.1038/ajgsup.2016.11.
- Bachir Y.N., Zafour A., Medjkane M. Formulation of stable microcapsules suspensions content Salvia officinalis extract for its antioxidant activity preservation. Journal of Food Processing and Preservation. 2018;42(2):13446. doi: 10.1111/jfpp.13446. [DOI] [Google Scholar]
- Bai Y.F., Wang S.W., Wang X.X., Weng Y.Y., Fan X.Y., Sheng H.…Zhang F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutrition & Diabetes. 2019;9(1):30–41. doi: 10.1038/s41387-019-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides S., Cortés P., Parada J., Franco W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chemistry. 2016;204:77–83. doi: 10.1016/j.foodchem.2016.02.104. [DOI] [PubMed] [Google Scholar]
- Blacher, E., Levy, M., Tatirovsky, E., & Elinav, E. (2017). Microbiome-Modulated Metabolites at the Interface of Host Immunity. Journal of Immunology (Baltimore, Md.: 1950), 198(2), 572-580. 10.4049/jimmunol.1601247. [DOI] [PubMed]
- Chang S., Qin D., Wang L., Zhang M., Yan R., Zhao C. Preparation of novel cinnamaldehyde derivative-BSA nanoparticles with high stability, good cell penetrating ability, and promising anticancer activity. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2021;624 doi: 10.1016/j.colsurfa.2021.126765. [DOI] [Google Scholar]
- Dunn L.L., Davidson P.M., Critzer F.J. Antimicrobial efficacy of an array of essential oils against lactic acid bacteria. Journal of Food Science. 2016;81(2):438–444. doi: 10.1111/1750-3841.13181. [DOI] [PubMed] [Google Scholar]
- Simicic D., Cudalbu C., Pierzchala K. Overview of oxidative stressfindings in hepatic encephalopathy: From cellular and ammonium-based animal models to human data. Analytical Biochemistry. 2022;114795 doi: 10.1016/j.ab.2022.114795. [DOI] [PubMed] [Google Scholar]
- Gibiino G., Lopetuso L.R., Scaldaferri F., Rizzatti G., Binda C., Gasbarrini A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Digestive and Liver Disease. 2018;50(7):635–639. doi: 10.1016/j.dld.2018.03.016. [DOI] [PubMed] [Google Scholar]
- He B., Moreau R. Lipid-regulating properties of butyric acid and 4-phenylbutyric acid: Molecular mechanisms and therapeutic applications. Pharmacological Research. 2019;144:116–131. doi: 10.1016/j.phrs.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Hill L.E., Gomes C., Taylor T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans -cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT – Food Science and Technology. 2013;51(1):86–93. doi: 10.1016/j.lwt.2012.11.011. [DOI] [Google Scholar]
- Hou J.J., Wang X., Li Y., Su S., Wang Y.M., Wang B.M. The relationship between gut microbiota and proteolytic activity in irritable bowel syndrome. Microbial Pathogenesis. 2021;157 doi: 10.1016/j.micpath.2021.104995. [DOI] [PubMed] [Google Scholar]
- Hou Q.H., Ye L., Liu H., Huang L., Yang Q., Turner J.R., Yu Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death and Differentiation. 2018;25(9):1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateen S., Shahzad S., Ahmad S., Naeem S.S., Khalid S., Akhtar K.…Moin S. Cinnamaldehyde and eugenol attenuates collagen induced arthritis via reduction of free radicals and pro-inflammatory cytokines. Phytomedicine. 2018;53:70–78. doi: 10.1016/j.phymed.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Milad A., Vahid L., Arezoo A., Maryam E., Masjedian J.F., Mahdi R., Malihe T. Interesting probiotic traits of mother's milk Lactobacillus isolates; from bacteriocin to inflammatory bowel disease improvement. Microbial Pathogenesis. 2021;158 doi: 10.1016/j.micpath.2021.104998. [DOI] [PubMed] [Google Scholar]
- Mitesh, D., Ansarullah, Ilian, R., & Helen, K. E. (2017). Alteration of Immune-Mechanisms by Human Microbiota and Development and Prevention of Human Diseases. Journal of Immunology Research, 2017, 6985256. 10.1155/2017/6985256. [DOI] [PMC free article] [PubMed]
- Mohammadzamani Z., Khorshidi A., Khaledi A., Shakerimoghaddam A., Moosavi G.A., Piroozmand A. Inhibitory effects of Cinnamaldehyde, Carvacrol, and honey on the expression of exoS and ampC genes in multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infections. Microbial Pathogenesis. 2020;140(C) doi: 10.1016/j.micpath.2019.103946. [DOI] [PubMed] [Google Scholar]
- Mostafa, O., Hammoda, H., Elsaygh, M., & Abdelwahab, I. (2017). Efficacy of the Clove Oil, Cinnamon Oil, Thyme Oil and Origanum Oil against Multidrug Resistant Pseudomonas aeruginosa and Burkholderia cepacia Complex. International Journal of Current Microbiology and Applied Sciences, 6(1), 29-35. 10.20546/ijcmas.2017.601.004.
- Nakajima, A., Nakatani, A., Hasegawa, S., Irie, J., Ozawa, K., Tsujimoto, G., Suganami, T., Itoh, H., & Kimura, I. (2017). The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS One, 12(7), e0179696. https://doi: 10.1371/journal.pone.0179696. [DOI] [PMC free article] [PubMed]
- Pang J., Ma S., Xu X., Zhang B., Cai Q. Effects of rhizome of Atractylodes koreana (Nakai) Kitam on intestinal flora and metabolites in rats with rheumatoid arthritis. Journal of Ethnopharmacology. 2021;281 doi: 10.1016/j.jep.2021.114026. [DOI] [PubMed] [Google Scholar]
- Pei L.Y., Ke Y.S., Zhao H.H., Wang L., Jia C., Liu W.Z.…Li S.C. Role of colonic microbiota in the pathogenesis of ulcerative colitis. BMC Gastroenterology. 2019;19(1):10. doi: 10.1186/s12876-019-0930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragyanshu, K., Sneha, J., Yachna, J., K, B. R., Priyanka, M., K, B. R., K, B. K., S, S. S., S, P. L., K, K. K., Kanwaljit, C., & Mahendra, B. (2016). Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice. BioFactors (Oxford, England), 42(2), 201-211. 10.1002/biof.1265. [DOI] [PubMed]
- Pratap S.D., Pragyanshu K., Vandana B., Kumar B.R., Jagdeep S., Kiran K.K.…Mahendra B. Coadministration of isomalto-oligosaccharides augments metabolic health benefits of cinnamaldehyde in high fat diet fed mice. BioFactors (Oxford, England) 2017;43(6):821–835. doi: 10.1002/biof.1381. [DOI] [PubMed] [Google Scholar]
- Qian J.Q., Chen Y., Wang Q., Zhao X.H., Yang H.Y., Gong F., Guo H. Preparation and antimicrobial activity of pectin-chitosan embedding nisin microcapsules. European Polymer Journal. 2021;157 doi: 10.1016/j.eurpolymj.2021.110676. [DOI] [Google Scholar]
- Sanbhal, N., Saitaer, X., Li, Y., Mao, Y., Zou, T., Sun, G., & Wang, L. (2018). Controlled Levofloxacin Release and Antibacterial Properties of β-Cyclodextrins-Grafted Polypropylene Mesh Devices for Hernia Repair. Polymers, 10(5), 493-493. doi: 10.3390/polym10050493. [DOI] [PMC free article] [PubMed]
- Shelley H., Babu R.J. Role of Cyclodextrins in Nanoparticle-Based Drug Delivery Systems. Journal of Pharmaceutical Sciences. 2018;107(7):1741–1753. doi: 10.1016/j.xphs.2018.03.021. [DOI] [PubMed] [Google Scholar]
- Soumen R., Giorgio T. Microbiota: A key orchestrator of cancer therapy. Nature Reviews Cancer. 2017;17(5):271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- Sun X.C., Zhao C.X., Hu X.Y., Zhang J.N., Xu S.Y., Li X.Y.…Wang Z.C. Body weight regulation of a low molecular weight xanthan gum on normal mice via gut microbiota. Journal of Functional Foods. 2022;88 doi: 10.1016/j.jff.2021.104874. [DOI] [Google Scholar]
- Suryanti V., Wibowo F.R., Khotijah S., Andalucki N. Antioxidant activities of cinnamaldehyde derivatives. IOP Conference Series: Materials Science and Engineering. 2018;333(1) doi: 10.1088/1757-899X/333/1/012077. [DOI] [Google Scholar]
- Tan H., Zhao J., Zhang H., Zhai Q., Chen W. Novel strains of Bacteroides fragilis and Bacteroides ovatus alleviate the LPS-induced inflammation in mice. Applied Microbiology and Biotechnology. 2019;103(5):2353–2365. doi: 10.1007/s00253-019-09617-1. [DOI] [PubMed] [Google Scholar]
- Wen, C. K., Hua, T. C., Ju, C. I., Cheng, H. B., Ju, L. H., Wen, H. K., Wei, L. W., Hung, C. C., Yii, H. M., Lin, L. Y., R, R. S., Yuan, W. J., Long, C. Y., & Lu, C. T. (2022). Inhibition of gut microbial β-glucuronidase effectively prevents carcinogen-induced microbial dysbiosis and intestinal tumorigenesis. Pharmacological Research, 177(prepublish). 10.1016/j.phrs.2022.106115. [DOI] [PubMed]
- Yang F., Chen H., Gao Y., An N., Li X., Pan X.…Xing Y. Gut microbiota-derived short-chain fatty acids and hypertension: Mechanism and treatment. Biomedicine & Pharmacotherapy. 2020;130 doi: 10.1016/j.biopha.2020.110503. [DOI] [PubMed] [Google Scholar]
- Yang W., Wang L., Ban Z., Yan J., Lu H., Zhang X.…Li L. Efficient microencapsulation of Syringa essential oil; the valuable potential on quality maintenance and storage behavior of peach. Food Hydrocolloids. 2019;95:177–185. doi: 10.1016/j.foodhyd.2019.04.033. [DOI] [Google Scholar]
- Zhang J., Song L., Wang Y., Liu C., Zhang L., Zhu S.…Duan L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. Journal of Gastroenterology and Hepatology. 2019;34(8):1368–1376. doi: 10.1111/jgh.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.B., Wang Y., Zhao X.Q., Liu C., Wang B.Z., Zhou J. Mechanistic basis and preliminary practice of butyric acid and butyrate sodium to mitigate gut inflammatory diseases: A comprehensive review. Nutrition Research. 2021;95:1–18. doi: 10.1016/j.nutres.2021.08.007. [DOI] [PubMed] [Google Scholar]
- Zhao H., Xie Y., Yang Q., Cao Y., Tu H., Cao W., Wang S. Pharmacokinetic study of cinnamaldehyde in rats by GC–MS after oral and intravenous administration. Journal of Pharmaceutical and Biomedical Analysis. 2014;89:150–157. doi: 10.1016/j.jpba.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Zou Y.Y., Yuan C., Cui B., Wang J.L., Yu B., Guo L., Dong D. Mechanical and antimicrobial properties of high amylose corn starch/konjac glucomannan composite film enhanced by cinnamaldehyde/β-cyclodextrin complex. Industrial Crops & Products. 2021;170 doi: 10.1016/J.INDCROP.2021.113781. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.