Abstract
Sacha Inchi (Plukenetia Volubilis L.), SI, is the oleaginous plant of the Euphorbiaceous family originally cultivated in the Amazonian forest. It is traditionally appreciated and consumed as the healthful food. In vivo, in vitro and clinical studies have suggested the beneficial effects of SI for a variety of neuroprotection, dermatology, antidyslipidaemic, antioxidant and anti-inflammatory, antiproliferative and antitumor modulation activities. Many of these potential impacts are related to its bioactive compounds, particularly essential fatty acids, proteins and phytochemicals. However, there are some scientific evidences underlying the risk of toxicity associated with the high doses of SI seed oils. With the aforementioned, this review outlines a narrative review of SI, including its ethnobotanical components, phytochemistry profile, organoleptic and sensory evaluations. The essential development of its latest applications in the field of medicine, pharmacology, safety and toxicological issues, are laconically demonstrated. Moreover, the underlying challenges and upcoming prospective for the integration of SI use are detailed.
Keywords: Complementary medicine, Ethnopharmacology, Functional food, Sacha Inchi, Precision, Nutrition
Complementary medicine; Ethnopharmacology; Functional food; Sacha Inchi; Precision; Nutrition.
1. Introduction
Sacha Inchi (SI) is a perennial, oleaginous plant of the Euphorbiaceous family, which grows in the Amazonian forest. The plant, widely cultivated in Peru and Northwestern Brazil, has endured a long history among various native tribal groups of the region (del-Castillo et al., 2019). It is known as “Inca Peanut”, “wild peanut”, “Inca Inchi” or “mountain peanut”. The cultivation of SI requires a warm climate (10–36 °C) and considerable annual rainfall ranged between 850–1000 mm, with well-drained acidic soil (sandy or clay loams) between the altitude of 200–1500 m acclimatised to high-light growing conditions (Cai, 2011; Gillespie, 2007) (Table 1). The plant is semi-woody with an approximate height of 2 m, and the cultivation produces both flowers and fruit capsules after 8 months of planting (Kodahl, 2020). It has a star-shaped fruit capsule, measuring approximately 3–5 cm. As the fruit matures, the colour turns from green to blackish brown. The fruit capsule contains 4–6 edible dark brown oval seeds ranging from 1.5 to 2 cm (Wang, 2018). The dried fruit capsule consists of 30–35% shell (non-edible) and 65–70% kernels (edible seeds) (Chirinos et al., 2016) (Figure 1).
Table 1.
Crop features of SI.
| Factor | Features |
|---|---|
| Altitude | Reaches a height of 200 m above sea level in the low jungle and 1500 m in the high jungle. |
| Temperature | Adjusts well in a wide range of temperatures (from 10 to 36 °C). |
| High temperatures are undesirable, which resulting in the wilting of flowers and fruits, especially those that are still fresh. | |
| Water | Requires constant access to water in order to thrive, and it will grow more effectively if the rainy season is consistent throughout the year (850–1000mm). |
| During the dry season, continuous irrigation is vital. | |
| Drought or cold temperatures for extended periods induce slow and difficult growth. | |
| Light | When exposed to low light intensity, the crop needs additional days to complete its vegetative cycle. |
| Flowering is reduced in instances where the shade is particularly severe. | |
| Soil | Able to grow in a variety of soil conditions, including acidic soils and areas with high aluminium concentrations. |
| Location selection is vital to allow proper growth and productivity. | |
| It thrives in clay soil, sandy loam, and acidic soils. | |
| Drainage | In order to rid surplus water both superficially and deeply, this crop requires proper drainage system. |
| The texture of the soil is addressed for proper drainage. |
Adapted from (Gillespie, 2007).
Figure 1.
Plukenetia volubilis L.; Photo A – Habitus of plant with fresh capsules; B – Whole fruit; driedmcapsules; C – Raw seeds, with testa (shells); D – Dried testa; E – Seeds, without testa; and F –Consumer products; oil and roasted, salted seeds.
Sacha Inchi oil (SIO), which extracted from the seeds, is used in the preparation of various meals. Traditionally, the seeds are roasted, while the leaves are cooked and consumed as part of the routine diet. SI seeds are also used as traditional remedy in the Amazon region to treat rheumatic problems and aching muscles (Fanali et al., 2011). Pharmaceutically, SIO has also been used traditionally in skincare treatment, mainly to soften skin, healing wounds, treating insect bites and skin infections. Women from several Peruvian ethnic groups, such as the Mayoruna, Campas, Huitotas, Shipibas, Yaguas, and Bora tribes mix SI ground seeds and SIO with flour to produce skin creams for cosmetic purposes, with the ultimate aim to revitalise their skin (Hanssen, 2011). Commercially, SIO is valued for its beneficial health properties and unique sensory profiles (taste and flavour) (Garmendia et al., 2011). In recent years, it has gained a surge of recognition and popularity in international markets, typically in the form of encapsulated oil (Kodahl and Sørensen, 2021). In Peru, SI is declared as an endangered species. Consequently, a few projects have been launched to support the sustainable cultivation of the SI plant (Krist, 2020). In parallel, the indigenous plant is also being cultivated widely in other parts of the world (e.g., Southeast Asia) due to its great potential as an economic crop (Medina-Mendoza et al., 2021). SI is now cultivated as a valuable oilseed crop owing to its elevated amount of α-linolenic acid (ALA, ω-3), protein and other bioactive components (Cachique et al., 2018). As a crop of economic importance for the food, pharmaceutical and cosmetic industries, it is grown industrially in the regions of San Martín, Loreto, Lamas, Moyobamba, and El Dorado of tropical America (Ocelák, 2016). Lately, its cultivation has also expanded to the Cambodia, Thailand, Laos, including China regions (Kodahl and Sørensen, 2021). In the recent ‘World Edible Oil’ contest, SIO has been awarded with gold medal, witnessing its superior organoleptic qualities (Ocelák, 2016).
Of major interest, SI has been proposed to present various pharmacological properties. Although the biological function of SI has not been fully delineated, its beneficial impact in modulating non-communicable diseases has gained popularity worldwide. In view with the matter, this paper attempts to postulate an initial platform to arise the unique implications of ethnobotanical uses of the SI plant. The phytochemical constituents isolated from useful parts of the plants, and its organoleptic and sensory profiles are elucidated. The present work also providing an up to date picture of the prominent roles of SI for neuroprotection, skin care, antidyslipidaemic, antioxidant and anti-inflammatory, antiproliferative and antitumor modulations. The comprehensive literature has been summarised to familiarise the readers with pertinent information regarding the safety and toxicity perspectives of SI consumption. The literature searching and work compilation were performed using Scopus, ScienceDirect, PubMed and Google Scholar databases. The index terms relating to Sacha Inchi, Plukenetia Volubilis, pharmacology, ethnobotany and phytochemical were used to capture the entirety of the literature. English search term were employed and articles most pertinent to the theme were selected.
2. Ethnobotanical components
The seeds, seed kernels and leaves of SI carry the most ethnobotanical components in the plant. The female seeds of SI were found to be the most expressive of unigenes involved in ALA biosynthesis and fatty acid (FA) catabolism pathways. Most of the unigenes related to ALA biosynthesis metabolism were up-regulated, whereas majority of the enzymes related to FA catabolism were down-regulated in the seeds of SI. In particular, the up-regulation of fatty acid desaturase 3 (FAD3) and fatty acid desaturase 7 (FAD7) may play an important role for higher level ALA accumulation in the SI seeds. Some transcription factors are up-regulated in seeds, which are potentially related to triacylglycerol accumulation (Hu et al., 2018).
SIO is identified as an essential source of the healthy omega-3 and omega-6 linoleic acyl groups. These polyunsaturated fatty acids (PUFAs) are beneficial in controlling cardiometabolic syndrome, namely coronary heart disease and hypertension, and demonstrating hypocholesterolaemic effect when it is used as food supplements (Follegatti-Romero et al., 2009). In addition, SIO-contained cosmetic products also exhibited potential antibacterial, anti-inflammatory, skin tightening and anti-ageing effects (Wang, 2018). SI leaves were reported to demonstrate antioxidant and anti-inflammatory properties. As a result, the leaves are often roasted or processed for human consumption. Nascimento et al. (2013) conducted laboratory extraction procedures using SI fresh leaves, followed by antioxidant and antiproliferative assays testing towards the normal versus tumour cells. The results showed that some of the leave extracts presented antioxidant and antiproliferative activities against HeLa cells. The same leave extracts were also observed to be able to stimulate the cell proliferation in fibroblast cells-3T3.
3. Phytochemistry profile
3.1. SIO extract
Throughout the years, SIO has gained popularity among the scientific communities owing to its rich content of unsaturated fatty acids. SI's chemical composition varies depending on its seed-associated factors (subspecies, quality, growth, geographical and climate circumstances, harvesting time, and storage settings), as well as associated extraction methods and efficiency considerations (Sánchez et al., 2021). At both the commercial and artisanal levels, cold and screw expeller pressings are the most common processes for the extraction of oil from SI kernels. Although screw pressing procedure yields more oil, however, cold pressing yields superior quality oils, as the thermolabile component, such as the tocopherol, is preserved in higher proportions (Goyal et al., 2022). SIO is mainly composed of lipids and proteins, carbohydrates, including dietary fibre, as shown in Table 2.
Table 2.
Proximate composition of SI seed (kernel) oil and powdered SI.
| Component | SI seed∗ | Powdered SI∗∗ |
|---|---|---|
| Moisture (g/100g) | 3.30–8.32 | 4.08 ± 0.03 |
| Fat (g/100g) | 33.4–54.70 | 5–11.2 |
| Protein (g/100 g) | 24.20–33.30 | 57.60–61 |
| Total fibre (g/100 g) | 6.59–13.86 | 5.72–12 |
| Carbohydrates (g/100g) | 6.00–30.90 | 15.62–22 |
| Ash (g/100g) | 2.70–6.46 | NR |
| Minerals (mg/100g) | ||
| Calcium | 126.30 ± 0.69 | NR |
| Phosphorus | 519.70 ± 2.77 | NR |
| Sodium | 0.30 ± 0.00 | NR |
| Potassium | 489.30 ± 10.7 | NR |
| Magnesium | 344.20 ± 2.1 | NR |
| Copper | 0.80 ± 0.0 | NR |
| Iron | 4.20 ± 0.0 | NR |
| Manganese | 1.00 ± 0.0 | NR |
| Zinc | 4.10 ± 0.4 | NR |
Source: (Organic Crops E.I.R.L, 2017; Quinteros et al., 2016).
3.2. SI raw seeds
The raw seeds of SI contain approximately 22–30% protein, while the defatted seeds of pressed cake after oil extraction are rich with approximately 53–59% protein (Follegatti-Romero et al., 2009). The major soluble protein fractions are albumins, glutelins, globulins and prolamins. The seeds also contain several essential amino acids, including leucine, tyrosine, isoleucine, lysine, and tryptophan (approximately 64, 55, 50, 43, and 43 mg/g of protein, respectively), with a particularly larger amount of sulfur-containing amino acids as compared to other oilseed crops (Sathe et al., 2012). The carbohydrates content in SI seed range from 12.1% to 30.9% (Ruiz et al., 2013; Takeyama and Fukushima, 2013; Gutiérrez, 2011). Although these components have been barely investigated, the total dietary fibre in the SI seed is estimated at 72.4% insoluble dietary fibre, and 9.0% soluble dietary fibre (Takeyama and Fukushima, 2013).
The extract of SI reveals mostly lipid fraction in the seeds, while the presence of phenols, flavonoids, tannin, cardiac glycosides, steroids and terpenoids were mainly dominating the seed shells and leaves (Kodahl and Sørensen, 2021; Wuttisin et al., 2020). The lipid fractions are composed of approximately 77.5–84.4% PUFAs, 8.4–13.2% monounsaturated fatty acids (MUFAs), and 6.8–9.1% saturated fatty acids (SFAs). In SI, the PUFA fraction is composed of two types of fatty acids, the ALA (C18:3 n-3) and linoleic acid (C18:2 n-6, LA). These essential fatty acids must be obtained through the diet as they cannot be synthesised in the body due to the lack of delta-12 and delta-15 desaturases (Czumaj and Ś ledzi ń ski, 2020). The detailed fatty acid profile of SI seed and oil is presented in Table 3.
Table 3.
Fatty acids content (% of total fatty acids) and bioactive compounds in SI seed and SIO.
| Component | SI seed∗ | SI oil∗∗ |
|---|---|---|
| Fatty acida | ||
| Palmitic (C16:0) | 1.6–2.1 | 4.7 ± 0.2 |
| Stearic (C18:0) | 1.1–1.3 | 3.3 ± 0.1 |
| Oleic (C18:1, ω-9) | 3.5–4.7 | 8.9 ± 0.1 |
| Linoleic (C18:2, ω-6) | 12.4–34.98 | 34.1 ± 0.1 |
| ⍺-linolenic acid (C18:3, ω-3) | 12.8–47.04 | 48.2 ± 0.4 |
| Total SFAs | 2.6–3.2 | NR |
| Total UFAs | 30.6–34.3 | NR |
| Tocopherols | ||
| ⍺-tocopherol (mg/100g) | 1.13–1.27 | 0.4 |
| β-tocopherol (mg/100g) | 0.75–0.95 | NR |
| γ-tocopherol (mg/100g) | 57.4–68.2 | 125.7 |
| δ-tocopherol (mg/100g) | 29.2–47.6 | 86.9 |
| Total flavonoids (mg rutin eq./g oil extract) | NR | 0.34 |
| Total carotenoids (mg/kg) | 0.7–0.9 | NR |
| Total phenols (mg GAE/100g) | 64.6–80.0 | 6.20 |
| Phytosterols (mg/100g) | ||
| Campesterol | 4.5–8.8 | 15.0–15.3 |
| Stigmasterol | 21.2–32.3 | 36.11–58.70 |
| β-Sitosterol | 46.6–63.1 | 43.46–127.40 |
| Total antioxidant activity (μmol TE/g) | 6.5–9.8 | 18.2–95.0 |
Data are presented as % total fatty acids.
Source: (Carillo et al., 2018; Chirinos et al., 2013).
3.3. SI seed oils
The antioxidant properties of SI seed oils are attributed to its phenols, tocopherols, and carotenoids content. Fanali et al. (2011) detected 21 phenolic compounds in the SI seed oil, and the amount increases with the roasting intensity. The total antioxidant capacity (TAC) of the seed oil pressed from unroasted seeds was 18.2 mcg Trolox equivalent (TE)/g oil, and the value increases with roasting degree to 95.0 mcg TE/g oil for highly roasted seeds (Cisneros et al., 2014). Meanwhile, the total tocopherol contents of SI seeds ranged from 78.6 to 137.0 mg/100g seed, with the seed oils content were 2.39 (using Soxhlet extraction) and 2.79 g/kg oil (using cold pressing), respectively. Recently, the application of supercritical carbon dioxide extraction at 40 °C was found to slightly increase the tocopherol content of SI seed oils (3.07 g/kg oil).
The total carotenoid content of seed oils from 17 SI cultivars has recorded a range of 0.07–0.09mg of ß-carotene equivalent per 100g of seed (Gutiérrez, 2011). Sitosterol (45.2–53.5 mg/100g seed) is the predominant phytosterol found in the seed oils, followed by stigmasterol (21.2–26.9 mg/100g seed) and campesterol (7.1–8.8mg/100g seed). The sum of these 3 phytosterols ranged from 73.5 to 89 mg/100g seed (Follegatti-Romero et al., 2009). The total phenolic content (TPC) of SI seed oils varies over a wide range (64.6–80.0 mg gallic acid equivalent (GAE)/100g seed, wet basis). Phenyl alcohol, flavonoid, secoridoid, and lignan type phenolics have been identified in SI seed oil. The TPC varied when the seeds were processed using different thermal treatments, particularly with the applications of open boiling, pressure boiling, vacuum boiling, low temperature (125 °C), high-temperature (197 °C), and honey roasting (175 °C) (Follegatti-Romero et al., 2009).
3.4. SI seed shells and leaf
Chirinos and labmates (2016) have successfully extracted 1.24% of total lipid fraction from the SI seed shell. The laboratory investigations reported that 74.56 mg GAE/g total phenolics were found in the SI seed kernel, predominantly the condensed tannins (69.42 mg cyanidin equivalents/g). Besides, the presence of hydrolysable tannins (3.28 mg GAE/g), lignans (0.84 mg secoisolaricirecinol diglucoside/g), bound phenolic acids (0.40 mg GAE/g), flavonoids (0.36 mg quercetin equivalents/g), flavonoids (0.15 mg CE (catechin)/g), and free phenolic acids (0.11 mg GAE/g) were also reported. Other phenolic acid subtypes, such as the cinnamic protocatechuic, hydroxycinnamic and p-coumaric acid, were found in the seed kernels (Chirinos et al., 2016). The leaf of SI contains terpenoids, saponins, phenolic compounds (flavonoids) and other components responsible for its antioxidant activity. The TAC and 2,2-diphenylpicrylhydrazyl (DPPH) values of SI leaf extract were reported as 59.31–97.76 ascorbic acid equivalent (AAE)/g and 62.8–88.3%, respectively (Nascimento et al., 2013).
4. Pharmacological activities
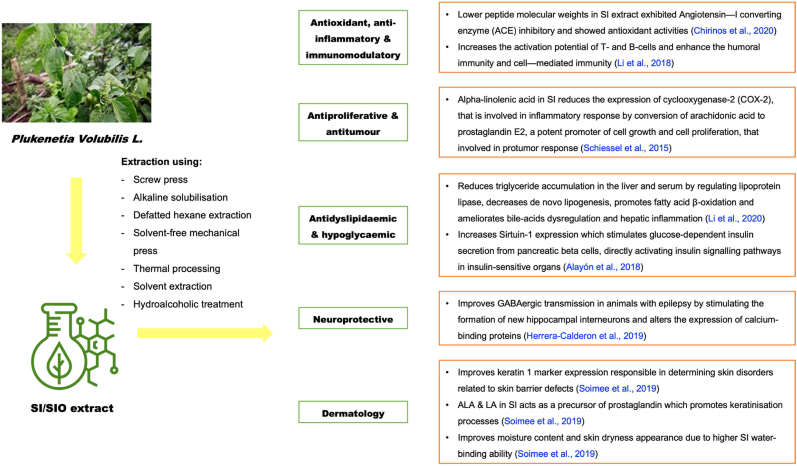
The effects of SI (seed, protein hydrolysate or oil substitutes) intake and its relation with the pharmacological activities remains a global debate. A number of in vitro, in vivo and human clinical trials have been undertaken to explore the prominent roles of SI (and its derivatives) for modulating chronic disease, particularly the non-communicable diseases (Table 4). The mechanism of SI for the amelioration of disease is simplified in Figure 2.
Table 4.
Pharmacological activities of SI.
| Pharmacological Activity | Part | Methods and Outcomes | Reference |
|---|---|---|---|
| Antioxidant | Seed | SI seeds from 16 cultivars were analysed for different phytochemical, and the content of the evaluated compounds exhibit high variations. | (Chirinos et al., 2013) |
| The total phenolic and total carotenoid contents were linked to the hydrophilic and lipophilic antioxidant capabilities, respectively. | |||
| PUFAs, tocopherols, phytosterols, and phenolic compounds in the seed showed potent antioxidant properties. | |||
| Seed (oil) | ABTS and DPPH assays were used to determine the antioxidant activity of the oil's lipophilic and hydrophilic extracts in vitro. | (Jáuregui et al., 2010) | |
| Lipophilic extract demonstrated higher antioxidant activity than the hydrophilic extract. | |||
| Seed and seed kernels (raw and honey-coated) | The changes of total phenolic content using several processing methods (open boiling, pressure boiling, low and high temperature roasting, and honey roasting) were tested on SI kernels. The DPPH value was strongly affected by the process temperature and the water activity of the seeds. | (Št ě rbov á et al., 2017) | |
| Leaf (leaf extract and leaf extract-based silver nanoparticles) | AgNPs (silver nanoparticles) showed a stronger antioxidant activity against DPPH radicals than leaf extracts | (Kumar et al., 2014) | |
| Antidyslipidaemic | Seed (roasted) | In 28 volunteers, the effect of consuming 30 g/d SI seeds for 6 weeks were evaluated. 30g confit wheat (Triticum aestivum) was given to the control group. Significant decreases in cholesterol, triglycerides, and LDL-C, as well as an increase in HDL-C level was observed in the SI group. | (Saavedra et al., 2010) |
| Seed (oil) | SIO consumption resulted in decreases in mean total cholesterol and non-esterified fatty acid readings, as well as an increase in HDL-C. | (Garmendia et al., 2011) | |
| Antitumour and antiproliferative | Leaf (leaf extracts) | Several leaf extracts were used to address HeLa (cervix) and A549 (lung) tumour cell lines. Methanol and hexane extraction methods inhibited HeLa cell proliferation by 54.3 and 48.5%, respectively. | (Nascimento et al., 2013) |
| Seed (oil) | In Walker 256 tumour-bearing rats, SIO was found to exhibit anticancer action. Ex vivo, a SIO-based diet (1kg BW/d for four weeks) reduced tumour bulk and proliferation in Walker 256 tumour cells. A higher lipoperoxidation level was found in Walker 256 tumour tissues, aligned with lower levels of glycaemia, triglycerides, and plasma inflammatory cytokines. | (Schiessel et al., 2015) |
ABTS = 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate); DPPH assay = 2,2-diphenyl-1-picryl-hydrazyl-hydrate.
Figure 2.
Simplified mechanisms of SI bioactivity.
4.1. Antioxidant, anti-inflammatory and immunomodulatory properties
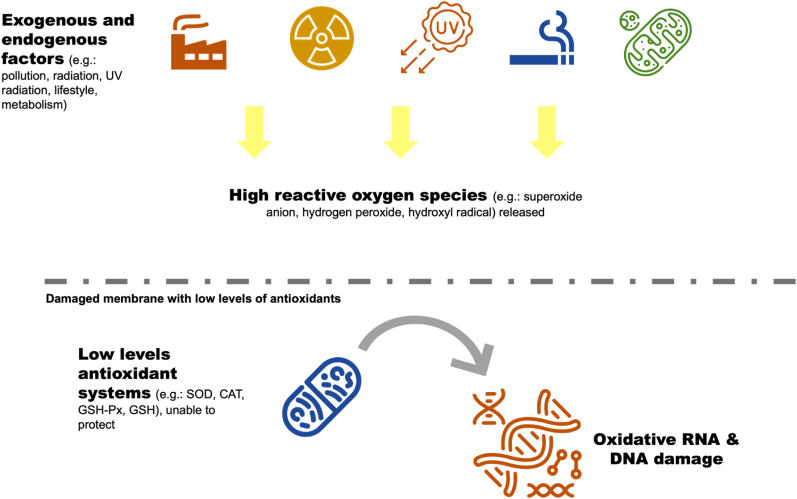
Since the past decades, replacing synthetic antioxidants with natural antioxidants has become the great interests among the scientific communities (Lourenço et al., 2019). The interests in these natural components are not only due to their biological value, but also to their economic impact, as most of them may be extracted from food by-products and under-exploited plant species, such as SI. Human body produces a lot of by-products from the normal cellular energy production and functional activities known as reactive oxygen species (ROS). In an ideal mechanism, the ROS such as superoxide anion, singlet oxygen, lipid peroxides and hydroxyl radical are well-regulated (Rajendran et al., 2014). However, ROS levels may increase intensely due to endogenous and exogenous sources, which may lead to the damage of many molecules, notably proteins, lipids, RNA and DNA (Figure 3). Higher production of ROS and their removal by biological antioxidant defences are called oxidative stress, which has been linked to increased risk of cancer, diabetes, atherosclerosis, arthritis, neurodegenerative diseases and premature ageing (Lin et al., 2019).
Figure 3.
Mechanism of ROS that can damage RNA and DNA.
Antioxidants act as hydrogen and electron donor, radical scavenger, oxygen quencher and decreasing the localised oxygen concentrations (Oroian and Escriche, 2015). Several studies analysed parts of SI (such as the leaves and shells), and reported that these plant structures exhibited antioxidant, anti-inflammatory and immunomodulatory properties (Chirinos et al., 2016; Nascimento et al., 2013; Wuttisin et al., 2020). The properties, and its benefits are divided accordingly to its protein hydrolysate, extract treatment, flavonoid and tocopherol content.
4.1.1. SI protein hydrolysate
SI protein hydrolysate showed antioxidant action by activating the radical scavenging activity [measured by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay] (Chirinos et al., 2020). The study examined various types of enzyme-assisted SI protein hydrolysis degree, and reported that the highest ABTS antioxidant activity (AOX) of 1.7 folds higher, in hydrolysate of seed, is obtained from the combination of food grade enzymes Alcalase-Flavourzyme and Alcalase-Flavourzyme-Thermolysine. These may be attributed to the release of bioactive peptides, with its radical scavenging, lipid peroxidation inhibition and metal ion chelating properties. Another study investigated the screw press extraction of SI seeds oil also yielded similar finding (Muangrat et al., 2018). The research team suggested that the antioxidant capacity values using ABTS radical assays were more effective in scavenging ABTS radicals than the DPPH radicals, with the IC50 values of 215.48 and 317.33 mg/mL, respectively in the non-dried SI seeds. The IC50 value denotes the concentration of the sample required to scavenge 50% of DPPH radicals. Low IC50 values indicate high scavenging radical activity. Therefore, it can be concluded that SIO seeds, including its protein hydrolysates, contained natural phenolic compounds that demonstrated antioxidant capacity. In an in vitro study, the use of 1000 μg/ml SI protein isolate under acidic conditions demonstrated 78.1% of anti-inflammatory activity (Quinteros et al., 2016).
Meanwhile, Li et al. (2018) evaluated the albumin fraction of SI seed protein at various concentrations. At the concentrations of 5–320 μg/mL, SI-albumin fraction stimulated spleen lymphocytes. SI-albumin fraction at 320 μg/mL also declined cell viability and intracellular ROS, while elevating nitrogen oxide (NO) and hydrogen peroxide (H2O2) productions of RAW 264.7 cells.
4.1.2. SI extract treatment
Potent antioxidant activity is also reported in the less studied part of SI plant, the seed kernels and leaves. In a study comparing the effects of different thermal processing of SI seed kernels, a significant radical scavenging capacity has been reported following water treatment (Št ě rbov á et al., 2017). The highest mean value was recorded at 256.6 mmol TE/100g, while the lowest capacity was recorded for low-temperature roasting (186.4 mmol TE/100g). However, the team demonstrated a significant reduction trend in radical scavenging capacity after the seed kernels were thermally processed at 45 min. In the same vein, Nascimento et al. (2013) extracted SI leaf composition, and the TAC for 5 different solvent extraction methods (methanol, ethanol, chloroform, hexane and aqueous) were measured. The TAC ranged between 59.31 to 97.76 EAA/g, and higher antioxidant performance were observed for methanol-, hexane- and chloroform-extractions at 97.76, 83.42, and 89.21 EAA/g, respectively.
4.1.3. SI flavonoid and tocopherol content
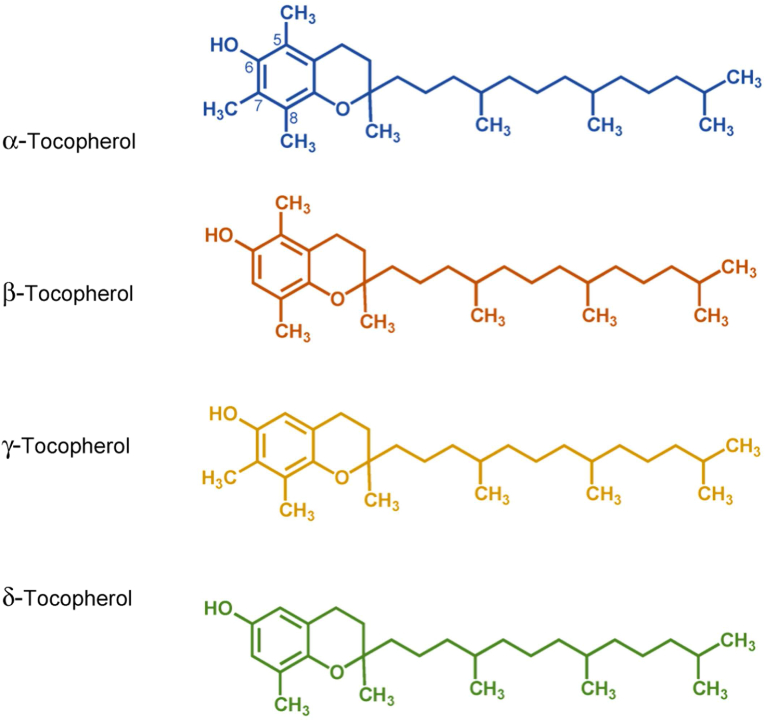
Literature also reported the potential of SI flavonoids for the prevention, formation and elimination of free radicals (Dıaz-Araya et al., 1998; Shahidi et al., 1992). The compounds, similarly identified in the hydroalcoholic extract of SI leaf along with tannins, showed significant in vitro inhibition of the lipid peroxidation (measured as the formation of malondialdehyde) induced by iron (II) ascorbate in hepatic tissue of Rattus Albinus rats after concomitant treatment of hepatocyte homogenate using a dosage of 70 and 140 mg/L extracts (Saavedra et al., 2010). de Souza et al., 2013 assessed the total tocopherol content in seed kernels and SI seed. The average tocopherol content in the shell and SI seed were 3.06 and 8.99 mg/100g, respectively, notably higher than that of other oleaginous plants like rye (0.1 mg/100g), including several tocopherol isomers, the ⍺-, β- and γ-tocopherol (Ryan et al., 2007) (Figure 4). Tocopherols were known as natural antioxidants that inhibit lipid oxidation in biological systems by stabilising hydroperoxyl and other free radicals. Apart from increasing oil stability, tocopherols are also essential for humans as they have been associated with delayed cellular ageing, reduced risk of cardiovascular diseases and regression of several cancers in cell culture (Aguirrezábal et al., 2015).
Figure 4.
Chemical structure of tocopherol isomers.
4.2. Antiproliferative and antitumor properties
Exogenic factors such as stress, radiation, pollution, pesticides and industrial chemicals may disturb the normal balance of ROS in human bodies, which are highly associated with the development of cancer (Lin et al., 2019). To combat cancer, antiproliferative and antitumor substances are being used in the treatment to kill cancer cells (Choudhari et al., 2020). The Euphorbiacea is formed by more than 6,000 species with extreme diversity of secondary compounds. The variability of compounds may explain the different uses of the plants from this family (Mwine and Damme, 2011).
However, scarce information is known to explain the mechanisms of which the SI derivatives are protective towards neoplasia or tumour prevention. In 2015, a pioneer animal trial in Peru suggested that SIO showed potential anticancer activity. The SIO based diet (1g/kgBW/d for 4 weeks) has successfully reduced tumour mass and proliferation of Walker 256 tumour cells ex vivo, and lowered the expression of cyclooxygenase-2 (COX-2) in the tissue. The diet has increased the lipoperoxidation in the tumour tissues, reduced hypertriacylglycerolaemia, and improved hypoglycaemia and plasma levels of inflammatory cytokines TNF-⍺ and interleukin-6 (IL-6) in tumour-bearing rats (Schiessel et al., 2015).
In parallel, the extracts from SI leaves have been demonstrated to induce apoptosis (both in early and late stages) in cell cultures. The SI leaf extracts inhibited the HeLa (cervical cancer cells) and A549 (lung tissue tumour cells) cancerous cells. Significant reductions of 48.5, and 54.3% of their proliferation rate after 48h of treatment with methanol and hexane-extracted SI leaf fractions were reported. These fractions also induced early apoptosis of the HeLa cells by 10.2 and 13.3%, respectively. Terpenoids, saponins, and phenolic compounds (flavonoids) were the main bioactive compounds found in the leaf with antiproliferative activity against the cancer cells (Nascimento et al., 2013).
The polysaccharides extracted from SI seeds are also believed to show a significant immunostimulatory activities. Tian et al. (2020) extracted and purified polysaccharides from SI seeds to test the viability and phagocytic activities against the RAW264.7 cells (a type of monocyte/macrophage-like cells). In the study, SI extract was shown to exhibit a concentration-dependent radical-scavenging activities against anion, hydroxyl, ABTS and DPPH radicals ranges between 78.6% to 89%, corresponding to the concentrations between 106 – 198 μg/mL. Similar study also reported an improved viability of RAW 264.7 cells, suggesting that SI is non-toxic. It was also suggested that SI extract increases the pinocytosis of the cells while reporting an increase in secretion of cytokines particularly nitric oxide (NO), TNF-⍺, IL-1β and IL-6 at 100–200 μg/mL extraction. The research, however, suggested that the immunomodulatory activities of SI is attributed to its polysaccharide structural features such as glycosidic-bonds, conformations, molecular weights, and functional groups. Higher molecular weight polysaccharide demonstrated triple-helix conformations with high-branching degree, which is connected to significant immunostimulatory activity and antioxidant capacity.
4.3. Antidyslipidaemic and hypoglycemic properties
The chronic consumption of diet rich in saturated fats and cholesterol can induce hyperlipidaemia, and accumulation of lipid fractions in the liver, lipid peroxidation, inflammation and hepatotoxicity. In individuals with high fat diet, alterations in the lipid profile have been observed, and shown as increased triglycerides (TG), total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C) and a decrease in high density lipoprotein-cholesterol (HDL-C) (Ambulay et al., 2020). The alteration of the levels of these lipoproteins in serum could cause an accumulation of lipids and change of cellular metabolism, which is expressed as inflammation, oxidative stress, and cellular atrophy (Savini et al., 2013). Some food components with adipogenesis suppressive profile could be useful in the prevention of dyslipidaemia. The long-chain omega-3 PUFAs (Chang and Kim, 2019), which is highly abundant in SIO, demonstrated potential health benefits for the modulation of balanced lipid profile.
Garmendia and coworkers (2011) have conducted the first human clinical trial using SIO in hypercholesterolaemic patients. Twenty-four patients were given SIO up to 4 months, and the TC, LDL-C, non-HDL cholesterol, TG, very low-density lipoprotein (VLDL) and non-esterified fatty acids (NEFAs) were significantly reduced. In 2010, Gorriti et al. (2010) administered 0.5ml/kgBW of SIO to fed the Holtzman rats up to 60 days. Results depicted that supplementation of SIO improved liver function, as indicated by reductions of cholesterol levels and TG, and an elevation of HDL-C level. By using an organoleptic study design, Alayón et al. (2019) demonstrated the positive effect of applying SIO into a high-fat meal diet in 20 metabolically healthy individuals. SIO-added high fat diet successfully attenuated the increase of TC, in addition to a meaningful IL-6 reduction in circulating bloods as compared to control group. IL-6 is often studied as a measure of low-grade transient inflammatory state, which is closely associated with cardiovascular diseases (Borén et al., 2014). The addition of SIO into the daily diet was capable to modulate postprandial lipids and inflammation, subjective to the individual's metabolic state.
Very recently, the hypolipidemic effects of SIO (0.5–1.5 ml/kg/d) as compared to fish oil (1.0 ml/kg/d) were conducted, and results confirmed that SIO alleviated gut microbiota dysbiosis and improves hepatic lipid dysmetabolism in high-fat diet-fed rats. According to Li et al. (2020), SIO acts by reducing serum and TG accumulation in the liver, and decreasing de novo lipogenesis. SIO also regulates the activities of lipoprotein lipase and fatty acids β-oxidation, indirectly monitoring the bile-acids secretion and hepatic inflammation.
Alayón et al. (2018) conducted the pioneer double-blind, randomised, crossover human clinical trial to investigate the effect of 15 ml SIO supplementation into the high fat breakfast on postprandial glycemic state in metabolically healthy versus metabolically unhealthy individuals. The study showed an attenuation in the elevation of blood glucose, and remarkable increase in the expression of Sirtuin-1 (SIRT1) in the SIO group. SIRT1, also known as NAD-dependent deacetylase sirtuin-1, is a vital protein in human body to stimulate glucose-dependent insulin secretion from the pancreatic beta cells, and directly activates the insulin signaling pathways in insulin-sensitive organs (Liang et al., 2009). However, the result was contradicted with another 4 months interventional trial, where supplementation of either 10 or 15 ml of SIO did not exhibit any significant change in regular and fasting blood glucose levels among the healthy adults (Gonzales and Gonzales, 2014). The discrepancy findings hinting the needs for a larger sample size human trial, and future studies are needed to unravel the complex interrelationship between SIO consumption and the Type 2 Diabetes Mellitus risk.
4.4. Neuroprotective properties
The International League against Epilepsy (ILAE) proposed a new definition of epilepsy as a chronic brain illness defined by a lasting proclivity for recurring unprovoked seizures (Fisher et al., 2014). Approximately 50 million individuals worldwide have been diagnosed with epilepsy, representing a serious problem of public health for all ages, gender and social groups (Xu et al., 2016). Disorders of the neural depolarisation membrane, neural anatomy, and ionic environment are all recognised to perform roles in epilepsy pathophysiology. These changes result in an imbalance of excitatory and inhibitory neurotransmitters (glutamate and aspartate), and gamma amino butyric acid (GABA) (Gupta et al., 2014). Evidences to elaborate the possible anticonvulsant properties of omega-3 PUFAs, including ⍺-linolenic, eicosapentanoic, and docosahexaenoic acids, have been involving the cell cultures and ex vivo preparations (Taha et al., 2010).
SIO was firstly presented with anticonvulsant effect in an experimental model of epilepsy. Thirty male Balb/C albino mice were seizure-induced with pentylenetetrazole (PTZ) and SIO. A protective effect against the seizure, which was very similar to diazepam, was demonstrated using SIO administered at 1000mg/kgBW. The researchers inferred that the mechanism of SIO against PTZ-induced seizures involves the protection pathway via the generation of the neurotransmitter GABA and its cumulative antioxidant effects (Herrera-Calderon et al., 2019). The core explanation involves the mechanism by which omega-3 PUFAs extending the refractory period in neurons, thus protect against the seizure episodes. This mechanism appears to result from a partial inhibition of sodium and calcium voltage-gated channels (Taha et al., 2010).
4.5. Dermatology properties
In Peru, SI is naturally grown in Mariscal Ramón Castilla, Loreto, Maynas, Loreto, Lamas, San Martin and Bellavista. The community uses the SI plant as daily skin care oil, which is regularly applied to preserve skin softness and healthiness (Gonzalez-Aspajo et al., 2015). Commercially available SIO is also formulated as packaged day-care skin creams in Europe, together with other substances (Swiss Import Promotion Programme, 2012).
With the risen popularity, the efficacy of SI has been tested in several dermatological studies. Staphylococcus aureus is the predominant causative agent of skin disorders, such as impetigo, scalded skin syndrome and septicaemia (Marques, 2015; Pereira, 2014). An in vitro study reported that commercially available virgin SIO was not bactericide S. aureus. However, the oils were capable of preventing the attachment of S. aureus to keratinocytes, and efficiently detaching S. aureus from human skin explants (Gonzalez-Aspajo et al., 2015). The beneficial effect could be due to the rich content of PUFAs in SIO, which might increase the membrane fluidity of S. aureus, and hence, affect their adherence to the skin layer (Arsic et al., 2012).
Later, Soimee and her colleagues examined the moisturising and irritative effects of SIO in an ex vivo skin tissue culture, and conducted a clinical research using 13 volunteers. In skin tissue cultures, there was no induced secretion of TNF-⍺ and interleukin-1 alpha (IL-1⍺), or loss of keratin 1 integrity in the stratum corneum layer of SIO-treated cultures compared to non-treated skin tissues. Meanwhile, the human clinical study demonstrated improvements in moisture content and skin dryness appearance at the SIO-applied sites. Such improvements were comparable with that observed at the olive oil-applied sites (Soimee et al., 2020).
5. Organoleptic, sensory evaluations and techno-functional properties
In the early human history, sesame oil and olive oil were commonly used as edible plant-based oil (EPO) in food preparations and consumption (Zhou et al., 2020). The U.S Department of Agriculture (USDA) reported that the EPO market was close to 203 million tons in 2019 (USDA Foreign Agricultural Service, 2021). Edible oils in general are capable to change the sensory properties of food such as colour, fragrance, and taste during processing while enhancing flavour diversity and intensifies the sense of satiety (Tan et al., 2014). With the development of agriculture, processing and inspection technologies, a wide selections of EPO have been incorporated in the food industry. It provides a substantial promise as a renewable source for food industrial applications. EPO is widely studied using agricultural machinery and advanced methods, such as cell engineering, genome editing, and tissue culture (Zhou et al., 2020). The products include plant-based meat alternatives to the oil incorporation into dairy products as a substitute for those with lactose intolerance.
Few researches have looked into the sensory characteristics and acceptance of SI-added products, while some evaluated the enrichment of well-known foodstuffs with SI. In Ecuador, Clavijo et al. (2015) substituted 10% pork fat with ground SI seeds in the production of hamburger patties. Result revealed that the patties contained higher protein and PUFAs content, which resulted with greater consumer acceptability. In Peru, researcher mixed a blend of milk with SI seed suspension for the innovation of new cheese (Fernández et al., 2015). In Colombia, Vanegas-Azuero and Gutié rrez, 2018 conducted the first attempt by incorporating grounded SI seeds into the yogurt blend. The final product showed higher PUFA content and yielded better sensory acceptability. While the acceptability of SI products seem to be good after incorporation into the foods, the acceptability of pure SIO consumption was found to be low after a single week of daily consumption (37.5%). Nevertheless, prolonged SIO consumption (after 6 weeks) yielded a higher acceptability (81.25–93.75%) (Gonzales and Gonzales, 2014). Similarly, partial substitution of the cocoa butter (CB) with SIO increased the rheological behavior and the texture of SIO-incorporated dark chocolates. The substitution of CB with SIO in chocolates also improved consumer preferences towards sensory analysis (Medina-Mendoza et al., 2021).
Potent techno-functional properties of protein products are highly dependent on the solubility, emulsification, foaming and gelation. Few initiatives have investigated the techno-functional properties of SI and its fractions (Table 5). However, the application of protein techno-functional properties of SI is rarely experimented. Manipulation of techno-functional changes in polymers, for example between starches and proteins could contribute to the three-dimensional stability of network of proteins, and thus improve the techno-functional and nutritional properties of a new biopolymeric network (Té llez-Morales et al., 2020). Proteins recovered from SI agroindustrial by-products could be reused as ingredient for fortified foods and dietary supplements as techno-functional constituent, due to its gelling, emulsifying, foaming, and water and oil-binding properties, as biopolymer material, and as source of bioactive peptides (Pojić et al., 2018).
Table 5.
Techno-functional properties of SI proteins.
| Outcomes | Reference |
|---|---|
| Low solubility and water adsorption capacity (7.96% and 2.16 g/g, respectively) | (Alcívar et al., 2020) |
| Water absorption index (3.2–4.4 g/g), water solubility index (12.4–26.2%), and solubility (10.7–38.0 mg/g) | (Jagersbeger, 2013) |
| Albumin fraction extracted with saline solution displayed notable protein solubility (63%), water-holding capacity (1.6 g/g), oil retention capacity (1.7 g/g), foaming (350%) and emulsifying (13.0 mL/g) abilities Heating at temperatures lower than 100 °C improves solubility, oil-holding capacity, foaming and emulsifying capacities |
|
| Increased protein solubility up to 63% as pH increased from 3.0 to 7.0 and significantly dropped (up to approximately 18%) at pH 10.0 | (Li et al., 2018) |
| 2% salt addition reduces solubility of protein fraction | (Mercado et al., 2015) |
| Oil absorption capacity (1.4 g/g), foaming capacity (55% at 1% concentration and pH 8.0), foam stability (33.7% at 1% of concentration, pH 8.0 at 120 min), and emulsifying capacity (59.1%), generally higher than soy protein isolates | |
| Lower water holding (1.8 g/g) and gelling capacities (15%) compared to soy protein isolates | |
| Protein isolates extracted by alkaline water (pH 12.0) presented 84.4% solubility, foam stability of 30% at pH 8.0, and emulsifying, water retention, oil absorption, gelling, and foaming (pH 8.0) capacities of 53.5%, 4.7 g/g, 267.1%, 13%, and 49%, respectively | (Cuñaña, 2018) |
Adapted from (Sánchez et al., 2021).
6. Safety and toxicity perspectives
Food plants in the human diet contain a considerably high concentrations of secondary metabolites that can manifest adverse effects if consumed in large amounts. Although many of the toxins are effective against insect herbivores, they may not be present in sufficient concentration to cause acute toxic effects in human who consume a modest and varied diet (Colegate et al., 2015). However, the evaluation of the safety and toxicity aspects of any ingested food remains the utmost important. While they are not always harmful, anti-nutritional components of some plant-based foods might induce adverse consequences by reducing the effective absorption of dietary inorganic micronutrients and the digestion of macronutrients (Rousseau et al., 2020). For example, polyphenols (tannins) in some cereal grains and legumes, including red sorghum and red beans, respectively, can inhibit the absorption of nonheme iron and vitamin B12, directly affecting the digestibility of dietary starches, proteins and lipids. Similarly, oxalic acid and oxalates, which can exert renal toxicity at high concentrations, can induce antinutritional effects at lower concentrations by chelating with dietary calcium (Colegate et al., 2015).
Consumption of raw SI seeds may cause mild to severe toxicity to human due to the presence of phytotoxins (alkaloids, lectins and saponins) (Sánchez et al., 2021). In 2018, Srichamnong et al., 2018 have discovered mild cytotoxicity in hepatic cells administered with raw SI seed, where the presence of saponins, alkaloids and lectins were detected. The concentrations of these phytotoxins were significantly reduced following heat processing, suggesting that roasting is mandatory before consuming SI seeds and leaves. In contrast, no morbidity and mortality signs were detected in mice and rats fed with de-oiled SI cake at the concentration of 2000 mg/kgBW, sub-chronic toxicity (50, 250 and 500 mg/kgBW/d for 90 days) and genotoxicity (Rodeiro et al., 2018).
A number of studies have investigated the toxicity of SIO and reported rare instances of toxicity (Choudhari et al., 2020). This is in line with the Food Safety Authority of Ireland (FSAI) substantive equivalence opinion, which concluded that SIO is highly comparable to linseed oil in terms of composition, nutritional value, metabolism, and levels of undesirable chemicals (FSAI, 2012). Furthermore, Gorriti et al. (2010) also failed to demonstrate a median fatal dose (LD50) of SIO in rodents. However, the research team expected that the LD50 could be beyond 37g/kgBW, which represented the highest dosage examined in the study.
Despite this, a rare case of occupational allergy, combined with bronchial asthma has been documented in a cosmetic industry worker assigned to crush the seeds of SI (Sastre et al., 2010). In an attempt to identify the allergen, complexes with ca. 8, 10, 27 and 73 kDa were discovered (Bueso et al., 2010). While no further occurrences of allergy to SI have been reported, this case highlighted the need for more investigations into the species allergenicity.
7. Major challenges and future prospects
SI is an undervalued indigenous plant and consumed as traditional medicine, and emerging to offer humongous opportunities for the development of novel value-added nutraceutical and pharmaceutical products. Compelling evidence from the laboratory, preclinical and clinical studies has suggested the use of SI for macro- and micronutrients, α-linolenic acid and phytochemicals extraction, organoleptic enhancement, antiproliferative and antitumor modulation, neuroprotection, dermatology, antidyslipidaemic, antioxidant and anti-inflammatory activities. Despite the promising results from the advanced studies, the huge concentration range is remained obstacles for both nutraceutical and drug evaluation. Furthermore, the targeted mechanism actions of SI with its functional constituents are still not conclusive, and the entity is still remaining unknown. Amidst these challenges, the urgency to conduct further-in-depth studies on the nature and mechanisms of the bioactive constituents ought to be an immediate action. The bioavailability of the different compounds in the SI, and its appropriate dose levels need to be discovered. Ultimately, the clinical intervention trials would provide reliable evidences to support the sustainable use of SI in medicine.
8. Conclusion
SI is an excellent source of PUFAs, particularly ALA, protein and phytochemical constituents. A growing research interest has been directed towards the beneficial use of SI in the health science and food applications development. SI has emerged to be a unique candidate in the regulation of antioxidant and anti-inflammatory status, antiproliferative and antitumor modulation, neuroprotection, dermatology and antidyslipidaemic activities. It also becomes imperative and crucial to verify the safety and toxicity concern to ascertain safe consumption. In future, it is plausible that major investigations will be made to utilize the discovery of nutritional interventions on SI's bioactive compounds and its expression into the health preventive and management strategies.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
The authors acknowledge the financial support provided by the Double Tax Deduction Grant Scheme [Project code: 304/PTEKIND/6501176/O107].
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge the support provided by Universiti Sains Malaysia.
References
- Aguirrezábal L., Martre P., Pereyra-Irujo G., Echarte M.M., Izquierdo N. Crop Physiology. Elsevier; 2015. Improving grain quality: ecophysiological and modeling tools to develop management and breeding strategies; pp. 423–465. [Google Scholar]
- Alayón A.N., Ortega Avila J.G., Echeverri Jiménez I. Carbohydrate metabolism and gene expression of sirtuin 1 in healthy subjects after Sacha inchi oil supplementation: a randomized trial. Food Funct. 2018;9:1570–1577. doi: 10.1039/c7fo01956d. [DOI] [PubMed] [Google Scholar]
- Alayón A.N., Ortega Ávila J.G., Echeverri Jiménez I. Metabolic status is related to the effects of adding of sacha inchi (Plukenetia volubilis L.) oil on postprandial inflammation and lipid profile: randomized, crossover clinical trial. J. Food Biochem. 2019;43 doi: 10.1111/jfbc.12703. [DOI] [PubMed] [Google Scholar]
- Alcívar J., Martínez Pérez M., Lezcano P., Scull I., Valverde A. Technical note on physical-chemical composition of Sacha inchi (Plukenetia volubilis) cake. Cuban J. Agric. Sci. 2020;54(1) [Google Scholar]
- Ambulay J.P., Rojas P.A., Timoteo O.S., Barreto T.V., Colarossi A. Effect of the emulsion of Sacha Inchi (Plukenetia huayabambana) oil on oxidative stress and inflammation in rats induced to obesity. J. Funct.Foods. 2020;64 [Google Scholar]
- Arsic B., Zhu Y., Heinrichs D.E., McGavin M.J. Induction of the staphylococcal proteolytic cascade by antimicrobial fatty acids in community acquired methicillin resistant staphylococcus aureus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borén J., Matikainen N., Adiels M., Taskinen M.R. Postprandial hypertriglyceridemia as a coronary risk factor. Clin. Chim. Acta. 2014;431:131–142. doi: 10.1016/j.cca.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Bueno-Borges L.B., Sartim M.A., Gil C.C., Sampaio S.V., Rodrigues P.H.V., Regitano-d’Arce M.A.B. Sacha inchi seeds from sub-tropical cultivation: effects of roasting on antinutrients, antioxidant capacity and oxidative stability. J. Food Sci. Technol. 2018;55:4159–4166. doi: 10.1007/s13197-018-3345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueso A., Rodriguez-Perez R., Rodriguez M., Dionicio J., Perez-Pimiento A., Caballero M.L. Occupational allergic rhinoconjunctivitis and bronchial asthma induced by Plukenetia volubilis seeds. Occup. Environ. Med. 2010;67:797–798. doi: 10.1136/oem.2010.057224. [DOI] [PubMed] [Google Scholar]
- Cachique D.H., Solsol H.R., Sanchez M.A.G., López L.A.A., Kodahl N. Vegetative propagation of the underutilized oilseed crop sacha inchi (Plukenetia volubilis L.) Genet. Resour. Crop. 2018;65:2027–2036. [Google Scholar]
- Cai Z.Q. Shade delayed flowering and decreased photosynthesis, growth, and yield of Sacha Inchi (Plukenetia volubilis L.) plants. Ind. Crop. Prod. 2011;34:1235–1237. [Google Scholar]
- Carillo W., Quinteros M.F., Carpio C., Morales D., Vásquez G., Álvarez M., Silva M. Identification of fatty acids in sacha inchi oil (Cursive Plukenetia volubilis L.) from Ecuador. Asian J. Pharmaceut. Clin. Res. 2018;11:379–381. [Google Scholar]
- Chang E., Kim C. Natural products and obesity: a focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules. 2019;24:1157. doi: 10.3390/molecules24061157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasquibol N.A., Gallardo G., Gómez-Coca R.B., Trujillo D., Moreda W., Pérez-Camino M.C. Glyceridic and unsaponifiable components of microencapsulated Sacha Inchi (Plukenetia huayllabambana L. and Plukenetia volubilis L.) edible oils. Foods. 2019;8:671. doi: 10.3390/foods8120671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos R., Necochea O., Pedreschi R., Campos D. Sacha inchi (Plukenetia volubilis L.) shell: an alternative source of phenolic compounds and antioxidants. Int. J. Food Sci. Technol. 2016;51:986–993. [Google Scholar]
- Chirinos R., Pedreschi R., Campos D. Enzyme-assisted hydrolysates from sacha inchi (Plukenetia volubilis) protein with in vitro antioxidant and antihypertensive properties. J. Food Process. Preserv. 2020;44 [Google Scholar]
- Chirinos R., Zuloeta G., Pedreschi R., Mignolet E., Larondelle Y., Campos D. Sacha inchi (Plukenetia volubilis): a seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem. 2013;141:1732–1739. doi: 10.1016/j.foodchem.2013.04.078. [DOI] [PubMed] [Google Scholar]
- Choudhari A.S., Mandave P.C., Deshpande M., Ranjekar P., Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front. Pharmacol. 2020;10:1614. doi: 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros F.H., Paredes D., Arana A., Cisneros-Zevallos L. Chemical composition, oxidative stability and antioxidant capacity of oil extracted from roasted seeds of Sacha-Inchi (Plukenetia volubilis L.) J. Agric. Food Chem. 2014;62:5191–5197. doi: 10.1021/jf500936j. [DOI] [PubMed] [Google Scholar]
- Clavijo D., Velásquez Rodríguez F., Castellanos Estupiñán J.E. Utilización de plukenetia volubilis (sacha inchi) para mejorar los componentes nutricionales de la hamburguesa. Enfoque UTE. 2015;6:59–76. [Google Scholar]
- Colegate S.M., Gardner D.R., Lee S.T. In: Handbook of Food Chemistry. Cheung P.C.K., Mehta B.M., editors. Springer Berlin Heidelberg; 2015. Plant-associated natural food Toxins; pp. 753–783. [Google Scholar]
- Cuñaña P. Universidad Nacional de San Martin; 2018. Obtención y caracterización de un aislado proteico a partir de la torta desengrasada de sacha inchi (Plukenetia volubilis L.) [Google Scholar]
- Czumaj A., Śledziński T. Biological role of unsaturated fatty acid desaturases in health and disease. Nutrients. 2020;12:356. doi: 10.3390/nu12020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza A.H.P., Gohara A.K., Rodrigues Â.C., Souza N.E. de, Visentainer J.V., Matsushita M. Sacha inchi as potential source of essential fatty acids and tocopherols: multivariate study of nut and shell. Acta Sci. Technol. 2013;35:757–763. [Google Scholar]
- del-Castillo Á.M.R., Gonzalez-Aspajo G., de Fátima Sánchez-Márquez M., Kodahl N. Ethnobotanical knowledge in the Peruvian amazon of the neglected and underutilized crop sacha inchi (Plukenetia volubilis L.) Econ. Bot. 2019;73:281–287. [Google Scholar]
- Dıaz-Araya G., Godoy L., Naranjo L., Squella J.A., Letelier M.E., Nunez-Vergara L.J. Antioxidant effects of 1,4-dihydropyridine and nitroso aryl derivatives on the fe3+/ascorbate-stimulated lipid peroxidation in rat brain slices. Gen. Phamacol. 1998;31:385–391. doi: 10.1016/s0306-3623(98)00034-2. [DOI] [PubMed] [Google Scholar]
- Fanali C., Dugo L., Cacciola F., Beccaria M., Grasso S., Dachà M., Dugo P., Mondello L. Chemical characterization of sacha inchi (plukenetia volubilis L.) oil. J. Agric. Food Chem. 2011;59:13043–13049. doi: 10.1021/jf203184y. [DOI] [PubMed] [Google Scholar]
- Fernández A.O., Medina M., Martínez E., Navarro E. Obtaining cheese with milk mixture and Inca peanut (plukenetia volubilis) J. Chemist. Chem Engin. 2015;9:533–537. [Google Scholar]
- Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., Engel J., Forsgren L., French J.A., Glynn M., Hesdorffer D.C., Lee B.I., Mathern G.W., Moshé S.L., Perucca E., Scheffer I.E., Tomson T., Watanabe M., Wiebe S. ILAE Official Report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Follegatti-Romero L.A., Piantino C.R., Grimaldi R., Cabral F.A. Supercritical CO2 extraction of omega-3 rich oil from Sacha inchi (Plukenetia volubilis L.) seeds. J. Supercrit. Fluids. 2009;49:323–329. [Google Scholar]
- Food Safety Authority of Ireland . 2012. Inca Inchi Virgin Oil Substantial Equivalence Opinion. [Google Scholar]
- Garmendia F., Pando R., Ronceros G. Efecto del aceite de sacha inchi (Plukenetia volúbilis L) sobre el perfil lipídico en pacientes con hiperlipoproteinemia. Rev. Peru. Med. Exp. Salud Pública. 2011;28:628–632. [PubMed] [Google Scholar]
- Gillespie L.J. A revision of paleotropical plukenetia (euphorbiaceae) including two new species from Madagascar. Syst. Bot. 2007;32:780–802. [Google Scholar]
- Gonzales G.F., Gonzales C. A randomized, double-blind placebo-controlled study on acceptability, safety and efficacy of oral administration of Sacha Inchi oil (Plukenetia volubilis L.) in adult human subjects. Food Chem. Toxicol. 2014;65:168–176. doi: 10.1016/j.fct.2013.12.039. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aspajo G., Belkhelfa H., Haddioui-Hbabi L., Bourdy G., Deharo E. Sacha Inchi Oil (Plukenetia volubilis L.): effect on adherence of Staphylococus aureus to human skin explant and keratinocytes in vitro. J. Ethnopharmacol. 2015;171:330–334. doi: 10.1016/j.jep.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Gorriti A., Arroyo J., Quispe F., Cisneros B., Condorhuamán M., Almora Y., Chumpitaz V. Plukenetia volubilis L.) Y LINAZA (Linum usitatissimum L.) Rev. Peru. Med. Exp. Salud Pública. 2010;27(3):9. doi: 10.1590/s1726-46342010000300007. [DOI] [PubMed] [Google Scholar]
- Goyal A., Tanwar B., Kumar Sihag M., Sharma V. Sacha inchi (Plukenetia volubilis L.): an emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131459. [DOI] [PubMed] [Google Scholar]
- Gupta G., Dua K., Kazmi I., Anwar F. Anticonvulsant activity of Morusin isolated from Morus alba: modulation of GABA receptor. Biomed. Aging Pathol. 2014;4:29–32. [Google Scholar]
- Gutiérrez L.F. Composición química de las semillas de Sacha Inchi (Plukenetia volubilis L.) y características de su fracción lipídica. Grasas Aceites. 2011;62:76–83. [Google Scholar]
- Hanssen H.P. Academic Press; 2011. Chapter 117—Sacha Inchi (Plukenetia volubilis L.) Nut Oil and its Therapeutic and Nutritional Uses. Nuts and Seeds in Health and Disease Prevention; pp. 991–994. [Google Scholar]
- Herrera-Calderon O., Yuli-Posadas R.A., Tinco-Jayo J.A., Enciso-Roca E., Franco-Quino C., Chumpitaz-Cerrate V., Figueroa-Salvador L. Neuroprotective effect of Sacha Inchi Oil (Plukenetia volubilis L.) in an experimental model of epilepsy. Phcog. J. 2019;11:1591–1596. [Google Scholar]
- Hu X.D., Pan B.Z., Fu Q., Niu L., Chen M.S., Xu Z.F. De novo transcriptome assembly of the eight major organs of Sacha Inchi (Plukenetia volubilis) and the identification of genes involved in α-linolenic acid metabolism. BMC Genom. 2018;19:380. doi: 10.1186/s12864-018-4774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagersbeger J. Universität Wien; 2013. Development of Novel Products on Basis of Sacha Inchi – Use of Press Cakes and Hulls [MSc. Thesis] [Google Scholar]
- Jáuregui A.M., Escudero F.R., Ortiz-Ureta C.A., Castañeda B.C., Mendoza E.B., Farfán J.Y., Asencios D.C. Evaluation of the content of phytosterols, phenolic compounds and chemical methods to determine the antioxidant activity in seeds of Sacha Inchi (Plukenetia volubilis L.) (In Spanish) Rev. Soc. Quim. Peru. 2010;8 [Google Scholar]
- Kim D.S., Joo N. Nutritional composition of Sacha inchi (Plukenetia Volubilis L.) as affected by different cooking methods. Int. J. Food Prop. 2019;22:1235–1241. [Google Scholar]
- Kodahl N. Sacha inchi (Plukenetia volubilis L.)—from lost crop of the Incas to part of the solution to global challenges? Planta. 2020;251:80. doi: 10.1007/s00425-020-03377-3. [DOI] [PubMed] [Google Scholar]
- Kodahl N., Sørensen M. Sacha Inchi (Plukenetia volubilis L.) is an underutilized crop with a great potential. Agronomy. 2021;11:1066. [Google Scholar]
- Krist S. first ed. Springer International Publishing; 2020. Vegetable Fats and Oils. [Google Scholar]
- Kumar B., Smita K., Cumbal L., Debut A. Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts. Saudi J. Biol. Sci. 2014;21:605–609. doi: 10.1016/j.sjbs.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Huang J., Xiao N., Cai X., Yang Y., Deng J. Sacha inchi oil alleviates gut microbiota dysbiosis and improves hepatic lipid dysmetabolism in high-fat diet-fed rats. Food Funct. 2020;11:5827–5841. doi: 10.1039/d0fo01178a. [DOI] [PubMed] [Google Scholar]
- Li P., Wen J., Ma X., Lin F., Jiang Z., Du B. Structural, functional properties and immunomodulatory activity of isolated Inca peanut (Plukenetia volubilis L.) seed albumin fraction. Int. J. Biol. Macromol. 2018;118:1931–1941. doi: 10.1016/j.ijbiomac.2018.07.046. [DOI] [PubMed] [Google Scholar]
- Liang F., Kume S., Koya D. SIRT1 and insulin resistance. Nat. Rev. Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- Lin L.S., Wang J.F., Song J., Liu Y., Zhu G., Dai Y., Shen Z., Tian R., Song J., Wang Z., Tang W., Yu G., Zhou Z., Yang Z., Huang T., Niu G., Yang H.H., Chen Z.Y., Chen X. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics. 2019;9:7200–7209. doi: 10.7150/thno.39831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço S.C., Moldão-Martins M., Alves V.D. Antioxidants of natural plant origins: from sources to food industry applications. Molecules. 2019;24:4132. doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques L.M. Sepsis induced by Staphylococcus aureus: participation of biomarkers in a murine model. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2015;21:345–355. doi: 10.12659/MSM.892528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Mendoza M., Rodriguez-Pérez R.J., Rojas-Ocampo E., Torrejón-Valqui L., Fernández-Jeri A.B., Idrogo-Vásquez G., Cayo-Colca I.S., Castro-Alayo E.M. Rheological, bioactive properties and sensory preferences of dark chocolates with partial incorporation of Sacha Inchi (Plukenetia volubilis L.) oil. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado R.,J.L., Elías P.,C.C.A., Pascual C.,G.J. Obtención de UN AISLADO PROTEICO de torta de sacha INCHI (Plukenetia volubilis L.) Y evaluación de SUS PROPIEDADES TECNO-FUNCIONALES. An. Cient. (La Molina) 2015;76(1):160. [Google Scholar]
- Muangrat R., Veeraphong P., Chantee N. Screw press extraction of Sacha inchi seeds: oil yield and its chemical composition and antioxidant properties. J. Food Process. Preserv. 2018;42 [Google Scholar]
- Mwine J.T., Damme P.V. Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. J. Med. Plants Res. 2011;5:652–662. [Google Scholar]
- Nascimento A.K.L., Melo-Silveira R.F., Dantas-Santos N., Fernandes J.M., Zucolotto S.M., Rocha H.A.O., Scortecci K.C. Antioxidant and antiproliferative activities of leaf extracts from Plukenetia volubilis Linneo (Euphorbiaceae). Evid. Based Complement. Alternative Med. 2013;2013:1–10. doi: 10.1155/2013/950272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocelák M. Czech University of Life Sciences Prague; 2016. Molecular Characterization of Plukenetia volubilis L. And Analysis of Seed Storage Protein Pattern and Protein Fractions. [Google Scholar]
- Organic Crops E.I.R.L. Organic Crops E.I.R.L; 2017. Product Specification Sacha Inchi Powder. [Google Scholar]
- Oroian M., Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res. Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Pereira L.B. Impetigo—review. An. Bras. Dermatol. 2014;89:293–299. doi: 10.1590/abd1806-4841.20142283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pojić M., Mišan A., Tiwari B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018;75:93–104. [Google Scholar]
- Quinteros M., Vilcacundo R., Carpio C., Carillo W. Isolation of proteins from sacha inchi Plukenetia volubilis L. in presence of water and salt. Asian J. Pharmaceut. Clin. Res. 2016;9:193–196. [Google Scholar]
- Rajendran P., Nandakumar N., Rengarajan T., Palaniswami R., Gnanadhas E.N., Lakshminarasaiah U., Gopas J., Nishigaki I. Antioxidants and human diseases. Clin. Chim. Acta. 2014;436:332–347. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Rodeiro I., Remirez D., Flores D. Safety assessment of sacha inchi powder. J. Pharma Pharmacogn Res. 2018;6:10. [Google Scholar]
- Rousseau S., Kyomugasho C., Celus M., Hendrickx M.E.G., Grauwet T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2020;60:826–843. doi: 10.1080/10408398.2018.1552243. [DOI] [PubMed] [Google Scholar]
- Ruiz C., Díaz C., Anaya J., Rojas R. Análisis proximal, antinutrientes, perfil de ácidos grasos Y de aminoácidos de semillas Y Tortas de 2 especies de sacha inchi (Plukenetia volubilis y. Rev. Soc. Quim. Peru. 2013;79:29–36. [Google Scholar]
- Ryan E., Galvin K., O’Connor T.P., Maguire A.R., O’Brien N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- S.I.P.O. 2012. Market Brief for Sacha Inchi.Pdf. (2012). OSEC Zurich - Swiss Import Promotion Programme. [Google Scholar]
- Saavedra C., Felix E., Viera C., Felix S., Alfaro R., Elizabeth C. Estudio fitoquímico de Plukenetia volubilis L. y su efecto antioxidante en la lipoperoxidación inducida por Fe3+/ascorbato en hígado de Rattus rattus var. Albinus. 2010;2:11. [Google Scholar]
- Sánchez E.G., Hernández-Ledesma B., Gutiérrez L.-F. Sacha inchi oil press-cake: physicochemical characteristics, food-related applications and biological activity. Food Rev. Int. 2021:1–12. [Google Scholar]
- Sastre J., Garcia del Potro M., Aguado E., Fernandez-Nieto M. Occupational asthma due to 5-aminosalicylic acid. Occup. Environ. Med. 2010;67:798–799. doi: 10.1136/oem.2010.058008. [DOI] [PubMed] [Google Scholar]
- Sathe S.K., Kshirsagar H.H., Sharma G.M. Solubilization, fractionation, and electrophoretic characterization of Inca Peanut (Plukenetia volubilis L.) proteins. Plant Foods Hum. Nutr. 2012;67:247–255. doi: 10.1007/s11130-012-0301-5. [DOI] [PubMed] [Google Scholar]
- Savini I., Catani M., Evangelista D., Gasperi V., Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int. J. Mol. Sci. 2013;14:10497–10538. doi: 10.3390/ijms140510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiessel D.L., Yamazaki R.K., Kryczyk M., Coelho I., Yamaguchi A.A., Pequito D.C.T., Brito G.A.P., Borghetti G., Fernandes L.C. α-linolenic fatty acid supplementation decreases tumor growth and cachexia parameters in walker 256 tumor-bearing rats. Nutr. Cancer. 2015;67:839–846. doi: 10.1080/01635581.2015.1043021. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Janitha P.K., Wanasundara P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Soimee W., Nakyai W., Charoensit P., Grandmottet F., Worasakwutiphong S., Phimnuan P., Viyoch J. Evaluation of moisturizing and irritation potential of sacha inchi oil. J. Cosmet. Dermatol. 2020;19:915–924. doi: 10.1111/jocd.13099. [DOI] [PubMed] [Google Scholar]
- Srichamnong W., Ting P., Pitchakarn P., Nuchuchua O., Temviriyanukul P. Safety assessment of Plukenetia volubilis (Inca peanut) seeds, leaves, and their products. Food Sci. Nutr. 2018;6:962–969. doi: 10.1002/fsn3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štěrbová L., Hlásná Čepková P., Viehmannová I., Huansi D.C. Effect of thermal processing in Sacha Inchi kernels. J. Food Process. Preserv. 2017;41 [Google Scholar]
- Taha A.Y., Burnham W.M., Auvin S. Polyunsaturated fatty acids and epilepsy. Epilepsia. 2010;51:1348–1358. doi: 10.1111/j.1528-1167.2010.02654.x. [DOI] [PubMed] [Google Scholar]
- Takeyama E., Fukushima M. Physicochemical properties of Plukenetia volubilis L. seeds and oxidative stability of cold-pressed oil (green nut oil) Food Sci. Technol. Res. 2013;19:875–882. [Google Scholar]
- Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- Téllez-Morales J.A., Herman-Lara E., Gómez-Aldapa C.A., Rodríguez-Miranda J. Techno-functional properties of the starch-protein interaction during extrusion-cooking of a model system (corn starch and whey protein isolate) LWT (Lebensm.-Wiss. & Technol.) 2020;132 [Google Scholar]
- USDA Foreign Agricultural Service . United States Department of Agriculture; 2021. Oilseeds: World Market and Trades. [Google Scholar]
- Vanegas-Azuero A.M., Gutiérrez L.F. Physicochemical and sensory properties of yogurts containing sacha inchi (Plukenetia volubilis L.) seeds and β-glucans from Ganoderma lucidum. J. Dairy Sci. 2018;101:1020–1033. doi: 10.3168/jds.2017-13235. [DOI] [PubMed] [Google Scholar]
- Wang S. Sacha inchi (Plukenetia volubilis L.): nutritional composition, biological activity, and uses. Food Chem. 2018;265:316–328. doi: 10.1016/j.foodchem.2018.05.055. [DOI] [PubMed] [Google Scholar]
- Wuttisin N., Nararatwanchai T., Sarikaputi A. Total phenolic, flavonoid, flavonol contents and antioxidant activity of Inca peanut (Plukenetia volubilis L.) leaves extracts. Food Res. 2020;5:216–224. [Google Scholar]
- Xu Y., Nguyen D., Mohamed A., Carcel C., Li Q., Kutlubaev M.A., Anderson C.S., Hackett M.L. Frequency of a false positive diagnosis of epilepsy: a systematic review of observational studies. Seizure. 2016;41:167–174. doi: 10.1016/j.seizure.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhao W., Lai Y., Zhang B., Zhang D. Edible plant oil: global status, health issues, and perspectives. Front. Plant Sci. 2020;11:1315. doi: 10.3389/fpls.2020.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.