Abstract
Dyke-Davidoff-Masson syndrome (DDMS) was first described in 1933 as a cerebral condition of hemispheric atrophy characterized clinically by contralateral hemiparesis, facial-asymmetry, seizures, and mental retardation. Neuroimaging findings include asymmetric thickening of the calvarium and enlargement of frontal and ethmoid sinuses. There have been 21 reported cases described in the literature with the syndrome undiagnosed until adult age, likely due to less severe or absent clinical findings or symptoms as described in the case presented in this report. This article describes a case where the Dyke-Davidoff-Masson imaging features were identified as an incidental finding on a CT scan of the brain performed for non-seizure related symptoms. A 54-year-old woman presented with weakness and gait difficulty and only upon further evaluation was she found to have cranial deformities. CT and MRI demonstrate encephalomalacia in the right frontal lobe anteriorly with gliosis and moderate unilateral cerebral atrophy, and extensive hypertrophy of the right frontal calvarium, right ethmoid cells and frontal sinuses.
Keywords: Dyke-Davidoff-Masson syndrome, Neuroimaging findings, Calvarial Hypertrophy
Introduction
Dyke-Davidoff-Masson syndrome (DDMS) was first described in 1933 as a cerebral condition of hemispheric atrophy characterized clinically by contralateral hemiparesis, facial-asymmetry, seizures, and mental retardation. Neuroimaging findings include asymmetric thickening of the calvarium and enlargement of frontal and ethmoid sinuses [1]. This neurological disorder is usually diagnosed during childhood due to abundant clinical symptoms. There have been 21 reported cases described in the literature however were the syndrome was not diagnosed until adult age [2], due to less severe or absent clinical findings or symptoms as described in the case presented in this report. The age range at the time of diagnosis was from 8 to 75 years of age, with the mean at 29.6 and the median at 26 [8], [9], [10], [11], [12], [13], [14]. Nine patients were asymptomatic or had non-specific symptoms at the time of diagnosis. In one case the patient was asymptomatic and the diagnosis of Dyke-Davidoff-Masson syndrome was made post-mortem at the age of 75 years [3].
This article describes a case where the Dyke Davidoff-Masson imaging features were identified as an incidental finding on a CT scan of the brain performed for non-seizure related symptoms. A 54-year-old woman presented with weakness and gait difficulty and only upon further evaluation was she found to have cranial deformities. The incidental nature of these findings with lack of classic symptoms indicates that DDMS represents a spectrum with variable degree of clinical expression.
Case
A 54-year-old female patient presented to the ED with a 2-day history of nausea, vomiting, and diplopia. She was noted to have ptosis and medial and vertical gaze palsy of the right eye. She presented with elevated temperature and leukocytosis in the 20,000s. Her initial CT and MRI demonstrate a suprasellar mass causing mass effect on the optic chiasm. Both studies demonstrate encephalomalacia with gliosis and atrophy in the right frontal lobe anteriorly and prominence of the right frontal calvarium and adjacent paranasal sinuses. She was emergently taken to surgery for transsphenoidal suprasellar mass resection. Pathologic examination revealed a propionibacterium acne containing mucocele. During the first postsurgical day her CNIII palsy resolved suggesting that her neurologic symptoms were caused by the suprasellar mass, rather than to her right frontal lobe cerebral lesions. The patient was diagnosed with central diabetes insipidus in the postsurgical course and was discharged with medical treatment.
CT (Fig. 1) and MRI scans (Figs. 2 and 3) demonstrate incidental findings with a combination of encephalomalacia in the right frontal lobe anteriorly with gliosis and moderate unilateral cerebral atrophy, and extensive hypertrophy of the right frontal calvarium, right ethmoid cells and frontal sinuses, consistent with Dyke-Davidoff-Masson syndrome. She denied any past medical history of seizures and on physical exam had 5/5 strength bilaterally. There was no known history of intellectual or developmental delay. In summary, on neuroimaging, this patient is positive for DDMS but negative clinically.
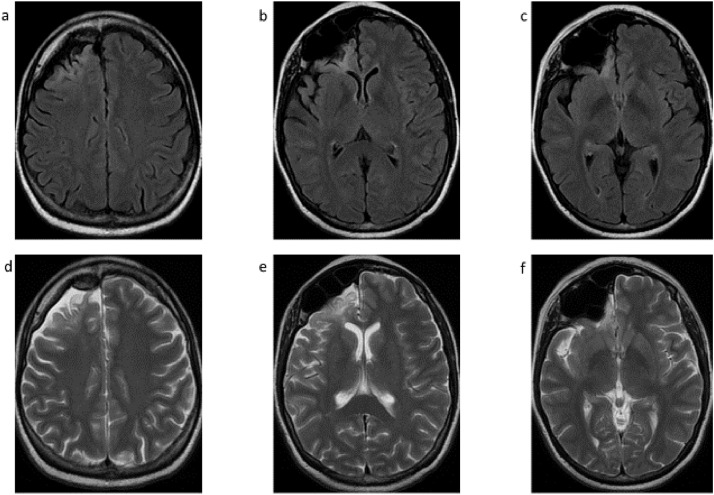
Fig. 1.
Non-contrast axial head CT soft tissue (A-C) and bone (D-F) filter images demonstrate right frontal lobe atrophy and encephalomalacia with multiple tiny cortical calcifications, and hypertrophy of the right frontal calvarium and marked asymmetric enlargement of the right paranasal sinuses.
Fig. 2.
Non-contrast axial brain MRI FLAIR (A-C) and T2-weighted (D-F) images demonstrate right frontal lobe atrophy and encephalomalacia with and gliosis at this level, hypertrophy of the right frontal calvarium, and marked asymmetric enlargement of the right paranasal sinuses.
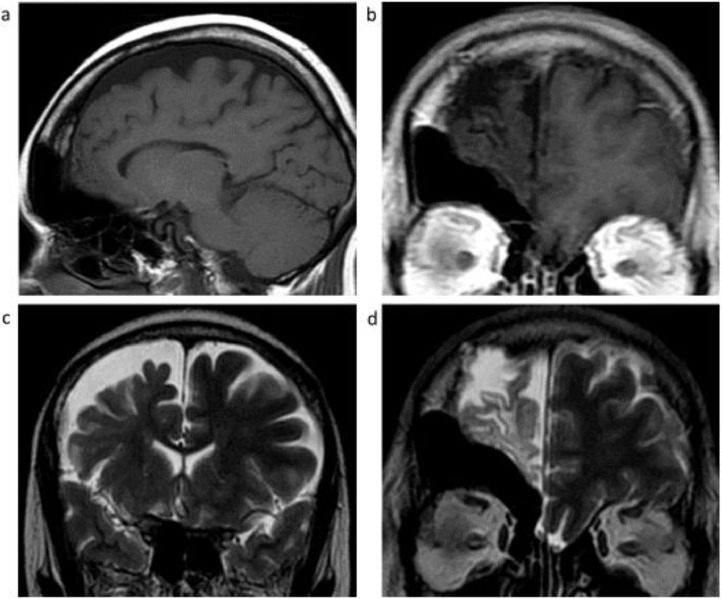
Fig. 3.
Non-contrast MRI T1 sagittal (A) and coronal (B), and coronal T2-weighted images of the right frontal lobe posteriorly (C) and anteriorly (D) demonstrate right frontal lobe atrophy and encephalomalacia with and gliosis, hypertrophy of the right frontal calvarium, and marked asymmetric enlargement of the right frontal sinus and ethmoid cells.
Discussion
DDMS is classified as congenital or acquired. The congenital type is likely due to intrauterine vascular occlusion, which typically results in an entirely hypoplastic cerebral hemisphere with consecutive hypertrophy of the adjacent calvarium and paranasal sinuses filling the vacant intracranial space [1,4]. Causes of congenital DDMS in the prenatal period are congenital malformation, infection and vascular cerebral insult. These injuries can occur silently. In a study by Atalar et al. a group of patients who were considered to have congenital DDMS, the parents did not report any problems during pregnancy [5]. Etiologies of acquired DDMS include trauma, tumor, infections, prolonged febrile seizures, ischemia and hemorrhagic states, particularly in premature infants [5]. Acquired type DDMS can include relatively mild hemi-atrophy and mild clinical symptoms.
The lack of clinical symptoms in some cases of DDMS, while demonstrating the corresponding anatomic deformities and classic imaging findings, calls into question the limits of neuroplasticity. The cases previously reported were found due to the refractory nature of their seizures or in pursuit of investigating the cause of their hemiparesis. The development phase at the time of the cerebral insult and the severity, extent and anatomical level of the cerebral damage, as well as a possible variable amount of recovery are the factors that determine the degree of severity of future imaging findings and clinical outcome, with variable compensation through neuroplasticity. Tatlidede et al. noted that children with cerebral hemi-atrophy due to DDMS with severe symptoms were able to lateralize dorsal visual pathways responsible for spatial processing to the right hemispheres [6]. The extent of functional reorganization achievable by the plastic capacity of the brain requires further investigation. However, a minor insult in conjunction with sufficient amount of neurotoxicity could conceivably result in clinically inconspicuous cases with no loss of function identified as noted in this case.
The 3 primary clinical features of DDMS are unilateral weakness, seizures, and mental retardation [1]. The most common symptoms found in the 21 reported adult cases of DDMS was unilateral weakness and either seizures (76.1%) or mental retardation (61.1%) [2]. Our case lacks this clinical picture but does fulfill the anatomic criteria as demonstrated on neuroimaging. This apparent disconnect between imaging and clinical findings with the lack of neurological symptoms argues for the grouping of cerebral hemi-atrophy, calvarial thickening, and dilation of frontal and ethmoid sinuses to be defined as asymptomatic variant of DDMS as opposed to cases of DDMS with variable degree of expression of clinical symptoms depending on the severity and impact of the initial cerebral insult, the patient's age at the time of the incident and potential recovery of function through neuroplasticity.
An incidentally found case as described in this article is suggestive of the actual prevalence of DDMS being much higher than currently reported in the literature. Conceivably, the previously reported symptomatic cases were caused by more severe insults or during more vulnerable development stages resulting in more extensive cerebral damage that was beyond the limits of compensation of neuroplasticity as often seen in patients after strokes [7]. In milder or asymptomatic cases, the initial cerebral damage and hemi-atrophy are potentially less severe and cerebral neuroplasticity might result in complete functional compensation.
Patient consent
Informed consent for publication has been obtained. We are using entirely anonymized images from pathology slides, CT scans, and MRI. These do not contain any identifying marks and are not accompanied by text that might identify the individual concerned.
Footnotes
Competing Interests: None.
References
- 1.Sharma S, Goyal D, Negi A, Sood RG, Jhobta A, Surya M. Dyke-Davidoff-Masson syndrome. Indian J Radiol Imaging. 2006;16:165–166. [Google Scholar]
- 2.Diestro JDB, Dorotan MKC, Camacho AC, Perez-Gosiengfiao KT, Cabral-Lim LT. Clinical spectrum of Dyke-Davidoff-Masson syndrome in the adult: an atypical presentation and review of literature. Case Rep. 2018;2018 doi: 10.1136/bcr-2018-224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoevesandt D, Stock K, Spielmann RP, Heine HJ, Paulsen F, Bräuer L. Postmortal diagnosis of a Dyke-Davidoff-Masson syndrome in a 75-year-old woman: a case report. Ann Anat. 2009;191:225–227. doi: 10.1016/j.aanat.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Kumar NV, Gugapriya TS, Guru AT, Kumari SN. Dyke-Davidoff-Masson syndrome. Int J Appl Basic Med Res. 2016;6:57–59. doi: 10.4103/2229-516X.174016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atalar MH, Icagasioglu D, Tas F. Cerebral hemiatrophy (Dyke-Davidoff-Masson syndrome) in childhood: clinicoradiological analysis of 19 cases. Pediatr Int. 2007;49(1):70–75. doi: 10.1111/j.1442-200X.2007.02299.x. [DOI] [PubMed] [Google Scholar]
- 6.Demirtas-Tatlidede A, Yalcin AD, Uysal E, Forta H. Right cerebral hemiatrophy: neurocognitive and electroclinical features. Epilepsy Behav. 2010;17(4):536–540. doi: 10.1016/j.yebeh.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Dimyan M, Cohen L. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7(2):76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biçici V, Ekiz T, Bingol I, Hatipoglu C. Dyke-Davidoff-Masson syndrome in adulthood. Neurology. 2014;83:1121. doi: 10.1212/WNL.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R, Joshi S, Mittal A, Luthra I, Mittal P, Verma V. Magnetic resonance imaging depiction of acquired Dyke-Davidoff-Masson syndrome with crossed cerebro-cerebellar diaschisis: report of two cases. J Pediatr Neurosci. 2015;10(3):294–296. doi: 10.4103/1817-1745.165730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Kim E, Kim W, Kim Y, Mo E, Moon S, et al. A case of Dyke-Davidoff-Masson syndrome associated with central hypothyroidism and secondary adrenal insufficiency. Hormones. 2013;12:461–465. doi: 10.1007/BF03401312. [DOI] [PubMed] [Google Scholar]
- 11.Park S, Lee M, Kim J, Kim S, Shin J, Shin Y, et al. A case of Dyke-Davidoff-Masson syndrome associated with hypopituitarism and diabetes mellitus. Korean J Med. 2010;79(3):316–320. [Google Scholar]
- 12.Aguiar PH, Liu CW, Leitao H, Issa F, Lepski G, Figueiredo EG, et al. MR and CT imaging in the Dyke-Davidoff-Masson syndrome. Report of three cases and contribution to pathogenesis and differential diagnosis. Arq Neuropsiquiatr. 1998;56:803–807. doi: 10.1590/s0004-282x1998000500016. [DOI] [PubMed] [Google Scholar]
- 13.Romeo AD, Pego RR, Branas FF, Martínez VF, Cortes LJA. Contralateral cerebellar atrophy in the Dyke-Davidoff-Masson syndrome. Neurologia. 1999;14:320–321. [PubMed] [Google Scholar]
- 14.Tasdemir HA, Incesu L, Yazicioglu AK, Belet U, Gungor L. Dyke-Davidoff-Masson syndrome. Clin Imaging. 2002;26:13–17. doi: 10.1016/s0899-7071(01)00318-7. [DOI] [PubMed] [Google Scholar]