Abstract
Spices are an affluentpool of polyphenolcompounds that possessgigantic medicinal peculiarities such as remedying microbial infections, oxidative stress, inflammation, diabetes, cancers, neurodegenerative disorders, cardiac disorders, etc. On that account, thepresent review illustrates the therapeutic potential, mechanism of action, and different procedures for conscious extraction of polyphenols. The various ethnopharmacological properties; reasons for their diverse pharmacological actions and the mechanism of action of spices-derived phenolics have also been discussed. The findings of this review may be utilized by the food and pharmaceutical industries for developing suitable alternatives to synthetic antioxidants and can be developed into effective food supplements. Further in-depth scientific studies are needed to find out their actual and exact relevance as natural health boosters. Moreover, clinical and toxicological studies are also required for harnessing the full therapeutic potential of polyphenols derived from dietary spices.
Keywords: Spices, Secondary metabolites, Polyphenols, Pharmacology, Oxidative stress, Inflammation
Graphical abstract
Highlights
-
•
Spices are the treasure house of polyphenols and other useful bioactive compounds.
-
•
Clove, oregano, thyme and rosemary contains highest amount of phenolic compounds.
-
•
They provide protection from microbial infection and oxidative stress related disorders.
-
•
Polyphenols obtained from spices improve the functioning of beneficial gut microbiota.
The chemical compounds studied in this article.
Eugenol (Pubchem CID: 3314); Curcumin (Pubchem CID: 839564); Kaempferol (Pubchem CID: 4444395); Quercetin (Pubchem CID: 4444051); Apigenin (Pubchem CID: 4444100); Thymol (Pubchem CID: 21105998); Gallic acid (Pubchem CID: 361); D-(+)-Catechin (Pubchem CID: 8711); Caffeic acid (Pubchem CID: 2423); Rosmarinic acid (Pubchem CID: 4445104).
1. Introduction
The present generation is facing the twin challenges of a stressful lifestyle and an unbalanced diet (de Araújo et al., 2020). These factors are badly impacting human health and leading to many life-threatening metabolic disorders and epigenetic modifications associated with deaths at an alarming rate (Gupta et al., 2020). Thus, consumers nowadays are continuously seeking food items that meet nutritional requirements and that have positive impacts on human health, mental well-being, and decreased disease occurrence (Oliviero et al., 2018). Scientific and technological advancements have led to many novel innovations both in the field of food and medications. Lutz et al. (2019) reported that 30% of demise can be avoided through dietary modification, particularly enriched with plant-based biomass. Biomass represents a good source of food, timber, and several valuable chemicals (Samfira et al., 2013). Such plant-based diets present an excellent source of bioactive entities such as polyphenols, alkaloids, etc., which contribute a lot to human health at every stage of life (Fraga et al., 2019; Singh et al., 2022). Plenty of plant-based food products with a high amount of phenolic compounds are already in use in different parts of the globe. Over 500 diverse polyphenols have been recognized in dietary products. Coffee, tea, wine (particularly red wine), cocoa, fruits, vegetables, spices, and culinary herbs are considered excellent sources of phenolic compounds having a positive effect on human health (Issaoui et al., 2020).
Spices and culinary herbs are considered one of the richest reservoirs of polyphenols (Yashin et al., 2017; Kaundal et al., 2022). Spices are dried plant parts, specifically used to improve culinary delicacies such asthe taste, flavor, color, and aroma of food items (Vázquez-Fresno et al., 2019; Singh et al., 2021). The essential oil obtained from spices are also a complex mixture of different chemical constituents such as aliphatic and aromatic hydrocarbons, aldehydes, ketones, esters, and other secondary metabolites such as terpenoids, polyphenols, etc. (Samfira et al., 2015). Due to the advancement in plant biochemistry research, the investigation and the innovation of more and more chemical constituents from plants have become more feasible. Moreover, the bioprocesses involved during the synthesis of these phytoconstituents that take place at the inner molecular level can also be explored (Bostan et al., 2013). These chemical constituents can be used for maintaining good health, treating routine maladies (antiseptic, antimicrobial, anti-inflammatory, decongestants), and boosting immunity (Butnariu andBostan, 2011; Samfira et al., 2015; Ogbunugafor et al., 2017; Rani et al., 2022).
Polyphenolsare supposed to be associated with the tactile sensation of astringency and add value to the flavor, color, and aroma of food items. These compounds are one of the major secondary metabolites consisting of a diverse pool of biologically active compounds (Rajendran et al., 2021). Recently, these compounds have engrossed much attention in diet-based therapy due to their cost-effectiveness, easy access, acceptability, long administration, and omnipresence properties (Imran et al., 2021; Usman et al., 2022). Being a rich reservoir of antioxidant compounds, these compounds impart an excellent positive effect on overall health and well-being (Lucci et al., 2017; Ali et al., 2021). The antioxidant property of polyphenols is mainly attributed to the presence of hydroxyl groups (Piccolella et al., 2019). Besides, they are well-established natural therapeutic agents used to combat cancer, cardiovascular, neurodegenerative diseases, nephrotoxicity, and numerous other chronic or non-communicable diseases (Mark et al., 2019; Piccolella et al., 2019). Pharmacological effects viz. Antimicrobial, antioxidant, antidepressant, anti-inflammatory, anticancer, antilipidemic, and so on, rendered by polyphenols are also well documented in the literature (Kumar and Goel, 2019; Wang et al., 2020; Usman et al., 2022). These compounds also possess the ability to downregulate the angiogenic growth factors, thus altering the signalings involved in tumor progression and ultimately suppressing tumorigenesis (Rani et al., 2022). Owing to the enormous therapeutic efficacy of phenolics (derived from spices), the present study is planned to club the up-to-date information on their different pharmacological aspects such as microbial infection, oxidative stress, inflammation, diabetes, cancer, and cardiovascular disorders. Classification of natural polyphenols along with their different extraction procedure is also discussed. The possible mechanism of action is elicited in figures to ease the understanding of signaling pathways. Ultimately, the study provides solid shreds of evidence on the various pharmacological attributes of polyphenols and useful baseline for researchers and academia. Hence, the article would be a great contribution to the discipline of Food Science and chemistry.
2. Methodology
The major publication databases viz. Web of Science, Scopus, Google Scholar, PubMed, and Science Direct were searched for critical scrutinizing of the literature. Polyphenols, phenolics derived from spices, health benefits of polyphenols, dietary polyphenols, etc. were used as the keywords. The bibliographies of the primary publications were also used as a source to find additional relevant literature. The articles were selected by adopting comprehensive inclusion and exclusion criteria. The scientific studies dealing with the polyphenols, polyphenols extracted particularly from spices, extraction of polyphenols, health benefits of polyphenols, etc., were selected for the present review while articles focused on some other perspectives like phenolic compounds extracted from other than spices such as beverages and other plant sources, their role in meat preservation and dairy products, phenolic nano-encapsulation, the role of tissue culture in phenolic production, stress and temperature effect on phenolic composition, etc. were excluded.
3. Phenolic compounds
Phenolic compounds, characterized by the presence of one or more phenolic hydroxyl groups, are the most abundant heterogeneous group of secondary metabolites of plants (Basli et al., 2017; Hussain et al., 2019). These compounds can be found in free form or bound form. Free phenolics (FPs) do not interact with other molecules and are soluble in the polar aqueous/organic solvents and are easy to isolate. Bound phenolics (BPs) remain entrapped with other macromolecules via covalent bonds. BPs possesses strong therapeutic potential such as antioxidant, anti-inflammation, anticancer, antiobesity, antidiabetic, anti-mutagenic, probiotic as well as exerts good health effect on central nervous system disorders (Kumar and Goel, 2019; Takó et al., 2020). The therapeutic potential of BPs is greater than FPs. In food matrixes, BPs occur comparatively in higher proportion as compared to FPs (Wang et al., 2020). In the food industry, these can be utilized as food preservatives, food dyes, bioactive packaging, hydrogels, and nanocomplexes. BPs can alter the physicochemical properties of starch in a positive direction. Despite their great applications in the food industry, these compounds can also be used for the development of cosmetic products, fertilizers, surfactants, textiles, paints, rubber, plastics and curing agents, etc. (de Araújo et al., 2020). They also act as good metal chelators and directly act upon the inhibition of Fe3+ reduction to reduce the formation of reactive OH• during the Fenton reaction (Zhang and Tsao, 2016).
4. Classification of polyphenols
Over 8000 phenolic compounds are synthesized via the pentose phosphate, shikimate phenylpropanoid pathway (de Araújo et al., 2020; Rajendran et al., 2021). These compounds can be classified into different groups such as phenolic acids, flavonoids, stilbenes, and lignans (Lucci et al., 2017). Flavonoids (flavonols, flavonones, flavanols, flavones, isoflavonoids, anthocyanins, and isoflavons) represent the largest group of phenolic compounds, with more than 8000compounds (Mark et al., 2019). The different classes of polyphenol compounds along with their structures are presented in Table 1.
Table 1.
Classification of phenolic compounds.
| S. No. | Main class | Sub-class | Name of the compound | Structure of the compound |
|---|---|---|---|---|
| 1. | Phenolic acids | Hydobezoates | Gallic acid (Pubchem CID: 361) | 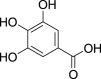 |
| Ellagic acid (Pubchem CID: 4445149) | 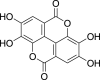 |
|||
| Hydroxycinnamates | p-Coumaric acid (Pubchem CID: 553148) | 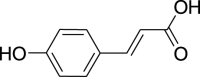 |
||
| Caffeic acid (Pubchem CID: 2423) | 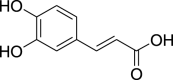 |
|||
| Ferulic acid (Pubchem CID: 689) | 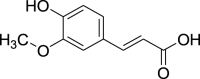 |
|||
| 2. | Tannins | Condensed tannins | Catechin (Pubchem CID: 8711) | 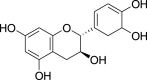 |
| Epicatechin (Pubchem CID: 65230) | 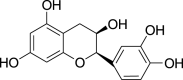 |
|||
| Hydrolyzable tannins | Ellagitannins (Pubchem CID: NR) | 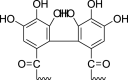 |
||
| Gallotannins (Pubchem CID: 398744) | 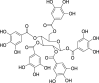 |
|||
| 3. | Lignans | Dibenzylbutanediol lignans | Secoisolariciresinol (Pubchem CID: 58845) | 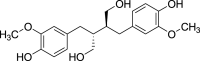 |
| Dibenzylbutyrolactone lignans | Matairesinol (Pubchem CID: 106491) | 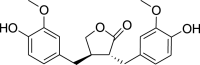 |
||
| Furanoid lignans | Medioresinol (Pubchem CID: 158029) | 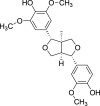 |
||
| 4. | Flavonoids | Flavones | Apigenin (Pubchem CID: 4444100) | 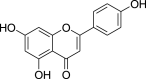 |
| Baicalin (Pubchem CID: 58507) | 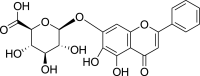 |
|||
| Chrysin (Pubchem CID: 4444926) | 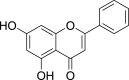 |
|||
| Luteolin (Pubchem CID: 4444102) | 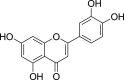 |
|||
| Isoflavones | Genistin (Pubchem CID: 4444736) | 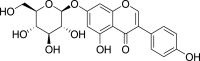 |
||
| Daidzein (Pubchem CID: 4445025) | 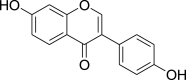 |
|||
| Glycitein (Pubchem CID: 4476508) | 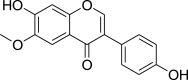 |
|||
| Flavonols | Quercetin (Pubchem CID: 4444051) | 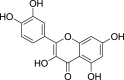 |
||
| Kaempferol (Pubchem CID: 4444395) | 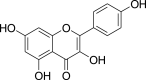 |
|||
| Myricetin (Pubchem CID: 4444991) | 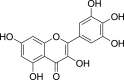 |
|||
| Flavan-3-ols | D-(+)-Catechin (Pubchem CID: 8711) | 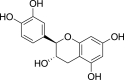 |
||
| (−)-Epicatechin (Pubchem CID: 65230) | 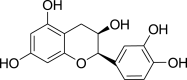 |
|||
| (−)-Epigallocatechin gallate (Pubchem CID: 65231) | 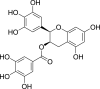 |
|||
| Flavanones | Naringenin (Pubchem CID: 388383) | 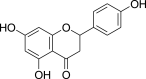 |
||
| Hesperetin (Pubchem CID: 65234) | 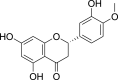 |
|||
| Eriodictyol (Pubchem CID:389606) | 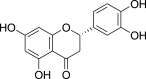 |
|||
| Anthocyanidins | Cyanidin (Pubchem CID: 114193) | 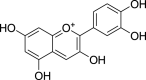 |
||
| Delphinidin (Pubchem CID: 114185) |  |
|||
| 5. | Stilbens | Phytoalexin | Resveratrol (Pubchem CID: 392875) |  |
| Catechols/resorcinols | Piceatannol (Pubchem CID: 581006) |  |
||
| Stilbene glycosides | Astringin (Pubchem CID: 4445028) |  |
||
| 6. | Coumarins | 7-hydroxycoumarins | Umbelliferone (Pubchem CID: 4444774) | 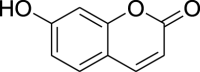 |
| 6,7-dihydroxycoumarins | Esculietin (Pubchem CID: NR) | 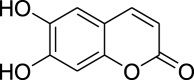 |
||
| 7. | Curcuminoids | Diarylheptanoids | Curcumin (Pubchem CID: 839564) | 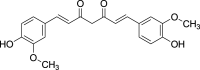 |
| Demethoxycurcumin (Pubchem CID: 4579941) | ||||
| Bisdemethoxycurcumin (Pubchem CID: 4474770) | 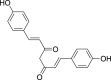 |
NR* = Not reported.
5. Extraction of phenolic compounds
Free phenolics (FP's) are not bounded to other biological molecules and are soluble. Due to their simple nature, the extraction of FP's is quite easy and can be extracted efficiently with aqueous/organic solvents but due to their insoluble and bounded nature, the same procedure of extraction is not applicable in the case of BPs. Therefore, some chemical, physical and biological treatments have been developed to facilitate the efficient and precise extraction of BPs, as discussed as follows:
5.1. Chemical methods
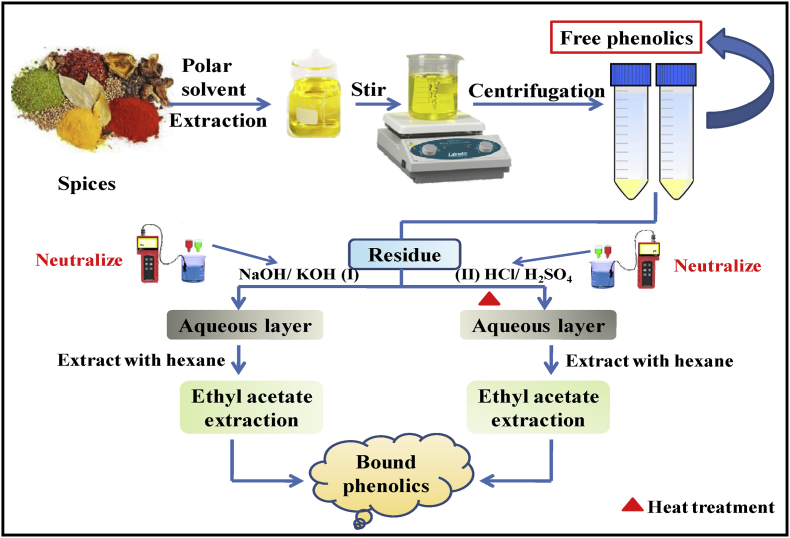
The chemical method involves the use of chemicals for the efficient extraction of phenolic compounds. The method is further divided into two different parts based on chemicals used for the extraction of phenolic compounds i.e., alkali method of extraction and acidic method of extraction as depicted in Fig. 1.
Fig. 1.
Alkali and acidic method of phenolics extraction.
5.1.1. Alkali method of extraction
The alkali method involves the use of alkalis such as sodium hydroxide (NaOH) for the extraction procedure. The alkali treatment leads to the cleavage of the ester bond and consequently releases the polyphenols entrapped into the cell wall components. For instance, the bound phenolics such as caffeic, chlorogenic acids, ferulic, vanillic, p-coumaric, syringic etc. can be extracted by this method. In this method, though NaOH is primarily used for the extraction procedures but KOH is also used for the decomposition of cell-wall structure. The alkali method is somewhat complicated due to its requirement of the pretreatment process. The method also requires ascorbic acid and ethylenedinitrilotetraacetic acid (EDTA) to prevent BPs' oxidation. In addition to this, hydrochloric acid (HCl) is also required to adjust the pH because, in a strong alkali environment, phenolic hydroxyl groups become susceptible to the deprotonation while in strongly acidic conditions, quinone formation is inhibited. Moreover, in this method, a large proportion of protein becomes insoluble and washed out as protein precipitation occurs in strongly basic conditions. Such limitations of this method restrict the frequent use of this method (Wang et al., 2020). It is also recommended that this method should be conducted in an inert gas atmosphere under darkness.
5.1.2. Acidic method of extraction
This is another widely used chemical method for the extraction of phenolics compounds. Though this method usually employs strong acids like hydrochloric acid (HCl) and sulfuric acid (H2SO4) some weak acids like oxalic acids can also be utilized for the extraction of some bound phenolics viz. ferulic acid. Acid hydrolysis helps in the breakage of ester and glycosidic bonds present between polyphenols and the cell wall components. Though this acidic method is considered more reliable and efficient as compared to the alkali method but there are also some disadvantages of this method such as the requirement of elevated temperature which can denature some phenolic compounds at low pH. Sometimes, the chemical treatment also led to the morphological alteration of phenolics compounds (Wang et al., 2020).
5.2. Biological methods
This method is based upon the use of biological entities such as enzymes for the extraction procedure (Panja, 2018). Carbohydrate-hydrolyzing enzymes such as pectinase, amylase, xylanase, cellulose can be recruited for the extraction of BPs. For example, the ferulic acid esterase enzyme is used to disrupt the interaction between ferulic acid and oligosaccharides components of the cell walls. The enzymatic method is comparatively more specific, effective and reliable than chemical methods. The biological method also overcomes the limitations of chemical methods (Wang et al., 2020).
5.3. Physical methods
This method of extraction is highly cost-effective as compared to other extraction procedures. It applies elevated heat treatment, microwave, ultrasound and ultrahigh-pressure for the breakage of covalent, hydrogen and hydrophobic interactions between the phenolics and macromolecules which resultantly release the phenolics from the microstructural forces. This method is highly used at the industrial level (Panja, 2018).
6. Spices as the source of phenolics compounds
Everyone seeks tasty foods that can elevate cognition and mood, with many health benefits viz. Antimicrobial, antioxidant, anti-inflammatory, anticarcinogenic, antidiabetic and so forth. Spices contribute a lot to this imprint and are specifically selected according to sensorial aspects (Jiang, 2019; Vázquez-Fresno et al., 2019). Cloves, dried peppermint, chili, star anise, dried oregano, turmeric, celery seeds, common sage, rosemary, spearmint, dried and fresh thyme, capers, sweet basil, curry, ginger, lemon verbena, cumin, cinnamon, caraway, parsley and marjoram are most common seasoning ingredients used to improve the food taste and visualization. These spices are also the rich reservoir of many biologically active compounds such as phenolic compounds. These compounds attributes enormous therapeutic benefits to spices (Saraswathi et al., 2020). For instance; Clove is positioned at the top and contains about 15 188 mg/100 g as total phenols, attributed to their strong antioxidant property. Oregano is rich in flavonoids and is potentially used to cure respiratory, inflammatory, digestive disorders, headaches, rheumatism, diabetes, oxidative stress and cancer (Issaoui et al., 2020). Oregano, thyme and rosemary respectively contain the highest amount of polyphenols i.e., 23.8, 24.2 and 23.4 mg GAE g-1 dry matter (Slimestad et al., 2020). Parsley, coriander, dill and thyme were observed with the richest level of flavonoids. Ghafoor et al. (2020) studied the total phenolic content, antioxidant capacity and total carotenoids of ginger rhizomes dried via different techniquesviz. oven, microwave, freeze and room-air drying. The freeze-dried gingerwas foundwith comparatively higher total phenolics, antioxidant capacity and total carotenoids i.e., 931.94 mgGAE/100 g, 82.00%, 13.17 μg/g, respectively as compared to those dried using other methods. It was also noticed that (+)-catechin, gallic acid and 3, 4-dihydroxybenzoic acid were the principal phenolic compounds of dried ginger and occur in a proportion of 250.02 mg/100 g, 197.03 mg/100 g and 116.07 mg/100 g, respectively. The total phenolic content was positively correlated with total antioxidant activity (r2 = 0.973, p < 0.001). Specific phenolic compounds and their associated pharmacological effects of some spices are presented in Table 2.
Table 2.
Summary of some spice derived phenolic compounds and pharmacological effects.
| S. No. | Botanical name | Common name | Name of the compound | Structure of the compound | Pharmacological effects | Ref. | |
|---|---|---|---|---|---|---|---|
| 1. |
Syzygiumaromaticum (L.) Merr.& Perry |
Clove | Eugenol (Pubchem CID: 3314) | 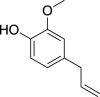 |
|
Issaoui et al. (2020); Saraswathi et al. (2020) | |
| 2. | Coriandrum sativum L. | Coriander | Stigmasterol (Pubchem CID: 4444352) | 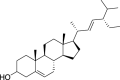 |
|
Ge et al. (2017); Bi et al., 2017; Issaoui et al. (2020) | |
| Sitosterol (Pubchem CID: 192962) | 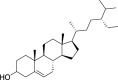 |
||||||
| 3. | Crocus sativus L. | Saffron | Safranal (Pubchem CID: 55000) | 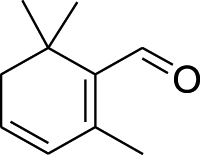 |
|
Issaoui et al. (2020) | |
| 4. | Thymus vulgaris L. | Thyme | Rosmarinic acid (Pubchem CID: 4445104) | 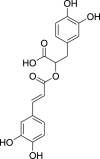 |
|
Issaoui et al. (2020) | |
| Caffeic acid (Pubchem CID: 2423) | 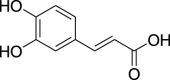 |
||||||
| 5. | Origanum vulgare L. | Oregano | Narigenin (Pubchem CID: NR) | 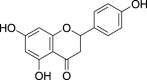 |
|
Issaoui et al. (2020) | |
| Pinocembrin (Pubchem CID: 580917) | 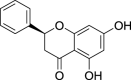 |
||||||
| 6. | Rosmarinus officinalis L. | Rosemary | Rosmarinic acid (Pubchem CID: 4445104) | 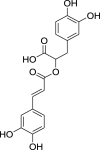 |
|
Elansary et al. (2020); Issaoui et al. (2020) | |
| 7. | Mentha piperita L. | Mint | |||||
| 8. | Zingiber officinale Rosc. | Ginger | (+)-Catechin (Pubchem CID: 8711) | 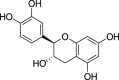 |
|
Yashin et al. (2017); Ghafoor et al. (2020); Takó et al. (2020) | |
| Gallic acid (Pubchem CID: 361) | 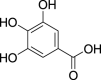 |
||||||
| Gingerol (Pubchem CID: 391126) | 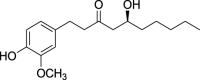 |
||||||
| 9. | Cinnamomum verum J. Presl | Cinnamon | Hydroxycinnamic acids (Pubchem CID: NR) | 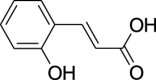 |
|
Rashid et al. (2020); Singh et al., (2020); Takó et al., (2020) | |
| 10. | Curcuma longa Linn. | Turmeric | Curcumin (Pubchem CID: 839564) | 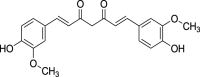 |
|
Oliviero et al. (2018); Ahangarpour et al. (2019); Mughal (2019) | |
| 11. | Piper nigrum L. | Black pepper | p-Hydroxybenzoic acid (Pubchem CID: 132) | 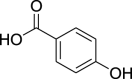 |
|
Feng et al. (2020) | |
| 12. | Nigella sativa L. | Black cumin | Thymol (Pubchem CID: 21105998) | 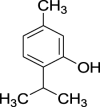 |
|
Chahal et al. (2017); Mughal (2019); Feng et al. (2020) | |
| 13. | Trachyspermumammi L. | Ajwain | |||||
| 14. | Amomum subulatum | Black cardamom | Diosmin (Pubchem CID: 4444932) | 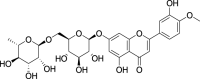 |
|
Feng et al. (2020) | |
| 15. | Petroselinum crispumMill. | Parsley | Apigenin (Pubchem CID: 4444100) | 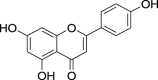 |
|
Yashin et al. (2017) | |
| 16. | Apium graveolens L. | Celery | Luteolin (Pubchem CID: 4444102) | 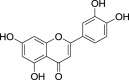 |
|
Yashin et al. (2017) | |
| 17. | Salvia officinalis L. | Sage | Carnosol (Pubchem CID: 390568) | 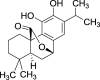 |
|
Oliviero et al. (2018) | |
| 18. | Anethum graveolens L. | Dill | Quercetin (PubchemCID: 4444051) | 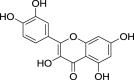 |
|
Yashin et al. (2017) | |
| 19. | Foeniculum vulgare Mill. | Fennel | |||||
| 20. | Allium sativum L. | Garlic | |||||
| 21. | Brassica junceaL. Czern | Mustard | Kaempferol (Pubchem CID: 4444395) | 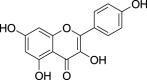 |
|
Yashin et al. (2017) | |
| 22. | Trigonella foenum-graecum L. | Fenugreek | Rhaponticin (Pubchem CID: 552853) | 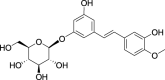 |
|
Li et al. (2018) | |
| 23. | Pimpinella anisum L. | Aniseed | Chlorogenic acid (Pubchem CID: 1405788) | 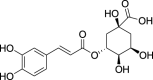 |
|
BettaiebRebey et al. (2018) | |
| 24. | Carum carvi L. | Caraway | Carvacrol (Pubchem CID: 21105867) | 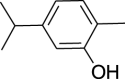 |
|
Sachan et al. (2016) | |
NR* = Not reported.
From Table 2, it can be inferred that a single phytocompound can exhibit more than one pharmacological effect, therefore, a single phytocompound or spice can be employed for treating several ailments. Moreover, the phytocompound which is still unexplored or least explored, can be further explored against several diseases.
7. Health benefits of phenolics
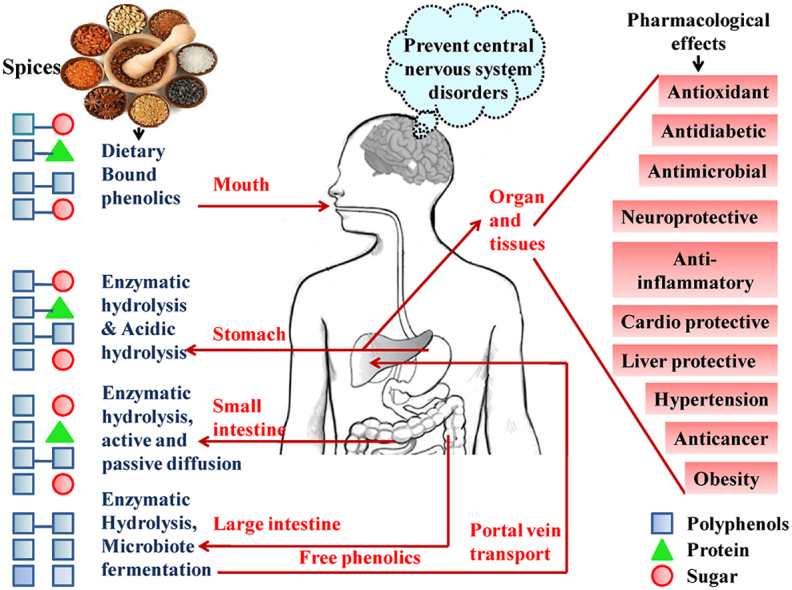
Spices and herbs are the treasure house of useful bioactive compounds. Many such compounds possess significant antimicrobial, antioxidant, anti-inflammatory, antidiabetic, anticancer properties (Guldiken et al., 2018). Phenolics compounds derived from spices are considered as natural therapeutic agents. The BPs ingested by humans is not directly absorbed by the body tissues. Rather, reach the intestine via the stomach and further survive to the colon in their intact form, followed by their enzymatic digestion and colonic fermentation, in order to make them simple to use (Wang et al., 2020). The released simple form of BPs circulated and effortlessly absorbed by body tissues and eventually perform their multiple therapeutic effects like combating oxidative stress, microbial infection, hypertension, inflammation, obesity, hyperglycemic, cardiovascular disorder, cancer, neurodegeneration, Parkinson's, Alzheimer's and many more (Basliet al., 2017; Hussain et al., 2019). Slimestad et al. (2020) reported that the polyphenolic mechanisms of actions are associated with the proper management of oxidative stress. In another study, Kumar et al. (2019) reported the role of dietary polyphenols in improving the composition and functioning of beneficial gut microbiota while antimicrobial action against pathogenic gut microbiota, help in combating a broad range of health disorders. Some of the phenolics mediated pharmacological effects are discussed as follows:
7.1. Antimicrobial activity
The continuous use of antibiotics consequences the antibiotic-resistant pathogen as well as their resistant genes (Gaşpar et al., 2021). The microbial pathogen widespread their infection via quorum sensing. Quorum sensing is a density-dependent pathway of pathogenic cell-to-cell communication which eases the way of biofilm synthesis, antibiotic resistance, expression of virulence gene, sporulation, conjugation, bioluminescence, toxin production, bacterial cell survival, and proliferation. These events lead to serious contamination of food items. Thus, there is an urgent need for some biological control to suppress these signaling pathways, to avoid food deterioration and poisoning, thus, necessitates the innovation of novel antibiotics. Recently, phenolic compounds from spices have achieved much attention in the food industry due to their natural potential of growth inhibition of food deteriorating pathogens (Chan et al., 2018; Vetrani et al., 2020). These compounds possess significant anti-quorum sensingpotential. In this regard, these entities can well suppress the superficial adhesion, biofilm synthesis, bacterial motility, toxic release from food pathogens and finally induce their microbicide effect. Kumar et al. (2019) also reported the antiquorum activity of dietary polyphenols. Different polyphenols exhibit a different mechanism of action; however, the flavonoid catechin was reported to significantly invade the bacterial cell lipid bilayer, resulting in the release of intramembranous cellular contents outside the cell followed by subsequently cell death. Furthermore, the synergistic effects with the antibioticswas also noticed, associated with enhanced effectiveness and reduced dose uptake. Thus, polyphenols suit well for the development of natural food additives and preservatives (Takó et al., 2020). Zhang et al. (2019) studied the antibacterial activity of 67 spices ethanolic extract against foodborne antibiotic-resistant bacterial strains i.e.,Staphylococcus aureus and Salmonella enteritidis through agar well diffusion assay. Out of 67 different spices extracts, 38 extracts were effective against S. aureus while only four extracts were effective against S. enteritidis. It was reported that thetotal phenolic content of spices might be responsiblefor the antimicrobial activity of spices. Chan et al. (2018) evaluated the antimicrobial activity of some dietary spices viz. Cinnamomum burmannii (Ness) Blume, Origanum vulgare L. and Syzygiumaromaticum (L.) Merr. & L. M. Perry, Cinnamomum cassiaPresl. It was suggested that the polyphenolic content of spices is primarily responsible for the spices' antimicrobial efficacy. The spices showed good antimicrobial action against all the studied microbial strains (Bacillus cereus (QAP D15), Escherichia coli (ATCC 25922), Salmonella enterica subsp. enterica serovar typhimurium (ATCC 14028; S. typhimurium), Shigella flexneri (QC 5820) and Staphylococcus aureus (ATCC 25923) except five lactic acid bacteria (LAB) including Lactobacillus acidophilus (CSCC 2400), Lactobacillus delbrueckii subsp. bulgaricus (ASCC 859; L. bulgaricus), Lactobacillus casei (ASCC 290), Lactobacillus plantarum (WCFS1) and Lactobacillus rhamnosus (ATCC 53103). The antimicrobial effect of polyphenols was insignificant on LAB. Therefore, spices can be used for improving the survival of probiotic bacterial diversity having positive health effects.
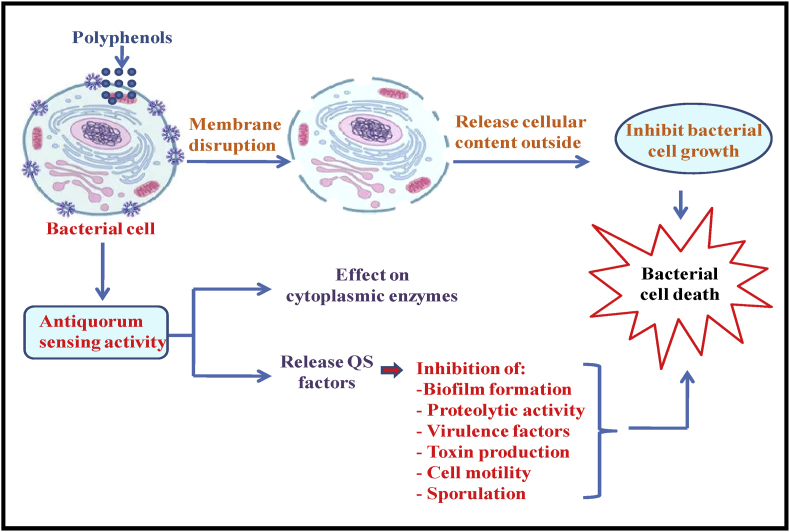
7.1.1. Mechanism of antimicrobial activity
Polyphenols can invade the pathogenic cell and exert their antimicrobial effects (Takó et al., 2020). Chan et al. (2018) described the various events such as penetration to the bacterial cell, inhibition of biofilm synthesis, the release of cellular contents outside the cell and resultantly cell mortality, as triggered by phenolic compounds. However, the decreased cell mobility was also reported. Apart from this, polyphenolic compounds were reported with pronounced anti-quorum sensing properties (Kumar et al., 2019). Hence, it can interfere that polyphenols possess significant antimicrobial activity and can be used for the preservation of the food products. Based on the above data, the antimicrobial mechanism of action of polyphenols is depicted in Fig. 2.
Fig. 2.
Mechanism of antimicrobial activity of polyphenols.
7.2. Antioxidant activity
Oxidative stress is the major cause of a variety of chronic diseases and cellular aging (Martins et al., 2016). Antioxidants are the compounds used for neutralizing the adverse effects of free radicals and offsetting resulted in oxidative stress (Yashin et al., 2017). Although, biological systems also possess the endogenous antioxidants to neutralize the negative effect of free radicals but sometimes they are insufficient to counteract these free radicals, hence urges the need of exogenous antioxidants. Because of the adverse effects associated with the use of synthetic antioxidants, the use of natural antioxidants found in the food products is increasing rapidly (Sokamte et al., 2019). Being a rich source of phenolics and polyphenols, spices possess natural radical scavenging activity (RSA) (Yashin et al., 2017; Hussain et al., 2019). Škrovánková et al. (2017) also demonstrated that polyphenols of spices exhibit significant antioxidant activity. The polyphenolic content and antioxidant activity of aqueous and ethanolic extract of Capsicum annuum (paprika) and Piper nigrum (pepper) was assessed via Folin-Ciocaulteu reagent and 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, respectively. In the case of paprika, the total phenol content was higher than pepper and ranged from 14.67 to 28.78 mg GAE. g-1, while it ranged from 12.03 to 22.88 mg GAE. g-1, in case of pepper. The total antioxidant activity of paprika was higher in ethanolic extract (8.73–16.17 mg AAE. g-1) than aqueous extract (4.45–16.24 mg AAE. g-1). In the case of pepper, the total antioxidant activity was 7.07–15.81 mg AAE. g-1 and 8.25–15.93 mg AAE. g-1 for aqueous and ethanol extracts, respectively. Piccolella et al. (2019) also opined that polyphenols help in promoting human health via scavenging the reactive oxygen species (ROS). In another study by Ali et al. (2021), 12 commonly used spices (clove, cinnamon, cumin, black cumin, cardamom, black cardamom, allspices, turmeric, fennel, nutmeg, black pepper, star-anise) were examined for their total phenolic content and antioxidant efficacy. Results revealed clove and allspice with highest total phenolic content. The antioxidant assay showed clove with highest antioxidant capacity; therefore, it was postulated that there is positive correlation between phenolic content and antioxidant efficacy. Basli et al. (2017) demonstrated that polyphenols can inhibit the enzyme xanthine oxidaseinvolved in superoxide ion production; therefore, can alter the redox status of a cell suffering from oxidative stress. In other studies, it was confirmed that the antioxidant power of phenolic compounds is decided by the number and position of hydroxyl group in a particular phenolic compound (Kumar and Goel, 2019; Olszowy, 2019). Assefa et al. (2018) carried out a comprehensive study on 39 spices, to evaluate their total phenolic content and antioxidant capacity via DPPH, 2,2′-azino-bis(3- ethylbenzothiazoline-6-sulphonic acid) (ABTS) and cupric reducing antioxidant capacity (CUPRAC) assays. The total antioxidant capacity was obtained from 1.42 to 112.94 mg ascorbic acid equivalents (AAE)/g, 1.14–91.09 mg Trolox equivalents (TE)/g, and 0.52–54.47 mg TE/g with ABTS, DPPH and CUPRAC assay, respectively. The total phenolic content was measured through folin–ciocalteu assay and was ranged from 2.93 to 160.55 mg of gallic acid equivalents (GAE)/g DW. Syzygiumaromaticum (flower buds of cloves) was found as the most potent antioxidant agent followed by Pimenta dioica (allspice fruits) and Cinnamomum verum (cinnamon bark). Results also showed that total phenolic content is directly correlated with antioxidant potential. Mastur et al. (2017) examined the total phenolic content and total antioxidant capacity of six instant mix spices used for cookery art for the preparation of different food items. The total phenolic content was quantified by Folin-Ciocalteu assay while the total antioxidant capacities were measured by DPPH, ABTS and FRAP assays. Results showed a good quantity of total phenolic content i.e., ranged from 246.25 to 370.57 mg GAE/100 g. The total antioxidant capacities ranged from 728.54 to 1267.66 μmol TE/100 g, 833.19–1589.40 μmol TE/100 g and 1247.15–1886.89 μmol TE/100 g through DPPH, ABTS and FRAP assays, respectively. Apositive correlation was observed between the total phenolic content and total antioxidant potential. Sepahpour et al. (2018) compared the total antioxidant capacity and total phenolic compounds of different solvent extracts of turmeric, curry leaf, torch ginger and lemongrass. The antioxidant activity was assessed via DPPH and FRAP assay while total phenolic compounds were quantified by high-performance liquid chromatography (HPLC). Turmeric was noticed with the highest antioxidant activity followed by curry leaf, torch ginger and lemongrass. The maximum total phenolic compounds were obtained with 80% solvent in turmeric (221.68 mg GA/g freeze-dried crude extract), torch ginger (98.10 mg GA/g freeze-dried crude extract) and lemongrass (28.19 mg GA/g freeze-dried crude extract). Their results indicated the direct correlation between the total phenolic compounds and their antioxidant capacity. Wang et al. (2020) reported that individual polyphenol compounds such as hydroxycinnamic acid, ferulic acid, coumaric acid and syringic acid also exhibit good RSA.
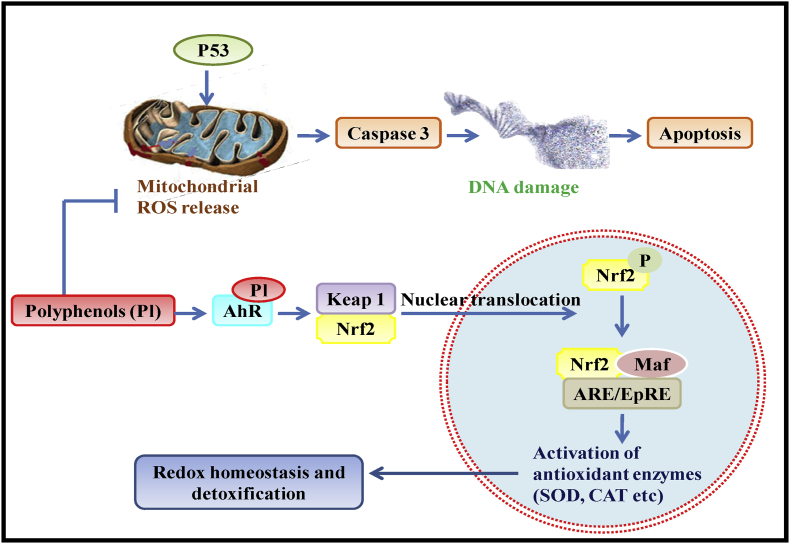
7.2.1. Mechanism of antioxidant activity
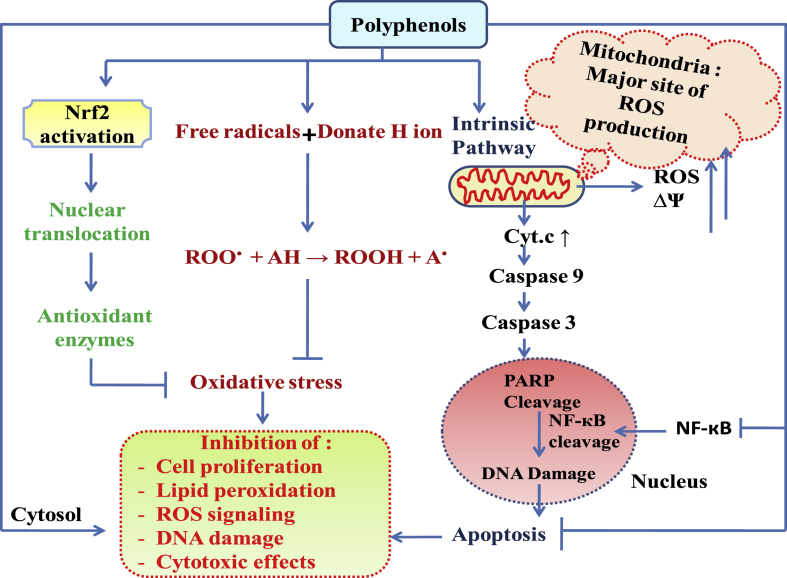
Zhang and Tsao (2016) explained various signaling pathways mediated by phenolic compounds to sequester ROS and to protect the cell from oxidative damage. It was proposed that phenolic compounds can donate hydrogen atoms to the free radicals to stabilize them and suppress the process of mitochondrial ROS generation, associated with the activation of pro-apoptotic factor; Caspase-3 and programme cell death (PCD). Phenolic compounds mediate the nuclear translocation of nuclear factor-erythroid 2-related factor 2 (Nrf2), a key factor involved in the activation of both constitutive and inducible expression of antioxidant genes and associated enzymes (Serafini and Peluso, 2016). Phenolic compounds stimulate the dissociation and ubiquitination kelch-like protein-1 (Keap1), the negative regulator of the Nrf2 transcription factor. Keap1 dissociation will facilitate the Nrf2 nuclear translocation, to activate antioxidant response elements (ARE) such as various antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), heme oxygenase-1 (HO-1) and release of the endogenous antioxidant glutathione (GSH) etc. to eventually protects the cell from oxidative stress and PCD. The various signals activated by polyphenols to exert their antioxidative effect are depicted in Fig. 3.
Fig. 3.
Mechanism of antioxidative activity of polyphenols.
7.3. Anti-inflammatory activity
Inflammation is the result of dysregulated immune homeostasis and oxidative imbalance, associated with the activation of various transcription factors and inflammatory genes (Hussain et al., 2016). It also occurs due to an elevated level of nitric oxide production (NO). NO production is stimulated via pro-inflammatory cytokines such as tumor necrosis factor- α (TNF- α), interleukin (IL)-1β, and IL-6, IL-8 and IFN-γ etc. Thus, the reduction of NO production is a mandatory approach to inflammation treatment. The downregulation of pro-inflammatory mediators will lead to the reduction in NO production and eventually the prevention of inflammation (Singh et al., 2020). Phenolic compounds are actively involved in the reduction of NO production (Zhang and Tsao, 2016). Hussain et al. (2019) reported that phenolic compounds can scavenge the reactive nitrogen species (RNS) formed as a result of inflammation. The polyphenols can also downregulate the expression of pro-inflammatory enzymes. In another study, Lutz et al. (2019) opined that phenolic compounds downregulate the expression of various pro-inflammatory mediators such as cyclooxygenases-1 (COX-1) and COX-2 while Fraga et al. (2019) stated that polyphenols alter the synthesis of inflammatory cytokines such as TNF-α, IL-1β, and IL-6, involved in NO. Tressera-Rimbau et al. (2017) also reported the pro-inflammatory markers (TNF-α, IL-1, IL-6, and IL-8) reducing the potential of polyphenols as a major pathway to inflammation treatment. Xu et al. (2020) examined the immunomodulatory effect of 15 spices methanolic extracts. The effect was measured in terms of their NO and TNF-α synthesis preventing efficacy in lipopolysaccharide (LPS) induced RAW 264.7 macrophages. It was noticed that all the spices have significantly inhibited the NO and TNF-α synthesis, attributed to their polyphenols. Serafini and Peluso (2016) reported the role of curcuminoids capsules (from curcumin) in reducing the concentration of pro-inflammatory cytokines and lipid oxidation resulted in improved redox and inflammatory markers.
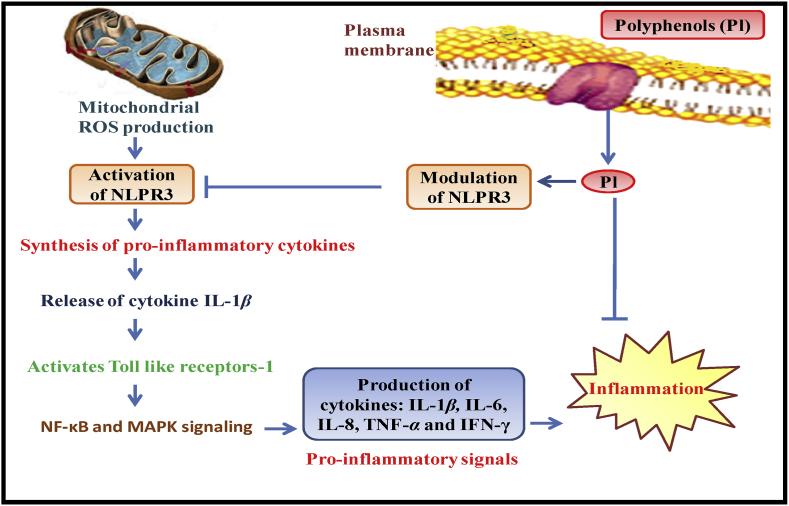
7.3.1. Mechanism of anti-inflammatory activity
NO is the major factor involved in inflammation and polyphenols interact with nitric oxide synthases to alter the NO production (Hussain et al., 2016; Oliviero et al., 2018). Some flavonoids such as quercetin, silibin, and luteolinwere reported with natural efficacy of Xanthine oxidaseinhibition, a key enzyme involved in the ROS production. Zhang and Tsao (2016) also studied the anti-inflammatory mechanism of phenolic compounds. Thepro-inflammatory cytokines and oxidative stress were observed as the leading cause of inflammation, so the suppression ofpro-inflammatory cytokines and oxidative stress can be the primary focus to ultimately halt the occurrence of inflammation. During inflammation, nucleotide-binding oligomerization domain, leucine-rich repeat-containing gene family, and pyrin domain-containing 3 (NLPR3) signaling play a key role to induce inflammation. Mitochondrial ROS is majorly responsible to upregulates the expression of NLPR3, which in turn will subsequently stimulate the synthesis of the pro-inflammatory cytokine, the release of IL-1β cytokine, activation of toll-like receptors-1 (TLR-1), NF-kB and MAPK signalings. These signalings will trigger the transductions of pro-inflammatory signaling and releasing of cytokines such as IL-1β, IL-6, IL-8, TNF-α and IFN-γ etc. (Serafini and Peluso, 2016). This series of pro-inflammatory signalings will amplify inflammatory responses and eventually results in systematic inflammation. Since antiquity, phenolic compounds are well reported as good anti-inflammatory agents as these compounds can significantly suppress the production and activation of pro-inflammatory cytokines via the modulation of NLPR3 signaling; to reduce inflammation (as depicted in Fig. 4).
Fig. 4.
Mechanism of anti-inflammatory activity of polyphenols.
7.4. Antidiabetic activity
Diabetes is an oxidative stress disorder characterized by the condition of insulin imbalance and improper glucose levels (Singh et al., 2020). It is the leading metabolic disorder of the world and 592 million may be affected till 2035 (Ahangarpour et al., 2019). Phenolic acid plays a crucial role in combating oxidative stress and associated malfunctioning of insulin and glucose receptors. Mughal (2019) reported the insulin-mimetic and activation potential of spices polyphenols. The polyphenolic compounds can upregulate the expression of glucose transporter 2 (GLUT2) in pancreatic β-cells to increase insulin production (Vetrani et al., 2020), while the translocation of GLUT4 is enhanced via phosphoinositide-3-kinase MAPK (P13 K/Akt) activated protein kinase pathways. Chlorogenic and ferulic acids were reported with natural transporter stimulation mechanism, thus can be used as a natural antidiabetic agent. Apart from this, phenolic compounds were also accounted for their α-amylase (converts complex starch to oligosaccharides) and α-glucosidase (converts polysaccharides to glucose) inhibitory potential. These two key enzymes play a major role during the glycemic load (Kumar and Goel, 2019). Bi et al. (2017) reviewed the role of spices in controlling hyperglycemia. It was opined that the polyphenol content of spices is primarily responsible for compelling the antidiabetic potential of spices. The complex mixture of polyphenols helps in improving glucose homeostasis and insulin resistance. Kumar et al. (2019) also supported the modulation of hyperglycemia and increased glucose uptake under the influence of polyphenols. Haldar et al. (2018) stated that Glucagon-like peptide-1 (GLP-1) plays a key role in glucose balancing. It was proposed that dietary polyphenols rich mixed spices have the ability to stimulate the secretion of postprandial GLP-1. Therefore, a randomized, controlled, dose-dependent clinical study on 20 young, healthy, Chinese men, supplemented with test meals enriched with mixed spices and vegetables was conducted. A significant upregulation of plasma in-vivo GLP-1 concentrations and justified the role of polyphenols rich spices in combating high glucose level was noticed.
7.4.1. Mechanism of antidiabetic activity
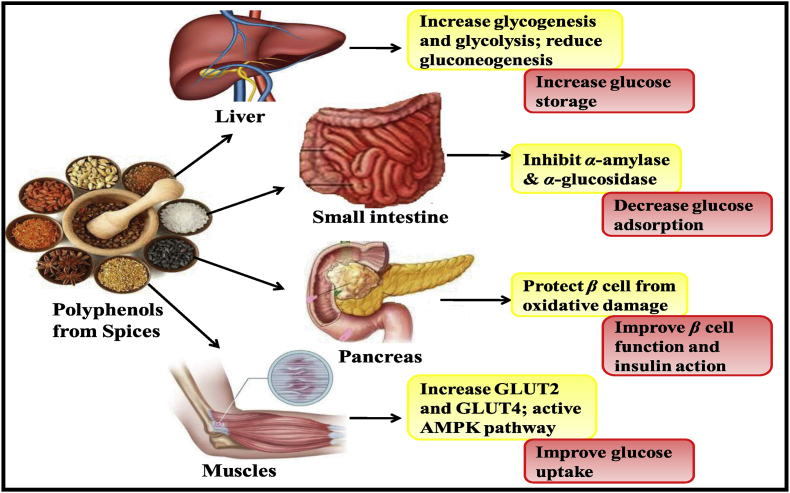
Ge et al. (2017) described the antidiabetic mechanism of polyphenols from spices. It was reported that phenols and polyphenols from spices actively interrupt the glucose metabolism signaling and regulates the glucose absorption (intestinal glucose uptake), glucose production (intrahepatic glucose output) and insulin release (from islet β cells) with enhanced activity of insulin-receptors sensitive tissues. All these events rendered by phenolic compounds will ultimately lead to a balanced sugar level, aid in the prevention of hyperglycemia. The various pathways activated via spices polyphenols in different body parts, for combating diabetes are well shown in Fig. 5.
Fig. 5.
Mechanism of antidiabetic activity of polyphenols.
7.5. Anticancer activity
Cancer is a multifactorial, most dreadful dynamicdisorder characterized by uncontrolled cell proliferation and metastasis. It is one of the leading causes of demise and more than 22.2 million may die by 2030 (Basli et al., 2017; Guldiken et al., 2018). Polyphenols are supposed to have the ability to interrupt the multiple key steps of cell proliferation, inflammation, differentiation, apoptosis, angiogenesis and metastasis etc. involved during cancer occurrence. The anticancer effect of polyphenols is mainly attributed to their antioxidant potential because oxidative stress plays a pivotal role in carcinogenesis. Golonko et al. (2019) opined that phenolic compounds can alter the signaling of the ubiquitin-proteasome system (UPS) pathway, as one of the most appropriate approaches to cancer treatment. The polyphenols exert their antioxidative potential and reduce the lipid peroxidation, ROS signaling, DNA damage and impart their cytoprotective effect. Xu et al. (2020) screened the antineoplastic effect of 15 spices methanolic extracts. The study was carried out in six cancer cell lines including liver (HepG2), colon (HT29), breast (MCF7), pancreas (MIA PaCa2), lung (A549) and blood (Raji). It was observed that all spice extracts showed significant antineoplastic activity. The spices containing rich polyphenols content showed better antineoplastic results.
7.5.1. Mechanism of anticancer activity
As discussed earlier, spices derived polyphenols exhibit excellent antioxidant capacity. Basli et al. (2017) reported the anticancer mechanism rendered by polyphenols. The antioxidant capacity of polyphenols plays a crucial role in the prevention of cell proliferation and carcinogenesis. The polyphenolic compounds donate the hydrogen atom to neutralize the effect of free radicals. These compounds have the capacity to stimulate the activation of phase I enzymes involved in mitigating oxidative stress and is directly linked to the prevention of carcinogenesis. The polyphenolic compounds also facilitate the process of cancer cell apoptosis via intrinsic and extrinsic pathways. Based on the literature survey, the following diagram is provided to depict the anticancer mechanism of polyphenols (as depicted in Fig. 6).
Fig. 6.
Mechanism of anticanceractivity of polyphenols.
7.6. Application of polyphenols in cardiovascular diseases
Cardiovascular disease is also the leading cause of death. Age, high cholesterol (HC), high blood pressure (HBP), LDL oxidation, inflammation, hyperglycemia, urinary isoprostanesand inflammation etc. Are considered as the major risk factors for cardiometabolic health impairment. Platelet hyperactivation and their subsequent accumulation ease the way to thrombus formation. Apart from this, dysregulated endothelium contributes to the development of atherothrombosis and cardiovascular diseases (Lutz et al., 2019). It was opined that the intake of phenolic rich diet may limit the occurrence of coronary heart diseases viz. Atherosclerosis. These secondary metabolites can significantly inhibit the oxidation of low-density lipoprotein (LDL) and perform their anti-platelet aggregation activity, a key mechanism involved in atherosclerosis propagation. Fraga et al. (2019) reported that quercetin (flavonoid) and resveratrol (stilbene) exert a beneficial effect on cardiometabolic health. Tressera-Rimbau et al. (2017) reported that consumption of dietary polyphenols inversely relates to the cause of cardiovascular and cerebrovascular disorders caused morbidity. It was also described that the polyphenols can modulate the various risk factors viz. reduced pro-inflammatory markers, oxidative stress and induction of apoptosis. This series of modulated risk factors will also reduce the neurological, cardiovascular and cerebrovascular disorders. Vetrani et al. (2020) suggested that polyphenols can improve the composition of gut microbiota, as an advanced strategy for curing cardiovascular disorders.
8. Reasons for the diverse pharmacological actions of phenolic compounds
Oxidative stressis the major cause of the occurrence of many human ailments (Martins et al., 2016). Hussain et al. (2016) also reported that oxidative stress caused inflammation triggers plenty of chronic disorders. There is directproportional relationship between the occurrence of inflammation and oxidative stress. It was proposed that the antioxidant potential of polyphenols is beneficial to counteract inflammation as well as inflammation caused carcinogenesis. Oliviero et al. (2018) reported inflammation as a key factor responsible for the occurrence of osteoarthritis. Therefore, prevention of oxidative stress can prevent the occurrence of most of these biological disorders such as osteoporosis, inflammation, aging, obesity, neurodegenerative diseases, cardiovascular diseases, diabetes, cancer and so forth (de Araújo et al., 2020; Singh et al., 2020). Polyphenols can significantly sequester the ROS and RNS, attributed to their most biological benefits. Zhang and Tsao (2016) demonstrated the role of polyphenols in combating oxidative stress and inflammation and showed that the signaling pathways activated via polyphenols to prevent oxidative stress were also involved during anti-inflammatory signaling. The polyphenols lead to the activation of antioxidant enzymes during both the conditions, essentially affect similar biomarkers. Therefore, curing oxidative stress will automatically lead to the prevention of inflammation. Anti-inflammatory and antiatherogenic properties of dietary polyphenols reduce the risk and morbidity associated with cardiovascular diseases (Tressera-Rimbau et al., 2017). Thus, curing of one disorder will automatically lead to the prevention of other associated disorders. Polyphenols can interact with other biological molecules too, to enhance their pharmacological effectiveness and impart synergistic effects (Fraga et al., 2019; de Araújo et al., 2020). Therefore, polyphenols enriched spices could potentially be utilized as ameliorating or preventing agent for curing more than one ailment at a single time and overall well-being (Yashin et al., 2017).
9. Concluding remarks and future perspectives
It can be concluded that among different spices, clove, thyme, oregano and rosemary are rich source of polyphenol content. Owing to their rich polyphenol content, these spicescanreduce the risk of many diseases up to a great extent. They majorly act upon ROS scavenging and combating oxidative stress, associated with the occurrence of multiple disorders such as diabetes, inflammation and cancer, etc. This is the basic reason for a broad spectrum of polyphenol's pharmacological attributes. Moreover, these compounds also help in achieving the sustainability goals by supplementing the nutritious and healthy food at affordable costs. Therefore, these secondary metabolites can be used as an alternative to synthetic antioxidants and can be developed into effective food supplements. Though recommendations are warranted to intake polyphenols enriched diet but cautions must be exercised at high doses. Clinical research should also be practiced to confirm the real efficacy of polyphenols and spices.
Ethics approval
Not Applicable.
Funding
Council for Scientific and Industrial Research (CSIR), New Delhi and Haryana State Council for Science and Technology (HSCST), Panchkula, SERB-DST, New Delhi, Fund for Improvement of S&T infrastructure in universities & higher educational institutions (FIST), Department of Science and Technology, Govt. of India, New Delhi.
Code availability
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Yes.
Availability of data and material
Not Applicable.
CRediT authorship contribution statement
Neetu Singh: Writing – original draft, Conceptualization, Investigation, Data curation. Surender Singh Yadav: Conceptualization, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Financial assistance from the Council for Scientific and Industrial Research (CSIR), New Delhi and Haryana State Council for Science and Technology (HSCST), Panchkula, Fund for Improvement of S&T infrastructure in universities & higher educational institutions (FIST), Department of Science and Technology, Govt. of India, New Delhi is thankfully acknowledged.
Handling Editor: Dr. Quancai Sun
Abbreviations
- AAE
: Ascorbic acid equivalents
- ABTS
: 2,2′-Azino-bis(3- ethylbenzothiazoline-6-sulphonic acid)
- ARE
: Antioxidant response elements
- BPs
: Bound phenolics
- CAT
: Catalase
- COX
: Cyclooxygenases
- CUPRAC
Cupric reducing antioxidant capacity
- DPPH
: 1,1-Diphenyl-2-picrylhydrazyl
- EDTA
: Ethylenedinitrilotetraacetic
- FPs
: Free phenolics
- FRAP
: Ferric reducing antioxidant power
- GAE
: Gallic acid equivalents
- GLP-1
: Glucagon-like peptide-1
- GPx
: Glutathione peroxidase
- GSH
: Glutathione
- HCl
: Hydrochloric acid
- HO-1
: Heme oxygenase-1
- HPLC
: High-performance liquid chromatography
- IL
: Interleukin
- KEAP1
: Kelch-like protein-1
- LDL
: Low-density lipoprotein
- LPS
: Lipopolysaccharide
- NaOH
: Sodium hydoxide
- NO
: Nitric oxide
- Nrf2
: Nuclear factor-erythroid 2-related factor 2
- PCD
: Programme cell death
- PI3K
: Phosphoinositide-3-kinase MAPK
- RNS
: Reactive nitrogen species
- ROS
: Reactive oxygen species
- RSA
: Radical scavenging activity
- SOD
: Superoxide dismutase
- TE
: Trolox equivalents
- TNF-α
: Tumor necrosis factor- α
- UPS
: Ubiquitin-proteasome system
- FP's
: Free phenolics
References
- Ahangarpour A., Sayahi M., Sayahi M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: a review study. Diabetes Metab.: Clin. Res. Rev. 2019;13:854–857. doi: 10.1016/j.dsx.2018.11.051. [DOI] [PubMed] [Google Scholar]
- Ali A., Wu H., Ponnampalam E.N., Cottrell J.J., Dunshea F.R., Suleria H.A. Comprehensive profiling of most widely used spices for their phenolic compounds through lc-esi-qtof-ms2 and their antioxidant potential. Antioxidants. 2021;10:721. doi: 10.3390/antiox10050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa A.D., Keum Y.S., Saini R.K. A comprehensive study of polyphenols contents and antioxidant potential of 39 widely used spices and food condiments. J. Food Meas. Char. 2018;12:1548–1555. doi: 10.1007/s11694-018-9770-z. [DOI] [Google Scholar]
- Basli A., Belkacem N., Amrani I. Marcos Soto-Hernández, Rosario García-Mateos and Mariana Palma-Tenango. IntechOpen; London, UK: 2017. Health benefits of phenolic compounds against cancers; pp. 193–210. (In Phenolic Compounds–Biological Activity). [DOI] [Google Scholar]
- BettaiebRebey I., Bourgou S., Aidi Wannes W., HamrouniSelami I., SaidaniTounsi M., Marzouk B., Ksouri R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosystems-an International Journal Dealing with All Aspects of Plant Biology. 2018;152:971–978. doi: 10.1080/11263504.2017.1403394. [DOI] [Google Scholar]
- Bi X., Lim J., Henry C.J. Spices in the management of diabetes mellitus. Food Chem. 2017;217:281–293. doi: 10.1016/j.foodchem.2016.08.111. [DOI] [PubMed] [Google Scholar]
- Bostan C., Butnariu M., Butu M., Ortan A., Butu A., Rodino S., Parvu C. Allelopathic effect of Festuca rubra on perennial grasses. Rom. Biotechnol. Lett. 2013;18:8190–8196. [Google Scholar]
- Butnariu M., Bostan C. Antimicrobial and anti-inflammatory activities of the volatile oil compounds from Tropaeolum majus L.(Nasturtium) Afr. J. Biotechnol. 2011;10:5900–5909. [Google Scholar]
- Chahal K.K., Dhaiwal K., Kumar A., Kataria D., Singla N. Chemical composition of Trachyspermumammi L. and its biological properties: a review. J. Pharmacogn. Phytochem. 2017;6:131–140. [Google Scholar]
- Chan C.L., Gan R.Y., Shah N.P., Corke H. Polyphenols from selected dietary spices and medicinal herbs differentially affect common food-borne pathogenic bacteria and lactic acid bacteria. Food Control. 2018;92:437–443. doi: 10.1016/j.foodcont.2018.05.032. [DOI] [Google Scholar]
- de Araújo F.F., de Paulo Farias D., Neri-Numa I.A., Pastore G.M. Polyphenols and their applications: an approach in food chemistry and innovation potential. Food Chem. 2020;338 doi: 10.1016/j.foodchem.2020.127535. [DOI] [PubMed] [Google Scholar]
- Elansary H.O., Szopa A., Kubica P., Ekiert H., Klimek-Szczykutowicz M., El-Ansary D.O., Mahmoud E.A. Polyphenol profile and antimicrobial and cytotoxic activities of natural Mentha× piperita and Mentha longifolia populations in Northern Saudi Arabia. Processes. 2020;8:479. doi: 10.3390/pr8040479. [DOI] [Google Scholar]
- Feng Y., Dunshea F.R., Suleria H.A. LC-ESI-QTOF/MS characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020;57:4671–4687. doi: 10.1007/s13197-020-04504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga C.G., Croft K.D., Kennedy D.O., Tomás-Barberán F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10:514–528. doi: 10.1039/c8fo01997e. [DOI] [PubMed] [Google Scholar]
- Gaşpar C.M., Cziszter L.T., Lăzărescu C.F., Ţibru I., Pentea M., Butnariu M. Antibiotic resistance among Escherichia coli isolates from hospital wastewater compared to community wastewater. Water. 2021;13:3449. doi: 10.3390/w13233449. [DOI] [Google Scholar]
- Ge Q., Chen L., Chen K. Treatment of diabetes mellitus using iPS cells and spice polyphenols. J. Diabetes Res. 2017:1–11. doi: 10.1155/2017/5837804. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafoor K., Al Juhaimi F., Özcan M.M., Uslu N., Babiker E.E., Ahmed I.A.M. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. Lebensm. Wiss. Technol. 2020;126 doi: 10.1016/j.lwt.2020.109354. [DOI] [Google Scholar]
- Golonko A., Pienkowski T., Swislocka R., Lazny R., Roszko M., Lewandowski W. Another look at phenolic compounds in cancer therapy the effect of polyphenols on ubiquitin-proteasome system. Eur. J. Med. Chem. 2019;167:291–311. doi: 10.1016/j.ejmech.2019.01.044. [DOI] [PubMed] [Google Scholar]
- Guldiken B., Ozkan G., Catalkaya G., Ceylan F.D., Yalcinkaya I.E., Capanoglu E. Phytochemicals of herbs and spices: health versus toxicological effects. Food Chem. Toxicol. 2018;119:37–49. doi: 10.1016/j.fct.2018.05.050. [DOI] [PubMed] [Google Scholar]
- Gupta J., Sharma S., Sharma N.R., Kabra D. Phytochemicals enriched in spices: a source of natural epigenetic therapy. Arch Pharm. Res. (Seoul) 2020;43:171–186. doi: 10.1007/s12272-019-01203-3. [DOI] [PubMed] [Google Scholar]
- Haldar S., Chia S.C., Henry C.J. Polyphenol-rich curry made with mixed spices and vegetables increases postprandial plasma GLP-1 concentration in a dose-dependent manner. Eur. J. Clin. Nutr. 2018;72:297–300. doi: 10.1038/s41430-017-0069-7. [DOI] [PubMed] [Google Scholar]
- Hussain M.B., Hassan S., Waheed M., Javed A., Farooq M.A., Tahir A. Plant Physiological Aspects of Phenolic Compounds. In Marcos Soto-Hernández, Rosario García-Mateos and Mariana Palma-Tenango. 2019. Bioavailability and metabolic pathway of phenolic compounds. IntechOpen. [DOI] [Google Scholar]
- Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell. Longev. 2016:1–9. doi: 10.1155/2016/7432797. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran A., Arshad M.U., Sherwani H., Shabir Ahmad R., Arshad M.S., Saeed F., Anjum F.M. Antioxidant capacity and characteristics of theaflavin catechins and ginger freeze-dried extract as affected by extraction techniques. Int. J. Food Prop. 2021;24:1097–1116. doi: 10.1080/10942912.2021.1953524. [DOI] [Google Scholar]
- Issaoui M., Delgado A.M., Caruso G., Micali M., Barbera M., Atrous H., Chammem N. Phenols, flavors, and the mediterranean diet. J. AOAC Int. 2020;103:1–10. doi: 10.1093/jaocint/qsz018. [DOI] [PubMed] [Google Scholar]
- Jiang T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019;102:395–411. doi: 10.5740/jaoacint.18-0418. [DOI] [PubMed] [Google Scholar]
- Kaundal R., Kumar M., Kumar S., Singh D., Kumar D. Polyphenolic profiling, antioxidant, and antimicrobial activities revealed the quality and adaptive behavior of viola species, a dietary spice in the himalayas. Molecules. 2022;27:3867. doi: 10.3390/molecules27123867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019;24 doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.A., Cabral C., Kumar R., Ganguly R., Kumar R.H., Gupta A., Pandey A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients. 2019;11:2216. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Luan G., He Y., Tie F., Wang Z., Suo Y., Wang H. Polyphenol stilbenes from fenugreek (Trigonella foenum-graecum L.) seeds improve insulin sensitivity and mitochondrial function in 3T3-L1 adipocytes. Oxid. Med. Cell. Longev. 2018:1–9. doi: 10.1155/2018/7634362. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucci P., Saurina J., Núñez O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. Trends Anal. Chem. 2017;88:1–24. doi: 10.1016/j.trac.2016.12.006. [DOI] [Google Scholar]
- Lutz M., Fuentes E., Ávila F., Alarcón M., Palomo I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules. 2019;24:366. doi: 10.3390/molecules24020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark R., Lyu X., Lee J.J., Parra-Saldívar R., Chen W.N. Sustainable production of natural phenolics for functional food applications. J. Funct.Foods. 2019;57:233–254. doi: 10.1016/j.jff.2019.04.008. [DOI] [Google Scholar]
- Martins N., Barros L., Ferreira I.C. In vivo antioxidant activity of phenolic compounds: facts and gaps. Trends Food Sci. Technol. 2016;48:1–12. doi: 10.1016/j.tifs.2015.11.008. [DOI] [Google Scholar]
- Mastur Y.H., Hasnah H., Yap Y.T. Total phenolic content and antioxidant capacities of instant mix spices cooking pastes. Int. Food Res. J. 2017;24:68–74. [Google Scholar]
- Mughal M.H. Spices: a review on diabetes mellitus. Biomed. J. Sci. Technol. Res. 2019;15:11651–11657. doi: 10.26717/BJSTR.2019.15.002770. [DOI] [Google Scholar]
- Ogbunugafor H.A., Ugochukwu C.G., Kyrian-Ogbonna A.E. The role of spices in nutrition and health: a review of three popular spices used in Southern Nigeria. Food Qual. Saf. 2017;1:171–185. doi: 10.1093/fqsafe/fyx020. [DOI] [Google Scholar]
- Oliviero F., Scanu A., Zamudio-Cuevas Y., Punzi L., Spinella P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018;98:1653–1659. doi: 10.1002/jsfa.8664. [DOI] [PubMed] [Google Scholar]
- Olszowy M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019;144:135–143. doi: 10.1016/j.plaphy.2019.09.039. [DOI] [PubMed] [Google Scholar]
- Panja P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018;23:173–182. doi: 10.1016/j.cofs.2017.11.012. [DOI] [Google Scholar]
- Piccolella S., Crescente G., Candela L., Pacifico S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. 2019;175 doi: 10.1016/j.jpba.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Rajendran S., Rajagopal P., Selvaraj Jayaraman J.M., Kasturi R., Nagarajan V., Karuppan M. A review on dietary poly phenols: herbal neutraceuticals to combat nephrotoxicity. Nat. Volatiles Essent. Oils. 2021;8:7584–7597. [Google Scholar]
- Rani V., SR P., Prabhu A. Nutraceuticals derived from dietary spices as candidate molecules in targeting glioma signaling pathways. Rev. Bras. Farmacogn. 2022;32:1–13. doi: 10.1007/s43450-022-00285-3. [DOI] [Google Scholar]
- Rashid Z., Khan M.R., Mubeen R., Hassan A., Saeed F., Afzaal M. Exploring the effect of cinnamon essential oil to enhance the stability and safety of fresh apples. J. Food Process. Preserv. 2020;44 doi: 10.1111/jfpp.14926. [DOI] [Google Scholar]
- Sachan A.K., Das D.R., Kumar M. Carum carvi-An important medicinal plant. J. Chem. Pharmaceut. Res. 2016;8:529–533. [Google Scholar]
- Samfira I., Butnariu M., Rodino S., Butu M. Structural investigation of mistletoe plants from various hosts exhibiting diverse lignin phenotypes. Dig. J. Nanomater. Biostructures. 2013;8:1679–1686. [Google Scholar]
- Samfira I., Rodino S., Petrache P., Cristina R.T., Butu M., Butnariu M. Characterization and identity confirmation of essential oils by mid infrared absorption spectrophotometry. Dig. J. Nanomater. Biostructures. 2015;10:557–566. [Google Scholar]
- Saraswathi K., Arumugam P., Sivaraj C. Pharmacological activities of differential parts of selected Essential Indian Spices. J. Pharmacogn. Phytochem. 2020;9:2024–2033. doi: 10.22271/phyto.2020.v9.i2ag.11151. [DOI] [Google Scholar]
- Sepahpour S., Selamat J., Abdul Manap M.Y., Khatib A., AbdullRazis A.F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules. 2018;23:402. doi: 10.3390/molecules23020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini M., Peluso I. Functional foods for health: the interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr. Pharmaceut. Des. 2016;22:6701–6715. doi: 10.2174/1381612823666161123094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Rao A.S., Nandal A., Kumar S., Yadav S.S., Ganaie S.A., Narasimhan B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2020;338 doi: 10.1016/j.foodchem.2020.127773. [DOI] [PubMed] [Google Scholar]
- Singh N., Yadav S.S., Kumar S., Narashiman B. A review on traditional uses, phytochemistry, pharmacology, and clinical research of dietary spice Cuminum cyminum L. Phytother Res. 2021;35:5007–5030. doi: 10.1002/ptr.7133. [DOI] [PubMed] [Google Scholar]
- Singh N., Yadav S.S., Kumar S., Narashiman B. Ethnopharmacological, phytochemical and clinical studies on Fenugreek (Trigonella foenum-graecum L.) Food Biosci. 2022;46 doi: 10.1016/j.fbio.2022.101546. [DOI] [Google Scholar]
- Škrovánková S., Mlček J., Orsavová J., Juríková T., Dřímalová P. Polyphenols content and antioxidant activity of paprika and pepper spices. Potr. S. J. F. Sci. 2017;11:52–57. doi: 10.5219/695. [DOI] [Google Scholar]
- Slimestad R., Fossen T., Brede C. Flavonoids and other phenolics in herbs commonly used in Norwegian commercial kitchens. Food Chem. 2020;309 doi: 10.1016/j.foodchem.2019.125678. [DOI] [PubMed] [Google Scholar]
- Sokamte T.A., Mbougueng P.D., Tatsadjieu N.L., Sachindra N.M. Phenolic compounds characterization and antioxidant activities of selected spices from Cameroon. South Afr. J. Bot. 2019;121:7–15. doi: 10.1016/j.sajb.2018.10.016. [DOI] [Google Scholar]
- Takó M., Kerekes E.B., Zambrano C., Kotogán A., Papp T., Krisch J., Vágvölgyi C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants. 2020;9:165. doi: 10.3390/antiox9020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tressera-Rimbau A., Arranz S., Eder M., Vallverdú-Queralt A. Dietary polyphenols in the prevention of stroke. Oxid. Med. Cell. Longev. 2017:1–10. doi: 10.1155/2017/7467962. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman I., Hussain M., Imran A., Afzaal M., Saeed F., Javed M., Saewan S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022;25:1215–1233. doi: 10.1080/10942912.2022.2074030. [DOI] [Google Scholar]
- Vázquez-Fresno R., Rosana A.R.R., Sajed T., Onookome-Okome T., Wishart N.A., Wishart D.S. Herbs and spices-biomarkers of intake based on human intervention studies–a systematic review. Genes & nutrition. 2019;14:18. doi: 10.1186/s12263-019-0636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrani C., Costabile G., Vitale M., Giacco R. (Poly) phenols and cardiovascular diseases: looking in to move forward. J. Funct.Foods. 2020;71 doi: 10.1016/j.jff.2020.104013. [DOI] [Google Scholar]
- Wang Z., Li S., Ge S., Lin S. Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J. Agric. Food Chem. 2020;68:3330–3343. doi: 10.1021/acs.jafc.9b06574. [DOI] [PubMed] [Google Scholar]
- Xu B., Ganesan K., Mickymaray S., Alfaiz F.A., Thatchinamoorthi R., Al Aboody M.S. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. Bioact. compd. health dis. 2020;3:15–31. doi: 10.31989/bchd.v3i2.687. [DOI] [Google Scholar]
- Yashin A., Yashin Y., Xia X., Nemzer B. Antioxidant activity of spices and their impact on human health: a review. Antioxidants. 2017;6:70. doi: 10.3390/antiox6030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Gan R.Y., Farha A.K., Kim G., Yang Q.Q., Shi X.M., Corke H. Discovery of antibacterial dietary spices that target antibiotic-resistant bacteria. Microorganisms. 2019;7:157. doi: 10.3390/microorganisms7060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.