Abstract
Objectives
This study identified different multimorbidity patterns among adults with subjective cognitive decline (SCD) and examined their association with SCD-related functional difficulties.
Methods
Data were obtained from the 2019 Behavioral Risk Factor Surveillance System. Latent class analysis was applied to identify different patterns of chronic conditions. Logistic regression was implemented to examine relationships between multimorbidity patterns and risk of SCD-related functional difficulties.
Results
Five multimorbidity patterns were identified: severely impaired (14.6%), respiratory/depression (18.2%), obesity/diabetes (18.6%), age-associated (22.3%), and minimal chronic conditions group (26.3%). Compared with minimal chronic conditions group, severely impaired group was most likely to report SCD-related functional difficulties, followed by respiratory/depression and obesity/diabetes group.
Discussions
Individuals in the three multimorbidity groups had elevated risk of SCD-related functional difficulties compared with minimal chronic conditions group. Characteristics of the high-risk groups identified in this study may help in development and implementation of interventions to prevent serious consequences of having multiple chronic conditions.
Keywords: subjective cognitive decline, multimorbidity patterns, subjective cognitive decline-related functional difficulties
Background
The percentage of US residents living with at least one chronic disease increased from 40% to 60% in 2009 and the percentage of those with two or more chronic diseases (i.e., multimorbidity) increased from one third to 40% in 2021 (CDC, 2009, 2021). Individuals with chronic diseases have an increased risk of death (Nunesa et al., 2016), disability (Anesetti-Rothermel & Sambamoorthi, 2011), adverse events (Barile et al., 2015; Laires et al., 2021; Salive, 2013), and impaired functional status (Caracciolo et al., 2013), as well as lower health-related quality of life (Barile et al., 2015). Beyond these individual consequences, multimorbidity can lead to increased medical service utility via more emergency department visits, hospitalizations, etc., (Zheng et al., 2020a).
Previous studies examining the impact of multimorbidity on health outcomes considered chronic diseases as multiple covariates, measured multimorbidity as a cumulative count of diseases or treated multimorbidity as a binary indicator (Barile et al., 2015; Caracciolo et al., 2013; Laires et al., 2021; Nunesa et al., 2016; Salive, 2013). However, more recently there has been a call for studies examining the synergistic effects of multimorbidity (Barile et al., 2015). One such study classified the US general population into five subgroups according to their distinct combination of chronic diseases: healthy, hypertensive, respiratory conditions, heart disease, and severely impaired group (Zheng et al., 2020a). The authors found that heart condition group had no difference in the risk of visual impairment compared to the hypertension group despite the heart condition group having a larger mean number of chronic diseases. Another study also classified the US general population into five subgroups (i.e., healthy, vascular risk, anxiety, heart disease, and severely impaired group) and found the health-related quality of life was significantly higher in the vascular risk group compared to the anxiety group, even though the average number of chronic diseases was larger in the former group (Zheng et al., 2020b). These studies demonstrate the importance of studying multimorbidity as unique subgroups rather than using a binary or cumulative approach.
Patterns of multimorbidity vary by subpopulations and disease groups. Among US residents aged 65 years and older, Zheng et al. (2021) identified five patterns of multimorbidity (healthy, age-associated chronic conditions, respiratory conditions, cognitively impaired, and complex cardiometabolic group) and found the cognitive impaired group had significantly higher mortality than the respiratory group, despite the number of reported chronic disease/conditions being similar between the two groups. Among patients diagnosed with multiple myeloma, Fillmore et al. (2021) identified five multimorbidity groups (minimal comorbidity, cardiovascular and metabolic, psychiatric and substance use, chronic lung disease and multisystem impairment group), and found that chronic lung disease group had higher risk of mortality than the cardiovascular and metabolic group, though the median number of chronic conditions were the same. Among acutely hospitalized patients, eight multimorbidity patterns were found (Juul-Larsen et al., 2020), and the pattern with the highest number of chronic conditions did not show the highest healthcare utilization.
Subjective cognitive decline (SCD) is a self-reported experience of confusion or memory loss that is happening more often or getting worse (CDC, 2019b). It is one form of cognitive impairment and can be considered one of the earliest noticeable symptoms of Alzheimer’s disease (AD) (Neto & Nitrini, 2016) (Murphy et al., 2013). Individuals with SCD may unconsciously give up daily activities such as cooking and cleaning, or be unable to manage medical appointments or medication regimens, resulting in poor health outcomes (Taylor et al., 2018). In the US, one in nine adults aged 45 years and older reported having SCD, with half (50.6%) of whom reporting SCD-related functional difficulties (Taylor et al., 2018). Among those with SCD, 66.2% had two or more chronic diseases (CDC, 2019b). To date, only one study has examined multimorbidity patterns within a population with subjective memory complaints (i.e., a measure similar to SCD) (Yap et al., 2020). However, this study was not nationwide, with only 6179 participants and eight chronic diseases considered. Other studies examining the relationship between multimorbidity and cognitive function found that adults with multimorbidity had a higher risk of SCD-related, or more broadly, cognitive-related functional impairment (Caracciolo et al., 2013; Jindai et al., 2016; Melis et al., 2013; Taylor et al., 2020), but, the synergistic effect of chronic diseases was not considered. In addition to chronic diseases/conditions, health-related risk behaviors including sedentary life style, excessive alcohol consumption, smoking, and unhealthy diet are associated with cognitive-related functional impairment (Alzheimer’s Association International Conference, 2019; National Institute on Aging, 2020). Therefore, the purpose of this study was to identify different multimorbidity patterns among adults with SCD and examine their association with SCD-related functional difficulties.
Methods
Study Data and Study Population
Data from the Behavioral Risk Factor Surveillance System (BRFSS) 2019 survey were used for this study. The BRFSS—a national premier system of health-related telephone surveys—is administered and supported by the Centers for Disease Control and Prevention (CDC) (CDC., 2014). BRFSS collects data about noninstitutionalized US adults (≥18) regarding their health-related risk behaviors, chronic diseases and health conditions, access to health care, and use of preventive services (CDC, 2019a). Data were obtained from the Core Component and the Cognitive Decline Optional Module. The core component contains a standard set of questions that all states use, inquiring about demographic information, health-related perceptions, conditions and health-related behaviors. The cognitive decline module targets residents aged 45 years and older and was developed in 2011 to understand the effect and burden of SCD in the population (CDC, 2018).
Participants who responded to the BRFSS 2019 survey were eligible to be included in this study. The inclusion criteria were (a) those 32 states implemented the cognitive decline model in 2019, (b) residents aged 45 years and older, and (c) reported “yes” to the question of SCD. After using the inclusion criteria, 15,621 observations were retained for this study.
Study Variables
SCD was obtained from the cognitive decline optional module and is assessed using the question “During the past 12 months, have you experienced confusion or memory loss that is happening more often or is getting worse?” If participants answered “yes,” further questions regarding SCD-related functional difficulties were asked, including “During the past 12 months, as a result of confusion or memory loss, how often have you given up day-to-day household activities or chores you used to do, such as cooking, cleaning, taking medications, driving or paying bills?” and “During the past 12 months, how often has confusion or memory loss interfered with your ability to work, volunteer, or engage in social activities outside the home?“ An individual was considered as experiencing SCD-related functional difficulties if at least one answer to the two questions is “always,” “usually,” “sometime,” or “rarely,” or as not experiencing SCD-related functional difficulties with the answer of “never” to the two questions. Chronic diseases/conditions were obtained from the core component of the BRFSS survey. 17 chronic diseases/conditions were used to identify different patterns of multimorbidity: hypertension, diabetes, high cholesterol, myocardial infarction/coronary heart diseases (MI/CHD), stroke, asthmas, arthritis, chronic obstructive pulmonary disease (COPD)/emphysema/chronic bronchitis, depression, kidney disease, hepatitis B, hepatitis C, deaf/serious difficulty hearing, blind/serious difficulty seeing, obesity, skin cancer, and other cancer. All conditions except obesity were determined via the questions: “Have you ever been told you have … by a doctor, nurse, or other health professionals?“ Obesity was defined as body mass index (BMI) equal or larger than 30 (WHO, 2021), where BMI was calculated by dividing body weight in kilograms by the square of height in meters.
12 variables were used to examine the relationships of different patterns of multimorbidity and SCD-related functional difficulties, including seven demographic variables (age group, sex, urban/rural, educational level, marital status, employment, and household income) and, five health-related risk behaviors (heavy drinker, physical exercise except job, fruit consumption at least one time per day, vegetable consumption at least one time per day, smoking status). Observations that coded as “refused,” “not sure,” “not asked,” or “don’t know” were recoded as missing.
Statistical Analyses
All the statistical analyses were conducted using R with main packages poLCA for latent class analysis (LCA), mice for multiple imputation, twang for propensity score weighting, survey for logistic regression with weights.
LCA was applied to classify individuals to homogeneous subgroups of multimorbidity patterns, with the reported chronic diseases or conditions as indicators (Lazarsfeld, 1950). The large number of variables in the LCA resulted in about 97% of cells being small (i.e., containing less than five observations) in the joint tabulation of the 17 chronic diseases, which leads to low power of chi-square goodness-of-fit tests and numerical problems in estimating parameters and the asymptotic variance-covariance matrix (Galindo Garre & Vermunt, 2006; Langeheine et al., 1996; Wurpts & Geiser, 2014). Therefore, composite variables including cancer (35%), hepatitis (5.5%), blind/deaf (35.8%), and asthma/COPD (33.5%) were created based on variables with similar traits including skin/other cancer (17.2%/18.7%), hepatitis B/C (2.2%/4.1%), blind or serious difficulty seeing/deaf or serious difficulty hearing (17.2%/25%), and asthma/COPD or emphysema or chronic bronchitis (19.2%/23.4%). Hepatitis was removed because of large percent of missing (85.3%) (see Supplementary Material Appendix 1). Finally, 12 chronic conditions were included in the LCA with the percent of small cells decreased to 73%. The missing percentages for the 12 variables ranged from 0.3% to 8.9%. Further details of the frequency table and missing percentage for all the variables in this study can be found in Supplementary Material Appendix 1. The correlation matrix for variables in LCA was examined using the tetrachoric method (Pearson, 1900); no extremely high correlations were observed (see Supplementary Material Appendix 2). A series of LCA models with one to eight subgroups were fit to the data and the optimal number of subgroups was determined based on Bayesian information criteria (BIC), consistent Akaike information Criteria (CAIC), adjusted BIC (ABIC) and clinical significance. Smaller BIC, CAIC and ABIC indicate a better goodness-of-fit. Average posterior class probabilities (AvePP) and odds of correct classification ratio (OCC) were calculated to assess the degree of subgroup separation of the selected LCA model. AvePPs values lager than 0.7 and OCCs 5 or larger were used to define an acceptable degree of subgroup separation (Nagin, 2005). Meaningful labels were given according to the characteristics of the combination of chronic diseases and existing literature on clustering of multimorbidity using population-based data. The predicted subgroup membership for each individual was estimated based on the probability of having chronic conditions; it was then treated as a predictor in the subsequent logistics regression analyses.
Descriptive statistics including the percentage of observations for multimorbidity subgroup by demographic factors and percentage of observations for SCD-related functional status by multimorbidity groups and health-related risk factors were generated taking the sample weights into account. Chi-square tests adjusting for sample weights were used to compare the distributions of demographic factors across multimorbidity groups and compare the distributions of multimorbidity groups and the five health-related risk factors across SCD-related functional status (Kariya, 1984; Lumley, 2020).
After classifying participants into subgroups, multiple imputation (MI) was implemented for missing data, including age, race, educational level, household income, employment status, and the five health-related risk factors (Sterne et al., 2009). 20 imputations were generated to ensure accurate standard errors (Von Hippel, 2020). Logistic regression was then used to examine the association of the defined multimorbidity subgroups with SCD-related functional difficulties. For each imputed data set, inverse probability of treatment weighting (IPTW) with propensity score (PS) method was used to control confounding effects of age, sex, race, urban/rural, education, income, and employment status (Chen & Moskowitz, 2016; GW, 2000; Leite, 2019b). Generalized boosted modeling (GBM), which is a data mining method that does not require a specification of statistical model and is able to automatically take interactions and nonlinear effects into account, was applied to estimate the IPTWs (Leite, 2020; Strobl et al., 2009). Distribution of propensity scores and overlap between groups (i.e., common support) were evaluated using the Box-and-Whiskers plots (Leite, 2019a). Covariate balance was evaluated by comparing the absolute standardized difference between unweighted and weighted means or proportions across groups (Leite, 2019b); a value below 0.1 indicates an adequate covariate balance (Austin, 2011). Summary statistics (means, standard deviation, and range of the estimated PSs) were generated to assess the existence of extreme values. Final weights were derived by multiplying the IPTWs with the CDC sample weights, which incorporate the complex survey design and adjusted of characteristics of the population to make the collected data more representative of the population (CDC, 2020). Logistic regression results were then combined across all 20 imputations.
Results
Patterns of Multimorbidity Among People with SCD
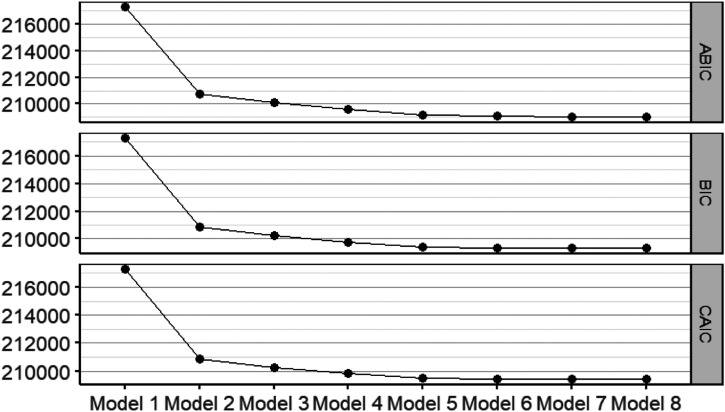
The LCA model with five subgroups was selected, as minimum model improvements (i.e., lower BIC, CAIC, and ABIC values) were observed beyond this model (Figure 1). In addition, results from this model were the most interpretable and clinically meaningful according to relevant literature (Collerton et al., 2016; Marengoni et al., 2020; Zheng et al., 2021).
Figure 1.
BIC, CAIC, and ABIC of the Eight Latent Class Analysis Models.
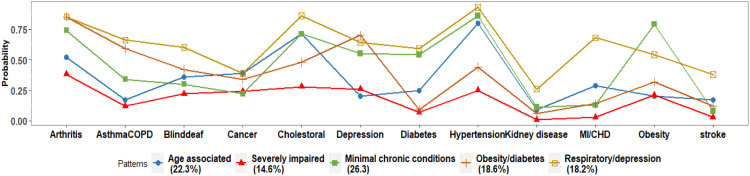
The five subgroups were labeled according to their specific multimorbidity patterns (Table 1). Classification of the SCD population is shown in Table 1 and Figure 2. Twenty-six percent of the study population were classified into the minimal chronic conditions group with low probabilities of having any of the chronic diseases; 22.3% were classified into age-associated group with high probability of having hypertension, high cholesterol level, and arthritis; 18.6% were classified into obesity or diabetes group with high probabilities of having obesity, diabetes, age-associated chronic conditions; 18.2% were classified into respiratory or depression group with high probabilities of having asthma or COPD, depression and arthritis; and 14.6% were classified into the severely impaired group with high probability of having the majority of the chronic diseases.
Table 1.
Probabilities of Having Chronic Conditions and Mean Number of Chronic Diseases for Each Multimorbidity Pattern Group.
| Age-associated (22.3%) | Severely impaired(14.6%) | Minimal chronic conditions (26.3%) | Obesity/diabetes(18.6%) | Respiratory/depression(18.2%) | |
|---|---|---|---|---|---|
| Hypertension | 0.80 | 0.93 | 0.25 | 0.86 | 0.44 |
| Diabetes | 0.25 | 0.59 | 0.07 | 0.54 | 0.09 |
| Cholesterol | 0.71 | 0.86 | 0.28 | 0.71 | 0.48 |
| MI/CHD | 0.29 | 0.68 | 0.03 | 0.13 | 0.14 |
| stroke | 0.17 | 0.38 | 0.03 | 0.08 | 0.12 |
| Asthma/COPD | 0.17 | 0.66 | 0.12 | 0.34 | 0.59 |
| Arthritis | 0.52 | 0.85 | 0.38 | 0.74 | 0.85 |
| Blind/deaf | 0.36 | 0.60 | 0.22 | 0.30 | 0.42 |
| Depression | 0.20 | 0.64 | 0.26 | 0.55 | 0.70 |
| Kidney disease | 0.09 | 0.26 | 0.01 | 0.11 | 0.06 |
| Obesity | 0.20 | 0.54 | 0.21 | 0.79 | 0.32 |
| Cancer | 0.39 | 0.39 | 0.24 | 0.22 | 0.34 |
| Mean number of chronic diseases | 4.1 | 7.7 | 1.8 | 5.3 | 4.6 |
Note. CHD—coronary heart disease; MI—myocardial infarction; COPD—chronic obstructive pulmonary disease. The number in bold indicates higher probability of having that chronic disease/condition in that multimorbidity subgroup.
Figure 2.
Probabilities of Having Chronic Conditions for Each Latent Class.
Factors Associated with Multimorbidity Patterns
Demographic factors across groups are shown in Table 2. The eight demographic factors were distributed differently across the five groups. Age-associated group had the lowest percentage of participants in the age range of 45–49; more than 60% of this group was aged 65 years and older and mostly retired. Minimal chronic condition group was mostly not-white, living in urban counties, better-educated, having higher income, mostly working, married or a member of unmarried couple comparing with other groups.
Table 2.
Distribution of Demographic Factors by Multimorbidity Patterns.
| Age associated | Severely impaired | Minimal chronic conditions | Obesity/diabetes | Respiratory/depression | p | |
|---|---|---|---|---|---|---|
| n | 3365 | 2231 | 4222 | 2987 | 2816 | |
| Age group | <0.001 | |||||
| 45–49 | 4.3% | 8.1% | 14.3% | 12.2% | 13.2% | |
| 50–54 | 12.4% | 11.4% | 17.4% | 16.4% | 20.2% | |
| 55–59 | 11.3% | 12.6% | 13.0% | 16.5% | 19.4% | |
| 60–64 | 11.2% | 21.2% | 14.3% | 18.7% | 16.3% | |
| 65–69 | 10.5% | 14.1% | 9.1% | 12.2% | 9.9% | |
| 70–74 | 12.7% | 12.8% | 8.4% | 11.7% | 8.1% | |
| 75–79 | 14.7% | 11.2% | 8.6% | 6.4% | 5.9% | |
| 80+ | 22.9% | 8.7% | 14.9% | 5.9% | 7.0% | |
| Sex male | 52.3% | 46.2% | 45.7% | 43.3% | 35.1% | <0.001 |
| Race Not-white | 69.1% | 68.2% | 74.4% | 67.7% | 76.3% | 0.002 |
| Urban status—rural | 7.7% | 11.0% | 6.8% | 9.7% | 10.0% | <0.001 |
| Education | <0.001 | |||||
| < high school | 21.3% | 30.7% | 15.1% | 21.7% | 23.0% | |
| = high school | 31.6% | 31.1% | 28.1% | 31.2% | 34.5% | |
| < college/technical school | 26.0% | 28.2% | 30.8% | 30.0% | 28.2% | |
| >= college/technical school | 21.1% | 10.0% | 26.0% | 17.1% | 14.3% | |
| Income | <0.001 | |||||
| <$15,000 | 14.6% | 31.8% | 13.9% | 20.0% | 27.8% | |
| $15,000–$25,000 | 26.4% | 33.0% | 18.5% | 25.6% | 26.2% | |
| $25,000–$35,000 | 14.2% | 11.6% | 10.7% | 13.6% | 10.0% | |
| $35,000–$50,000 | 13.5% | 8.3% | 13.2% | 12.2% | 13.1% | |
| $50,000 + | 31.3% | 15.3% | 43.7% | 28.6% | 22.9% | |
| Employment | <0.001 | |||||
| Not work | 31.8% | 56.8% | 25.5% | 44.5% | 54.6% | |
| Retired | 49.1% | 36.2% | 34.7% | 32.8% | 26.8% | |
| Working | 19.1% | 7.0% | 39.8% | 22.7% | 18.6% | |
| Married/couple | 51.1% | 43.2% | 56.4% | 50.1% | 42.2% | <0.001 |
Note. The percentages were calculated with sample weights. For binary variables, only one category is shown, where the percentage for the other category can be derived by using one substitute the value in the table. p-values is for the chi-square tests with sample weights.
Association Between Multimorbidity Patterns and Functional Difficulties
Physical exercise, vegetable consumption, smoking status, and multimorbidity patterns were distributed differently when comparing those with and without SCD-related functional difficulties groups (Table 3). All larger proportion of participants in the minimal chronic conditions group reported no SCD-related functional difficulties compared to other groups. A larger proportion of people without SCD-related functional difficulties reported doing physical exercise, consuming fruit or vegetables at least one time per day, and having never smoked or formerly smoked comparing with people with SCD-related functional difficulties.
Table 3.
Distribution of Multimorbidity Patterns and Health-related Risk Behaviors by SCD-related Functional Difficulties.
| SCD-related functional difficulties | p | ||
|---|---|---|---|
| Yes, % | No, % | ||
| Multimorbidity patterns | <0.001 | ||
| Age associated | 16.71 | 23.20 | |
| Severely impaired | 9.74 | 19.59 | |
| Minimal chronic conditions | 18.92 | 33.51 | |
| Obesity/diabetes | 21.88 | 18.37 | |
| Respiratory/depression | 22.91 | 15.18 | |
| Heavy drinker (Yes) | 5.40 | 5.97 | 0.45 |
| Physical exercise (Yes) | 47.93 | 61.48 | <0.001 |
| Fruits ≥1 time per day (Yes) | 55.44 | 58.06 | 0.11 |
| Vegetables ≥1 time per day (Yes) | 67.39 | 76.20 | <0.001 |
| Smoke status | <0.001 | ||
| Never smoked | 36.92 | 44.23 | |
| Former smoker | 28.33 | 38.21 | |
| Current smoker | 34.75 | 17.56 | |
Note. The percentages were calculated with sample weights. For binary variables, only one category is shown, where the percentage for the other category can be derived by using one substitute the value in the table. p-values is for the chi-square tests with sample weights.
After implementing the IPTW propensity score method, the absolute standardized difference of unweighted and weighted means and proportions for each pair of the groups on all imputed data sets are below 0.1 (Supplementary Material Appendix 3 Figure 1–20). This indicates that the covariate balance was achieved for all measured covariates. Extreme propensity scores were not observed (Supplementary Material Appendix 3 Table 1).
Results from logistic regression models found that the multimorbidity pattern groups had higher odds of reporting SCD-related functional difficulties when compared to the minimal chronic conditions group, except the age-associated group (Table 4). Compared with minimal chronic conditions group, the severely impaired group was most likely to report SCD-related functional difficulties (OR = 2.43, 95% CI: 1.92–3.08), followed by respiratory/depression group (OR = 1.72, 95% CI: 1.39–2.12), and obesity/diabetes group (OR = 1.57, 95% CI: 1.29–1.92), controlling for other factors. Further, paired comparisons found that the odds of reporting SCD-related functional difficulties were the highest in the severely impaired group compared with the age-associated (OR = 2.08, 95% CI: 1.60–2.69), obesity/diabetes (OR = 1.55, 95% CI: 1.24–1.93) and respiratory/depression group (OR = 1.42, 95% CI: 1.13–1.78). The odds of reporting SCD-related functional difficulties in obesity/diabetes and respiratory/depression groups did not significantly differ (OR = 1.09, 95% CI: 0.90–1.32), but both were significantly higher than age-associated group with OR equals 1.34 (95% CI: 1.07–1.68) and 1.47 (95% CI: 1.17–1.84), respectively.
Table 4.
Odds Ratios of SCD-related Functional Difficulties.
| Variable | Odds ratio (95%CI) | p-value |
|---|---|---|
| Intercept | 0.33 (0.28,0.40) | <.001 |
| Multimorbidity pattern (ref = Minimal chronic conditions) | ||
| Age-associated | 1.17 (0.92, 1.49) | 0.2 |
| Severely impaired | 2.43 (1.92, 3.08) | <.001 |
| Obesity/diabetes | 1.57 (1.29, 1.92) | <.001 |
| Respiratory/depression | 1.72 (1.39, 2.12) | <.001 |
| Severely impaired versus age-associated | 2.08 (1.60, 2.69) | <.001 |
| Obesity/diabetes versus age-associated | 1.34 (1.07, 1.68) | 0.01 |
| Respiratory/depression versus age-associated | 1.47 (1.17, 1.84) | <.001 |
| Severely impaired versus obesity/diabetes | 1.55 (1.24, 1.93) | <.001 |
| Respiratory/depression versus obesity/diabetes | 1.09 (0.90, 1.32) | 0.37 |
| Severely impaired versus respiratory/depression | 1.42 (1.13, 1.78) | 0.003 |
| Heavy drinker (ref = Yes) | 0.86 (0.64, 1.16) | 0.33 |
| Physical exercise (ref = Yes) | 1.47 (1.27, 1.69) | <.001 |
| Fruit consumption (ref = Yes) | 0.93 (0.80, 1.08) | 0.34 |
| Vegetable consumption (ref = Yes) | 1.32 (1.10, 1.57) | 0.002 |
| Smoking status (ref = never smoked) | ||
| Former smoker | 0.86 (0.73, 1.01) | 0.07 |
| Current smoker | 2.11 (1.75, 2.54) | <.001 |
Note. ref—reference group; Odds ratio indicate the odds ratio of having SCD-related functional difficulties in that group and the reference group.
The odds of reporting SCD-related functional difficulties in people reported not having physical exercise was 47% (95% CI: 27%–69%) higher than those who did have. Vegetable intake at least one time per day was significantly related to a 32% (95% CI: 10%–57%) increase of the odds of reporting SCD-related functional difficulties. Compared to participants who never smoked, current smoker had 111% (95% CI: 75%–154%) increased odds of reporting SCD-related functional difficulties. Heavy drinker and fruit consumption were not significantly related to the odds of reporting SCD-related functional difficulties. The difference of odds between former smokers and never smokers was also not significant.
Conclusions and Discussions
This research is the first nationwide study to investigate multimorbidity patterns among the US noninstitutionalized residents aged 45 years and older who reported experiencing SCD. Five multimorbidity patterns were revealed by latent class analysis—age-associated (22.3%), severely impaired (14.6%), minimal chronic conditions (26.3%), obesity or diabetes (18.6%) and respiratory or depression (18.2%).
Previous research identified five groups among the noninstitutionalized general population aged 50 years and older in the US (Zheng et al., 2021). There are several differences in the multimorbidity patterns observed in the SCD population within this study and general population in previous literature. First, the five groups in our study had more chronic conditions on average compared with the groups with a similar rank of mean number of chronic conditions in their study. For example, both studies found a group with the smallest probability of having almost all the chronic diseases, but the group in this study (minimal chronic condition group) had an average number of chronic conditions of 1.8, whereas the group in the other study (healthy group) has an average number of 0.6. Both studies found a group with the highest probability of having almost all the chronic diseases. However, in this study, that group (severely impaired group) had an average number of chronic conditions of 7.7 compared to (complex cardiometabolic group) an average number of 1.2 in the other study. Second, 85.1% of the general population were classified into the two groups with the smallest and second smallest mean number of chronic conditions (healthy and age-associated), while only 40.8% of people with SCD were classified into the minimal chronic conditions and age-associated groups. Third, 14.6% of people with SCD were in the most severe group (severely impaired group), while only 3.2% of the general population were classified into the most severe group (cardiometabolic group). Moreover, the most severe group among the SCD population was more acute than it in the general population.
Comparing with the minimal chronic conditions group, the severely impaired group has the greatest increased risk of reporting SCD-related functional difficulties, followed by respiratory/depression group and obesity/diabetes group. Previous studies examining the association of chronic diseases and SCD-related functional difficulties found that a greater number of chronic disease was related to more severe SCD-related functional difficulties (Dunlop et al., 2002; Jindai et al., 2016; Taylor et al., 2020; Yap et al., 2020; Yokota et al., 2017); however, these studies treated chronic diseases as a cumulative count, categorical indicator or multiple covariates. Results here found the age-associated and respiratory/depression groups reported similar mean number of chronic diseases but the odds of having SCD-related functional difficulties in the respiratory/depression group was 1.47 (95% CI: 1.17, 1.84) times the odds in the age-associated group. Moreover, the mean number of reported chronic diseases in age-associated group (4.1) was much higher than in the minimal chronic condition group (1.8) but the odds of reporting SCD-related functional difficulties in these two groups was not significantly different (OR = 1.17, 95% CI: 0.92, 1.49).
Our findings support that people can benefit from doing physical exercise and eating vegetables at least one time per day, as this was associated with decreased odds of reporting SCD-related functional difficulties regardless of multimorbidity group. Current smokers had a higher risk SCD-related functional difficulties compared to never smoker.
This study has several limitations. First, only noninstitutionalized adults and individuals from the 32 states that included the cognitive decline optional module were included. Therefore, caution is needed when attempting to generalize findings to the whole population with SCD in the US. Measurements of cognitive decline and SCD-related functional difficulties in this study are self-reported at only one time point of a year and rely on only one or two questions. As such, recall bias and misclassification may be present. However, it has been shown that SCD occurs at the preclinical state of AD which cannot be detected by objective tests (Jessen et al., 2014, 2020). Future studies could consider other validated multiple-item questionnaires to measure SCD-related functional difficulties such as those in Diaz-Galvan et al., (2021) and Rabin et al., (2020). Third, the sample weighting used to reflect the complex survey design were not considered in the LCA models. It is recommended to use sample weights when the sampling weights are related to the class membership (Vermunt, 2002). Demographic factors such as age has been shown to be associated with chronic disease status (Anesetti-Rothermel & Sambamoorthi, 2011; Barile et al., 2015) and thus could affect the class participants belong to; however, the “poLCA” package used in this study does not allow the use of weights (Linzer & Lewis, 2013). Incorporating sample weights in the LCA should be considered for future research. Fourth, measurement invariance for age group, and sex was not tested which could affect the estimated item response probabilities (Vermunt, 2002). However, 73% of small cells were still present after combining conditions with low prevalence and the chi-square test for measurement invariance is not advised when having more than 20% small cells (Linzer & Lewis, 2013). Fifth, the conclusions based on the two discrimination ability indices of the selected LCA model are not consistent. Only two out of five AvePPs are above 0.7 indicating that the subgroups were not well separated (Supplementary Material Appendix 4 Table 1). In contrast, all the OCCs for the five subgroups were above 5 indicating good latent class separation and high assignment accuracy. This may indicate the existence of overlap among the five latent subgroups, potentially, due to the high heterogeneity among older adults within groups (Zheng et al., 2020a). Nevertheless, there were enough differences among the five multimorbidity groups to distinguish them. For example, the probability of having obesity is the highest in obesity/diabetes group while low in other groups, and the probability of having depression is the highest in the respiratory/depression group while low in the minimal chronic condition and age-associated groups. Six, the estimated PSs may not have adequate common support across the five multimorbidity classes as the boxplots not well overlapped (Supplementary Material Appendix 4 Figure 1). However, the lack of common support may or may not result in covariates balance in propensity score weighting analysis (Leite, 2019a). Moreover, the absolute standardized difference in the means and proportions for each pair of the subgroups were below 0.1, indicating the covariates balance was achieved. Lastly, the predicted classes in the LCA are used as a predictor in the subsequent analysis; misclassification may introduce bias in the estimation of following analysis (Croon, 2002). Advanced methods such as bias-adjusted three-step methods (Asparouhov & Muthén, 2014; Z. Bakk et al., 2013) and two-step method (Bakk & Kuha, 2018) were proposed in recent years and can be explored in the future.
Missing observations were assumed to be missing at random, which is likely a reasonable assumption according to Supplementary Material Appendix 5. Multiple imputation was not applied on the variables in the LCA because the Expectation–Maximization algorithm used in the “poLCA” package allowed missing data in the observed variables (Linzer & Lewis, 2013). Only 8.9% of observations were missing of data used in the LCA.
This study may provide valuable information to help understand multimorbidity patterns among the SCD population and contributes to the evidence of vulnerable populations of SCD-related functional difficulties. The results may provide insights for clinicians and others to improve health resources allocation and managing patient complexity. The characteristics of high-risk groups identified by this study may help the relevant organizations to develop and implement interventions to prevent more serious consequences of multimorbidity. The relevant organizations and media could promote healthy lifestyle especially doing physical exercise, eating vegetables and stopping smoking by educating the public of the decreased risk of SCD-related functional difficulties benefitted from these behaviors.
Supplemental Material
Supplemental Material, sj-pdf-1-jah-10.1177_08982643221080287 for Multimorbidity Patterns in US Adults with Subjective Cognitive Decline and Their Relationship with Functional Difficulties by Yixiu Liu and Depeng Jiang in Journal of Aging and Health
Acknowledgments
We would like to thank Dr. Lisa Lix, Olawale Ayilara and Ali Neshati in the Visual and Automated Disease Analytics Graduate Training Program foundation course for their valuable feedbacks in completing this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Visual and Automated Disease Analytics (VADA) Graduate Training Program of Natural Science and Engineering Research Council of Canada (NSERC), and Children’s Hospital Research Institute of Manitoba (CHRIM) Foundation.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Yixiu Liu https://orcid.org/0000-0002-1989-9832
References
- Alzheimer’s Association International Conference (2019). Lifestyle interventions provide maximum memory benefit when combined, may offset elevated Alzheimer’s risk due to genetics, pollution. Alzheimer’s Association International Conference, San Diego, USA, July 31–Aug 4, 2022. https://www.alz.org/aaic/releases_2019/sunLIFESTYLE-jul14.asp [Google Scholar]
- Anesetti-Rothermel A., Sambamoorthi U. (2011). Physical and mental illness burden: Disability days among working adults. Population Health Management, 14(5), 223–230. 10.1089/pop.2010.0049 [DOI] [PubMed] [Google Scholar]
- Asparouhov T., Muthén B. (2014). Auxiliary variables in mixture modeling: three-step approaches using Mplus. Structural Equation Modeling, 21(3), 329–341. 10.1080/10705511.2014.915181 [DOI] [Google Scholar]
- Austin P. C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research, 46(3), 399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakk Z., Kuha J. (2018). Two-step estimation of models between latent classes and external variables. Psychometrika, 83(4), 871–892. 10.1007/s11336-017-9592-7 [DOI] [PubMed] [Google Scholar]
- Bakk Z., Tekle F. B., Vermunt J. K. (2013). Estimating the association between latent class membership and external variables using bias-adjusted three-step approached. American Sociological Association, 43(1), 272–311. 10.1177/0081175012470644 [DOI] [Google Scholar]
- Barile J. P., Mitchell S. A., Thompson W. W., Zack M. M., Reeve B. B., Cella D., Smith A. W. (2015). Patterns of chronic conditions and their associations with behaviors and quality of life, 2010. Preventing Chronic Disease, 12(12), 1–11. 10.5888/pcd12.150179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo B., Gatz M., Xu W., Marengoni A., Pedersen N. L., Fratiglioni L. (2013). Relation of multimorbidity to subjective and objective cognitive impairment: a population-based twin study. Journal of Alzheimer’s Disease, 36(2), 275–284. 10.3233/JAD-122050.Relation [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2009). The power of prevention. CDC. 10.1111/idh.12067 [DOI] [Google Scholar]
- CDC (2014). About BRFSS. https://www.cdc.gov/brfss/about/index.htm [Google Scholar]
- CDC (2018). BRFSS statistical brief: Cognitive decline pptional module (1–16). https://www.cdc.gov/aging/data/BRFSS-statistical-brief-cognitive-decline-508.pdf [Google Scholar]
- CDC (2019. a). Behavioral risk factor surveillance system. 10.1097/00005768-199706001-00025 [DOI] [Google Scholar]
- CDC (2019. b). Subjective cognitive decline — a public health issue. https://www.cdc.gov/aging/aginginfo/subjective-cognitive-decline-brief.html [Google Scholar]
- CDC (2020). The behavioral risk factor surveillance system complex sampling weights and preparing 2019 BRFSS module. Data for analysis. https://www.cdc.gov/brfss/annual_data/2019/pdf/Complex-Smple-Weights-Prep-Module-Data-Analysis-2019-508.pdf [Google Scholar]
- CDC (2021). Chronic diseases in America. https://www.cdc.gov/chronicdisease/pdf/infographics/chronic-disease-H.pdf [Google Scholar]
- Chen K. P., Moskowitz A. (2016). Comparative effectiveness: propensity score analysis Secondary analysis of electronic health records (pp. 339–349). 10.1007/978-3-319-43742-2_23 [DOI] [PubMed] [Google Scholar]
- Collerton J., Jagger C., Yadegarfar M. E., Davies K., Parker S. G., Robinson L., Kirkwood T. B. L. (2016). Deconstructing complex multimorbidity in the very old: findings from the Newcastle 85+ Study. BioMed Research International, 2016, 8745670. 10.1155/2016/8745670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croon M. (2002). Using predicted latent scores in general latent structure models. Latent variable and latent structure models (1st ed., pp. 195–223). Psychology Press. [Google Scholar]
- Diaz-Galvan P., Ferreira D., Cedres N., Falahati F., Hernández-Cabrera J. A., Ames D., Barroso J., Westman E. (2021). Comparing different approaches for operationalizing subjective cognitive decline: impact on syndromic and biomarker profiles. Scientific Reports, 11(1), 4356. 10.1038/s41598-021-83428-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore N. R., DuMontier C., Yildirim C., La J., Epstein M. M., Cheng D., Cirstea D., Yellapragada S., Abel G. A., Gaziano J. M., Do N., Brophy M., Kim D. H., Munshi N. C., Driver J. A. (2021). Defining multimorbidity and its impact in older United States veterans newly treated for multiple myeloma. J Natl Cancer Inst., 113(8), 1084–1093. 10.1093/jnci/djab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop D. D., Manheim L. M., Sohn M. W., Liu X., Chang R. W. (2002). Incidence of functional limitation in older adults: The impact of gender, race, and chronic conditions. Archives of Physical Medicine and Rehabilitation, 83(7), 964–971. 10.1053/apmr.2002.32817 [DOI] [PubMed] [Google Scholar]
- Galindo Garre F., Vermunt J. K. (2006). Avoiding boundary estimates in latent class analysis by bayesian posterior mode estimation. Behaviormetrika, 33(1), 43–59. 10.2333/bhmk.33.43 [DOI] [Google Scholar]
- GW I. (2000). The role of the propensity score in estimating dose-response functions. Biometrika. [Google Scholar]
- Jessen F., Amariglio R. E., Buckley R. F., van der Flier W. M., Han Y., Molinuevo J. L., Rabin L., Rentz D. M., Rodriguez-Gomez O., Saykin A. J., Sikkes S. A. M., Smart C. M., Wolfsgruber S., Wagner M. (2020). The characterisation of subjective cognitive decline. The Lancet Neurology, 19(3), 271–278. 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Amariglio R. E., Van Boxtel M., Breteler M., Ceccaldi M., Chételat G., Dubois B., Dufouil C., Ellis K. A., Van Der Flier W. M., Glodzik L., Van Harten A. C., De Leon M. J., McHugh P., Mielke M. M., Molinuevo J. L., Mosconi L., Osorio R. S., Perrotin A., Wagner M. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s and Dementia, 10(6), 844–852. 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindai K., Nielson C. M., Vorderstrasse B. A., Quiñones A. R. (2016). Multimorbidity and functional limitations among adults 65 or older, NHANES 2005-2012. Preventing Chronic Disease, 13(11), 1–11. 10.5888/pcd13.160174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul-Larsen H. G., Christensen L. D., Bandholm T., Andersen O., Kallemose T., Jørgensen L. M., Petersen J. (2020). Patterns of multimorbidity and differences in healthcare utilization and complexity among acutely hospitalized medical patients (≥65 years) – a latent class approach. Clinical Epidemiology, 12, 245–259. 10.2147/CLEP.S226586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya T. (1984). On chi-squared tests for multiway contingency tables with proportions estimated from survey data. Annals of Statistics, 12(1), 46–60. 10.1214/aos/1176346391 [DOI] [Google Scholar]
- Laires P. A., Dias S., Gama A., Moniz M., Pedro A. R., Soares P., Aguiar P., Nunes C. (2021). The association between chronic disease and serious COVID-19 outcomes and its influence on risk perception: Survey study and database analysis. JMIR Public Health and Surveillance, 7(1), 1–14. 10.2196/22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeheine R., Pannekoek J., Van De Pol F. (1996). Bootstrapping goodness-of-fit measures in categorical data analysis. Sociological Methods and Research, 24(4), 492–516. 10.1177/0049124196024004004 [DOI] [Google Scholar]
- Lazarsfeld P. F. (1950). The logical and mathematical foundations of latent strature analysis. Princeton University Press. [Google Scholar]
- Leite W. (2019. a). Propensity score estimation. Practical propensity score methods using R (pp. 19–46). SAGE Publication, Inc. 10.4135/9781071802854.n2 [DOI] [Google Scholar]
- Leite W. (2019. b). Propensity score weighting. Practical propensity score methods using R (pp. 47–68). SAGE Publication, Inc. 10.4135/9781071802854.n3 [DOI] [Google Scholar]
- Leite W. (2020). Propensity score methods for multiple treatments. Practical Propensity score methods using R (pp. 111–129). SAGE Publication, Inc. 10.4135/9781071802854.n6 [DOI] [Google Scholar]
- Linzer D. A., Lewis J. (2013). poLCA: Polytomous variable latent class analysis. Journal of Statistical Software, 42(10), 1–29. http://dlinzer.github.io/poLCA/ [Google Scholar]
- Lumley A. T. (2020). Survey: Analysis of complex survey samples. http://r-survey.r-forge.r-project.org/survey/ [Google Scholar]
- Marengoni A., Roso-Llorach A., Vetrano D. L., Fernández-Bertolín S., Guisado-Clavero M., Violán C., Calderón-Larrañaga A. (2020). Patterns of multimorbidity in a population-based cohort of older people: Sociodemographic, lifestyle, clinical, and functional differences. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 75(4), 798–805. 10.1093/gerona/glz137 [DOI] [PubMed] [Google Scholar]
- Melis R. J. F., Marengoni A., Rizzuto D., Teerenstra S., Kivipelto M., Angleman S. B., Fratiglioni L. (2013). The influence of multimorbidity on clinical progression of dementia in a population-based cohort. Plos One, 8(12), e84014. 10.1371/journal.pone.0084014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. L., Xu J., Kochanek K. D. (2013). Deaths: Final data for 2010. National Vital Statistics Reports, 61(4), 1–117. [PubMed] [Google Scholar]
- Nagin D. S. (2005). Group-based modeling of development. Harvard University Press. [Google Scholar]
- National Institute on Aging (2020). Cognitive health and older adults. National Institute on Aging. https://www.nia.nih.gov/health/cognitive-health-and-older-adults [Google Scholar]
- Neto A. S., Nitrini R. (2016). Subjective cognitive decline: The first clinical manifestation of alzheimer’s disease? Dementia e Neuropsychologia, 10(3), 170–177. 10.1590/s1980-5764-2016dn1003002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunesa B. P., Floresb T. R., Mielkeb G. I., Thuméa E., Facchinib L. A. (2016). Multimorbidity and mortality in older adults: A systematic review and meta-analysis. Gerontology and Geriatrics, 67, 130–138. 10.1016/j.archger.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Pearson K. (1900). Mathematical contributions to the theory of evolution. —VII. On the correlation of characters not quantitatively measurable. Philosophical Transactions of the Royal Society of London, 195(262–273), 1–47. 10.1098/rsta.1900.0022 [DOI] [Google Scholar]
- Rabin L. A., Wang C., Mogle J. A., Lipton R. B., Derby C. A., Katz M. J. (2020). An approach to classifying subjective cognitive decline in community-dwelling elders. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring, 12(1), 1–13. 10.1002/dad2.12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salive M. E. (2013). Multimorbidity in older adults. Epidemiologic Reviews, 35(1), 75–83. 10.1093/epirev/mxs009 [DOI] [PubMed] [Google Scholar]
- Sterne J. A. C., White I. R., Carlin J. B., Spratt M., Royston P., Kenward M. G., Wood A. M., Carpenter J. R. (2009). Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ (Online), 339(7713), 157–160. 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl C., Malley J., Tutz G. (2009). An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychological Methods, 14(4), 323–348. 10.1037/a0016973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. A., Bouldin E. D., Greenlund K. J., McGuire L. C. (2020). Comorbid chronic conditions among older adults with subjective cognitive decline, United States, 2015–2017. Innovation in Aging, 4(1), 145–210. 10.1093/geroni/igz045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. A., Bouldin E. D., McGuire L. C. (2018). Subjective cognitive decline among adults aged ≥45 years — United States, 2015–2016. CDC, MMWR. Morbidity and Mortality Weekly Report, 67(27), 753–757. 10.15585/mmwr.mm6727a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt J. K. (2002). Latent class analysis of complex sampling survey data. Journal of the American Statistical Association, 94(459), 736–737. [Google Scholar]
- Von Hippel P. T. (2020). How many imputations do you need? A two-stage calculation using quadratic rule. Sociological Methods & Research, 49(3), 699–718. 10.1177/0049124117747303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2021). Obesity. https://www.who.int/health-topics/obesity#tab=tab_1 [Google Scholar]
- Wurpts I. C., Geiser C. (2014). Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a Monte-Carlo study. Frontiers in Psychology, 5, 120–215. 10.3389/fpsyg.2014.00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap K. H., Warren N., Allotey P., Reidpath D. D. (2020). Chronic disease profiles of subjective memory complaints: A latent class analysis of older people in a rural Malaysian community. Aging and Mental Health, 24(5), 709–716. 10.1080/13607863.2018.1550632 [DOI] [PubMed] [Google Scholar]
- Yokota R. T. C., Nusselder W. J., Robine J. M., Tafforeau J., Deboosere P., Moura L., Andrade S. S. C. A., Castro S. S., Van Oyen H. (2017). Contribution of chronic conditions to functional limitations using a multinomial outcome: Results for the older population in Belgium and Brazil. Archives of Public Health, 75(1), 1–12. 10.1186/s13690-017-0235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D. D., Christ S. L., Lam B. L., Feaster D. J., McCollister K., Lee D. J. (2020. a). Patterns of chronic conditions and their association with visual impairment and health care use. JAMA Ophthalmology, 138(4), 387–394. 10.1001/jamaophthalmol.2020.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D. D., McCollister K. E., Christ S. L., Lam B. L., Feaster D. J., Lee D. J. (2020. b). Chronic condition patterns in the US population and their association with health related quality of life. Preventive Medicine, 136, 106102. 10.1016/j.ypmed.2020.106102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D. D., Loewenstein D. A., Christ S. L., Feaster D. J., Lam B. L., McCollister K. E., Curiel-Cid R. E., Lee D. J. (2021). Multimorbidity patterns and their relationship to mortality in the US older adult population. Plos One, 16(1), 1–15. 10.1371/journal.pone.0245053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-jah-10.1177_08982643221080287 for Multimorbidity Patterns in US Adults with Subjective Cognitive Decline and Their Relationship with Functional Difficulties by Yixiu Liu and Depeng Jiang in Journal of Aging and Health