Summary
Background
Advanced imaging modalities have helped elucidate the cerebral alterations associated with neurological impairment caused by degenerative cervical myelopathy (DCM), but it remains unknown how brain functional network changes at different stages of myelopathy severity in DCM patients, and if patterns in network connectivity can be used to predict transition to more myelopathic stages of DCM.
Methods
This pilot cross-sectional study, which involves the collection of resting-state functional MRI (rs-fMRI) images and the modified Japanese Orthopedic Association (mJOA) score, enrolled 116 participants (99 patients and 17 healthy controls) from 2016 to 2021. The patient cohort included 21patients with asymptomatic spinal cord compression, 48 mild DCM patients, and 20 moderate or severe DCM patients. Functional connectivity networks were quantified for all participants, and the transition matrices were quantified to determine the differences in network connectivity through increasingly myelopathic stages of DCM. Additionally, a link prediction model was used to determine whether more severe stages of DCM can be predicted from less symptomatic stages using the transition matrices.
Findings
Results indicated interruptions in most connections within the sensorimotor network in conjunction with spinal cord compression, while compensatory connectivity was observed within and between primary and secondary sensorimotor regions, subcortical regions, visuospatial regions including the cuneus, as well as the brainstem and cerebellum. A link prediction model achieved an excellent predictive performance in estimating connectivity of more severe myelopathic stages of DCM, with the highest area under the receiver operator curve (AUC) of 0.927 for predicting mild DCM from patients with asymptomatic spinal cord compression.
Interpretation
A series of predictable changes in functional connectivity occur throughout the stages of DCM pathogenesis. The brainstem and cerebellum appear highly influential in optimizing sensorimotor function during worsening myelopathy. The link predication model can inclusively estimate brain alterations associated with myelopathy severity.
Funding
NIH/NINDS grants (1R01NS078494-01A1, and 2R01NS078494).
Keywords: Cervical, Myelopathy, Network, Connectivity, Functional
Research in context.
Evidence before this study
Degenerative cervical myelopathy (DCM) is the leading cause of spinal cord impairment in older individuals, resulting from progressive degeneration of the cervical spinal canal leading to cord compression and, eventually, neurological impairment. Advanced imaging modalities have helped elucidate cerebral alterations associated with neurological impairment caused by DCM, with increasing evidence suggests that neural plasticity through brain reorganization acts to preserve function in DCM patients.
We searched PubMed from January 2014, to December 2021, for relevant articles in English relating to brain functional connectivity associated with progressive myelopathy in DCM patients. Search terms included: “progressive degenerative cervical myelopathy”, “asymptomatic cervical spondylosis myelopathy”, “fMRI”, “brain functional network”, “brain functional connectivity”, “graph theory”, “link prediction”, “severity of degenerative cervical myelopathy prediction”. Previous studies have reported altered functional connectivity with within sensorimotor regions as a response to DCM, but no study has analyzed the brain functional network in asymptomatic DCM patients. We have also found that majority of studies associated functional connectivity changes with symptom severity evaluated by mJOA score, but no study has characterized the cortical pattern of functional brain network at different stages of myelopathy severity, ranging from asymptomatic spinal cord compression (mJOA=18) myelopathy to moderate/severe myelopathy (mJOA≤14), and it remains unknown if patterns in network connectivity be used to predict transition to more myelopathic stages of DCM. Additionally, some studies have suggested that the brain helps to compensate for functional deficits during progressive stages of DCM, but the importance of cerebellum and brainstem has been understudied.
Added value of this study
This study examined and characterized the cerebral patterns of the functional connectivity network for each stage of DCM symptom severity, as well as healthy controls and those with asymptomatic spinal cord compression. Results demonstrate that a series of predictable changes in functional connectivity occur as DCM patients transition from asymptomatic spinal cord compression through moderate/severe myelopathy. Interestingly, the brainstem and cerebellum appear highly influential in optimizing sensorimotor function during worsening myelopathy. The study also established a link prediction model based on a graph embedding method and a light GBM algorithm to estimate strengthening and/or weakening functional connectivity during transition from one stage of DCM severity to the next. The ROC AUC for transitions between symptomatic DCM stages ranged from 0.884 to 0.927, with the highest AUC for predicting mild from asymptomatic DCM.
Implications of all of the available evidence
This is the first cross-sectional observational study to characterize brain functional networks in DCM patients with varying degrees of myelopathy severity; and use the functional connectivity within the functional brain network exhibited during a particular myelopathy stage of DCM to estimate the network present in the next stage with higher symptom severity. The study has also evidenced a widespread cerebral network differences between the healthy and the asymptomatic stage, which may serve as an important predictive biomarker for monitoring DCM. Neurological function appears preserved through large-scale cerebral plasticity in cases of increased severity of myelopathy, and compensatory input from subcortical regions undergoes adaptations in response to increasing neurological deterioration. The proposed link predication model can inclusively estimate brain changes associated with myelopathy severity by both adding and removing connections within the functional network. Network features extracted via graph embedding method can contribute to future automated modeling by using a graph convolution network in simulating results of other image modalities, classifying myelopathy severity, predicting surgical outcomes, and monitoring disease progression.
Alt-text: Unlabelled box
Introduction
Degenerative cervical myelopathy (DCM) is the leading cause of spinal cord impairment in patients over the age of 55, often with profound negative effects on their quality of life.1 Progressive degeneration of the cervical spinal canal can lead to spinal cord compression and ultimately degenerative cervical myelopathy (DCM), manifesting as gait disturbance, limb dyscoordination, and sensory abnormalities. Damage to cervical neurons travelling both to and from the brain affects upstream cerebral structural and functional networks.
Increasing evidence suggests neural plasticity through brain reorganization acts to preserve function in DCM patients.2, 3, 4, 5, 6, 7 Using functional MRI, studies have showed widespread adaptive changes in bilateral primary motor cortex, supplementary motor area, premotor area, cingulate motor area, parietal cortex, and contralateral primary somatosensory cortex in cases of spinal cord injury and cervical compressive myelopathy due to spondylosis.2,6,8 Additionally, increased functional connectivity within sensorimotor networks, as well as decreased functional connectivity between subcortical regions and frontal lobe executive regions were found to be associated with worsened neurological impairment in DCM.3,5 However, most of these studies capture specific regional differences, but do not examine the global patterns in brain function with the goal of predicting severity of myelopathy. Thus, novel methods are needed to analyze and understand the entire brain functional network, which may be useful for predicting disease progression, understanding which brain regions are involved in adaptation and compensation, and predicting post-surgical outcomes.
A promising approach for modeling whole brain connectivity changes is the “link prediction model”, in which alterations in “links” within the network are predicted based on features intrinsic to the network itself.9 This technique has previously been applied to social and biological networks, and more recently to brain network alterations in Alzheimer's disease.10,11 We theorize that a link prediction model may be of significant value for describing brain connectivity in patients with DCM, as we hypothesize brain networks are continuously evolving as myelopathic symptoms intensify. Therefore, the current study characterized brain functional networks in healthy controls, asymptomatic patients with spinal cord compression, and DCM patients with varying degrees of myelopathy severity. We then used a link prediction model to quantify whole brain connectivity at different stages of myelopathy severity, as defined by their modified Japanese Orthopedic Association (mJOA) score. We hypothesized sensorimotor regions would drive widespread network changes in the brain associated with worsening myelopathic severity. We also theorized that subcortical regions would be the first to exhibit functional reorganization associated with neurological deterioration, presumably to preserve sensorimotor function. Lastly, we postulated that functional connectivity within the functional brain network exhibited during a particular symptomatic stage of DCM could be estimated from the network present in the preceding stage with lower symptom severity using a link prediction model.
Methods
Patient population
A total of 116 participants, including 78 DCM patients, 21 patients with asymptomatic spinal cord compression (SCC), and 17 healthy controls, were prospectively enrolled from 2016 to 2021 in a cross-sectional study involving observational MRI and evaluation of neurological dysfunction. DCM patients were recruited from an outpatient neurosurgery clinic, and each had spinal cord compression with evidence of spinal cord deformation and no visible cerebrospinal fluid signal around the spinal cord at the site of maximal compression on MRI. The mJOA score was used as a measure of neurological function,12 where lower value of mJOA represents a worse neurological impairment.
Ethics
This study was approved by the UCLA Office of the Human Research Protection Program, IRB # 11-001876. All patients signed Institutional Review Board-approved consent forms, and all analyses were performed in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
MR imaging acquisition
To evaluate functional connectivity and construct the functional network networks, resting-state functional MR images (rs-fMRI) were acquired using a Siemens Prisma 3T MRI scanner (Siemens Healthcare, Erlangen, Germany) with repetition time (TR)=2000 ms; echo time (TE)=28 ms; slice thickness of 4 mm with no interslice gap; field of view (FOV)=220 mm with an acquisition matrix of 64 × 64 for an in-plane resolution of 3.4 mm, and flip angle of 77°. Additionally, a 1 mm 3D isotropic MPRAGE sequence was acquired for alignment with functional MRI data using standard acquisition parameters (TR=2300–2500 ms, a minimum TE, inversion time (TI)=900–945 ms, flip angle 9–15°, FOV=240 × 320 mm and matrix size of 240 × 320, slice thickness=1 mm).
Image preprocessing
All functional MR images were pre-processed using the default built-in pre-processing pipeline within the CONN Toolbox (https://www.nitrc.org/projects/conn),13 which implements functions from the Statistic Parametric Mapping (SPM, http://www.fil.ion.ucl.ac.uk/spm/) toolbox (see detailed pre-processing steps in appendix p 1);.
Individual functional connectivity network construction
Two region of interest (ROI)-to-ROI functional connectivity networks (FCNs) were constructed for each individual participant. The brain atlas for both FCNs is based on the Montreal Neurological Institute and Hospital (MNI) coordinate system: 1) whole brain FCN with all the brain regions selected, including 86 cortical regions and 19 subcortical regions. For subcortical regions, we further divided the cerebellum into 26 sub-regions and the brainstem into 6 sub-regions according to Automated Anatomical Labeling (AAL).14 2) DCM representative brain FCN with regions seeded from anatomical regions that are known to often be explicitly associated with DCM,3, 4, 5, 6,15 as well as from regions that have been demonstrated in previous studies to undergo cortical morphological changes as a result of DCM (appendix p 3).16
Functional connectivity network associated with varying degrees of myelopathy severity
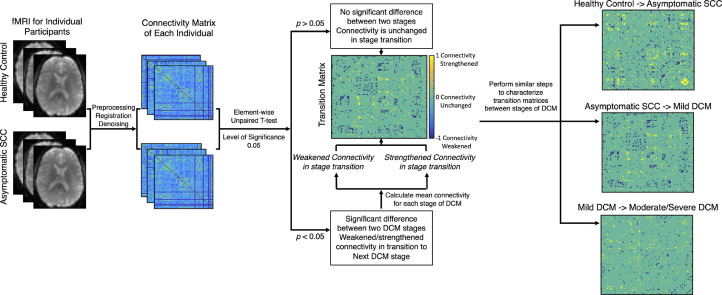
Characterizing the FCNs associated with varying degrees of myelopathy severity requires matrices containing information of FCs strengthening and/or weakening during transition from one stage of DCM severity to the next. As illustrated in Figure 1, after verifying that the FC distribution of each stage was approximately normal, matrices containing the transition information were computed by performing unpaired t-tests (with age included as a covariate) between FCNs at each stage, defined as the healthy control stage, asymptomatic SCC stage, mild DCM stage and the moderate/severe DCM stage. When results failed to show statistical significance (p > 0.05), this was interpreted no change in connectivity between the two stages. For statistical differences in FCs between two stages, the mean FC was calculated for the two diagnostic stages. Using the healthy control stage and the asymptomatic SCC stage as an example (Figure 1), if the healthy control stage had a higher mean FC than the asymptomatic SCC stage, the link in FC was interpreted as weakening as the healthy control stage transitions to the asymptomatic SCC stage. In contrast, if the healthy control stage had a lower mean FC compared with the asymptomatic SCC stage, the link in FC was interpreted as strengthening as the healthy control stage transitions to the asymptomatic SCC stage.
Figure 1.
Image processing pipeline for functional network analyses and the link prediction model. fMRI images were preprocessed through CONN and correlation coefficients among different regions were extracted from symmetric adjacency matrices to construct brain functional networks. Transitional matrices representing disease progression between different myelopathy stages matrices were calculated, which were further used in the topological analyses and link prediction. Significance was set at p < 0·05.
Following the calculation of link matrices representing differences between myelopathy stages, the matrix representing each DCM stage was initialized. To do this, the average connectivity matrix across subjects was calculated for each sequential stage, then converted elements larger than 0.25 were set to “1” and elements equal to or smaller than 0.25 were set to “0”.17 To take the stage transition information into consideration, we subtracted the matrices that represent differences between DCM stages from the matrix representing each DCM stage. This subtraction was propagated and enacted for 1000 iterations until reaching a steady state solution.
Representative brain functional connectivity network analysis
The Graph Theory GLM (GTG) Matlab Toolbox (www.nitrc.org/projects/metalab_gtg) and in-house Matlab workflow scripts were used to calculate and analyze the properties of representative brain FCN. Specifically, two indices of centrality metrics were compute: 1) Degree centrality, reflecting the number of neighbors a brain region interacts with (see detailed information and mathematical formula in appendix p 1); 2) Betweenness centrality, reflecting the ability of a region to influence information flow between two other regions (see detailed information and mathematical formula in appendix p 1). Regions with both high degree and betweenness centrality are considered as brain hubs, which are highly influential within a network, communicate with many other regions, and facilitate functional integration.
Link prediction model using whole brain functional connectivity network
Graph embedding method was implemented to build the link prediction model.18 Compared to topological properties characterized by graph theory, which only carry features of node related information, but also captures information about the surrounding nodes by representing every node within FCN as a fixed-length vector. To achieve this, algorithmic framework node2vec18 was used to learn and extract continuous features of each node in the whole brain FCN representing different DCM stages of severity (appendix p 2), then those features were fed into the light gradient boosting machine (light GBM) algorithm for further classification of connectivity as strengthening, weakening, or unchanging. Light GBM is an ensemble algorithm, which uses a special type of decision trees, also called weak learners, to capture complex, non-linear patterns. To prevent model from overfitting, early stopping is enabled for light GBM if the predictions haven't improved for the last 50 rounds (appendix p 2). The AUC (area under the receiver operating characteristic (ROC) curve) was used to evaluate the model performance, where higher AUC indicates better performance of the link prediction model in classifying FCs associated with sequential DCM stages.
Role of funders
The funders had no role in study design, data collection, data analyses, interpretation, or writing of report.
Results
The patient cohort consisted of 61 men and 38 women, ranging in age from 31 to 82 with an average age of 59. Twenty-one asymptomatic SCC patients (mJOA=18) were included in the cohort, 8 of whom had upper motor neuron signs elicited on physical examination. Hyperreflexia and a positive Hoffman's sign were each encountered in 5 of these patients. Two of the asymptomatic SCC compression patients had both hyperreflexia and a positive Hoffman's sign. The remainder of the study cohort consisted of 48 DCM patients with mild myelopathy (mJOA 15–17), and 20 DCM patients with moderate or severe myelopathy (mJOA ≤ 14). Additionally, a cohort of 17 healthy control volunteers (nine men and 8 women), ranging in age from 25 to 62 and with an average age of 41, underwent the same MRI protocol. The patient and healthy control volunteer demographics are summarized in Table 1.
Table 1.
Demographics of study participants.
| Subject Population | Subgroup | N | Ages (mean ± SD) | Sex | mJOA (mean ± SD) |
|---|---|---|---|---|---|
| Patients | Asymptomatic SCC | 21 | 57.0 ± 14.2 [31, 76] | 13M/8F | 18.0 |
| Mild DCM | 48 | 60.3 ± 10.3 [39, 82] | 29M/19F | 16.1 ± 0.9 [15, 17] | |
| Moderate/Severe DCM | 30 | 58.4 ± 8.6 [42, 74] | 19M/11F | 12.1 ± 1.6 [9, 14] | |
| Overall | 99 | 59.0 ± 10.7 [31, 82] | 61M/38F | 15.3 ± 2.5 [9, 18] | |
| Healthy Control Volunteers | - | 17 | 41.0 ± 14.0 [25, 62] | 9M/8F | 18.0 |
mJOA denotes modified Japanese Orthopedic Association.
SCC denotes spinal cord compression.
N/A denotes not applicable, N denotes number, SD denotes standard deviation.
M denotes male, F denotes female.
Characterizing representative brain FCN of different myelopathy stage
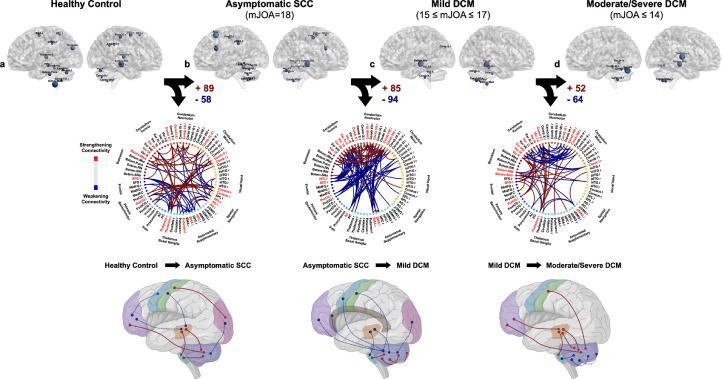
In healthy control volunteers (appendix p 4), 23 brain regions were identified as brain hubs, which corresponded to anatomic locations known to be important role in maintaining sensorimotor function. In the transition from healthy control to asymptomatic SCC (Figure 2a-b), 89 connections between the cerebellum and the brainstem as well as thalamus were strengthened, while 58 connections between the frontal lobe and the thalamus and supplementary regions were weakened. These changes in connectivity were driven by alterations in the right superior frontal gyrus (SFG r), the left precentral gyrus (PreCG l), bilateral thalamus, the left putamen, the left SMA, regions responsible for visual input and spatial navigation, and several subregions of cerebellum and brainstem.
Figure 2.
Patterns of functional connectivity (FC) during transition from (a) healthy control, (b) asymptomatic SCC (mJOA = 18), (c) mild DCM (15 ≤ mJOA ≤ 17), (d) to moderate/severe DCM (mJOA ≤ 14). Patterns were characterized using representative brain FCN with regions seeded from anatomical regions that are known to often be explicitly associated with DCM, as well as from regions that have been demonstrated in previous studies to undergo cortical morphological changes as a result of DCM. Top row of images denotes anatomic regions with high betweenness and degree centrality. Middle row denotes the transitional matrix associated with worsened severity, with red connections indicating strengthening connectivity and blue connections indicating weakening connectivity. Bottom row illustrates the anatomy of these changes in connectivity as DCM evolves and becomes more symptomatic.
When examining asymptomatic SCC patients (appendix p 5), 20 brain regions were identified as brain hubs within the functional network, corresponding to bilateral PreCG and the anterior cingulate (AC), as well as subregions located in cerebellum. During transition from asymptomatic SCC to DCM with mild myelopathy (Figure 2B-C), the connectivity corresponding to 85 connections with the cerebellum were strengthened, while connectivity in 94 connections between the cerebellum and the AC, right PreCG, bilateral SFG and SMA were weakened. These changes were driven by alterations in the centrality of the AC, the right PreCG, bilateral SFG and SMA, as well multiple subregions of cerebellum.
For both mild (appendix p 6) and moderate-to-severe DCM (appendix p 7), only 12 and 10 brain regions were identified as brain hubs, respectively. Additionally, for symptomatic patients with at least mild DCM, no cortical regions were identified as brain hubs, but several subregions of cerebellum and brainstem still maintained their influential role within network. In the transition from the mild myelopathy to moderate-to-severe DCM (Figure 2c-d), the connectivity for 52 connections between cerebellar vermis and brainstem to the left SFG, the left PreCG and thalamus strengthened, while 64 connections within the cerebellum weakened.
Link prediction model using whole brain FCN
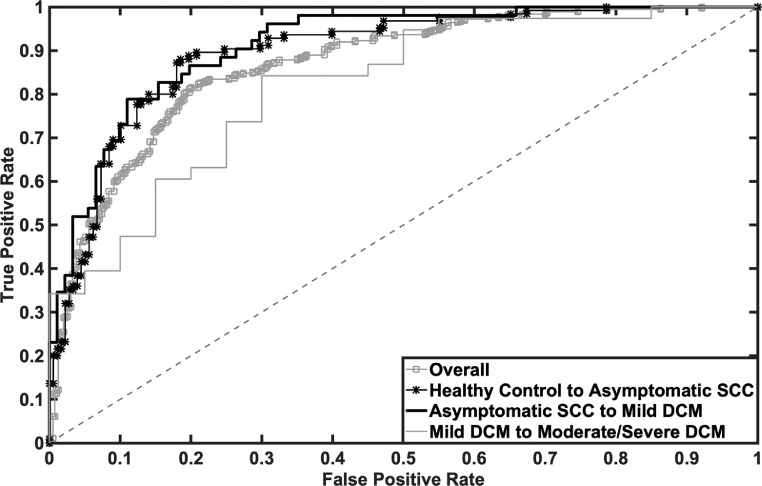
Lastly, the graph embedding method was used to extract features from the matrices representing different stages of DCM symptom severity, and then the light GBM algorithm was applied to classify strengthening and weakening connectivity. Using this approach, the ROC AUC for transitions between symptomatic DCM stages ranged from 0.884 to 0.927, with the highest AUC for predicting mild from asymptomatic SCC (Table 2 and Figure 3).
Table 2.
Summary of link prediction performance in classifying FC patterns associated with different stages of DCM symptom severity.
| Link Prediction of FC Patterns Associated with DCM Symptom Severity | ROC AUC | p Value |
|---|---|---|
| Predicting Asymptomatic SCC Stage from Healthy Control | 0.896 | <0.0001 |
| Predicting Mild DCM from Asymptomatic SCC | 0.927 | <0.0001 |
| Predicting Moderate/Severe DCM from Mild DCM | 0.884 | 0.0007 |
| Overall AUC score of Link Prediction Model | 0.885 | <0.0001 |
SCC denotes spinal cord compression.
ROC AUC denotes area under the receiver operator curve.
Figure 3.
Receiver operating characteristic curve (ROC) of the link prediction model using light GBM algorithm for overall prediction (gray line with square marker), predicting connectivity patterns in asymptomatic SCC from healthy control connectivity (black line with asterisk marker), predicting connectivity patterns for mild DCM from asymptomatic SCC (black line), and predicting connectivity patterns for moderate/severe DCM from mild DCM (gray line).
Discussion
In the past decade, upstream morphological abnormalities,16 and functional connectivity within sensorimotor regions as a response to spinal cord injury have been widely studied.2, 3, 4, 5, 6, 7 Consistent with these previous findings, we were able to confirm that large-scale functional network reorganization occurs in patients with DCM by characterizing the connectivity patterns associated with varying degrees of functional impairment in patients with DCM. These findings are consistent with previous reports demonstrating degenerative motor and sensory function,1 widespread cortical atrophy,6,16 and abnormal cerebral network connectivity in patients with DCM,3,6 dependent on symptom severity.
Results suggest that during asymptomatic SCC, when the spinal cord is compressed but the patient is not experiencing the typical symptoms of upper motor neuron damage caused by spinal cord compression,19 connections between the thalamus and sensorimotor network are interrupted, while new connections are strengthened between brainstem, subcortical regions, visuospatial areas, and regions within the cerebellum. The strengthening of connectivity observed between the cuneus and precentral gyrus suggests visual input may be used in assisting postural control and preserving motor function during initial stages of cord compression, while the strengthened connectivity between cerebellum and thalamus may indicate greater input from the cerebellothalamic tract, a fiber pathway responsible for motor adaptation and transmitting information for the sensory system.20 Additionally, the right SFG, the left PreCG, the left putamen, the left SMA and bilateral thalamus were identified as altered hub regions, suggesting asymptomatic spinal cord compression may in fact be resulting in chronic damage to sensorimotor neurons.21
As asymptomatic SCC transitions to mild DCM, a decrease in connectivity was observed between the cerebellum, brainstem, SMA and primary motor cortex, as well as the frontal lobe, anterior cingulate, thalamus, and cuneus. Together, this suggests possible white matter impairment to the bilateral corticospinal tracts extending through the corona radiata associated with the neurological deterioration.5 While connectivity between these regions decreased dramatically, connectivity within the cerebellum and between the brainstem and cerebellum appeared to strengthen. This was particularly the case between the cerebellar vermis, and subregions including the cerebellar second motor representation (lobule VIII) and cerebellar third non-motor representations (lobule X). The spinocerebellular tract, part of the cerebellar vermis, acts to receive somatosensory input from ascending spinal pathways, as well as associated bodily posture and locomotion information.22 The cerebellar second motor representation (lobule VIII) tends to be engaged in motor processes that require high attention, but less involved in pure motor information processing. Additionally, evidence suggests the cerebellar second motor representation might share functional similarities with the cerebellar third nonmotor representation.22 Together, this suggests increased cerebellar connectivity may serve in a compensatory capacity to preserve sensorimotor function in response to initial stages of neurological deterioration in DCM.
As mild DCM evolves to moderate/severe DCM, connectivity directly between the cerebellum and thalamus, sensorimotor areas, and frontal lobe strengthened sharply, and connectivity with the brainstem and within the cerebellum itself weakened. These findings strongly support the hypothesis that neurological function is preserved through large-scale cerebral plasticity in cases of increased severity of myelopathy, and that compensatory input from subcortical regions undergo adaptations in response to neurological deterioration. Previous studies have proposed that upper motor cortex neurons influence the spinal cord circuits by two routes: direct projections to the spinal cord via the corticospinal pathway, and indirect projections to brainstem centers via the corticoreticulospinal pathway.23 It is possible that during worsening myelopathy, patients may exhaust their localized functional compensatory mechanisms via the cerebellum, then subsequent adaptations are recruited to optimize residual neurological function and sustain motor behavior performance. This hypothesis is further supported by the observed reduction in the number of cortical regions identified as brain hubs during the more severe myelopathic stages of DCM, while several subregions of cerebellum and brainstem still maintained their influential role within these networks. Importantly, DCM patients with moderate/severe myelopathy have a greater number and severity of symptoms,24 and surgical guidelines have strongly recommended surgery for this cohort, whereas mild DCM can be treated nonoperatively.25 Therefore, understanding the transition between mild and moderate/severe DCM has direct clinical implications.
Graph analyses and corresponding algorithms have been widely used to understand the structure of functional brain networks by predicting future relationships and missing connections.26,27 One group has also utilized the link prediction model to estimate brain network changes in neurodegenerative disorders including Alzheimer's and Parkinson's disease.11 Rather than computing the node neighborhood similarity score using popular algorithms such as Common Neighbors (CN) and Preferential Attachments (PA) for direct use of graph vector features for each node,9 the current study implemented graph embedding and extracted vector features of each node.18 Graph embedding learns low-dimensional representations for nodes in a graph, which is able to capture information about the surrounding nodes other than the node itself. These features can also benefit future automated modeling via a graph convolution network28 to classify myelopathy severity, predict surgical outcomes, and monitor disease progression. Results out of the link prediction model highlighted the transition from asymptomatic SCC to mild DCM as having the strongest classification performance, whereas the transition from mild to moderate/severe DCM was the least predictive. This may be the case because previous studies have suggested link prediction models work best when the functional network has fewer changes at each stage,11 implying the transition from asymptomatic SCC to mild DCM may be subtle. This is consistent with previous studies showing that patients with significant spinal cord compression do not always have corresponding symptoms, in part due to supraspinal compensatory mechanisms.2,19,29,30
Limitations
It is important to note that the performance of the link prediction model is highly dependent on sample size. Future study with a larger group of severe DCM patients will allow for better stratification of the moderate/severe group, although this is partially mitigated as these patients are treated similarly from a clinical perspective. Future investigation utilizing comparisons between the mJOA subscores and the brain functional connectivity network could provide additional insights into the neural plasticity associated with DCM. Although the statistical analysis of connectivity differences between patients and the healthy control volunteer cohort accounted for the variation in age of each patient, inclusion of additional older healthy control volunteers would be useful in improving the performance of the predictive model. Furthermore, the matrix representing each DCM stage was calculated by binarizing functional connectivity correlation matrix. Although the current study iterated 1000 times over matrices representing differences between DCM stages until reaching a steady state solution, a more comprehensive algorithm that includes the weight of ROI-to-ROI connections would likely benefit the characterization of network representing each DCM state, thus the outcomes of the link prediction model. Finally, continuous collection of longitudinal imaging that tracks disease progression of individual patients is necessary to validate the current cross sectional model. This could also provide more information about impact of symptom duration on supraspinal reorganization. In addition, there is a small subset of DCM patients that improve neurologically with nonoperative treatment, and a longitudinal study design would allow for further investigation into this group. Lastly, we theorize a combination of structural and functional connectivity patterns could be employed to further understand how the brain compensates for neurological impairment during progressive DCM.
To summarize, a series of well defined, predictable changes in brain functional connectivity occur during DCM pathogenesis. Additionally, the proposed link prediction model may be beneficial in future studies for monitoring disease progression and/or predicting treatment response in patients with DCM.
Contributors
All authors had full access to the final dataset and approved the final submitted version of this report. BE and LH contributed to study conceptualization. CW, BE, and TO contributed to data curation. CW contributed to methodology and statistical analysis. BE and LH contributed to funding acquisition. BE and LH supervised the study. NS and LH validated the data. CW wrote the original draft. BE, TO, NS, and LH contributed to manuscript review and editing. LH has accessed and verified the data and were responsible for the decision to submit the manuscript.
Data sharing statement
Data collected for the study are legally unavailable. Additional related documents (study protocol and statistical analysis plan) will be made available on publication via the corresponding author.
Declaration of interests
All authors declare no conflicts of interest related to the subject matter in this publication.
Acknowledgments
We would like to thank all participants for their participation and all MR technicians for their contributions in this study. This study was funded by NIH/NINDS grants: 1R01NS078494-01A1 (to LTH, NS, and BME), and 2R01NS078494-06 (to LTH, NS, and BME).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104255.
Appendix. Supplementary materials
References
- 1.Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy - update and future directions. Nat Rev Neurol. 2020;16(2):108–124. doi: 10.1038/s41582-019-0303-0. [DOI] [PubMed] [Google Scholar]
- 2.Holly LT, Dong Y, Albistegui-DuBois R, Marehbian J, Dobkin B. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine. 2007;6(6):544–551. doi: 10.3171/spi.2007.6.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodworth DC, Holly LT, Salamon N, Ellingson BM. Resting-state functional magnetic resonance imaging connectivity of the brain is associated with altered sensorimotor function in patients with cervical spondylosis. World Neurosurg. 2018;119:e740–e749. doi: 10.1016/j.wneu.2018.07.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holly LT, Wang C, Woodworth DC, Salamon N, Ellingson BM. Neck disability in patients with cervical spondylosis is associated with altered brain functional connectivity. J Clin Neurosci. 2019;69:149–154. doi: 10.1016/j.jocn.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Laiwalla A, Salamon N, Ellingson BM, Holly LT. Compensatory brainstem functional and structural connectivity in patients with degenerative cervical myelopathy by probabilistic tractography and functional MRI. Brain Res. 2020;1749 doi: 10.1016/j.brainres.2020.147129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernabeu-Sanz A, Molla-Torro JV, Lopez-Celada S, Moreno Lopez P, Fernandez-Jover E. MRI evidence of brain atrophy, white matter damage, and functional adaptive changes in patients with cervical spondylosis and prolonged spinal cord compression. Eur Radiol. 2020;30:357–369. doi: 10.1007/s00330-019-06352-z. [DOI] [PubMed] [Google Scholar]
- 7.Lee CC. Exploring functions for the non-lemniscal auditory thalamus. Front Neural Circuits. 2015;9:69. doi: 10.3389/fncir.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Y, Zhou F, Wu L, et al. Alteration of regional homogeneity within the sensorimotor network after spinal cord decompression in cervical spondylotic myelopathy: a resting-state fMRI study. Biomed Res Int. 2015;2015 doi: 10.1155/2015/647958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannistraci CV, Alanis-Lobato G, Ravasi T. From link-prediction in brain connectomes and protein interactomes to the local-community-paradigm in complex networks. Sci Rep. 2013;3:1613. doi: 10.1038/srep01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng J, Lu G, Shang X. A survey of network representation learning methods for link prediction in biological network. Curr Pharm Des. 2020;26:3076–3084. doi: 10.2174/1381612826666200116145057. [DOI] [PubMed] [Google Scholar]
- 11.Sulaimany S, Khansari M, Zarrineh P, et al. Predicting brain network changes in Alzheimer's disease with link prediction algorithms. Mol Biosyst. 2017;13(4):725–735. doi: 10.1039/c6mb00815a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976) 2001;26(17):1890–1894. doi: 10.1097/00007632-200109010-00014. discussion 5. [DOI] [PubMed] [Google Scholar]
- 13.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 14.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 15.Bhagavatula ID, Shukla D, Sadashiva N, Saligoudar P, Prasad C, Bhat DI. Functional cortical reorganization in cases of cervical spondylotic myelopathy and changes associated with surgery. Neurosurg Focus. 2016;40(6):E2. doi: 10.3171/2016.3.FOCUS1635. [DOI] [PubMed] [Google Scholar]
- 16.Woodworth DC, Holly LT, Mayer EA, Salamon N, Ellingson BM. Alterations in cortical thickness and subcortical volume are associated with neurological symptoms and neck pain in patients with cervical spondylosis. Neurosurgery. 2019;84(3):588–598. doi: 10.1093/neuros/nyy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akoglu H. User's guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grover A, Leskovec J. node2vec: scalable feature learning for networks. KDD. 2016;2016:855–864. doi: 10.1145/2939672.2939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical myelopathy: an updated predictive model. Eur Spine J. 2008;17(3):421–431. doi: 10.1007/s00586-008-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakayori N, Kato S, Sugawara M, et al. Motor skills mediated through cerebellothalamic tracts projecting to the central lateral nucleus. Mol Brain. 2019;12(1):13. doi: 10.1186/s13041-019-0431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou JM, Yan RB, Xiang ZM, et al. Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience. 2014;266:208–215. doi: 10.1016/j.neuroscience.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci USA. 2011;108(38):16068–16073. doi: 10.1073/pnas.1107904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2nd ed. Sinauer Associates; Sunderland (MA): 2001. Neuroscience. [Google Scholar]
- 24.Tetreault L, Kopjar B, Nouri A, et al. The modified Japanese orthopaedic association scale: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2017;26(1):78–84. doi: 10.1007/s00586-016-4660-8. [DOI] [PubMed] [Google Scholar]
- 25.Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(3 Suppl):70S–83S. doi: 10.1177/2192568217701914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazrooyisebdani M, Nair VA, Garcia-Ramos C, et al. Graph theory analysis of functional connectivity combined with machine learning approaches demonstrates widespread network differences and predicts clinical variables in temporal lobe epilepsy. Brain Connect. 2020;10(1):39–50. doi: 10.1089/brain.2019.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirakhorli J, Amindavar H, Mirakhorli M. A new method to predict anomaly in brain network based on graph deep learning. Rev Neurosci. 2020;31:681–689. doi: 10.1515/revneuro-2019-0108. [DOI] [PubMed] [Google Scholar]
- 28.Chen SH, Cerda F, Rizzo P, Bielak J, Garrett JH, Kovacevic J. Semi-supervised multiresolution classification using adaptive graph filtering with application to indirect bridge structural health monitoring. Ieee T Signal Proces. 2014;62(11):2879–2893. [Google Scholar]
- 29.Cao JM, Zhang JT, Yang DL, Yang YP, Xia HH, Yang L. Imaging factors that distinguish between patients with asymptomatic and symptomatic cervical spondylotic myelopathy with mild to moderate cervical spinal cord compression. Med Sci Monit. 2017;23:4901–4908. doi: 10.12659/MSM.906937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical cord compression. Spine (Phila Pa 1976) 2004;29(20):2260–2269. doi: 10.1097/01.brs.0000142434.02579.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.