Abstract
Background
Pyroptosis has been attracting much attention recently. We have briefly compared its differences and similarities with other programmed deaths and the process of its study. With further exploration of the caspase family, including caspase-1/3/4/5/8/11, new insights into the molecular pathways of action of pyroptosis have been gained. It is also closely related to the development of many cancers, which at the same time provides us with new ideas for the treatment of cancer.
Scope of Review
We describe what is known regarding the impact of pyroptosis on anticancer immunity and give insight into the potential of harnessing pyroptosis as a tool and applying it to novel or existing anticancer strategies.
Major Conclusions
Pyroptosis, a caspase-dependent cell death, causes pore formation, cell swelling, rupture of the plasma membrane, and release of all intracellular contents. The role of pyroptosis in cancer is an extremely complex issue. There is growing evidence that tumor pyroptosis has anti-tumor and pro-tumor roles. It should be discussed in different cancer periods according to the characteristics of cancer occurrence and development. In cancer treatment, pyroptosis provides us with some potential new targets. For the existing drugs, the study of pyroptosis also helps us make better use of existing drugs for anticancer treatment. Immunotherapy is a hot research direction in the field of cancer treatment.
Keywords: Pyroptosis, Cancer, Caspase, Gasdermin
1. Introduction
Cell death has numerous benefits on the health and function of the body, especially in cancer treatment [1]. There are mainly two types of cell death, necrosis and programmed cell death (PCD). Necrosis is a passive type of cell death that occurs as a result of pathological stimulation. The permeability of the plasma membrane of necrotic cells increases, which causes the cells to swell and rupture, thereby releasing the cellular content and leading to inflammation. Pyroptosis, apoptosis, autophagy, and necroptosis are manifestations of PCD. PCD is a process in which cells receive certain signals or are stimulated by certain factors to maintain the stability of the internal environment (Table 1) [[2], [3], [4]]. Apoptosis is a type of PCD in which genes control the mechanical self-destruction of cells, but the cell membrane remains intact, and the process does not induce an inflammatory reaction [5]. Recently, pyroptosis, a necrotic and lytic PCD, has attracted considerable attention for its capacity to elicit inflammatory response [6]. It was first studied in the context of immune defense against pathogens [7]. Pyroptosis is a critical immune response mechanism in organisms that inhibits infections and endogenous injury signals. It plays a significant role in the development of tumors, infectious diseases, metabolic diseases, neurological-related diseases, and atherosclerotic diseases [8] (Tables 2 and 3).
Table 1.
Comparison of several modes of cell death.
| Apoptosis | Autophagy | Pyroptosis | Necroptosis | Necrosis | |
|---|---|---|---|---|---|
| Type | PCD | PCD | PCD | PCD | Non-PCD |
| Causes | Gene regulation under physiological conditions | Nutritional deficiency or hormonal induction | Inflammasome stimulation | Pathological irritation or violent injury | Pathological process |
| Morphology | Dying cell shrinks\ nucleus condense and fragments\ apoptotic bodies | Accumulation of autophagic vacuoles | Cell swelling and deformation with violent inflammatory response | Swelling of cytoplasmic organelles | Cell swelling and lysis |
| Cell Membrane | Integrity | Integrity | Rupture | Rupture | Rupture |
| DNA | Degradation to 180–200 bp and its integer multiples | Random degradation and no chromatin condensation | Random degradation | Random degradation | Random degradation |
Table 2.
(A) Expression of pyroptotic components in cancers. (B) The pathway of different cancers.
| Cancer type | Level of pyroptotic components | Associated consequences | References |
|---|---|---|---|
| (A) | |||
| Esophagus cancer | NLRP3↑ | cell death and Barrett's esophagus | [61] |
| Upregulated GSDME | transit the apoptosis to pyroptotic-signaling pathways | [60] | |
| Gastric cancer | GSDMD↓ | cancer cell proliferation | [62] |
| GSDMA↓ | anti-oncogenic factor | [70,71] | |
| GSDMB↑ | control tumor infringement | [49] | |
| GSDMC | cancer suppressor gene | [73] | |
| DFNA5/GSDME signaling pathway | pyroptosis facilitates GC progression | [62] | |
| Hepatocellular carcinoma | Caspase-1 protein↓ | Tumor proliferation | [80,84] |
| NLRP3↓ | Increase HCC etiology and progression | [81,82] | |
| AIM2↑ | Promote pyroptosis and antitumor effects | [85,87] | |
| Colorectal cancer | Caspase-1↑ | Promote epithelial cell proliferation and apoptosis | [54] |
| Lack of AIM2 | Poor prognosis in CRC | [89] | |
| NLRP1↓ | Tumor progression | [88,91] | |
| GSDMC↓ | Tumor cell proliferation | [73] | |
| GSDMD↓ | Poor prognosis in CRC | [93] | |
| GSDME↑ | Increase proliferation | [94] | |
| Lung cancer | GSDMD↑ | Tumor proliferation | [95,96] |
| Knocking out GSDME | Flip from apoptosis to pyroptosis | [97] | |
| Cervical cancer | Caspase-1↑ | Lower tumor weight and volume | [105] |
| GSDMD↑ | Lower tumor weight and volume | [92,105] | |
| NLRP3↑ | Lower tumor weight and volume | [105,106] | |
| Ovarian cancer | GSDME↓ | OC progression | [109] |
| GSDMD↓ | OC progression | [110] | |
| Caspase-4↓ | OC progression | [110] | |
| Breast cancer | GSDMB↑ | Metastasis | [117] |
| GSDME↓ | Metastasis | [78] | |
| GSDMD↑ | Caustic death | [119] | |
| Skin cancer | GSDMC↓ | Invasion and metastasis | [73] |
| Cancer types | The canonical inflammasome pathway | The noncanonical inflammasome pathway | Other pathways |
|---|---|---|---|
| (B) | |||
| Esophagus cancer | √ | ||
| Gastric cancer | √ | ||
| Hepatocellular carcinoma | √ | ||
| Colorectal cancer | √ | ||
| Lung cancer | √ | √ | |
| Cervical cancer | √ | √ | |
| Ovarian cancer | √ | √ | |
| Breast cancer | √ | √ | |
| Skin cancer | √ | ||
Table 3.
Synthetic and natural products that mediate their anticancer effects.
| Compounds | Targeting pathways | Cancer types | References |
|---|---|---|---|
| Metformin | MiR-497/proline-, glutamic acid-, and leucine-rich protein-1(PELP1) axis/GSDMD | Esophageal cancer | [128] |
| PLK1 inhibitors | Bax/Caspase-3/GSDME | Esophagus cancer | [60] |
| LDC7559 | GSDMD | Esophagus cancer | [146] |
| Chemotherapeutic medicines such actinomycin-D, doxorubicin (DOXO), topotecan, and bleomycin | Caspase-1, Caspase-3, GSDMD/GSDME/DFNA/eEF-2K | Lung cancer | [18] |
| Paclitaxel and cisplatin | Caspase-3/GSDME | Lung cancer | [130] |
| Chalcone derivative-8 | GSDME/ROS | Lung cancer | [131] |
| N-substituted EF24 analog 13d | NF-kB/caspase-3 | Lung cancer | [132] |
| L61H10 | NF-kB | Lung cancer | [133] |
| 4-hydroxybenzoic acid (4-HBA) | Caspase-1/IL-1β/and IL-18 | Lung cancer | [147] |
| Lobaplatin | ROS/JNK/Caspase-3/GSDME | Colon cancer | [122] |
| 5-fluorouracil | caspase-3/GSDME | Gastric cancer | [62] |
| Piperlongumine-analogue (PL-a) L50377 | NF-kB/ROS | NSCLC cell | [134] |
| Alpha-NETA | GSDMD/Caspase-4 | Ovarian cancer | [110] |
| Decitabine (DAC) | Caspase-3/GSDME | Malignant cells | [135] |
| Anthocyanidins (ANTH) | NLRP3/caspase-1/IL-1β/GSDMD | Oral squamous cell carcinoma (OSCC) | [137] |
| Galanin | GSDME | Glioblastoma | [138] |
| Omega-3 fatty acids | Caspase-1/IL-1β/GSDMD | Breast cancer | [114] |
| Dipeptidyl peptidases (DPPs) | Caspase-1 | Acute myeloid leukemia (AML) | [142] |
| Necrosulfonamide | GSDMD | Myeloid leukemia | [145] |
Although the precise mechanism by which pyroptosis participates in the tumor process is unknown, it is thought to have a dual role in tumor pathogenesis. The various signaling pathways and inflammatory mediators released during pyroptosis are directly associated with carcinogenesis and drug resistance to chemotherapeutic medicines. Pyroptosis acts as a type of cell death that prevent the occurrence and growth of tumors [9]. The role of pyroptosis in tumors is increasingly being studied. The purpose of this study is to discuss and assess the potential effects of pyroptosis on cancer as well as its role in anticancer treatment.
2. Characteristics of pyroptosis
The term pyroptosis is derived from a Greek root “pyro”, meaning fire or fever, and “ptosis”, meaning decline. This term is used to describe a new type of proinflammatory PCD [7]. Pyroptosis, also known as gasdermin-mediated programmed necrosis, is a new type of PCD triggered by innate immunity-related disruptions of extracellular or intracellular homeostasis [5,10].
Pyroptotic cells are similar and yet distinct from apoptotic and necrotic cells in certain aspects. While pyroptosis and apoptotic cells share chromatin condensation and DNA fragmentation, they can be distinguished by their intact nucleus, cell swelling, pore formation, and osmotic lysis [11]. Pyroptosis, like necrosis, results in cell membrane pore formation, membrane rupture, and cellular swelling, all of which trigger the release of cellular contents and proinflammatory mediators, including IL-1β and IL-18 [12].
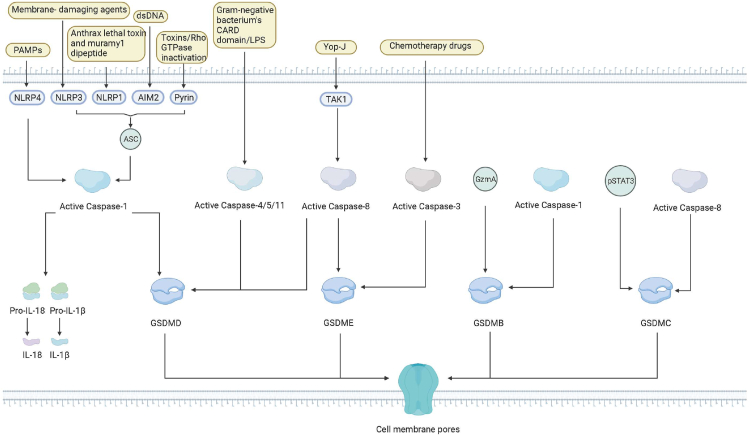
Pyroptosis is a type of caspase-1 mediated PCD that is morphologically distinct from apoptosis, and it was first discovered by Arturo et al., in 1992 [13]. In 1997, Shigella dysenteriae was found to activate caspase-1 in host cells [14]. Another study in 1999 demonstrated that inhibiting caspase-1 prevented Salmonella-induced cell death [15]. It was until 2001 that Cookson et al. referred to such a proinflammatory PCD as “pyroptosis” [7]. Numerous studies have since been conducted on pyroptosis. In 2015, gasdermin D (GSDMD) was discovered to be a target for caspase-1 and caspase-4, 5, and 11 cleavages [16,17]. Additionally, emerging evidence demonstrated that other members of the gasdermin superfamily protein like gasdermin E (GSDME) are activated by caspase-3 to cause pyroptosis [18,19]. Recent studies have demonstrated that pathogen-induced or pharmacological inhibition of the kinase transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) or the inhibitor of apoptosis (IAP) can stimulate caspase-8 production and GSDMD cleavage to elicit pyroptosis [[20], [21], [22]]. Recent studies have discovered that activated caspases and granzyme proteases can cleave members of the gasdermin family, including GSDMA/B/C/D/E, and that the N-terminal oligomerizes in membranes, thereby generating holes that contribute to pyroptosis [23] (Figure 1).
Figure 1.
Timeline of discovery of pyroptosis. [Created with BioRender.com].
3. Mechanisms of pyroptosis
3.1. The canonical inflammasome pathway
Pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) activate cells’ respective canonical inflammasomes in response to infection or immunological challenges, thereby forming a cytoplasmic-multiprotein oligomeric complex that ultimately activates an innate immune response. The inflammasomes are composed of the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family (NLRP3, NLRP1, NLRC4), AIM2, or pyrin proteins, which contain N-terminal caspase recruitment (CARD) or pyrin domain (PYD) [12,24,25]. The NLRP1 inflammasome recognizes only Bacillus lethal toxins and muramyl dipeptide [26]. The NLRP3 inflammasome can be activated by a variety of membrane-damaging agents, including bacteria, viruses, reactive oxygen species (ROS), DAMPs, and pore-forming toxins [[27], [28], [29]]. NLRC4 inflammasomes are activated only by PAMPs such as type III secretion apparatus and bacterial flagellin [30,31]. The AIM 2 inflammasome was reported to be specifically activated by endogenous or pathogen-derived double-stranded DNA (dsDNA) [32,33]. Pyrin inflammasomes indirectly detect inactivating modifications to the host Rho GTPases [33].
These studies indicate that for the innate immunity of cells, although cells have specific perception factors for different heterologous substances or antigens, the formation of immune bodies is essential for the initiation of pyroptosis. Therefore, further investigations are needed whether there is a conserved molecular structure and mechanism basis in the process of immune corpuscle formation to generate ideas for developing therapeutic targets for pyroptosis-related diseases.
When activated, many of these sensors interact with the receptor complex apoptosis-associated speck-like protein containing CARD (ASC), which recruits and cleaves procaspase-1 and activates caspase-1. Caspases-1 converts pro-IL-1 and pro-IL-18 into IL-1 and IL-18, respectively, which are then released through the necrotic pores of the membrane created by GSDMD-N, in addition to releasing and activating the lethal N-terminal domain of GSDMD (GSDMD-N) [34]. NLRC4 can specifically recruit pro-caspase-1 without forming ASC [35,36]. Caspases are a family of proteases that are activated by inflammasomes and regulate the innate immune system.
3.2. The noncanonical inflammasome pathway
Human caspases 4 and 5 and mouse caspase 11 are involved in noncanonical pyroptotic cell death [37,38]. The gram-negative bacterium's CARD domain binds directly to lipopolysaccharide (LPS), thus triggering the noncanonical inflammasome pathway. Caspase-4/5/11 can cleave GSDMD directly into an N-terminal fragment and a C-terminal fragment. By lysing on liposomes containing phosphoinositide or cardiolipin or liposomes comprising natural polar lipid mixtures, the N-terminal domain of GSDMD can generate large gasdermin pores that release free IL-1 and IL-18 outside the cell [10,16,17,39,40]. These pathways activate the pyroptotic process, which results in the efflux of K+, activating the NLRP3 inflammasome and upregulating caspase-1 activity [17]. Caspases 4/5/11 can also cleave the pannexin1 (Panx-1) channel protein, resulting in ATP synthesis and activation of P2X purinoceptor 7 (P2RX7), which activates the NLRP3 inflammasome, leading to pyroptosis [41,42].
3.3. Other caspase pathways
Recent studies have also established that Caspase-3 and/or Caspase-8 signaling pathways play a critical role in pyroptosis [8,43]. Caspase-1/2/4/5/11 has previously been demonstrated to activate pyroptosis through GSDMD cleavage, whereas caspase-3 is an important hallmark of apoptosis. More recently, caspase-3 has been shown to influence and activate GSDME to induce pyroptosis [18]. Caspase-3, which is involved in pyroptosis and expresses high levels of GSDME through Apaf-1 can be activated by chemotherapy drugs in cancer and normal cells [18,19,44]. As a proapoptotic protease, caspase-3 may rapidly identify and cleave GSDMD stimulating pore formation and cell death by pyroptosis [19,45]. Pyroptosis is rapidly induced in the presence of high GSDME levels, whereas apoptosis occurs in the low GSDME levels [18].
Caspase-8 induces the cleavage of gasdermin C (GSDMC) and GSDME to elicit pyroptosis. TAK-1 is an essential factor in regulating NF-κB signaling mechanisms [46]. It has been demonstrated that inhibiting TAK-1 with Yersinia outer protein-J (Yop-J) or other relatively minor inhibitors facilitates caspase-8-mediated GSDMD cleavage to induce pore opening and cell membrane rupture and hence pyroptosis [21,22].
Apart from pyroptosis, GSDMD cleavage has also been observed in many physiological activities such as vesicle traffic, secretion of neurotransmitter, and anti-inflammation.
As mentioned above, casp3 and casp8 are important regulatory factors in apoptosis and cell necrosis. Under specific pathological conditions, these regulatory factors can also contribute to pyroptosis. This suggests that there could be important crosstalk between different types of cell death, which needs to be further investigated.
3.4. GSDMD is a pyroptotic effector
Gasdermins are a family of pore-forming proteins that contribute to pyroptosis. Of note, GSDMA-E and Pejvakin (PJVK) are expressed in humans, whereas mice have three GSDMA homologs (GSDMA1-3), four GSDMC homologs (GSDMC1–4), one GSDMD or GSDME homolog, and PJVK. All GSDM proteins, except for PJVK, contain two predicted domains and a variable linker region [10]. GSDMA-E and GSDMA3 are cytotoxic and induce pyroptosis in cells when their N-terminal domains are cleaved, although their full-length fragments do not exhibit such effects [10,47]. Thus far, one GSDMD protein has been identified as the primary pyroptosis mediator of inflammatory caspases [16,17]. Subsequent research has revealed that the ability of GSDMD's N-terminal fragment (GSDMD-NT) to form pores, which contributes to pyroptotic cell death [16]. The GSDMD-NT binds to phosphatidylinositol, phosphatidic acid, and phosphatidylserine in cell membranes, causing them to oligomerize and resulting in the formation of pores [40,48]. GSDMD can only cause cell lysis in mammalian cells due to the asymmetric distribution of phosphoinositides on the plasma membrane [10,48].
Several other alternative pyroptosis pathways have been reported, including caspase-3 activation of GSDME [18], caspase-1 [49] or granzyme A (GzmA) and cleavage of GSDMB [50], caspase-8 cleavage of GSDMC and transcriptional upregulation by hypoxia-activated programmed death-ligand 1 (PD-L1) and pSTAT3 [51], and GSDMA pore formation through an unknown mechanism [52].
The abovementioned mechanisms of pyroptosis are summarized in Figure 2.
Figure 2.
Molecular mechanisms of pyroptosis. [Created with BioRender.com.].
Abbreviations: PAMPs: Pathogen-associated molecular patterns, DAMPs: danger-associated molecular patterns, NLRP: nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family, ROS: reactive oxygen species, dsDNA: double-stranded DNA, ASC: apoptosis-associated speck-like protein containing CARD, LPS: lipopolysaccharide, P2RX7: P2X purinoceptor 7, GSDMC: cleaving gasdermin C, Yop-J: Yersinia outer protein-J, CARD: caspase recruitment, PYD: pyrin domain, AIM2: Absent In Melanoma 2, GZMA: granzyme A, TAK1: TGF-Beta Activated Kinase-1
4. Pyroptosis and cancer
The enigmatic involvement of pyroptosis in cancer appears to be contingent on the cell type, genetics, and the duration of pyroptosis induction. GSDMs, inflammasomes, and/or pro-inflammatory cytokines can contribute to tumor pathology by inducing immunosuppressive cells, which may promote epithelial-to-mesenchymal transition and/or upregulate matrix metalloproteinases for extracellular matrix remodeling following abnormal expression and prolonged activity [53]. Carcinogenesis is influenced by proto- and anticogene activity, immunological microenvironment, oxidative stress, and chronic inflammation. The risk of cancer is increased when tissues and/or cells are exposed to an inflammatory environment for an extended period. Activation of pyroptosis is accompanied by the release of inflammatory mediators IL-1 and IL-18, which may contribute to the development of cancer [[54], [55], [56]].
4.1. Relationship between pyroptosis and cancer
4.1.1. Esophagus and gastric cancer
Alcohol buildup has been reported to inhibit caspase-1 and increase the production of IL-18 and IL-1 in esophageal epithelial cells, both of which are associated with pyroptosis [57]. Alcohol can hasten the etiology and development of esophagitis by activating pyroptosis-signaling pathways [57]. Gastroesophageal reflux disease (GERD) destroys the esophageal mucosa membrane and exposes the esophageal epithelium for an extended period, allowing stimuli such as alcohol accumulation to enter the body and induce chronic inflammatory responses. Additionally, GERD has been associated with Barrett's esophagus and esophageal cancer [58,59]. In Barrett's cell lines, lipopolysaccharide (LPS) activated the NLRP3 inflammasome and pyroptosis-signaling pathways, which enhanced pro-inflammatory factors [59]. LPS stimulates TLR-4, priming inflammasome-signaling pathways, and enhancing the release of pro-inflammatory mediators and the NLRP3 inflammasome. LPS can also activate the NLRP3 inflammasome leading to the activation of caspase-1 and release of pro-inflammatory molecules such as IL-18, IL-1, and LDH by increasing mitochondrial ROS production (an indicator of pyroptosis). As a result, LPS induces pyroptosis-regulated cell death thereby aggravating Barrett's esophagus [60,61]. Wu and colleagues reported that GSDME expression was upregulated in esophageal squamous cell carcinoma (ESCC) cell lines [60]. Additionally, increased GSDME expression induced apoptosis and signaling pathways associated with pyroptosis [60].
However, it remains unclear whether high GSDME expression is a driver of ESCC. In addition, whether LPS regulates GSDME expression is unknown and deserves further investigation.
Pyroptosis is vital in the pathogenesis and progression of gastric cancer (GC). The expression of GSDMD protein, a pyroptosis marker, is decreased in GC cells, thus promoting cancer cell proliferation [62]. Suppression of activated GSDMD molecules through the PI3K/AKT, ERK1/2, and STAT3 signaling pathways has also been shown to inhibit cell cycle protein A2 and cell cycle protein-dependent kinase (CDK2) expression levels in GC cells [[63], [64], [65]]. Cdk2/cyclin A2 complexes regulate the phosphorylation of proteins involved in DNA synthesis and promote S phase advancement, whereas the downregulation of cyclinA2/CDK2 complexes causes S to G2/M transition arrest. These studies suggest that GSDMD may be a driving factor in GC development. However, it has also been reported that in addition to its role in pyroptosis, GSDMs also play this regulatory role in biological behaviors such as vesicle transport and immune resistance, which are not dependent on the function of pyroptosis effectors. Therefore, further rigorous experimental studies are needed to investigate whether GSDMD regulates GC by affecting pyroptosis [66,67].
The NLRP4 inflammasome initiates a variety of inflammatory responses [68]. GC cells express significantly higher levels of NLRP4 inflammasome than normal gastric epithelial cells [69]. GSDMA expression was downregulated in GC cells, and the GSDMA protein molecule functions as an anti-oncogenic factor [70,71]. Recent research indicates that abundant GSDMB expression has been detected in a few normal GC tissues [72]. Surprisingly, another study discovered that overexpression of GSDMB can inhibit tumor invasion [49]. According to Saeki et al., GSDMC acts as a tumor suppressor gene [69]. According to Miguchi et al. [73]. GSDML is a potential subgroup of the GSDMC protein molecule, which is linked to cancer [74]. GSDML has previously been shown to promote the development of several cancers, including gastric, hepatic, colorectal, and nonlesion tissues [75]. The GSDML gene was found to be upregulated in human colon, stomach, and liver cell lines; neoplasms; and nonlesion tissues. Another study found that the GSDML protein was linked to the cell's cytoplasm in both tumors and nonlesional tissues [75]. GSDML protein expression was shown to be greater in tumor tissues. GSDML exhibited a characteristic vesicular staining pattern in mucous cells on the colon surface, gastric chief cells in the apical area, and the basal part of neuroendocrine cells [75]. According to previous research, GSDMD expression levels are increased in both GC cell lines and experimental animal models [70,71]. The silencing of DFNA5/GSDME was initially observed in primary GC cell lines [76]. Additionally, DFNA5/GSDME transfection into GC cell lines decreased the number of colonies and inhibited cell growth as compared to cells transfected with an empty vector [77,78]. The p-53 gene regulates the expression of DFNA5/GSDME, indicating that DFNA5 is a tumor suppressor gene [79]. Caspase-3-mediated pyroptosis enhances GC formation through the DFNA5/GSDME signaling pathways [62].
As mentioned above, GSDMs have diverse effects on the development of GC. It can be a tumor suppressor (such as GSDMA, GSDMB) or a tumor promoter (such as GSDML). This may be caused by different regulatory factors that act on GSDMs. Therefore, identification of the upstream regulatory factors of GSDMs in GC is the key to achieve comprehensive understanding of the influence of GSDMs on the occurrence and development of GC. In addition, it remains unclear whether the effect of these GSDMs on GC is related to cell pyroptosis. Therefore, the effect of GSDMs on GC is not sufficient to evaluate the effect of pyroptosis on GC.
4.1.2. Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is a cancer that affects hepatocytes. The role of pyroptosis in human HCC is not well understood. Chu and colleagues previously reported that caspase-1 protein and mRNA expression levels were decreased in both human tumor tissues and cell lines, indicating that HCC growth and progression may be promoted by heat-induced cell death [80]. Wei et al. conducted an ex vivo study and found that the downregulation of the NLRP3 inflammasome was associated with the genesis and development of HCC [81,82]. Additionally, Wei et al. observed that 17β-estradiol possesses anticancer properties, which they attributed to its potential to induce pyrogenesis via the NLRP3 inflammasome [81]. Wei and colleagues reported increasing caspase-1 dosages and IL-1 expression in HCC cells [83]. Chen and colleagues recently revealed that caspase-1, IL-1, and IL-18 expression was much lower in HCC tissues than in surrounding normal tissues [84]. Previous research has demonstrated that the AIM2 inflammasome can inhibit S6K1 activation and hence limit cancer cell progression by targeting mTOR and that its accumulation in HCC cells can result in pyroptosis, which has antitumor effects [85,86]. In a groundbreaking study, IF and western blotting analyses demonstrated an obvious decrease in downregulated AIM2 expression in HepG2215 cells [87]. On the basis of these findings, it could be stated that pyroptosis plays a role in the pathophysiology and development of HCC; however, its molecular mechanism needs to be further explored.
4.1.3. Colorectal cancer
According to Hu et al., caspase-1 gene deficiency promotes tumorigenesis in azoxymethane- and dextran sodium sulfate colitis-related colorectal cancer mice models [54]. The putative role in carcinogenesis has not been linked to the control of colonic inflammation, but in colon cells, it is involved in epithelial cell proliferation and death [54]. Animal models with caspase-1-gene deletion showed increased colonic epithelial cell proliferation and inhibited apoptosis in advanced tumors during the early stages of injury-stimulated tumor growth. Additionally, caspase-1 promotes the tumor suppressor activity of STAT1 in colitis-associated carcinogenesis [88]. AIM2 expression was previously determined in a subset of colorectal tumors with an exacerbated mutation in a study investigating the potential involvement of AIM2 in tumor cells and patient prognosis. Lower AIM2 expression was found to be closely associated with a poor prognosis in colorectal cancer [89]. Williams et al. found that NLRP1 expression was significantly reduced in IBD and colon cancer [90]. Inflammation, cancer, and morbidity may be promoted in NLRP1b-knockout mice [90]. As previously demonstrated, inflammasome formation to NLRs, misdirected inflammation, and tumor progression were associated with decreased levels of IL-1 and IL-18 pro-inflammatory mediators. Chen et al. demonstrated that NLRP1 expression was associated with tumor progression in colon cancer [88,91].
Tang et al. showed that NLRP3 and caspase-1 inflammasome pathways participate in the development of colorectal cancer [92]. FL118 induces pyroptosis in colorectal cancer cells by activating NLRP3-ASC-Caspase-1-IL-18 and IL-1 signaling pathways [92]. Previous research demonstrated that mRNA and protein expression of NLRP3 and Caspase-1 was downregulated in colon cancer patients, indicating a relationship between pyroptosis and colon cancer [92]. GSDMC expression is downregulated in colorectal tumors [73]. Additionally, the decreased expression levels of GSDMC may promote the proliferation and development of tumor cells. GSDMC has the potential to open up a new therapeutic window for treating and regulating colorectal cancer in the near future [73]. Wu et al. demonstrated a significant correlation between GSDMD expression levels in human colorectal cancer (CRC) tissues and a poor prognosis in CRC patients. LPS, but not TNF-, induced pyroptosis in HT29 cells by promoting GSDMD and GSDMD-N membrane translocation and increasing chemosensitivity in response to L-OHP. Additionally, the overexpression of GSDMD in HT29 cells decreased cell survival and resulted in cell death. Reduced GSDMD expression is associated with a poor prognosis in CRC, and LPS-induced pyroptosis may increase the anticancer activity of L-anti-cancer OHP’ by activating the GSDMD signaling pathway, hence suppressing CRC carcinogenesis. Our findings lay the foundation for future exploitation of GSDMD as a predictive biomarker and a feasible target for colorectal cancer treatment [93]. Recent research indicates that GSDME may be a potential biomarker for the detection of colorectal cancers [94]. Additional research is required to reveal the underlying mechanism of GSDME-mediated pyroptotic cell death in colorectal cancer.
4.1.4. Lung cancer
According to a recent study, pyroptosis mediated by GSDMD is critical to the genesis and progression of lung cancer (LC) [95]. GSDMD deficiency has been shown to significantly slow tumor development. Reduced epidermal growth factor receptor signaling, increased caspase-3 breakdown, and accelerated apoptosis in GSDMD-silenced NSCLC cells result in the suppression of tumor growth in transplanted animals. Stimulation of NLRP3/caspase-1 signaling induces apoptosis rather than pyroptosis in tumor cells lacking GSDMD [95]. GSDMD knockdown inhibits cell growth in NSCLC by promoting apoptosis and inhibiting the EGFR/Akt pathway. High GSDMD expression has been associated with aggressive characteristics such as larger tumors and advanced tumor-node-metastatic stages [96]. Surprisingly, high expression level of GSDMD in lung adenocarcinoma (LAD) resulted in a worse prognosis (SCC) when compared with that for squamous cell carcinoma [96]. Lu et al. demonstrated that knockdown of DFNA5/GSDME causes caspase-3-activated cells to flip from apoptosis to pyroptosis, thus confirming that the expression level of DFNA5/GSDME regulates the death mode of caspase-3-activated cells. In LC, the deletion of the DFNA5/GSDME genes increases resistance to drugs, whereas overexpression of DFNA5/GSDME enhances drug sensitivity [97]. According to Hoechst33324/PI staining, flow cytometry analysis, and toss real-time live-cell imaging analysis, the downregulation of inflammasome components promotes the progression of NSCLC in A549 and H1299 cell lines [98]. A previous study discovered increased NLRP3, ASC, Caspase-1, IL-1, IL-18, and GSDMD mRNA and protein expression in A549 and H1299. Therefore, inhibiting the ROS/NF–B/NLRP3/GSDMD signaling axis promotes NSCLS progression by downregulating caspase-1-mediated pyroptosis. According to Liu et al., the upregulation of lncRNA-XIST may promote the development of NSCLC by inhibiting pyroptotic cell death mediated by the miR-335/SOD2/ROS/NLRP3 signaling pathway, thus implying that NLRP3 inflammasome-mediated pyroptosis provides new insight and knowledge on the pathogenesis and development of lung cancer [99].
4.1.5. Cervical cancer
Cervical cancer is linked to infection with a DNA virus called human papillomavirus (HPV) in more than 90% of cases [100]. HPV transforms infective cervical cells into cancer cells by integrating the viral DNA and evading host antiviral immunity [101]. SIRT1 (Sir-tuin 1) is the mammalian ortholog of silent information regulatory 2 (Sir2), and it has been established that SIRT1 regulates cell death by deacetylating the NF–B subunit RelA/p65 [102]. SIRT1 is overexpressed in HPV-infected cervical cancer cells as compared to normal cervical cancer cells, and it contributes to cancer cell development and proliferation [103]. SIRT1 expression in cervical cancer cells suppresses NF–B-induced transcription of absent in melanoma 2 (AIM2) genes by disrupting the mRNA of the AIM2 gene transcription factor RelB. SIRT1 knockdown, on the other hand, increased RelB stability and activated AIM2 inflammasome-related genes, resulting in AIM2 inflammasome-regulated pyroptosis [104]. AIM2 is a receptor that recognizes cytosolic double-stranded DNAs (dsDNA), including HPV, and forms a large supramolecular complex with ASC to activate caspase-1-induced pyroptosis [33]. Additionally, extracellular vesicles transport AIM2 inflammasomes and AIM2 inflammasome-regulated pyroptosis between cells [104]. These data suggest that pyroptosis induced by the AIM2 inflammasome contributes to the development of CC.
Yang et al. established that the protein expression levels of caspase-1, GSDMD, and NLRP3 were reduced in human and mouse tumor tissues and cell lines [105]. In a previous study, researchers used the GSDMD si-RNA lentivirus to transfect endometrial cancer cells to investigate the potential role of the GSDMD protein molecule in pyroptosis-regulated cell death [105]. GSDMD knockdown induced pyroptosis in endometrial cancer tissues and cell lines derived from xenograft mice. Additionally, increased expression levels of caspase-1, GSDMD, and NLRP3 decreased tumor weight and volume [105].
Overexpression of miR-214, which targets the NLRP3 inflammasome in CCs, may activate pyroptosis-signaling pathways. Cervical cancer patients have decreased levels of miR-214, NLRP3, and caspase-1. Pyroptosis can be induced in CC cells by upregulating miR-214 and increasing NLRP3 inflammasome expression [106]. Tong and colleagues identified the miR-145/GSDMD signaling pathway as a key mediator of tanshinone II's action on HeLa cells [90]. Thus, CC formation requires the miR-145/GSDMD signaling pathway. Additionally, the miR-145/GSDMD signaling axis may be a target for CC treatment, and there is a relationship between miR-145 and pyroptosis, which provides fresh insights into the pathophysiology of CC and potential preventative medicine for its treatment and regulation [92]. As a result, additional research is necessary to elucidate the critical role of pyroptosis-dependent cell death in CC.
4.1.6. Ovarian cancer
Recent research has examined the role of the lncRNA GAS5 in ovarian cancer suppression. The lncRNA GAS5 induces apoptosis and pyroptosis in ovarian cancer cells and thus acts as a tumor suppressor. Overexpression of lncRNA GAS5 was associated with caspase-1 activation, which increased the expression levels of IL-18 and IL-1β in a time-dependent manner, whereas lncRNA GAS5 knockdown decreased the expression level of these factors [107]. lncRNA GAS5 influenced inflammasome development and triggered an inflammatory response by disrupting the glucocorticoid receptor (GR), which is distributed throughout the body and is required for the regulation of genes involved in growth, metastasis, and the immune system [107,108].
Liang et al. suggested that decreased GSDME-dependent pyroptosis is a key factor in OC progression [109]. Additionally, Qiao and colleagues found that GSDMD/caspase-4 expression was downregulated in epithelial ovarian cancer (EOC) cell lines such as Ho8910PM, A2780, and Ho8910 [110]. They used alpha-NETA to induce pyroptosis in those cell lines and produced a variety of microbubbles [110]. Alpha-NETA increased the expression of pyroptosis-related proteins in EOC cells, including caspase-4 and GSDMD [110]. Caspase-4 and GSDMD knockdown significantly reduced alpha-NETA cell invasion activity in EOC cells, thus indicating that alpha-NETA activated pyroptosis signaling pathways [110]. Luborsky et al. reported that the mRNA and protein expression levels of NLRP3, Caspase-1, IL1, and IL18 were significantly decreased in ovarian cancers, indicating that pyroptosis plays a critical role in the progression of OC [111].
HOTTIP, an lncRNA, is an important regulator in multiple cancers, including OC. Tan et al. recently demonstrated an increase in HOTTIP expression in OC tissue samples and cell lines [112]. In this study, the authors revealed that silencing HOTTIP inhibited cell growth and NLRP1 inflammasome-mediated pyroptosis. Additionally, HOTTIP increased AKT2 expression by inhibiting the ASK1/JNK signaling pathway and negatively regulating miR-148a-3p. Further rescue experiments revealed that the upregulation of AKT2 and downregulation of miR-148a-3p expression in OC cells mitigated the effects of HOTTIP downregulation [112]. These findings suggest a novel pyroptosis-related mechanism through which HOTTIP contributes to the development of OC, suggesting that HOTTIP may be therapeutic target.
4.1.7. Breast cancer
Breast cancer is a complex, heterogeneous illness with a diverse histological characteristics and clinical manifestations [113]. Pizato et al. demonstrated that a reduction in GSDMD-regulated pyroptosis contributes significantly to BC progression [114]. Decreased caspase-1, GSDMD, HMGB1, and IL-1 expression was also found to increase BC cell proliferation and expansion. Cytoplasm and membrane hole development were also observed in BC cells. Guo and colleagues discovered that retinoic acid-inducible gene (RIG-I) signaling induces pyroptosis in ER + BC cells by activating the caspase-3, caspase-1, and GSDMD signaling pathways [113]. The formation of a cellular membrane pore in BC cells results in lysosomal damage and generation of reactive oxygen species (ROS) [115]. The formation of a cellular membrane pore in BC cells results in lysosomal damage and generation of ROS [114]. According to Wu and colleagues, IL-1β expression was significantly elevated during the development and progression of breast neoplasms, and variations in IL-18 and IL-1β have been associated with breast tumor progression [116]. Increased GSDMB expression levels have been associated with shorter survival and higher rates of metastasis in BC [117]. Hergueta and colleagues found that GSDMB enhanced invasion and metastasis in BC cells [117]. As a result, GSDMD may be considered a robust biomarker for monitoring the prognosis of BC. The expression of DFNA5/GSDME (formerly known as ICERE-1) is elevated in ER-negative cell lines, which promotes BC-specific carcinogenesis of hormone-unresponsiveness. Kim et al. reported that DFNA5 methylation is associated with lymph node metastases in human BC [78]. Reduced-DFNA5/GSDME expression levels in MCF-7 cells are also sensitive to paclitaxel treatment, as shown in a new study. Additionally, Masuda et al. revealed that p53 may induce DFNA5/GSDME expression through a unique-binding regional intron-1 of DFNA5 [79]. p-63 is a member of the p53 family that promotes DFNA5 expression, indicating that it is a novel transcriptional member of the p53 family [118].
Wu et al. demonstrated that the expression of caspase-1, IL-1β, and GSDMD proteins was positively correlated in BC tissues, which was consistent with the development of the caustic death pathway [119]. Overexpression of IL-1β and NLRP3 in the breast tumor microenvironment has been associated with the accumulation of MDSCs and TAMs. An in silico meta-analysis of caspase-1, NLRP3, and IL-1β revealed the expression levels of these factors was correlated with longer overall survival of BC. NLRP3 demonstrated a favorable association with survival across all molecular subtypes in the TCGA BC dataset. The levels of NLRP3 and IL-1β in TAM are associated with survival, lymph node invasion, and metastasis in patients with HER2+ BC. This indicates that pyroptosis has a positive impact on the progression, invasion, and metastasis of BC. There is an urgent need for additional experimental research into the pathophysiology of pyroptosis.
4.1.8. Skin cancer
Miguchi et al. showed that the expression level of GSDMC was lower in malignant melanoma cells, possibly due to cancer cell invasion and metastasis [73]. A previous study also showed that DFNA5/GSDME expression was significantly higher in the nonresistant MeWo cell line than in the etoposide-resistant MeWo ETO 1 cell line [120]. Furthermore, DFNA5/GSDME knockdown increased etoposide resistance in etoposide-resistant melanoma cells, whereas DFNA5/GSDME upregulation improved etoposide sensitivity, implying that low expression of DNFA5 is associated with increased etoposide resistance in melanoma cells [121].
Eukaryotic elongation factor-2 kinase (eEF-2K) regulates protein synthesis to trigger autophagy and pyroptosis in tumor cells [122]. Yu et al. recently reported that eEF-2K signaling is crucial in pyroptosis of doxorubicin-stimulated human MCs [122]. These findings demonstrate that GSDME-dependent pyroptosis is crucial in melanoma.
4.1.9. Other cancers
The expression of mammalian STE20-like kinase 1 (MST1) is reduced in pancreatic ductal adenocarcinoma (PDAC) cells. However, restoration of MST1 expression increases PDAC cell death and suppresses PDAC cell proliferation, migration, and invasion through ROS-driven pyroptosis [123]. Recently, it was found that microRNA (miRNA) is associated with pyroptosis in certain cancers [124]. miRNA-214 reduces glioma cell migration and proliferation by altering the caspase-1 pathway [125]. Moreover, Kay et al. showed that the NAIP-NLRC4 inflammasome contributes to the development and progression of glioma [126].
4.2. Triggering pyroptosis in tumors for therapeutic purposes
4.2.1. Chemotherapeutic drugs
4.2.1.1. Metformin
Metformin is a biguanide derivative commonly used to treat type 2 diabetes and can inhibit cancer proliferation. Many retrospective data and experimental studies have shown that metformin has antineoplastic activity. However, the specific mechanisms are unknown [127]. Through in vitro and in vivo, Wang et al. demonstrated that metformin might promote GSDMD-induced pyroptosis of esophageal squamous cell carcinoma (ESCC) by targeting the miR-497/proline-, glutamic acid-, and leucine-rich protein-1 (PELP1) axis [128]. Another study suggested that metformin can be an alternative treatment for chemo- and radiotherapy-resistant ESCC or other malignancies with similar pyroptosis pathways [128].
4.2.1.2. Doxorubicin
A recent study revealed that eukaryotic elongation factor-2 kinase (eEF-2K), a negative regulator of protein synthesis, plays a significant role in DOXO-induced pyroptosis of human melanoma cells [122]. The silencing of eEF-2K increases pyroptosis and enhances the sensitivity of melanoma cells to doxorubicin. Therefore, targeting eEF-2K may enhance the anti-tumor efficacy of doxorubicin, providing a novel insight into tumor chemotherapy [122]. Yu et al. also found that eEF-2K knockdown increases GSMDE levels, indicating that eEF-2K can alter pyroptosis by decreasing GSDME formation. The precise molecular mechanism for this phenomenon should be investigated [122].
4.2.1.3. Lobaplatin
Yu et al. revealed that lobaplatin (LPT) can reduce the viability of HT-29 and HCT116 colon cells in a dose-dependent manner. Moreover, HT-29 and HCT116 cells show microscopic features of cell enlargement, huge bubbles from the plasma membrane, and many pores in the membrane after lobaplatin treatment, as revealed by transmission electron microscopy (TEM) [129]. Mechanistically, lobaplatin elevates the level of ROS and c-Jun N-terminal kinase (JNK) phosphorylation. Activated JNK triggers caspase-3/9 cleavage and GSDME-dependent pyroptosis in colon cancer cells by recruiting Bax to mitochondria, thereby promoting the release of cytochrome c into the cytosol and leading to GSDME-dependent pyroptosis [129]. Lobaplatin eliminates colon cancer cells via GSDME-dependent pyroptosis and thus can be used as an anticancer drug [129].
4.2.1.4. Paclitaxel and cisplatin
Paclitaxel and cisplatin activate caspase-3 and GSDME in A549 lung cancer cells to induce pyroptosis. Cisplatin has a stronger promoting effect on pyroptosis than paclitaxel. Similarly, cisplatin triggers stronger caspase-3 activation and production of the N-terminal segment of GSDME than paclitaxel [130]. These findings show that cisplatin can induce higher pyroptosis of A549 cells as compared to paclitaxel, indicating that cisplatin may have additional benefits on lung malignancies with high GSDME expression [130].
4.2.1.5. 5-Fluorouracil
Treatment with 5-fluorouracil causes the production of cleaved caspase-3 and accumulation of the N-terminal segment of GSDME in gastric cancer cells, leading to pyroptosis [62]. CRISPR-Cas9-mediated deletion of GSDME transforms 5-fluorouracil-induced pyroptosis into apoptosis, suggesting that GSDME might transform 5-fluorouracil-induced caspase-3-dependent apoptosis into pyroptosis in gastric cancer cells [62].
4.2.1.6. Others
Other pyroptosis-related chemicals have also been investigated. For instance, Chalcone derivative-8 with the α,β-unsaturated-ketone unit inhibits the proliferation of NCI–H460 cancer cell lines. Zhu et al. reported that these derivatives trigger GSDME-mediated pyroptosis. Moreover, N-substituted EF24 analog 13d decreases xenograft tumor development by inhibiting cell viability, arresting cell cycle in the G2/M phase, promoting cell apoptosis, and inhibiting cell viability. Compound 13d can also cause pyroptosis due to the suppression of the nuclear transcription factor-κB (NF-kB) and caspase-3 signaling pathways in lung cancer [132]. L61H10 is a heterocyclic ketone derivative with anticancer properties [133]. L61H10 can convert NF-kB-stimulated apoptosis into pyroptosis in LC cells [133]. Li et al. showed that the novel piperlongumine-analogue (PL-a) L50377 can affect NSCLC cells, thus causing pyroptosis. ROS-induced NF-κB regulation is involved in the L50377-stimulated NSCLC cell pyroptosis [134].
4.2.1.7. Alpha-NETA
Alpha-NETA is a reversible choline acetylcholine-transferase inhibitor. A recent study showed that alpha-NETA can activate GSDMD/caspase-4-induced pyroptosis, thus inhibiting EOC cell growth [110], consistent with the results of in vivo tests. Pyroptosis induction may be induce a therapeutic effect for OC treatment [110].
4.2.1.8. PLK1 inhibitors
A previous study showed that PLK1 inhibitors can enhance ESCC cell sensitivity to cisplatin (CP) by suppressing the DNA damage repair mechanism and enhancing the Bax/Caspase-3/GSDME signaling pathway-dependent pyroptosis [60]. Therefore, PLK1 inhibitors are promising therapeutic agents for ESCC-targeted treatment, especially when combined with CP [60].
4.2.1.9. Decitabine (DAC)
Fan et al. developed a decitabine (DAC) and nano-drug combination therapy for generating pyroptosis in malignant cells by targeting epigenetics [135]. DAC pretreatment demethylated the DFNA5 gene in malignant cells of tumor-bearing mice. The researchers used a tumor-specific nanoliposome loaded with CP (Lipo-DDP) to activate caspase-3 and pyroptosis in tumor cells. Decitabine combined with chemotherapeutic nanodrugs induced pyroptosis of tumor cells through epigenetics, thus enhancing the immunological impact of chemotherapy [135]. Furthermore, GSDME inhibition in malignant cells induces pyroptosis [135].
4.2.1.10. Anthocyanidins (ANTH)
Anthocyanidins (ANTH) are natural colorants belonging to the flavonoid family. They have attracted much attention because of their anticancer properties [136]. A recent research study showed that anthocyanins can lower the viability of oral squamous cell carcinoma (OSCC) cells, thus limiting their migration and invasion. Anthocyanins can lead to pyroptosis activation in OSCC due to the increased expression of NLRP3, caspase-1, and IL-1β. Furthermore, western blot assay revealed that anthocyanin can increase GSDMD protein expression, indicating that anthocyanin induces its anti-cancer effect through pyroptosis induction. A recent study showed that anthocyanins can lower the viability of OSCC cells, thus limiting their migration and invasion. Anthocyanins can lead to pyroptosis activation in OSCC due to increased expression of NLRP3, caspase-1, and IL-1β. Western blotting assay showed that anthocyanin can increase GSDMD protein expression, indicating that anthocyanin induces its anticancer effect through pyroptosis induction [137]. However, caspase-1 inhibitors can reduce anthocyanin-activated pyroptosis, while increasing cell survival, migration, and invasion rates [137].
4.2.1.11. Galanin
Yang et al. discovered that galanin treatment increased the N-terminal fragment of GSDME in a dose-dependent manner. They also showed that GSDME knockdown can exacerbate nuclear DNA damage in glioblastoma multiforme cells, thus implying that pyroptosis may influence the extent of apoptosis after galanin treatment [138].
4.2.1.12. Omega-3 fatty acids
Numerous research studies have demonstrated that omega-3 fatty acids can control inflammation and exhibit anti-cancer properties [139]. The omega-3 fatty acid docosahexaenoic acid (DHA) can cause cancer cell death because of its anticancer properties [140]. Pizato et al. explored the potential inhibitory effect of DHA on triple-negative breast cancer cells and the underlying molecular pathways. They discovered that DHA increases caspase-1 and GSDMD activation, increases IL-1 production, and translocates human high mobility group box 1 (HMGB1) to the cytoplasm to form holes in the membrane in breast cancer cells [114].
4.2.1.13. Dipeptidyl peptidases (DPPs) inhibitor
Dipeptidyl peptidases (DPPs) are serine proteases that are phylogenetically related. DPP8 and DPP9 belong to the S9b subfamily of serine proteases, including DPP IV [141]. Val-boroPro, a dipeptidyl peptidase 8 and 9 (DPP8/9) inhibitor, is associated with pyroptosis. Gaiet al. also found that the CARD-containing protein CARD8 can mediate DPP8/9 inhibitor-induced pro-caspase-1-dependent pyroptosis in sensitive human acute myeloid leukemia (AML) cells. Therefore, Val-boroPro can induce GSDMD cleavage, indicating that small molecule DPP8/9 inhibitors are potential AML therapeutics [142].
Arsenic trioxide-NPs suppress tumor growth in HCC by upregulating GSDME-N and inhibiting caspase-3 [143].
4.2.1.14. GSDMD targeting compounds
GSDMD is a target for several small compounds, including necrosulfonamide and LDC7559. Necrosulfonamide is a necroptosis inhibitor that can block necrosome production in several cancer cells by preferentially targeting the mixed lineage kinase domain-like protein (MLKL) [144]. Rathkey et al. reported that necrosulfonamide can treat septic shock by binding to cysteine 191 and inhibiting the oligomerization of GSDMD dimers [145]. LDC7559 can effectively prevent the development of neutrophil extracellular traps (NETs) and GSDMD-mediated pyroptotic cell death in human cells [146].
4.2.1.15. 4-Hydroxybenzoic acid (4-HBA)
Sannino et al. found that 4-hydroxybenzoic acid (4-HBA) can induce pyroptosis-regulated cell death in LC A549 cell lines [147]. Moreover, 4-HBA can upregulate caspase-1, IL-1β, and IL-18 in LAD cells [147]. The 4-HBA treatment can also activate the transcription of caspase-1, IL-1, and IL-18 regulatory genes in A549 cells. However, additional research is required to assess how 4-HBA induces pyroptosis-regulated death in cancer cells [147].
4.2.2. Immunotherapy
The use of a biorthogonal system consisting of the cancer-imaging probe phenylalanine trifluoroborate (Phe-BF3) and gold nanoparticle (NP) delivery to GSDMA3 in tumor cells has revealed that pyroptosis can enhance anticancer immune response to improve the efficiency of immune checkpoint blockade [148].
Zhou et al. reported that natural killer cells and cytotoxic T lymphocytes destroy gasdermin B (GSDMB)-positive cells through pyroptosis. Lymphocyte-derived granzyme A (GZMA) kills GSDMB by cleaving it and releasing its pore-forming function. Interferon (IFN) induces pyroptosis by upregulating GSDMB expression. GZMA-cleavable GSDMB can increase tumor clearance in mice when introduced into mouse cancer cells. Gasdermin-mediated pyroptosis is a cytotoxic lymphocyte-killing mechanism which may improve anti-tumor immunity [50].
MEK inhibitors combined with BRAF inhibitors can cause pyroptosis of melanoma cells with high expression of HMGB1, high number of tumor-associated T cells, and low dendritic cell infiltration. However, GSDME deficiency inhibits anti-tumor immunity [149,150]. Another study showed that GSDME knockdown in GSDME-expressing tumors enhances tumor development. However, ectopic expression in GSDME-repressed tumors decreases tumor growth in mice through killer cytotoxic lymphocytes. However, this suppression does not occur in perforin-deficient or killer lymphocyte-depleted animals. GSDME expression increases the quantity and activity of tumor-infiltrating natural killer and CD8+ T lymphocytes, and their ability to phagocytose tumor cells through tumor-associated macrophages. Killer-cell granzyme B promotes caspase-independent pyroptosis in target cells by directly cleaving GSDME at the same location as caspase 3. GSDME proteins that are not soluble or have pore defects cannot suppress tumor development. Consequently, tumor GSDME functions as a tumor suppressor by increasing antitumor immunity through pyroptosis induction [151]. However, Gao Tan showed that HMGB1, a proinflammatory factor secreted by GSDME-mediated pyroptotic epithelial cells, promotes colorectal cancer growth through the ERK1/2 pathway [152]. Furthermore, Liu discovered that pyroptosis-released components from tumor cells can stimulate pyroptosis in macrophages resulting in cytokine release and cytokine release syndrome (CRS) [153].
These findings show that pyroptosis mediates the tumoricidal effects of certain inhibitors or cytolytic immune cells. Pyroptosis-induced release of inflammatory factors may either induce anti-tumor immunity, promote tumor growth, or generate inflammatory cascades. Therefore, the relationship between pyroptosis and anti-tumor immunity should be assessed.
A previous study showed that human umbilical cord mesenchymal stem cells release substances that trigger MCF7 cell pyroptosis. The substances can also significantly alter the expression of NLRP1 and CAPS4 and the pathways associated with inflammation [154]. Colunga et al. discovered that calpain, caspase-7, and caspase-3 activation in many malignant melanoma cells inoculated with the herpes simplex virus type 2 (HSV-2) mutant PK (HSV-2 mutant PK) can increase the oncolytic impact of PK. The △PK can also increase expression level of heat shock proteins, such as Beclin-1 and H11/Hsp B8. The corresponding immunohistochemical results also revealed that TNF-αis activated and participates in the formation of the NLRP3 inflammasome mediated by caspase-1, thus inducing apoptosis and pyroptosis [155]. Zhou et al. indicated that increased ROS may oxidize Tom20, an outer mitochondrial membrane protein, after iron therapy, thereby allowing Bax recruitment to mitochondria and accelerating caspase-3/GSDME-mediated pyroptosis. Iron triggers ROS to promote DFNA5/GSDME-dependent pyroptosis and selectively enhances DFNA5/GSDME expression in melanoma cells. Therefore, it may be a promising therapy for melanoma. Iron supplements can enhance the anticancer function of clinical ROS-induced medicines and suppress the proliferation and spread of xenografted melanoma cells via DFNA5/GSDME-dependent pyroptosis [156].
5. Conclusions and perspectives
Pyroptosis, a caspase-dependent cell death, causes pore formation, cell swelling, rupture of the plasma membrane, and release of all intracellular contents. This paper discusses the molecular mechanisms of pyroptosis to generate ideas for developing therapeutic strategies for disease treatment. A number of studies have shown that tumor cells undergo pyroptosis in vivo, suggesting a potential role for pyroptosis in the regulation of tumorigenesis. Previous studies have extensively investigated approaches for promoting apoptosis of cancer cells as a strategy to eliminate malignant cells. However, this approach is hampered by the revelation that tumor cells have mechanisms of evading apoptosis. Based on theories of inflammation–cancer transformation and chronic inflammation-induced cell carcinogenesis, pyroptosis, as a pro-inflammatory mode of death, can create a microenvironment suitable for tumor cell growth.
The role of pyroptosis in cancer is an extremely complex issue. This review argues that it should be discussed in different cancer periods according to the characteristics of cancer occurrence and development. At the stage of cancer occurrence, the current evidence points out that pyroptosis promotes cancer more than it inhibits cancer. For example, it promotes cancer occurrence by promoting the release of IL-1, IL-18, or NLRP inflammatory bodies, which can be confirmed in gastric cancer and liver cancer. It should be noted that the pathway of pyroptosis-induced cancer may be different in different cancers, which needs further exploration. However, interestingly, we also found that the decrease of pyroptosis could promote the development of cancer in the process of cancer progression. For example, in gastric cancer, the downregulation of GSDMD was significantly positively correlated with the proliferation of cancer cells, or the decrease of NLRP3 inflammasome could promote the development of liver cancer, including the decrease in caspase-1 and IL-1 expression in HCC tissues. This conclusion can also be more or less evident in other cancers (such as lung cancer and cervical cancer). Throughout the whole process, the research of the Gasdermin family is also a very promising issue, such as the possibility of GSDMD and GSDME as biomarkers in colorectal cancer, and GSDML, a member of the Gasdermin family with future research prospects.
In cancer treatment, pyroptosis provides us with some potential new targets, such as HOTTIP. For the existing drugs, the study of pyroptosis also helps us make better use of existing drugs for anticancer treatment. Immunotherapy is a hot research direction in the field of cancer treatment. As mentioned above, we look forward to more studies on immunotherapy related to pyroptosis in the future.
In conclusion, there is growing evidence that tumor pyroptosis has antitumor and protumor roles. Failure of cancer therapy is largely caused by resistance to apoptosis. Focusing on answering the question “What Role Does Pyroptosis Play in Cancer?” will help to improve cancer diagnosis and treatment.
Ethical approval and consent to participate
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 32000533); Sichuan Science and Technology Program (grant number 2021YFS0215); Post-doctor Research Project, West China Hospital, Sichuan University (grant number 2020HXBH113).
Authors’ contributions
CLZ contributed to the guidance of this review and gave the final approval of the version to be published; CH drafted the manuscript; and JL prepared the figures. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Availability of supporting data
Not applicable
Acknowledgments
Not applicable
Conflict of interests
The authors declare that they have no competing interests.
Abbreviations
- PCD
programmed cell death
- GSDMD
gasdermin D
- GSDME
gasdermin E
- TAK1
transforming growth factor-β activated kinase 1
- IAP
inhibitor of apoptosis
- GERD
Gastroesophageal reflux disease
- ESCC
esophageal squamous cell carcinoma
- CDK2
cycle protein-dependent kinase
- HCC
Hepatocellular carcinoma
- CRC
colorectal cancer
- LC
lung cancer
- LAD
lung adenocarcinoma
- Sir2
Silent information regulatory 2
- GR
glucocorticoid receptor
- EOC
epithelial ovarian cancer
- eEF-2K
Eukaryotic elongation factor-2 kinase
- PDAC
pancreatic ductal adenocarcinoma
- LPT
lobaplatin
- TEM
transmission electron microscopy
- JNK
Jun N-terminal kinase
- PL-a
piperlongumine-analogue
- CP
cisplatin
- DAC
Decitabine
- ANTH
Anthocyanidins
- OSCC
oral squamous cell carcinoma
- HMGB1
human high mobility group box 1
- DPPs
Dipeptidyl peptidases
- DPP8/9
dipeptidyl peptidase 8 and 9
- AML
acute myeloid leukemia
- NETs
neutrophil extracellular traps
- 4-HBA
4-hydroxybenzoic acid
- NP
nanoparticle
- Phe-BF3
phenylalanine trifluoroborate
- IFN
Interferon
- CRS
cytokine release syndrome
- HSV-2
herpes simplex virus type 2
- PAMPs
Pathogen-associated molecular patterns
- DAMPs
danger-associated molecular patterns
- NLRP
nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family
- ROS
reactive oxygen species
- dsDNA
double-stranded DNA
- ASC
apoptosis-associated speck-like protein containing CARD
- LPS
lipopolysaccharide
- P2RX7
P2X purinoceptor 7
- GSDMC
cleaving gasdermin C
- Yop-J
Yersinia outer protein-J
- CARD
caspase recruitment
- PYD
pyrin domain
- AIM2
Absent In Melanoma 2
- GZMA
granzyme A
- TAK1
TGF-Beta Activated Kinase-1
Data availability
No data was used for the research described in the article.
References
- 1.Crowley L.C., Marfell B.J., Scott A.P., Boughaba J.A., Chojnowski G., Christensen M.E., et al. Dead cert: measuring cell death. Cold Spring Harbour Protocols. 2016;2016(12) doi: 10.1101/pdb.top070318. [DOI] [PubMed] [Google Scholar]
- 2.Bedoui S., Herold M.J., Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nature Reviews Molecular Cell Biology. 2020;21(11):678–695. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 3.Kim K.H., Lee M.-S. Autophagy-a key player in cellular and body metabolism. Nature Reviews Endocrinology. 2014;10(6):322–337. doi: 10.1038/nrendo.2014.35. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen I., Rayamajhi M., Miao E.A. Programmed cell death as a defence against infection. Nature Reviews Immunology. 2017;17(3):151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death and Differentiation. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariathasan S., Weiss D.S., Dixit V.M., Monack D.M. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. Journal of Experimental Medicine. 2005;202(8):1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cookson B.T., Brennan M.A. Pro-inflammatory programmed cell death. Trends in Microbiology. 2001;9(3):113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 8.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: mechanisms and diseases. Signal Transduction and Targeted Therapy. 2021;6(1):128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagarajan K., Soundarapandian K., Thorne R.F., Li D., Li D. Activation of pyroptotic cell death pathways in cancer: an alternative therapeutic approach. Translational Oncology. 2019;12(7):925–931. doi: 10.1016/j.tranon.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Fink S.L., Cookson B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cellular Microbiology. 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 12.Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nature Reviews Immunology. 2016;16(7):407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 13.Zychlinsky A., Prevost M.C., Sansonetti P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 14.Hilbi H., Chen Y., Thirumalai K., Zychlinsky A. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infection and Immunity. 1997;65(12):5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hersh D., Monack D.M., Smith M.R., Ghori N., Falkow S., Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 17.Kayagaki N., Stowe I.B., Lee B.L., O’Rourke K., Anderson K., Warming S., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Gao W., Shi X., Ding J., Liu W., He H., et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661) doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 19.Rogers C., Fernandes-Alnemri T., Mayes L., Alnemri D., Cingolani G., Alnemri E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nature Communications. 2017;8 doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orning P., Weng D., Starheim K., Ratner D., Best Z., Lee B., et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science (New York, N.Y.) 2018;362(6418):1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarhan J., Liu B.C., Muendlein H.I., Li P., Nilson R., Tang A.Y., et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during infection. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(46):E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K.W., Demarco B., Heilig R., Shkarina K., Boettcher A., Farady C.J., et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. The EMBO Journal. 2019;38(10) doi: 10.15252/embj.2019101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X., Xia S., Zhang Z., Wu H., Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nature Reviews Drug Discovery. 2021;20(5):384–405. doi: 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao C., Zhao W. NLRP3 inflammasome-A key player in antiviral responses. Frontiers in Immunology. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aachoui Y., Sagulenko V., Miao E.A., Stacey K.J. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Current Opinion in Microbiology. 2013;16(3):319–326. doi: 10.1016/j.mib.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunology. 2009;10(3):241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Vanaja S.K., Rathinam V.A.K., Fitzgerald K.A. Mechanisms of inflammasome activation: recent advances and novel insights. Trends in Cell Biology. 2015;25(5):308–315. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rathinam V.A.K., Fitzgerald K.A. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165(4):792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y., Yang J., Shi J., Gong Y.-N., Lu Q., Xu H., et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477(7366):596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 31.Miao E.A., Mao D.P., Yudkovsky N., Bonneau R., Lorang C.G., Warren S.E., et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(7):3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts T.L., Idris A., Dunn J.A., Kelly G.M., Burnton C.M., Hodgson S., et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science (New York, N.Y.) 2009;323(5917):1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 33.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K., Sun Q., Zhong X., Zeng M., Zeng H., Shi X., et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180(5) doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Case C.L., Roy C.R. Asc modulates the function of NLRC4 in response to infection of macrophages by Legionella pneumophila. mBio. 2011;2(4) doi: 10.1128/mBio.00117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broz P., von Moltke J., Jones J.W., Vance R.E., Monack D.M. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host and Microbe. 2010;8(6):471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonopoulos C., Russo H.M., El Sanadi C., Martin B.N., Li X., Kaiser W.J., et al. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. Journal of Biological Chemistry. 2015;290(33):20167–20184. doi: 10.1074/jbc.M115.652321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K.W., Demarco B., Broz P. Beyond inflammasomes: emerging function of gasdermins during apoptosis and NETosis. The EMBO Journal. 2020;39(2) doi: 10.15252/embj.2019103397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M., Chen X., Zhang Y. Biological functions of gasdermins in cancer: from molecular mechanisms to therapeutic potential. Frontiers in Cell and Developmental Biology. 2021;9 doi: 10.3389/fcell.2021.638710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aglietti R.A., Estevez A., Gupta A., Ramirez M.G., Liu P.S., Kayagaki N., et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(28):7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duez H., Pourcet B. Nuclear receptors in the control of the NLRP3 inflammasome pathway. Frontiers in Endocrinology. 2021;12 doi: 10.3389/fendo.2021.630536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang D., He Y., Muñoz-Planillo R., Liu Q., Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43(5):923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu B., He R., Zhang L., Hao B., Jiang W., Wang W., et al. Inflammatory caspases drive pyroptosis in acute lung injury. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.631256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuchiya K. Switching from apoptosis to pyroptosis: gasdermin-elicited inflammation and antitumor immunity. International Journal of Molecular Sciences. 2021;22(1) doi: 10.3390/ijms22010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuchiya K. Inflammasome-associated cell death: pyroptosis, apoptosis, and physiological implications. Microbiology and Immunology. 2020;64(4):252–269. doi: 10.1111/1348-0421.12771. [DOI] [PubMed] [Google Scholar]
- 46.Kim S.I., Choi M.E. TGF-β-activated kinase-1: new insights into the mechanism of TGF-β signaling and kidney disease. Kidney Research and Clinical Practice. 2012;31(2) doi: 10.1016/j.krcp.2012.04.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan J., Xia S., Liu X., Lieberman J., Wu H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature. 2018;557(7703):62–67. doi: 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H., et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L., Li Y., Bai Y. Role of GSDMB in pyroptosis and cancer. Cancer Management and Research. 2020;12:3033–3043. doi: 10.2147/CMAR.S246948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Z., He H., Wang K., Shi X., Wang Y., Su Y., et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells New York, N.Y.) Science. 2020;368(6494) doi: 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- 51.Hou J., Zhao R., Xia W., Chang C.-W., You Y., Hsu J.-M., et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nature Cell Biology. 2020;22(10):1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang L., Lu C., Zheng G., Burgering B.M. Emerging insights on the role of gasdermins in infection and inflammatory diseases. Clinical and Translational Immunology. 2020;9(10):e1186. doi: 10.1002/cti2.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M., Jiang S., Zhang Y., Li P., Wang K. The multifaceted roles of pyroptotic cell death pathways in cancer. Cancers. 2019;11(9) doi: 10.3390/cancers11091313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu B., Elinav E., Huber S., Booth C.J., Strowig T., Jin C., et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen I.C., TeKippe E.M., Woodford R.-M.T., Uronis J.M., Holl E.K., Rogers A.B., et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. Journal of Experimental Medicine. 2010;207(5):1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaki M.H., Lamkanfi M., Kanneganti T.-D. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends in Immunology. 2011;32(4):171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F., Li G., Ning J., Chen L., Xu H., Kong X., et al. Alcohol accumulation promotes esophagitis via pyroptosis activation. International Journal of Biological Sciences. 2018;14(10):1245–1255. doi: 10.7150/ijbs.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herbella F.A.M., Neto S.P., Santoro I.L., Figueiredo L.C. Gastroesophageal reflux disease and non-esophageal cancer. World Journal of Gastroenterology. 2015;21(3):815–819. doi: 10.3748/wjg.v21.i3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoofs N., Bisschops R., Prenen H. Progression of Barrett’s esophagus toward esophageal adenocarcinoma: an overview. Annals of Gastroenterology. 2017;30(1):1–6. doi: 10.20524/aog.2016.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu M., Wang Y., Yang D., Gong Y., Rao F., Liu R., et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. EBioMedicine. 2019;41:244–255. doi: 10.1016/j.ebiom.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nadatani Y., Huo X., Zhang X., Yu C., Cheng E., Zhang Q., et al. NOD-like receptor protein 3 inflammasome priming and activation in barrett’s epithelial cells. Cellular and Molecular Gastroenterology and Hepatology. 2016;2(4):439–453. doi: 10.1016/j.jcmgh.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Yin B., Li D., Wang G., Han X., Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochemical and Biophysical Research Communications. 2018;495(1):1418–1425. doi: 10.1016/j.bbrc.2017.11.156. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh F.F., Barnett L.A., Green W.F., Freedman K., Matushansky I., Skoultchi A.I., et al. Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27(Kip1) and inactivation of cdk2 kinase. Blood. 2000;96(8):2746–2754. [PubMed] [Google Scholar]
- 64.Martín A., Odajima J., Hunt S.L., Dubus P., Ortega S., Malumbres M., et al. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1) Cancer Cell. 2005;7(6):591–598. doi: 10.1016/j.ccr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Oakes V., Wang W., Harrington B., Lee W.J., Beamish H., Chia K.M., et al. Cyclin A/Cdk2 regulates Cdh1 and claspin during late S/G2 phase of the cell cycle. Cell Cycle. 2014;13(20):3302–3311. doi: 10.4161/15384101.2014.949111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karmakar M., Minns M., Greenberg E.N., Diaz-Aponte J., Pestonjamasp K., Johnson J.L., et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nature Communications. 2020;11(1):2212. doi: 10.1038/s41467-020-16043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kambara H., Liu F., Zhang X., Liu P., Bajrami B., Teng Y., et al. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Reports. 2018;22(11):2924–2936. doi: 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis B.K., Wen H., Ting J.P.Y. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual Review of Immunology. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moon S.W., Son H.J., Mo H.Y., Yoo N.J., Lee S.H. Somatic mutation of genes in gastric and colonic cancers. Pathology and Oncology Research. 2021;27 doi: 10.3389/pore.2021.607385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiu S., Liu J., Xing F. Hints in the killer protein gasdermin D: unveiling the secrets of gasdermins driving cell death. Cell Death and Differentiation. 2017;24(4):588–596. doi: 10.1038/cdd.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saeki N., Usui T., Aoyagi K., Kim D.H., Sato M., Mabuchi T., et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes, Chromosomes and Cancer. 2009;48(3):261–271. doi: 10.1002/gcc.20636. [DOI] [PubMed] [Google Scholar]
- 72.Saeki N., Komatsuzaki R., Chiwaki F., Yanagihara K., Sasaki H. A GSDMB enhancer-driven HSV thymidine kinase-expressing vector for controlling occult peritoneal dissemination of gastric cancer cells. BMC Cancer. 2015;15:439. doi: 10.1186/s12885-015-1436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miguchi M., Hinoi T., Shimomura M., Adachi T., Saito Y., Niitsu H., et al. Gasdermin C is upregulated by inactivation of transforming growth factor β receptor type II in the presence of mutated apc, promoting colorectal cancer proliferation. PLoS One. 2016;11(11):e0166422. doi: 10.1371/journal.pone.0166422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Q., Yang J., Xing G., Sun Q., Zhang L., He F. Expression of GSDML associates with tumor progression in uterine cervix cancer. Translational Oncology. 2008;1(2):73–83. doi: 10.1593/tlo.08112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carl-McGrath S., Schneider-Stock R., Ebert M., Rocken C. Differential expression and localisation of gasdermin-like (GSDML), a novel member of the cancer-associated GSDMDC protein family, in neoplastic and non-neoplastic gastric, hepatic, and colon tissues. Pathology. 2008;40(1):13–24. doi: 10.1080/00313020701716250. [DOI] [PubMed] [Google Scholar]
- 76.Saeki N., Kuwahara Y., Sasaki H., Satoh H., Shiroishi T. Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mammalian Genome : Official Journal of the International Mammalian Genome Society. 2000;11(9):718–724. doi: 10.1007/s003350010138. [DOI] [PubMed] [Google Scholar]
- 77.Kim M.S., Chang X., Yamashita K., Nagpal J.K., Baek J.H., Wu G., et al. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene. 2008;27(25):3624–3634. doi: 10.1038/sj.onc.1211021. [DOI] [PubMed] [Google Scholar]
- 78.Kim M.S., Lebron C., Nagpal J.K., Chae Y.K., Chang X., Huang Y., et al. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochemical and Biophysical Research Communications. 2008;370(1):38–43. doi: 10.1016/j.bbrc.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masuda Y., Futamura M., Kamino H., Nakamura Y., Kitamura N., Ohnishi S., et al. The potential role of DFNA5, a hearing impairment gene, in p53-mediated cellular response to DNA damage. Journal of Human Genetics. 2006;51(8):652–664. doi: 10.1007/s10038-006-0004-6. [DOI] [PubMed] [Google Scholar]
- 80.Chu Q., Jiang Y., Zhang W., Xu C., Du W., Tuguzbaeva G., et al. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget. 2016;7(51):84658–84665. doi: 10.18632/oncotarget.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei Q., Zhu R., Zhu J., Zhao R., Li M. E2-Induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncology Research. 2019;27(7):827–834. doi: 10.3727/096504018X15462920753012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li P., Liu Y., He Q. Anisodamine suppressed the growth of hepatocellular carcinoma cells, induced apoptosis and regulated the levels of inflammatory factors by inhibiting NLRP3 inflammasome activation. Drug Design, Development and Therapy. 2020;14:1609–1620. doi: 10.2147/DDDT.S243383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei Q., Mu K., Li T., Zhang Y., Yang Z., Jia X., et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Laboratory Investigation; a Journal of Technical Methods and Pathology. 2014;94(1):52–62. doi: 10.1038/labinvest.2013.126. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y.F., Qi H.Y., Wu F.L. Euxanthone exhibits anti-proliferative and anti-invasive activities in hepatocellular carcinoma by inducing pyroptosis: preliminary results. European Review for Medical and Pharmacological Sciences. 2018;22(23):8186–8196. doi: 10.26355/eurrev_201812_16511. [DOI] [PubMed] [Google Scholar]
- 85.Ma X., Guo P., Qiu Y., Mu K., Zhu L., Zhao W., et al. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget. 2016;7(24):36185–36197. doi: 10.18632/oncotarget.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao W., Yang J., Liu W., Wang Y., Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(33):E4857–E4866. doi: 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi X., Wang L., Ren L., Li J., Li S., Cui Q., et al. Dihydroartemisinin, an antimalarial drug, induces absent in melanoma 2 inflammasome activation and autophagy in human hepatocellular carcinoma HepG2215 cells. Phytotherapy Research : PTR. 2019;33(5):1413–1425. doi: 10.1002/ptr.6332. [DOI] [PubMed] [Google Scholar]
- 88.Flood B., Manils J., Nulty C., Flis E., Kenealy S., Barber G., et al. Caspase-11 regulates the tumour suppressor function of STAT1 in a murine model of colitis-associated carcinogenesis. Oncogene. 2019;38(14):2658–2674. doi: 10.1038/s41388-018-0613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dihlmann S., Tao S., Echterdiek F., Herpel E., Jansen L., Chang-Claude J., et al. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. International Journal of Cancer. 2014;135(10):2387–2396. doi: 10.1002/ijc.28891. [DOI] [PubMed] [Google Scholar]
- 90.Williams T.M., Leeth R.A., Rothschild D.E., Coutermarsh-Ott S.L., McDaniel D.K., Simmons A.E., et al. The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis Baltimore, Md. Journal of Immunology. 2015;194(7):3369–3380. doi: 10.4049/jimmunol.1402098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen C., Wang B., Sun J., Na H., Chen Z., Zhu Z., et al. DAC can restore expression of NALP1 to suppress tumor growth in colon cancer. Cell Death and Disease. 2015;6:e1602. doi: 10.1038/cddis.2014.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tong W., Guo J., Yang C. Tanshinone II A enhances pyroptosis and represses cell proliferation of HeLa cells by regulating miR-145/GSDMD signaling pathway. Bioscience Reports. 2020;40(4) doi: 10.1042/BSR20200259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu L.-S., Liu Y., Wang X.-W., Xu B., Lin Y.-L., Song Y., et al. LPS enhances the chemosensitivity of oxaliplatin in HT29 cells via GSDMD-mediated pyroptosis. Cancer Management and Research. 2020;12:10397–10409. doi: 10.2147/CMAR.S244374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ibrahim J., Op de Beeck K., Fransen E., Croes L., Beyens M., Suls A., et al. Methylation analysis of Gasdermin E shows great promise as a biomarker for colorectal cancer. Cancer Medicine. 2019;8(5):2133–2145. doi: 10.1002/cam4.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao J., Qiu X., Xi G., Liu H., Zhang F., Lv T., et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer. Oncology Reports. 2018;40(4):1971–1984. doi: 10.3892/or.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xi G., Gao J., Wan B., Zhan P., Xu W., Lv T., et al. GSDMD is required for effector CD8 T cell responses to lung cancer cells. International Immunopharmacology. 2019;74 doi: 10.1016/j.intimp.2019.105713. [DOI] [PubMed] [Google Scholar]
- 97.Lu H., Zhang S., Wu J., Chen M., Cai M.-C., Fu Y., et al. Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor cell death. Clinical Cancer Research : An Official Journal of the American Association For Cancer Research. 2018;24(23):6066–6077. doi: 10.1158/1078-0432.CCR-18-1478. [DOI] [PubMed] [Google Scholar]
- 98.Teng J.-F., Mei Q.-B., Zhou X.-G., Tang Y., Xiong R., Qiu W.-Q., et al. Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal Axis in non-small cell lung cancer. Cancers. 2020;12(1) doi: 10.3390/cancers12010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J., Yao L., Zhang M., Jiang J., Yang M., Wang Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging. 2019;11(18):7830–7846. doi: 10.18632/aging.102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crosbie E.J., Einstein M.H., Franceschi S., Kitchener H.C. Human papillomavirus and cervical cancer. Lancet (London, England) 2013;382(9895):889–899. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 101.Woodman C.B.J., Collins S.I., Young L.S. The natural history of cervical HPV infection: unresolved issues. Nature Reviews Cancer. 2007;7(1):11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 102.Yeung F., Hoberg J.E., Ramsey C.S., Keller M.D., Jones D.R., Frye R.A., et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. The EMBO Journal. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Velez-Perez A., Wang X.I., Li M., Zhang S. SIRT1 overexpression in cervical squamous intraepithelial lesions and invasive squamous cell carcinoma. Human Pathology. 2017;59:102–107. doi: 10.1016/j.humpath.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 104.So D., Shin H.-W., Kim J., Lee M., Myeong J., Chun Y.-S., et al. Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense. Oncogene. 2018;37(38):5191–5204. doi: 10.1038/s41388-018-0339-4. [DOI] [PubMed] [Google Scholar]
- 105.Yang Y., Liu P.Y., Bao W., Chen S.J., Wu F.S., Zhu P.Y. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer. 2020;20(1):28. doi: 10.1186/s12885-019-6491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu S., Zhao N., He M., Zhang K., Bi X. MiRNA-214 promotes the pyroptosis and inhibits the proliferation of cervical cancer cells via regulating the expression of NLRP3. Cellular and Molecular Biology (Noisy-le-Grand, France) 2020;66(6):59–64. [PubMed] [Google Scholar]
- 107.Li J., Yang C., Li Y., Chen A., Li L., You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Bioscience Reports. 2018;38(2) doi: 10.1042/BSR20171150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Conzen S.D. Recent advances in understanding glucocorticoid receptor function in cancer. Clinical Advances in Hematology and Oncology. 2017;15(5):338–340. [PMC free article] [PubMed] [Google Scholar]
- 109.Liang J., Zhou J., Xu Y., Huang X., Wang X., Huang W., et al. Osthole inhibits ovarian carcinoma cells through LC3-mediated autophagy and GSDME-dependent pyroptosis except for apoptosis. European Journal of Pharmacology. 2020;874 doi: 10.1016/j.ejphar.2020.172990. [DOI] [PubMed] [Google Scholar]
- 110.Qiao L., Wu X., Zhang J., Liu L., Sui X., Zhang R., et al. α-NETA induces pyroptosis of epithelial ovarian cancer cells through the GSDMD/caspase-4 pathway. FASEB Journal : Official Publication of the Federation of American Societies For Experimental Biology. 2019;33(11):12760–12767. doi: 10.1096/fj.201900483RR. [DOI] [PubMed] [Google Scholar]
- 111.Luborsky J., Barua A., Edassery S., Bahr J.M., Edassery S.L. Inflammasome expression is higher in ovarian tumors than in normal ovary. PLoS One. 2020;15(1):e0227081. doi: 10.1371/journal.pone.0227081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tan C., Liu W., Zheng Z.-H., Wan X.-G. LncRNA HOTTIP inhibits cell pyroptosis by targeting miR-148a-3p/AKT2 axis in ovarian cancer. Cell Biology International. 2021;45(7):1487–1497. doi: 10.1002/cbin.11588. [DOI] [PubMed] [Google Scholar]
- 113.Badve S., Dabbs D.J., Schnitt S.J., Baehner F.L., Decker T., Eusebi V., et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Modern Pathology : An Official Journal of the United States and Canadian Academy of Pathology, Inc. 2011;24(2):157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- 114.Pizato N., Luzete B.C., Kiffer L.F.M.V., Corrêa L.H., de Oliveira Santos I., Assumpção J.A.F., et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Scientific Reports. 2018;8(1):1952. doi: 10.1038/s41598-018-20422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo B., Fu S., Zhang J., Liu B., Li Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Scientific Reports. 2016;6 doi: 10.1038/srep36107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu T., Hong Y., Jia L., Wu J., Xia J., Wang J., et al. Modulation of IL-1β reprogrammes the tumor microenvironment to interrupt oral carcinogenesis. Scientific Reports. 2016;6 doi: 10.1038/srep20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hergueta-Redondo M., Sarrió D., Molina-Crespo Á., Megias D., Mota A., Rojo-Sebastian A., et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fujikane T., Nishikawa N., Toyota M., Suzuki H., Nojima M., Maruyama R., et al. Genomic screening for genes upregulated by demethylation revealed novel targets of epigenetic silencing in breast cancer. Breast Cancer Research and Treatment. 2010;122(3):699–710. doi: 10.1007/s10549-009-0600-1. [DOI] [PubMed] [Google Scholar]
- 119.Wu X., Mao X., Huang Y., Zhu Q., Guan J., Wu L. Detection of proteins associated with the pyroptosis signaling pathway in breast cancer tissues and their significance. International Journal of Clinical and Experimental Pathology. 2020;13(6):1408–1414. [PMC free article] [PubMed] [Google Scholar]
- 120.Grottke C., Mantwill K., Dietel M., Schadendorf D., Lage H. Identification of differentially expressed genes in human melanoma cells with acquired resistance to various antineoplastic drugs. International Journal of Cancer. 2000;88(4):535–546. doi: 10.1002/1097-0215(20001115)88:4<535::aid-ijc4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 121.Lage H., Helmbach H., Grottke C., Dietel M., Schadendorf D. DFNA5 (ICERE-1) contributes to acquired etoposide resistance in melanoma cells. FEBS Letters. 2001;494(1-2):54–59. doi: 10.1016/s0014-5793(01)02304-3. [DOI] [PubMed] [Google Scholar]
- 122.Yu P., Wang H.-Y., Tian M., Li A.-X., Chen X.-S., Wang X.-L., et al. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharmacologica Sinica. 2019;40(9):1237–1244. doi: 10.1038/s41401-019-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cui J., Zhou Z., Yang H., Jiao F., Li N., Gao Y., et al. MST1 suppresses pancreatic cancer progression via ROS-induced pyroptosis. Molecular Cancer Research : MCR. 2019;17(6):1316–1325. doi: 10.1158/1541-7786.MCR-18-0910. [DOI] [PubMed] [Google Scholar]
- 124.Tezcan G., Martynova E.V., Gilazieva Z.E., McIntyre A., Rizvanov A.A., Khaiboullina S.F. MicroRNA post-transcriptional regulation of the NLRP3 inflammasome in immunopathologies. Frontiers in Pharmacology. 2019;10:451. doi: 10.3389/fphar.2019.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang Z., Yao L., Ma H., Xu P., Li Z., Guo M., et al. miRNA-214 inhibits cellular proliferation and migration in glioma cells targeting caspase 1 involved in pyroptosis. Oncology Research. 2017;25(6):1009–1019. doi: 10.3727/096504016X14813859905646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kay C., Wang R., Kirkby M., Man S.M. Molecular mechanisms activating the NAIP-NLRC4 inflammasome: implications in infectious disease, autoinflammation, and cancer. Immunological Reviews. 2020;297(1):67–82. doi: 10.1111/imr.12906. [DOI] [PubMed] [Google Scholar]
- 127.Gong T.-T., Wu Q.-J., Lin B., Ruan S.-K., Kushima M., Takimoto M. Observational studies on the association between post-diagnostic metformin use and survival in ovarian cancer: a systematic review and meta-analysis. Frontiers In Oncology. 2019;9:458. doi: 10.3389/fonc.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang L., Li K., Lin X., Yao Z., Wang S., Xiong X., et al. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Letters. 2019;450:22–31. doi: 10.1016/j.canlet.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 129.Yu J., Li S., Qi J., Chen Z., Wu Y., Guo J., et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death and Disease. 2019;10(3):193. doi: 10.1038/s41419-019-1441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang C.-C., Li C.-G., Wang Y.-F., Xu L.-H., He X.-H., Zeng Q.-Z., et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis : An International Journal On Programmed Cell Death. 2019;24(3-4):312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 131.Zhu M., Wang J., Xie J., Chen L., Wei X., Jiang X., et al. Design, synthesis, and evaluation of chalcone analogues incorporate α,β-Unsaturated ketone functionality as anti-lung cancer agents via evoking ROS to induce pyroptosis. European Journal of Medicinal Chemistry. 2018;157:1395–1405. doi: 10.1016/j.ejmech.2018.08.072. [DOI] [PubMed] [Google Scholar]
- 132.Chen L., Li Q., Zheng Z., Xie J., Lin X., Jiang C., et al. Design and optimize N-substituted EF24 as effective and low toxicity NF-κB inhibitor for lung cancer therapy via apoptosis-to-pyroptosis switch. Chemical Biology and Drug Design. 2019;94(1):1368–1377. doi: 10.1111/cbdd.13514. [DOI] [PubMed] [Google Scholar]
- 133.Chen L., Weng B., Li H., Wang H., Li Q., Wei X., et al. A thiopyran derivative with low murine toxicity with therapeutic potential on lung cancer acting through a NF-κB mediated apoptosis-to-pyroptosis switch. Apoptosis : An International Journal On Programmed Cell Death. 2019;24(1-2):74–82. doi: 10.1007/s10495-018-1499-y. [DOI] [PubMed] [Google Scholar]
- 134.Li Q., Chen L., Dong Z., Zhao Y., Deng H., Wu J., et al. Piperlongumine analogue L50377 induces pyroptosis via ROS mediated NF-κB suppression in non-small-cell lung cancer. Chemico-Biological Interactions. 2019;313 doi: 10.1016/j.cbi.2019.108820. [DOI] [PubMed] [Google Scholar]
- 135.Fan J.-X., Deng R.-H., Wang H., Liu X.-H., Wang X.-N., Qin R., et al. Epigenetics-based tumor cells pyroptosis for enhancing the immunological effect of chemotherapeutic nanocarriers. Nano Letters. 2019;19(11):8049–8058. doi: 10.1021/acs.nanolett.9b03245. [DOI] [PubMed] [Google Scholar]
- 136.de Sousa Moraes L.F., Sun X., Peluzio M.d.C.G., Zhu M.-J. Anthocyanins/anthocyanidins and colorectal cancer: what is behind the scenes? Critical Reviews in Food Science and Nutrition. 2019;59(1):59–71. doi: 10.1080/10408398.2017.1357533. [DOI] [PubMed] [Google Scholar]
- 137.Yue E., Tuguzbaeva G., Chen X., Qin Y., Li A., Sun X., et al. Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology. 2019;56:286–294. doi: 10.1016/j.phymed.2018.09.223. [DOI] [PubMed] [Google Scholar]
- 138.Kong Y., Feng Z., Chen A., Qi Q., Han M., Wang S., et al. The natural flavonoid galangin elicits apoptosis, pyroptosis, and autophagy in glioblastoma. Frontiers In Oncology. 2019;9:942. doi: 10.3389/fonc.2019.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tan R.-H., Wang F., Fan C.-L., Zhang X.-H., Zhao J.-S., Zhang J.-J., et al. Algal oil rich in n-3 polyunsaturated fatty acids suppresses B16F10 melanoma lung metastasis by autophagy induction. Food and Function. 2018;9(12):6179–6186. doi: 10.1039/c8fo01617h. [DOI] [PubMed] [Google Scholar]
- 140.Jeong S., Kim D.Y., Kang S.H., Yun H.K., Kim J.L., Kim B.R., et al. Docosahexaenoic acid enhances oxaliplatin-induced autophagic cell death via the ER stress/sesn2 pathway in colorectal cancer. Cancers. 2019;11(7) doi: 10.3390/cancers11070982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gai K., Okondo M.C., Rao S.D., Chui A.J., Ball D.P., Johnson D.C., et al. DPP8/9 inhibitors are universal activators of functional NLRP1 alleles. Cell Death and Disease. 2019;10(8):587. doi: 10.1038/s41419-019-1817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johnson D.C., Taabazuing C.Y., Okondo M.C., Chui A.J., Rao S.D., Brown F.C., et al. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nature Medicine. 2018;24(8):1151–1156. doi: 10.1038/s41591-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu J., Dong Y., Ding L., Dong Y., Wu Z., Wang W., et al. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduction and Targeted Therapy. 2019;4:28. doi: 10.1038/s41392-019-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang H., Sun L., Su L., Rizo J., Liu L., Wang L.-F., et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Molecular Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 145.Rathkey J.K., Zhao J., Liu Z., Chen Y., Yang J., Kondolf H.C., et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Science Immunology. 2018;3(26) doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sollberger G., Choidas A., Burn G.L., Habenberger P., Di Lucrezia R., Kordes S., et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Science Immunology. 2018;3(26) doi: 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- 147.Sannino F., Sansone C., Galasso C., Kildgaard S., Tedesco P., Fani R., et al. Pseudoalteromonas haloplanktis TAC125 produces 4-hydroxybenzoic acid that induces pyroptosis in human A459 lung adenocarcinoma cells. Scientific Reports. 2018;8(1):1190. doi: 10.1038/s41598-018-19536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Q., Wang Y., Ding J., Wang C., Zhou X., Gao W., et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579(7799):421–426. doi: 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 149.Erkes D.A., Cai W., Sanchez I.M., Purwin T.J., Rogers C., Field C.O., et al. Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discovery. 2020;10(2):254–269. doi: 10.1158/2159-8290.CD-19-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Long G.V., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. New England Journal of Medicine. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Z., Zhang Y., Xia S., Kong Q., Li S., Liu X., et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579(7799):415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tan G., Huang C., Chen J., Zhi F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. Journal of Hematology and Oncology. 2020;13(1):149. doi: 10.1186/s13045-020-00985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Y., Fang Y., Chen X., Wang Z., Liang X., Zhang T., et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Science Immunology. 2020;5(43) doi: 10.1126/sciimmunol.aax7969. [DOI] [PubMed] [Google Scholar]
- 154.Jiao Y., Zhao H., Chen G., Sang X., Yang L., Hou Z., et al. Pyroptosis of MCF7 cells induced by the secreted factors of hUCMSCs. Stem Cells International. 2018;2018 doi: 10.1155/2018/5912194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Colunga A.G., Laing J.M., Aurelian L. The HSV-2 mutant DeltaPK induces melanoma oncolysis through nonredundant death programs and associated with autophagy and pyroptosis proteins. Gene Therapy. 2010;17(3):315–327. doi: 10.1038/gt.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou B., Zhang J.-Y., Liu X.-S., Chen H.-Z., Ai Y.-L., Cheng K., et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Research. 2018;28(12):1171–1185. doi: 10.1038/s41422-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable
No data was used for the research described in the article.