Abstract
In accordance with Article 43 of Regulation (EC) No 396/2005, the European Commission requested EFSA to assess whether the existing temporary maximum residue levels (MRLs) for nicotine in rose hips, teas and capers are sufficiently protective for European consumers, based on the risk assessment model currently used for pesticide risk assessments (EFSA PRIMo rev. 3.1). In its assessment, EFSA noted potential acute exposure risks for the intake of nicotine through rose hips and tea; EFSA recommended to lower the existing MRLs for these two commodities to levels that do not lead to an exceedance of the ARfD. For capers, EFSA concluded that the existing MRL is unlikely to lead to acute intake concerns. The risk assessment for the three food commodities under assessment is affected by additional, non‐standard uncertainties.
Keywords: nicotine, rose hips, teas, capers, pesticides, MRL, acute risk assessment
Background
Nicotine is the main alkaloid in tobacco and other tobacco species; it is found in low concentrations also in other crops belonging to the family of Solanaceae. Due to its action as an agonist for acetylcholine receptors, nicotine exhibits insecticidal activities, and the compound was used as an active substance in plant protection products in the past.
The use of nicotine as an insecticide was evaluated in the framework of Directive 91/414/EEC. 1 Based on the Draft Assessment Report (DAR) prepared by the Rapporteur Member State, the European Commission concluded that the existing evidence is not sufficient to demonstrate a safe use of nicotine as a plant protection product with respect to operators, workers, bystanders and consumers. Thus, the evaluation under Directive 91/414/EEC resulted in a decision not to include nicotine in Annex I (Commission Decision 2009/9/EC 2 ) and all plant protection products containing nicotine as active substance had to be withdrawn by 8 June 2009. At that time, no specific maximum residue levels (MRLs) for nicotine were set, nor was that substance included in Annex IV to Regulation (EC) No 396/2005 3 , and therefore the default MRL of 0.01 mg/kg applied to all products.
In 2009 and 2011, the European Commission asked the European Food Safety Authority (EFSA) to provide advice on the setting of temporary MRLs for nicotine for a number of commodities, in which residue levels greater than the default MRL of 0.01 mg/kg were repeatedly identified in the framework of controls performed by food business operators and/or national competent authorities (EFSA, 2009, 2011).
Based on the assessment of EFSA, specific temporary MRLs were set in Annex IIIA of Regulation (EC) No 396/2005 for nicotine in wild fungi (Commission Regulation (EU) No 765/2010 4 ) and for nicotine in rose hips, herbs and edible flowers, teas, herbal infusions and spices (Commission Regulation (EU) No 812/2011 5 ).
The source of these residues in the commodities concerned has not been elucidated. Contaminations during harvest, drying, storage or transport were considered as possible sources of nicotine residues in the concerned products. Other explanations for the contamination were suggested, e.g. cultivation of the crops in fields previously used for tobacco cultivation or the natural occurrence of nicotine in certain plant products.
However, as scientific evidence was not conclusive to demonstrate whether nicotine occurs naturally in the concerned crop, risk managers decided to review the temporary MRLs after 10 years, taking into account additional information that became available in this period.
The levels of nicotine in these products, for which temporary MRLs are established, are periodically reviewed, considering results of official controls of competent national authorities, in view of the possible lowering of the temporary MRLs.
The Commission is currently drafting a Commission Regulation aiming at lowering some of the temporary MRLs for nicotine according to monitoring data and presented a draft for discussion with Member States to the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF), section Phytopharmaceuticals – Pesticides residues in April 2022. On that occasion, one Member State had informed the Commission that the current temporary MRL of 4 mg/kg for nicotine in capers might pose acute risks to consumers according to intake calculations performed with the most recent version of PRIMo (rev. 3.1). A potential acute risk for consumers was also identified for the current temporary MRLs for nicotine in rose hips and teas, taking into account the recent food consumption data for these products representative for European consumers.
Terms of reference (as provided by the requestor)
EFSA is requested, according to Article 43 of Regulation (EC) No 396/2005,
-
•
to assess the acute (short‐term) risk for European consumers related to nicotine exposure via residues in rose hips (code 0154050), teas (Camellia sinensis, 0610000) and capers (0850020) at the level equal to the current temporary MRLs established under Regulation (EC) No 396/2005 (i.e. 0.3 mg/kg for rose hips, 0.6 mg/kg for teas and 4 mg/kg for capers). The risk assessment shall be performed with the newest version of the PRIMo model, based on the available residue definitions, using the acute reference dose derived by EFSA in 2009 (EFSA, 2009);
-
•
to consider that capers are normally not eaten raw but are consumed in a certain processed state, e.g. salted or in brine, and to verify the basis of the reported monitoring data and consumption data by the Member States (if necessary with their assistance), in order to be able to match monitoring data and consumption data correctly;
-
•
to recommend, in case acute risk for consumers is identified for one or more the products listed above, MRLs that do not pose an unacceptable risk to consumers, and/or advise risk managers on alternative options.
The deadline for delivering a statement according to Article 43 of Regulation (EC) No 396/2005 on the safety of the proposed MRLs for consumers was agreed to be 2 months from receipt of this mandate.
EFSA accepted the mandate and included it in the EFSA Register of Questions with the reference number EFSA‐Q‐2022‐0499 and committed to provide the statement by 14 September 2022.
Assessment
EFSA performed the targeted short‐term dietary risk assessment for the existing temporary MRLs for nicotine in the three commodities concerned, focussing on the question whether the existing temporary MRLs are sufficiently protective for European consumers. Under the current mandate, EFSA was not requested to assess whether the MRLs which were derived in the past for the crops under consideration and for other crops on the basis of monitoring data are still appropriate, considering the most recent monitoring data. The review of temporary MRLs in view of their possible lowering is performed in the framework of a separate mandate.
1. Toxicological reference values
The toxicological profile of nicotine was assessed by EFSA in the framework of the setting of temporary MRLs for nicotine in mushrooms (EFSA, 2009). The information available in published literature was considered sufficient to derive toxicological reference values. An acute reference dose (ARfD) of 0.0008 mg/kg body weight was derived, based on the lowest observed adverse effect level (LOAEL) of 0.0035 mg/kg body weight for pharmacological effects after intravenous application of nicotine (i.e. slight, transient and rapidly reversible increase of the heart rate in humans), using an overall uncertainty factor of 10 and a correction factor of 0.44 for oral bioavailability of nicotine (extrapolation from the intravenous route to the oral route). The LOAEL is considered to be close to the no observed adverse effect level (NOAEL) and the overall uncertainty factor of 10 would be sufficient to cover the intraspecies variability and the extrapolation from the LOAEL to NOAEL for the pharmacological effects. The acceptable daily intake (ADI) was proposed at the same level (0.0008 mg/kg body weight per day).
Although the toxicological reference values have not been formally adopted according to the current procedures, the ARfD is considered appropriate for the current risk assessment.
2. Residue levels and residue definition
The enforcement residue definition established in Regulation (EC) No 396/2005 comprises the parent compound nicotine only.
The temporary MRLs for rose hips, teas and capers and the relevant descriptions of the part of the crop to which the MRL refers to are reported in Table 1.
Table 1.
Existing MRLs for nicotine
| Code(a) | Commodity | Existing tMRL | Part of the product to which MRLs apply |
|---|---|---|---|
| Residue definition for enforcement: Nicotine | |||
| 0154050 | Rose hips | 0.3 | Whole product after removal of caps, crown and stems (description for the group of berries and small fruits) |
| 0610000 | Teas | 0.6 | Dried leaves, stalk and flowers, whether fermented or otherwise treated |
| 0850020 | Capers | 4 | Dried product whole, crushed or ground (description for spices) |
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
As requested in the second bullet point of the Terms of Reference, EFSA investigated the origin of the current MRL for capers, which was derived in 2011 from monitoring data (EFSA, 2011). Re‐evaluating the monitoring data that were used as the basis for the MRL, EFSA noted that no specific monitoring data were available for bud spices, e.g. capers. Therefore, the MRL proposal for capers was derived by extrapolation from monitoring data from other spices (barks, roots and rhizome of spices). In total, results for 89 samples (40 samples on cinnamon bark, 39 samples for liquorice roots, 9 samples for ginger roots and 1 sample for curcuma) were available.
Among the more recent monitoring data submitted to EFSA, no information on nicotine residues in capers was available.
Hence, overall, in the pesticide monitoring database provided by the national competent authorities, no data are available on the magnitude of nicotine in capers.
3. Acute (short‐term) exposure assessment
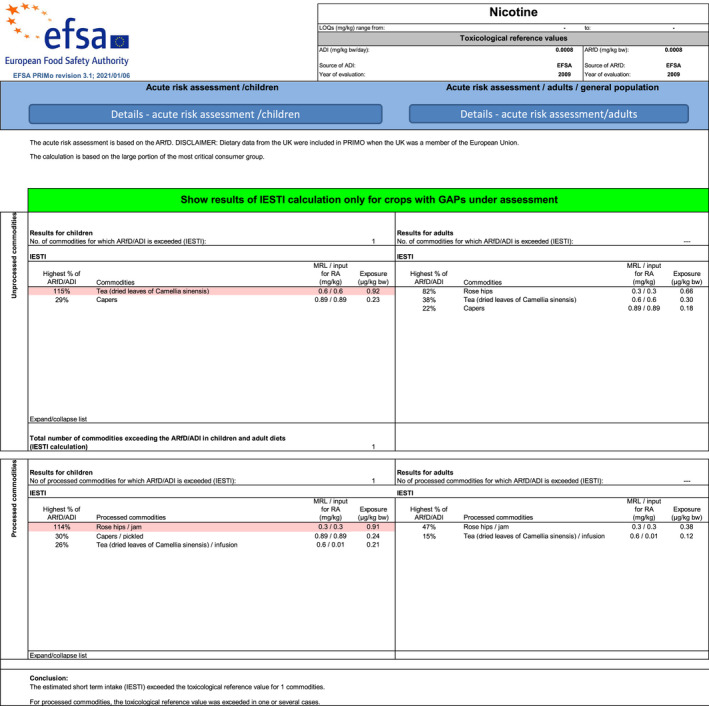
The consumer exposure assessment was performed using the revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo). This dietary exposure assessment model contains the European food consumption data for raw and of processed commodities (large portion 6 , 7 , LP) for different subgroups of the EU population (EFSA, 2018, 2019b). The acute exposure calculations in EFSA PRIMo rev. 3.1 are performed in accordance with the internationally estimated short‐term intake (IESTI) methodology. A screenshot of the report sheet of the PRIMo is presented in Appendix A.
It should be highlighted that the IESTI methodology was developed to assess the short‐term exposure to pesticide residues resulting from the intake of commodities that contain residues from the use of pesticides according to the intended/existing authorised use of pesticides according to Good Agricultural Practices. Although the risk management question to be addressed in the current assessment is slightly different from the question for which the IESTI methodology and PRIMo were designed, the methodology can provide indications whether the MRLs are sufficiently protective for consumers.
3.1. Rose hips
3.1.1. Exposure/risk assessment for unprocessed rose hips
In PRIMo rev. 3.1, the acute exposure calculations for unprocessed rose hips are performed for adults using the highest large portion food consumption data (expressed in terms of unprocessed rose hips) as reported for Finish women:
-
•
LP for Finish women (P97.5): 156.6 g/person, equivalent to 2.2 g/kg body weight.
Assuming consumption of a large portion of rose hips containing residues at the legal limit of 0.3 mg/kg, the exposure is calculated to be 0.66 μg/kg body weight, corresponding to 82% of the ARfD.
For children, no consumption data are available for unprocessed rose hips.
3.1.2. Exposure/risk assessment for processed rose hips
Information on the consumption of processed rose hips is available in EFSA PRIMo (large portion (P97.5)) for rose hip jam, with the highest intake reported for the following Dutch diets:
-
•
LP for Dutch children (P97.5): 55.7 g/person (equivalent to 3.03 g/kg body weight).
-
•
LP for Dutch adults (general population) (P97.5): 82.3 g/person (equivalent to 1.25 g/kg body weight).
Assuming consumption of a large portion of jam containing residues equal to the legal limit for unprocessed rose hips, the estimated exposure amounts for 0.91 μg/kg body weight (114% of the ARfD) for Dutch children and 0.38 μg/kg body weight (47% of ARfD) for Dutch adults.
As no processing studies are available that could give an indication of the effect of processing on the magnitude of residues in processed products (e.g. production of jam), the default conservative assumption used in the calculation is that the residue levels in jam are equal to the residue level in unprocessed products. Further refinements would be possible if appropriate processing studies for rose hip jam or comparable processed products are provided.
Assuming that the MRL for unprocessed rose hips is equally applicable to rose hips jam, the calculations give an indication that the existing temporary MRL of 0.3 mg/kg for rose hips is not sufficiently protective for European consumers. The calculation is affected by additional non‐standard uncertainties, due to the lack of information on the impact of processing (production of jam) on the residue level, compared to residues in unprocessed rose hips.
The residue concentration for nicotine in rose hips jam that leads to an exposure not exceeding the ARfD (threshold residue concentration) was calculated to be 0.26 mg/kg.
3.2. Tea (Camellia sinensis)
In the framework of the data collection to develop EFSA PRIMo, some Member States reported consumption data expressed as equivalents of the unprocessed, dried tea leaves (e.g. Germany, Ireland) while other Member States reported the large portion of (diluted) tea infusions. The exposure calculations in PRIMo rev. 3.1 are performed for both types of consumption data (see Sections 3.2.1 and 3.2.2), although it is acknowledged that even if consumption data are reported for dried tea leaves, tea is consumed in a form of infusions.
3.2.1. Exposure/risk assessment calculated on the basis of unprocessed tea
In PRIMo rev. 3.1, the acute exposure calculations for unprocessed tea leaves are performed using the large portion food consumption data for tea (Camellia sinensis) available for adults and children.
The highest consumption data expressed as dried tea leaves have been reported for the following diets:
-
•
Adults: Large portion for German women (14–50 years) (P97.5): 33.74 g/person, equivalent to 0.5 g/kg body weight;
-
•
Children: Large portion for Irish children (P97.5): 30.6 g/person, equivalent to 1.53 g/kg body weight.
Assuming consumption of a large portion of tea leaves containing residues equal to the legal limit (0.6 mg/kg), the estimated exposure amounts for 0.3 μg/kg body weight (38% of the ARfD) for adults and 0.92 μg/kg body weight (115% of the ARfD) for children.
The exposure calculations give an indication that the existing temporary MRL for tea is not sufficiently protective for children.
The threshold residue concentration for nicotine in teas (dry leaves) that leads to an exposure not exceeding the ARfD was calculated to be 0.52 mg/kg.
3.2.2. Exposure/risk assessment calculated on the basis of processed tea (consumption of tea infusion)
The highest consumption expressed on the basis of tea infusion (processed tea leaves) have been reported for the following diets:
-
•
Adults: Large portion for Dutch general population (P97.5): 1335.3 g/person, equivalent to 20.29 g/kg body weight;
-
•
Children: Large portion for Dutch children (P97.5): 645.7 g/person, equivalent to 35.09 g/kg body weight.
Using a default dilution factor of 0.01 for tea, 8 the residue concentration in tea infusion (corresponding to the existing EU MRL of 0.6 mg/kg) is estimated to amount for up to 0.006 mg/kg. For tea infusions the calculated exposure amounted for 0.12 μg/kg body weight for adults and 0.21 μg/kg body weight for children. This exposure corresponds to 15% and 26% of the ARfD for adults and children, respectively.
In 2011, when EFSA derived MRL proposals for nicotine in tea, herbal infusions, spices, rose hips and fresh herbs (EFSA, 2011), processing studies were assessed in view of deriving processing factors (transfer rates from tea and herbal infusions to the aqueous extracts) that would allow to replace the default dilution factors. However, as the worst‐case transfer rate was close to 100%, EFSA decided not to include the empirical processing factors in the short‐term exposure assessment. As no additional information is available to EFSA as regards the processing factor/dilution rate/transfer rate from tea leaves to tea infusion, no further refinements of the calculations are performed.
3.3. Capers
During the development of EFSA PRIMo, consumption data on capers submitted for the unprocessed product and for the processed capers. In Sections 3.3.1 and 3.3.2, the exposure/risk assessment is presented for the two forms of caper consumptions data.
3.3.1. Exposure/risk assessment calculated for unprocessed capers
In PRIMo rev. 3.1, the highest large portion food consumption data for unprocessed capers are identified for German adults and children.
-
•
Adults: Large portion for German women (P97.5): 13.49 g/person, equivalent to 0.2 g/kg body weight;
-
•
Children: Large portion for German children (P97.5): 4.2 g/person (equivalent to 0.26 g/kg body weight).
As it was unclear what was the basis of the reported large portions for German diets, EFSA contacted the German competent authorities to verify whether the consumption data refer to
-
•
dried capers (in compliance with the commodity description in Annex I of Regulation No 396/2005, see Section 2, Table 1),
-
•
fresh capers,
-
•
pickled capers or
-
•
any other form of processed capers.
According to information provided by the German competent authorities to EFSA, nearly all capers are consumed as pickled capers. The large portions for pickled capers have therefore been recalculated by German authorities to fresh capers (raw agricultural commodities, RACs) using a factor of 1.037 (pickled capers × 1.037 = capers RAC) 9 before submitting the consumption figures to EFSA.
Hence, the reported large portion for German adults and children used in EFSA PRIMo rev. 3.1 (see bullet points below) refer to fresh capers (RAC). Germany also noted that in a few months, more updated consumption information may become available.
For performing a short‐term dietary exposure assessment for German adults and children, it is necessary to re‐calculate the MRL (established at 4 mg/kg for dried capers) to the equivalent residue concentration in unprocessed capers (RAC) to match with the consumption data implemented in PRIMo rev. 3.1. Based on the assumption of the dry matter (DM) content of 90% for dried capers 10 and 20% for fresh capers (Giuferida et al., 2002, 11 ), the drying factor is calculated according to equation 1 (European Commission, 2022):
Equation 1:
This drying factor means that for producing 1 kg of dried capers, 4.5 kg of fresh capers are required. It also means that the residue concentration in the unprocessed RAC is significantly lower, considering the weight loss resulting from the drying process. The corresponding MRL for unprocessed capers is calculated according to equation 2.
Equation 2:
Hence, the MRL of 4 mg/kg for dried capers would correspond to an MRL of 0.89 mg/kg for fresh capers.
Assuming consumption of a large portion of fresh capers (equivalent to the P97.5 reported for German women) containing residues of 0.89 mg/kg (corresponding to the legal limit of 4 mg/kg for dried capers), the exposure is calculated to be 0.18 μg/kg body weight. This exposure corresponds to 22% of the ARfD. The estimated exposure for German children amounts for 0.23 μg/kg body weight (29% of the ARfD).
The calculations are affected by additional, non‐standard uncertainties, resulting from the lack of a clearly defined dry matter content for the product ‘dried capers’ included in Annex I of Regulation (EC) No 396/2005.
3.3.2. Exposure/risk assessment calculated for processed capers
In addition, PRIMo rev. 3.1 contains separate consumption data for processed commodities; Dutch children have been identified as the critical consumers of pickled capers.
-
•
Children: Large portion for Dutch children (percentile was not reported): 5 g/person (equivalent to 0.27 g/kg body weight).
For performing a short‐term dietary exposure assessment, the MRL (established for dried capers) was recalculated to the corresponding MRL for pickled capers. As empirical data on the residue levels in pickled capers (processing studies) are not available, EFSA used the default processing factor of 1 derived from the EFSA raw primary commodity (RPC) model (EFSA, 2019a). This default processing factor does not differ significantly from the processing factor that can be derived from the yield factor reported by Germany (see footnote 9), considering that the default processing factor is the inverse value of the yield factor.
The estimated exposure for Dutch children consuming a large portion of pickled capers containing 0.89 mg/kg of nicotine amounts for 0.24 μg/kg body weight, corresponding to 30% of the ARfD.
The exposure calculations are affected by additional, non‐standard uncertainties resulting from the lack of empirically derived processing factors and the uncertainties resulting from the recalculation of the MRL set for dry capers to the RAC (see Section 3.3.1).
The threshold residue concentration for nicotine in capers (dry product) that would lead to an exposure not exceeding the ARfD was calculated to be 12 mg/kg, which is higher than the existing MRL.
From the exposure calculations reported for capers (Sections 3.3.1 and 3.3.2), EFSA concludes that the existing MRL is sufficiently protective for European consumers.
4. Conclusions and recommendations
The exposure calculations performed by EFSA give an indication that the existing temporary MRLs for nicotine may not be sufficiently protective for rose hips and tea, assuming the consumption of a large portion of the products concerned which contain residue concentrations at the legal limit. The acute risk assessment, however, could be further refined if additional information on the residue levels in processed products was available. Lacking this information, it is recommended to lower of the existing temporary MRLs for these two commodities to levels that do not exceed the ARfD. EFSA did not identify a consumer health risk for capers. The results of the assessment and the recommendations are summarised below (Table 2).
Table 2.
Summary table
| Code (a) | Commodity | Existing tMRL (b) | Threshold residue concentration (c) | Conclusion/recommendation |
|---|---|---|---|---|
| Residue definition for enforcement: Nicotine | ||||
| 0154050 | Rose hips | 0.3 | 0.26 |
Consumption of a large portion of unprocessed rose hips containing residues at the level of 0.3 mg/kg leads to an exposure equivalent to 82% of the ARfD (adults). Consumption of a large portion of processed rose hips in a form of rose hips jam containing residues at the level of 0.3 mg/kg leads to an exposure equivalent to 114% of the ARfD (children) and 47% of the ARfD (adults). The calculation is affected by additional non‐standard uncertainties, due to the lack of information on the impact of processing (production of jam) on the residue level, compared to residues in unprocessed rose hips. The threshold residue concentration for rose hips jam is 0.26 mg/kg (residue concentration that leads to an exposure equal to the ARfD). EFSA recommends the lowering of the existing MRL to a level equal or lower than the threshold concentration of 0.26 mg/kg calculated for rose hips jam. |
| 0610000 | Teas | 0.6 | 0.52 |
Consumption of a large portion expressed on the basis of unprocessed dried tea leaves, which contain residues at the level of 0.6 mg/kg leads to an exposure equivalent to 38% of the ARfD (adults) and 115% of the ARfD (children). The threshold residue concentration for unprocessed dried tea lives is 0.52 mg/kg (residue concentration that leads to an exposure equal to the ARfD). Consumption of a large portion of tea infusion leads to an exposure equivalent to 15% and 26% of the ARfD for adults and children, respectively. The calculation is performed with a default, conservative dilution factor of 100, assuming a complete transfer of nicotine residues from dry leaves to the infusion. EFSA recommends the lowering of the existing MRL to a level equal or lower than the threshold concentration of 0.52 mg/kg. |
| 0850020 | Capers | 4 | > 4 |
The existing MRL set for dried capers was recalculated to the corresponding concentration in fresh capers (i.e. 0.89 mg/kg), taking into account a drying factor, in order to match the residue levels with the consumption data which are available for fresh and/or pickled capers in EFSA PRIMo. Consumption of a large portion of capers (raw commodity), containing residues at the level of 0.89 mg/kg leads to 22% of the ARfD (adults) and 29% of the ARfD (children). The calculation is affected by additional non‐standard uncertainties, related to the lack of a clearly defined dry matter content for the product defined in Annex I of Regulation (EC) No 396/2005 (i.e. dried product whole, crushed or ground), to which MRL applies. Consumption of a large portion of processed capers (pickled capers) containing residues at the level of 0.89 mg/kg leads to 30% of the ARfD (children). A default processing factor of 1 was applied for estimating residues in pickled capers (meaning that the level in pickled capers is equal to the residue level in fresh capers). Due to the lack of an empirical processing factor, the calculation is affected by additional non‐standard uncertainties. The threshold for dried capers which does not lead to an exceedance of the ARfD is 12 mg/kg. A modification of the legal limit is not required, as the existing MRL is unlikely to lead to an exposure exceeding the ARfD. |
MRL: maximum residue level; ARfD: acute reference dose.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Existing temporary MRL (in mg/kg).
Threshold residue concentration (in mg/kg) is the residue level that leads to a short‐term exposure equal to the ARfD.
Risk managers are invited to consider the following additional recommendations:
-
•
A formal adoption of the toxicological reference values for nicotine derived by EFSA in 2009 is recommended to increase the transparency of the risk assessment for nicotine.
-
•
Considering that capers are usually not consumed as dried product, risk managers may re‐consider the modification of the commodity description in Annex I of Regulation (EC) No 396/2005 and define that MRLs for capers refer to the fresh product. If such a modification is agreed, the existing MRLs for capers need to be revised (lowered) by a factor of 4.5 (drying factor, see Section 3.3.1).
Abbreviations
- ADI
acceptable daily intake
- ARfD
acute reference dose
- DM
dry matter
- IESTI
international estimated short‐term intake
- LOAEL
lowest observed adverse effect level
- LP
large portion
- MRL
maximum residue level
- NOAEL
no observed adverse effect level
- PAFF
Standing Committee on Plants, Animals, Food and Feed
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RA
risk assessment
- RAC
raw agricultural commodity
- SCoPAFF
Standing Committee on Plants, Animals, Food and Feed (formerly: Standing Committee on the Food Chain and Animal Health; SCFCAH)
Appendix A – Pesticide Residue Intake Model (PRIMo) – acute risk assessment only
Suggested citation: EFSA (European Food Safety Authority) , 2022. Statement on the short‐term (acute) dietary risk assessment for the temporary maximum residue levels for nicotine in rose hips, teas and capers. EFSA Journal 2022;20(9):7566, 13 pp. 10.2903/j.efsa.2022.7566
Requestor European Commission
Question number EFSA‐Q‐2022‐00499
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Approved: 5 September 2022
Footnotes
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, pp. 1–32.
Commission Decision 2009/9/EC of 8 December 2008 concerning the non‐inclusion of nicotine in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing that substance. OJ L 5, 9.1.2009, pp. 7–8.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, pp. 1–16.
Commission Regulation (EU) No 765/2010 of 25 August 2010 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for chlorothalonil clothianidin, difenoconazole, fenhexamid, flubendiamide, nicotine, spirotetramat, thiacloprid and thiamethoxam in or on certain products. OJ L 226, 28.8.2010, pp. 1–37.
Commission Regulation (EU) No 812/2011 of 10 August 2011 amending Annex III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for dimethomorph, fluopicolide, mandipropamid, metrafenone, nicotine and spirotetramat in or on certain products. OJ L 208, 13.8.2011, pp. 1–22.
The information on the large portions were provided by national competent authorities in the framework of the development of the EFSA risk assessment model EFSA PRIMo rev. 3 and 3.1 (EFSA, 2018, 2019b).
Usually, the exposure calculations are performed for the large portion derived from a food survey, considering the 97.5th percentile (P97.5) of those consumers who have reported the consumption of the respective food product (‘consumers‐only’). More details on the large portions used in EFSA PRIMo can be found in EFSA (2018, 2019b).
This default dilution factor is calculated by assuming the use of 2.5 g of tea leaves to produce 250 g of tea infusion.
This factor implies that for the production of 1 kg of pickled capers, 1.037 kg of unprocessed capers are required (corresponding a yield factor of 0.96).
Although the water content for these dried spices is not explicitly defined in Regulation (EC) No 396/2005, EFSA assumes a dry matter content of approximately 90%, in line with information for other dried spices for which the MRL is established.
Giuferida et al. (2002) reported a water content for fresh capers ranging from 80% to 84%. Hence, the DM content is approximately 20%.
References
- EFSA (European Food Safety Authority) , 2009. Statement on the potential risks for public health due to the presence of nicotine in wild mushrooms. EFSA Journal 2009, 7(5), 286r, 47 pp. 10.2903/j.efsa.2009.286r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Reasoned opinion on the setting of temporary MRLs for nicotine in tea, herbal infusions, spices, rose hips and fresh herbs. EFSA Journal 2011, 9(3), 2098, 50 pp. 10.2903/j.efsa.2011.2098 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018, 16(1), 5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Dujardin B and Kirwan L, 2019a. Technical report on the raw primary commodity (RPC) model: strengthening EFSA's capacity to assess dietary exposure at different levels of the food chain, from raw primary commodities to foods as consumed. EFSA supporting publication 2019:EN‐1532, 30 pp. 10.2903/sp.efsa.2019.EN-1532 [DOI]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A, Verani A, 2019b. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019:EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI]
- European Commission , 2022. Information note on Article 20 of Regulation (EC) No 396/2005 as regards processing factors, processed and composite food and feed. SANTE/10704/2021, 22 February 2022.
- Giuferida D, Salvo F, Ziino M, Toscano G and Dugo G, 2002. Initial investigations on some chemical constituents of capers (Capparis spinosa L.) from the Island of Salina. Italian Journal of Food Science, 14, 25–33. [Google Scholar]


