Abstract
Background:
Measures of handgrip strength may show promise for detecting cognitive erosion during aging.
Objective:
To determine the associations between lower handgrip strength and poorer cognitive functioning for aging Americans.
Methods:
There were 13,828 participants aged at least 50 years from the 2006 wave of the Health and Retirement Study included and followed biennially for 8 years. Handgrip strength was assessed with a hand-held dynamometer and cognitive functioning was assessed with a modified version of the Mini-Mental State Examination. Participants aged <65 years with scores 7–11 had a mild cognitive impairment, ≤6 had a severe cognitive impairment, and ≤11 had any cognitive impairment. Respondents aged ≥65 years with scores 8–10 had a mild cognitive impairment, ≤7 had a severe cognitive impairment, and ≤10 had any cognitive impairment. Separate covariate-adjusted multilevel logistic models examined the associations between lower handgrip strength and any or severe cognitive impairment. A multilevel ordered logit model analyzed the association between lower handgrip strength and poorer cognitive functioning.
Results:
Every 5-kg lower handgrip strength was associated with 1.10 (95% confidence interval (CI): 1.04, 1.15) and 1.18 (CI: 1.04, 1.32) greater odds for any and severe cognitive impairment, respectively. Similarly, every 5-kg lower handgrip strength was associated with 1.10 (CI: 1.05, 1.14) greater odds for poorer cognitive functioning.
Conclusions:
Measurement of handgrip strength is a simple, risk-stratifying method for helping healthcare providers determine poorer cognitive functioning. Interventions aiming to prevent or delay cognitive dysfunction should also implement measures of handgrip strength as an assessment tool for determining efficacy.
Keywords: Alzheimer’s disease, cognition, dementia, frailty, geriatrics, muscle strength, muscle weakness
INTRODUCTION
Millions of adults in the United States are living with a mild or severe cognitive impairment, which is a hallmark precursor for Alzheimer’s disease and other related dementias [1–3]. Those that have a cognitive impairment experience higher healthcare costs, poorer quality of life, and greater premature mortality risk [4–7]. Reduced neural and motor system functioning during aging is linked to the onset of cognitive deficits [8, 9]. Therefore, detecting neural and motor system dysfunction with biomarkers of aging that are simple to measure may be helpful for discovering early identification and progression of cognitive declines. Such biomarkers may help healthcare providers determine cognitive impairment risk in their patients, and enhance assessments for interventions aiming to delay or prevent cognitive morbidity [10].
Handgrip strength is an easy and inexpensive method for estimating overall muscle strength, and is a powerful biomarker of aging [11]. Age-related declines in handgrip strength are not only due to reductions in muscle mass and quality, but are also exquisitely sensitive to the integrity of the neural systems that support the control of coordinated movement, and handgrip strength has been suggested to serve as a discriminating measure of neurological function and brain health [12]. For example, due to reduced neural drive to the muscles, the muscle force generated during handgrip strength measurements in aging adults is approximately half of what would be expected if the skeletal musculature were fully activated by the nervous system [13–15]. Similarly, the cognitive demand for completing motor tasks increases during aging [9], and hand dexterity, which is partially mediated by nervous and motor system functioning, is a determinant for handgrip strength performance. Given that age-related reductions to the nervous and motor systems are mechanisms for cognitive declines and contribute to diminished handgrip strength, this may help to explain why decreased handgrip strength is associated with an increased risk for Alzheimer’s disease [16].
The population in the United States is projected to grow rapidly [17], and being that age is a primary risk factor for cognitive impairment [18], the prevalence of cognitive morbidity in the United States is also expected to drastically increase [19]. Therefore, research that improves assessments and identifies biomarkers with diagnostic tools that distinguish cognitive dysfunction from normal brain aging is a priority for helping to mitigate the prevalence and progression of cognitive impairment [10]. Although reduced neural and motor system functioning is linked to decreased handgrip during aging, it remains unclear how handgrip strength and cognitive health are connected [11]. Providing more clarity for the longitudinal associations between handgrip strength and cognitive functioning will help to advance how cognitive impairments are assessed. Accordingly, the purpose of this study was to determine the associations between lower handgrip strength and poorer cognitive functioning for aging Americans.
METHODS AND MATERIALS
Participants
Data from the Health and Retirement Study (HRS) were analyzed for this investigation. Publicly available HRS data files were joined with the cleaned and standardized RAND HRS dataset [20]. The HRS is designed to monitor the health and financial status of middle-aged and older Americans [21]. Since 1992, participants in the HRS have been re-interviewed biennially and followed longitudinally until death. New cohorts of participants are intermittently added to the original sample, thereby allowing the HRS to maintain a national representative sample of community-dwelling aging adults over time [21]. The HRS uses a multi-state probability design, including geographical stratification and oversampling of certain demographic groups. More information about the HRS is published elsewhere [22].
Those aged at least 50 years who participated in the 2006 wave of the HRS and could complete interviews without a proxy (n = 15,734) were followed for eight years (2008, 2010, 2012, and 2014 waves). Beginning in the 2006 wave, a mixed-mode design for follow-up was deployed, wherein half of the participants alternated completion of the core and detailed face-to-face interviews, which included physical and biological measures. The half-samples alternated the detailed interviews at each subsequent wave to reduce participant burden. Interview response rates for each wave of the HRS have been >80% [21]. Written informed consent was provided by each participant prior to entering the HRS and the University’s Behavioral Sciences Committee Institutional Review Board approved all protocols. Data used in this secondary analysis contained no direct identifiers, thereby ensuring participant anonymity.
Outcome variables
Cognitive functioning was assessed at each wave with a series of tests that were modified from the Telephone Interview of Cognitive Status, a validated screening tool from the Mini-Mental State Examination that was designed for population-based studies [23]. A 27-point composite scale was used for respondents aged under 65 years that included immediate and delayed word recall from a list of 10 words (0–20 points), serial sevens subtraction test starting with the number 100 (0–5 points), and counting backward at maximal speed for 10 consecutive numbers starting from 20 (0–2 points). Participants with scores ≤11 were considered as having any cognitive impairment, those with scores of 7–11 had a mild cognitive impairment, while respondents with scores ≤6 had a severe cognitive impairment [24].
Participants aged 65 years and older completed a 35-point composite scale. Additional assessments on the 35-point scale included object naming (0–2 points), date naming (0–4 points), and correctly identifying the current president and vice president of the United States (0–2 points). Those with scores ≤10 were considered as having any cognitive impairment, participants with scores of 8–10 had a mild cognitive impairment, and respondents with scores ≤7 had a severe cognitive impairment [25].
Exposure variable
Handgrip strength was measured with a Smedley spring-type hand-held dynamometer (Scandidact, Denmark). Before performing handgrip strength tests, interviewers explained the protocol and fit the dynamometer to the hand size of each person. Participants completed a practice trial with the arm at the side and elbow flexed at 90-degrees. Beginning with the non-dominant hand, participants completed two handgrip strength measures, alternating between hands. For each measure, participants squeezed the dynamometer with maximal effort, and then released the muscle contractions.
A 30-second break was allowed between assessments if only one hand could be used for testing. Participants unable to stand or position their arm while gripping the dynamometer could be seated and rest their upper arm on a supporting object. Those that had a surgical procedure, swelling, inflammation, severe pain, or an injury in both hands did not engage in handgrip strength testing. More details about handgrip strength testing are published elsewhere [26]. The maximal handgrip strength measurement from a single trial on either hand was included in the analyses.
Given that handgrip strength measures were conducted in the detailed face-to-face interviews, data for handgrip strength from either the 2006 and 2008 waves, or 2010 and 2012 waves were ad hoc imputed. For example, if a participant had their handgrip strength measured in the 2006 wave, the same handgrip strength value was used for the 2008 wave; whereas, if a participant had handgrip strength measured in the 2008 wave, the same handgrip strength value was used for the 2006 wave. Figure 1 shows a portrayal for how handgrip strength data from the 2006 and 2008 waves, or 2010 and 2012 waves were imputed.
Fig. 1.
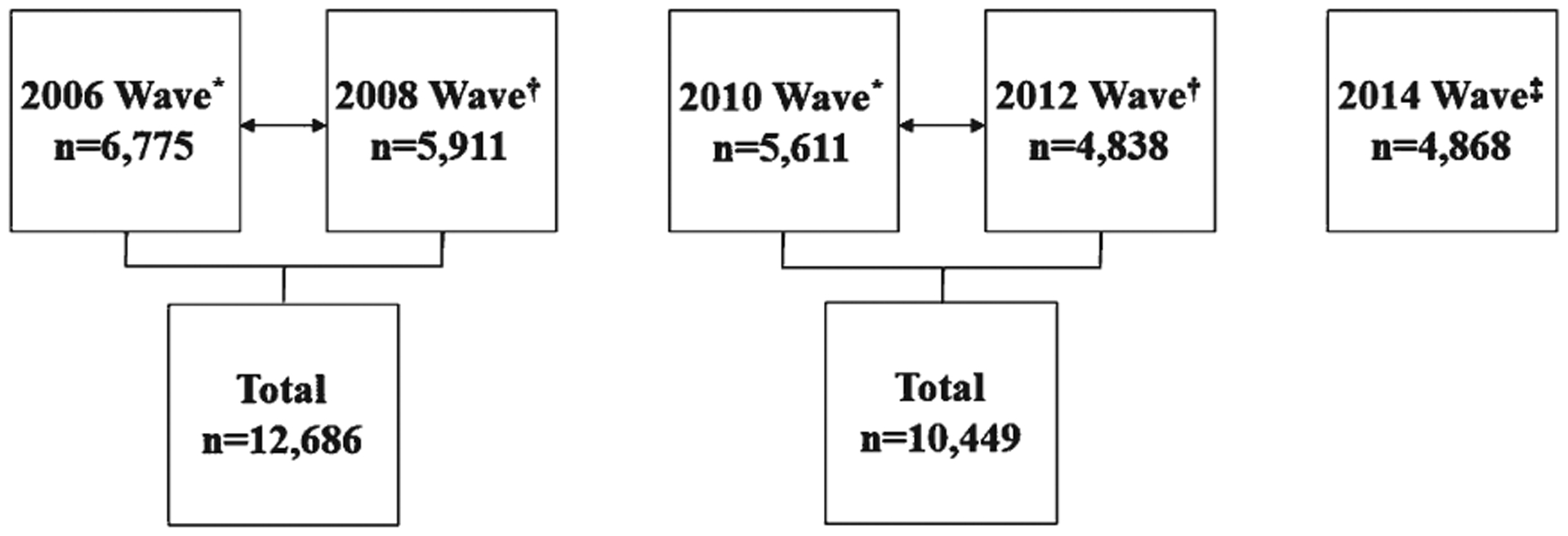
A Portrayal of the ad hoc Handgrip Strength Imputation. *Random half-sample “A”; †Random half-sample “B”; ‡handgrip strength data for the 2016 wave were not available at the time of analyses.
Covariates
Participants reported their age, sex, race, and ethnicity (Black, Hispanic, or White), height, and body mass at each wave. Time was measured as wave of participation in the HRS. Body mass index (BMI) was calculated as body mass in kilograms divided by height in meters-squared. Morbidity was collected by self-reported healthcare provider diagnosed hypertension, diabetes, cancer (excluding minor skin cancer), lung disease such as bronchitis or emphysema, heart condition, stroke, emotional or psychiatric problems, and arthritis or rheumatism. The number of affirmative morbid diagnoses were summed at each wave and included in the analyses.
The 8-item Center for the Epidemiologic Studies Depression (CES-D) scale was used for examining depressive symptoms [27]. Respondents indicated if they experienced any negative (felt depressed, everything was an effort, restless sleep, loneliness, sadness, and could not get going) or positive emotions (happiness, enjoyed life; reverse scored) during the week before the interview date. Scores ranged from 0–8, with higher values suggesting more depressive symptoms. Continuous scores were included in the analyses.
Participants indicated if they engaged in moderate or vigorous physical activity “every day”, “more than once a week”, “once a week”, “one to three times a month”, “never”. Those reporting any moderate or vigorous physical activity were considered as engaging in moderate-to-vigorous physical activity. Participants also reported their educational achievement and were categorized as either a college graduate or above, high school graduate or passing a high school equivalency test and completed some college, or did not graduate from high school.
Social engagement was examined by three variables: 1) volunteer activity at religious, educational, health-related, or other organization for at least one hour in the past year, 2) weekly or greater contact with parents or in-laws, and 3) current employment status. Scores ranged from 0–3 with higher scores suggesting more social engagement [28]. The continuous scores were included in the analyses.
Respondents told interviewers at each wave if they had ever smoked more than 100 cigarettes in their lifetime, and if they were currently smoking cigarettes. Likewise, a single-item measure of self-rated health was collected at each wave, wherein participants scored their health as either “excellent”, “very good”, “good”, “fair”, or “poor”.
Statistical analysis
All analyses were conducted with SAS 9.4 software (SAS Institute; Cary, NC). A multilevel logistic regression model examined the association between lower handgrip strength and any cognitive impairment using no cognitive impairment as the reference group. Another multilevel random effects logit model determined the association between lower handgrip strength and severe cognitive impairment using no cognitive impairment and mild cognitive impairment combined as the reference group. The outcomes from the cognitive functioning assessments (no cognitive impairment, mild cognitive impairment, severe cognitive impairment) were then categorized as ordinal data for evaluating worst cognitive functioning. A multilevel random effects ordinal logistic regression model analyzed the association between lower handgrip strength and poorer cognitive functioning. All models were adjusted for age, sex, race and ethnicity, BMI, morbidity, CES-D score, smoking history, current smoking status, moderate-to-vigorous physical activity, social engagement, time (wave), educational achievement, and self-rated health as fixed effects. Data were organized with each row representing a single wave for a participant. The models included a random intercept and random effect for time (slope), and unstructured covariance for each model. More details regarding multilevel models are published elsewhere [29, 30].
Sensitivity analyses were performed for the associations between lower handgrip strength and any cognitive impairment, severe cognitive impairment, and poorer cognitive functioning. Each regression model was stratified separately by sex and age (middle-aged adults: 50–64 years; older adults: ≥65 years). Moreover, proxy respondents who were originally removed from these data were included in each of our models after their cognitive functioning was scored [24], to determine how inclusion of proxies impacted the associations between lower handgrip strength and each of the outcomes. Multiple imputation was also performed as an additional sensitivity analysis to determine how missing covariates and sample attrition impacted our models. The imputation models were imputed five times using all the variables that were included in our models. The datasets were analyzed using the same multilevel logistic modeling methodologies. Rubin’s rules were used to combine the results [31]. Another sensitivity analysis examined how only analyzing handgrip strength measurements from the half-samples at each wave (i.e., no ad hoc imputation for the 2006 and 2008, and 2010 and 2012 waves) influenced the results. The results for sex and age stratification, inclusion of proxy respondents, multiple imputation, and individual wave handgrip strength measurements were reported as sensitivity analyses (Supplementary Tables) because we did not plan to perform these analyses a priori. An alpha level of 0.05 was used for all analyses.
RESULTS
After excluding 1,906 participants for missing covariates at all waves, 13,828 participants (87.9%) were included and a data flow diagram is depicted in Fig. 2. The descriptive characteristics of the participants are shown in Table 1. To make comparisons for the descriptive characteristics at each wave, the means and 95% confidence intervals (CI) are presented in Supplementary Table 1.
Fig. 2.
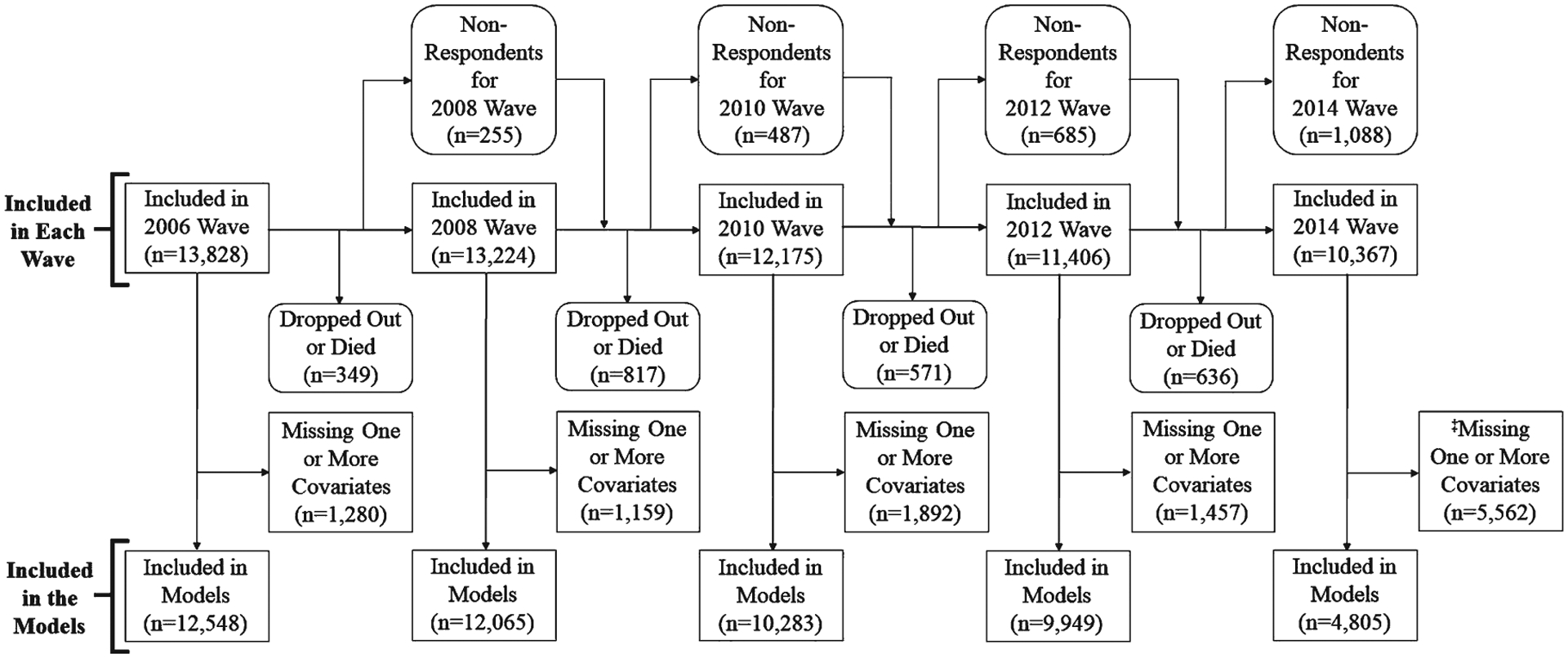
Data Flow Schematic for the Included Participants. ‡Handgrip strength data for the 2016 wave were not available at the time of analyses.
Table 1.
Baseline descriptive characteristics of the participants
| No Cognitive Impairment (n = 12,519) | Mild Cognitive Impairment (n = 1,045) | Severe Cognitive Impairment (n = 264) | Any Cognitive Impairment (n = 1,309) | |
|---|---|---|---|---|
| Handgrip Strength (kg) | 31.6 ± 11.0 | 32.0 ± 11.4 | 29.8 ± 11.0 | 31.6 ± 11.3 |
| Age (y) | 67.0 ± 10.1 | 59.1 ± 8.2 | 61.4 ± 10.1 | 59.6 ± 8.7 |
| Body Mass Index (kg/m2) | 28.1 ± 5.7 | 29.2 ± 6.6 | 28.1 ± 6.9 | 29.0 ± 6.6 |
| Morbidities | 1.8 ± 1.3 | 1.9 ± 1.5 | 2.2 ± 1.6 | 2.0 ± 1.5 |
| CES-D Score | 1.3 ± 1.8 | 2.3 ± 2.4 | 2.9 ± 2.3 | 2.4 ± 2.4 |
| Male (n (%)) | 5,069 (40.5%) | 376 (36.0%) | 98 (37.1%) | 474 (36.2%) |
| Black (n (%)) | 1,471 (11.8%) | 327 (31.3%) | 80 (30.3%) | 407 (31.1%) |
| White (n (%)) | 10,537 (84.2%) | 597 (57.1%) | 139 (52.6%) | 736 (56.2%) |
| Hispanic (n (%)) | 955 (7.6%) | 197 (18.9%) | 59 (22.3%) | 256 (19.6%) |
| Current Smoker (n (%)) | 1,601 (12.8%) | 282 (27.0%) | 76 (28.7%) | 358 (27.4%) |
| Previous Smoker (n (%)) | 7,093 (56.7%) | 615 (58.9%) | 166 (62.8%) | 781 (59.7%) |
| Any Moderate-to-Vigorous Physical Activity (n (%)) | 10,481 (83.8%) | 810 (77.5%) | 178 (67.4%) | 988 (75.5%) |
| Social Engagement | 1.4 ± 0.9 | 1.3 ± 0.8 | 0.9 ± 0.8 | 1.2 ± 0.8 |
| Educational Achievement (n (%)) | ||||
| Grad College and Above | 2,978 (23.8%) | 81 (7.8%) | 17 (6.4%) | 98 (7.5%) |
| Grad HS or Passed HS Equivalent | 7,476 (59.7%) | 580 (55.5%) | 101 (38.3%) | 681 (52.0%) |
| Test; Completed Some College | ||||
| Did Not Grad from HS | 2,064 (16.5%) | 383 (36.7%) | 146 (55.3%) | 529 (40.4%) |
| Self-Rated Health (n (%)) | ||||
| Excellent | 1,579 (12.6%) | 91 (8.7%) | 17 (6.4%) | 108 (8.4%) |
| Very Good | 4,082 (32.6%) | 209 (20.0%) | 33 (12.5%) | 242 (18.5%) |
| Good | 3,986 (31.8%) | 301 (28.8%) | 51 (19.3%) | 352 (26.9%) |
| Fair | 2,216 (17.7%) | 312 (29.9%) | 99 (37.5%) | 411 (31.4%) |
| Poor | 649 (5.2%) | 129 (12.3%) | 64 (24.3%) | 193 (14.7%) |
CES-D, Center for Epidemiologic Studies Depression; Grad, graduate; HS, high school; kg, kilogram; kg/m2, kilograms per meters-squared.
The results for the association between lower handgrip strength and any cognitive impairment are in Table 2. Every 5-kg lower handgrip strength was associated with a 1.10 (CI: 1.04, 1.15) greater odds for any cognitive impairment. Table 3 presents the results for the association between lower handgrip strength and severe cognitive impairment. Every 5-kg lower handgrip strength was associated with a 1.18 (CI: 1.04, 1.32) greater odds for severe cognitive impairment. The results for the association between lower handgrip strength and poorer cognitive functioning are shown in Table 4. Every 5-kg lower handgrip strength was associated with a 1.10 (CI: 1.05, 1.14) greater odds for poorer cognitive functioning.
Table 2.
Association between lower handgrip strength and any cognitive impairment
| Odds Ratio | 95% Confidence Interval | ||
|---|---|---|---|
| Handgrip Strength (5-kg Lower) | 1.10 | 1.04, | 1.15 |
| Body Mass Index | 0.98 | 0.96, | 0.99 |
| Depression Score | 1.13 | 1.09, | 1.17 |
| Male (Reference: Female) | 1.98 | 1.55, | 2.51 |
| Black (Reference: Not-Black) | 2.45 | 1.56, | 3.84 |
| White (Reference: Not-White) | 0.37 | 0.25, | 0.55 |
| Hispanic (Reference: Not-Hispanic) | 2.36 | 1.72, | 3.24 |
| Morbidity | 1.04 | 0.97, | 1.11 |
| Age | 0.90 | 0.89, | 0.91 |
| Current Smoker (Reference: Non-Smoker) | 1.67 | 1.31, | 2.12 |
| Previous Smoker (Reference: Non-Smoker) | 0.76 | 0.62, | 0.93 |
| Moderate-to-Vigorous Physical Activity | 0.82 | 0.69, | 0.97 |
| Social Engagement | 0.75 | 0.68, | 0.83 |
| Time | 0.98 | 0.96, | 0.99 |
| Educational Achievement (Reference: College Graduate and Above) | |||
| Graduated High School or Passed a High School Equivalency | 2.62 | 1.97, | 3.48 |
| Test; Completed Some College | |||
| Did Not Graduate from High School | 9.63 | 6.86, | 13.50 |
| Self-Rated Health (Reference: Excellent) | |||
| Very Good | 0.87 | 0.66, | 1.14 |
| Good | 1.06 | 0.80, | 1.41 |
| Fair | 1.32 | 0.97, | 1.78 |
| Poor | 2.36 | 1.64, | 3.38 |
Table 3.
Association between lower handgrip strength and severe cognitive impairment
| Odds Ratio | 95% Confidence Interval | ||
|---|---|---|---|
| Handgrip Strength (5-kg Lower) | 1.18 | 1.04, | 1.32 |
| Body Mass Index | 0.93 | 0.90, | 0.97 |
| Depression Score | 1.13 | 1.04, | 1.21 |
| Male (Reference: Female) | 2.40 | 1.40, | 4.11 |
| Black (Reference: Not-Black) | 1.41 | 0.60, | 3.33 |
| White (Reference: Not-White) | 0.51 | 0.24, | 1.08 |
| Hispanic (Reference: Not-Hispanic) | 1.20 | 0.62, | 2.30 |
| Morbidity | 1.03 | 0.89, | 1.19 |
| Age | 0.94 | 0.92, | 0.96 |
| Current Smoker (Reference: Non-Smoker) | 0.79 | 0.45, | 1.40 |
| Previous Smoker (Reference: Non-Smoker) | 0.72 | 0.45, | 1.15 |
| Moderate-to-Vigorous Physical Activity | 0.60 | 0.42, | 0.86 |
| Social Engagement | 0.65 | 0.51, | 0.84 |
| Time | 0.78 | 0.65, | 0.94 |
| Educational Achievement (Reference: College Graduate and Above) | |||
| Graduated High School or Passed a High School Equivalency | 1.52 | 0.70, | 3.28 |
| Test; Completed Some College | |||
| Did Not Graduate from High School | 4.13 | 1.83, | 9.35 |
| Self-Rated Health (Reference: Excellent) | |||
| Very Good | 0.38 | 0.18, | 0.78 |
| Good | 0.96 | 0.48, | 1.92 |
| Fair | 1.38 | 0.67, | 2.82 |
| Poor | 1.62 | 0.73, | 3.60 |
Table 4.
Association between lower handgrip strength and poorer cognitive functioning
| Odds Ratio | 95% Confidence Interval | ||
|---|---|---|---|
| Handgrip Strength (5-kg Lower) | 1.10 | 1.05, | 1.14 |
| Body Mass Index | 0.97 | 0.96, | 0.98 |
| Depression Score | 1.12 | 1.09, | 1.16 |
| Male (Reference: Female) | 1.94 | 1.54, | 2.45 |
| Black (Reference: Not-Black) | 2.33 | 1.51, | 3.59 |
| White (Reference: Not-White) | 0.36 | 0.25, | 0.54 |
| Hispanic (Reference: Not-Hispanic) | 2.09 | 1.54, | 2.84 |
| Morbidity | 1.05 | 0.98, | 1.12 |
| Age | 0.90 | 0.89, | 0.91 |
| Current Smoker (Reference: Non-Smoker) | 1.47 | 1.17, | 1.85 |
| Previous Smoker (Reference: Non-Smoker) | 0.78 | 0.64, | 0.95 |
| Any MVPA (Reference: No MVPA) | 0.80 | 0.68, | 0.93 |
| Social Engagement | 0.78 | 0.71, | 0.86 |
| Time | 0.80 | 0.73, | 0.86 |
| Educational Achievement (Reference: College Graduate and Above) | |||
| Graduated High School or Passed a High School Equivalency | 2.36 | 1.80, | 3.09 |
| Test; Completed Some College | |||
| Did Not Graduate from High School | 8.67 | 6.29, | 11.99 |
| Self-Rated Health (Reference: Excellent) | |||
| Very Good | 0.82 | 0.64, | 1.06 |
| Good | 1.05 | 0.81, | 1.37 |
| Fair | 1.34 | 1.01, | 1.77 |
| Poor | 2.20 | 1.57, | 3.07 |
MVPA, moderate-to-vigorous physical activity.
Supplementary Table 2 shows the sex and age stratified estimates for the associations between lower handgrip strength and any cognitive impairment, severe cognitive impairment, and poorer cognitive functioning. Results for the associations between lower handgrip strength and any cognitive impairment, severe cognitive impairment, and poorer cognitive functioning after adding 2,735 proxy respondents to the models are in Supplementary Table 3. Likewise, Supplementary Table 4 presents the estimates for the associations between lower handgrip strength and any cognitive impairment, severe cognitive impairment, and poorer cognitive functioning after multiple imputation. Supplementary Table 5 shows the estimates for the association between lower handgrip strength and any cognitive impairment, severe cognitive impairment, and poorer cognitive functioning when only examining handgrip strength measurements by the half-samples at each wave.
DISCUSSION
The principal results of this investigation revealed that lower handgrip strength was associated with cognitive dysfunction for aging Americans. Specifically, every 5-kg lower handgrip strength was associated with 10% increased odds for any cognitive impairment and 18% increased odds for severe cognitive impairment. Likewise, every 5-kg lower handgrip strength was associated with 10% increased odds for poorer cognitive functioning. Handgrip strength may serve as an important biomarker for detecting poorer cognitive functioning and the onset of cognitive morbidity. These findings suggest that it would be useful to include measures of handgrip strength in assessments of cognitive functioning for helping healthcare providers determine cognitive impairment risk and evaluations of interventions aiming to prevent or delay cognitive dysfunction. The findings also have implications for better understanding common neurodegenerative processes underlying reductions in both motor and cognitive function.
Although research examining the association between handgrip strength and cognitive functioning are mixed [32], previous cross-sectional and longitudinal investigations of have revealed that low handgrip strength was associated with mild cognitive impairment in older Koreans and Mexican Americans [33, 34]. These investigations have postulated that the association between low handgrip strength and mild cognitive impairment could be a product of pathogenic (e.g., high inflammatory markers, low sex steroid levels, high oxidative stress), skeletal muscle (e.g., sarcopenia and dynapenia), or neural factors. Our findings indicated that lower handgrip strength was associated with any cognitive impairment, but our interpretation of these results is that age-related neurodegeneration is critically involved in explaining this association. For example, the fine motor skills of the hands are often limited in those with a cognitive impairment, and the cortical and subcortical brain regions that control hand dexterity are also related to cognitive functions [35]. Therefore, the neural and motor intricacies for completing a grip force task may become compromised at the onset of cognitive impairment. Given that lower handgrip strength was associated with any cognitive impairment, likely as a result of age-related neural and motor system deficits, lower handgrip strength may factor into advanced cognitive impairments.
Severe cognitive impairment is indicative of dementia [25], and diminished neural and motor functioning are characteristics associated with dementia [8, 35]. The age-related neural and motor system deficits that contribute to decreased handgrip strength may help to explain our results that revealed lower handgrip strength was associated with severe cognitive impairment. Our results are compatible with those of another investigation that showed decreased handgrip strength was associated with an increase in Alzheimer’s disease risk [16]. During a handgrip strength assessment, each digit on the hand differentially contributes to the force produced on the dynamometer [36, 37], and when digits are not functioning uniformly in grasping tasks declines in handgrip strength occur, and reduced output in the sensorimotor integration process implies reduced neural and motor system function [12, 38]. These factors are diminished in those with a more advanced cognitive impairment, which may help to explain our findings.
Cognitive dysfunction, in itself, should be viewed as a temporal sequence because a distinct characteristic of Alzheimer’s disease is the progressive loss of memory and cognition [39]. The Reisberg scale provides healthcare providers with an overview for the stages of cognitive deterioration [40]. Such scales highlight that cognitive morbidity is progressive, and certain health factors may relate to cognitive erosion. Our finding that lower handgrip strength was associated with poorer cognitive functioning demonstrates that handgrip strength declines are linked to cognitive erosion. This finding is consistent with the “common cause hypothesis”, which states that common factors are responsible for age-related deterioration in cognitive and non-cognitive processes [41, 42]. Progressive declines in handgrip strength may lead to advancements in severity for cognitive impairment stages. Healthcare providers with aging adult patients should include measures of handgrip strength for detecting poorer cognitive functioning and the onset of cognitive impairment. Likewise, testing handgrip strength in assessments of interventions targeting the prevention and treatment of cognitive dysfunction may help to determine an intervention’s efficacy. Beginning such assessments earlier may also help to improve intervention integration and participant adherence.
It should be noted that our sensitivity analyses revealed females and older adults had slightly elevated odds ratios for the association of handgrip strength and any cognitive impairment, and poorer cognitive functioning relative to the odds ratios for males and middle-aged adults, respectively. Many have suggested that cognitive impairment is more prevalent and impactful in females due to health factors such as hormones and genetics [43]. The apolipoprotein E4 allele may also be influential for cognitive functioning in females and its role in the association between weakness and cognitive impairment should continue to be investigated [43]. Reduced muscle strength is also higher in females that are 80 years of age and over [44], which may help to explain our findings in older adults. Healthcare providers and targeted interventions should consider the role of sex and age for the association between muscle strength and cognitive function.
Although measures of handgrip strength are often used for identifying age-related changes to the musculoskeletal system such as sarcopenia and dynapenia [11], the role of neural and motor system deficits during aging should also be acknowledged as factors that may contribute to lower handgrip strength and frailty. For example, diminished nervous system functioning during aging is a limiting aspect for the generation of neural signals that are responsible for muscle contractions, which in turn, affect motor performance, muscle mass, and strength [45]. This may help to explain why mechanisms of frailty and composite measures of muscle strength are associated with reduced cognitive functioning [39, 46]. Future research should continue evaluating methodologies and measures of muscle strength, including handgrip strength, and developing diagnostic tools that may better identify the onset and progression of cognitive dysfunction. Further, interventions seeking to delay or prevent cognitive deterioration should focus on preserving the integrity of the neural and motor systems. For example, diet and physical activity participation have shown promise for preserving cognitive functioning during aging [47, 48]. Also, examining the bidirectional associations between handgrip strength and cognitive impairments may help to parse out how handgrip strength and cognition influence one another.
Some limitations should be mentioned. Our sample included those aged at least 50 years, therefore, dynamic measures of neural and motor system functioning such as gait speed could not be included in our models because those HRS data were only available for participants aged at least 65 years. Further, the smaller number of cases for severe cognitive impairment likely explained why the odds ratios for each of our multilevel logistic regression models were similar. Data for handgrip strength measures were ad hoc imputed because such assessments were a part of the detailed face-to-face interviews that occurred in the half-samples at alternating waves. Handgrip strength data for the 2016 wave of the HRS were not yet publicly available at the time of analyses and thereby could not be merged with data from the 2014 wave. Multilevel models have the ability to overcome intermittent missing data between waves and participant drop-out was sparse between waves [49]. The number of missing observations at each wave for the covariates are in Supplementary Table 6. Despite these limitations, the sensitivity analyses helped to strengthen our results and provided insights into how certain demographic (i.e., sex and age) and methodological factors may have influenced the findings.
Conclusions
Lower handgrip strength was associated with poorer cognitive functioning for aging Americans. Our findings suggest that declines in handgrip strength during aging are attributed, in part, to reduced neural and motor system functioning, which are mechanisms for reduced cognition. Handgrip strength is an inexpensive and simple to measure biomarker of aging that may help to identify the onset and deterioration of cognitive functioning in clinical and epidemiological settings. Healthcare providers with aging adult patients should incorporate measures of handgrip strength in their geriatric assessments for helping to determine cognitive impairment risk. Similarly, handgrip strength measurements may help to enhance evaluations of cognitive functioning for interventions aiming to prevent or delay cognitive morbidity. Incorporating measures of handgrip strength in such assessments may help to mitigate the projected prevalence of cognitive dysfunction in the United States.
Supplementary Material
ACKNOWLEDGMENTS
RM was supported in part by funding from the College of Human Development and Education at North Dakota State University. BCC was supported in part by a grant from the National Institutes of Health and National Institute on Aging (R01AG044424).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0042r3).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-190042.
REFERENCES
- [1].Centers for Disease Control and Prevention. Cognitive impairment: A call for action, now. https://www.cdc.gov/aging/pdf/cognitive_impairment/cogimp_poilicy_final.pdf, Posted February 2011, Accessed 14 January 2019.
- [2].Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, McGuire LC (2019) Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement 15, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Campbell NL, Unverzagt F, LaMantia MA, Khan BA, Boustani MA (2013) Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med 29, 873–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhu CW, Sano M, Ferris SH, Whitehouse PJ, Patterson MB, Aisen PS (2013) Health-related resource use and costs in elderly adults with and without mild cognitive impairment. J Am Geriatr Soc 61, 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hill NL, McDermott C, Mogle J, Munoz E, DePasquale N, Wion R, Whitaker EJ (2017) Subjective cognitive impairment and quality of life: A systematic review. Int Psychogeriatr 29, 1965–1977. [DOI] [PubMed] [Google Scholar]
- [6].Perna L, Wahl H-W, Mons U, Saum K-U, Holleczek B, Brenner HJ (2014) Cognitive impairment, all-cause and cause-specific mortality among non-demented older adults. Age Ageing 44, 445–451. [DOI] [PubMed] [Google Scholar]
- [7].Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM (2013) Monetary costs of dementia in the United States. N Engl J Med 368, 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010) Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci Biobwehav Rev 34, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].National Institute on Aging. Aging Well in the 21st Century: Strategic Directions for Research on Aging. https://www.nia.nih.gov/sites/default/files/2017-07/nia-strategic-directions-2016.pdf. Posted 2016. Accessed 14 January 2019.
- [11].McGrath RP, Kraemer WJ, Al Snih S, Peterson MD (2018) Handgrip strength and health in aging adults. Sports Med 48, 1993–2000. [DOI] [PubMed] [Google Scholar]
- [12].Carson RG (2018) Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol Aging 71, 189–222. [DOI] [PubMed] [Google Scholar]
- [13].Clark BC (2019) Neuromuscular changes with aging and sarcopenia. J Frailty Aging 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shinohara M, Latash ML, Zatsiorsky VM (2003) Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. J Appl Physiol 95, 1361–1369. [DOI] [PubMed] [Google Scholar]
- [15].Ohtsuki T (1981) Inhibition of individual fingers during grip strength exertion. Ergonomics 24, 21–36. [DOI] [PubMed] [Google Scholar]
- [16].Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA (2007) Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology 29, 66–73. [DOI] [PubMed] [Google Scholar]
- [17].Colby SL, Ortman JM (2015) Projections of the Size and Composition of the US Population: 2014 to 2060. Current Population Reports, https://www.census.gov//content/dam/Census/library/publications/2015/demo/p25-1143.pdf 25–1143, Posted March 2015, Accessed 14 January 2019. [Google Scholar]
- [18].Harada CN, Love MCN, Triebel KL (2013) Normal cognitive aging. Clin Geriatr Med 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Health and Retirement Study. HRS Data Products. https://hrs.isr.umich.edu/data-products. Posted 2018. Accessed 14 January 2019. [Google Scholar]
- [21].Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR (2014) Cohort profile: The health and retirement study (HRS). Int J Epidemiol 43, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Health and Retirement Study. HRS Data Book. https://hrs.isr.umich.edu/about/data-book. Posted January 2017. Accessed 14 January 2019. [Google Scholar]
- [23].Plassman BL, Newman TT, Welsh KA, Helms M, Breitner JC (1994) Application in epidemiological and longitudinal studies. Cogn Behav Neurol 7, 235–241. [Google Scholar]
- [24].Crimmins EM, Kim JK, Langa KM, Weir DR (2011) Assessment of cognition using surveys and neuropsycho-logical assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 66, i162–i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, Rosen AB (2008) Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement 4, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Crimmins E, Guyer H, Langa K, Ofstedal M, Wallace R, Weir D (2008) Documentation of physical measures, anthropometrics and blood pressure in the Health and Retirement Study. HRS Documentation Report DR-011 14, 47–59. [Google Scholar]
- [27].Turvey CL, Wallace RB, Herzog R (1999) A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr 11, 139–148. [DOI] [PubMed] [Google Scholar]
- [28].Social Engagement and Cognitive Function of Older Adults in Mexico and the United States: How Universal is the Health Concordance in Couples? http://paa2019.populationassociation.org/uploads/191664. Accessed 16 April 2019. [DOI] [PMC free article] [PubMed]
- [29].Woltman H, Feldstain A, MacKay JC, Rocchi M (2012) An introduction to hierarchical linear modeling. Tutor Quant Methods Psychol 8, 52–69. [Google Scholar]
- [30].Silvia PJ (2007) An introduction to multilevel modeling for research on the psychology of art and creativity. Empir Stud Arts 25, 1–20. [Google Scholar]
- [31].Rubin DB (1976) Inference and missing data. Biometrika 63, 581–592. [Google Scholar]
- [32].Fritz NE, McCarthy CJ, Adamo DE (2017) Handgrip strength as a means of monitoring progression of cognitive decline–a scoping review. Ageing Res Rev 35, 112–123. [DOI] [PubMed] [Google Scholar]
- [33].Alfaro-Acha A, Al Snih S, Raji MA, Kuo Y-F, Markides KS, Ottenbacher KJ (2006) Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci 61, 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jang JY, Kim J (2015) Association between handgrip strength and cognitive impairment in elderly Koreans: A population-based cross-sectional study. J Phys Ther Sci 27, 3911–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Buchman AS, Bennett DA (2011) Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother 11, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Methot J, Chinchalkar S, Richards RJ (2010) Contribution of the ulnar digits to grip strength. Can J Plast Surg 18, 10–14. [PMC free article] [PubMed] [Google Scholar]
- [37].MacDermid JC, Lee A, Richards RS, Roth JH (2004) Individual finger strength: Are the ulnar digits “powerful”? J Hand Ther 17, 364–367. [DOI] [PubMed] [Google Scholar]
- [38].Carteron A, McPartlan K, Gioeli C, Reid E, Turturro M, Hahn B, Benson C, Zhang WJ (2016) Temporary nerve block at selected digits revealed hand motor deficits in grasping tasks. Front Hum Neurosci 10, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ní Mhaoláin AM, Gallagher D, Crosby L, Ryan D, Lacey L, Coen R, Bruce I, Walsh JB, Cunningham C, Lawlor BA (2011) Correlates of frailty in Alzheimer’s disease and mild cognitive impairment. Age Ageing 40, 630–633. [DOI] [PubMed] [Google Scholar]
- [40].The Global Deterioration for Assessment of Primary Degenerative Dementia. https://www.fhca.org/members/qi/clinadmin/global.pdf. Accessed 14 January 2019. [Google Scholar]
- [41].Christensen H, Mackinnon AJ, Korten A, Jorm AF (2001) The” common cause hypothesis” of cognitive aging: Evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging 16, 588–599. [DOI] [PubMed] [Google Scholar]
- [42].Salthouse TA, Hambrick DZ, McGuthry KE (1998) Shared age-related influences on cognitive and noncognitive variables. Psychol Aging 13, 486–500. [DOI] [PubMed] [Google Scholar]
- [43].Laws KR, Irvine K, Gale TM (2016) Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry 6, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Looker AC, Wang C-Y (2015) Prevalence of reduced muscle strength in older US adults: United States, 2011–2012. https://www.medpagetoday.com/upload/2015/1/28/db179.pdf. Accessed 3 June 2019. [PubMed]
- [45].Kwon YN, Yoon SS (2017) Sarcopenia: Neurological point of view. J Bone Metab 24, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA (2009) Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol 66, 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wanigatunga AA, Manini TM, Cook DR, Katula JA, Fielding RA, Kramer AF, Verghese J, Rapp SR, Sink KM, King AC (2018) Community-based activity and sedentary patterns are associated with cognitive performance in mobility-limited older adults. Front Aging Neurosci 10, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Smith P, Blumenthal JJ (2016) Dietary factors and cognitive decline. J Prev Alzheimers Dis 3, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Teerenstra S, Moerbeek M (2016) Power analysis of trials with multilevel data, 1st Ed. CRC Press, Boca Raton, FL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


