Abstract
The activity of ςB in Listeria monocytogenes is stimulated by high osmolarity and is necessary for efficient uptake of osmoprotectants. Here we demonstrate that, during cold shock, ςB contributes to adaptation in a growth phase-dependent manner and is necessary for efficient accumulation of betaine and carnitine as cryoprotectants.
Listeria monocytogenes is a ubiquitous, psychrotrophic food-borne pathogen capable of causing highly invasive infections in humans resulting in septicemia, meningitis, and spontaneous abortions (29). This organism is problematic for the food production industry due to its ubiquitous distribution in nature and its ability to grow at temperatures ranging from 4 to 45°C (14, 32, 40) and in salt concentrations ranging from 10 to 20% (12, 14, 24).
Two different types of adaptive responses to low temperature have been described thus far for L. monocytogenes. One of the responses is accomplished by adjusting membrane fluidity through alteration of the membrane fatty acid composition (3). A second response is manifest by the accumulation of compatible solutes such as glycine betaine, carnitine, and proline by uptake from the environment (7, 21). L. monocytogenes accumulates betaine and carnitine via biochemically distinct transporters (7, 17, 21, 26, 34, 35, 36). Genes encoding two different betaine transporters, GbuA (22) and BetL (33), have recently been cloned and demonstrated to encode homologues of ATP-dependent and ion-driven transporters, respectively. The structure of the carnitine transporter(s) is unknown, but biochemical studies demonstrate the existence of an ATP-dependent system (36).
Accumulation of compatible solutes is a common response to osmotic upshift in many species (7, 15, 18, 21, 23, 25), and these solutes presumably alleviate the consequences of osmotic stress by maintaining favorable osmotic pressure without altering the structure of intracellular proteins and other cellular machinery (4, 42). It is unclear if the solutes play the same roles in adaptation to low temperature or if the same machinery governs their accumulation under the physiologically distinct osmotic and temperature stress conditions.
One regulatory factor that could coordinate both osmotic and low-temperature stress responses in L. monocytogenes is the general stress sigma factor ςB, whose activity is stimulated in response to osmotic upshift and temperature downshift (5). In the related species Bacillus subtilis, ςB is known to control transcription of a large general stress regulon, whose products include different classes of proteins that alleviate the physiological consequences of environmental and nutritional stress conditions (19). The activity of ςB is stimulated by a wide variety of physical signals and is coupled to these signals by a complex cascade of eight different Rsb proteins that modulate the binding of ςB to its primary regulator through a series of protein-protein interactions and phosphate transfers (1, 2, 6, 8, 9, 13, 20, 37, 38, 41, 43).
The homology that is observed in the amino acid sequences of the ςB and Rsb protein homologues in L. monocytogenes and B. subtilis suggests that ςB is regulated similarly in each organism. However, activation of ςB in L. monocytogenes is acutely responsive to temperature and osmotic stress, whereas its activity in B. subtilis is only modestly induced in the case of osmolarity and not detectable in the case of rapid cold shock (5, 9). This indicates that the activity and function of the ςB regulon may be fine-tuned to the physiology of the host organism. In the present study, we sought to characterize the unique role that ςB plays in adaptation of L. monocytogenes to low temperatures.
Low-temperature activation of ςB in L. monocytogenes.
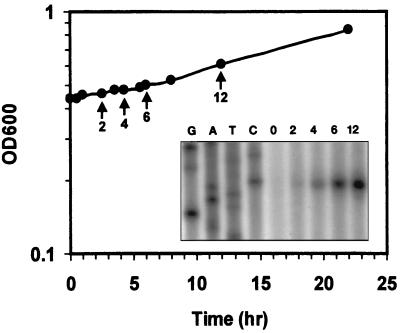
Since the growth pattern of L. monocytogenes after a temperature downshift is complex, we first compared growth and the appearance of ςB activity after the cells were subjected to a temperature downshift from 37 to 8°C (Fig. 1). When logarithmically growing cells were temperature downshifted, the cells rapidly ceased growing and did not assume a new growth rate until about 6 h after the shift. Primer extension analysis of transcripts originating from the ςB-dependent rsbV promoter (5) showed that the appearance of detectable transcript corresponded with the new growth rate (Fig. 1). RNA samples extracted from cells prior to the temperature downshift were devoid of detectable transcript. The transcript was also absent from samples after 15, 30, and 60 min of incubation (data not shown) but began to accumulate after 2 h with a substantial increase at the 6-h time point, the time at which the cells assumed a new growth rate.
FIG. 1.
Primer extension analysis of the pattern of ςB induction in L. monocytogenes after temperature downshift. Strain 10403S was grown in brain heart infusion to mid-logarithmic phase at 37°C followed by temperature downshift to 8°C. Growth was determined by measuring OD600, and values obtained after cold shock are shown on the graph. Primer extension analysis was used to measure the activity of ςB at the rsbV promoter. At times indicated by arrows on the graph, RNA was extracted from an aliquot of cells and 50 μg was used as a template for extension of the labeled VPROM2 primer (5). The numbers over the lanes in the autoradiograph correspond to the times indicated in the graph. A sequencing ladder generated with the same primer is shown to the left of the extension products.
Because ςB activity usually appears within 20 min of a stress signal in either L. monocytogenes or B. subtilis (5, 9), the delay in activation after cold shock was unanticipated. The cold-induced lag could be caused by the absence of ςB activity; however, data presented below are not consistent with this explanation. Alternatively, the function of one or more proteins necessary to elicit ςB activity may be impaired by cold shock. In support of the latter hypothesis, ribosome integrity or function is required for the relay of environmental stress to ςB in B. subtilis (30, 31). If ςB activity is linked to ribosome function in L. monocytogenes, then the delay in activity could be a consequence of the time necessary to reassemble, repair, and/or resynthesize cold-damaged ribosomes. Preliminary pulse-labeling experiments with L. monocytogenes are consistent with this explanation since the rate of translation falls 10-fold during the lag period following a cold shock and does not approach preshock levels until the cells assume a new growth rate, much like the appearance of ςB activity (L. A. Becker and A. K. Benson, unpublished data).
Role of ςB in low-temperature adaptation.
To determine whether ςB activity is necessary for growth at low temperatures, growth rates of log-phase cultures of the wild-type strain 10403S and the isogenic sigB::Km null mutant LMA2B (5) were measured in a defined medium (DM) (5, 7, 28) before and after a temperature downshift. Both strains displayed comparable lag periods after the downshift, and as shown in Table 1, their growth rates were nearly identical after growth was reinitiated at the lower temperature. Since the lag periods were nearly identical in both strains, it is unlikely that delayed activation of ςB in the wild-type strain (Fig. 1) is the cause of the lag. Thus, even though the appearance of ςB activity coincides with the pattern of growth after temperature downshift, its function is not essential for adaptation of log-phase cells.
TABLE 1.
Effect of betaine and carnitine on growth rate of cold-shocked L. monocytogenes 10403S and LMA2B
| Strain | Temp (°C) | Cryoprotectant (1 mM) | Growth rate (h−1 ± SD) |
|---|---|---|---|
| 10403Sa | 32 | None | 0.150 ± 0.010 |
| 8 | None | 0.009 ± 0.001 | |
| 8 | Betaine | 0.018 ± 0.004 | |
| 8 | Carnitine | 0.017 ± 0.001 | |
| LMA2Bb | 32 | None | 0.120 ± 0.001 |
| 8 | None | 0.008 ± 0.002 | |
| 8 | Betaine | 0.011 ± 0.003 | |
| 8 | Carnitine | 0.010 ± 0.001 |
Wild type.
sigB::Km.
Since we had previously shown that ςB function was required for osmotically induced accumulation of betaine, we next tested whether ςB activity contributes to uptake and utilization of betaine and carnitine as cryoprotectants at low temperatures. When 1 mM betaine or carnitine was added to the cultures of 10403S at the time of temperature downshift, the growth rate increased from 0.09 to 0.18 and 0.17 h−1, respectively (Table 1). Addition of betaine or carnitine to the sigB::Km strain, LMA2B (Table 1), did not have a statistically significant effect on growth, indicating that ςB activity contributes to utilization of betaine and carnitine as cryoprotectants.
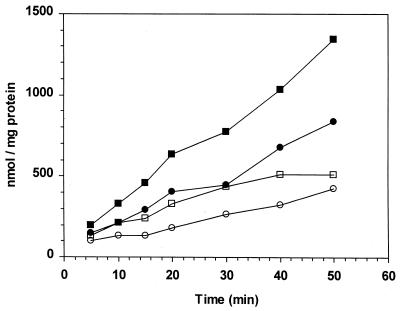
To test whether betaine and carnitine uptake is defective in the sigB mutant, accumulation of radiolabeled betaine and carnitine was measured before and after a temperature downshift. Cultures were grown at 37°C in DM to mid-logarithmic phase (optical density at 600 nm [OD600], ∼0.4), and then aliquots were removed and immediately shifted to 25 and 8°C for 5 min followed by addition of 1 mM [14C]betaine (65 μCi/mmol) or 1 mM [3H]carnitine (118 μCi/mmol). As shown in Fig. 2, betaine accumulated in the wild-type cells to comparable levels at both temperatures whereas carnitine accumulation was higher at 25°C. In contrast, accumulation of both betaine and carnitine was impaired in LMA2B at both temperatures. We conclude that, in log-phase cells, the principal contribution of ςB to low-temperature growth is to modulate the accumulation of compatible solutes.
FIG. 2.
Cryoprotectant accumulation in 10403S and LMA2B at room temperature and immediately following temperature downshift. Cells were grown in DM at 37°C to mid-logarithmic phase (OD600, ∼0.4), followed by immediate temperature downshift to 8°C. Prior to temperature shift and after acclimating for 5 min at 8°C, cell suspensions were collected and adjusted to approximately 0.12 mg of protein/ml. Uptake assays were performed at either 25°C (A) or 8°C (B) and started by addition of [14C]betaine or [3H]carnitine to each sample. Aliquots were removed over the time of the assay, harvested by centrifugation through oil, and counted by scintillation counting. Symbols: ■, 10403S plus [14C]betaine; ●, 10403S plus [3H]carnitine; □, LMA2B plus [14C]betaine; ○, LMA2B plus [3H]carnitine.
Role of ςB in low-temperature adaptation of stationary-phase cells.
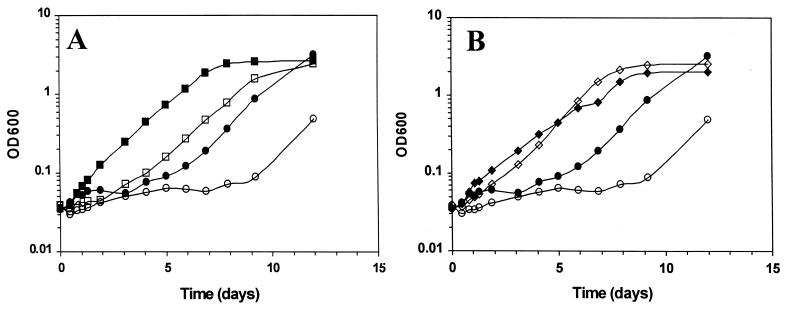
Because ςB activity is stimulated by onset of stationary phase, we next tested whether it might play unique roles in adaptation of stationary-phase cells to low-temperature environments. Overnight cultures of 10403S and LMA2B were grown at 37°C in DM and subsequently inoculated into fresh DM and incubated at 8°C. The resulting growth curves showed that the absence of ςB impaired adaptation of stationary-phase cells to growth at the lower temperature (Fig. 3). The parental strain 10403S exhibited a lag phase lasting approximately 4 days, while that of LMA2B was nearly twice as long, lasting approximately 8 days. These results imply that, in contrast to log-phase cells, ςB plays an important role in adaptation of stationary-phase cells to low-temperature growth.
FIG. 3.
Growth of 10403S and LMA2B at 8°C in the presence of cryoprotectants. Overnight stationary-phase cultures of 10403S and LMA2B growing in DM at 37°C were used to inoculate fresh DM supplemented with 1 mM betaine (A) or 1 mM carnitine (B) and incubated at 8°C. Symbols: ●, 10403S with no cryoprotectant; ○, LMA2B with no cryoprotectant; ■, 10403S with 1 mM betaine; □, LMA2B with 1 mM betaine; ⧫, 10403S with 1 mM carnitine; ◊, LMA2B with 1 mM carnitine.
Given the different phenotypes of log- and stationary-phase cells of the sigB::Km strain, we suggest that L. monocytogenes has independent pathways for adaptation to growth at low temperature. One pathway is ςB independent and can be entered into by log-phase cells, while the second pathway has a ςB-dependent component and is the pathway of choice in stationary-phase cells. Alternatively, a single pathway for adaptation may exist, with genes that participate in the pathway having ςB-independent modes of expression during log phase and ςB-dependent modes of expression that operate during stationary phase. Growth phase-dependent roles of ςB have also been reported for B. subtilis (16), and together, these data suggest that ςB may constitute a general device for controlling growth phase-dependent pathways of stress response.
When 1 mM betaine was added at the time of inoculation, the lag phase of both 10403S and LMA2B decreased (Fig. 3A); however, the decrease was more pronounced in the wild-type strain. Once LMA2B resumed growth in the presence of betaine, its rate was indistinguishable from that of the wild type. Addition of carnitine decreased the lag phase of both 10403S and LMA2B to nearly identical times (Fig. 3B). Thus, although ςB is necessary for efficient utilization of carnitine and betaine in log-phase cells, it is apparently necessary only for utilization of betaine in stationary-phase cells.
To test whether ςB is necessary for efficient uptake of betaine and carnitine in stationary-phase cells, accumulation was measured in cells that were grown from inoculation at 8°C. Overnight cultures growing at 37°C in DM were used to inoculate fresh DM followed by incubation at 8°C. Upon reaching mid-logarithmic growth, aliquots were removed and uptake of radiolabeled betaine or carnitine was measured. Figure 4 shows that there was considerably less accumulation of both betaine and carnitine in LMA2B compared to the wild-type strain, 10403S. Surprisingly, however, carnitine accumulation was defective in the sigB mutant growing at 8°C, even though the mutant's ability to utilize carnitine as a cryoprotectant (Fig. 3B) did not reflect its diminished ability to accumulate it. This supports the conclusion that residual carnitine transport in stationary-phase cells in the absence of ςB is sufficient to provide cryoprotection and implies that carnitine is a more efficient cryoprotectant than betaine.
FIG. 4.
Cryoprotectant accumulation in 10403S and LMA2B growing at 8°C. Overnight stationary-phase cultures of 10403S and LMA2B growing in DM at 37°C were used to inoculate fresh DM followed by incubation at 8°C. Cells in mid-logarithmic growth (OD600, ∼0.4) were collected, and cell suspensions were adjusted to approximately 0.12 mg of protein/ml. Uptake assays were performed at 8°C and started by addition of [14C]betaine or [3H]carnitine to each sample. Aliquots were removed over the time of the assay, harvested by centrifugation through oil, and counted by scintillation counting. Symbols: ■, 10403S plus [14C]betaine; ●, 10403S plus [3H]carnitine; □, LMA2B plus [14C]betaine; ○, LMA2B plus [3H]carnitine.
The relative efficiencies of carnitine and betaine as osmoprotectants and cryoprotectants have been examined with Escherichia coli and L. monocytogenes. Betaine is a better osmoprotectant than carnitine in E. coli, presumably because the longer carbon chain length of carnitine decreases its osmoprotective function (27). For wild-type L. monocytogenes, betaine has been reported to be more effective than carnitine in providing tolerance to low temperature (34), despite the fact that more carnitine accumulates at low temperatures than does betaine (21). It may be that a low threshold of carnitine accumulation is necessary to promote low-temperature growth of stationary-phase L. monocytogenes cells and that residual uptake in the sigB::Km strain is enough to sustain wild-type levels of growth. Betaine and carnitine may also have different roles in cryoprotection. In eukaryotic cells, carnitine stimulates beta-oxidation of lipids by serving as a carrier across the mitochondria (4). It is conceivable that carnitine could also function as a carrier in the process of fatty acid alteration that occurs during low-temperature adaptation of L. monocytogenes. If it were to play a role as a cofactor, then it might be needed only at modest concentrations.
Since two different betaine transport systems have been described biochemically and genetically (17, 22), it is possible that ςB could exert its effects by influencing the activity of one or both systems. To address this question, we used a physiological approach to determine the sensitivity of the residual betaine transport to metabolic inhibitors in the absence of ςB. The BetL system of betaine transport is sodium dependent and sensitive to the effects of monensin, a sodium ionophore (17), whereas the GbuA transport system is ATP dependent (22) and therefore insensitive to the immediate effects of monensin. When 0.02 mM monensin was added to cold-grown cell suspensions (Table 2), the rate of betaine transport by the wild-type strain, 10403S, was reduced by only 20%, indicating that, under these growth conditions, the majority of the transport was mediated by the GbuA-dependent system (assuming that GbuA is the only monensin-resistant betaine transport system in L. monocytogenes). Addition of monensin to the sigB mutant, however, inhibited betaine transport by more than 90%. This result suggests that residual betaine transport in the sigB mutant is BetL dependent and implies that ςB influences betaine transport primarily through the GbuA transporter. Although the promoter regions of betL and gbuA have not been identified experimentally, a sequence similar to ςB-dependent promoters was identified upstream of the betL gene (33), while no ςB-like element could be found upstream of gbuA (22). We have identified a substrate-inducible, ςB-independent promoter upstream of betL but have not been able to observe a transcript that originates from the putative ςB-dependent promoter under any condition tested (M. S. Cetin and A. K. Benson, unpublished). We are currently examining transcription from the gbuA and betL promoters to precisely determine how ςB influences betaine transport.
TABLE 2.
Effect of monensin on betaine and carnitine transport
| Treatment | Transport ratea (%)
|
|
|---|---|---|
| Betaine | Carnitine | |
| 10403Sb | 20.8 (100) | 34.7 (100) |
| 10403S + monensinc | 16.8 (81) | 3.6 (10) |
| LMA2B4d | 6.4 (100) | 12.7 (100) |
| LMA2B + monensinc | 0.6 (8) | 1.2 (9) |
Rates are expressed as nanomoles accumulated per milligram of protein per minute (and as percentages of the control rate).
Wild type.
Monensin was added at 0.02 mM.
sigB::Km.
The role of ςB in carnitine accumulation is less clear than that of betaine, since no carnitine transport systems have been cloned from L. monocytogenes. Although Verheul et al. (36) reported the presence of an ATP-dependent carnitine transport system in L. monocytogenes strain Scott A, carnitine transport in our experiments was inhibited by monensin to the same extent in both wild-type and mutant cells, leading us to conclude that a sodium-dependent system may be primarily responsible for transport of this cryoprotectant.
Acknowledgments
This research was supported, in part, by funds from USDA HATCH project no. NEB-16-077 and the Midwest Alliance for Food Manufacturing. S.N.E. is supported by USDA National Needs Fellowship no. 96-38420-3050.
Footnotes
Paper no. 13140, Journal Series Nebraska Agricultural Experimental Station, Lincoln, NE 69583-0919.
REFERENCES
- 1.Akbar S, Kang C M, Gaidenko T A, Price C W. Modulator protein RsbR regulates environmental signaling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24:567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- 2.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in Bacillus subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 3.Annous B A, Becker L A, Bayles D O, Labeda D P, Wilkinson B J. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperature. Appl Environ Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthoni U, Christophersen C, Hougaard L, Nielsen P H. Quaternary ammonium compounds in the biosphere—an example of a versatile adaptive strategy. Comp Biochem Physiol. 1991;99B:1–18. [Google Scholar]
- 5.Becker L A, Cetin M S, Hutkins R W, Benson A K. Identification of the gene encoding the alternative sigma factor ςB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson A K, Haldenwang W G. Bacillus subtilis ςB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc Natl Acad Sci USA. 1993;90:2330–2334. doi: 10.1073/pnas.90.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beumer R R, Te Giffel M C, Cox L J, Rombouts F M, Abee T. Effect of exogenous proline, betaine, and carnitine on growth of Listeria monocytogenes in a minimal medium. Appl Environ Microbiol. 1994;60:1359–1363. doi: 10.1128/aem.60.4.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan S A, Redfield A R, Price C W. Transcription factor ςB of Bacillus subtilis controls a large stationary-phase regulon. J Bacteriol. 1993;175:3957–3963. doi: 10.1128/jb.175.13.3957-3963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boylan S A, Redfield A R, Brody M S, Price C W. Stress-induced activation of the ςB transcription factor of Bacillus subtilis. J Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Christensen D P, Benson A K, Hutkins R W. Cloning and expression of the Listeria monocytogenes Scott A ptsH and ptsI genes, coding for Hpr and enzyme I, respectively, of the phosphotransferase system. Appl Environ Microbiol. 1998;64:3147–3152. doi: 10.1128/aem.64.9.3147-3152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole M B, Jones M V, Holyoak C. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J Appl Bacteriol. 1990;69:63–72. doi: 10.1111/j.1365-2672.1990.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 13.Dufour A, Haldenwang W G. Interactions between a Bacillus subtilis anti-sigma factor (RsbW) and its antagonist (RsbV) J Bacteriol. 1994;176:1813–1820. doi: 10.1128/jb.176.7.1813-1820.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farwick M, Siewe R M, Krämer R. Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum. J Bacteriol. 1995;177:4690–4695. doi: 10.1128/jb.177.16.4690-4695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaidenko T A, Price C W. General stress transcription factor ςB and sporulation sigma factor ςH contribute to survival of Bacillus subtilis under extreme growth conditions. J Bacteriol. 1998;180:3730–3733. doi: 10.1128/jb.180.14.3730-3733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhardt P N M, Smith L T, Smith G M. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J Bacteriol. 1996;178:6105–6109. doi: 10.1128/jb.178.21.6105-6109.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham J E, Wilkinson B J. Staphylococcus aureus osmoregulation: roles for choline, glycine betaine, proline, and taurine. J Bacteriol. 1992;174:2711–2716. doi: 10.1128/jb.174.8.2711-2716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the ςB regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 20.Kang C M, Brody M S, Akbar S, Yang X, Price C W. Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor ςB in response to environmental stress. J Bacteriol. 1996;178:3846–3853. doi: 10.1128/jb.178.13.3846-3853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko R, Smith L T, Smith G M. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J Bacteriol. 1994;176:426–431. doi: 10.1128/jb.176.2.426-431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko R, Smith L T. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl Environ Microbiol. 1999;65:4040–4048. doi: 10.1128/aem.65.9.4040-4048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landfald B, Strom A R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986;165:849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller A J. Combined water activity and solute effects on growth and survival of Listeria monocytogenes Scott A. J Food Prot. 1992;55:414–418. doi: 10.4315/0362-028X-55.6.414. [DOI] [PubMed] [Google Scholar]
- 25.Molenaar D, Hagting A, Alkema H, Driessen A J M, Konings W N. Characterististics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J Bacteriol. 1993;175:5438–5444. doi: 10.1128/jb.175.17.5438-5444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patchett R A, Kelly A F, Kroll R G. Transport of glycine-betaine by Listeria monocytogenes. Arch Microbiol. 1994;162:205–210. doi: 10.1007/BF00314476. [DOI] [PubMed] [Google Scholar]
- 27.Peddie B A, Lever M, Hayman C M, Randall K, Chambers S T. Relationship between osmoprotection and the structure and intracellular accumulation of betaines by Escherichia coli. FEMS Microbiol Lett. 1994;120:125–132. doi: 10.1111/j.1574-6968.1994.tb07018.x. [DOI] [PubMed] [Google Scholar]
- 28.Pine L, Malcom G B, Brooks J B, Daneshvar M I. Physiological studies on the growth and utilization of sugars by Listeria species. Can J Microbiol. 1989;35:245–254. doi: 10.1139/m89-037. [DOI] [PubMed] [Google Scholar]
- 29.Schuchat A, Swaminathan B, Broome C V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott J M, Haldenwang W G. Obg, an essential GTP binding protein of Bacillus subtilis, is necessary for stress activation of transcription factor ςB. J Bacteriol. 1999;181:4653–4660. doi: 10.1128/jb.181.15.4653-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott J M, Ju J, Mitchell T, Haldenwang W G. The Bacillus subtilis GTP binding protein Obg and regulators of the ςB stress response transcription factor cofractionate with the ribosomes. J Bacteriol. 2000;182:2771–2777. doi: 10.1128/jb.182.10.2771-2777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeliger H P R, Jones D. Listeria. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1235–1245. [Google Scholar]
- 33.Sleator R D, Gahan C G M, Abee T, Hill C. Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl Environ Microbiol. 1999;65:2078–2083. doi: 10.1128/aem.65.5.2078-2083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith L T. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl Environ Microbiol. 1996;62:3088–3093. doi: 10.1128/aem.62.9.3088-3093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verheul A, Glaasker E, Poolman B, Abee T. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J Bacteriol. 1997;179:6979–6985. doi: 10.1128/jb.179.22.6979-6985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verheul A, Rombouts F M, Beumer R R, Abee T. An ATP-dependent l-carnitine transporter in Listeria monocytogenes Scott A is involved in osmoprotection. J Bacteriol. 1995;177:3205–3212. doi: 10.1128/jb.177.11.3205-3212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vijay K, Brody M S, Fredlund E, Price C W. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the ςB transcription factor of Bacillus subtilis. Mol Microbiol. 2000;35:180–188. doi: 10.1046/j.1365-2958.2000.01697.x. [DOI] [PubMed] [Google Scholar]
- 38.Voelker U, Voelker A, Maul B, Hecker M, Dufour A, Haldenwang W G. Separate mechanisms activate ςB of Bacillus subtilis in response to environmental and metabolic stress. J Bacteriol. 1995;177:3771–3780. doi: 10.1128/jb.177.13.3771-3780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waite B L, Siragusa G R, Hutkins R W. Bacteriocin inhibition of two glucose transport systems in Listeria monocytogenes. J Appl Microbiol. 1998;84:715–721. doi: 10.1046/j.1365-2672.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- 40.Walker S J, Archer P, Banks J G. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990;68:157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 41.Wise A A, Price C W. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor ςB in response to environmental signals. J Bacteriol. 1995;177:123–133. doi: 10.1128/jb.177.1.123-133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero G N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Kang C M, Brody M S, Price C W. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 1996;10:2265–2275. doi: 10.1101/gad.10.18.2265. [DOI] [PubMed] [Google Scholar]