Abstract
The devastating chronic central post stroke pain is associated with variety of comorbidities. Disrupted sleep is a severe comorbidity, causing an increase in the suicide rate, due to CPSP’s pain symptom. Melatonin is a well-known jet-lag compound, which helps in entrainment of sleep cycle. Accordingly, whether melatonin as a therapeutic measurement for the regulation of sleep disturbance related to central post stroke pain remains unclear. Exogenous melatonin administration entrained the disrupted 24 h circadian cycle, more effectively after 2 and 3 week of administration. The effect of melatonin was persisted on 4th week too, when melatonin administration was discontinued. Also, melatonin ameliorated the pain due to distorted sleep-activity behavior after melatonin administration for 3 weeks. The low levels of melatonin in blood plasma due to CPSP were restored after 3 weeks of melatonin administration. After 30 mg/kg melatonin administrations for 3 weeks, all the disrupted resting and activity behaviors were reduced during light and dark periods. The results suggested that melatonin significantly ameliorated CPSP’s pain symptoms and comorbid sleep disturbance showing in activity behavior.
Keywords: hemorrhagic stroke, sleep, activity behavior, hyper-excitability, melatonin, pain
Introduction
Central Post Stroke Pain (CPSP) is characterized by constant or intermittent pain, and CPSP is associated with sensory abnormalities, particularly of thermal sensation. It is known that each year, out of 700,000 new and recurrent cases of stroke, in the United States; at least 56,000 cases are of CPSP.1,2 Recently it has been found that the estimated prevalence of CPSP ranges widely from 8% to 55% of stroke patients. 3 Previously, it was called as thalamic pain by Dejerine and Roussy in 1906; CPSP was thought to be similar to thalamic injury (Thalamic syndrome). 4 However, the precise pathophysiology of CPSP is unclear; but the positions of lesions causing CPSP are found to be along the spinothalamocortical pathway.5,6 Typically, the spinothalamocortical pathway is associated with evoked abnormal sensations. 7 For example, our previous study has shown that thalamic hemorrhage caused an excessive amount of intracellular ATP release, and it promoted IL-1β secretion from the reactive microglia leading to enhanced glutamate release and resulting in a higher frequency of neuron bursting along the spinothalamocortical pathway.8,9 Moreover, BDNF expression was enhanced in ablation of the medial thalamus (MT) in the CPSP group than the control group. In control animals, enhanced GABA activity inhibited neuronal activity in the MT. In CPSP animals, the inhibitory GABA system appeared to be reversed, suggesting that neuronal plasticity in the MT that was induced by BDNF overexpression after the thalamic MT lesion. 10
Amounts of 67–88% chronic pain patients suffer from sleep abnormalities. 11 For example, higher than 50% of the stroke patients experience insomnia, sleep-related breathing disorders, or sleep-wake cycle disorders. 12 Disrupted sleep did not only induce patients feel more pain but also interfered with post-stroke recovery.13,14 Earlier studies suggested that sleep disturbance may impair key processes that contribute to the development and maintenance of chronic pain, including endogenous pain inhibition and joint pain. 15 The development of sleep disturbance comes as a side effect of pain development, and acute experimentally-induced sleep deprivation increases pain sensitivity. 16 Moreover, the sleep deprivation alone could cause neuronal hyperexcitability, and this symptom is likely to occurrence at CPSP patients. 17 A nice cycle of sleep regiments improved neuroplasticity, and the brain is able to re-construct and create new neural connections in healthy parts of the brain. 18 Therefore, the sleep disturbance is a crucial comorbid symptom of CPSP patients, and the sleep disturbance is always distressed to CPSP patients.
Melatonin is a neuro-hormone secreted by pineal gland and extra pineal tissues, and it governs various physiological phenomenon such as circadian rhythm and mood behaviors. 19 Circadian and seasonal rhythms (reproduction, diapause, hibernation, fur color changes, and migration etc.) are a fundamental feature of all living organisms, reflecting the need to ensure that biological functions occur at a given time of the day or year. Precise timing is required at all levels from behavior to gene expression, and its dysregulation causes malfunction. 20 Melatonin plays an important role to regulate circadian rhythms or other seasonal rhythms.21,22 Chronic exposures to melatonin synchronize the timing of activity behavior. 23 Additionally, melatonin also plays a pivotal role in pain regulation. Melatonin administrations have demonstrated to be effective for the treatment of fibromyalgia, migraine, and irritable bowel syndrome. 24 Therefore, melatonin may be effective for amelioration in sleep disruption and pain perception, and it is possible that there might be a link between melatonin signaling and sleep and pain regulation.
In summary, previously, no research has ever explored the issue that whether melatonin can reduce CPSP, pain perception and comorbid sleep disturbance. Therefore, our present study addressed this issue that whether melatonin administrations can ameliorate pain and comorbid sleep disturbance in activity behaviors in rodents. This study would provide the knowledge of the novel treatment of exogenous melatonin administration to reduce pain due to CPSP and comorbid sleep disturbance in an animal model.
Materials and methods
Animals
Twenty-four male Sprague Dawley rats (approximately 8 weeks of age) were purchased from the laboratory animal supplier, BioLASCO, Taiwan. They were individually housed in an animal room at a constant 12-h light-dark cycle (light on: 06:00–18:00) in the colony room with 60% humidity. All rats were allowed to eat food chow and drink water adlibitum. All of the experiments were performed in accordance with the guidelines of the Academia Sinica Institutional Animal Care and Utilization Committee.
CPSP surgery procedure
The surgical procedures for the thalamic lesion was conducted by the method as described by the previous study. 8 During the surgery, the animals were maintained at 1% isoflurane anesthesia during surgery. Body temperature was maintained at 36.5–37.5°C with a homeothermic blanket system (Model 50-7079, Harvard Apparatus, Holliston, MA, USA). The animals were injected with type IV collagenase (C5138, SIGMA, Saint Louis, USA; 0.125 U/0.5 μL saline) into the right ventral posterior medial nucleus (VPM)/ventral posterior lateral nucleus (VPL) of the thalamus (coordinates: 3.0–3.5 mm posterior, 3.0–3.4 mm lateral to bregma, 5.7–6.0 mm depth). Further, the rehabilitation procedure was performed for 3 weeks after surgery.
Home cage behavior scans recording and analysis
Animals exhibit pain in different behaviors like immobility, low activity (less exploratory behavior) and high activity behavior. To assess behaviors, home cage behavioral analysis was conducted. For this approach, 80 × 80 × 80 cm transparent acrylic home cages were used with food and water adlibitum to record day-night sleep cycle. A camera was installed above the cages and was connected to the computer. The SMART v3.0.0.6 software was installed and employed (Pan lab Harvard Apparatus, Barcelona, Spain). An infrared light source (20W power) was used at night, and it was recorded in the “dark period.” The SMART v3.0.0.6 software is designed to record discrete activity behaviors in rats, which includes, immobility (resting behavior) low activity and high activity. Immobility is achieved when the subject is continuous resting for 30 s. Sleep/rest or paradoxical sleep patterns represents immobile behavior. The discrete animal activities were estimated by the pre-defined and activity threshold [i.e. the detected movement in an area (cm2) per second]. Low activity is considered as decreased exploratory behavior; it defines that when its global activity is greater than equal to the user-defined low activity threshold setting (2.5 cm2/s) and less than to the user-defined high activity threshold setting (25 cm2/s). Similarly, the subject is considered in high activity; it defines that when its global activity is greater than equal to define high activity threshold setting (i.e. 25 cm2/s). Another behavior parameter is wakefulness, which is defined as total activity behavior (low activity and high activity) per 24 h.
Von Frey task for pain behavior tests
In Von Frey task, the mechanical pain behavior was tested. In this task, the animals were placed on an elevated mesh and allowed to explore for 30 min. The specific force was imparted by an elastic filament, in compression and fairly constant way. The shorter the filament, the higher the force require to buckle it. The diameters of filaments provide a wide range of forces. The task recorded the minimal force/pressure at which the animal reacts (limb withdrawal) to the painful stimulus. Each hind limb and average of the minimal pressure was recorded for three trials and then it denoted as threshold. There would be 5 min interval between each trial.
Measurement of serum concentration of melatonin
The concentration of melatonin was measured by Melatonin ELISA kit (abcam, ab213978). All the materials were equilibrated and prepared reagents to room temperature prior to use. The assay procedure was below. 100 μL of standards and 100 μL of the samples were added into the appropriate wells. Then to the above wells, 50 μL of the melatonin tracer was added to all wells except for the blank. Later, 50 μL of the melatonin antibody was added to all wells except for the NSB and blank. Then sealed and incubated at room temperature on a plate shaker for 1 h at ∼500 r/min. The wells were washed by adding 400 μL of wash buffer thrice. Then, 200 μL of the melatonin conjugate solution was added to each well except the blank. Sealed the plate and incubated at room temperature on a plate shaker for 30 min at ∼500 r/min. The wells were washed by adding 400 μL of wash buffer thrice. Then, 200 μL of TMB substrate solution was added into each well. Sealed the plate and incubated for 30 min at room temperature on a plate shaker at ∼500 r/min. To this, 50 μL of the stop solution was added into each well and read the OD at 450 nm.
Experimental design
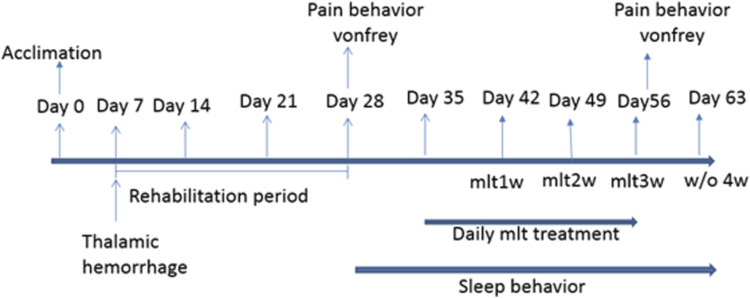
All rats were divided into sham (n = 8), lesion (n = 16), and lesion+melatonin groups (n = 8). The melatonin treatments were conducted for weeks 1–3 and discontinuing treatment for week 4. Melatonin was obtained from Sigma-Aldrich, USA. A daily dose of 30 mg/kg, i.p. was used. 25 The experiments were performed as indicated in Figure 1. After 1 week of acclimation period, the animals were subjected to stereotaxic microinjection for thalamic lesions. On Day 28, Von Frey test was conducted to test pain behavior. Melatonin was administrated between 6 p.m. to 8 p.m. daily for 3 weeks, and it was tested the effect of melatonin weekly on activity behavior. The sleep recording was conducted for a 12:12 h light-dark cycle (8 p.m. to next day 8 p.m.) after 2 h of melatonin treatment, and the activity behavior was evaluated with melatonin for 3 weeks and fourth week (from Day 57 to Day 63) without melatonin treatment. The effect of melatonin on CPSP rats was evaluated by Von Frey test after day 57.
Figure 1.
Experimental design.
Statistics
In Von Frey pain test, independent t-test was performed between the control and CPSP groups. Two-way mixed analysis of variance (ANOVA) was performed for the factors of group and phase (light vs dark). Furthermore, one-way ANOVA was conducted for group in the duration of immobility, low activity and high activity. When appropriate, the post hoc Dunett or Tukey’s test was conducted. *p value indicates lower than 0.05.
Results
Levels of endogenous melatonin
Levels of endogenous melatonin were measured, before lesion, after lesion and after 3 weeks of melatonin treatment. There was drastic dip in levels of endogenous melatonin production after lesion. The results in our study suggest the neurons got damaged and therefore, production of endogenous melatonin from pineal gland got hampered. The blood-serum concentration of melatonin was found lowered in CPSP rats (1.9085 ng/mL) (16.99 ± 2.728) than the control (18.900 ng/mL); which after the 3 weeks of exogenous melatonin administration was significantly restored (10.4683 ng/mL) (8.559 ± 2.728) [F(2, 7) = 19.54, p < 0.05)].
Effect of CPSP on total sleep duration and wakefulness in 24 h cycle
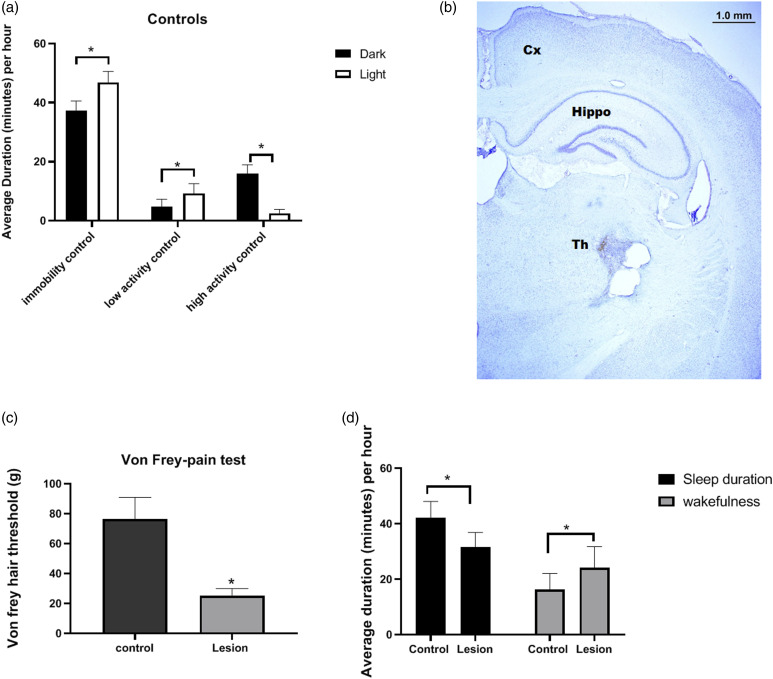
Rats (control) being nocturnal showed significantly high resting (immobile) behavior during light phase (t = 5.52, p < 0.05) and significantly higher exploratory behavior (high activity) during dark phase (t = 3.09, p < 0.05). Moreover, the low activity was higher during light period (t = 11.91, p < 0.05) (Figure 2a). Thalamic lesion for CPSP was successfully induced that was shown by Nissl stain of lesioned rat brain (Figure 2b). Mechanical hyperalgesia was revealed by Von Frey test on 28th day (t = 21.28, p < 0.05; Figure 2c). The total average immobile duration and activity duration (wakefulness) of CPSP rats as well as control rats were measured in 24 h. Concerning the pain behavioral test, both sleep duration and wakefulness were affected. The total average sleep duration per hour was decreased (t = 5.26, p < 0.05) while the wakefulness was increased in CPSP rats (t = 3.31, p < 0.05; Figure 2d).
Figure 2.
(a) Discrete activity behavior of Control (normal) rats during light and dark periods. Immobility: (control) rats being nocturnal showed significantly high resting behavior during day. Low activity: The normal rats did not show any difference in low activity. High activity: control rats have significantly higher exploratory behavior during night. Two-way ANOVA post Bonferroni test. (b) CPSP induction: showing Nissl stain of CPSP rat after 3 weeks of lesion. (c) shows Von Frey mechanical hyperalgesia in control and lesioned rats. (d) shows total amount of sleep and wakefulness in 24 h in control and lesioned rats. Two-way ANOVA followed by Bonferroni’s post hoc test (p < 0.05) control (n = 8), CPSP (n = 8).
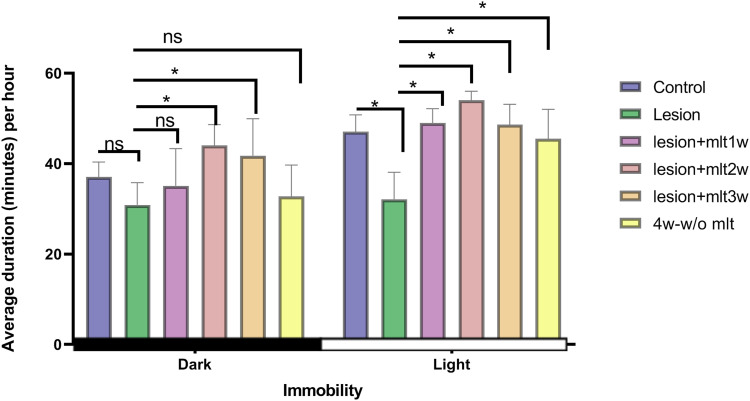
Effect of exogenous melatonin on rest activity (immobile behavior) during light and dark periods
Rats being nocturnal are more immobile during light period. CPSP impairs the resting behavior significantly during light time in rats (Figure 3). Lesion causes disruption in circadian rhythm, and thus interferes with the ability to rest in rats. The two-way ANOVA, group and phase values of immobile behavior were: [F(5, 84) = 18.50, p < 0.05] and [F(1, 84) = 65.67, p < 0.05] respectively. The post hoc Tukey test was [F(5, 42) =5.24, p < 0.05] for the dark period and [F(5, 42) = 20.86, p < 0.05] noted for the light period. The effect after 1 week of melatonin administration was non-significant during dark period while it was significantly different during light period (p < 0.05*). After 2 week of melatonin injection; there was increased resting behavior during light period (p < 0.005). However, rats shown some immobile behavior during dark period too (p < 0.05), but this behavior during dark period, was subsided on 4 week (the week without melatonin administration). Three week melatonin injection, showed similar effects to 2 week injection (p < 0.05) with some drowsiness during dark time disrupted immobility behavior was restored that was seen after the 4th week without melatonin, which suggests the effect of melatonin was persisted (p < 0.05).
Figure 3.
Effect of exogenous melatonin on rest activity (immobility) during light and dark periods. Immobility data during the 12:12 h-dark period in control, lesion, mlt, and without mlt groups. Two-way ANOVA followed by the Tukey’s post-test (p < 0.05). n = 8, dose of mlt:30 mg/kg (i.p.) O.D.
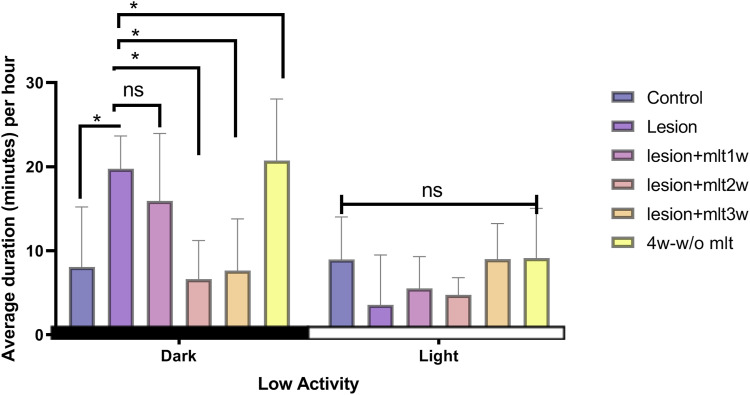
Effect of exogenous melatonin on low activity behavior during light and dark periods
The normal rats show higher “less exploratory behavior” during light period than dark period. Whereas, CPSP rats shown higher “reduced exploratory behavior” during dark period (Figure 4). The two-way ANOVA, group and phase values of immobile behavior were: [F(5, 84) = 5.23, p < 0.05] and [F (1, 84) = 30.41, p < 0.05] respectively. The F value post hoc Tukey test was [F(5, 42) = 12.09, p < 0.05] for the dark period and [F(5, 42) = 2.66, p < 0.05] noted for the light period. There was no significant change in low activity of rats in both light period and dark period, after 1 week of melatonin administration. After 2 week of melatonin injection, at dark time––less exploratory behavior (low activity) was significantly reversed by melatonin 2 week (p < 0.05). The effect of melatonin 3 week was same as 2 week melatonin (p < 0.05). On 4 week without melatonin “Low activity behavior” was significantly increased during dark period suggesting altered “low activity behavior due to CPSP” came back after the melatonin discontinued (p < 0.05).
Figure 4.
Effect of exogenous melatonin on “decreased exploratory behavior” (low activity). Low activity data during the 12:12 h-dark period in control, lesion, mlt, and without mlt groups. Two-way ANOVA followed by the Tukey’s post-test (p < 0.05). n = 8, dose of mlt:30 mg/kg (i.p.) O.D.
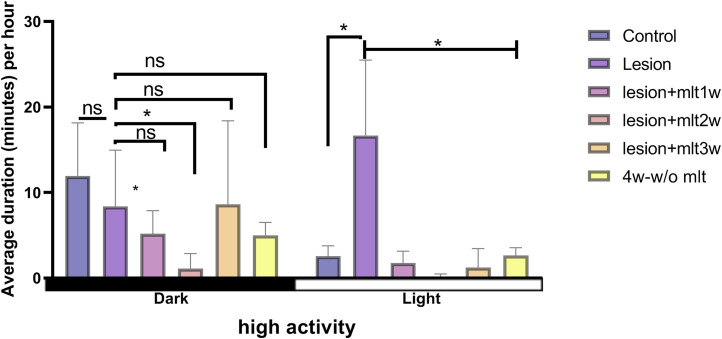
Effect of melatonin on high activity behavior during light and dark periods
To test the effects of melatonin on high activity on light and dark periods, two-way mixed ANOVA was conducted. The results showed that significant differences occurred in the factor of group [F(5, 84) = 6.60, p < 0.05] and the phase value of immobile behavior [F(1, 84) = 6.71, p < 0.05], respectively. Post hoc Tukey’s test indicated that significant differences occurred in the dark period and the light period (p < 0.05). Melatonin after 1 week of administration, helped to decrease high activity during light period in CPSP rats. The administration of melatonin for 1 week did not show any significant effect during dark hours. However, high activity behavior was reduced by 2 week melatonin administration significantly during light period (p < 0.05). High activity behavior was significantly reduced during light period. Interestingly, melatonin 3 week has shown increased high activity during dark period, and lower during light time, suggesting melatonin after 3 weeks of administration has neuroprotective effects (p < 0.05). After the 3 week of melatonin administration, High activity was significantly reversed by melatonin, we can see the effect of melatonin on 4th week too (p < 0.05; Figure 5).
Figure 5.
Effect of melatonin on high activity behavior during light and dark periods. High activity data during the 12:12 h -dark period in control, lesion, mlt, and without mlt groups. Two-way ANOVA followed by the Tukey’s post-test (p < 0.05). n = 8, dose of mlt:30 mg/kg (i.p.) O.D.
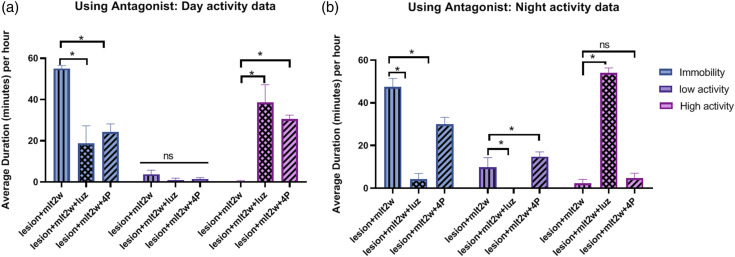
Effect of melatonin antagonists on activity behavior
In order to see the efficacy of melatonin, melatonin antagonists were used. When MT1 non selective antagonist Luzindole (Luz) and selective MT2 antagonist 4P- PDOT (4P) were used after 2 week of melatonin, treatment, both Luz and 4P significantly blocked immobility and high activity effects of melatonin, while there was no difference in low activity effect, during light period [F(4, 27) = 82.05] (p < 0.05) (Figure 6a). However, during the dark period, both Luz and 4P significantly blocked immobility and low activity effects of melatonin, while high activity was only blocked by Luz significantly. From the dark period activity data, results shown Luz has effectively blocked the effects of melatonin than 4P [F(4, 27) = 348.1] (p < 0.05) (Figure 6b).
Figure 6.
(a) Effect of MT1 non selective antagonist Luzindole (Luz) and selective MT2 antagonist 4P-PDOT (4P) on Day activity in melatonin treated (2 week) animals: Both Luz and 4P have significantly blocked immobility and high activity effects of melatonin, while there was no difference in low activity effect. (b) Effect of MT1 non selective antagonist Luzindole (Luz) and selective MT2 antagonist 4P-PDOT (4P) on Night activity in melatonin treated (2 week) animals: Both Luz and 4P have significantly blocked immobility and low activity effects of melatonin, while high activity was only blocked by Luz significantly. From the night activity data, we have seen Luz has effectively blocked the effects of melatonin than 4P.
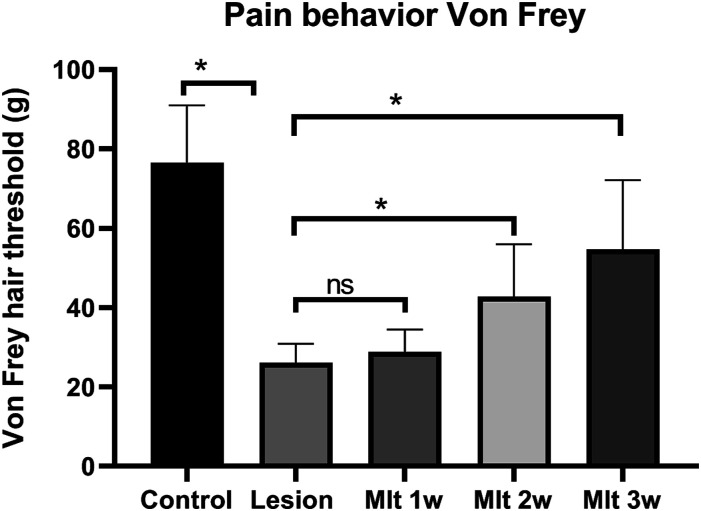
Effect of melatonin on pain behavior
With regard to the effect of melatonin on pain behavioral tests, one-way ANOVA was used to analyze. The mechanical hyperalgesia threshold in Von Frey test was significantly increased by melatonin administration and the data indicated reduction in pain perception [F(4, 60) = 41.68, p < 0.05)]. Post hoc Tukey’s test showed, reduction effect of melatonin on the pain sensitivity of CPSP on the 1, 2, and 3 week after 30 mg/kg of melatonin administration (Figure 7).
Figure 7.
Pain behavior test, mechanical hyperalgesia, done after 1, 2, 3 week of melatonin injection. Two-way ANOVA followed by the Tukey’s post-test (p < 0.05). n = 8, dose of mlt:30 mg/kg (i.p.) O.D.
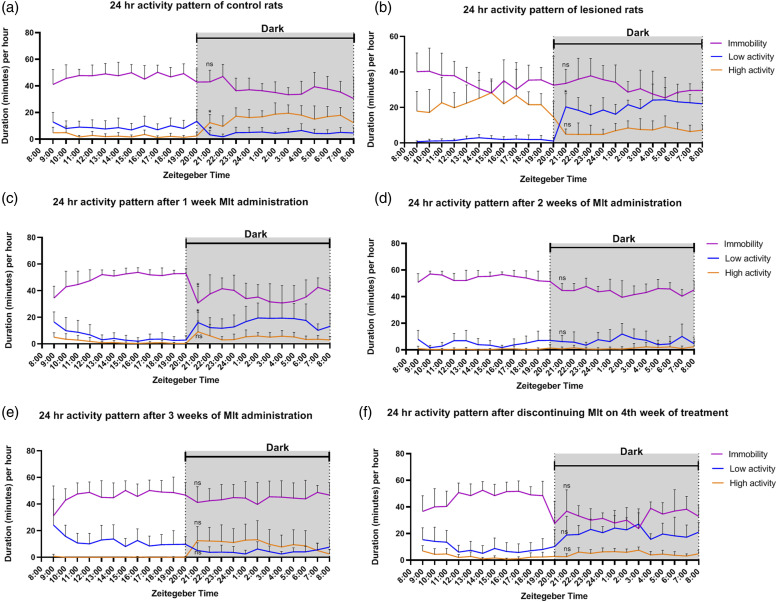
Entrainment of sleep behavior
To measure the effect of melatonin on sleep behavior in the control group, two-way ANOVA was conducted. The results showed that melatonin during night enhanced the night-time state of that animal (entrainment) in sleep or wake [F(46, 483) = 9.04, p < 0.05] (Figure 8a). However, after the stroke, the rats appeared to reduce the resting/immobile behavior and higher active behavior compared to control [F(46, 483) = 13.06, p < 0.05] (Figure 8b), indicating the stroke rat suffered from the sleep disturbance. Further, the lesioned animal’s immobility was increased at night due to the inability of rats to entrain their normal sleep behavior. While rats normally have high activity behaviors at night, but the lesioned rats reduced their high active behavior at night. Instead, the low active behavior was due to pain perception (Figure 8b). Interestingly, melatonin was found to recover the distorted immobility and high activity behavior induced by CPSP. in rats after 3 weeks of melatonin administration, during 12 h light period (p < 0.05; Figure 8c–f).
Figure 8.
Figure showing 24-h activity (per hour) in control (a), CPSP (b) and melatonin treated 1 week (c), 2 week (d), 3 week (e) animals, 4 week-without melatonin treatment (f).
Discussion
Sleeping behavior of nocturnal species like rats is quite different from diurnal human beings. 26 Rats often exhibit polyphasic sleep–wake behavior in which they alternate between states of wake and sleep on a time scale of tens of minutes, the characteristic of nocturnal rats in 12L:12D phase conditions. 27 The complex process of the sleep-wake cycle is controlled by the body’s circadian rhythm. Our endogenous pacemaker, the suprachiasmatic nucleus (SCN) (or the master clock), lies in the hypothalamus, is known to be responsible for circadian sleep/wake cycle. 28 In both diurnal and nocturnal species, there is a daily rhythm in the firing of neurons in the central circadian clock, SCN. The SCN receives information about light levels (an exogenous zeitgeber) from the optic nerve, which sets the circadian rhythm so that it is in synchronization with the outside world, e.g. day and night. The neurons tend to fire most during the day and least during the night. Low firing rates are therefore associated with sleep in diurnal animals, but wake in nocturnal animals. It sends signals to the pineal gland, which leads to an increased production of melatonin at night.
The effects of melatonin on sleep are in part mediated via the SCN, since SCN neurons have melatonin receptors. Melatonin suppresses the firing of SCN neurons. It is notable that melatonin is released during the night in both diurnal and nocturnal species, but it is not sleep-promoting in nocturnal species. 29 The SCN and pineal gland work together as endogenous pacemakers; however, their activity is responsible to the external cue of light. 30
The results of our study suggest that the neurons got damaged and therefore, production of endogenous melatonin from pineal gland got hampered. The blood-serum concentration of melatonin was found lowered in CPSP rats; which after the 3 weeks of exogenous melatonin administration were significantly restored. Hence treatment with exogenous melatonin has proven to be efficient. Further we all know that thalamus has a strong nonphotic influence on circadian rhythmicity, pineal melatonin production, and secretion. 31 CPSP due to intra-thalamic lesion, may damage the neurons firing in thalamus. In turn, damage to the thalamus might affect endogenous melatonin production, causing abrupt changes in rest/sleep and activity behavior of animals. Since one of the common outcomes of the biological clock is the activity and rest cycles, which follows the circadian rhythm with subjective day and night which, in nocturnal rodents, are characterized by immobility and low activity. 32
We have found in our study; the disrupted activity behaviors were associated with disrupted sleep cycle after CPSP. Animals in pain due to CPSP tend to sleep less and shown less immobility (resting) behavior and more high active behavior during their sleeping hours of the 24 h circadian cycle. While they tend to shown more low-activity behavior instead of high activity behaviors during the waking hours of the sleep cycle. However exogenous melatonin administration was able to entrain the disrupted 24 h circadian cycle, more effectively after 2 and 3 week of administration. Also, the effect of melatonin was quite persisted on 4th week too, when melatonin administration was discontinued. The use of melatonin, was able of keep immobility and low activity (during dark), non-significant with the control, almost for the 3 weeks. But we have seen, discontinuing melatonin on 4th week, caused the CPSP related low activity disruption again, probably due to “phase-shift” caused by the exogenous melatonin in the circadian rhythms of rats.
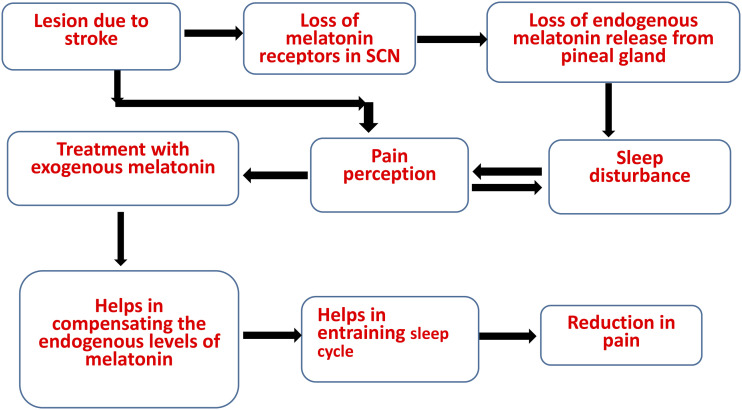
As per our data, we speculate, there might be two mechanisms involved with effect of exogenous melatonin. First, administration of 30 mg/kg i.p. melatonin may be compensating the effect of endogenous melatonin production. Second, entrainment of circadian rhythms, helped in reducing pain perception as chronic pain is also circadian in nature (Figure 9). Moreover, it is postulated that, opioidergic signaling and dopaminergic signaling may have a role in sleep and pain regulation, because of the presence of opioid receptors in suprachiasmatic nucleus and periaqueductal gray and dopamine receptors in reticular activating system. 33 Further, melatonin receptors have also been found in reticular nucleus as well as SCN. 34 We believe exogenous melatonin not only helped in improvement of activity behaviors but also pain associated with CPSP and pain associated with disrupted sleep cycle and vice versa (Figure 9). This was consistent with the pain behavior Von Frey test in our study. Therefore, considering the effect of melatonin on pain and on sleep, the melatonin might have role in pain-sleep bidirectional pathway. However, further studies are needed in this context.
Figure 9.
Flow chart showing effect of exogenous melatonin on sleep disruption and central post stroke pain.
Conclusion
In the present study, CPSP could induce pain behavior and disturbed circadian activity as a comorbidity. The activity behavior for CPSP rats showed differences in light and dark periods.
Chronic melatonin administrations for 3 weeks recover all the hampered activity behaviors in rats with CPSP. Moreover, melatonin administrations present persisted effect; however, disrupted low activity was returned in absence of melatonin. Melatonin might be alternative treatments for the stroke patients suffering with pain and sleep disturbance. The present findings might offer some implications for clinical aspects. The issue of melatonin for CPSP treatments should be examined in the further clinical studies.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Science and Technology (108-2320-B-001-024).
ORCID iDs
Andrew Chih Wei Huang https://orcid.org/0000-0001-9794-7302
Bai-Chuang Shyu https://orcid.org/0000-0001-5619-2281
References
- 1.Henry JL, Lalloo C, Yashpal K. Central poststroke pain: an abstruse outcome. Pain Res Manage 2008; 13: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilron I, Watson CPN, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. CMAJ 2006; 175: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer J, Conigliaro A, Spina E, Law SW, Levine SR. Central poststroke pain: a systematic review. Int J Stroke 2017; 12: 343–355. [DOI] [PubMed] [Google Scholar]
- 4.Jahngir MU, Qureshi AI. Dejerine Roussy Syndrome. 2018. [PubMed] [Google Scholar]
- 5.Leijon G, Boivie J, Johansson I. Central post-stroke pain—neurological symptoms and pain characteristics. Pain 1989; 36: 13–25. [DOI] [PubMed] [Google Scholar]
- 6.Jensen TS, Lenz FA. Central post-stroke pain: a challenge for the scientist and the clinician. Pain 1995; 61(2): 161–164. [DOI] [PubMed] [Google Scholar]
- 7.Hansson P. Post‐stroke pain case study: clinical characteristics, therapeutic options and long‐term follow‐up. Eur J Neurol 2004; 11: 22–30. [DOI] [PubMed] [Google Scholar]
- 8.Kuan Y-H, Shih H-C, Tang S-C, Jeng J-S, Shyu B-C. Targeting P2X7 receptor for the treatment of central post-stroke pain in a rodent model. Neurobiol Dis 2015; 78: 134–145. [DOI] [PubMed] [Google Scholar]
- 9.Kuan Y-H, Shih H-C, Shyu B-C. Involvement of P 2 X 7 receptors and BDNF in the pathogenesis of central poststroke pain. Adv Pain Res 2018; 2018: 211–227. [DOI] [PubMed] [Google Scholar]
- 10.Shih H-C, Kuan Y-H, Shyu B-C. Targeting brain-derived neurotrophic factor in the medial thalamus for the treatment of central poststroke pain in a rodent model. Pain 2017; 158: 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004; 8: 119–132. [DOI] [PubMed] [Google Scholar]
- 12.Wallace DM, Ramos AR, Rundek T. Sleep disorders and stroke. Int J Stroke 2012; 7: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause AJ, Prather AA, Wager TD, Lindquist MA, Walker MP. The pain of sleep loss: a brain characterization in humans. J Neurosci 2019; 39: 2291–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duss SB, Seiler A, Schmidt MH, Pace M, Adamantidis A, Müri RM, Bassetti CL. The role of sleep in recovery following ischemic stroke: a review of human and animal data. Neurobiol Sleep Circadian Rhythms 2017; 2: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drewes AM, Rössel P, Arendt-Nielsen L, Nielsen KD, Hansen LM, Birket-Smith L, Stengaard-Pedersen K. Sleepiness does not modulate experimental joint pain in healthy volunteers. Scand J Rheumatol 1997; 26: 399–400. [DOI] [PubMed] [Google Scholar]
- 16.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 2013; 14: 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014; 81: 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M-Q, Li R, Wang Y-Q, Huang Z-L. Neural plasticity is involved in physiological sleep, depressive sleep disturbances, and antidepressant treatments. Neural Plasticity 2017; 2017: 5870735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reproduction 1998; 3: 13–22. [DOI] [PubMed] [Google Scholar]
- 20.Schibler U. The daily timing of gene expression and physiology in mammals. Dial Clin Neurosci 2007; 9: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reproduction 1998; 3: 13–22. [DOI] [PubMed] [Google Scholar]
- 22.Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, Reiter R, Hardeland R, Rol MA, Madrid JA. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci 2014; 15: 23448–23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeffer M, Korf H-W, Wicht H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen Comparative Endocrinol 2018; 258: 215–221. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I. Analgesic effects of melatonin: a review of current evidence from experimental and clinical studies. J Pineal Res 2011; 51: 270–277. [DOI] [PubMed] [Google Scholar]
- 25.Mickle A, Sood M, Zhang Z, Shahmohammadi G, Sengupta JN, Miranda A. Antinociceptive effects of melatonin in a rat model of post-inflammatory visceral hyperalgesia: a centrally mediated process. Pain 2010; 149: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubbard J, Ruppert E, Calvel L, Robin-Choteau L, Gropp C-M, Allemann C, Reibel S, Sage-Ciocca D, Bourgin P. Arvicanthis ansorgei, a novel model for the study of sleep and waking in diurnal rodents. Sleep 2015; 38: 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleshner M, Booth V, Forger DB, Diniz Behn CG. Circadian regulation of sleep–wake behaviour in nocturnal rats requires multiple signals from suprachiasmatic nucleus. Philos Trans R Soc A: Math Phys Eng Sci 2011; 369: 3855–3883. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez M. Lighting conditions that jeopardize the health and wellbeing of laboratory animals. Front Neurol 2018; 9: 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi AV, Mosser EA, Oikonomou G, Prober DA. Melatonin is required for the circadian regulation of sleep. Neuron 2015; 85: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans J, Silver R. The suprachiasmatic nucleus and the circadian timekeeping system of the body. Neurosci 21st Century 2016; 2016: 1–49. [Google Scholar]
- 31.Jan JE, Reiter RJ, Wasdell MB, Bax M. The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res 2009; 46: 1–7. [DOI] [PubMed] [Google Scholar]
- 32.Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health 2001; 25: 85–93. [PMC free article] [PubMed] [Google Scholar]
- 33.Finan PH, Buenaver LF, Runko VT, Smith MT. Cognitive-behavioral therapy for comorbid insomnia and chronic pain. Sleep Med Clin 2014; 9: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-Canul M, Min SH, Posa L, De Gregorio D, Bedini A, Spadoni G, Gobbi G, Comai S. Melatonin MT1 and MT2 receptors exhibit distinct effects in the modulation of body temperature across the light/dark cycle. Int J Mol Sci 2019; 20: 2452. [DOI] [PMC free article] [PubMed] [Google Scholar]