Abstract
Stress alters both cognitive and emotional function, and increases risk for a variety of psychological disorders, such as depression and posttraumatic stress disorder. The prefrontal cortex is critical for executive function and emotion regulation, is a target for stress hormones, and is implicated in many stress-influenced psychological disorders. Therefore, understanding how stress-induced changes in the structure and function of the prefrontal cortex are related to stress-induced changes in behavior may elucidate some of the mechanisms contributing to stress-sensitive disorders. This review focuses on data from rodent models to describe the effects of chronic stress on behaviors mediated by the medial prefrontal cortex, the effects of chronic stress on the morphology and physiology of the medial prefrontal cortex, mechanisms that may mediate these effects, and evidence for sex differences in the effects of stress on the prefrontal cortex. Understanding how stress influences prefrontal cortex and behaviors mediated by it, as well as sex differences in this effect, will elucidate potential avenues for novel interventions for stress-sensitive disorders characterized by deficits in executive function and emotion regulation.
Keywords: Prefrontal cortex, stress, prelimbic, infralimbic, sex difference
Introduction
Stressful experiences, which broadly can be considered to be challenges to an organism’s wellbeing or allostasis (McEwen, 2017), can vary in their intensity, duration, and chronicity. Further, cognitive processing of potentially stressful events can vary across individuals. Thus, it is not surprising that such challenges can have a range of effects, varying from beneficial to detrimental (Sapolsky, 2003). Nonetheless, excessive, chronic, or repeated stress can produce cognitive and emotional dysfunction. For instance, stressful life events can precipitate episodes of major depression (Risch et al., 2009), traumatic stressors can trigger posttraumatic stress disorder (Lechin, Van der Dijs, & Benaim, 1996; Turner & Lloyd, 2004), and risk for mood and anxiety disorders increases with repeated exposure to stress (Risch et al., 2009). Such chronic stressors have adverse effects on many behaviors in the absence of overt psychopathology. For instance, disrupted frontoparietal connectivity and associated deficits in attentional control have been found in medical students following one month of academic stress (Liston, McEwen, & Casey, 2009). Similarly, in animal models, stress produces deficits on a variety of cognitive and emotion-regulation tasks, including extinction of conditioned fear, attentional set-shifting, spatial learning and recognition, and working memory in animal models (reviewed in Conrad, Ortiz, & Judd, 2017; Hurtubise & Howland, 2017; Maren & Holmes, 2016; Wellman & Moench, 2019). Interestingly, many of the behaviors that are susceptible to stress effects are mediated by the prefrontal cortex.
As in primates, the prefrontal cortex in rodents can be subdivided into several major subregions (Figure 1). The medial prefrontal cortex includes anterior cingulate, prelimbic, and infralimbic cortex. This region is functionally homologous to the primate dorsolateral and ventromedial prefrontal cortices, and plays a role in autonomic and HPA axis regulation (McKlveen, Myers, & Herman, 2015), emotion regulation (Wellman & Moench, 2019), working memory (Kesner & Churchwell, 2011), and cognitive flexibility (Hamilton & Brigman, 2015). Orbitofrontal cortex, which includes the medial, ventral, and lateral orbitofrontal subregions, is functionally homologous to primate orbitofrontal cortex and appears to play a role in modulating behavioral responses based on changing incentive values of reward-related stimuli (Hamilton & Brigman, 2015; Holmes & Wellman, 2009).
Figure 1.

Schematic diagram of coronal sections through the rostral forebrain of the rat, with prefrontal brain regions likely to play a role in stress effects on extinction identified. Coordinates given are relative to Bregma in rat brain. AC, anterior cingulate; PL, prelimbic; IL, infralimbic; MO, medial orbitofrontal; VO, ventral orbitofrontal; LO, Lateral Orbitofrontal. Adapted from Paxinos and Watson (1998).
While stress influences the structure and function of orbitofrontal cortex (e.g., Godar et al., 2015; Gourley, Swanson, & Koleske, 2013; Liston et al., 2006), these effects have been most extensively studied in the medial prefrontal cortex. Thus, in this chapter we focus on the medial prefrontal cortex. First, we provide a brief overview of the effects of chronic stress on behaviors mediated by the medial prefrontal cortex, as assessed in animal models. We then focus on the effects of chronic stress on the morphology and physiology of the medial prefrontal cortex, and mechanisms that may mediate these effects. Because the vast majority of research on the effects of stress on the prefrontal cortex has focused on males, the bulk of this chapter will likewise focus on males. However, we end with a discussion of emerging evidence for sex differences in the effects of stress on the prefrontal cortex.
Effects of Chronic Stress on Prefrontally-Mediated Behaviors
In male rodents, chronic stress produces deficits in a variety of behaviors mediated by the medial prefrontal cortex, and these deficits are remarkably consistent across a range of stressors and chronicities. For instance, extinction of conditioned fear is heavily dependent on the infralimbic cortex, and studies using a variety of stress manipulations (e.g., restraint stress, unpredictable mild stress, elevated platform stress), either acute or chronic (varying in duration from seven to twenty-one days) have demonstrated that stress impairs extinction learning (Baran, Armstrong, Niren, Hanna, & Conrad, 2009; Garcia, Spennato, Nilsson-Todd, Moreau, & Deschaux, 2008; Goswami, Cascardi, Rodriguez-Sierra, Duvarci, & Pare, 2010; Izquierdo, Wellman, & Holmes, 2006; Knox, George, et al., 2012; Knox, Nault, Henderson, & Liberzon, 2012; Knox, Perrine, George, Galloway, & Liberzon, 2010; M. Maroun et al., 2013; Miracle, Brace, Huyck, Singler, & Wellman, 2006; Wilber et al., 2011). Likewise, chronic stress of varying intensities and chronicities impairs behavioral flexibility as assessed via attentional set-shifting. The extradimensional shift in this task is mediated by the prelimbic cortex (Birrell & Brown, 2000; Hamilton & Brigman, 2015), and is preferentially disrupted by chronic stress in male rats (Jett, Bulin, Hatherall, McCartney, & Morilak, 2017; Liston et al., 2006; McKlveen et al., 2016; Moench, Breach, & Wellman, 2020; Nikiforuk & Popik, 2011, 2013). Likewise, working memory is impaired after chronic stress (Hains et al., 2009; Kim et al., 2017; Mika et al., 2012; Mizoguchi et al., 2000). On the other hand, effects of acute stress on working memory are mixed, with reports of either facilitation (Yuen et al., 2009) or impairment (Devilbiss, Jenison, & Berridge, 2012), perhaps dependent on the timing of the acute stressor.
Effects of Chronic Stress on Structure and Function of the Prefrontal Cortex
Effects of Chronic Stress on the Medial Prefrontal Cortex: Morphology
The medial prefrontal cortex is a target for hormones involved in the stress response, such as the glucocorticoids corticosterone and cortisol (Meaney & Aitken, 1985). Stress-induced alterations in neuronal morphology are perhaps the best-documented effects of stress on the prefrontal cortex (Figure 2). In male rats, chronic restraint stress produces retraction of apical dendrites of pyramidal neurons in the prelimbic cortex (Cook & Wellman, 2004; Garrett & Wellman, 2009; Liston et al., 2006; Martin & Wellman, 2011; Moench & Wellman, 2017; Radley et al., 2005; Radley et al., 2006; Radley et al., 2004), an effect that is mimicked with chronic corticosterone administration (Cerqueira et al., 2005; Cerqueira, Taipa, Uylings, Almeida, & Sousa, 2007; Wellman, 2001). A similar pattern of stress-induced retraction is seen in apical dendritic branches of neurons within the infralimbic region of the medial prefrontal cortex (Izquierdo et al., 2006; Moench, Maroun, Kavushansky, & Wellman, 2016; Shansky, Hamo, Hof, McEwen, & Morrison, 2009). Even shorter, milder episodes of stress are sufficient to produce dendritic atrophy in the medial prefrontal cortex: 10 minutes of restraint stress for 10 days (Brown, Henning, & Wellman, 2005) or 3 weeks of vehicle injection alone (Wellman, 2001) reduce dendritic arborization within the medial prefrontal cortex, again with retraction occurring only in distal portions of the apical arbor. These findings suggest that the morphology of the medial prefrontal cortex is exquisitely sensitive to stress. Indeed, even acute stress, such as a single episode of forced swimming in mice (Izquierdo et al., 2006) or exposure to an elevated platform stressor in rats (Moench et al., 2016) produces reductions in apical dendritic branch length in the infralimbic cortex.
Figure 2.
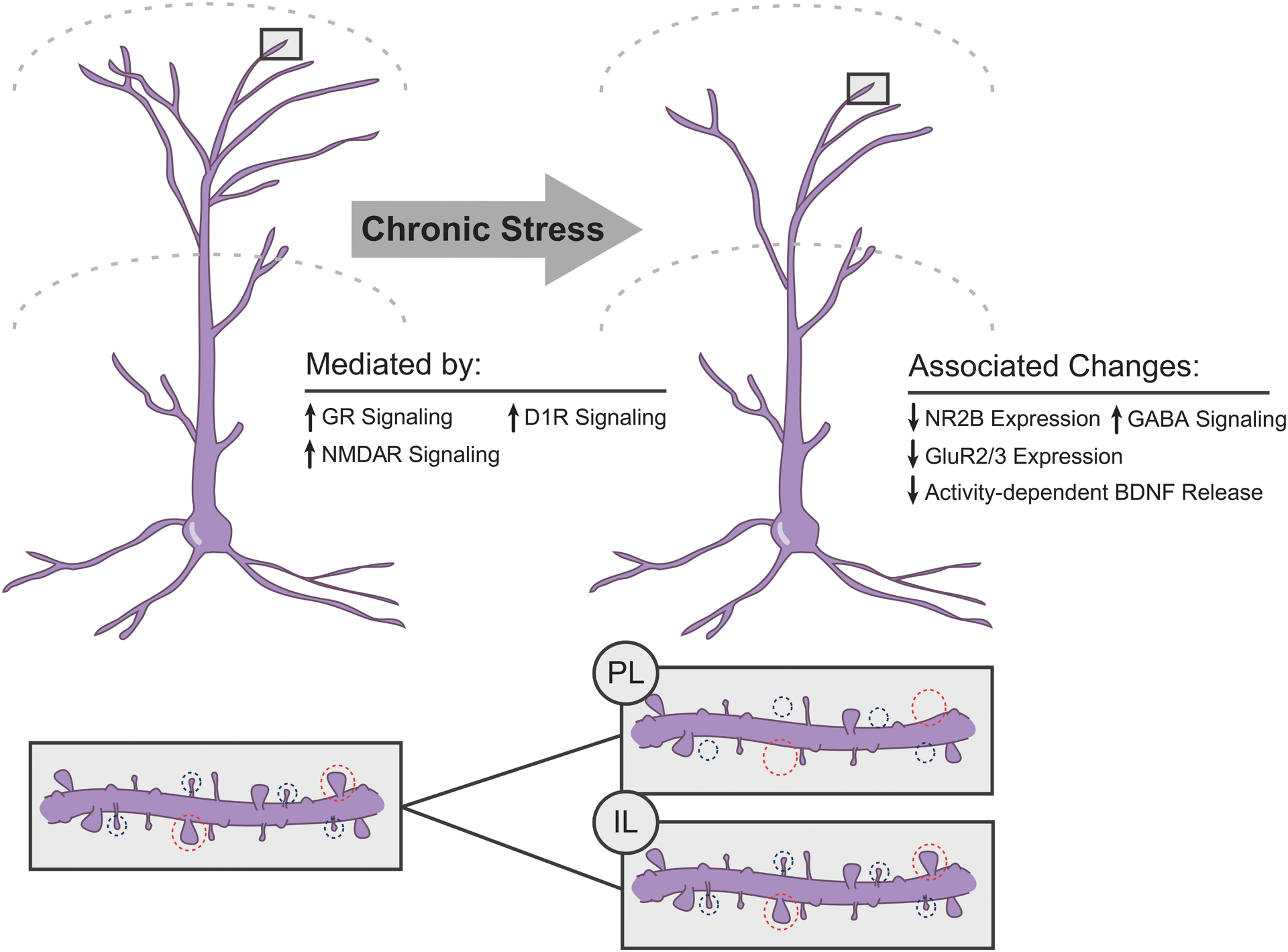
Summary of chronic stress-induced neuronal remodeling in medial prefrontal cortex of male rodents. Above. Chronic stress produces retraction of apical dendrites of pyramidal neurons in both prelimbic and infralimbic cortex. Blockade of either glucocorticoid (GR), NMDA (NMDAR), or dopaminergic D1 family receptors prevents the stress-induced dendritic remodeling. Below. Chronic stressors decrease spine density in prelimbic cortex and the ratio of mushroom (circled in blue, large heads) to thin spines (circled in red, small heads). The effect of stress on spine density in infralimbic cortex is less studied, but may be less robust.
Variations in dendritic structure are critical determinants of neuronal activity (e.g., Rall et al., 1992; Spruston, 2008). In addition, within a neuron, dendritic compartments (e.g., apical versus basilar, distal tufts versus proximal trunk; Gordon, Polsky, & Schiller, 2006; Han & Heinemann, 2013) are functionally distinct, with different inputs across dendritic compartments (e.g., Groenewegen, 1988; L. W. Swanson & Cowan, 1977; Urban-Ciecko & Barth, 2016). Thus, stress-induced structural alterations, with their specificity to the apical dendrite and their distinctive spatial distribution within the apical arbor, may have important functional implications. Consistent with this notion, in stressed rats, apical dendritic debranching and retraction in the infralimbic region of medial prefrontal cortex predict poorer extinction memory (Moench et al., 2016).
Dendritic spines are the major sites of excitatory inputs onto prefrontal pyramidal cells; thus, stress-induced alterations in dendritic spine density could also contribute to alterations in prefrontally-mediated behaviors. Indeed, stress- and corticosterone-induced dendritic retraction is coupled with a decrease in spine density in the anterior cingulate and prelimbic cortices (Barfield et al., 2017; Hains et al., 2009; Liu & Aghajanian, 2008; Radley et al., 2006; Radley et al., 2008; A. M. Swanson, Shapiro, Whyte, & Gourley, 2013). Importantly, this decrease in spine density correlates with impairment of working memory, a cognitive function mediated by dorsal medial prefrontal cortex, and prevention of the stress-induced spine loss via inhibition of protein kinase C prevents the working memory deficit (Hains et al., 2009).
Variations in spine morphology may also reflect differences in function. For instance, larger, mushroom-type spines are perhaps more mature, less labile, so-called “memory spines,” whereas thin spines are thought to be more plastic (Segal, 2017). Accordingly, numerous alterations in dendritic spine morphology after acute or chronic stress have been reported. For instance, a shift in spine morphology from large mushroom spines to smaller thin spines, and thus an overall reduction in spine volume and surface area, has been demonstrated in the dorsal medial prefrontal cortex following either three weeks of daily restraint stress (Barfield et al., 2017; Radley et al., 2008; though cf effects of chronic corticosterone administration) or two weeks of chronic variable stress (Radley, Anderson, Hamilton, Alcock, & Romig-Martin, 2013).
On the other hand, the effects of stress and glucocorticoids on dendritic spines in infralimbic cortex are less clear. In mice, administration of corticosterone via drinking water at a dose that produces restraint-stress levels of corticosterone in plasma significantly reduced dendritic spine density on apical branches of deep-layer pyramidal cells in the infralimbic cortex. However, chronic restraint stress (6 h/day for 3 weeks) did not significantly alter spine density in the infralimbic cortex of rats (Shansky et al., 2009). These disparate results may reflect sustained high levels of corticosterone in the former study versus habituation to restraint in the latter, which suggests that subregions of the medial prefrontal cortex may be differentially sensitive to stress. However, alterations in spine morphology or density accompanying sub-chronic or acute stress may nonetheless contribute to stress-induced deficits in extinction, as density of thin spines and estimates of total numbers of spines on apical terminal branches are strongly negatively correlated with freezing during extinction in rats exposed to one episode of elevated platform stress (Moench et al., 2016). Important areas for future research include understanding how subregion-specific stress-induced neuronal remodeling influences the physiology of the medial prefrontal cortex and related circuitry; and how these influences are expressed in prefrontally-mediated behaviors.
Effects of Chronic Stress on the Medial Prefrontal Cortex: Physiology
Stress-induced changes in the activity of the medial prefrontal cortex may underlie deficits in prefrontally-mediated behaviors. For instance, in male rats, the deficit in extinction retrieval induced by seven days of prior chronic restraint stress is accompanied by alterations in activity of infralimbic cortex neurons (Wilber et al., 2011): neurons in unstressed rats exhibited increased firing in response to the conditioned stimulus, whereas in stressed rats, this increase in firing was absent. In addition, chronic restraint stress impaired induction of long-term potentiation in the hippocampus-to-medial prefrontal cortex pathway (Cerqueira, Mailliet, Almeida, Jay, & Sousa, 2007). Given that hippocampal projections to the prefrontal cortex play a critical role in extinction learning (Garcia et al., 2008; Knapska et al., 2012; Knapska & Maren, 2009), this impaired plasticity could contribute to the stress-induced impairment of extinction (Garcia et al., 2008). Similarly, even an acute elevated platform stressor impairs the induction of long-term potentiation in the amygdala-to-medial prefrontal cortex pathway (Mouna Maroun & Richter-Levin, 2003), and application of the Single Prolonged Stressor model decreases functional connectivity of the ventral medial prefrontal cortex and the basolateral amygdala, as assessed with expression of the immediate early gene c-Jun during extinction retrieval (Knox et al., 2018). Finally, acute stress exposure during a working memory task altered the firing of prelimbic neurons, and this altered activity was associated with deficits on the task (Devilbiss et al., 2012). Taken together, these studies provide support for the notion that stress-induced alterations in the medial prefrontal cortex contribute to deficits in prefrontally-mediated behaviors.
Effects of Chronic Stress on Medial Prefrontal Cortex: Neurochemistry
Stress-induced changes in neuronal morphology and function are accompanied by pronounced alterations in the prefrontal glutamatergic system. For instance, two weeks of exposure to stress levels of corticosterone administered via drinking water markedly decreased expression of the NR2B subunit of the NMDA receptor and the GluR2 and 3 subunits of the AMPA receptor in the ventral medial prefrontal cortex (comprised of infralimbic cortex and ventral portions of prelimbic cortex), and this was associated with impaired extinction of contextual fear (Gourley, Kedves, Olausson, & Taylor, 2009). Likewise, seven days of daily restraint or variable stress altered expression of the NR1, NR2A, and NR2B subunits of the NMDA receptor and the GluR1 and GluR1 subunits of the AMPA receptor in adolescent (1-month-old) rats, and impaired AMPA- and NMDA-receptor mediated neuronal excitability in the medial prefrontal cortex (Wei et al., 2014; Yuen et al., 2012). Similar stress-induced decreases in glutamatergic receptor expression and transmission have been reported in the medial prefrontal cortex of adult rats and mice (Jett et al., 2017; Shepard & Coutellier, 2018). Given the well-documented role for NMDA receptor-dependent activity in the prefrontal cortex in prefrontally-mediated behaviors such as extinction (Burgos-Robles, Vidal-Gonzalez, Santini, & Quirk, 2007; Santini, Muller, & Quirk, 2001; Vieira et al., 2015), it is likely that this stress-induced dysfunction of the prefrontal glutamatergic system contributes to the stress-induced impairment of prefrontal behaviors.
Interestingly, changes in the glutamatergic system may be driven in part by stress-induced increases in GABAergic inhibition of pyramidal cells. Using patch-clamp recordings, Herman and colleagues (McKlveen et al., 2016) recently demonstrated that 2 weeks of chronic variable stress (rotation on a platform orbital shaker, warm swim, cold swim, cold exposure, brief hypoxia, overnight social isolation, overnight social crowding) produced increased miniature inhibitory postsynaptic currents in pyramidal neurons in infralimbic cortex, an effect that was blocked by the application of a GABAA receptor antagonist. This increased inhibition may have been due to increased GABAergic innervation of the pyramidal cells, as the number of Gad65-positive puncta on CAMKII-positive cells was markedly increased in rats subjected to chronic variable stress (McKlveen et al., 2016).
Long-Term Sequelae of Chronic Stress
Recent data suggest that chronic stress can have relatively long-lasting effects on morphology and neurochemistry in medial prefrontal cortex. For instance, dendritic remodeling in prelimbic cortex of chronically stressed male rats is highly dynamic during the post-stress rest period. Following a 7-day post-chronic stress rest period, chronically stressed male rats have apical dendritic outgrowth relative to unstressed males, despite initial chronic stress-induced dendritic retraction (Moench & Wellman, 2017). In this same time period, expression of the genes coding for the NR1 and GluR1 subunits of the NMDA and AMPA receptors, respectively, along with Gad67 and parvalbumin, is increased (Moench et al., 2020). It is interesting to speculate that the upregulation of glutamatergic genes may reflect the increase in dendritic material seen one week post-stress, while the upregulation of the GABAergic genes may be indicative of compensatory changes involved in maintaining an optimal excitation-inhibition balance in prelimbic cortex. Interestingly, this same study demonstrated a chronic stress-induced deficit in extradimensional set-shifting one day following stress, which was no longer present one week post-stress. This suggests that the delayed changes in gene expression may also play a role in the functional recovery of prelimbic cortex following the cessation of chronic stress (Moench et al., 2020). It is interesting to speculate that that these lasting changes may confer some resilience to subsequent stressors, as chronically stressed male rats have persistent reductions in novel stress-induced c-Fos expression in mPFC (Moench, Breach, & Wellman, 2019; Ostrander et al., 2009) and a blunted neuroendocrine response to a novel stress challenge (Ostrander et al., 2009).
Mechanisms of Chronic Stress-Induced Alterations in the Medial Prefrontal Cortex
A growing body of literature has begun to elucidate the mechanisms underlying stress-induced changes in structure and function in the medial prefrontal cortex. For instance, similar to stress, three weeks of daily injections of stress levels of corticosterone remodels apical dendrites of pyramidal neurons in the dorsal medial prefrontal cortex (Cerqueira et al., 2005; Wellman, 2001). Further, systemic administration of the glucocorticoid receptor blocker RU38486 during either chronic restraint (10 days) or chronic unpredictable stress (14 days) prevents stress-induced apical dendritic remodeling (Liu & Aghajanian, 2008) and spine loss (Horchar & Wohleb, 2019) in the medial prefrontal cortex. This is consistent with the finding that, in vitro, glucocorticoid receptors mediate the stress-induced decreases in functional glutamatergic receptors and impairment of glutamatergic transmission in medial prefrontal cortex (Yuen et al., 2012). However, it is not known whether, in vivo, glucocorticoid receptors contribute to stress-induced dendritic retraction via direct actions in prefrontal cortex, or by modulation of inputs to prefrontal cortex.
In addition, systemic administration of a competitive NMDA receptor blocker during daily restraint stress also prevents stress-induced dendritic retraction in medial prefrontal cortex (Martin & Wellman, 2011). Again, this could be due either to a direct effect in the medial prefrontal cortex or alteration of inputs to the medial prefrontal cortex. Although the aforementioned study did not assess spine density, a small literature implicates NMDARs and AMPA receptors (AMPARs) in chronic stress-induced loss of spines. For instance, a single dose of either the NMDAR blocker ketamine or the specific NMDAR 2B blocker Ro25–6981 following 21 days of chronic unpredictable mild stress (including cold, disruption of light-dark cycle, crowding, shaking, and exposure to an aversive odor) rescues spines, particularly in layer V of the prelimbic cortex (Li et al., 2011). This effect involved activation of the mTOR (mammalian target of rapamaycin) signaling pathway, as the beneficial effect of NMDAR blockade was prevented by administration of rapamycin. Additionally, NMDAR- and AMPAR-excitatory post-synaptic currents (EPSCs) are reduced following 5 or more days, but not 3 or fewer days, of repeated behavioral stressors (Li et al., 2011; Yuen et al., 2012). Further examination of the mechanisms underlying decreased activity at these glutamatergic receptors demonstrated that the reduction in EPSC amplitude is dependent on ubiquitin/proteasome degradation of GluR1 and NR1 subunits (Yuen et al., 2012). Given the profound and multifaceted influence of glucocorticoids and glucocorticoid receptors on glutamatergic transmission, including both classical and nongenomic effects on glutamate release and clearance as well as NMDAR and AMPAR responses (reviewed in Popoli, Yan, McEwen, & Sanacora, 2012), it is interesting to speculate that glutamatergic receptors on dendritic spines may be the final common pathway by which stress-induced increases in glucocorticoids alter both dendritic morphology in the medial prefrontal cortex and prefrontally-mediated behaviors.
Consistent with this notion, modulators of glutamatergic activity, such as dopaminergic signaling and brain derived neurotrophic factor (BDNF), are critical for several prefrontally-mediated behaviors and have also been implicated in stress-induced alterations in the medial prefrontal cortex. For instance, dopamine modulates NMDA receptor-mediated currents via D1 receptors (reviewed in Tritsch & Sabatini, 2012), and D1 receptors in the medial prefrontal cortex have been implicated in extinction (Fiorenza, Rosa, Izquierdo, & Myskiw, 2012; Hikind & Maroun, 2008), attentional set-shifting (Nikiforuk, 2012), and working memory (Vijayraghavan, Wang, Birnbaum, Williams, & Arnsten, 2007). Given the interactions between glucocorticoids and D1 receptors (reviewed in Sinclair, Purves-Tyson, Allen, & Weickert, 2014), it is not surprising that infusion of the D1 blocker SCH23390 into the medial prefrontal cortex prevents stress-induced dendritic retraction (G. L. Lin, Borders, Lundewall, & Wellman, 2015) and attenuates stress-facilitated, priming-induced drug seeking after extinction (Ball et al., 2018).
BDNF signaling is likewise critical for extinction (McGinty, Whitfield, & Berglind, 2010; Peters, Dieppa-Perea, Melendez, & Quirk, 2010; Rosas-Vidal, Do-Monte, Sotres-Bayon, & Quirk, 2014) and behavioral flexibility (Barfield & Gourley, 2017; Otis, Fitzgerald, & Mueller, 2014; Papaleo et al., 2011). However, although BDNF appears to play a key role in synaptic remodeling in hippocampus (An et al., 2008) and amygdala (Govindarajan et al., 2006) following stress, evidence for its role in the medial prefrontal cortex is mixed, with some reports of stress-induced downregulation of BDNF mRNA (reviewed in Calabrese, Molteni, Racagni, & Riva, 2009), and other studies failing to find stress-induced changes (Chiba et al., 2012; Y. Lin et al., 2009). On the other hand, infusion of BDNF into the infralimbic cortex can reverse some of the behavioral effects of stress (Graybeal et al., 2011). Further, Yu and colleagues (2012) demonstrated that 7 days of chronic restraint stress reduced spine density in the medial prefrontal cortex of Val66Met knock-in mice, in which activity-dependent release of BDNF is reduced, compared to stressed wild type (WT) mice. Interestingly, although the behavioral and neuroendocrine profile of these knock-in mice was comparable to that of WT prior to stress exposure, spine density was not different in unstressed WT versus knock-in mice. Nonetheless, stress-induced increases in plasma corticosterone and adrenocorticotropic hormone (ACTH) were greater in knock-in mice relative to WT. Thus, low basal levels of BDNF are not sufficient to decrease spine density, but could contribute to the development of a heightened response to stress, leading to downstream effects on spinogenesis. This finding illustrates the importance of considering gene × environment interactions in the pathophysiology of stress-sensitive disorders. In addition, administration of ketamine, an NMDA antagonist, did not ameliorate the effects of BDNF deficiency in Val66Met knock-in mice, but does ameliorate stress-induced prefrontal spine loss in WT mice and rats, indicating that BDNF plays a crucial role in synaptogenesis in the medial prefrontal cortex. Liu and colleagues (2012) suggest a mechanism by which glucocorticoids, NMDARs, and BDNF interact to mediate synaptogenesis: 1) ketamine transiently increases the presynaptic release of glutamate, leading to a burst of action potentials at the synapse; 2) this bursting increases AMPAR stimulation, facilitating the release of BDNF; 3) BDNF binds to its receptor (TrkB), activating mTOR, which has the downstream effect of the translation of synaptic proteins, thus leading to synaptogenesis (Liu et al., 2012). Therefore, if BDNF is down-regulated as in Val66Met knock-in mice or by chronic stress, the downstream events of ketamine administration or natural rises in glutamate will be blocked, resulting in either synaptic stasis or potentially, synaptic pruning in cases where excess levels of glucocorticoids are present (see Figure 3). This synaptic pruning in turn could contribute to stress-induced deficits in extinction.
Figure 3.
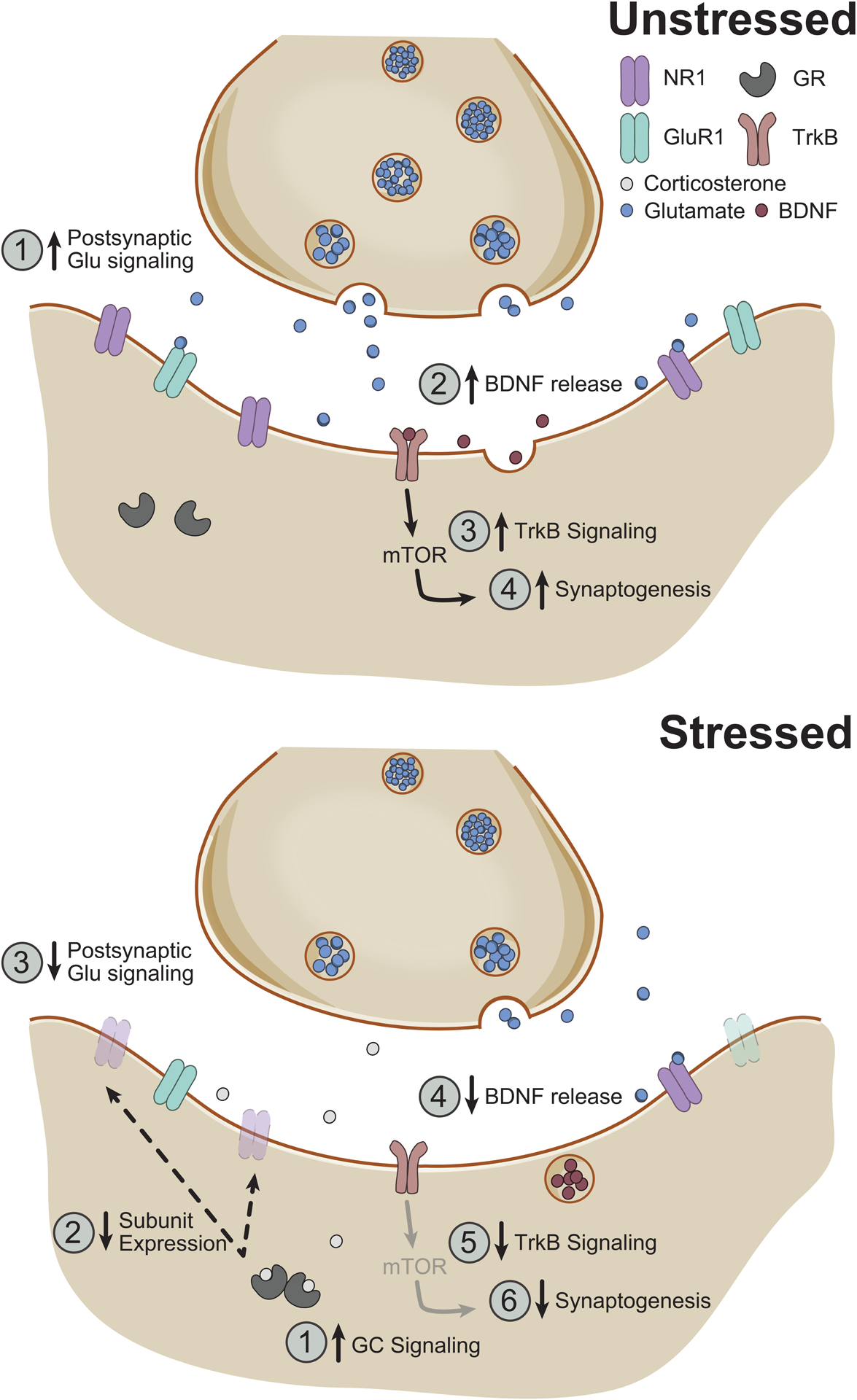
Possible neurochemical/molecular pathways for stress-induced synaptic remodeling in medial prefrontal cortex in males. Above. In an unstressed rodent, increases in postsynaptic glutamatergic signaling (1) are associated with release of brain-derived neurotrophic factor (BDNF) (2); BDNF’s activity at its TrkB receptor (3) results in increased synaptogenesis via a mammalian target of rapamaycin (mTOR)-dependent pathway (4). Below. During chronic stress, increased glucocorticoid signaling (1) results in loss of functional NMDA and AMPA receptors (2), which decreases postsynaptic glutamatergic signaling (3). Concurrent reduction in BDNF release (4) in medial prefrontal cortex decreases TrkB signaling (5), resulting in decreased synaptogenesis (6). NR1, NR1 subunit of the NMDA receptor; GluR1, GluR1 subunit of the AMPA receptor; GR, glucocorticoid receptor.
Interestingly, emerging evidence suggests a novel potential mechanism for the effects of stress on prefrontal structure and function in males: activation of microglia. Microglia, the immunocompetent cells of the brain, can sculpt neuronal morphology through the release of neurotrophic factors, cytokines, and chemokines, engagement with complement proteins, and direct microglia-neuron contact. These cells can modulate neuronal activity, prune synapses, stimulate spine outgrowth, and alter behavioral function (reviewed in Salter & Beggs, 2014; Salter & Stevens, 2017). Chronic stress increases microglial cell density and heightens microglia-neuron interaction, as reflected in cellular morphology and reciprocal increases in neuronal colony stimulating factor 1 (CSF1) and microglial CSF1R expression in the medial prefrontal cortex (Bollinger, Bergeon Burns, & Wellman, 2016; Bollinger, Salinas, Fender, Sengelaub, & Wellman, 2019; Walker, Nilsson, & Jones, 2013; Wohleb, Terwilliger, Duman, & Duman, 2018). These changes in microglial activation are associated with deficits in spatial working memory and temporal object recognition (Hinwood, Morandini, Day, & Walker, 2012; Horchar & Wohleb, 2019; Wohleb et al., 2018). Moreover, recent findings indicate that chronic unpredictable stress increases microglial engagement with dendritic spines and phagocytosis of dendritic elements, while decreasing apical dendritic spine density in the medial prefrontal cortex. Importantly, local knockdown of CSF1 prevented these effects (Wohleb et al., 2018), suggesting that neuron-microglia signaling may orchestrate stress-induced dendritic remodeling. In line with prior findings, glucocorticoids appear to initiate these interactions: blocking glucocorticoid receptors with daily injections of RU38486 prevents chronic stress-induced, microglia-mediated dendritic remodeling in the medial prefrontal cortex (Horchar & Wohleb, 2019).
Sex Differences in the Effect of Stress on the Medial Prefrontal Cortex
Despite dramatic sex differences in the rates and expression of stress-related psychological disorders (Cover, Maeng, Lebrón-Milad, & Milad, 2014), the vast majority of the research on the neurobiological mechanisms underlying stress effects on emotional behavior has focused on males, as have the preceding sections of this chapter. Investigations of the mechanisms underlying potential stress-induced plasticity of corticolimbic structures in females are critical for providing the groundwork necessary to develop sex-specific treatment for stress-related psychopathology. A small number of studies have begun to address this issue, and have demonstrated that stress does in fact produce very different effects on the structure and function of the prefrontal cortex. For example, while chronic stress impairs temporal object recognition and fear extinction in male rats, similar deficits are not observed in female rats (Baran et al., 2009; Hoffman, Armstrong, Hanna, & Conrad, 2010; Wei et al., 2014). Likewise, chronic stress impairs attentional set-shifting in male but not female rats (Moench et al., 2020). Further, whereas male rats exhibit dendritic retraction in medial prefrontal cortex following stress, female rats either exhibit no dendritic changes or may even exhibit dendritic hypertrophy (Figure 4; Garrett & Wellman, 2009; Moench & Wellman, 2017; Shansky et al., 2010).
Figure 4.

Sex differences in stress effects on dendritic morphology and recovery in the medial prefrontal cortex. Pyramidal neurons in the medial prefrontal cortex have more complex apical dendritic arbors in unstressed male rats compared to unstressed females. Following 10 days of chronic restraint stress (0 D), males show robust reductions in dendritic arborization, whereas females show either no change in arborization or dendritic outgrowth. Males exhibit dendritic outgrowth at 7 days post-stress (7 D), with dendritic complexity returning to baseline by 10 days post-stress (10 D). Females show baseline dendritic complexity at both 7- and 10-days post-stress. Together, these findings indicate sex differences in dendritic arborization in the medial prefrontal cortex, alongside sex-specific stress effects on dendritic complexity in this region. The physiological and behavioral consequences of these sex-dependent patterns remain largely unexplored.
In males, chronic stress results in a downregulation of the NMDA receptor subunits NR1, NR2A, and NR2B (Lee & Goto, 2011; Shepard & Coutellier, 2018). These changes are associated with deficits in temporal order working memory in adolescent rats (Wei et al., 2014). Downregulation of the AMPA receptor subunit GluR1 also has also been reported following chronic stress and is associated with depressive-like behaviors in male rats (Li et al., 2011). Thus, reduced glutamatergic transmission likely plays an important role in stress-induced deficits in behaviors that are mediated by the medial prefrontal cortex. Notably, the effect of stress on glutamatergic neurotransmission appears to be more pronounced in males than females (Wei et al., 2014).
In contrast to the effects of chronic stress on glutamatergic neurotransmission, recent studies have suggested that chronic stress-induced changes in GABAergic neurotransmission may be more pronounced in the medial prefrontal cortex of females. For instance, female mice exposed to 2 weeks of chronic unpredictable mild stress have an increase in the expression of parvalbumin mRNA in the medial prefrontal cortex, which corresponded to a decrease in overall neuronal activation as measured by c-Fos expression (Shepard, Page, & Coutellier, 2016). Further, chronically stressed female mice have greater glutamatergic transmission onto parvalbumin-expressing neurons in the medial prefrontal cortex (Shepard & Coutellier, 2018). Thus, inhibitory tone in the medial prefrontal cortex may be enhanced in chronically stressed female mice, which may contribute to deficits on an object-context mismatch test (Shepard & Coutellier, 2018; Shepard et al., 2016). Together, these studies suggest that chronic stress-induced changes in the prefrontal GABAergic system may be pronounced in females, while changes in glutamatergic neurotransmission may be pronounced in males. This pattern likely contributes to sex differences in performance on tasks mediated by the medial prefrontal cortex following chronic stress.
Differential activation of microglia may contribute to these sex-dependent effects of stress on the prefrontal cortex and behavior. For instance, acute restraint stress induced microglial morphological activation in medial prefrontal cortex in males, but deactivation in females (Bollinger et al., 2016). Microglial morphology returned to baseline following 10 days of restraint in males, whereas microglial deactivation persisted in females (Bollinger et al., 2016). These findings demonstrate both opposite effects of stress on microglia activation in males and females and differing temporal patterns of stress-induced microglial remodeling. Consistent with the notion that these differences may contribute to sex-dependent stress effects on neuronal morphology, chronic unpredictable stress induced microglia-neuron interaction and synaptic pruning in medial prefrontal cortex in males, but not females (Wohleb et al., 2018).
Interestingly, the lasting effects of chronic stress are also sex-dependent. For instance, while male rats undergo dynamic dendritic remodeling in the week following the cessation of chronic restraint stress, females do not (Moench & Wellman, 2017). Such differences may produce functional differences in subsequent responses to stress. For example, there are sex differences in neuronal activation across a number of corticolimbic brain regions in chronically stressed rats that are exposed to a novel stress challenge (Moench et al., 2019). Notably, males have a persistent reduction in novel stress-induced neuronal activation in prelimbic cortex. In contrast, prior chronic stress does not modulate neuronal activation in prelimbic cortex in response to a novel stress challenge in females (Moench et al., 2019). Consistent with this, while parvalbumin mRNA expression was increased in males one week post chronic stress and sustained through a novel stress challenge, this novel stress challenge reduced parvalbumin expression in females. Together, these data suggest that males may have enhanced inhibitory signaling in prelimbic cortex following a novel stress challenge that was not present immediately following chronic stress. In contrast, while females appear to have greater inhibitory tone after chronic stress, this inhibition may be reduced following a novel acute stress challenge, resulting in behavioral deficits that are not found in males (Moench et al., 2020). These differential responses in the medial prefrontal cortex coincide with sex-dependent effects of the subsequent novel stressor on attentional set-shifting, with females but not males showing deficits in extradimensional set-shifting (Moench et al., 2020).
Further systematic investigation of the phenomenology, functional implications, and mechanisms underlying the immediate and longer-term sex-dependent effects of stress is critical for developing sex-specific treatments for stress-sensitive psychopathologies.
Conclusions
Extensive evidence gathered from animal models demonstrates that prior stress—either chronic or acute—alters the prefrontal cortex and behaviors mediated by it. Indeed, in males, these behavioral changes correlate nicely with alterations in prefrontal structure and function. However, much work remains to be done, both at the level of neurochemical mechanisms and localization of these mechanisms to specific structures, to directly test the hypothesis that these changes mediate stress-induced changes in prefrontally-dependent behaviors. Ultimately, a full understanding of the neural mechanisms underlying stress-induced impairments in prefrontal function will require investigations at the circuit level of analysis, to account for differential and opposing effects of stress on the neural circuitry underlying these complex behaviors (Wellman & Moench, 2019). Finally, investigation of the neural basis for individual differences, especially sex differences in vulnerability to stress-induced alterations in extinction and behavioral flexibility, will be instrumental in identifying avenues for novel interventions for stress-sensitive disorders characterized by alterations in prefrontally-mediated behaviors.
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- An JJ, Gharami K, Liao G-Y, Woo NH, Lau AG, Vanevski F, … Xu B (2008). Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell, 134(1), 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Stone E, Best O, Collins T, Edson H, Hagan E, … Woodlen K (2018). Chronic restraint stress during withdrawal increases vulnerability to drug priming-induced cocaine seeking via a dopamine D1-like receptor-mediated mechanism. Drug and Alcohol Dependence, 187, 327–334. doi: 10.1016/j.drugalcdep.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, & Conrad CD (2009). Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of Learning and Memory, 91(3), 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield ET, Gerber KJ, Zimmermann KS, Ressler KJ, Parsons RG, & Gourley SL (2017). Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure. PLoS biology, 15(11). doi:ARTN e2003000 10.1371/journal.pbio.2003000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield ET, & Gourley SL (2017). Adolescent Corticosterone and TrkB Pharmaco-Manipulations Sex-Dependently Impact Instrumental Reversal Learning Later in Life. Frontiers in Behavioral Neuroscience, 11. doi:ARTN 237 10.3389/fnbeh.2017.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, & Brown VJ (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci, 20(11), 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Bergeon Burns CM, & Wellman CL (2016). Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain, Behavior, and Immunity, 52, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Salinas I, Fender E, Sengelaub DR, & Wellman CL (2019). Gonadal hormones differentially regulate sex-specific stress effects on glia in the medial prefrontal cortex. Journal of Neuroendocrinology, 31(8), e12762. doi: 10.1111/jne.12762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Henning S, & Wellman CL (2005). Short-term, mild stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex, 15, 1714–1722. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, & Quirk GJ (2007). Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron, 53(6), 871–880. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, & Riva MA (2009). Neuronal plasticity: A link between stress and mood disorders. Psychoneuroendocrinology, 34, Supplement 1(0), S208–S216. doi: 10.1016/j.psyneuen.2009.05.014 [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, & Sousa N (2007). The prefrontal cortex as a key target of the maladaptive response to stress. Journal of Neuroscience, 27(11), 2781–2787. doi: 10.1523/jneurosci.4372-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OFX, & Sousa N (2005). Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. Journal of Neuroscience, 25(34), 7792–7800. doi: 10.1523/jneurosci.1598-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HBM, Almeida OFX, & Sousa N (2007). Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cerebral Cortex, 17(9), 1998–2006. [DOI] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, & Kunugi H (2012). Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 39, 112–119. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Ortiz JB, & Judd JM (2017). Chronic stress and hippocampal dendritic complexity: Methodological and functional considerations. Physiology & Behavior, 178, 66–81. doi: 10.1016/j.physbeh.2016.11.017 [DOI] [PubMed] [Google Scholar]
- Cook SC, & Wellman CL (2004). Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology, 60, 236–248. [DOI] [PubMed] [Google Scholar]
- Cover KK, Maeng LY, Lebrón-Milad K, & Milad MR (2014). Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Translational Psychiatry, 4, e422. doi: 10.1038/tp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Jenison RL, & Berridge CW (2012). Stress-Induced Impairment of a Working Memory Task: Role of Spiking Rate and Spiking History Predicted Discharge. PLoS Comput Biol, 8(9), e1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza NG, Rosa J, Izquierdo I, & Myskiw JC (2012). Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behavioural Brain Research, 232(1), 210–216. doi: 10.1016/j.bbr.2012.04.015 [DOI] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau J-L, & Deschaux O (2008). Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiology of Learning & Memory, 89(4), 560–566. [DOI] [PubMed] [Google Scholar]
- Garrett JE, & Wellman CL (2009). Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience, 162, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Bortolato M, Richards SE, Li FG, Chen K, Wellman CL, & Shih JC (2015). Monoamine oxidase A is required for rapid dendritic remodeling in response to stress. International Journal of Neuropsychopharmacology, 18(9), 1–12. doi:http://dx.doi.org.proxy.ulib.uits.iu.edu/10.1093/ijnp/pyv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon U, Polsky A, & Schiller J (2006). Plasticity compartments in basal dendrites of neocortical pyramidal neurons. Journal of Neuroscience, 26(49), 12717–12726. doi: 10.1523/Jneurosci.3502-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Cascardi M, Rodriguez-Sierra OE, Duvarci S, & Pare D (2010). Impact of predatory threat on fear extinction in Lewis rats. Learning & Memory, 17(10), 494–501. doi: 10.1101/lm.1948910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, & Taylor JR (2009). A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology, 34(3), 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, & Koleske AJ (2013). Corticosteroid-Induced Neural Remodeling Predicts Behavioral Vulnerability and Resilience. The Journal of Neuroscience, 33(7), 3107–3112. doi: 10.1523/jneurosci.2138-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, & Chattarji S (2006). Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proceedings of the National Academy of Science, 103, 13208–13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, & Holmes A (2011). Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nature Neuroscience, 14(12), 1507–1509. doi: 10.1038/nn.2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ (1988). Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience, 24(2), 379–431. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MAT, Maciejewski PK, van Dyck CH, Gottron M, & Arnsten AFT (2009). Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proceedings of the National Academy of Sciences of the United States of America, 106(42), 17957–17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, & Brigman JL (2015). Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Genes Brain and Behavior, 14, 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EB, & Heinemann SF (2013). Distal Dendritic Inputs Control Neuronal Activity by Heterosynaptic Potentiation of Proximal Inputs. The Journal of Neuroscience, 33(4), 1314–1325. doi: 10.1523/jneurosci.3219-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikind M, & Maroun M (2008). Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiology of Learning and Memory, 90, 217–222. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, & Walker FR (2012). Evidence that Microglia Mediate the Neurobiological Effects of Chronic Psychological Stress on the Medial Prefrontal Cortex. Cerebral Cortex, 22(6), 1442–1454. doi: 10.1093/cercor/bhr229 [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Armstrong CE, Hanna JJ, & Conrad CD (2010). Chronic stress, cyclic 17β-estradiol, and daily handling influences on fear conditioning in the female rat. Neurobiology of Learning & Memory, 94, 422–433. [DOI] [PubMed] [Google Scholar]
- Holmes A, & Wellman CL (2009). Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and Biobehavioral Reviews, 33, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horchar MJ, & Wohleb ES (2019). Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress. Brain, Behavior, and Immunity, 81, 329–340. [DOI] [PubMed] [Google Scholar]
- Hurtubise JL, & Howland JG (2017). Effects of stress on behavioral flexibility in rodents. Neuroscience, 345, 176–192. doi: 10.1016/j.neuroscience.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, & Holmes A (2006). Brief Uncontrollable Stress Causes Dendritic Retraction in Infralimbic Cortex and Resistance to Fear Extinction in Mice. Journal of Neuroscience, 26(21), 5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett JD, Bulin SE, Hatherall LC, McCartney CM, & Morilak DA (2017). Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience, 346, 284–297. doi: 10.1016/j.neuroscience.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, & Churchwell JC (2011). An analysis of rat prefrontal cortex in mediating executive function. Neurobiology of Learning and Memory, 96(3), 417–431. [DOI] [PubMed] [Google Scholar]
- Kim DJ, St Louis N, Molaro RA, Hudson GT, Chorley RC, & Anderson BJ (2017). Repeated unpredictable threats without harm impair spatial working memory in the Barnes maze. Neurobiology of Learning and Memory, 137, 92–100. doi: 10.1016/j.nlm.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, … Kaczmarek L (2012). Functional anatomy of neural circuits regulating fear and extinction. Proceedings of the National Academy of Sciences of the United States of America, 109(42), 17093–17098. doi: 10.1073/pnas.1202087109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, & Maren S (2009). Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learning & Memory, 16(8), 486–493. doi: 10.1101/lm.1463909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, & Liberzon I (2012). Single prolonged stress disrupts retention of extinguished fear in rats. Learning & Memory, 19(2), 43–49. doi: 10.1101/lm.024356.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, & Liberzon I (2012). Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience, 223, 163–173. [DOI] [PubMed] [Google Scholar]
- Knox D, Perrine SA, George SA, Galloway MP, & Liberzon I (2010). Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neuroscience Letters, 480(1), 16–20. doi: 10.1016/j.neulet.2010.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Stanfield BR, Staib JM, David NP, DePietro T, Chamness M, … Lawless C (2018). Using c-Jun to identify fear extinction learning-specific patterns of neural activity that are affected by single prolonged stress. Behavioural Brain Research, 341, 189–197. doi: 10.1016/j.bbr.2017.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechin F, Van der Dijs B, & Benaim M (1996). Stress versus depression. Progress in neuro-psychopharmacology & biological psychiatry, 20(6), 899–950. [DOI] [PubMed] [Google Scholar]
- Lee Y-A, & Goto Y (2011). Chronic stress modulation of prefrontal cortical NMDA receptor expression disrupts limbic structure–prefrontal cortex interaction. European Journal of Neuroscience, 34(3), 426–436. doi:doi: 10.1111/j.1460-9568.2011.07750.x [DOI] [PubMed] [Google Scholar]
- Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, … Duman RS (2011). Glutamate N-methyl-D-aspartate Receptor Antagonists Rapidly Reverse Behavioral and Synaptic Deficits Caused by Chronic Stress Exposure. Biological Psychiatry, 59, 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin GL, Borders CB, Lundewall LJ, & Wellman CL (2015). D1 receptors regulate dendritic morphology in normal and stressed prelimbic cortex. Psychoneuroendocrinology, 51, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu AH, Li XJ, & Westenbroek C (2009). Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cerebral Cortex, 19(9), 1978–1989. doi: 10.1093/cercor/bhn225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, & Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America, 106(3), 912–917. doi: 10.1073/pnas.0807041106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, … McEwen BS (2006). Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience, 26(30), 7870–7874. doi: 10.1523/jneurosci.1184-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R-J, & Aghajanian GK (2008). Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proceedings of the National Academy of Sciences, 105(1), 359–364. doi: 10.1073/pnas.0706679105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R-J, Lee FS, Li X-Y, Bambico F, Duman RS, & Aghajanian GK (2012). Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biological Psychiatry, 71(11), 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, & Holmes A (2016). Stress and fear extinction. Neuropsychopharmacology Reviews, 41, 58–79. doi: 10.1038/npp.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Ioannides PJ, Bergman KL, Kavushansky A, Holmes A, & Wellman CL (2013). Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. European Journal of Neuroscience, 38, 2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, & Richter-Levin G (2003). Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. Journal of Neuroscience, 23(11), 4406–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KP, & Wellman CL (2011). NMDA Receptor Blockade Alters Stress-Induced Dendritic Remodeling in Medial Prefrontal Cortex. Cerebral Cortex, 21(10), 2366–2373. doi: 10.1093/cercor/bhr021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2017). Allostasis and the epigenetics of brain and body health over the life course: The brain on stress. JAMA Psychiatry, 74(6), 551–552. doi: 10.1001/jamapsychiatry.2017.0270 [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, & Berglind WJ (2010). Brain-derived neurotrophic factor and cocaine addiction. Brain Research, 1314, 183–193. doi: 10.1016/j.brainres.2009.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, … Herman JP (2016). Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biological Psychiatry, 80(10), 754–764. doi: 10.1016/j.biopsych.2016.03.2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, & Herman JP (2015). The Medial Prefrontal Cortex: Coordinator of Autonomic, Neuroendocrine and Behavioural Responses to Stress. Journal of Neuroendocrinology, 27(6), 446–456. doi: 10.1111/jne.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, & Aitken DH (1985). [3H]Dexamethasone binding in rat frontal cortex. Brain research, 328(1), 176–180. doi:0006–8993(85)91340-X [pii] [DOI] [PubMed] [Google Scholar]
- Mika A, Mazur GJ, Hoffman AN, Talboom JS, Bimonte-Nelson HA, Sanabria F, & Conrad CD (2012). Chronic stress impairs prefrontal cortex-dependent response inhibition and spatial working memory. Behavioral Neuroscience, 126(5), 605–619. doi: 10.1037/a0029642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, & Wellman CL (2006). Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of Learning & Memory, 85, 213–218. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui D-H, & Tabira T (2000). Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. Journal of Neuroscience, 20(4), 1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench KM, Breach MR, & Wellman CL (2019). Chronic stress produces enduring sex- and region-specific alterations in novel stress-induced c-Fos expression. Neurobiology of Stress, 10, 100147. doi: 10.1016/j.ynstr.2019.10014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench KM, Breach MR, & Wellman CL (2020). Prior stress followed by a novel stress challenge results in sex-specific deficits in behavioral flexibility and changes in gene expression in rat medial prefrontal cortex. Hormones and Behavior, 117, 104615. doi: 10.1016/j.yhbeh.2019.104615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench KM, Maroun M, Kavushansky A, & Wellman CL (2016). Alterations in neuronal morphology in infralimbic cortex predict resistance to fear extinction following acute stress. Neurobiology of Stress, 3, 23–33. doi: 10.1016/j.ynstr.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench KM, & Wellman CL (2017). Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neuroscience, 357, 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A (2012). Dopamine D1 receptor modulation of set shifting: the role of stress exposure. Behavioural Pharmacology, 23(4), 434–438. doi: 10.1097/FBP.0b013e328356522f [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, & Popik P (2011). Long-lasting cognitive deficit induced by stress is alleviated by acute administration of antidepressants. Psychoneuroendocrinology, 36(1), 28–39. doi: 10.1016/j.psyneuen.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, & Popik P (2013). Neurochemical modulation of stress-induced cognitive inflexibility in a rat model of an attentional set-shifting task. Pharmacological Reports, 65(6), 1479–1488. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Flak JN, Richtand NM, & Herman JP (2009). Chronic stress produces enduring decreases in novel stress-evoked c-fos mRNA expression in discrete brain regions of the rat. Stress-the International Journal on the Biology of Stress, 12(6), 469–477. doi: 10.3109/10253890802641966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Fitzgerald MK, & Mueller D (2014). Infralimbic BDNF/TrkB Enhancement of GluN2B Currents Facilitates Extinction of a Cocaine-Conditioned Place Preference. Journal of Neuroscience, 34(17), 6057–6064. doi: 10.1523/Jneurosci.4980-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Silverman JL, Aney J, Tian Q, Barkan CL, Chadman KK, & Crawley JN (2011). Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learning & Memory, 18(8), 534–544. doi: 10.1101/lm.2213711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (1998). The rat brain in stereotaxic coordinates (4 ed.). New York: Academic Press. [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, & Quirk GJ (2010). Induction of fear extinction with hippocampal-infralimbic BDNF. Science, 328(5983), 1288–1290. doi: 10.1126/science.1186909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, & Sanacora G (2012). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience, 13(1), 22–37. doi:http://www.nature.com/nrn/journal/v13/n1/suppinfo/nrn3138_S1.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Hamilton BA, Alcock JA, & Romig-Martin SA (2013). Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33(36), 14379–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, & Morrison JH (2005). Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol, 196(1), 199–203. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, … Morrison JH (2006). Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex, 16(3), 313–320. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, … Hof PR (2008). Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. The Journal of Comparative Neurology, 507(1), 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, … Morrison JH (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience, 125(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, & Segev I (1992). Matching dendritic neuron models of experimental data. Physiological Reviews, 72, S159–S186. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, … Merikangas KR (2009). Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression A Meta-analysis. Jama-Journal of the American Medical Association, 301(23), 2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, & Quirk GJ (2014). Hippocampal-Prefrontal BDNF and Memory for Fear Extinction. Neuropsychopharmacology, 39(9), 2161–2169. doi: 10.1038/npp.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, & Beggs S (2014). Sublime microglia: expanding roles for the guardians of the CNS. Cell, 58, 15–24. [DOI] [PubMed] [Google Scholar]
- Salter MW, & Stevens B (2017). Microglia emerge as central players in brain disease. Nature Medicine, 23, 1018. doi: 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, & Quirk GJ (2001). Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. Journal of Neuroscience, 21(22), 9009–9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM (2003). Stress and plasticity in the limbic system. Neurochemical Research, 28(11), 1735–1742. [DOI] [PubMed] [Google Scholar]
- Segal M (2017). Dendritic spines: Morphological building blocks of memory. Neurobiology of Learning and Memory, 138, 3–9. doi: 10.1016/j.nlm.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, & Morrison JH (2010). Estrogen promotes stress sensitivity in a prefrontal cortex–amygdala pathway. Cerebral Cortex, 20(11), 2560–2567. doi: 10.1093/cercor/bhq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, & Morrison JH (2009). Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral Cortex, 19(10), 2479–2484. doi: 10.1093/cercor/bhp003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard R, & Coutellier L (2018). Changes in the Prefrontal Glutamatergic and Parvalbumin Systems of Mice Exposed to Unpredictable Chronic Stress. Molecular Neurobiology, 55(3), 2591–2602. [DOI] [PubMed] [Google Scholar]
- Shepard R, Page CE, & Coutellier L (2016). Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience, 332, 1–12. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Purves-Tyson T, Allen K, & Weickert C (2014). Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology, 231(8), 1581–1599. doi: 10.1007/s00213-013-3415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N (2008). Pyramidal neurons: dendritic structure and synaptic integration. Nature reviews. Neuroscience, 9(3), 206–221. doi: 10.1038/nrn2286 [DOI] [PubMed] [Google Scholar]
- Swanson AM, Shapiro LP, Whyte AJ, & Gourley SL (2013). Glucocorticoid receptor regulation of action selection and prefrontal cortical dendritic spines. Communicative & Integrative Biology, 6(6), e26068. doi: 10.4161/cib.26068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, & Cowan WM (1977). An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. Journal of Comparative Neurology, 172(1), 49–84. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, & Sabatini BL (2012). Dopaminergic Modulation of Synaptic Transmission in Cortex and Striatum. Neuron, 76(1), 33–50. doi: 10.1016/j.neuron.2012.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, & Lloyd DA (2004). Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Archives of general psychiatry, 61(5), 481–488. doi: 10.1001/archpsyc.61.5.481 [DOI] [PubMed] [Google Scholar]
- Urban-Ciecko J, & Barth AL (2016). Somatostatin-expressing neurons in cortical networks. Nature Reviews Neuroscience, 17(7), 401–409. doi: 10.1038/nrn.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira PA, Corches A, Lovelace JW, Westbrook KB, Mendoza M, & Korzus E (2015). Prefrontal NMDA receptors expressed in excitatory neurons control fear discrimination and fear extinction. Neurobiology of Learning and Memory, 119(0), 52–62. doi: 10.1016/j.nlm.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, & Arnsten AFT (2007). Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience, 10(3), 376–384. doi:http://www.nature.com/neuro/journal/v10/n3/suppinfo/nn1846_S1.html [DOI] [PubMed] [Google Scholar]
- Walker FR, Nilsson M, & Jones K (2013). Acute and Chronic Stress-Induced Disturbances of Microglial Plasticity, Phenotype and Function. Current Drug Targets, 14(11), 1262–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, … Yan Z (2014). Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry, 19(5), 588–598. doi: 10.1038/mp.2013.83 [DOI] [PubMed] [Google Scholar]
- Wellman CL (2001). Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology, 49(3), 245–253. [DOI] [PubMed] [Google Scholar]
- Wellman CL, & Moench KM (2019). Preclinical studies of stress, extinction, and prefrontal cortex: intriguing leads and pressing questions. Psychopharmacology, 236, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, & Wellman CL (2011). Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience, 174, 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Terwilliger R, Duman CH, & Duman RS (2018). Stress-induced neuronal CSF1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biological Psychiatry, 83, 38–49. doi: 10.1016/j.biopsych.2017.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang D-D, Wang Y, Liu T, Lee FS, & Chen Z-Y (2012). Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32(12), 4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, & Yan Z (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America, 106(33), 14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, & Yan Z (2012). Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron, 73, 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]


