Abstract
Background:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), an entity in children initially characterized by milder case presentations and better prognoses as compared with adults. Recent reports, however, raise concern for a new hyperinflammatory entity in a subset of pediatric COVID-19 patients.
Methods:
We report a fatal case of confirmed COVID-19 with hyperinflammatory features concerning for both multi-inflammatory syndrome in children (MIS-C) and primary COVID-19.
Results:
This case highlights the ambiguity in distinguishing between these two entities in a subset of pediatric patients with COVID-19-related disease and the rapid decompensation these patients may experience.
Conclusions:
Appropriate clinical suspicion is necessary for both acute disease and MIS-C. SARS-CoV-2 serologic tests obtained early in the diagnostic process may help to narrow down the differential but does not distinguish between acute COVID-19 and MIS-C. Better understanding of the hyperinflammatory changes associated with MIS-C and acute COVID-19 in children will help delineate the roles for therapies, particularly if there is a hybrid phenotype occurring in adolescents.
Keywords: coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, inflammatory syndrome, pediatric, adolescent, multi-inflammatory syndrome in children
Children account for 1%–5% of diagnosed coronavirus disease 2019 (COVID-19) cases worldwide.1,2 In comparison to adults, children with COVID-19 experience few if any symptoms and represent less than 2% of hospitalizations and deaths, although adolescents seem to experience more severe disease than younger children do.1-3 Severe COVID-19 in children typically manifests as respiratory distress requiring supplemental oxygen, noninvasive positive pressure, or mechanical ventilation like the presentation seen in adults. In mid-April of 2020, however, pediatricians internationally began reporting varying features of a severe hyperinflammatory syndrome uniquely affecting children connected to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at least temporally.4-7 The various hyperinflammatory presentations included children with features of Kawasaki disease (KD) shock syndrome, atypical KD, macrophage activation syndrome or myocarditis. Some children with multi-inflammatory syndrome in children (MIS-C) may present without features of any of these entities.3-6,8 The definition of MIS-C is broad and encompasses children and adolescents <21 years old with evidence of current or recent SARS-CoV-2, fever for ≥24 hours, laboratory evidence of inflammation and involvement of ≥2 systems or organs without other plausible explanation.9 Notably, many of the features of acute COVID-19 illness also overlap with MIS-C.
This report describes the epidemiologic, clinical, pathologic and virologic features of a fatal case of COVID-19-associated disease with key distinguishing features in a previously healthy adolescent girl. While this case report does not require Johns Hopkins Institutional Review Boards approval, her mother consented to share it.
CASE REPORT
In May 2020, a community hospital transferred to our hospital a 15-year-old African American female with 1 week of progressively worsening epigastric pain and loss of appetite, without nausea, emesis or diarrhea. Two days before the presentation, she developed nasal congestion and rhinorrhea without dyspnea or cough. She also endorsed loss of smell and taste, but she minimized these symptoms. The day before the presentation, she developed myalgias but denied fevers and had been afebrile at the community hospital before transfer. She reported sheltering in place with her family at home, no recent travel, and no known exposures to COVID-19, although her 2-year-old sibling recovered from a fever and congestion the prior week. Her medical history was only notable for obesity (BMI 31 kg/m2, 97.0%).
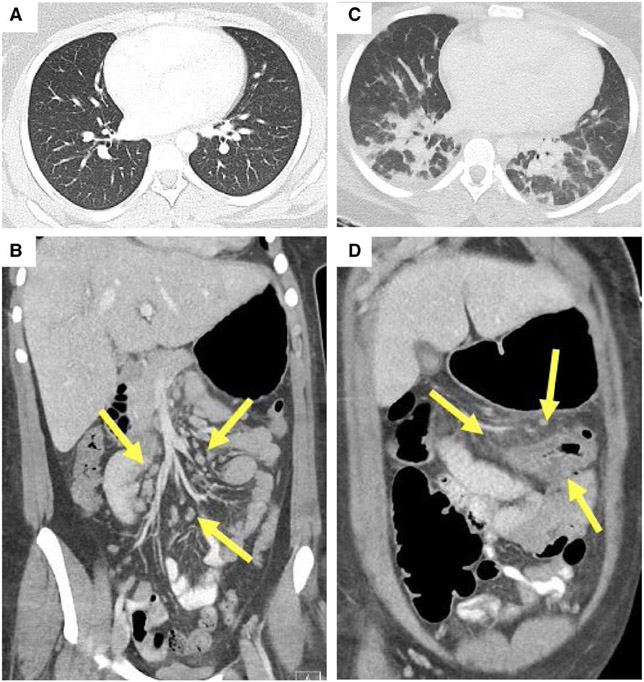
Febrile to 38.9°C and tachycardic at presentation to our hospital, her physical examination revealed epigastric and periumbilical tenderness without peritoneal signs. The remainder of her examination was normal. Her initial laboratory results (Table 1) were notable for leukocytosis with neutrophilia and lymphopenia, elevated C-reactive protein (CRP) to 24.3 mg/dL and erythrocyte sedimentation rate (ESR) to 74 mm/h. Nasopharyngeal (NP) swabs for SARS-CoV-2 on day of illness 7 (from community hospital) and 8 (at our hospital) were negative. A computed tomography (CT) examination with contrast of the abdomen and pelvis performed at the community hospital showed an area of fat stranding and prominent lymph nodes in the epigastric region, mildly prominent lymph nodes in the left retroperitoneum and unremarkable bowel (Fig. 1B).
TABLE 1.
Clinical Signs and Laboratory Values
| Measure | Reference Range |
Illness day 8, Hospital day 1 Admission |
Illness day 10, Hospital day 3 |
Illness day 11, Hospital day 4 |
Illness day 12, Hospital day 5 12 am |
Illness day 12, Hospital day 5 Morning |
|---|---|---|---|---|---|---|
| Clinical signs | ||||||
| Fever (maximum temperature) (°C) | <38.1 | 38.9† | 37.7 | 39.9† | 39.6† | 39.7† |
| Rash/mucocutaneous inflammation | Absent | Absent | Present | Fading | Absent | Absent |
| Hypotension or shock | Absent | Absent | Absent | Present | Absent | Present |
| Echocardiogram | … | … | … | … | Mild mitral and tricuspid regurgitation, LVEF 66% | Severely diminished LVEF‡ |
| Laboratory values | ||||||
| C-reactive protein (mg/dL) | <0.5 | 24.3† | 30.4† | 33.7† | 30.9† | … |
| Erythrocyte sedimentation rate (mm/h) | 4–25 | 74† | … | 107† | 118† | … |
| D-dimer (mg/L) | 0.00–0.49 | … | 1.09† | … | 1.55† | … |
| Ferritin (ng/mL) | 13–150 | … | 137 | 136 | … | … |
| Interleukin-6 (pg/mL) | <10 | … | … | … | 239.3† | … |
| White blood cell count (K/mm3) | 4.5–13.0 | 16.3† | 18.8† | 27.8† | 37.5† | 55.0† |
| Absolute neutrophil count (K/mm3) | 1.5–8.0 | 14.9† | 18.0† | 27.2† | 36.0† | 47.3† |
| Absolute lymphocytes (K/mm3) | 1.30–6.30 | 0.79* | 0.19* | 0.28* | 0.75* | 3.30 |
| Band % (%) | 2–7 | … | 24† | 43† | 28† | 7 |
| Hemoglobin (g/dL) | 12.4–14.8 | 12.2* | 12.4 | 10.7* | 10.1* | 10.4* |
| Hematocrit (%) | 36.0–46.0 | 39.6 | 40.1 | 34.0* | 31.5* | 33.8* |
| Platelet count (K/mm3) | 150–350 | 260 | 272 | 282 | 280 | 280 |
| Troponin (ng/mL) | <0.04 | … | <0.04 | <0.04 | … | 2.48† |
| Creatine kinase (U/L) | 24–170 | … | 43† | … | 301† | … |
| Pro-brain natriuretic peptide (pg/mL) | 0–125 | … | … | 652† | 8328† | … |
| Total protein (g/dL) | 6.0–8.2 | 7.2 | 7 | 6.3 | 6 | 7.4 |
| Albumin (g/dL) | 3.5–5.3 | 3.9 | 3.5 | 3.3 | 2.9* | 2.6* |
| Total bilirubin (mg/dL) | 0.0–1.2 | 0.7 | 1.9† | 1.9† | 2.2† | 1.9† |
| Direct bilirubin (mg/dL) | 0.0–0.4 | … | 1.4† | 1.8† | … | … |
| Aspartate aminotransferase (U/L) | 0–31 | 20 | 33† | 29 | 54† | 79† |
| Alanine aminotransferase (U/L) | 0–31 | 13 | 32† | 26 | 24 | 32† |
| Gamma-glutamyl transferase (U/L) | 8–51 | 82† | 139† | 132† | … | … |
| Fibrinogen (mg/dL) | 170–422 | … | … | 965† | … | 825† |
| Prothrombin time (s) | 9.3–11.7 | … | … | 12.2† | 12.5† | 14.0† |
| Activated partial thromboplastin time (s) | 23.1–30.9 | … | … | 34.8† | 30.3 | 35.7† |
| Urine bacterial culture | No growth | … | … | No growth | … | … |
| Blood bacterial culture | No growth | … | … | No growth | … | … |
| SARS-CoV-2 testing | ||||||
| SARS-CoV-2 NAT nasopharyngeal swab | No RNA detected | No RNA detected | ·· | ·· | RNA detected† | ·· |
| SARS CoV-2 S1 IgA (arb’U) | <2.80 | … | 9.87† | 7.17† | … | ·· |
| SARS CoV-2 S1 IgG (arb’U) | <0.80 | … | 10.85† | 8.71† | … | … |
LVEF, left ventricular ejection fraction; SARS CoV-2, severe acute respiratory syndrome coronavirus 2.
The value in the patient was below normal.
The value in the patient was above normal.
Obtained by point of care ultrasound without quantification of ejection fraction.
FIGURE 1.
Imaging at hospital admission (A and B) and at PICU admission (C and D). Axial chest CT showed normal lungs (A) with coronal IV contrast-enhanced abdominal CT (B) showing enlarged mesenteric and retroperitoneal nodes (arrows) and normal bowel. On HD 4, repeat chest CT (C) showed bilateral lower lobe consolidation without pulmonary embolism and coronal IV contrast-enhanced abdominal CT (D) demonstrated new wall thickening of the distal transverse colon, gallbladder wall edema and mesenteric stranding (arrows). Both chest and abdominal CT at that time showed prominent diffuse supraclavicular, hilar, mediastinal, abdominal and retroperitoneal lymphadenopathy (C and D). PICU, pediatric intensive care unit.
The patient’s abdominal pain and oral fluid intake initially improved on hospital day (HD) 1, but she continued to have fever and tachycardia. Over the course of her first 3 days of hospitalization, she developed worsening abdominal pain, myalgias, sore throat, diffuse paraspinal tenderness and pleuritic chest pain. A coalescing faintly erythematous maculopapular rash appeared on HD 3, most prominently on her chest and palms but with additional involvement of her upper extremities, soles and back. Worsening leukocytosis with bandemia and rising CRP (Table 1) developed. Her D-dimer was mildly elevated to 1.09 mg/L, ferritin was normal at 137 ng/mL and troponin was <0.04 ng/mL. An extensive workup for rheumatologic and other infectious etiologies was negative.
PEDIATRIC INTENSIVE CARE UNIT COURSE
On HD 4, she developed increased work of breathing, unrelenting fevers, tachycardia and hypotension despite fluid boluses, requiring transfer to the pediatric intensive care unit (PICU). The PICU initiated heated high flow nasal cannula at 15 L/min and 0.50 FiO2 and norepinephrine infusion for warm shock with a blood pressure nadir of 81/52 mm Hg. An electrocardiogram showed sinus tachycardia. Repeat CT imaging revealed mesenteric and peripancreatic stranding, prominent diffuse lymphadenopathy and interstitial pulmonary opacities (Fig. 1C, D). Echocardiogram showed normal left ventricle (LV) systolic function (ejection fraction 66.3%), normal left coronary artery (right not visualized), and mild tricuspid and mitral valve regurgitation. Her CRP, ESR and bandemia increased (Table 1). Her pro-brain natriuretic peptide (pro-BNP) was 652 pg/mL. She received cefepime, metronidazole and doxycycline while awaiting culture and serologic data. She initially improved and briefly weaned from vasoactive support for approximately 3 hours.
After 12 hours in the PICU, SARS-CoV2 IgG and IgA (EUROIMMUN) resulted positive (drawn on HD 3, day 10 of illness), increasing concern for MIS-C and prompting intravenous immunoglobulin (2 g/kg) administration. Simultaneously, the PICU staff and infectious disease team attempted to procure remdesivir. The team received approval to treat her with it, but she never received the medication due to her rapid demise. She also did not receive dexamethasone as her illness occurred before the publication of data revealing the benefits of dexamethasone for patients with severe or critical disease.10
Overnight between HD 4 and 5, her hypoxemic respiratory failure worsened leading to initiation of noninvasive bi-level positive pressure ventilation and ultimately intubation the morning of HD 5 due to acute respiratory distress syndrome. Hypotension immediately developed postintubation, requiring norepinephrine and vasopressin infusion. Point of care ultrasound revealed severely diminished LV ejection fraction and adequate right ventricle filling with normal septal position. Pro-BNP increased to 8328 pg/mL. Her hypotension persisted despite bolus epinephrine, and she progressed to a pulseless electrical activity (PEA) arrest. She received high-quality cardiopulmonary resuscitation (CPR)11 with a return of spontaneous circulation after less than 2 minutes. Following her first arrest, troponins increased to 2.48 ng/mL, interleukin-6 resulted at 239 mg/dL and a third NP polymerase chain reaction (PCR) returned positive for SARS-CoV-2. She suffered a second PEA arrest. She again developed a brief perfusing rhythm after less than 2 minutes of CPR.
A third PEA arrest occurred as the PICU initiated venoarterial extracorporeal membrane oxygenation for cardiac dysfunction and refractory hypotension. Multiple femoral percutaneous attempts for cannulation occurred without success. Ultimately, a left femoral cutdown revealed an engorged vein, which the surgeons cannulated without difficulty. The surgical team performed a right femoral cutdown for arterial access and found absent arterial blood flow despite numerous cannula manipulations. The surgeons noted retrograde flow into the femoral artery. After 90 minutes of CPR following the third arrest, the team ceased resuscitative efforts.
PATHOLOGY
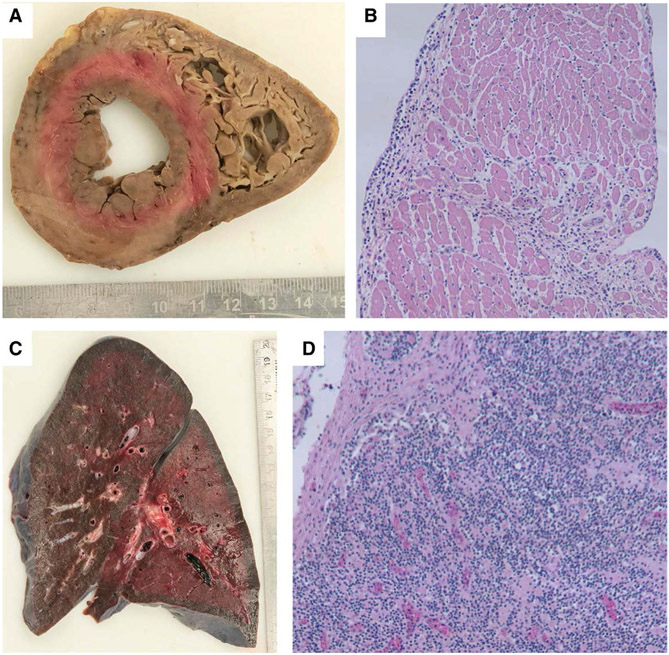
The autopsy was performed according to our institutional protocol for SARS-CoV-2 positive patients, including removal and full examination of chest organs and in situ sampling of all other organs except for the central nervous system. The heart was within normal range by body weight (Fig. 2A), but the lungs were heavy (right 880 g, reference range 360–570 g; left 770 g, reference range 325–480 g). Microscopically, the heart showed a diffuse lymphoplasmacytic inflammatory infiltrate, most marked in the septum (Fig. 2B). The inflammation clustered around and partially involved small arterioles, venules and lymphatics but did not involve capillaries (Fig. 2C). There were only very focal areas of visible myocyte damage in the right ventricle (Fig. 2D). It is possible that the myocardial injury was in fact more extensive and occurred too acutely to show diffuse histologic evidence. The lungs demonstrated vascular congestion without inflammation, thrombi or other abnormality. One kidney showed possible focal apoptosis of tubular cells but without frank inflammation, damage or microthrombi. The enlarged lymph nodes showed normal appearing populations of lymphocytes. There were no remarkable abdominal findings.
FIGURE 2.
Biventricular cross-section of heart (A) with areas of pallor. Cardiac myocytes with interstitial lymphoplasmacytic infiltrate (B, H&E stain, 100x). Mixed inflammation surrounding and involving a cardiac vessel (C, H&E stain, 100x). Focal area of myocyte damage in the right ventricle (D, H&E stain, 100x).
VIROLOGY
The NP swab from HD 1 was negative by the NeuMoDx SARS-CoV-2 assay. Repeat NP swab obtained on HD 5 was positive for the N gene at a cycle threshold of 30.66. Culturable, infectious SARS-CoV-2 virus was successfully isolated from the NP specimen obtained on HD 5 using Vero-TMPRSS2 cells.12
DISCUSSION
Inflammation is a prominent feature of both acute COVID-19 disease and MIS-C. With the generally mild and relatively infrequent presentation of acute COVID-19 in children, pediatricians and the public have focused on MIS-C. MIS-C seems to affect children following infection with SARS-CoV-2. Almost all children reported in the literature have demonstrated positive serologies (ranging from 80% to 100%), while only a minority have a positive NP PCR (ranging from 0% to 50%)4-7 suggesting that MIS-C occurs as a postinflammatory syndrome rather than as a result of active infection. Cardiovascular injury appears to result from this postinflammatory response in some children although acute COVID-19 can result in cardiovascular complications as well.4,13
Our patient is one of the few reported pediatric patients who died from COVID-19. Several key features distinguish our patient from the pediatric patients reported to date in the literature, including (1) her rapid clinical deterioration considering our patient did not meet the MIS-C case definition and was clinically stable requiring no respiratory support until <36 hours before she died; (2) discordant inflammatory marker profile on presentation to our hospital with lower ferritin, mildly elevated D-dimer, normal albumin; (3) symptoms of diffuse inflammation including pleuritic chest pain and paraspinal tenderness without a source; (4) lack of fever until late in her clinical course; (5) atypical diffuse maculopapular rash; (6) diffuse supraclavicular, hilar, mediastinal, abdominal and retroperitoneal adenopathy and (7) both PCR and serology positive with culturable, infectious virus isolated. While many MIS-C and acute COVID-19 patients have had prominent gastrointestinal symptoms,5,6 our patient had prominent postprandial abdominal pain with lymphadenitis without concomitant vomiting or diarrhea. She did not manifest features of either classic or atypical KD apart from a subtle macular rash that did not appear until HD 3. Her relatively normal ferritin, D-dimer and albumin made a COVID-19-related diagnosis seem less likely before SARS-CoV-2 serology results. While her first 2 NP SARS-CoV-2 swabs were negative, her third sampling was positive for culturable virus, although the viral load was low.14,15
Similar to the DeBiasi case series, where adolescents and young adults represented 66% of the critical care admissions, her age may have contributed to her severe presentation.3 Like both her adult and pediatric counterparts, her comorbid obesity likely increased her vulnerability to the disease.16,17 Indeed, her clinical manifestations may represent the inflammatory and cardiac manifestations seen in stage III (hyperinflammation stage) of acute COVID-19 affecting many adults during this pandemic.13,18 There may be an adolescent phenotype, representing an intermediate between the adult and school-age phenotypes. While her clinical course may have more closely paralleled the adult phenotype, her pathology findings showed none of the classic pulmonary fibrosis, diffuse alveolar damage or pulmonary hemorrhage findings seen on adult patients’ histology highlighting that there are significant differences as well.19,20
In retrospect, although her initial PCR results and serology positivity along with her symptomatology suggested MIS-C, the finding of culturable virus raises our suspicion for acute COVID-19 infection, recrudescence in viral replication in the setting of decreased antiviral response, or prolonged shedding in the setting of severe disease. These findings highlight the difficulty of differentiating between acute COVID-19 and MIS-C. Ideally distinguished by their time course, NP PCR and serologic findings, and symptomatology, the lack of obvious preceding illness in many possible MIS-C cases, the limitations of standard laboratory testing and the overlapping features of the 2 entities makes differentiating them challenging. Determining whether a patient has acute COVID-19 or MIS-C has important treatment implications. Treatment for MIS-C includes IVIG, aspirin, steroids and immunomodulating agents while acute COVID-19 treatment includes antiviral medications.
In conclusion, our patient underscores the ambiguities and the lack of full understanding of SARS-CoV-2 infection presentations. Her course emphasizes the heterogeneous and often non-specific presentation of MIS-C versus acute COVID-19 infection in adolescents. Importantly and sadly, her case also illustrates the potential for rapid clinical deterioration. Appropriate clinical suspicion is necessary for both acute disease and MIS-C. SARS-CoV-2 serologic tests obtained early in the diagnostic process may help to narrow down the differential but does not distinguish between acute COVID-19 and MIS-C. Better understanding of the hyperinflammatory changes associated with MIS-C and acute COVID-19 in children will help delineate the roles for therapies, particularly if there is a hybrid phenotype occurring in adolescents. Rapid communication and dissemination of data is important to better understand and treat the emerging complications of SARS-CoV-2.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC COVID-19 Response Team. Coronavirus disease 2019 in children – United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBiasi RL, Song X, Delaney M, et al. Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J Pediatr. 2020;223:199–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.048360. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed June 4, 2020.
- 10.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. 2020;NEJMoa2021436. doi: 10.1056/NEJMoa2021436. [Epub ahead of print] [DOI] [Google Scholar]
- 11.Hunt EA, Jeffers J, McNamara L, et al. Improved cardiopulmonary resuscitation performance with CODE ACES2: a resuscitation quality bundle. J Am Heart Assoc. 2018;7:e009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuyama S, Nao N, Shirato K, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci USA. 2020;117:7001–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long B, Brady WJ, Koyfman A, et al. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wölfel R, Corman VM, Guggemos W, et al. Virologic assessment of hospitalized patients with COVID-19. Nature. 2020;581:465–469. [DOI] [PubMed] [Google Scholar]
- 16.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York city, New York. JAMA Pediatr. 2020;e202430. doi: 10.1001/jamapediatrics.2020.2430. [Epub ahead ofprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaller T, Hirschbühl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. LancetRespir Med. 2020;8:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]