Abstract
Background.
Metabolic and bariatric surgery (MBS) is a safe and effective treatment option for adolescents with severe obesity, but no long-term studies are available with >10 years of follow-up data to document sustained improved outcomes.
Methods.
A total of 96 patients who completed MBS at ≤21 years of age in a tertiary academic center 2002 to 2010 were contacted for a telehealth visit. Body weight, co-morbidity status, social/physical function status, and long-term complications were evaluated 10-to-18 years after surgery.
Results.
Mean participant (83% female, 75%- Hispanic) age at MBS was 18.8 (±1.6) years (median age 19 years, range 15–21 years), and median pre-MBS body mass index was 44.7 kg/m2 (SD 6.5). At follow-up (mean 14.2 [±2.2] years) post-MBS (90.6% Roux-en-Y gastric bypass [RYGB] or 8.3% laparoscopic adjustable gastric banding [LAGB]) mean total body weight decreased by 31.3% (IQR 20.0%−38.9%); 32.0% (IQR, 21.3%−40.1%) among RYGB participants and 22.5% (IQR, 0.64%−28.3%) among LAGB participants. Patients with pre-MBS hyperlipidemia (14.6%), asthma (10.4%), and diabetes/hyperglycemia (5.2%) reported 100% remission at follow-up (p<0.05 for all). Pre-post decrease in hypertension (13.5% vs. 1%, p=0.001), sleep apnea (16.7% vs. 1.0%, p<0.001), gastroesophageal reflux disease (13.5% vs. 3.1%, p=0.016), anxiety (7.3% vs. 2.1%, p=0.169), and depression (27.1% vs. 4.2%, p<0.001) were also found.
Conclusions.
Significant sustained reductions in weight and co-morbidities, and low rates of long-term complications, a decade or more after completing MBS as an adolescent were found. These findings have important implications for adolescents who may be considering MBS for weight reduction and overall health improvements that extend into adulthood.
Precis
Little information is present on long-term outcomes of adolescent bariatric surgery. This study shows the lasting positive impact of bariatric surgery, even decades after.
Introduction
In the US, almost 12% of non-Hispanic Black (NHB), 9% of Hispanic, and 7% of non-Hispanic White (NHW) adolescents ages 12-to-19 years old have severe obesity (defined as having a body mass index [BMI] ≥35 kg/m2 or ≥120% of the 95th percentile for age and sex).1 Evidence suggests that children with severe obesity are at increased risk for developing chronic diseases including cardiometabolic disorders2 and cancers,3 which may result in long-term disability and premature death.4
Metabolic and bariatric surgery (MBS) has emerged as a safe and effective treatment modality for adolescents with severe obesity with similar perioperative complication rates as for adults who have undergone MBS.5–8 The American Academy of Pediatrics (AAP) recently released a policy statement that recommended early referral to multi-disciplinary pediatric-focused MBS programs for adolescents with severe obesity.9 MBS in adolescents has been an underutilized treatment modality, due to barriers including referral,access,10,11 and a lack of long-term evidence of MBS outcomes for adolescents. Recent reviews stress the urgent need for longitudinal studies demonstrating durable and sustainable weight loss.12–14
MBS patients are routinely lost-to-follow-up, thus limiting the availability of weight, co-morbidities and other outcomes. In adults, the Swedish Obesity Study documented 20-year post-MBS follow-up compared to usual care and showed a long-term reduction in incident diabetes, myocardial infarction, stroke, and mortality.15 Although there have been short and medium term studies in adolescent MBS patients with similar outcomes,16 >10-year follow-up studies do not exist. Major trials involving adolescents undergoing MBS, including the Teen-LABS cohort (NCT00474318, 161 patients)17 the AMOS trial (NCT02378259, 81 patients),18 and the Follow-up of Adolescent Bariatric Surgery (NCT00776776, 58 patients)19 have demonstrated similar decreases in post-MBS weight loss and reductions in co-morbid conditions at 3-to-8 or more years post-MBS. A recently published study in 632 in patients who received MBS at 5-to-18 years of age showed excellent maintenance of weight loss and co-morbidity resolution 7–10 years post-surgery.20
While clinical trials with low-attrition offer insights into postoperative outcomes, this may limit overall generalizability as post-MBS loss-to-follow-up has been as high as 60%.21,22 Long-term follow-up of adolescents who have completed MBS outside of a formal research protocol and were subsequently lost-to-follow-up has never been described in the literature. This is a novel area of research as these community-based-practice patients may better represent the majority of MBS patients. We report post-MBS weight and co-morbidity outcomes of adolescent patients who completed surgery between 10 and 18 years previously, most of whom had been lost-to-follow-up for multiple years. We hypothesized that despite long-term loss-to-follow-up, these patients would have sustained weight-loss, co-morbidity resolution, and an overall improved quality-of-life as an adult.
Methods
Study Design.
A retrospective medical chart review was conducted at an academic tertiary referral center to identify patients who completed MBS at ≤21 years old and ≥10 years ago, (from January 2002-September 2010). The surgeries were performed by the lead surgeon when he was in a community-based practice from 2002–2009. A new dataset was created from identified patients with the following variables; demographic data (age, sex, race/ethnicity), pre-surgery weight, BMI, and pre-operative co-morbidities. The number of post-MBS follow-up visits, duration of follow-up, number of months lost-to-follow-up, peri-operative complications, and surgical interventions were also included. This information was subsequently used as the interview guideline to verify and capture new follow-up information among study participants. This study was approved by the institutional review board (IRB).
Participants.
A total of 130 patients who met inclusion criteria were identified.
Ninety-seven patients were successfully contacted and 96 agreed to participate in the study.
Study Procedures.
Patients were assigned a bariatric practice team clinical healthcare provider (MD, RD, ARNP, or LPN) who attempted telephone contact. Once contacted, the provider followed an IRB-approved verbal script to attempt to obtain consent for the study. All patients who were successfully contacted, except one, consented to participate. The providers then read from a two-page questionnaire (see Appendix) to obtain the follow-up data.
Up to 10 attempts were made to contact a patient. All phone numbers in the database and in a separate university health system database were used. For patients who could not be reached, a fee-based online national search was conducted to obtain the latest phone numbers and addresses on record. If there was still no successful contact with the patient by telephone, patients were mailed a packet that included the consent form, the study questionnaire, and paid return envelopes. Finally, a nationwide mortality search was completed for all patients not successfully contacted.
Measures.
Demographics.
Participants were asked about their current age, sex, race/ethnicity, highest level of education completed, current employment status, marital and relationship status (committed, long-term, etc.), pregnancy, and pregnancy outcomes.
Anthropometrics.
Participants were asked to report their current weight, current height, and lowest weight.
Co-morbidities and Complications.
Participants previously documented complications and co-morbidities were reviewed during the visit and the current status recorded. Participants were also asked about any new health conditions since having surgery.
Behavioral Outcomes.
Participants were asked about personal relationship status, and current and past alcohol consumption, physical activity, and dietary intake patterns.
Statistical Analysis
Categorical variables were presented as frequencies and percentages, and continuous variables were assessed for normality via the Shapiro-Wilk test. Normally distributed continuous variables were summarized as means (standard deviation [SD]) or were summarized as a median (interquartile [IQR]). We assessed the weight change from baseline to current weight and lowest weight post-MBS based on both actual weight and BMI. Wilcoxon signed-rank test was performed to examine the statistical significance of weight and BMI change. Furthermore, non-parametric statistics was employed at baseline to test if there were any significant differences between those that were contacted for a telehealth visit as compared to those that were not.
Total body weight loss percentage (TBWL%) was calculated by the formula below:
Fisher’s exact tests were used to compare patient co-morbidities including anemia, asthma, anxiety, back pain, depression, type 2 diabetes (T2D), gastroesophageal reflux disease (GERD), hyperlipidemia, hypertension, and sleep apnea from baseline to long-term follow-up. The association between baseline and current BMI was explored with a Pearson correlation. Since a positive and significant correlation was found, we analyzed the normalized BMI difference by dividing with the standard deviation of the current BMI in a multivariable linear regression model. This model was adjusted for demographic factors and co-morbidities to identify predictors for BMI. The equation for calculating the normalized BMI difference (i.e., the dependent variable of the linear model) is as follows:
The final model included 12 levels, resulting in 80% power (assuming 8 responses per participant).23 All analyses were conducted with SAS (version 9.4). Two-sided p-values <0.05 was considered as statistically significant.
Sensitivity Analysis
A post-hoc sensitivity analysis using Pearson chi-square test was performed to compare risk of further abdominal operations in patients had band vs. RYGB surgery. We found there is no difference between number of related abdominal surgeries and surgery types. 20.69% (n=18) and 37.50% (n=3) RYGB and Lap band patients had other abdominal surgeries, respectively (P=0.273).
Results
Thirty-two potential participants could not be reached; additionally, one did not consent, and one was deceased 9 months after surgery (from unrelated medical condition). Thus, the final analytical sample included a total of 96 consented patients (73.9% follow-up). Table 1 shows the mean (±SD) pre-MBS age was 18.8 (±1.6) years (median age 19 years, range 15–21 years). The majority (83.3%, n=80) were female and 73.9% (n=71) were Hispanic followed by NHW (16.7%, n=16) and NHB (9.4%, n=9). Most patients (90.6%, n=87) completed RYGB. Of the 8 band patients, two underwent band removal for reflux and slippage, one underwent repositioning for slippage, and one had a spontaneous band deflation that has been left in place. The other four patients still have their bands intact but they have not been adjusted in over 10 years. There was no difference between participants and non-participants in age at time of surgery, gender, insurance type, or baseline BMI.
Table 1.
Baseline Characteristics among Those Who Had Metabolic and Bariatric Surgery at ≤21 Years of Age (n=130) by Contact Status
| Characteristic | Patients consented (n=96) | Patients not consented (n=34) | p Value* |
|---|---|---|---|
| Age at surgery, y, mean (SD) † | 18.8 (1.6) | 19.1 (1.4) | 0.269 |
| Sex, n (%) | |||
| Male | 16 (16.7) | 8 (23.5) | 0.376 |
| Female | 80 (83.3) | 26 (76.5) | |
| Race/ethnicity, n (%) | |||
| NHW | 16 (16.7) | 2 (5.9) | |
| NHB | 9 (9.4) | 0 (0) | 0.023 |
| Hispanic | 71 (73.9) | 31 (91.2) | |
| Native American | 0 (0) | 1 (2.9) | |
| Procedure type, n (%) | |||
| RYGB | 87 (90.6) | 30 (88.2) | 0.642 |
| Lap band | 8 (8.3) | 4 (11.8) | |
| Sleeve gastrectomy | 1 (1.0) | 0 (0) | |
| Insurance type, n (%) | |||
| Commercial | 65 (67.7) | 20 (58.8) | 0.724 |
| Government | 9 (9.4) | 4 (11.8) | |
| Self-pay | 20 (20.8) | 9 (26.5) | |
| Not available | 2 (2.1) | 1 (2.9) | |
| BMI at surgery, median [kg/m2, (IQR)] | 45.0 (41.0–49.0) | 45.5 (42–49.0) | 0.758 |
Mann-Whitney U test for continuous variables; Pearson Chi-square or Fisher’s exact test for categorical variables.
Patients consented: median age 19 years (range 15–21 years); Patients not consented: median age 19 years (range 16–21 years).
NHB, non-Hispanic Black; NHW, non-Hispanic White; IQR, interquartile range; RYGB, Roux-en-Y gastric bypass.
The three most common patient responses to the question “why did you get lost to follow up” were changes in insurance and coverage, immaturity and not realizing the importance of follow up, and moving away from the area. Although lost to the bariatric clinic, many patients were followed by their pediatricians and primary care physicians.
Table 2 summarizes the changes of weight status from baseline to long-term follow-up. The baseline median (IQR) weight was 278.5 (241.5 to 324) lbs. At present, current median weight was 195 (IQR, 160–240) pounds converting to a TBWL% of 31.3% (IQR, 20.0% to 38.9%) in the total cohort, 32.0% (IQR, 21.3% to 40.1%) among participants who completed RYGB, and 22.5% (IQR, 0.64% to 28.3%) among those who completed LAGB surgery (p<0.001 for all comparisons). The one patient who underwent a sleeve gastrectomy has a current TBWL% of 33%. (Data not shown). When calculating with lowest weight after surgery, the results were even more striking with a TBWL% of 44.4% (IQR, 40.1% to 48.6%). Patients’ BMI values were also significantly decreased from 44.9 (IQR, 41.5–50.1) before surgery to the lowest BMI of 25.2 (IQR, 23.4–27.5) equating to a 44.4% (IQR 40.1% to 48.6%) decrease after surgery, and 31.7 (IQR, 27.3 −37.3) at present equating to a 31.4% (IQR 21.3% to 39.8%) decrease (p<0.001 for all comparisons). The long-term weight loss responder rate (defined as having a TBWL >20% after 5 years) was 74.7% in RYGB patients and 62.5% in LAGB patients.23
Table 2.
Weight Change from Baseline to Long-Term Follow-Up among Those Who Had Metabolic and Bariatric Surgery at ≤21 Years Old (n=96)
| Variable | Baseline/at MBS | Long-term follow-up | |
|---|---|---|---|
| Current weight | Lowest weight | ||
| Weight change | |||
| Weight, lbs, median (IQR) | 278.5 (241.5–324) | 195 (160–240) | 155 (135–179) |
| Weight loss, lbs, median (IQR) | – | 83 (54–117) | 125 (98–147) |
| Total body weight loss, %, median (IQR) | – | 31.3 (20.0–38.9) | 43.4 (38.9–48.3) |
| p Value* | – | <0.001 | <0.001 |
| BMI change | |||
| BMI, median (IQR), kg/m2 | 44.9 (41.5–50.1) | 31.7 (27.3–37.3) | 25.2 (23.4–27.5) |
| BMI absolute change, kg/m2, median (IQR) | – | 14.5 (9.3–18.5) | 19.9 (17.4–24.5) |
| BMI change, %, median (IQR) | – | 31.4 (21.3–39.8) | 44.4 (40.1–48.6) |
| p Value* | – | <0.001 | <0.001 |
Wilcoxon Signed-Rank test for weight and BMI change from baseline to long-term follow-up.
Table 3 lists co-morbid conditions and resolution rates. Remission was defined as off medicine and not undergoing any medical management or treatment. Patients with hyperlipidemia (14.6%), asthma (10.4%), and T2D/hyperglycemia (5.2%) reported 100% remission at follow-up (p<0.05 for all). Remission of hypertension (13.5% vs. 1%, p=0.001), sleep apnea (16.7% vs. 1.0%, p<0.001), GERD (13.5% vs. 3.1%, p=0.016), anxiety (7.3% vs. 2.1%, p=0.169) and depression (27.1% vs. 4.2%, p<0.001) were also found.
Table 3.
Patient Comorbidities from Baseline (pre-MBS) to Long-Term Follow-Up (post-MBS) among Those Who Had Metabolic and Bariatric Surgery at ≤21 Years Old (n=96).
| Comorbidityh | Pre-MBS, n (%) | Post-MBS, n (%) | p valuea |
|---|---|---|---|
| Anemia | 3 (3.1) | 65 (67.7) | <0.001 |
| Asthma | 10 (10.4) | 0 | 0.002 |
| Anxiety | 7 (7.3) | 2 (2.1) | 0.169 |
| Back pain | 32 (33.3) | 4 (4.2) | <0.001 |
| Depression | 26 (27.1) | 4 (4.2) | <0.001 |
| Diabetes or hyperglycemia | 5 (5.2) | 0 | 0.059 |
| GERD | 13 (13.5) | 3 (3.1) | 0.016 |
| Hyperlipidemia | 14 (14.6) | 0 | <0.001 |
| Hypertension | 13 (13.5) | 1 (1.0) | 0.001 |
| Sleep apnea | 16 (16.7) | 1 (1.0) | <0.001 |
| Transfusion | 0 | 23 (24.0) | <0.001 |
Fisher’s exact test.
MBS, metabolic and bariatric surgery; GERD, gastroesophageal reflux disease
Since surgery, 19.8% of participants were readmitted for various complications, with anemia (4.2%) and intussusception (3.1%) being the most common. Thirty-eight (39.6%) underwent post-MBS abdominal surgical interventions, cosmetic surgery (18.8%) and cholecystectomy (8.3%) being the most common (Supplemental Table 1). While 3.1% had anemia pre-operatively, the rate was 67.7% in the post-operative period. 24% of the patients required transfusion. (Table 3)
Other post-MBS outcomes are reported in Supplementary Table 2. In summary, the mean number of years since MBS was 14.3 (2.2). Over half (59%) had graduated college or pursued a graduate degree and 84.2% reported current employment. Over half (52.2%) reported currently being married and 67.1% females had a successful pregnancy and birth. About half (52.1%) reported regular alcohol consumption, while 8.3% reported having a drinking problem at some point post-operatively. Over half (60.4%) reported engaging in regular physical activity and 86.5% reported improved dietary habits since surgery. Almost all (88.5%) of participants were satisfied with their MBS results and 91.7% said they would undergo MBS again.
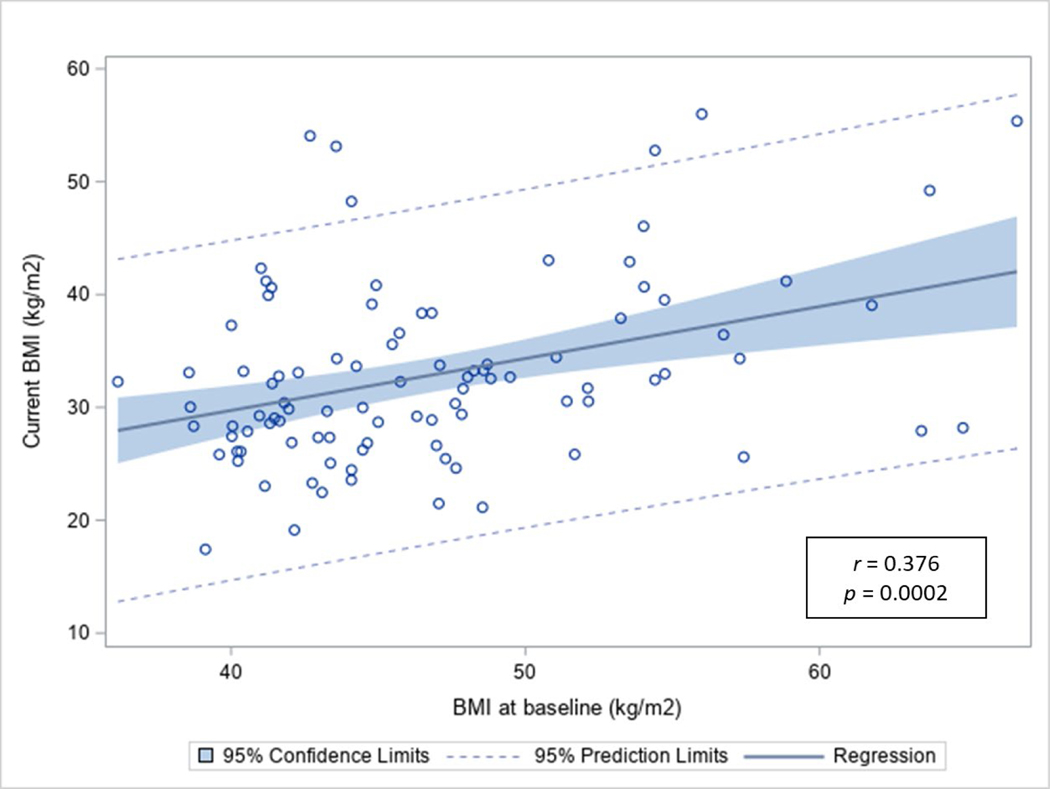
Figure 1 shows a significant but modest association between current BMI and baseline BMI (r = 0.376, p<0.001). The multivariable linear regression adjusting for age at surgery, sex, race/ethnicity, education, surgery type, insurance, and co-morbidities showed that NHB had significantly more weight loss compared to NHW (β=−0.95, SE=0.47, p=0.047) (Table 4). All other predictors were insignificant.
Figure 1.
Pearson correlation of BMI at baseline with current BMI among those who had metabolic and bariatric surgery at ≤21 years old (n=96).
Table 4.
Multivariable Linear Regression to Explore Possible Predictors for BMI among Those Who Had Metabolic and Bariatric Surgery at ≤21 Years Old (n=96).
| Variable | Beta-coefficient | SE | p Value* |
|---|---|---|---|
| Age at surgery | 0.09 | 0.07 | 0.220 |
| Sex | |||
| Male | 0 (ref) | N/A (ref) | |
| Female | 0.414 | 0.311 | 0.188 |
| Race/ethnicity | |||
| NHW | 0 (ref) | N/A (ref) | |
| NHB | −0.95 | 0.47 | 0.047 |
| Hispanic | −0.18 | 0.32 | 0.581 |
| Education | |||
| Some high school | 0 (ref) | N/A (ref) | |
| High school graduate | −1.73 | 1.10 | 0.118 |
| Some college | −1.77 | 1.05 | 0.095 |
| College graduate | −1.45 | 1.05 | 0.174 |
| Postgraduate | −1.63 | 1.08 | 0.133 |
| Surgery type | |||
| RYGB | 0 (ref) | N/A (ref) | |
| Lap band | 0.76 | 0.39 | 0.057 |
| Sleeve gastrectomy | 0.86 | 1.22 | 0.445 |
| Insurance type | |||
| Commercial | 0 (ref) | N/A (ref) | |
| Government | −0.57 | 0.39 | 0.147 |
| Self-pay | −0.15 | 0.28 | 0.581 |
| Comorbidity at baseline | |||
| No | 0 (ref) | N/A (ref) | |
| Yes | 0.27 | 1.06 | 0.801 |
The dependent variable of the linear regression model is normalized BMI difference between current and baseline BMI divided by the standard deviation of current BMI.
Multivariable linear regression adjusted for BMI at baseline, age, sex, race/ethnicity, education, surgery type, insurance type, and co-morbidity at baseline.
Statistically significant.
RYGB, Roux-en-Y gastric bypass
Discussion
We report here the longest follow-up data currently available in the literature on adolescent MBS patients. A portion of this cohort has been previously reported with four-year outcomes showing a median BMI of approximately 31 kg/m2.24 Long-term weight loss was sustained and significant, up to almost two decades and without consistent clinical follow-up with their surgeon. Co-morbidity resolution was equally significant with several conditions completely resolving via self-report. This is an important area of inquiry as these patients may represent the majority of MBS patients who are not enrolled in a formal research protocol, are frequently lost-to-follow-up, and may be indicative of real-world outcomes and the sustainability of adolescent MBS. Our cohort had a lower prevalence of pre-surgery comorbid conditions versus other published studies17–19 because the majority of adolescents were not referred by a physician, but rather by a parent. Moreover, our program did not actively engage referring physicians. Most of the patients had parents who were successful MBS completers, and had experienced the benefits as a result, and thus did not want their child to experience the same life challenges.
The AAP supports greater access to MBS for the nearly 4 million US youth suffering with severe obesity.9 While MBS is an invasive treatment option, and perhaps not an agreeable weight loss solution for many youths and their families, it is an evidence-based, safe and effective tool that healthcare providers can introduce as an option to their patients. Patients with psychiatric or emotional conditions, such as eating disorders, severe depression or anxiety, may need to address these issues before completing MBS. The potential disadvantages, beyond the complications of any surgery, can include vitamin deficiencies and/or weight regain. Moreover, Woolford et al. found in 2007 that 48% of physicians would not ever refer an adolescent for MBS and 46% would not make a referral until the patient was 18 years old. 25 Results here show that completing MBS as an adolescent has durable weight loss and co-morbidity resolution that last at least up to almost two decades. These positive health effects may also influence patients’ decision to pursue education, employment, and social relationships. The majority of the female patients had successful pregnancies, confirming findings that MBS decreases pregnancy complications compared to patients with obesity, in particular, hypertensive disorders and gestational diabetes and its complications.26
Inadequately treated or poorly managed obesity can have significant psychological consequences in adolescents and young adults.27 Many teens with extreme obesity struggle with depression and conflicts within their peer groups. Our findings suggest that the psychological, social and overall quality of life outcomes reported by patients favor surgery over no intervention. The percentage of patients undergoing treatment for depression significantly decreased from 27.1% to 4.2% (as defined by either pharmacotherapy and/or counseling). Overall, participants were satisfied with the decision to undergo MBS and 91% reporting they would undergo surgery again. These findings show holistic health improvements as a result of MBS that are critically important for both pediatricians and families to understand when considering this procedure for their patient or child.
The AAP policy statement9 highlights “watchful waiting”, defined as long-term lifestyle management, as a barrier of access to care with MBS since adolescents with severe obesity are unlikely to benefit from this treatment approach. A simulation study that used nationally representative data assessed the risk for adult obesity based on childhood obesity status showed that if a child suffered from severe obesity at age 19 years, their chances of being normal weight at age 35 years was 3.5% among boys, and 8.2% among girls.28 Studies show very low MBS utilization rates among adolescents.29,30 Recent population-level analyses by our group showed that adolescents are referred later than adults with a higher proportion entering MBS with a BMI >50 kg/m2.10 There is additional disparity in that ethnic minority patients are even less likely to undergo MBS.25 Recent data shows that there may be a protective effect of MBS on youth, as adolescents undergoing these operations have greater improvements on their comorbid conditions than compared to adults.31 MBS can prevent years of suffering from the cumulative impact of multiple comorbid diseases. Our findings show that there is long-term weight loss, reduced rates of co-morbidities, and improved quality of life as a result of MBS. Additionally, long-term post-MBS complication, readmission, and reoperation rates were comparable to those reported in adult patients.32,33 In summary, there is a greater benefit to having MBS at a younger age than waiting until the patient is older.
Reporting outcomes on patients lost-to-follow-up has not been previously documented in the adolescent literature. A sustained TBWL% of 31.3% and a mean decrease in BMI from 45.4 mg/m2 to 31.7 mg/m2 at 10-to-18 years post-MBS is remarkable, especially given that this was not a controlled research study. Pre-MBS preparation included completing extensive psychological, nutritional, and medical evaluations as part of a rigorous screening process because of their age. We stressed that successful outcomes require a lifelong commitment to significant changes in diet and vitamin supplementation. Most patients are currently taking at least one of the recommended supplements with many are taking all of them. Most admitted to being poorly compliant in their early 20’s; consistent with the history of anemia that many experienced. Even with a lack of formal post-surgical follow-up, the absence of significant long-term complications is reassuring.
It should be noted that about half (52.1%) of the sample reported regular alcohol consumption, and almost 10% (8.3%) reported having a drinking problem at some point post-operatively. This is consistent with previous studies that suggest that some patients develop progressive alcohol use disorder several years following RYGB.34 Our team has previously reported that 4.2% of an ethnically diverse sample of young MBS patients developed a post-MBS alcohol use disorder with 14.5% of respondents reporting binge drinking and 42% reported drinking until intoxication.35 Qualitative findings showed four major themes prompting an increase in post-MBS alcohol use: (1) increased sensitivity to alcohol intoxication, (2) utilizing alcohol as a replacement self-soothing mechanism for food, (3) increase in socialization, and (4) utilizing alcohol as a coping mechanism.36 Risk factors for problematic postoperative alcohol use include regular or problematic alcohol use pre-MBS, male gender, younger age, tobacco use, and symptoms of attention deficient and hyperactivity disorder.37
Another important finding was that 4.2% of participants were readmitted with anemia (4.2%) and while 3.1% had anemia pre-operatively, the rate was 67.7% in the post-operative period. 24% of the patients required transfusion. Anemia has been a well-documented highly prevalent post-MBS complication.38,39 Indeed, a recent meta-analysis showed that patients undergoing RYGB had a higher risk of postoperative vitamin B12 deficiency than those undergoing SG (relative risk, 1.86; 95% confidence interval, 1.15–3.02; p = 0.012; high level of evidence).40 The authors suggest that that patients undergoing RYGB require more stringent vitamin B12 supplementation and surveillance than those undergoing SG. These results have important pre-operative implications for pre-MBS discussions concerning appropriate procedure type. While our sample included only 1 sleeve and 8 lap bands given that some participants had their surgeries almost 20 years ago, our practice now conducts primarily sleeve gastrectomy procedures and no lap bands.
Regression analysis showed no significant differences in age at surgery, sex, race/ethnicity, education level, insurance type, and baseline co-morbidities in predicating long-term BMI status. The model also showed that LAGB had a less favorable sustained BMI outcome vs. RYGB, which is consistent with the literature.41,42 These findings are encouraging in that MBS is a safe and effective long-term weight loss option for all patients who qualify, regardless of these many factors.
Limitations and Strengths
Some study limitations should be noted. The study includes a retrospective medical chart review combined with a questionnaire. The absence of a matched non-MBS control group with similar severe obesity limits the rigor of study conclusions. Although the results of this population-based observational study should be validated in a large randomized controlled trial of adolescent MBS vs. optimal medical therapy to conclusively establish the impact of MBS on reducing clinically significant outcomes, this will not occur for the foreseeable future due to a lack of international collaboration to study the long-term effects of these rare procedures. Second, patient height, weight and other data were self-reported. However, many studies have reported that self-reported weight in bariatric surgery candidates is equally valid as in-person measurement.43,44 While use of a survey cannot medically include or exclude comorbidities, these findings are valuable because they are patient perceived health assessments in a real-world setting. In some ways, that is more valuable than the sub clinical laboratory assessments of an objective measure. In addition, we were unable to reach a quarter of the cohort, thus there may be an inclusion bias. However, a sensitivity analysis showed no substantial baseline differences between long-term follow-up participants vs. those not reached, indicating no systematic bias. In addition, all subjects, except one, who were successfully contacted consented for the study. A strength was the inclusion of a high percentage of Hispanic and NHB participants given this that these populations are disproportionately impacted by many of the severe obesity-related co-morbidities38 and less likely to undergo MBS than their NHW peers.45
Conclusions
Our results show sustained and remarkable weight loss up to almost two decades post-adolescent MBS without weight regain. Co-morbidity resolution was equally notable with several conditions completely resolving. Participants also reported positive quality-of-life outcomes. This is an important area of inquiry as MBS patients who do not return for follow-up visits represent the majority of those who complete surgery each year in the US. These results can encourage health care providers to consider MBS as a viable treatment option to prevent and reduce the risk of clinically significant events developing in adolescents with severe obesity.
Supplementary Material
Support:
Drs Messiah, Atem, Xie, and Mr Mathew were funded by the National Institutes of Health, National Institute on Minority Health and Health Disparities (grant #R01MD011686) and #R01MD011686-S1). Dr Lipshultz’s work was supported in part by the US Department of Health and Human Services Health Resources and Services Administration, Rockville, MD (HRSA-C76HF15614–10679 Pediatric Integrative Medicine Research Center), the National Center for Toxicological Research (NCTR-E0728711), the National Institutes of Health (HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, CA127642, CA068484, HD052102, HD052104, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL109090, HL111459, HL095127, HD80002, HD028820), the Laura Coulter-Jones Foundation (Division of Pediatric Clinical Research), the Batchelor Foundation (Batchelor Children’s Research Institute), the Children’s Cardiomyopathy Foundation, Sofia’s Hope, Inc., the Kyle John Rymiszewski Foundation, the Children’s Hospital of Michigan Foundation, the Scott Howard Fund, and the Michael Garil Fund.
List of Abbreviations:
- AAP
American Academy of Pediatrics
- BMI
body mass index
- GERD
gastroesophageal reflux disease
- LAGB
laparoscopic adjustable gastric band
- MBS
metabolic and bariatric surgery
- NHB
non-Hispanic Black
- NHW
non-Hispanic White
- IRB
institutional review board
- IQR
interquartile
- RYGB
Roux-en-Y gastric bypass
- SD
standard deviation
- TBWL%
total body weight loss percentage
- T2D
Type 2 diabetes
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.
References
- 1.Skinner AC, Ravanbakht SN, Skelton JA, et al. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 2018;141(3):e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Perrin EM, Moss LA, et al. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med 2015;373(14):1307–1317. [DOI] [PubMed] [Google Scholar]
- 3.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer--viewpoint of the IARC working group. N Engl J Med 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004;110(10):1245–1250. [DOI] [PubMed] [Google Scholar]
- 5.Messiah SE, Lopez-Mitnik G, Winegar D, et al. Changes in weight and co-morbidities among adolescents undergoing bariatric surgery: 1-year results from the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis 2013;9(4):503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poliakin L, Roberts A, Thompson KJ, et al. Outcomes of adolescents compared with young adults after bariatric surgery: an analysis of 227,671 patients using the MBSAQIP data registry. Surg Obes Relat Dis 2020;16(10):1463–1473. [DOI] [PubMed] [Google Scholar]
- 7.Lopez EH, Munie S, Higgins R, et al. Morbidity and mortality after bariatric surgery in adolescents versus adults. J Surg Res 2020;256:180–186. [DOI] [PubMed] [Google Scholar]
- 8.Inge TH, Coley RY, Bazzano LA, et al. Comparative effectiveness of bariatric procedures among adolescents: the PCORnet bariatric study. Surg Obes Relat Dis. 2018;14(9):1374–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolling CF, Armstrong SC, Reichard KW, et al. Metabolic and bariatric surgery for pediatric patients with severe obesity. Pediatrics 2019;144(6):e20193224. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong S AAP guidance calls for better access to bariatric surgery for teens with severe obesity. Accessed April 28, 2021. (https://www.aappublications.org/news/2019/10/27/bariatricsurgery102719) [Google Scholar]
- 10.Messiah SE, Xie L, Atem F, et al. Disparity between United States adolescent class II and III obesity trends and bariatric surgery utilization, 2015–2018. Ann Surg 2020; 10.1097/SLA.0000000000004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra S, Czepiel KS, Akam EY, Shaw AY, Sivasubramanian R, Seetharaman S, Stanford FC. Bariatric surgery in the treatment of adolescent obesity: current perspectives in the United States. Expert Rev Endocrinol Metab. 2021. Apr 21:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts CA. Physical and psychological effects of bariatric surgery on obese adolescents: a review. Front Pediatr 2021;8:591598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarno LA, Lipshultz SE, Harmon C, et al. Short- and long-term safety and efficacy of bariatric surgery for severely obese adolescents: a narrative review. Pediatr Res 2020;87(2):202–209. [DOI] [PubMed] [Google Scholar]
- 14.Sjöström L Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273(3):219–234. [DOI] [PubMed] [Google Scholar]
- 15.De La Cruz-Muñoz N, Lopez-Mitnik G, et al. Effectiveness of bariatric surgery in reducing weight and body mass index among Hispanic adolescents. Obes Surg 2013;23(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med 2016;374(2):113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olbers T, Beamish A, Gronowitz E, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: a prospective five-year Swedish nationwide study (AMOS). Lancet Diabetes Endocrinol 2017; 5(3): 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inge TH, Jenkins TM, Xanthakos SA, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol 2017;5(3):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alqahtani Aayed R., et al. Ten-Year Outcomes of Children and Adolescents Who Underwent Sleeve Gastrectomy: Weight Loss, Comorbidity Resolution, Adverse Events, and Growth Velocity, Journal of the American College of Surgeons, 2021. (In Press) 10.1016/j.jamcollsurg.2021.08.678 [DOI] [PubMed] [Google Scholar]
- 20.O’Brien PE, Hindle A, Brennan L, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg 2019;29(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moroshko I, Brennan L, O’Brien P. Predictors of attrition in bariatric aftercare: a systematic review of the literature. Obes Surg 2012;22(10):1640–1647. [DOI] [PubMed] [Google Scholar]
- 22.Derderian SC, Patten L, Kaizer AM, et al. Influence of weight loss on obesity-associated complications after metabolic and bariatric surgery in adolescents. Obesity (Silver Spring) 2020;28(12):2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hair JF, Black WC, Babin BJ, et al. Pearson new international edition. In Multivariate data analysis, Seventh Edition 2014. (Pearson Education Limited Harlow, Essex: ) [Google Scholar]
- 24.de la Cruz-Muñoz N, Messiah SE, Cabrera JC, et al. Four-year weight outcomes of laparoscopic gastric bypass surgery and adjustable gastric banding among multiethnic adolescents. Surg Obes Relat Dis 2010;6(5):542–547. [DOI] [PubMed] [Google Scholar]
- 25.Woolford SJ, Clark SJ, Gebremariam A, et al. To cut or not to cut: physicians’ perspectives on referring adolescents for bariatric surgery. Obes Surg 2010;20(7):937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong W, Tomlinson G, Feig D, Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? American Journal of Obstetrics and Gynecology 2018;218(6):573–580 [DOI] [PubMed] [Google Scholar]
- 27.Kansra AR, Lakkunarajah S, Jay MS. Childhood and adolescent obesity: a review. Front Pediatr 2021;8:581461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward ZJ, Long MW, Resch SC, et al. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med 2017;377(22):2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mocanu V, Lai K, Dang JT, et al. Evaluation of the trends, characteristics, and outcomes in North American youth undergoing elective bariatric surgery. Obes Surg 2021;31(5):2180–2187. [DOI] [PubMed] [Google Scholar]
- 30.Grant HM, Perez-Caraballo A, Romanelli JR, Tirabassi MV. Metabolic and bariatric surgery is likely safe, but underutilized in adolescents aged 13–17 years. Surg Obes Relat Dis 2021;17(6):1146–1151. [DOI] [PubMed] [Google Scholar]
- 31.Michalsky M, Inge T, Jenkins T, et al. Cardiovascular risk factors after adolescent bariatric surgery. Pediatrics 2018. Feb;141(2):e20172485. doi: 10.1542/peds.2017-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.English WJ, DeMaria EJ, Hutter MM, et al. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis 2020;16(4):457–463. [DOI] [PubMed] [Google Scholar]
- 33.Daigle CR, Brethauer SA, Tu C, et al. Which postoperative complications matter most after bariatric surgery? Prioritizing quality improvement efforts to improve national outcomes. Surg Obes Relat Dis 2018;14(5):652–657. [DOI] [PubMed] [Google Scholar]
- 34.Cuellar-Barboza AB, Frye MA, Grothe K, et al. Change in consumption patterns for treatment-seeking patients with alcohol use disorder post-bariatric surgery. J Psychosom Res. 2015;78(3):199–204. [DOI] [PubMed] [Google Scholar]
- 35.Spadola CE, Wagner EF, Accornero VH, Vidot DC, de la Cruz-Munoz N, Messiah SE. Alcohol use patterns and alcohol use disorders among young adult, ethnically diverse bariatric surgery patients. Subst Abus. 2017;38(1):82–87. [DOI] [PubMed] [Google Scholar]
- 36.Spadola CE, Wagner EF, Varga LM, Syvertsen JL, De La Cruz Munoz NF, Messiah SE. A Qualitative Examination of Increased Alcohol Use after Bariatric Surgery among Racially/Ethnically Diverse Young Adults. Obes Surg. 2018;28(6):1492–1497. [DOI] [PubMed] [Google Scholar]
- 37.Spadola CE, Wagner EF, Dillon FR, Trepka MJ, De La Cruz-Munoz N, Messiah SE. Alcohol and Drug Use Among Postoperative Bariatric Patients: A Systematic Review of the Emerging Research and Its Implications. Alcohol Clin Exp Res. 2015;39(9):1582–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis C-A, de Jersey S, Seymour M, Hopkins G, Hickman I, Osland E. Iron, vitamin B 12, folate and copper deficiency after bariatric surgery and the impact on anaemia: a systematic review. Obes Surg. 2020; 30(11):4542–4591. 5. [DOI] [PubMed] [Google Scholar]
- 39.Weng T-C, Chang C-H, Dong Y-H, Chang Y-C, Chuang L-M. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. BMJ Open. 2015;5(7):e006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon Y, Ha J, Lee YH, et al. Comparative risk of anemia and related micronutrient deficiencies after Roux-en-Y gastric bypass and sleeve gastrectomy in patients with obesity: An updated meta-analysis of randomized controlled trials. Obes Rev. 2022;23(4):e13419. [DOI] [PubMed] [Google Scholar]
- 41.Kang JH, Le QA. Effectiveness of bariatric surgical procedures: A systematic review and network meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(46):e8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C, Wang FG, Yan WM, Yan M, Song MM. Clinical outcomes of sleeve gastrectomy versus Roux-En-Y gastric bypass after failed adjustable gastric banding. Obes Surg 2019;29(10):3252–3263. [DOI] [PubMed] [Google Scholar]
- 43.White MA, Masheb RM, Burke-Martindale C, Rothschild B, Grilo CM. Accuracy of self-reported weight among bariatric surgery candidates: the influence of race and weight cycling. Obesity (Silver Spring) 2007;15(11):2761–2768. [DOI] [PubMed] [Google Scholar]
- 44.Ross KM, Eastman A, Wing RR. Accuracy of self-report versus objective smart-scale weights during a 12-week weight management intervention. Obesity (Silver Spring) 2019;27(3):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messiah SE, Lopez-Mitnik G, Winegar D, et al. Effect of ethnicity on weight loss among adolescents 1 year after bariatric surgery. World J Diabetes 2013;4(5):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.