Abstract
Respiratory infectious diseases (H1N1, H5N1, COVID-19, etc.) are pandemics that can continually spread in the air through micro-droplets or aerosols. However, the detection of samples in gaseous media is hampered by the requirement for trace amounts and low concentrations. Here, we develop a wearable bioelectronic mask device integrated with ion-gated transistors. Based on the sensitive gating effect of ion gels, our aptamer-functionalized transistors can measure trace-level liquid samples (0.3 μL) and even gaseous media samples at an ultra-low concentration (0.1 fg/mL). The ion-gated transistor with multi-channel analysis can respond to multiple targets simultaneously within as fast as 10 min, especially without sample pretreatment. Integrating a wireless internet of things system enables the wearable mask to achieve real-time and on-site detection of the surrounding air, providing an alert before infection. The wearable bioelectronic masks hold promise to serve as an early warning system to prevent outbreaks of respiratory infectious diseases.
Keywords: ion-gated transistors, ionic gel, respiratory infectious diseases, gaseous media, bioelectronic devices, biosensing
Graphical abstract
Progress and potential
Developing wearable electronics capable of detecting viruses directly from airborne media is crucial for rapidly diagnosing respiratory infectious diseases. However, wearable sensor platforms for analyzing airborne media remain underexplored. Here, a wearable bioelectronic mask device integrated with ion-gated transistors is developed for testing viral proteins. A stretchable ionic gel synthesized by a two-solvent system works as a dielectric layer for the ion-gated transistors. The device can detect trace liquid samples and gaseous media samples. Advantages of low detection limit (0.1 fg/mL), rapid response (10 min), and multi-channel analysis make the bioelectronic mask a versatile detection platform for various respiratory infectious diseases. The integration of internet of things technology facilitates real-time on-site detection of surrounding air. This wearable bioelectronic mask is expected to serve as an early warning system to prevent outbreaks of respiratory infectious diseases.
We demonstrate a wearable bioelectronic mask for detecting airborne respiratory infectious disease viruses. The device is developed based on multi-channel ion-gated transistors and integrated internet of things technology. It exhibits fast response, excellent selectivity, real-time on-site monitoring, and ultra-low detection limits for detecting target-containing trace solutions and gaseous media. The bioelectronic mask could be utilized as an early warning diagnostic tool to help prevent outbreaks of respiratory infectious diseases.
Introduction
Respiratory infectious diseases are caused by pathogens invading and infecting the respiratory system. These diseases spread worldwide and threaten human life and health, such as the avian influenza virus H5N1 in 2003, the influenza virus H1N1 in 2009, and the coronavirus disease 2019 (COVID-19).1 , 2 , 3 , 4 During the incubation period of human-to-human transmission, respiratory viruses can continuously transmit in air through droplets or aerosols.5 Studies indicate that a single cough or sneeze, or even a few minutes of speech, can produce thousands of infectious virus-containing droplets that can remain suspended in the air for a long time as transmission media.6 , 7 , 8 Hence, direct detection of viruses in the air may be the most appealing approach for the early diagnosis of major infectious diseases. However, the direct analysis of airborne media has been underutilized for the diagnosis of respiratory infectious disease. Current clinical diagnosis methods, such as quantitative polymerase chain reaction with reverse transcription (RT-PCR), enzyme-linked immunosorbent assay (ELISA), and isothermal nucleic acid amplification technologies,9 , 10 , 11 , 12 are mainly focused on analyzing the samples collected from infected/uninfected individuals. It is challenging for those diagnosis methods to pre-diagnose the presence of the virus in the surrounding air. Moreover, these diagnostic techniques require sample pretreatment and specialized technicians, which hardly meet the requirement of on-site and point-of-care detection.13 , 14 , 15 Hence, detection technologies for gaseous media that are real-time, fast, and portable would be conducive to slowing down the spread of respiratory diseases and alleviating the testing bottleneck.
Wearable electronics exhibit great potential in healthcare applications. Their advantages of portability and sustainable real-time monitoring can provide an early and timely alert, which is significant to avoiding the large-scale spread of respiratory infectious diseases. However, little work has been reported on using wearable bioelectronic devices to analyze those important gaseous media (droplets and aerosols before reaching the human respiratory system) during transmissions.16 In another aspect, wearable electronics for trace infectious virus detection in the gaseous media need to meet the challenging requirement of high-sensitivity response. Recently, several types of biosensors have been developed for virus detection by using electrochemical (voltammetry and impedance)17 , 18 , 19 and optical techniques (surface plasmon resonance, fluorescence, and luminescence).20 , 21 , 22 , 23 Among them, ion-gated transistors (IGTs) have attracted wide attention in the field of bioelectronics due to their output signal amplification effect, mechanical flexibility, easy manufacture, and miniaturization.24 , 25 , 26 , 27 , 28 Through the electric double layer (EDL) effect of the ionic gel, a slight change of the effective gate voltage can significantly induce the channel current change. The efficient field amplification of the input signal enables high sensitivity and low noise recording. Moreover, IGTs are all-solid transistors with ionic gel as the dielectric layer, which allows a high degree of electronic integration and has an advantage in liquid-free circuits over other electrolyte-gated transistors.29 , 30 From this perspective, IGTs are attractive for detecting respiratory infectious diseases based on gaseous media, although the research in this area is almost non-existent.
Herein, we demonstrate a wearable bioelectronic mask system integrated with IGTs. Stretchable polyvinyl alcohol (PVA)-based ionic gels were prepared as dielectric layers for flexible electronics. We adopted a double-solvent system to improve the compatibility of lipophilic ionic liquids and hydrophilic PVA to form a homogeneous ionic gel network, resulting in long-term stability, high capacitance (16.5 μF cm−2), and great ionic conductivity (5.95 × 10−3 S/cm at 25°C). With the strong carrier induction and significant gating effect of the ionic gel dielectric layer, the IGT device exhibits excellent field amplification performance, enabling low-concentration detection of 0.1–10 fg/mL for viral proteins of various respiratory infectious diseases (COVID-19, H1N1, and H5N1). Our device can be sensitive to trace-level liquid samples as low as 0.3 μL, which is much smaller than the volume of virus-containing droplets produced by coughing/talking. Notably, due to its high sensitivity and all-solid feature (without external ionic sources as electrolytes), the polymerized ionic liquid (PIL)-IGT can also detect gaseous media targets, such as atomizing gas and carrier gas samples containing viral proteins. Detection signals can be obtained as fast as 10 min, even without sample pretreatment. Moreover, integrated with the internet of things (IoT) system, our bioelectronic masks can be operated and monitored on mobile devices anytime and anywhere, enabling free movement and real-time monitoring of surrounding air. Thus, our bioelectronic masks are expected to be used for personal protection and early warning during a respiratory infectious disease pandemic.
Results and discussion
Overview of the bioelectronic mask
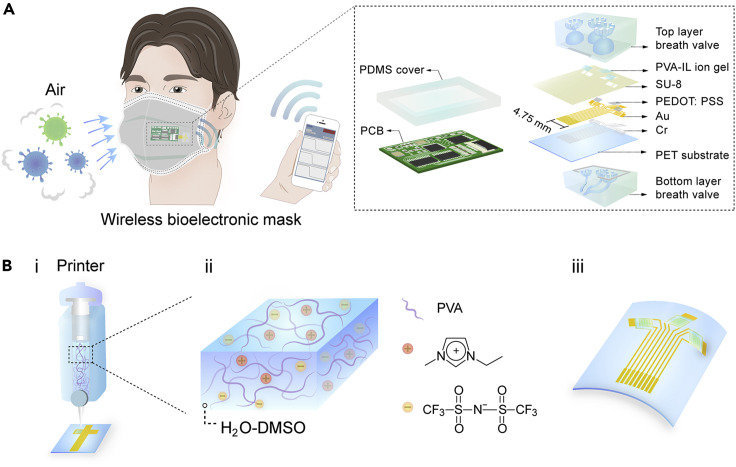
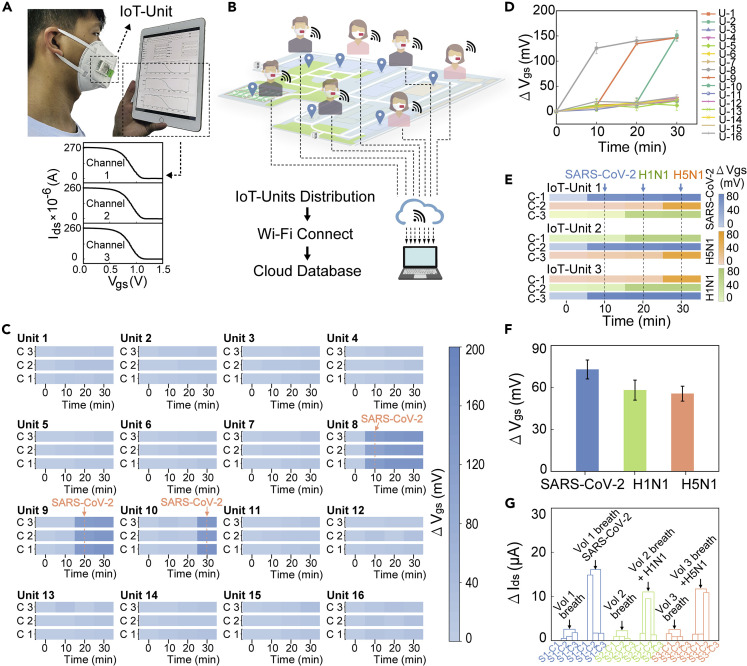
The wireless bioelectronic mask is shown in the schematic illustration in Figure 1A. When people wear the mask, the bioelectronic device mounted outside the mask can directly analyze infectious viruses in the air. Meanwhile, the wireless data feedback is acquired on the mobile device in real time. The bioelectronic mask incorporates an IGT device with poly(2,3-dihydrothieno-1,4-dioxin)-poly(styrenesulfonate) (PEDOT:PSS) as channel materials, together with a breath valve and a printed circuit board (PCB), as shown in the enlarged view of Figure 1A. The three-channel PIL-IGT is patterned with rhombohedral PVA-IL ion gel on the electrodes. The device is based on a thin and flexible polyethylene terephthalate (PET) substrate, making it well suited for the bending surface of the facemask. Moreover, a detachable breath valve is designed to encapsulate the detection ports of PIL-IGT, and protect the device from dust contamination and damage.
Figure 1.
Design and application of the wireless bioelectronic mask
(A) Schematic diagram of the bioelectronic mask device and the enlarged schematic illustration of a PIL-IGT, a breath valve, and a PCB.
(B) Schematic diagram of (i) the fabrication process of PVA-IL ion gel as a dielectric layer of PIL-IGT by 3D printing, (ii) composition of PVA-IL ion gel, and (iii) flexible PIL-IGT.
Studies have demonstrated that gas exchange in the human body occurs through exhalation and inhalation, with an average expiratory volume of approximately 0.5 L and a respiratory rate of 12 times per minute.31 , 32 Thus, through a breath valve composed of top and bottom layers, human breathing is utilized to form the pumping power and inhale the target gas outside the facemask. The fine flower-shaped tubes inside the top breath valve aim to introduce and gather gas in three semicircular chambers to increase the content of the gas target. The bottom breath valve serves as the supporting substrate of PIL-IGT, mainly through the multi-branch merged design of pipes to prolong the gas outflow time (see Experimental procedures for details). The PIL-IGT is controlled and driven by a customized PCB (connected through the zero insertion force connectors) with a wireless module and is protected by a flexible polydimethylsiloxane cover.
Fabrication, operational principles, and performance of PIL-IGT
Dielectric materials play a crucial role in controlling charge carriers in transistors. Thus, it is essential to design the ionic gel dielectric layer with large capacitance and high induction of carriers. In this work, PVA with good biocompatibility was employed as the main body of ionic gel. The ionic conductivity of the ionic gel was improved by incorporating 1-ethyl-3-methylimidazole bis(trifluoromethylsulfonyl) imide (EMIM:TFSI) ionic liquid (IL), denoted as PVA-IL (Figure 1Bi and 1Bii). To enhance the dispersibility of lipophilic EMIM:TFSI in PVA, a double-solvent system composed of water (H2O) and dimethyl sulfoxide (DMSO) was employed as the dispersion medium. As a suitable solvent for ionic liquid, binary solvents facilitate the formation of a homogeneous gel network and the migration of ions. Furthermore, the hydrogen bonding between the H2O/DMSO binary solvent and water molecules hinders the evaporation of free water and provides anti-drying stability for the PVA-IL ion gel. A PAV-IL ion gel of 0.2 mm was patterned on three independent channels of PIL-IGT via three-dimensional (3D) printing (Figures 1Biii and S1, and see Note S1 for details). In the high-resolution FT-IR spectra (Figure S2), these peaks (3,380, 1,349, and 1,100 cm−1) attributable to PVA presented small redshifts due to the weak hydrogen bonds between PVA and EMIM:TFSI. New peaks belonging to EMIM:TFSI appeared at 1,188, 1,057, and 654 cm−1, respectively.33 , 34 In the XPS patterns of PVA-IL ion gel (Figure S3), the characteristic peaks of F 1s, N 1s, and S 2p from EMIM:TFSI were found at 687.2, 400.4, 397.8, 168.5, and 167.3 eV, respectively. Especially, the peaks of C 1s (Figure 2A) could be deconvoluted into TFSI (291.2 eV), EMIM (283.4, 284.6, 284.0, and 285.2 eV), and PVA (284.8 eV), respectively.35 , 36 The above results indicate the successful synthesis of PVA-IL ion gel.
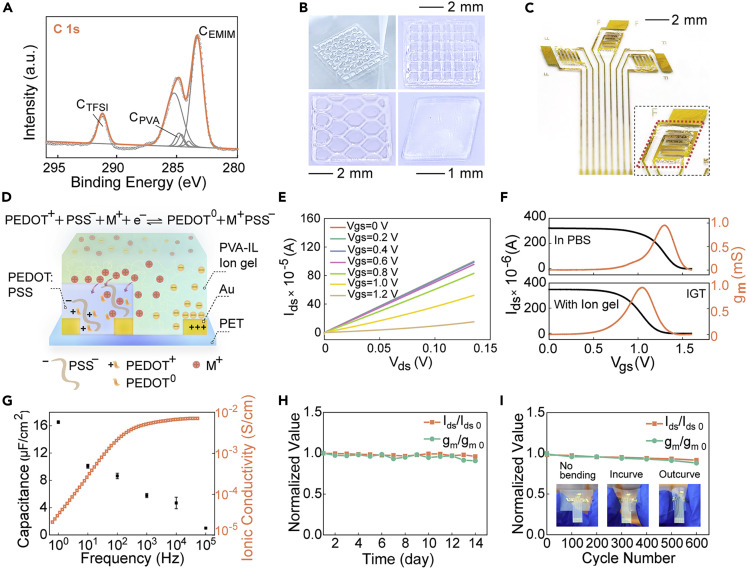
Figure 2.
Performance and operational principles of PIL-IGT
(A) High-resolution XPS spectra of O 1s in PVA-IL ion gel.
(B) PVA-IL ion gels of different sizes and patterns were formed through 3D printing. Scale bars denote 1 mm for the lower right panel and 2 mm for remaining two panels.
(C) Structure of three-channel PIL-IGT with PVA-IL ion gel. Inset: an enlarged view of the PVA-IL ion gel on the electrodes. Scale bar denotes 2 mm.
(D) Operation principles of PIL-IGT.
(E) Output characteristic curves of PIL-IGT.
(F) Transfer characteristic curves of PIL-IGT and ion-gel-free transistor with solution as dielectric layer (Vds = 0.04 V).
(G) Frequency dependence of capacitance and ionic conductivity for the PVA-IL ion gel. The error bars represented the standard deviation calculated from at least three sets of tests.
(H) Repeatability of PIL-IGT stored at 4°C in 14 days with stable normalized values of gm/gmo and Ids/Idso.
(I) Bending resistance of PIL-IGT in 600 cycles (with one inward bending and one outward bending as one round) with stable normalized values of gm/gmo and Ids/Idso. Inset: schematic diagram of PIL-IGT without bending, with inward bending, and with outward bending.
To optimize the field-effect performance of PIL-IGT, the appropriate ratio of each component in the PVA-IL ion gel was adjusted. Only a single solvent system of H2O or unbalanced ratios of gel and ionic liquid would lead to poor performance of PIL-IGT (including low currents or weak gating effects). This is probably attributed to the easy volatilization of the unitary solvent and weak ionic conductivity (Figure S4; Table S1). In addition, the printable feature of PVA-IL ion gel simplifies the fabrication of transistors. Using 3D printing to form the dielectric layer, the PVA-IL ion gel can be easily patterned into various shapes and sizes to match different transistor electrode designs, especially overcoming the limitation of the substrates (including glass, PET, etc.) (Figure 2B). Programmable printing can fabricate large-scale PIL-IGTs based on homogeneous PVA-IL ion gel (Figure S5A), which is conducive to reducing device-to-device variation. Furthermore, we can precisely control the coverage of PVA-IL ion gel through fixed-point printing. It was patterned only on a small area of gate electrodes (Figure 2C and the red rhombus dotted frame in the inset), leaving the rest exposed for detection.
The operating principles of PIL-IGT with PVA-IL ion gel as a dielectric layer are displayed in Figure 2D. When a positive voltage is applied to the gate electrode, the negative ions in the PVA-IL ion gel migrate and accumulate on the ion gel/gate electrode interface, and the positive ions migrate toward the ion gel/PEDOT:PSS channel layer to form EDL. Meanwhile, PEDOT:PSS can be injected and doped by partial positive ions in PVA-IL ion gel due to its ionic conductivity.37 There is a reaction existing in PEDOT:PSS as follows:
| (Equation 1) |
The doping of positive ions promotes the reaction to proceed in the positive direction. It competes for the negatively charged groups (PSS−), resulting in the conductive main body (PEDOT−) in the active layer returning to neutrality (PEDOT0),38 , 39 which manifests as a decrease in the current between the source electrode and the current electrode.
The electrical performance of PIL-IGT was assessed by the output and transfer characteristic curves. In the output curves (Figure 2E), the source-drain current (Ids) decreased with the increased gate-source voltage (Vgs) in steps of 0.2 V, which demonstrated an excellent gating effect of gate voltage in the presence of PVA-IL ion gel. Moreover, the relationship curve of source-drain current and source-drain voltage indicated a great ohmic contact between the PVA-IL ion gel and the channel materials, which may benefit from the fluidity of the ionic gel before molding and its flexibility after molding. Notably, the gating performance of PIL-IGT was more remarkable than that of the same electrodes replaced with phosphate-buffered saline (PBS) solution as a dielectric layer, with an enormous increase in the amplitude of current change (Figure S6). The PIL-IGT’s transfer curves and transconductance curves were compared with the ion-gel-free transistor utilizing solutions as the dielectric layer (Figure 2F). The gate voltage corresponding to the maximum transconductance of PIL-IGT was dropped by 0.29 V. The turn-off voltage of the PIL-IGT device was also reduced by 0.24 V, which is much smaller than that of the ion-gel-free transistor, indicating the lower operating voltage of the PIL-IGT. The transfer curves of PIL-IGT under dual sweep and the leakage current are presented in Figure S7. At the dual sweep mode, a slight hysteresis of the transfer curve was observed, attributed to the doping of the channel material by mobile ions in the dielectric layer.
In Figure S8, the device-to-device deviations of PIL-IGT are presented through the histograms of threshold voltage (Vth), transconductance (gm), and mobility (μ). Narrow distributions of Vth, gm, and μ are observed with relative standard deviations of 0.87%, 1.31%, and 0.83%, respectively (Table S2). The slight device-to-device deviation is attributed to the quantitative and uniform gel dielectric layers prepared by 3D printing on the PIL-IGT. The relationship between the specific capacitance and the frequency of a 3 mm thick PVA-IL ion gel was measured by being sandwiched between two electrodes (Figure 2G, see Nyquist diagram and the relationship diagram between phase angle and frequency in Figure S9). The capacitance of PVA-IL ion gel reached 16.5 μF cm−2 at 1 Hz and remained above 1.3 μF cm−2 at a high frequency (104 Hz), much higher than that of traditional dielectric materials (e.g., 11.5 nF cm−2 capacitance of 300 nm SiO2 40). In addition, the ionic conductivity as a function of frequency indicated the ability of the PVA-IL ion gel to store substantial charges.41 The high ionic conductivity, up to about 5.95 × 10−3 S/cm at 25°C (see comparison in Table S3), is beneficial for efficient ion transport and rapid fast switching response.42 , 43 Therefore, the large capacitance and high induced carrier concentration of the PVA-IL ion gel endow PIL-IGT with excellent field-effect performance.
Moreover, the transparent PVA-IL ion gel exhibited recoverable elasticity under a strain over 400% to satisfy the flexibility demands of wearable devices (Figures S10 and S11, and see Note S2 for details). We found that, compared with the hydrogel system without ionic liquid, the volume of PVA-IL ion gel decreased much less in the same period (Figure S12). This enables PVA-IL ion gel to remain stable as electronic layers on wearable IGT-based electronics. The almost constant normalized values of gm/gm0 and Ids/Ids0 further validated the long-term stability of PIL-IGT when tested for continuous 14 days (Figures 2H and S13, see comparison with other IGTs in Table S4). Moreover, in the 600 cycles of bending trials (with one inward bending and one outward bending as one round), we found that the normalized gm/gm0 and Ids/Ids0 of PIL-IGT had no apparent variation (Figures 2I and S14). This demonstrated the capacity of PIL-IGT to maintain stable electrical performance under bending deformation.
EMIM:TFSI, as the essential component in the ionic gel, has exhibited extremely low volatility and high thermal stability. The EMIM:TFSI showed almost no significant mass loss after being placed at different temperatures (25°C, 50°C, and 100°C) for as long as 24 h (Figure S15). Moreover, gas samples passing through the surface of the PVA-IL ion gel were further verified by GC-MS. For the volatiles of the freshly prepared PVA-IL ion gels, no characteristic signals of DMSO and EMIM were found in the GC-MS spectrum (Figure S16), which indicated that neither DMSO nor ionic liquid was detected in the gas samples. That is because the volume of ion gel patterned on each electrode of the PIL-IGT is as little as ∼1 μL (Figure S5B). Moreover, the content of volatile components in the PVA-IL ion gel is even more negligible after further filtering through the non-woven mask. Furthermore, we conducted the cell live/dead staining for the biocompatibility evaluation of ion gel. We first collected gases containing ion gel volatiles for cytotoxicity experiments, which simulates respiratory airflow across the gel surface. Human embryonic kidney 293T cells were added to the culture medium after volatile gas treatment and incubated at 37°C for 24 h (see supplemental information for details). The cells were in a healthy growth state (Figure S17A). The viability of cells with the addition of volatile gas remained at about 98.8% (Figure S17B). We further examined the cytotoxicity of ion gel when directly co-culturing with cells. Three milligrams of ion gel was directly added to the 3 mL culture medium containing cells and co-cultured for 24 h. Confocal images showed that cells co-cultured with ion gel were in a healthy growth state with a great survival rate of 98.0%. These results imply that the PVA-IL ion gel in our device has excellent biocompatibility.
Detection of targets in trace liquid
The PIL-IGT was used to analyze the protein target by immobilizing multiple aptamers as biological probes. Aptamers are known for their uniquid adaptabilities to temperature, pH, and chemical environments.44 , 45 The specific aptamers were immobilized on the gold gate electrodes of PIL-IGT via thiol bonds. The aptamers generated configuration changes when combined with the specific target, resulting in a detectable electrical signal (Figure 3A). Several proteins of respiratory infectious diseases, including SARS-CoV-2, H1N1, and H5N1, were used to evaluate the responsive capability of PIL-IGT as a universal detection platform. It was found that the transfer curve of PIL-IGT presented a noticeable shift to a lower gate voltage level as the concentrations of SARS-CoV-2 spike proteins increased from 0.1 fg/mL to 10 ng/mL (Figure S18A). The aptamers subscribed to conformational rearrangements after binding to the protein targets. This affected the concentration and distribution of surface charges on the gate electrode and changed the effective gate voltage on the gate circuit. Thus, the gate voltage of PIL-IGT generated a monotonic decrease as the SARS-CoV-2 aptamers bound to the corresponding proteins (see inset in Figure 3B). The gate voltage response (ΔVgs) for the low-concentration target (0.1 fg/mL) was approximately 25 mV, indicating the high sensitivity of the PIL-IGT detection platform.
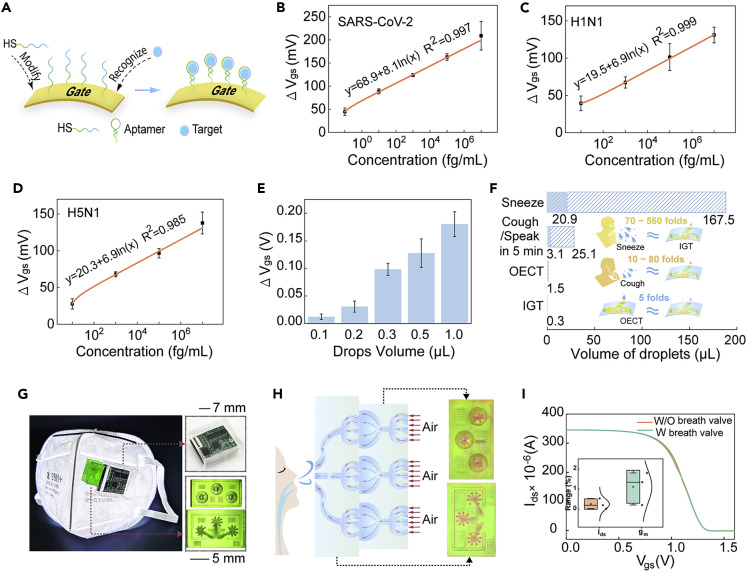
Figure 3.
Detection of targets in trace liquid
(A) Schematic diagram of gold gate electrodes functionalized with aptamers. Concentration-dependent voltage response curves of trace liquid (volume of 0.3 μL) containing (B) SARS-CoV-2 spike proteins, (C) H1N1 proteins, and (D) H5N1 proteins. (E) Voltage differences of PIL-IGT to SARS-CoV-2 spike proteins with different liquid volumes.
(F) Comparison between the volume of droplets produced by daily events (sneeze, cough, and talk) and the 0.3 μL droplets required for the test of PIL-IGT.
(G) Bioelectronic masks integrated with PIL-IGT, and the enlarged view of the PCB encapsulated in a soft PDMS cover, a bottom view of the top breath valve, and a top view of the bottom breath valve. Scale bars denote 7 mm for the upper right panel and 5 mm for the lower right panel.
(H) Schematic diagram of the airflow in the breath valve and the feasibility verification of the breath valve through the infiltration of red liquid.
(I) Transfer curves of PIL-IGT with and without breath valve. Inset: the deviation of Ids and gm of PIL-IGT with and without breath valve. The error bars in (B–E) represented the standard deviation calculated from at least three devices.
The logarithmic relationship between the ΔVgs and the SARS-CoV-2 spike protein concentration showed that a correlated response existed over a wide concentration range with a slope of 8.1 mV/decade (R2 = 0.997), which shows the sensitivity of PIL-IGT detection platform to SARS-CoV-2 spike protein. The detection of H1N1 and H5N1 proteins showed similar trends, with a gradual decrease in gate voltages over the target concentration range of 1 fg/mL to 10 ng/mL (Figures S18B and S18C). The logarithmic relationship curve of H1N1 proteins exhibited a R2 of 0.999 and a slope of 6.9 (Figure 3C). For the H5N1 proteins, the R2 was 0.985 with a similar slope of 6.9 (Figure 3D). The ultra-low limit of detection for each viral protein (Table S5) is attributed to the inherent amplification effect of the output signal of PIL-IGT. Furthermore, the specificity of PIL-IGT was verified by the absence of gate voltage shifts in the unfunctionalized device (Figure S19A).
The superiority of PIL-IGT to test targets in trace liquid was further demonstrated. In Figure 3E, the PIL-IGT showed an apparent gate voltage shift when subjected to the 0.3 μL of SARS-CoV-2 spike proteins at 10 fg/mL. Due to the sensitivity of PIL-IGT, even 0.1 and 0.2 μL of target liquid can evoke a specific voltage response (Figure S20). Respiratory infections can transmit through tiny droplets when people talk, cough, or sneeze. Small volumes prolong the suspension time of droplets in the air, thereby increasing the risk of propagation.46 Thus, the analysis of targets in trace liquid is significant for diagnosing respiratory infectious diseases. Droplet volumes under various conditions are compared in Figure 3F. One sneeze produces approximately 70–560 times6 , 47 more droplets than the 0.3 μL of liquid required for the PIL-IGT test (see Table S6 and Note S3 for details). The droplet volume generated within 5 min of talk is similar to that generated by coughing;8 both are about 10–80 times8 , 48 larger than 0.3 μL, far exceeding the detectable volume. Therefore, the PIL-IGT platform can measure virus-carrying droplets in the air to efficiently diagnose respiratory infectious diseases. Moreover, for the ion-gel-free device, the trace liquid target (0.3 μL) was insufficient to effectively connect the gate electrode and the channel layer to initiate the device operation; thus, the SARS-CoV-2 spike proteins cannot be detected (Figure S21A). In our results, the minimum volume required to operate an ion-gel-free transistor with the same electrode structure was 1.5 μL (Figure S22).
The PIL-IGT was integrated on a wearable mask to exhibit its function in more detection scenarios, as shown in Figure 3G. The detection unit comprises a micro-controller protected by a soft PDMS cover, a top breath valve layer, and a bottom breath valve layer with delicate internal structures (Figures S23 and S24). The device features plug-and-play and secondary regeneration (Figures S25 and S26). The test port of PIL-IGT is packaged in the breath valve, which is assembled outside the mask. The flow of air in the breath valve under human respiration is shown in Figure 3H. The target gas is inhaled from the top breath valve and accumulated in the semicircular chamber for sufficient contact with the PIL-IGT. The breathing process is simulated by the flow of red liquid from the top side to the bottom side of the breath valve (Figure S27). The filling of red liquid demonstrated the feasibility of fluid flowing through the internal pipeline structure of the breath valve (Figure S28, and see Note S4 for details). In addition, the average value deviations of Ids and gm remained almost the same in the absence or presence of the breath valve (Figure 3I). Thus, the breath valve could be used to encapsulate the device for protection without interfering with target detection.
Detection of targets in gaseous media
The long-distance transmission of respiratory viruses is through tiny droplets and aerogel in the air. Therefore, we evaluated the measurement in gaseous media (Figure 4A). An atomizer was utilized to simulate the propagation of aerosols in an open environment. The atomizing gas generated by the atomizer is as large as about 3–5 μm in size, approximating the size of droplets (<4.7 μm49) in the air. When PIL-IGT was exposed to the SARS-CoV-2 spike proteins in atomizing gas, the gate voltage shifts were consistent with those in trace liquid sample detection (Figure 4B). Similarly, the PIL-IGT also showed a gradient decrease of gate voltage to the atomizing gas containing H1N1 and H5N1 proteins, respectively (Figures 4C and 4D), indicating the ability of PIL-IGT to test targets in atomizing gas.
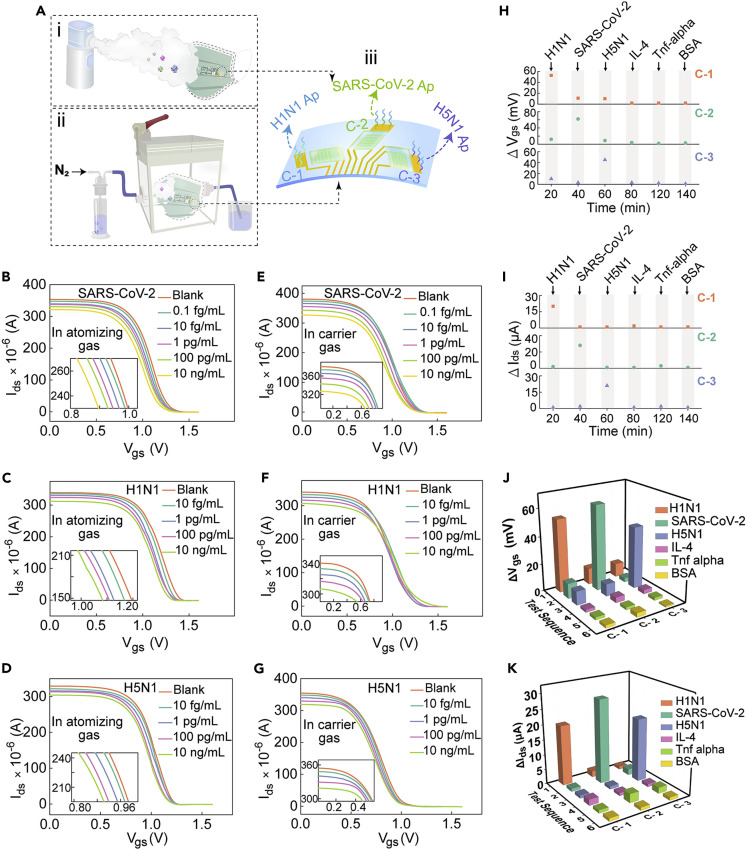
Figure 4.
Detection of targets in gaseous media
(A) Schematic diagram of PIL-IGT testing atomizing gas and carrier gas containing SARS-CoV-2 spike proteins, H1N1 proteins, and H5N1 proteins, and the functionalization of multi-channel with H1N1 aptamers, SARS-CoV-2 aptamers, and H5N1 aptamers, respectively (the Ap in the figure was the abbreviation of aptamer). Voltage responses of PIL-IGT to atomizing gas containing (B) SARS-CoV-2 spike proteins, (C) H1N1 proteins, and (D) H5N1 proteins of different concentrations, respectively (Vds = 0.04 V). Inset: partially enlarged views of the response curves. Current responses of PIL-IGT to carrier gas containing (E) SARS-CoV-2 spike proteins, (F) H1N1 proteins, and (G) H5N1 proteins of different concentrations, respectively (Vds = 0.04 V). Inset: partially enlarged views of the response curves. Specific selectivity and the simultaneous response of PIL-IGT with three channels to targets (H1N1, SARS-CoV-2, H5N1, IL-4, TNF-α, and BSA) in the form of (H) atomizing gas and (I) carrier gas. Comparison of the voltage/current changes of PIL-IGT with different targets in the form of (J) atomizing gas and (K) carrier gas.
A gas measuring chamber with good air tightness approximates to an indoor environment for testing, and the carrier gas was used to simulate the spread of viruses in the air. Nitrogen, as the main air component, was utilized as a carrier gas to flow through the viral protein-contained liquid and into the sealed chamber where the PIL-IGT device was placed. Figures 4E, 4F, and 4G correspond to the detection results of SARS-CoV-2 spike proteins, H1N1 proteins, and H5N1 proteins in the carrier gas by the PIL-IGT platform, respectively. The Ids of PIL-IGT decreased monotonically with the increase of target concentrations (from 0.1 fg/mL to 10 ng/mL). The test port of PIL-IGT was thoroughly combined with more targets due to the relatively closed detection environment and the continuous introduction of carrier gas, resulting in a decrease in the total channel current as an output signal. The relationship curves between the gate voltage/channel current and the target concentrations in atomizing gas or carrier gas are shown in Figures S29 and S30. No remarkable difference in output signal was observed when the aptamer-unfunctionalized PIL-IGT was used to measure the atomizing gas/carrier gas containing protein targets, indicating the specific recognition of the device (Figure S19B and S19C). Moreover, the bare transistors without the ionic gel layer failed to respond to atomizing gas or carrier gas targets (Figures S21B and S21C). In contrast, the all-solid PIL-IGT successfully detected targets in the form of droplets or aerosols, free from the limitation of liquid samples. The actual test setups are shown in the Figure S31.
Selectivity is one of the most important capabilities in evaluating the PIL-IGT detection platform. IL-4 and TNF-α, two inflammatory factors induced by respiratory infectious diseases, as well as BSA, one common protein applied in biological modification, were used as the control groups to verify the selectivity of PIL-IGT to these target (H1N1 proteins, SARS-CoV-2 spike proteins, and H5N1 proteins). The three-channel PIL-IGT exerted superiority in the simultaneous measurement of multiple targets. As shown in Figure 4Aiii, each gate electrode of PIL-IGT was functionalized with different biological probes (channels 1, 2, and 3 correspond to H1N1 aptamers, SARS-CoV-2 aptamers, and H5N1 aptamers, respectively). When PIL-IGT was sequentially exposed to multiple protein targets, including H1N1, SARS-CoV-2, H5N1, IL-4, TNF-α, and BSA in atomizing gas or carrier gas, the output signals of the three channels were generated simultaneously, and did not interfere with each other. The remarkable gate voltage shift or source-drain current occurred only when the channel with specific aptamers was bound to the corresponding protein target (Figures 4H and 4I). The H1N1 aptamer-functionalized channel 1 generated a more significant voltage difference (ΔVgs > 50 mV) for H1N1 than for control groups (Figure 4J). Similarly, channel 2 and channel 3 exhibited specific output signal changes to SARS-CoV-2 and H5N1, respectively. The detecting results from channel 2 and channel 3 exhibited several times or even ten times higher response-signal than the control groups. Moreover, the high selectivity of PIL-IGT was further demonstrated by testing various targets in the carrier gas, with reduced current values (ΔIds > 20 μA) far more than those of the control groups (Figure 4K). The multi-channel analysis enables PIL-IGT to detect multiple targets simultaneously, which is crucial for screening epidemiological sources of various respiratory pathogens.
A wireless bioelectronic mask integrated with an IoT unit
When respiratory infectious diseases transmit in the air, pathogens can be carried by air to a certain distance and inhaled by uninfected individuals, which is one of the critical reasons for the epidemic.50 , 51 Therefore, we demonstrate a bioelectronic mask incorporated with a wireless IoT-based PIL-IGT detection platform (called an IoT unit) for the real-time detection of surrounding gaseous media. When worn, the bioelectronic mask is connected to the wireless network through an internal microcontroller with a remote-communication microchip (Figure 5A). The programmable application (APP) written for the bioelectronic mask allows the wearer to set parameters and monitor the test status. Once the PIL-IGT detection platform on the mask starts the measurement, the transfer characteristic curves of three channels can be presented in real-time on personal mobile terminals, such as smartphones and pads (see Video S1). A framework for early warning of epidemic events was developed based on a system composed of numerous networked IoT units (Figure 5B). This consisted of three parts: a sensing layer for IoT-assisted PIL-IGT detection wearable mask; a network layer with Wi-Fi connection; and a cloud server layer for database construction (see Figure S32 for details). After the IoT unit framework is established, the detection area of IoT units can cover the environments around human daily activities, because the devices can be distributed to various sites along with moving users in the form of wearable masks. Meanwhile, the real-time detections by the IoT units are carried out rapidly upon exposure to environmental viral targets. The detecting results (including the viral concentrations in the surrounding air) can be displayed on personal mobile terminals and alert people.
Figure 5.
Wireless bioelectronic mask in combination with IoT
(A) A user who wears the bioelectronic mask monitors the test curves on the pad in real time (upper panel) and the corresponding test curves of the IoT unit’s three channels (lower panel).
(B) Architecture diagram of the wireless IoT unit.
(C) Gate voltage changes of three channels of sixteen wireless masks IoT units at 0, 10, 20, and 30 min, respectively. The color bars from light to dark represent the gate voltage shifts in 0–200 mV. IoT units 8, 9, and 10 were subsequently exposed to SARS-CoV-2 spike proteins in atomizing gas after 10, 20, and 30 min.
(D) Average gate voltage decreases of three channels of sixteen devices at each test.
(E) Simultaneous responses of three channels of three devices to different targets (SARS-CoV-2 spike proteins, H1N1 proteins, and H5N1 proteins). The blue, orange, and cyan color bar shades correspond to the gate voltage offsets of the channels that functionalized with SARS-CoV-2 aptamers, H5N1 aptamers, and H1N1 aptamers, respectively.
(F) Average gate voltage changes of different IoT unit to the atomizing gas containing the same target.
(G) Source-drain current changes of three IoT units to the pure exhaled breath from three volunteers and the exhaled breath doped with different targets. The error bars in (D and F) represent the standard deviation calculated from at least three sets of tests.
Sixteen IoT units (marked from 1 to 16) functionalized with SARS-CoV-2 aptamers were used to simulate the exposure events in epidemics (Figures S33–S35). The massive results of all IoT units were run simultaneously and collected remotely on a laptop, enabling real-time monitoring. The gate voltage changes of each IoT unit’s three channels were detected every 10 min and were presented by the shades of the blue color bar, where the color gradation from light to deep indicated the voltage shifts in the range of 0–200 mV (Figure 5C). When the IoT units were not exposed to any target, no significant voltage shifts were observed, resulting in a similar light blue color across the three channels of each IoT unit. The voltage differences of each channel floated around 10 mV, representing the IoT unit’s excellent repeatability and stability in multiple tests. After exposing all IoT units to SARS-CoV-2 spike proteins (10 pg/mL) in atomizing gas for 10 min, the channel color of IoT units 8 to 10 began to deepen significantly with voltage changes exceeding 100 mV, which alerted to the situation of the gradual spread of the epidemic. At this point, users can choose to share the results with healthcare institutions for immediate help via the cloud database and provide essential information for early epidemic control. Notably, the responses generated by a short 10-min exposure to the target demonstrated the IoT unit’s ability for rapid detection. Due to the quasi-liquid nature of gels, the IGT devices can maintain an ordered elastic state to provide the mobility of ions at room temperature (RT).52
The effective identification of the IoT unit of the target was confirmed by comparing average voltage responses in three channels of each IoT unit (Figure 5D). The performance of the IoT unit in monitoring multiple diseases simultaneously was further validated via immobilizing different aptamers on the IoT unit’s three channels (Figure S36). The outputs of the three channels were represented by different color bars. The shades of blue, orange, and cyan color bars correspond to the voltage offsets for the channels functionalized with SARS-CoV-2 aptamers, H5N1 aptamers, and H1N1 aptamers, respectively (Figure 5E). The exposure to a specific target resulted in a remarkable color change in the corresponding channel. The comparison of three IoT units’ voltage shifts to the same target is displayed in Figure 5F, with a standard deviation of 5–6 mV. Finally, breath samples of three healthy volunteers were collected to assess the interference with IoT unit detection (Figure S37). Simultaneously, the simulated patient sample was prepared by mixing the exhaled breath with three target proteins. The response current values of the simulated samples were much higher than those of the healthy samples, indicating the successful discrimination of viral targets in naturally exhaled breath (Figure 5G). All these results indicate that the portable bioelectronic mask with an integrated IoT unit can provide real-time monitoring for users without interfering with daily activities, and issue early warnings before infection has occurred.
Conclusion
Overall, we demonstrated a wearable bioelectronic mask device based on IGTs with ionic gel as the dielectric layer. In the synthesis of PVA-IL ion gel, a double-solvent system was developed to promote each component’s compatibility and reduce volatility. The PVA-IL ion gel exhibited stability, high capacitance (16.5 μF cm−2), and ionic conductivity (5.95 × 10−3 S/cm at 25°C). PVA-IL ion gel-based PIL-IGT combined the merits of all-solid-state, decent field amplification effect, and mechanical stability. With the gating effect of PVA-IL ion gel, PIL-IGT exhibited excellent performance in detecting trace-level liquid samples (0.3 μL) and gaseous media samples. These advantages of low detection limit (0.1 fg/mL), fast response (10 min), and multi-channel analysis enabled the bioelectronic mask to be a universal detection platform for various respiratory infectious diseases, including H1N1, H5N1, and COVID-19. Moreover, integrating IoT technology on the wearable mask would facilitate wireless and real-time monitoring for personal protection and prevent infectious diseases in advance.
Experimental procedures
Resource availability
Lead contact
Further information and requests for resources and materials should be directed to and will be fulfilled by the lead contact, Yin Fang (yin_fang@tongji.edu.cn).
Materials availability
This study did not generate new, unique reagents.
Materials
Bovine serum albumin (BSA), (3-glycidoxypropyl) trimethoxysilane (GOPS), sodium dodecylbenzene sulfonate, and ethylene glycol were all purchased from Sigma-Aldrich. PVA and DMSO were purchased from Sinopharm Chemical Reagent (Shanghai, China), and EMIM:TFSI was purchased from Saen Chemical Technology (Shanghai, China). PEDOT:PSS (PH1000) was obtained from Heraeus. H1N1-specific aptamer (sequence of the aptamer: 5′-HS-AATTAACCCTCACTAAAGGGCTGAGTCTCAAAACCGCAATAACTGGTTGTATGGTCGAATAAGTTAA-3′) and H5N1-specific aptamer (sequence of the aptamer: 5′-HS-AATTAACCCTCACTAAAGGGCTGAGTCGAATAAGTTAA-3′), PBS (pH 7.4) were purchased from Sangon Biotech (Shanghai, China). The aptamer of SARS-CoV-2 spike protein functionalized with sulfhydryl was purchased from Angpu Tomai Biotechnology (Anhui, China). The following viral target proteins were purchased from Sino-Biological: SARS-CoV-2 (2019-nCoV) spike protein (40591-V08H), influenza A (H1N1) HA protein (11684-V08H), and influenza A (H5N1) HA protein (11062-V08H1). All aqueous solutions were prepared with ultrapure water.
Fabrication of IGT
The preparation process of PIL-IGT is shown in Figure S38. The PIL-IGT electrodes with a channel length of 0.64 mm and width of 1.18 mm were fabricated through multilayer lithography technology. The photoresist (BP212-37S) was spin-coated on the PET substrate and then exposed to ultraviolet light. The photoresist pattern was formed by using a developer (KMPPD238-II). The Cr electrode layer (10 nm) and Au electrode layer (100 nm) were deposited by thermal evaporation (see Figure S39 for details). Then the first layer of photoresist was stripped off. A PEDOT:PSS thin film pattern was prepared between the source and drain electrodes by the second photolithography session. The PEDOT:PSS dispersion (containing 5 wt % ethylene glycol, 0.1 wt % sodium dodecylbenzene sulfonate, and 1 wt % GOPS) was spin-coated on the photoresist pattern to form the channel layer of the PIL-IGT, and annealed at 100°C for 1 h. Finally, the SU-8 photoresist was used to form an insulating layer on the Au electrodes for protection.
Biofunctionalization of IGT
The PIL-IGT was rinsed with deionized water and ethanol, then cleaned with plasma ozone. After that, it was dried with a nitrogen gun. A 20 μM aptamer solution was prepared in PBS (the same as for SARS-CoV-2, H1N1, and H5N1) and mixed with 10 mM TCEP at a volume ratio of 99:1. The solution was activated at RT for 1 h to reduce the disulfide bond of the aptamer. The reduced aptamer was directly dropped on the gate electrodes of the PIL-IGT. Then, the PIL-IGT was incubated at RT for 18–24 h. After that, 1 mg/mL BSA was added to eliminate unspecific binding. Finally, the PIL-IGT was rinsed with PBS and dried with nitrogen. The biofunctionalized PIL-IGT could be used for subsequent experiments or stored at 4°C.
Fabrication of PVA-IL ion gel
The PVA-IL ion gel was synthesized by a one-step hydrothermal method: 0.65 g of PVA was added to a solvent system composed of water (3.5 g) and DMSO (1 g) and reacted at 90°C for 1 h to completely dissolve the PVA; 1.3 g of EMIM:TFSI ionic liquid was added to the above system under stirring with the reaction temperature reducing to 70°C. After the reaction, the PVA-IL ion gel stock was used to form a pattern on the PIL-IGT as a dielectric layer by 3D printing (CELLINK Bio X). The obtained PIL-IGT was frozen at −20°C for several times to form the gel state.
Fabrication and assembly of the bioelectronic mask
The breath valve (overall size of 1.0 × 1.7 × 1.0 cm3) was made of photosensitive resin (ANYCUBIC) with good strength and flexibility by 3D printing. The internal structure of the top breath valve contained flower-like tubules (radius of 0.25 mm) and three semicircular chambers (maximum radius of 2 mm). Multiple flower-shaped pipes converged into three slender bends (radius of 0.35 mm) inside the bottom breath valve. Specifically, each channel of the PIL-IGT had a corresponding set of air-guiding structures in the breath valve. The two layers of the breath valve were tightly bonded with silica gel after assembling the PIL-IGT on the notch of the lower breath valve. The customized PCB (size of 2.3 × 2.9 × 0.3 cm3) was encapsulated by the soft protective cover obtained via reverse molding of PDMS (10:1 of base to curing agent). The PDMS was processed by ultrasound to remove bubbles and then was slowly poured into the clean mold. The PDMS cover was obtained after being placed at 80°C about 3 h after removing the mold. Then, the PIL-IGT was connected to the PBC soldered with a battery via (2.0 × 1.0 × 0.2 cm3) zero insertion force connectors. Finally, the whole device was assembled on the mask via silicon, which was driven by a lightweight rechargeable lithium battery.
Acknowledgments
We acknowledge support by the National Key Research and Development Program (2022YFE0100400), the National Natural Science Foundation of China (82001960), the Science and Technology Commission of Shanghai Municipality (21ZR1465200), the Shanghai Municipal Science and Technology Major Project (2021SHZDZX0100), and the Fundamental Research Funds for the Central Universities.
Author contributions
Y.F. conceived the project and designed the experiments. B.W. and J.D. synthesized the materials and carried out the material characterizations. B.W., D.Y., and R.Z. worked on the device design, manufacturing, biofunctionalization, and experiments. D.Y., Z.C., and B.W. developed the wireless testing platform and conducted the IoT experiments. B.W., D.Y., R.Z., and J.D. collected and analyzed the data. Y.F. and B.W. wrote the manuscript. All authors discussed the results and participated in manuscript input.
Declaration of interests
We have a pending patent related to this work, and the authors declare no competing interests.
Published: September 19, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.matt.2022.08.020.
Supplemental information
Data and code availability
All data required to evaluate the conclusions in this article are provided within the article and supplemental information.
References
- 1.Li Y., Wang R. Aptasensors for detection of avian influenza virus H5N1. Methods Mol. Biol. 2017;1572:379–402. doi: 10.1007/978-1-4939-6911-1_25. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14–18. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wielders C.C.H., Van Lier E.A., Van’T Klooster T.M., Van Gageldonk-Lafeber A.B., Van Den Wijngaard C.C., Haagsma J.A., Donker G.A., Meijer A., Van Der Hoek W., Lugnér A.K., et al. The burden of 2009 pandemic influenza A (H1N1) in Tthe Netherlands. Eur. J. Public Health. 2012;22:150–157. doi: 10.1093/eurpub/ckq187. [DOI] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. J. Am. Med. Assoc. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 7.Li F., Jiang H., Shen X., Yang W., Guo C., Wang Z., Xiao M., Cui L., Luo W., Kim B.S., et al. Sneezing reflex is mediated by a peptidergic pathway from nose to brainstem. Cell. 2021;184:P3762–P3773.e10. doi: 10.1016/j.cell.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvaraju S.B., Selvarangan R. Evaluation of three influenza A and B real-time reverse transcription-PCR assays and a new 2009 H1N1 assay for detection of influenza viruses. J. Clin. Microbiol. 2010;48:3870–3875. doi: 10.1128/JCM.02464-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakauchi M., Takayama I., Takahashi H., Tashiro M., Kageyama T. Development of a reverse transcription loop-mediated isothermal amplification assay for the rapid diagnosis of avian influenza A (H7N9) virus infection. J. Virol. Methods. 2014;204:101–104. doi: 10.1016/j.jviromet.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev. Mol. Diagn. 2020;20:453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler-Laporte G., Lawandi A., Schiller I., Yao M., Dendukuri N., McDonald E.G., Lee T.C. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern. Med. 2021;181:353–360. doi: 10.1001/jamainternmed.2020.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.H., McDevitt J.J., Hau B.J.P., Yen H.L., Li Y., Ip D.K.M., Peiris J.S.M., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra T., Wang M., Metwally A.A., Bogu G.K., Brooks A.W., Bahmani A., Alavi A., Celli A., Higgs E., Dagan-Rosenfeld O., et al. Pre-symptomatic detection of COVID-19 from smartwatch data. Nat. Biomed. Eng. 2020;4:1208–1220. doi: 10.1038/s41551-020-00640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu T., Watkinson P., Clifton D.A. Smartwatch data help detect COVID-19. Nat. Biomed. Eng. 2020;4:1125–1127. doi: 10.1038/s41551-020-00659-9. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen P.Q., Soenksen L.R., Donghia N.M., Angenent-Mari N.M., de Puig H., Huang A., Lee R., Slomovic S., Galbersanini T., Lansberry G., et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 2021;39:1366–1374. doi: 10.1038/s41587-021-00950-3. [DOI] [PubMed] [Google Scholar]
- 17.Butler D., Ebrahimi A. Rapid and sensitive detection of viral particles by coupling redox cycling and electrophoretic enrichment. Biosens. Bioelectron. 2022;208:114198–114199. doi: 10.1016/j.bios.2022.114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yousefi H., Mahmud A., Chang D., Das J., Gomis S., Chen J.B., Wang H., Been T., Yip L., Coomes E., et al. Detection of SARS-CoV-2 viral particles using direct, reagent-free electrochemical sensing. J. Am. Chem. Soc. 2021;143:1722–1727. doi: 10.1021/jacs.0c10810. [DOI] [PubMed] [Google Scholar]
- 19.Eissa S., Zourob M. Development of a low-cost cotton-tipped electrochemical immunosensor for the detection of SARS-CoV-2. Anal. Chem. 2021;93:1826–1833. doi: 10.1021/acs.analchem.0c04719. [DOI] [PubMed] [Google Scholar]
- 20.Chazot-Franguiadakis L., Eid J., Socol M., Molcrette B., Guégan P., Mougel M., Salvetti A., Montel F. Optical quantification by nanopores of viruses, extracellularvesicles, and nanoparticles. Nano Lett. 2022;22:3651–3658. doi: 10.1021/acs.nanolett.2c00253. [DOI] [PubMed] [Google Scholar]
- 21.Jin M., Tang S.J., Chen J.H., Yu X.C., Shu H., Tao Y., Chen A.K., Gong Q., Wang X., Xiao Y.F. 1/f-noise-free optical sensing with an integrated heterodyne interferometer. Nat. Commun. 2021;12:1973–1977. doi: 10.1038/s41467-021-22271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14:5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 23.Behrouzi K., Lin L. Gold nanoparticle based plasmonic sensing for the detection of SARS-CoV-2 nucleocapsid proteins. Biosens. Bioelectron. 2022;195:113669–113710. doi: 10.1016/j.bios.2021.113669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang Y., Meng L., Prominski A., Schaumann E.N., Seebald M., Tian B. Recent advances in bioelectronics chemistry. Chem. Soc. Rev. 2020;49:7978–8035. doi: 10.1039/D0CS00333F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian B., Liu J., Dvir T., Jin L., Tsui J.H., Qing Q., Suo Z., Langer R., Kohane D.S., Lieber C.M. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim H., Sim K., Ershad F., Yang P., Thukral A., Rao Z., Kim H.J., Liu Y., Wang X., Gu G., et al. Stretchable elastic synaptic transistors for neurologically integrated soft engineering systems. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax4961. eaax4961-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H., Yang A., Song J., Wang N., Lam P., Li Y., Law H.K.W., Yan F. Ultrafast, sensitive, and portable detection of COVID-19 IgG using flexible organic electrochemical transistors. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg8387. eabg8387-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo K., Wustoni S., Koklu A., Díaz-Galicia E., Moser M., Hama A., Alqahtani A.A., Ahmad A.N., Alhamlan F.S., Shuaib M., et al. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng. 2021;5:666–677. doi: 10.1038/s41551-021-00734-9. [DOI] [PubMed] [Google Scholar]
- 29.Seo D.G., Lee Y., Go G.T., Pei M., Jung S., Jeong Y.H., Lee W., Park H.L., Kim S.W., Yang H., et al. Versatile neuromorphic electronics by modulating synaptic decay of single organic synaptic transistor: from artificial neural networks to neuro-prosthetics. Nano Energy. 2019;65:104035. doi: 10.1016/j.nanoen.2019.104035. [DOI] [Google Scholar]
- 30.Lee H., Lee S., Lee W., Yokota T., Fukuda K., Someya T. Ultrathin organic electrochemical transistor with nonvolatile and thin gel electrolyte for long-term electrophysiological monitoring. Adv. Funct. Mater. 2019;29:1906982–1906989. doi: 10.1002/adfm.201906982. [DOI] [Google Scholar]
- 31.Folke M., Cernerud L., Ekström M., Hök B. Critical review of non-invasive respiratory monitoring in medical care. Med. Biol. Eng. Comput. 2003;41:377–383. doi: 10.1007/BF02348078. [DOI] [PubMed] [Google Scholar]
- 32.Reyes B.A., Reljin N., Kong Y., Nam Y., Chon K.H. Tidal volume and instantaneous respiration rate estimation using a volumetric surrogate signal acquired via a smartphone camera. IEEE J. Biomed. Health Inform. 2017;21:764–777. doi: 10.1109/JBHI.2016.2532876. [DOI] [PubMed] [Google Scholar]
- 33.Cho B.S., Choi J., Kim K.Y. Preparation and properties of solid polymer electrolyte based on imidazolium-based ionic liquids for structural capacitors. Fibers Polym. 2017;18:1452–1458. doi: 10.1007/s12221-017-7266-9. [DOI] [Google Scholar]
- 34.Hou Y., Chen C., Liu K., Tu Y., Zhang L., Li Y. Preparation of PVA hydrogel with high-transparence and investigations of its transparent mechanism. RSC Adv. 2015;5:24023–24030. doi: 10.1039/c5ra01280e. [DOI] [Google Scholar]
- 35.Verma Y.L., Singh R.K. Conformational States of Ionic Liquid 1-Ethyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide in bulk and confined silica nanopores probed by crystallization kinetics study. J. Phys. Chem. C. 2015;119:24381–24392. doi: 10.1021/acs.jpcc.5b06672. [DOI] [Google Scholar]
- 36.Gong M., Zhang L., Zuo Y., Zou Q., Wang Y., Wang L., Li Y. Investigation on the interpenetrating polymer networks (ipns) of polyvinyl alcohol and poly(N-vinyl pyrrolidone) hydrogel and its in vitro bioassessment. J. Appl. Polym. Sci. 2012;125:2799–2806. doi: 10.1002/app.36247. [DOI] [Google Scholar]
- 37.Mariani F., Conzuelo F., Cramer T., Gualandi I., Possanzini L., Tessarolo M., Fraboni B., Schuhmann W., Scavetta E. Microscopic determination of carrier density and mobility in working organic electrochemical transistors. Small. 2019;15:1902534–1902544. doi: 10.1002/smll.201902534. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.H., Hong K., Xie W., Lee K.H., Zhang S., Lodge T.P., Frisbie C.D. Electrolyte-gated transistors for organic and printed electronics. Adv. Mater. 2013;25:1822–1846. doi: 10.1002/adma.201202790. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y., Chortos A., Xu W., Liu Y., Oh J.Y., Son D., Kang J., Foudeh A.M., Zhu C., Lee Y., et al. A bioinspired flexible organic artificial afferent nerve. Science. 2018;360:998–1003. doi: 10.1126/science.aao0098. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H., Yu J., Yang X., Gao G., Qin S., Sun J., Ding M., Jia C., Sun Q., Wang Z.L. Ion gel capacitively coupled tribotronic gating for multiparameter distance sensing. ACS Nano. 2020;14:3461–3468. doi: 10.1021/acsnano.9b09549. [DOI] [PubMed] [Google Scholar]
- 41.Ren Y., Liu Z., Jin G., Yang M., Shao Y., Li W., Wu Y., Liu L., Yan F. Electric-field-induced gradient ionogels for highly sensitive, broad-range-response, and freeze/heat-resistant ionic fingers. Adv. Mater. 2021;33:2008486–2008499. doi: 10.1002/adma.202008486. [DOI] [PubMed] [Google Scholar]
- 42.Karnik R. Drug delivery: closed-loop dynamic dosing. Nat. Biomed. Eng. 2017;1:0072–0084. doi: 10.1038/s41551-017-0072. [DOI] [Google Scholar]
- 43.Khodagholy D., Curto V.F., Fraser K.J., Gurfinkel M., Byrne R., Diamond D., Malliaras G.G., Benito-Lopez F., Owens R.M. Organic electrochemical transistor incorporating an ionogel as a solid state electrolyte for lactate sensing. J. Mater. Chem. 2012;22:4440–4443. doi: 10.1039/c2jm15716k. [DOI] [Google Scholar]
- 44.Andersson Ersman P., Lassnig R., Strandberg J., Tu D., Keshmiri V., Forchheimer R., Fabiano S., Gustafsson G., Berggren M. All-printed large-scale integrated circuits based on organic electrochemical transistors. Nat. Commun. 2019;10:5053–5059. doi: 10.1038/s41467-019-13079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das J., Cederquist K.B., Zaragoza A.A., Lee P.E., Sargent E.H., Kelley S.O. An ultrasensitive universal detector based on neutralizer displacement. Nat. Chem. 2012;4:642–648. doi: 10.1038/nchem.1367. [DOI] [PubMed] [Google Scholar]
- 46.Giovannini G., Haick H., Garoli D. Detecting COVID-19 from breath: a game changer for a big challenge. ACS Sens. 2021;6:1408–1417. doi: 10.1021/acssensors.1c00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scharfman B.E., Techet A.H., Bush J.W.M., Bourouiba L. Visualization of sneeze ejecta: steps of fluid fragmentation leading to respiratory droplets. Exp. Fluids. 2016;57:24–29. doi: 10.1007/s00348-015-2078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole E.C., Cook C.E. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am. J. Infect. Control. 1998;26:453–464. doi: 10.1016/S0196-6553(98)70046-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fennelly K.P. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir. Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sareen S., Sood S.K., Gupta S.K. IoT-based cloud framework to control Ebola virus outbreak. J. Ambient Intell. Humaniz. Comput. 2018;9:459–476. doi: 10.1007/s12652-016-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., et al. Leung, Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 52.Zhu M., Xiao H., Yan G., Sun P., Jiang J., Cui Z., Zhao J., Zhang Z., Peng L.M. Radiation-hardened and repairable integrated circuits based on carbon nanotube transistors with ion gel gates. Nat. Electron. 2020;3:622–629. doi: 10.1038/s41928-020-0465-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data required to evaluate the conclusions in this article are provided within the article and supplemental information.