Abstract
Cancer stemness, defined as the self-renewal and tumor-initiation potential of cancer stem cells (CSCs), is a cancer biology property featuring activation of CSC signaling networks. Canonical WNT signaling through Frizzled and LRP5/6 receptors is transmitted to the β-catenin-TCF/LEF-dependent transcription machinery to up-regulate MYC, CCND1, LGR5, SNAI1, IFNG, CCL28, CD274 (PD-L1) and other target genes. Canonical WNT signaling causes expansion of rapidly cycling CSCs and modulates both immune surveillance and immune tolerance. In contrast, noncanonical WNT signaling through Frizzled or the ROR1/2 receptors is transmitted to phospholipase C, Rac1 and RhoA to control transcriptional outputs mediated by NFAT, AP-1 and YAP-TEAD, respectively. Noncanonical WNT signaling supports maintenance of slowly cycling, quiescent or dormant CSCs and promotes epithelial–mesenchymal transition via crosstalk with TGFβ (transforming growth factor-β) signaling cascades, while the TGFβ signaling network induces immune evasion. The WNT signaling network orchestrates the functions of cancer-associated fibroblasts, endothelial cells and immune cells in the tumor microenvironment and fine-tunes stemness in human cancers, such as breast, colorectal, gastric and lung cancers. Here, WNT-related cancer stemness features, including proliferation/dormancy plasticity, epithelial–mesenchymal plasticity and immune-landscape plasticity, will be discussed. Porcupine inhibitors, β-catenin protein–protein interaction inhibitors, β-catenin proteolysis targeting chimeras, ROR1 inhibitors and ROR1-targeted biologics are investigational drugs targeting WNT signaling cascades. Mechanisms of cancer plasticity regulated by the WNT signaling network are promising targets for therapeutic intervention; however, further understanding of context-dependent reprogramming trajectories might be necessary to optimize the clinical benefits of WNT-targeted monotherapy and applied combination therapy for patients with cancer.
Keywords: angiogenesis, cell proliferation, epithelial-to-mesenchymal transition, immunomodulation, transforming growth factors, Wnt proteins
Introduction
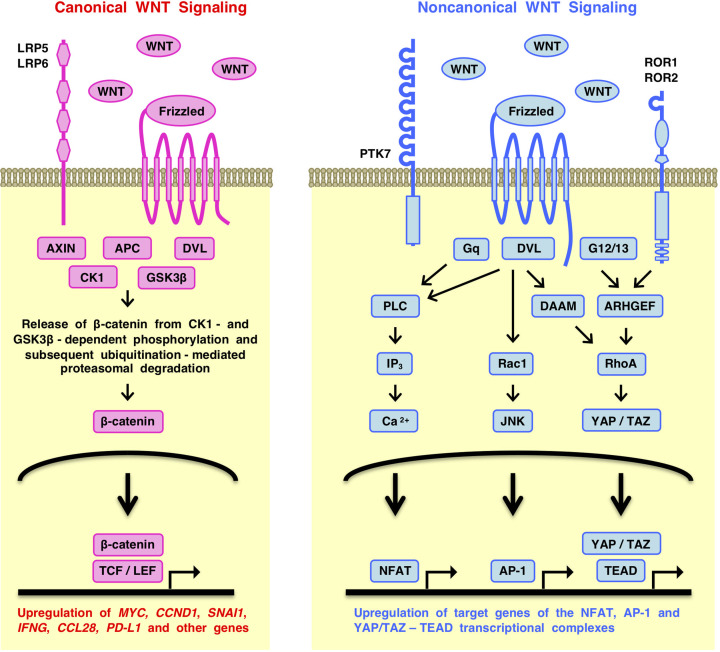
Canonical WNT signals are transmitted through Frizzled (atypical G-protein-coupled receptor, GPCR) and LRP5/6 (LDL receptor-related proteins) to the nuclear β-catenin-TCF/LEF (T-cell factor and lymphoid enhancer factor) complex to up-regulate the expression of target genes, such as MYC, CCND1 (cyclin D1) and LGR5 (leucine rich repeat-containing GPCR 5) [1–3] (Figure 1). In contrast, noncanonical WNT signals are transmitted through Frizzled or ROR1/2 (receptor tyrosine kinase-like orphan receptors) to the PLC (phospholipase C), Rac1 and RhoA branches to activate the NFAT (nuclear factor associated with T cells), AP-1 (activator protein 1) and YAP-TEAD (Yes-associated transcriptional regulator/TEA domain transcription factor) transcription factors, respectively [1,4,5] (Figure 1).
Figure 1. Simplified overview of the WNT signaling network.
WNT signals are transmitted to the canonical and noncanonical signaling cascades. Canonical WNT signaling is transmitted through Frizzled and LRP5/6 receptors to the transcriptional outputs depending on the β-catenin-TCF/LEF complex to up-regulate the expression of target genes, such as ALDH1A1, ASCL2, ATF3, AXIN2, BAMBI, CCL28, CCND1, CD274 (PD-L1), CLDN1, CTLA4, DKK1, EOMES, FGF20, FZD7, IL10, LEF1 (Lef-1), LGR5, MYC, NKD1, NOTUM, OPN, PROX1, RNF43, SNAI1 (Snail), TCF7 (Tcf-1), TNFRSP19 (Troy), WISP1 and ZNRF3. Noncanonical WNT signaling is transmitted through Frizzled or ROR1/2 receptors to the Gq-PLC-IP3-Ca2+, Rac1-JNK and RhoA-ROCK signaling branches to regulate transcriptional outputs depending on the NFAT, AP-1 and YAP-TEAD complexes. WNT signaling cascades cross-talk with the TGFβ (transforming growth factor-β), FGF (fibroblast growth factor), Hedgehog, Notch and YAP signaling cascades in the tumor microenvironment and affect cancer stem cells, cancer-associated fibroblasts, endothelial cells and immune cells.
Genetic alterations in WNT signaling molecules drive carcinogenesis in colorectal, gastric, liver, uterine and other cancers [6]. Loss-of-function alterations in the APC (adenomatous polyposis coli), AXIN1 and AXIN2 genes and gain-of-function mutations in the CTNNB1 (β-catenin) gene activate the canonical WNT signaling cascade owing to stabilization and nuclear accumulation of β-catenin [7], while loss-of-function alterations in the RNF43 (ring finger protein 43) gene can activate both canonical and noncanonical WNT signaling cascades through stabilization of plasma membrane Frizzled, LRP6 and ROR1 [8].
In the tumor microenvironment (TME), WNT ligands derived from tumor cells [9], cancer-associated fibroblasts (CAFs) [10] and immune cells [11] also activate both canonical and noncanonical WNT signaling cascades and affect the FGF (fibroblast growth factor) [1], Hedgehog [12], Notch [13] and TGFβ (transforming growth factor-β) [14] signaling cascades. The WNT signaling network in the TME regulates cancer biological behaviors, such as dormancy, drug resistance, epithelial–mesenchymal transition (EMT), immune evasion, proliferation and stemness.
Cancer stemness is defined as the self-renewal and tumor-initiation potential of cancer stem cells (CSCs) but also broadly as a cancer biological characteristic regulated by CSC signaling networks [15,16]. CSCs are involved in drug resistance, invasion, metastasis and recurrence of tumors, while evolution of CSC concepts from hierarchical organization model to cellular plasticity model have been described elsewhere. CD44, CD133 (prominin-1) and LGR5 are functional CSC markers. For example, LGR5 potentiates WNT signaling through R-spondin-mediated Frizzled stabilization [6], and Lgr5+ gastric CSCs are required for primary tumorigenesis, liver metastasis and drug resistance [17]. CD44+, CD133+ or LGR5+ CSCs show enhanced tumor-initiation potential; however, tumors can be reconstituted even after ablation of CD44+, CD133+ or LGR5+ CSCs owing to the plasticity of CSCs [18–20]. Therefore, it is reasonable to focus on CSC signaling networks rather than CSC markers for a mechanistic understanding of malignant processes, such as maintenance of dormancy, EMT and immune evasion.
Here, cancer stemness features related to the WNT signaling network, including proliferation/dormancy plasticity, epithelial–mesenchymal plasticity and immune-landscape plasticity, will be reviewed, and perspectives will be discussed.
Proliferation/dormancy plasticity
Tumor cells present a spectrum of proliferation/dormancy-related features, such as fast cycling (proliferative), slow cycling (shallow quiescence), reversible growth arrest (deep quiescence) and irreversible growth arrest (senescence) (Figure 2). Cyclin A (CCNA1 and CCNA2), cyclin B (CCNB1, CCNB2 and CCNB3), cyclin D (CCND1, CCND2 and CCND3), cyclin E (CCNE1 and CCNE2), cyclin-dependent kinase 1 (CDK1), CDK2, CDK4, CDK6 and E2F are representative cell cycle activators [21], whereas cyclin-dependent kinase inhibitor 1A (CDKN1A, p21 or CIP1), CDKN1B (p27 or KIP1), CDKN1C (p57 or KIP2), CDKN2A (p16 or ARF), CDKN2B (p15), CDKN2C, CDKN2D, RB and TP53 (p53) are representative cell cycle inhibitors [22]. Definitions of slow-cycling cells, quiescent cells, persistent cells, and dormant cells are confusing and controversial [23]; however, it is clear that the proliferation/dormancy-related features of cells are regulated by the balance between cell cycle activators and inhibitors [24].
Figure 2. Simplified view of proliferation/dormancy plasticity.
Tumor cells exist in a spectrum of cell cycle states: cells with fast cycling (proliferative), slow cycling (shallow quiescence), reversible growth arrest (deep quiescence) and irreversible growth arrest (senescence). These conditions are dynamically controlled based on the balance between cell cycle activators and inhibitors. Canonical WNT signaling induces proliferation of cancer stem cells (CSCs) through direct up-regulation of CCND1 and MYC and secondary up-regulation of CCNA2, CCNB1, CCND2, CCND3, CCNE2 and CDK4. In contrast, TGFβ (transforming growth factor-β) signaling induces CSC dormancy through up-regulation of CDKN1A, CDKN1B, CDKN1C and CNKN2B. TGFβ and YAP signaling converge to up-regulate WNT5A transcription and then WNT5A potentiates TGFβ and YAP signaling to constitute a TGFβ-WNT5A-YAP signaling loop. Noncanonical WNT signaling induces CSC dormancy through canonical WNT signaling inhibition and cross-talk with TGFβ signaling. Proliferation/dormancy plasticity is controlled by the canonical and noncanonical WNT signaling networks via cell cycle control.
Canonical WNT signals induce proliferation of tumor cells via direct transcriptional activation of CCND1 and MYC [1] and subsequent MYC-dependent up-regulation of CCNA2, CCNB1, CCND2, CCND3, CCNE2, CDK4 and CDK7 and down-regulation of CDKN1A, CDKN1B and CDKN2B [21]. In contrast, DKK1 inhibits canonical WNT signaling because of competitive binding to LRP5/6 coreceptors and induces dormancy of lung and breast cancer cells [25], and noncanonical WNT signaling via the ROR2 receptor inhibits canonical WNT signaling owing to β-catenin degradation and induces dormancy of prostate cancer cells [26]. Noncanonical WNT signaling through the ROR1 receptor induces RhoA-mediated YAP stabilization and nuclear translocation [5], and YAP reprograms LGR5+ proliferating CSCs into LGR5- dormant CSCs [27]. In contrast, TGFβ induces dormancy through SMAD-mediated up-regulation of CDKN1A, CDKN1C and CDKN2B and p38 MAPK-mediated CDKN2A up-regulation [28]. The canonical WNT signaling cascade induces CSC proliferation, whereas noncanonical WNT, TGFβ and YAP signaling cascades induce CSC dormancy (Figure 2).
Proliferative CSCs are vulnerable to chemotherapy-induced DNA damage, whereas nonproliferative CSCs are resistant to therapeutic insults [23]. Chemotherapy elicits DNA damage and TP53-mediated growth arrest or apoptosis, while WNT/β-catenin signaling activation induces reactivation of dormant cells and promotes relapse [29]. Canonical WNT signaling activation also induces resistance to targeted therapies, such as anti-HER2 monoclonal antibody (mAb, trastuzumab) [30] and MEK1/2 inhibitor (trametinib) [31] therapies. In contrast, WNT5A and receptor tyrosine kinases, such as AXL, EGFR, FGFR1, PDGFRB and NGFR, are up-regulated in BRAF inhibitor (vemurafenib)-resistant melanoma cells in part owing to YAP- and AP-1-dependent transcriptional activation [32], suggesting the possible involvement of noncanonical WNT5A signaling in drug resistance. Indeed, noncanonical WNT signaling through ROR1 elicits resistance to an anti-HER2 antibody–drug conjugate (ADC, T-DM1) in the treatment of HER2+ breast cancer patients [33]. Canonical and noncanonical WNT signaling causes therapeutic resistance and subsequent recurrence through expansion of proliferative CSCs and survival of dormant CSCs.
WNT-targeted investigational drugs in clinical development are classified into (i) Pan-WNT signaling inhibitors, (ii) canonical WNT signaling inhibitors, (iii) noncanonical WNT signaling inhibitors and (iv) biologics targeting WNT receptors and signaling modulators (Table 1). Porcupine inhibitors that abrogate the secretion of WNT ligands are pan-WNT signaling inhibitors [16]; β-catenin protein–protein interaction (PPI) inhibitors and β-catenin proteolysis targeting chimeras (PROTACs) are canonical WNT signaling inhibitors [34,35]; ROR1 inhibitors are noncanonical WNT signaling inhibitors [36]; and anti-ROR1 mAbs, anti-ROR1 ADCs, ROR1-targted chimeric antigen receptor-modified T cells (CAR-Ts) and anti-PTK7 ADCs are cutting-edge biologics targeting noncanonical WNT receptors [5,16,37,38]. Porcupine inhibitors are expected to be ideal drugs to eliminate canonical WNT signaling-dependent proliferative CSCs and noncanonical WNT signaling-dependent dormant CSCs. However, because WNT signaling cascades are involved in the homeostasis of various normal organs or tissues, such as bone, gastrointestinal tract and neural tissues, the clinical therapeutic window of pan-WNT and canonical WNT signaling inhibitors might be relatively narrow [39]. To broaden the therapeutic window of WNT-targeted therapy through prevention of treatment-induced bone loss, an anti-RANKL (RANK ligand or TNFSF11) mAb (denosumab) has been added to phase II clinical trials of the porcupine inhibitor RXC004 for the treatment of cancer patients (ClinicalTrials.gov identifier: NCT04907539 and NCT04907851). In contrast, because ROR1 is an oncofetal protein that is preferentially expressed in fetal tissues and cancers [36], ROR1 inhibitors and ROR1-targeted biologics are expected to show preferable risk-benefit ratios in clinical trials. WNT-targeted therapeutics are valuable research tools in basic and translational studies but are not yet approved for the treatment of patients with cancer.
Table 1. Therapeutics targeting WNT signaling cascades.
| Class | Mechanism of action | Drug | Phase | Clinical Trial # | Features | Recruitment status and ESCD |
|---|---|---|---|---|---|---|
| [1] Pan-WNT signaling inhibitors | Porcupine inhibitor | RXC004* | Phase II Phase II |
NCT04907851 NCT04907539 |
Monotherapy Combo ICI |
Recruiting (June, 2023) Recruiting (August, 2023) |
| CGX1321 | Phase I | NCT02675946 | Monotherapy & Combo ICI | Recruiting (March, 2023) | ||
| ETC-159 | Phase I | NCT02521844 | Monotherapy & Combo ICI | Recruiting (August, 2024) | ||
| LGK974 | Phase I | NCT01351103 | Monotherapy & Combo ICI | Recruiting (November, 2023) | ||
| [2] Canonical WNT signaling inhibitors | β-catenin PPI inhibitor | PRI-724 | Phase I & II | NCT01606579 | Monotherapy | Completed in 2016 |
| E7386 | Phase I & II | NCT05091346 | Combo ICI | Recruiting (May, 2024) | ||
| β-catenin PROTAC | xStAx-VHLL | Preclinical | ||||
| [3] Noncanonical WNT signaling inhibitors | ROR1 inhibitor | KAN0439834 | Preclinical | |||
| KAN0441571C | Preclinical | |||||
| [4] Biologics targeting WNT receptors and signaling modulators | Anti-ROR1 mAb | Cirmtuzumab | Phase I & II | NCT03088878 | Combo BTK inhibitor | Active NR (June, 2027) |
| Anti-ROR1 ADC | VLS-101 | Phase II | NCT04504916 | Monotherapy | Recruiting (March, 2024) | |
| NBE-002 | Phase I & II | NCT04441099 | Monotherapy | Recruiting (December, 2025) | ||
| CS5001 | Phase I | NCT05279300 | Monotherapy | Recruiting (March, 2024) | ||
| ROR1-targeted CAR-T | LYL797 | Phase I | NCT05274451 | Monotherapy | Recruiting (September, 2026) | |
| R12 CAR | Phase I | NCT02706392 | Monotherapy | Terminated in 2021 | ||
| Anti-PTK7 ADC | PF-06647020 | Phase I | NCT04189614 | Monotherapy | Recruiting (August, 2023) | |
| Anti-DKK1 mAb | DKN-01 | Phase II | NCT04363801 | Combo ICI and/or CAPOX | Recruiting (June, 2023) | |
| Anti-FZD1/2/5/7/8 mAb | OMP-18R5 | Phase I | NCT01345201 | Monotherapy | Completed in 2014 | |
| FZD8-Fc fusion protein | OMP-54F28 | Phase I | NCT01608867 | Monotherapy | Completed in 2017 | |
| Anti-RSPO3 mAb | OMP-131R10 | Phase I | NCT02482441 | Combo FOLFIRI | Completed in 2018 | |
| Anti-LGR5 mAb | BNC101 | Phase I | NCT02726334 | Monotherapy | Terminated in 2018 |
Anti-RANKL monoclonal antibody (mAb) has been added to RXC004 to prevent therapy-induced bone loss. Abbreviations: Active NR, Active, not recruiting; ADC, antibody-drug conjugate; CAPOX, chemotherapy with capecitabine and oxaliplatin; CAR-T, chimeric antigen receptor-modified T cells; Clinical Trial #, clinical trial identifier in the ClinicalTrials.gov database (https://clinicaltrials.gov); Combo, combination therapy; ESCD, Estimated Study Completion Date; ICI, immune checkpoint inhibitor; FOLFIRI, chemotherapy with leucovorin, fluorouracil and irinotecan; FZD8-Fc fusion protein, WNT-ligand trap; PF-06647020, cofetuzumab pelidotin; PPI, protein–protein interaction; PROTAC, proteolysis targeting chimera; VLS-101, zilovertamab vedotin; xStAx-VHLL, xStAx stapled helical peptide coupled with von Hippel-Lindau protein ligand.
Epithelial–mesenchymal plasticity
EMT and mesenchymal–epithelial transition are reciprocal processes accompanied by organized alteration of cell polarity, cell–cell contacts, cell–matrix contacts and the intracellular actin cytoskeleton [16,40]. Epithelial cells with apical-basal polarity interact via tight junctions, adherens junctions and desmosomes and spread over the basement membrane via hemidesmosomes, whereas mesenchymal cells with front-back polarity are not connected to each other via intercellular junctions and are not attached to the basement membrane and are thus motile (Figure 3). In contrast, a spectrum of hybrid epithelial–mesenchymal (hybrid E/M) cells, such as quasi-epithelial cells and quasi-mesenchymal cells, express both epithelial and mesenchymal markers and show intermediate phenotypes. Single-cell analyses of EMT processes have revealed that hybrid E/M cells go through sequential and parallel trajectories caused by context-dependent EMT signaling, chromatin modulation and transcriptional effects [41,42]. SNAI1 (Snail), SNAI2 (Slug), TWIST1, ZEB1 (zinc finger E-box binding homeobox 1) and ZEB2 are key transcription factors that reprogram epithelial cells toward hybrid E/M cells and mesenchymal cells under the control of WNT, FGF, Hedgehog, Notch, TGFβ and other signaling cascades (Figure 3).
Figure 3. Simplified view of epithelial–mesenchymal plasticity.
Tumor cells exist in a spectrum of epithelial, hybrid epithelial–mesenchymal (hybrid E/M, including quasi-epithelial and quasi-mesenchymal) and mesenchymal states. WNT, FGF (fibroblast growth factor), Hedgehog, Notch and TGFβ (transforming growth factor-β) signaling cascades induce epithelial–mesenchymal transition (EMT) via up-regulation of SNAI1 (Snail), SNAI2 (Slug), TWIST, ZEB1 and ZEB2. Hybrid E/M cells with intermediate phenotypes go through sequential and parallel trajectories during EMT. Canonical WNT signaling drives partial EMT and para-EMT programs depending on CCND1, MYC, SNAI1 and other target genes. Noncanonical WNT and TGFβ signaling drive complete EMT through ZEB1-, YAP- and AP-1-dependent transcription and potentiation of the mesenchymal signaling loop. Epithelial–mesenchymal plasticity is regulated by the balance between canonical WNT signaling in epithelial and hybrid E/M cells and noncanonical WNT, TGFβ and YAP signaling in hybrid E/M and mesenchymal cells; AJ, adherens junction; D, desmosome; HD, hemidesmosome; TJ, tight junction.
Colorectal cancers are classified into CMS1 (14%, microsatellite instable and immune hot), CMS2 (37%, canonical WNT and MYC signaling activation), CMS3 (13%, KRAS mutation) and CMS4 (23%, TGFβ and EMT signaling activation) subtypes [43]. Canonical WNT signaling is activated in CMS2 due to genetic alterations in the APC or RNF43 gene [7], which directly up-regulates SNAI1 via transcriptional and post-translational mechanisms [44] and induces partial EMT [43]. TGFβ signaling is activated in CMS4 owing to the processing of latent TGFβ precursor in the TME by glycoprotein A repetition predominant protein (GARP or LRRC32), integrins, metalloproteases (MMP9 and MMP14), mild acidic conditions, reactive oxygen species and TSP1 (thrombospondin 1; also known as THBS1), which up-regulates multiple EMT regulators, such as SNAI1, SNAI2, TWIST1 and ZEB1, and induces partial or complete EMT [28]. Canonical WNT signaling up-regulates the PROX1 transcriptional repressor in colorectal cancer [45] and down-regulates MMP14 to inhibit TGFβ-dependent EMT and extracellular matrix (ECM) remodeling [46], whereas TGFβ1 signaling down-regulates the WNT signaling potentiator Lgr5 to repress proliferation but induce EMT and CMS2-to-CMS4 conversion in colorectal cancer organoids [47]. Counteracting the canonical WNT and TGFβ signaling cascades shapes epithelial CMS2 and mesenchymal CMS4 phenotypes, respectively.
Breast CSCs are classified into canonical WNT-dependent epithelial-like CSCs and YAP-dependent mesenchymal-like CSCs [48], and gastric cancers are classified into an epithelial subtype with canonical WNT signaling activation and a mesenchymal subtype with Hedgehog signaling activation [49]. Noncanonical WNT and Hedgehog-Smoothened signaling promotes YAP stabilization and YAP-dependent transcription [5,50], and YAP up-regulates WNT5A and ITGAV (integrin αv) to establish a noncanonical WNT-YAP signaling loop [51] and activate TGFβ signaling [14], respectively. In contrast, noncanonical WNT, TGFβ and YAP signaling converges to induce ZEB1-dependent transcription [52–54] as well as AP-1-dependent transcription [1,28,53]. ZEB1 directly down-regulates ZEB1-only target genes, such as CDH1, CLDN3, CLDN4 and LLGL2, to promote EMT, while ZEB1 interacts with AP-1 and YAP and up-regulates target genes of ZEB1/AP-1/YAP (CTGF, EDN1, NRP1, TGFBI and others) to regulate para-EMT signaling [53]. ZEB1 and YAP bind to the AXIN2 promoter to inhibit canonical WNT signaling and maintain a quiescent or dormant state [55]. Noncanonical WNT, TGFβ and YAP signaling cascades constitute a mesenchymal signaling network that counteracts the canonical WNT signaling cascade. The epithelial CSC signaling network driven by the canonical WNT signaling cascade and the mesenchymal CSC signaling network driven by the noncanonical WNT, TGFβ and YAP signaling cascades compete with each other, and their interactions fine-tune the epithelial–mesenchymal plasticity (Figure 3).
Canonical WNT signaling is involved in proliferation and epithelial (or hybrid E/M) transition, whereas TGFβ and noncanonical WNT signaling are involved in dormancy and mesenchymal (or hybrid E/M) transition, as discussed above. The shared characteristics of epithelial/proliferative and mesenchymal/dormant phenotypes suggest mechanistic overlap in epithelial–mesenchymal and proliferative/dormant plasticity. In contrast, mammary tumor cells with dominant negative β-catenin mutation retained TGFβ-induced stemness but lost EMT, chemoresistance and metastasis potential [56], which indicates that the trajectories of epithelial/proliferative and mesenchymal/dormant reprogramming are not completely consistent with each other. Malignant phenotypes individually develop during sequential and parallel EMT trajectories depending on (i) context-dependent activation of WNT, TGFβ and other signaling cascades, (ii) combinatory effects of β-catenin-TCF/LEF, SMADs, NFAT, AP-1, YAP-TEAD and EMT-related transcription factors and (iii) the state of chromatin of target loci. Therefore, large-scale and multiomics single-cell analyses of primary or metastatic tumors are needed to understand how the canonical and noncanonical WNT signaling networks control cancer stemness, EMT, dormancy, drug resistance and relapse.
Immune-landscape plasticity
Tumor cells, CAFs, endothelial cells and immune cells are organized into inflammatory or noninflammatory TMEs [12,57–61]. Immune-stimulating CAFs, leukocyte-attracting endothelial cells and proinflammatory immune cells generate an inflammatory TME with elevated CCL2 (C-C motif chemokine ligand 2), CCL5, IFNγ (interferon-γ; also known as IFNG), IL1β (interleukin-1β) and TNF (tumor necrosis factor) levels (Figure 4A). In contrast, ECM-remodeling CAFs, angiogenic endothelial cells and suppressive immune cells give rise to a noninflammatory TME with elevated TGFβ, CCL28, IL4, IL10, lactate and VEGF (vascular endothelial growth factor) levels (Figure 4B). An inflammatory TME with active immune cells (featuring a hot immune environment with strong immune surveillance) preserves antitumor immunity to suppress tumorigenesis, whereas an inflammatory TME with exhausted immune cells (featuring a hot immune environment with strong immune evasion) and a noninflammatory TME (featuring a cold immune environment with strong immune evasion) lack antitumor immunity and thus promote tumorigenesis.
Figure 4. Simplified view of immune-landscape plasticity.
(A) Proinflammatory tumor microenvironment (TME). An inflammatory TME is generated by immune-stimulating cancer-associated fibroblasts (CAFs), leukocyte-attracting endothelial cells (ECs) and proinflammatory immune cells, such as antigen-presenting dendritic cells (DCs), M1-like tumor-associated macrophages (M1-TAMs), CD4+ helper T (Th1-like) cells, CD8+ cytotoxic T cells, natural killer (NK) cells and B cells. Immune surveillance is preserved in an inflammatory TME with active immune cells, whereas immune tolerance is elicited in an inflammatory TME with exhausted immune cells (featuring a hot immune environment with strong immune evasion); CCL2, C-C motif chemokine ligand 2; CSC, cancer stem cell; IFNγ, interferon-γ also known as IFNG; IL1β, interleukin-1β; TNF, tumor necrosis factor. (B) Immunosuppressive TME. A noninflammatory TME is established by angiogenic ECs, CAFs remodeling extracellular matrix (ECM), M2-TAMs, myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells. Transforming growth factor-β (TGFβ) gives rise to ECM-remodeling CAFs, M2-TAMs, monocytic MDSCs and Treg cells. Monocytic MDSCs produce IL10, TGFβ and vascular endothelial growth factor (VEGF). VEGF-induced tumor angiogenesis generates a hypoxic and acidic TME. Immune tolerance is elicited in a noninflammatory TME (featuring a cold immune environment with strong immune evasion). (C) WNT- and TGFβ-dependent immune regulation. (Left) Canonical WNT signaling activation in CD8+ T and Treg cells enhances antitumor immunity through production of proinflammatory cytokines, such as IFNγ and TNF. (Middle) Canonical WNT signaling activation in tumor cells promotes cold immune evasion through up-regulation of CCL28, CD274 (PD-L1, PD-1 ligand 1) and WISP1. (Right) TGFβ, noncanonical WNT and YAP signaling network establishes immunosuppressive TME through potentiation of the TGFβ signaling loop. Canonical WNT signaling promotes immune surveillance as well as immune tolerance in a context-dependent manner, whereas TGFβ signaling generates cold immune evasion.
PD-1 (programmed cell death 1, also known as PDCD1 or CD279) receptor and its ligand PD-L1 (PD-1 ligand 1; also known as CD274) constitute an immune checkpoint. The immunosuppressive PD-L1 ligand is up-regulated in tumor cells, CAFs, endothelial cells and monocytic myeloid-derived suppressor cells (MDSCs) [57–59,62] owing to IFNγ signaling, WNT/β-catenin-MYC signaling and hypoxia [62–65], while the PD-1 receptor is expressed on macrophages, natural killer cells, CD8+ (cluster of differentiation 8 positive) exhausted T cells and CD4+ follicular regulatory T (Treg) cells [66–68]. Anti-PD-L1 mAbs (atezolizumab, avelumab and durvalumab) and anti-PD-1 mAbs (cemiplimab, nivolumab and pembrolizumab) have been developed as immune checkpoint inhibitors (ICIs) to relieve immune exhaustion caused by PD-L1/PD-1 signaling [69,70]. Because PD-1 is expressed on both CD8+ cytotoxic T cells and CD4+ Treg cells [68], ICIs reinvigorate cytotoxic T cells to not only suppress tumorigenesis in some patients but also expand Treg cells to promote tumorigenesis in other patients.
Canonical WNT signaling affecting the β-catenin-TCF7 (Tcf-1) complex in CD8+ T and Treg cells enhances inflammation in part through up-regulation of proinflammatory cytokines, such as IFNγ and TNF [71,72]. In addition, WNT ligands, such as WNT3A, WNT5A, WNT10A and WNT10B, are up-regulated in inflamed tissues through proinflammatory signaling affecting the NF-κB (nuclear factor enhancer of immunoglobulin κ light chain of activated B cells) and STAT3 (signal transducer and activator of transcription 3) transcription factors [73–75]. Because WNT5A enhances NK-κB signaling to up-regulate IL1β and IL6 and these cytokines activate STAT3 signaling to up-regulate WNT5A, WNT signaling is incorporated into a proinflammatory feedforward loop and promotes immune surveillance. In contrast, canonical WNT signaling activation in dendritic cells (DCs) elicits immune evasion owing to IDO1 (indoleamine 2,3-dioxygenase 1)- and IL10-mediated Treg-cell expansion and cytotoxic T-cell impairment, respectively [76,77]. Canonical WNT signaling activation in tumor cells also promotes immune evasion through direct up-regulation of PD-L1 [64], the Treg-cell-attracting chemokine CCL28 [78] and the suppressive macrophage-supporting protein WISP1 (WNT-induced secreted protein 1) [79] and indirect down-regulation of the DC-attracting chemokines CCL4 [80] and CCL5 [81]. Taken together, these findings clearly indicate that WNT signaling activation induces immune surveillance as well as immune tolerance in a context-dependent manner (Figure 4C).
The response to pembrolizumab monotherapy in 1188 patients with cancer was positively correlated with a T-cell-inflamed signature (P = 3.6 × 10−12) and negatively correlated with angiogenesis, monocytic MDSCs and stroma/EMT/TGFβ signatures (P = 0.0001, 0.0001 and 0.0003, respectively) [82]. Because tumors with a hot environment with immune evasion are vulnerable to ICI-induced reinvigoration of CD8+ cytotoxic T cells, it is reasonable to select cancer patients for ICI monotherapy using the T-cell-inflamed signature [69]. TGFβ promotes generation of ECM-remodeling CAFs, M2-TAMs, monocytic MDSCs and Treg cells [61]; monocytic MDSCs produce IL10, TGFβ and VEGF [59]; and VEGF-dependent tumor angiogenesis induces a hypoxic and acidic TME, PD-L1 up-regulation and Treg-cell/MDSC expansion [58]. Because TGFβ signaling, monocytic MDSCs and tumor angiogenesis are immunosuppressive and together generate an ICI-resistant, cold environment with immune evasion (Figure 4), combination ICI therapies using angiogenesis inhibitors, monocytic MDSC inhibitors or TGFβ signaling inhibitors are promising options for cancer patients with a noninflamed environment featuring immune evasion.
The WNT expression signature was not significantly correlated with ICI resistance in the study mentioned above (P=0.10) [82], although an association between WNT-related genetic alterations and decreased T-cell inflammation was noted by others [83]. WNT-related genetic alterations occur in the CMS2 subtype of colorectal cancer, which shows canonical WNT and MYC signaling activation, as well as in the CSM1 subtype, which shows microsatellite instability (generally considered immune hot), and the CMS4 subtype, which shows stroma/EMT/TGFβ signaling activation (immune cold) [7,43]. It is not appropriate to predict ICI response based on WNT-related genetic alterations because such alterations are present in cases with the T-cell inflamed signature and stroma/EMT/TGFβ signature. Although the context-dependent immune regulation by WNT signaling cascades [71–81] and the unknown risk–benefit ratio of WNT signaling inhibitors [39] are limiting factors, phase I or II clinical trials of combination ICI therapy with WNT signaling inhibitors or WNT-related biologics (Table 1) are ongoing and aim to reveal new avenues for immunotherapy in cancer.
Summary
Canonical WNT signaling affecting the β-catenin-TCF/LEF cascade promotes CCND1/MYC-dependent proliferation and SNAI1-dependent partial EMT and context-dependently modulates immune surveillance and immune evasion.
Transcriptional outputs resulting from noncanonical WNT signaling depend on YAP, AP-1 and NFAT crosstalk with TGFβ signaling cascades to promote CDKN-dependent dormancy, ZEB1-dependent EMT and cold immune evasion.
The balance between the epithelial signaling network driven by the canonical WNT signaling cascade and the mesenchymal signaling network driven by noncanonical WNT, TGFβ and YAP signaling cascades modulates cancer stemness features.
WNT-related proliferation/dormancy plasticity, epithelial-mesenchymal plasticity and immune-landscape plasticity are promising targets for therapeutic intervention.
Further understanding of context-dependent reprogramming trajectories and plasticity-related chromatin remodeling are needed to optimize the clinical benefits of WNT-targeted therapy.
Abbreviations
- ADC
antibody–drug conjugate
- AP-1
activator protein 1
- APC
adenomatous polyposis coli
- CAF
cancer-associated fibroblast
- CCL
C-C motif chemokine ligand
- CCN
cyclin
- CD8+
cluster of differentiation 8 positive
- CDK
cyclin-dependent kinase
- CDKN
cyclin-dependent kinase inhibitor
- CSC
cancer stem cell
- CTNNB1
β-catenin
- DC
dendritic cell
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- FGF
fibroblast growth factor
- Frizzled
atypical G-protein-coupled receptor with Frizzled-like domain
- GARP
glycoprotein A repetition predominant protein or LRRC32
- GPCR
G-protein-coupled receptor
- ICI
immune checkpoint inhibitor
- IDO1
indoleamine 2,3-dioxygenase 1
- IFNγ
interferon-γ
- IL1β
interleukin-1β
- ITGAV
integrin αv
- LGR5
leucine rich repeat-containing G protein-coupled receptor 5
- LRP5/6
LDL receptor related proteins 5 and 6
- mAb
monoclonal antibody
- MDSC
myeloid-derived suppressor cell
- NF-κB
nuclear factor enhancer of immunoglobulin κ light chain of activated B cells
- NFAT
nuclear factor associated with T cells
- NK
natural killer
- PD-1
PDCD1 or CD279
- PD-L1
PD-1 ligand 1 or CD274
- PLC
phospholipase C
- PPI
protein–protein interaction
- PROTAC
proteolysis targeting chimera
- RNF43
ring finger protein 43
- ROR1/2
receptor tyrosine kinase-like orphan receptors 1 and 2
- SNAI1
Snail
- STAT3
signal transducer and activator of transcription 3
- TAM
tumor-associated macrophage
- TCF/LEF
T-cell factor 7 (TCF7 or Tcf-1) TCF7-like 1 (TCF7L1 or Tcf-3) TCF7-like 2 (TCF7L2 or Tcf-4) and lymphoid enhancer factor 1 (LEF1 or Lef-1)
- TGFβ
transforming growth factor-β
- TME
tumor microenvironment
- TNF
tumor necrosis factor
- Treg
regulatory T
- VEGF
vascular endothelial growth factor or VEGFA
- WISP1
WNT-induced secreted protein 1
- YAP-TEAD
Yes-associated transcriptional regulator and TEA domain transcription factor
- ZEB1
zinc finger E-box binding homeobox 1
- ZNRF3
zinc and ring finger 3
Competing Interests
The authors declare that there are no competing interests associated with this manuscript.
Funding
This work was supported in part by grants-in-aid from Masaru Katoh’s Fund for the Knowledge-Base Project and the Global Network Project.
Author Contribution
Masuko Katoh and Masaru Katoh designed and wrote this manuscript.
References
- 1.Katoh M. and Katoh M. (2007) WNT signaling pathway and stem cell signaling network. Clin. Cancer Res. 13, 4042–4045 10.1158/1078-0432.CCR-06-2316 [DOI] [PubMed] [Google Scholar]
- 2.Lagerström M.C. and Schiöth H.B. (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7, 339–357 10.1038/nrd2518 [DOI] [PubMed] [Google Scholar]
- 3.Katoh M. (2018) Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation. Int. J. Mol. Med. 42, 713–725 10.3892/ijmm.2018.3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M.T., Holderfield M., Galeas J., Delrosario R., To M.D., Balmain A.et al. (2015) K-Ras promotes tumorigenicity through suppression of non-canonical Wnt signaling. Cell 163, 1237–1251 10.1016/j.cell.2015.10.041 [DOI] [PubMed] [Google Scholar]
- 5.Zhang S., Zhang H., Ghia E.M., Huang J., Wu L., Zhang J.et al. (2019) Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc. Natl. Acad. Sci. U.S.A. 116, 1370–1377 10.1073/pnas.1816262116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons M.J., Tammela T. and Dow L.E. (2021) WNT as a driver and dependency in cancer. Cancer Discov. 11, 2413–2429 10.1158/2159-8290.CD-21-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugter J.M., Fenderico N. and Maurice M.M. (2021) Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat. Rev. Cancer 21, 5–21 10.1038/s41568-020-00307-z [DOI] [PubMed] [Google Scholar]
- 8.Radaszkiewicz T., Nosková M., Gömöryová K., Vondálová Blanářová O., Radaszkiewicz K.A., Picková M.et al. (2021) RNF43 inhibits WNT5A-driven signaling and suppresses melanoma invasion and resistance to the targeted therapy. Elife 10, e65759 10.7554/eLife.65759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tammela T., Sanchez-Rivera F.J., Cetinbas N.M., Wu K., Joshi N.S., Helenius K.et al. (2017) A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 545, 355–359 10.1038/nature22334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unterleuthner D., Neuhold P., Schwarz K., Janker L., Neuditschko B., Nivarthi H.et al. (2020) Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 23, 159–177 10.1007/s10456-019-09688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavan S., Mehta P., Xie Y., Lei Y.L. and Mehta G. (2019) Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J. Immunother. Cancer 7, 190 10.1186/s40425-019-0666-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh M. (2019) Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin. Sci. (London) 133, 953–970 10.1042/CS20180845 [DOI] [PubMed] [Google Scholar]
- 13.Katoh M. and Katoh M. (2020) Precision medicine for human cancers with Notch signaling dysregulation. Int. J. Mol. Med. 45, 279–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehghani-Ghobadi Z., Sheikh Hasani S., Arefian E. and Hossein G. (2022) Wnt5A and TGFβ1 converges through YAP1 activity and integrin alpha v up-regulation promoting epithelial to mesenchymal transition in ovarian cancer cells and mesothelial cell activation. Cells 11, 237 10.3390/cells11020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medema J.P. (2013) Cancer stem cells: the challenges ahead. Nat. Cell Biol. 15, 338–344 10.1038/ncb2717 [DOI] [PubMed] [Google Scholar]
- 16.Katoh M. (2017) Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity. Int. J. Oncol. 51, 1357–1369 10.3892/ijo.2017.4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatehullah A., Terakado Y., Sagiraju S., Tan T.L., Sheng T., Tan S.H.et al. (2021) A tumour-resident Lgr5+ stem-cell-like pool drives the establishment and progression of advanced gastric cancers. Nat. Cell Biol. 23, 1299–1313 10.1038/s41556-021-00793-9 [DOI] [PubMed] [Google Scholar]
- 18.Nakano M., Kikushige Y., Miyawaki K., Kunisaki Y., Mizuno S., Takenaka K.et al. (2019) Dedifferentiation process driven by TGF-beta signaling enhances stem cell properties in human colorectal cancer. Oncogene 38, 780–793 10.1038/s41388-018-0480-0 [DOI] [PubMed] [Google Scholar]
- 19.Shmelkov S.V., Butler J.M., Hooper A.T., Hormigo A., Kushner J., Milde T.et al. (2008) CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J. Clin. Invest. 118, 2111–2120 10.1172/JCI34401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Sousa e Melo F., Kurtova A.V., Harnoss J.M., Kljavin N., Hoeck J.D., Hung J.et al. (2017) A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 10.1038/nature21713 [DOI] [PubMed] [Google Scholar]
- 21.Bretones G., Delgado M.D. and León J. (2015) Myc and cell cycle control. Biochim. Biophys. Acta 1849, 506–516 10.1016/j.bbagrm.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 22.Muñoz-Espín D. and Serrano M. (2014) Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- 23.Phan T.G. and Croucher P.I. (2020) The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 10.1038/s41568-020-0263-0 [DOI] [PubMed] [Google Scholar]
- 24.Fujimaki K. and Yao G. (2020) Cell dormancy plasticity: quiescence deepens into senescence through a dimmer switch. Physiol. Genomics 52, 558–562 10.1152/physiolgenomics.00068.2020 [DOI] [PubMed] [Google Scholar]
- 25.Malladi S., Macalinao D.G., Jin X., He L., Basnet H., Zou Y.et al. (2016) Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 10.1016/j.cell.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren D., Dai Y., Yang Q., Zhang X., Guo W., Ye L.et al. (2019) Wnt5a induces and maintains prostate cancer cells dormancy in bone. J. Exp. Med. 216, 428–449 10.1084/jem.20180661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung P., Xiol J., Dill M.T., Yuan W.C., Panero R., Roper J.et al. (2020) Regenerative reprogramming of the intestinal stem cell state via Hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell 27, 590–604 10.1016/j.stem.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S., Ren J. and ten Dijke P. (2021) Targeting TGFβ signal transduction for cancer therapy. Signal Transduct. Target. Ther. 6, 8 10.1038/s41392-020-00436-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milanovic M., Fan D.N.Y., Belenki D., Däbritz J.H.M., Zhao Z., Yu Y.et al. (2018) Senescence-associated reprogramming promotes cancer stemness. Nature 553, 96–100 10.1038/nature25167 [DOI] [PubMed] [Google Scholar]
- 30.Choi H.J., Jin S., Cho H., Won H.Y., An H.W., Jeong G.Y.et al. (2019) CDK12 drives breast tumor initiation and trastuzumab resistance via WNT and IRS1-ErbB-PI3K signaling. EMBO Rep. 20, e48058 10.15252/embr.201948058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhan T., Ambrosi G., Wandmacher A.M., Rauscher B., Betge J., Rindtorff N.et al. (2019) MEK inhibitors activate Wnt signalling and induce stem cell plasticity in colorectal cancer. Nat. Commun. 10, 2197 10.1038/s41467-019-09898-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaffer S.M., Dunagin M.C., Torborg S.R., Torre E.A., Emert B., Krepler C.et al. (2017) Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 546, 431–435 10.1038/nature22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Islam S.S., Uddin M., Noman A.S.M., Akter H., Dity N.J., Basiruzzman M.et al. (2019) Antibody-drug conjugate T-DM1 treatment for HER2+ breast cancer induces ROR1 and confers resistance through activation of Hippo transcriptional coactivator YAP1. EBioMedicine 43, 211–224 10.1016/j.ebiom.2019.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada K., Hori Y., Inoue S., Yamamoto Y., Iso K., Kamiyama H.et al. (2021) E7386, a selective inhibitor of the interaction between β-catenin and CBP, exerts antitumor activity in tumor models with activated canonical Wnt signaling. Cancer Res. 81, 1052–1062 10.1158/0008-5472.CAN-20-0782 [DOI] [PubMed] [Google Scholar]
- 35.Liao H., Li X., Zhao L., Wang Y., Wang X., Wu Y.et al. (2020) A PROTAC peptide induces durable β-catenin degradation and suppresses Wnt-dependent intestinal cancer. Cell Discov. 6, 35 10.1038/s41421-020-0171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hojjat-Farsangi M., Moshfegh A., Schultz J., Norin M., Olin T., Österborg A.et al. (2021) Targeting the receptor tyrosine kinase ROR1 by small molecules. Handb. Exp. Pharmacol. 269, 75–99 10.1007/164_2021_535 [DOI] [PubMed] [Google Scholar]
- 37.Vaisitti T., Arruga F., Vitale N., Lee T.T., Ko M., Chadburn A.et al. (2021) ROR1 targeting with the antibody-drug conjugate VLS-101 is effective in Richter syndrome patient-derived xenograft mouse models. Blood 137, 3365–3377 10.1182/blood.2020008404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava S., Furlan S.N., Jaeger-Ruckstuhl C.A., Sarvothama M., Berger C., Smythe K.S.et al. (2021) Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell 39, 193–208 10.1016/j.ccell.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh M. and Katoh M. (2021) Grand challenges in molecular medicine for disease prevention and treatment through cyclical innovation. Front. Mol. Med. 1, 720577 10.3389/fmmed.2021.720577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J., Antin P., Berx G., Blanpain C., Brabletz T., Bronner M.et al. (2020) Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 10.1038/s41580-020-0237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshmukh A.P., Vasaikar S.V., Tomczak K., Tripathi S., den Hollander P., Arslan E.et al. (2021) Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc. Natl. Acad. Sci. U.S.A. 118, e2102050118 10.1073/pnas.2102050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Donaher J.L., Das S., Li X., Reinhardt F., Krall J.A.et al. (2022) Genome-wide CRISPR screen identifies PRC2 and KMT2D-COMPASS as regulators of distinct EMT trajectories that contribute differentially to metastasis. Nat. Cell Biol. 24, 554–564 10.1038/s41556-022-00877-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guinney J., Dienstmann R., Wang X., de Reyniès A., Schlicker A., Soneson C.et al. (2015) The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 10.1038/nm.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acebron S.P. and Niehrs C. (2016) β-catenin-independent roles of Wnt/LRP6 signaling. Trends Cell Biol. 26, 956–967 10.1016/j.tcb.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 45.Högström J., Heino S., Kallio P., Lähde M., Leppänen V.M., Balboa D.et al. (2018) Transcription factor PROX1 suppresses Notch pathway activation via the nucleosome remodeling and deacetylase complex in colorectal cancer stem-like cells. Cancer Res. 78, 5820–5832 10.1158/0008-5472.CAN-18-0451 [DOI] [PubMed] [Google Scholar]
- 46.Ragusa S., Prat-Luri B., González-Loyola A., Nassiri S., Squadrito M.L., Guichard A.et al. (2020) Antiangiogenic immunotherapy suppresses desmoplastic and chemoresistant intestinal tumors in mice. J. Clin. Invest. 130, 1199–1216 10.1172/JCI129558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flum M., Dicks S., Teng Y.H., Schrempp M., Nyström A., Boerries M.et al. (2022) Canonical TGFβ signaling induces collective invasion in colorectal carcinogenesis through a Snail1- and Zeb1-independent partial EMT. Oncogene 41, 1492–1506 10.1038/s41388-022-02190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sulaiman A., McGarry S., Han X., Liu S. and Wang L. (2019) CSCs in breast cancer-one size does not fit all: therapeutic advances in targeting heterogeneous epithelial and mesenchymal CSCs. Cancers (Basel) 11, 1128 10.3390/cancers11081128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh S.C., Sohn B.H., Cheong J.H., Kim S.B., Lee J.E., Park K.C.et al. (2018) Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat. Commun. 9, 1777 10.1038/s41467-018-04179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tariki M., Dhanyamraju P.K., Fendrich V., Borggrefe T., Feldmann G. and Lauth M. (2014) The Yes-associated protein controls the cell density regulation of Hedgehog signaling. Oncogenesis 3, e112 10.1038/oncsis.2014.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park H.W., Kim Y.C., Yu B., Moroishi T., Mo J.S., Plouffe S.W.et al. (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162, 780–794 10.1016/j.cell.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kröger C., Afeyan A., Mraz J., Eaton E.N., Reinhardt F., Khodor Y.L.et al. (2019) Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 116, 7353–7362 10.1073/pnas.1812876116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldker N., Ferrazzi F., Schuhwerk H., Widholz S.A., Guenther K., Frisch I.et al. (2020) Genome-wide cooperation of EMT transcription factor ZEB1 with YAP and AP-1 in breast cancer. EMBO J. 39, e103209 10.15252/embj.2019103209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pastushenko I., Mauri F., Song Y., de Cock F., Meeusen B., Swedlund B.et al. (2021) Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 589, 448–455 10.1038/s41586-020-03046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han Y., Villarreal-Ponce A., Gutierrez G. Jr., Nguyen Q., Sun P., Wu T.et al. (2022) Coordinate control of basal epithelial cell fate and stem cell maintenance by core EMT transcription factor Zeb1. Cell Rep. 38, 110240 10.1016/j.celrep.2021.110240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buechel D., Sugiyama N., Rubinstein N., Saxena M., Kalathur R.K.R., Lüönd F.et al. (2021) Parsing β-catenin’s cell adhesion and Wnt signaling functions in malignant mammary tumor progression. Proc. Natl. Acad. Sci. U.S.A. 118, e2020227118 10.1073/pnas.2020227118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson S., Coles M., Thomas T., Kollias G., Ludewig B., Turley S.et al. (2021) Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 21, 704–717 10.1038/s41577-021-00540-z [DOI] [PubMed] [Google Scholar]
- 58.Huinen Z.R., Huijbers E.J.M., van Beijnum J.R., Nowak-Sliwinska P. and Griffioen A.W. (2021) Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 18, 527–540 10.1038/s41571-021-00496-y [DOI] [PubMed] [Google Scholar]
- 59.Veglia F., Sanseviero E. and Gabrilovich D.I. (2021) Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 10.1038/s41577-020-00490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson M.J., Vignali P.D.A., Mullett S.J., Overacre-Delgoffe A.E., Peralta R.M., Grebinoski S.et al. (2021) Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 10.1038/s41586-020-03045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tauriello D.V.F., Sancho E. and Batlle E. (2022) Overcoming TGFβ-mediated immune evasion in cancer. Nat. Rev. Cancer 22, 25–44 10.1038/s41568-021-00413-6 [DOI] [PubMed] [Google Scholar]
- 62.Boussiotis V.A. (2016) Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 375, 1767–1778 10.1056/NEJMra1514296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casey S.C., Tong L., Li Y., Do R., Walz S., Fitzgerald K.N.et al. (2016) MYC regulates the antitumor immune response through CD47 and PD-L1. Science 352, 227–231 10.1126/science.aac9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu L., Fan J., Maity S., McFadden G., Shi Y. and Kong W. (2022) PD-L1 interacts with Frizzled 6 to activate β-catenin and form a positive feedback loop to promote cancer stem cell expansion. Oncogene 41, 1100–1113 10.1038/s41388-021-02144-2 [DOI] [PubMed] [Google Scholar]
- 65.Noman M.Z., Desantis G., Janji B., Hasmim M., Karray S., Dessen P.et al. (2014) PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon S.R., Maute R.L., Dulken B.W., Hutter G., George B.M., McCracken M.N.et al. (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu J., Hodgins J.J., Marathe M., Nicolai C.J., Bourgeois-Daigneault M.C., Trevino T.N.et al. (2018) Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 128, 4654–4668 10.1172/JCI99317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L., Yu X., Zheng L., Zhang Y., Li Y., Fang Q.et al. (2018) Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 564, 268–272 10.1038/s41586-018-0694-x [DOI] [PubMed] [Google Scholar]
- 69.Kraehenbuehl L., Weng C.H., Eghbali S., Wolchok J.D. and Merghoub T. (2022) Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 19, 37–50 10.1038/s41571-021-00552-7 [DOI] [PubMed] [Google Scholar]
- 70.Ramos-Casals M., Brahmer J.R., Callahan M.K., Flores-Chávez A., Keegan N., Khamashta M.A.et al. (2020) Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 6, 38 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sung B.Y., Lin Y.H., Kong Q., Shah P.D., Glick Bieler J., Palmer S.et al. (2022) Wnt activation promotes memory T cell polyfunctionality via epigenetic regulator PRMT1. J. Clin. Invest. 132, e140508 10.1172/JCI140508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quandt J., Arnovitz S., Haghi L., Woehlk J., Mohsin A., Okoreeh M.et al. (2021) Wnt-β-catenin activation epigenetically reprograms Treg cells in inflammatory bowel disease and dysplastic progression. Nat. Immunol. 22, 471–484 10.1038/s41590-021-00889-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rozovski U., Harris D.M., Li P., Liu Z., Jain P., Ferrajoli A.et al. (2019) STAT3-induced Wnt5a provides chronic lymphocytic leukemia cells with survival advantage. J. Immunol. 203, 3078–3085 10.4049/jimmunol.1900389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopez-Bergami P. and Barbero G. (2020) The emerging role of Wnt5a in the promotion of a pro-inflammatory and immunosuppressive tumor microenvironment. Cancer Metastasis Rev. 39, 933–952 10.1007/s10555-020-09878-7 [DOI] [PubMed] [Google Scholar]
- 75.Jang J., Song J., Lee H., Sim I., Kwon Y.V., Jho E.H.et al. (2021) LGK974 suppresses lipopolysaccharide-induced endotoxemia in mice by modulating the crosstalk between the Wnt/β-catenin and NF-κB pathways. Exp. Mol. Med. 53, 407–421 10.1038/s12276-021-00577-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeVito N.C., Sturdivant M., Thievanthiran B., Xiao C., Plebanek M.P., Salama A.K.S.et al. (2021) Pharmacological Wnt ligand inhibition overcomes key tumor-mediated resistance pathways to anti-PD-1 immunotherapy. Cell Rep. 35, 109071 10.1016/j.celrep.2021.109071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galluzzi L., Spranger S., Fuchs E. and López-Soto A. (2019) WNT signaling in cancer immunosurveillance. Trends Cell Biol. 29, 44–65 10.1016/j.tcb.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ji L., Qian W., Gui L., Ji Z., Yin P., Lin G.N.et al. (2020) Blockade of β-catenin-induced CCL28 suppresses gastric cancer progression via inhibition of Treg cell infiltration. Cancer Res. 80, 2004–2016 10.1158/0008-5472.CAN-19-3074 [DOI] [PubMed] [Google Scholar]
- 79.Tao W., Chu C., Zhou W., Huang Z., Zhai K., Fang X.et al. (2020) Dual role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat. Commun. 11, 3015 10.1038/s41467-020-16827-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spranger S., Bao R. and Gajewski T.F. (2015) Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 10.1038/nature14404 [DOI] [PubMed] [Google Scholar]
- 81.Ruiz de Galarreta M., Bresnahan E., Molina-Sánchez P., Lindblad K.E., Maier B., Sia D.et al. (2019) β-Catenin activation promotes immune escape and resistance to Anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 9, 1124–1141 10.1158/2159-8290.CD-19-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cristescu R., Nebozhyn M., Zhang C., Albright A., Kobie J., Huang L.et al. (2022) Transcriptomic determinants of response to pembrolizumab monotherapy across solid tumor types. Clin. Cancer Res. 28, 1680–1689 10.1158/1078-0432.CCR-21-3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luke J.J., Bao R., Sweis R.F., Spranger S. and Gajewski T.F. (2019) WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin. Cancer Res. 25, 3074–3083 10.1158/1078-0432.CCR-18-1942 [DOI] [PMC free article] [PubMed] [Google Scholar]