Abstract
This study describes the clinicopathological findings, diagnostic approach, treatment, and factors associated with non-survival of diarrheic horses admitted to 4 Canadian university teaching hospitals between 2015 and 2019. A total of 300 horses, ≥1-year-old, with acute diarrhea were included and represented 1.6% (300/18 481; range: 0.7 to 3%) of admissions during that period, 70% of the horses survived to discharge. Testing for enteropathogens was limited to a single fecal culture for Salmonella spp. in most cases. An enteropathogen was identified in 14% (42/300) of the horses, but in the hospital with higher testing rates enteropathogens were detected in 29% (16/55) of cases. Neorickettsia risticii was the pathogen most frequently detected (31%, 32/102). Antimicrobial drugs and plasma were administered to 57 and 8% of the cases, respectively. Laminitis occurred in 24/298 (8%) of the horses. A multivariable regression model identified an association between non-survival of diarrheic horses and colic signs, increased heart rate, packed cell volume, creatinine concentration, and decreased total protein concentration. A standardized approach for pathogen detection in diarrheic horses is not consistent among Canadian veterinary teaching hospitals, and testing for known pathogens is limited. Signs of colic, severe dehydration, endotoxemia, and hypoproteinemia are associated with non-survival of diarrheic horses.
Résumé
Diarrhée aiguë chez le cheval : une étude rétrospective canadienne multicentrique (2015 à 2019). Cette étude décrit les résultats clinicopathologiques, l’approche diagnostique, le traitement et les facteurs associés à la non-survie de chevaux diarrhéiques admis dans quatre hôpitaux universitaires canadiens entre 2015 et 2019. Un total de 300 chevaux, ≥1 an, atteints de diarrhée aiguë ont été inclus et représentaient 1,6 % (300/18 481; intervalle : 0,7 à 3 %) des admissions au cours de cette période. Soixante-dix pourcents des chevaux ont survécu jusqu’à leur congé. La recherche d’agents entéropathogènes était limitée à une seule culture fécale pour Salmonella spp. dans la plupart des cas. Un agent entéropathogène a été identifié chez 14 % (42/300) des chevaux, mais à l’hôpital avec des taux de dépistage plus élevés, des agents entéropathogènes ont été détectés dans 29 % (16/55) des cas. Neorickettsia risticii était l’agent pathogène le plus fréquemment détecté (31 %, 32/102). Des médicaments antimicrobiens et du plasma ont été administrés respectivement à 57 et 8 % des cas. Une fourbure est survenue chez 24/298 (8 %) des chevaux. Un modèle de régression multivarié a identifié une association entre la non-survie des chevaux diarrhéiques et les signes de coliques, l’augmentation de la fréquence cardiaque, l’hématocrite, la concentration de créatinine et la diminution de la concentration totale de protéines. Une approche normalisée pour la détection des agents pathogènes chez les chevaux diarrhéiques n’est pas uniforme dans les hôpitaux d’enseignement vétérinaires canadiens, et les tests pour les agents pathogènes connus sont limités. Des signes de coliques, de déshydratation sévère, d’endotoxémie et d’hypoprotéinémie sont associés à la non-survie des chevaux diarrhéiques.
(Traduit par Dr Serge Messier)
Introduction
Acute diarrhea in adult horses is an important cause of morbidity, although the global incidence of acute diarrhea in horses is unknown (1). The mortality rate of acute diarrhea varies depending on the causative agent (2) and geographic location (2,3). Horses suffering from Clostridioides (C.) difficile-associated diarrhea are more likely to die than horses suffering from other causes of acute diarrhea (3–5). Risk factors associated with mortality in cases of acute diarrhea include age, a history of antimicrobial therapy, azotemia during hospitalization, hemoconcentration, hypoproteinemia, and tachycardia (6,7). Currently, the incidence and risk factors associated with survival of diarrheic horses admitted to a veterinary teaching hospital (VTH) in Canada is unknown.
Canadian studies on equine diarrhea have mostly focussed on documenting the incidence of single pathogens in healthy and diarrheic horses or reporting the detection of specific pathogens in certain regions or VTH in the country (5,8–14). Information regarding the clinicopathological findings, diagnostic plan (e.g., pathogen testing, number of samples submitted, assays used for detection), and treatment approaches used in different hospitals is lacking. In addition, data regarding the most common pathogens detected in diarrheic horses throughout Canada are not available. This retrospective, multicenter study involving all Canadian VTH describes the epidemiology, clinicopathological findings, morbidity and mortality proportion, and risk factors associated with mortality of diarrheic horses. In addition, this study describes the etiology and treatment approaches to diarrheic horses in these hospitals. We hypothesized that the proportion of hospitalized horses with acute diarrhea and the survival proportion, regardless of etiology, varies among Canadian VTH. We also hypothesized that clinical and clinicopathologic findings associated with mortality in this population of horses with acute diarrhea would share similar risk factors previously reported for horses with gastrointestinal (GI) diseases. Lastly, we hypothesized that the diagnostic approaches, detected pathogens and treatment of horses with acute diarrhea varies among VTH across Canada.
Materials and methods
The medical records of all horses ≥1-year-of-age admitted to all 4 VTH in Canada between January 1, 2015 and December 31, 2019, were reviewed. Teaching hospitals included the Atlantic Veterinary College (AVC) of the University of Prince Edward Island, the Ontario Veterinary College (OVC) of the University of Guelph, the Centre hospitalier universitaire vétérinaire (CHUV) de l’Université de Montréal, and the Western College of Veterinary Medicine (WCVM) of the University of Saskatchewan. Horses admitted for evaluation of acute diarrhea of <48 h of onset or that developed diarrhea within the first 24 h after admission were included. Horses that underwent surgery and developed diarrhea after being admitted to the hospital were not included. The total number of horses admitted annually to each VTH was recorded. The proportion of horses with acute diarrhea was calculated as the number of new cases diagnosed in teaching hospitals in a specified period (i.e., 1 y and 5 y).
The following information was collected from each record: demographic data (sex, breed, age, month, and year of presentation) and historical data prior to admission (duration of clinical signs, history of administration of antimicrobial or anti-inflammatory drugs and reasons for administration, history of surgery or any disease before the onset of diarrhea and history of fever, diarrhea, or colic). Recorded admission physical examination findings determined by the attending clinician included demeanor (bright, obtunded, stuporous), temperature (T, Celsius), heart rate (HR, beats per minute, bpm), respiratory rate (RR, respirations per minute, rpm), mucous membranes (moist or tacky), capillary refill time (CRT, seconds), hydration status (estimated % of dehydration), and the presence of toxic line, diarrhea, colic, reflux, and laminitis (all recorded as present/absent).
From the complete blood (cell) count (CBC), the packed cell volume (PCV), the total white blood cell count (WBC), neutrophil, lymphocyte, and bands count, and the presence of toxic changes in the neutrophils (none, mild, moderate, or severe) were recorded. The following information was collected from the blood gas analysis: pH, pCO2, HCO3 −, and from the biochemistry profile: Na+, K+, Cl−, L-lactate, total protein (TP), and creatinine concentrations. When total protein was unavailable from the biochemistry, total solids (TS) were recorded.
The number and the types (feces, blood) of sample submitted for common pathogens (Salmonella, Clostridiodes difficile, equine coronavirus ECoV, Neorickettsia risticii) antemortem were recorded as well as the tests performed (e.g., PCR, culture, ELISA). Antimicrobial-associated diarrhea was defined as a horse being treated for a specific clinical diagnosis that developed diarrhea during therapy with antimicrobials.
Data on antimicrobial drug therapy administered during the first 24 h after admission and its duration was collected. Administration of plasma transfusion (type and volume), probiotics, and di- tri- octahedral (DTO) smectite during hospitalization were recorded. Complications during treatment (i.e., laminitis and phlebitis/thrombophlebitis) and outcome (survival to discharge or non-survival) were also recorded.
Statistical analysis
Normality of the data was assessed using the Kolmogorov-Smirnov test and data were analyzed accordingly. Descriptive statistics included mean and standard deviation (SD) or median and ranges. Comparison of categorical variables between institutions was performed using a X2 or Fisher exact tests, whereas numerical variables were compared with a 1-way ANOVA or the Steel-Dwass tests.
Variables associated with outcome (survivors and nonsurvivors) were analyzed with a Student’s t-test or the Mann-Whitney U-test. Spearman’s correlation coefficient was calculated to characterize associations between numerical variables. When 2 variables had a correlation coefficient > 0.6, only 1 variable was entered into the model (the variable of considered to have a major clinical relevance). The following selected variables were screened using a univariable analysis (Student’s t-test or Mann-Whitney U-test): institution, age (days), breed, sex, percentage of dehydration, presence of a toxic line, CRT, gastric reflux, diarrhea, colic signs, laminitis, HR, RR, T, PCV, TP, WBC, neutrophils, pH, pCO2, HCO3 −, Na+, K+, L-lac−, AG, and creatinine concentrations. The variables Na+ and Cl− and Cl and AG were correlated (0.73 and 0.64, respectively); therefore, Na+ and AG, but not Cl− were offered to the multivariate model. Transformation of non-normally distributed variables (HR, RR, WBC, neutrophils, L-lac−, and creatinine) was attempted to fit normal distribution but none of the used methods for transforming of the data improved the distributions. Variables with P <0.1 on univariable analysis were offered to the multivariable logistic model. The multivariable model was a backward stepwise model, whereby variables with the largest P-value were removed sequentially. Once the final model was determined, odds ratios (OR) and 95% confidence intervals (CI) were calculated. For the final model, interaction and quadratic terms for significant predictors were also investigated. Goodness-of-fit was evaluated using the Hosmer-Lemeshow goodness-of-fit X2 statistic. A P <0.05 was considered significant. Statistical analyses and figures were performed using statistical software (Minitab Software 19.2020.2.0, Philadelphia, Pennsylvania, USA) and JMP (JMP 16, SAS Institute, Cary, North Carolina, USA).
Results
Proportion of hospitalized horses with acute diarrhea
A total of 300 cases of acute diarrhea met the inclusion criteria during the 5-year study period. Fourteen (5%) were from AVC, 55 (18%) from CHUV, 204 (68%) from OVC, and 27 (9%) were from WCVM. The proportion of horses with acute diarrhea was 0.7% (14/1899) at AVC, 0.8% (55/6977) at CHUV, 3% (204/6825) at OVC, and 1% (27/2780) at WCVM. The proportion of diarrheic horses was higher for OVC compared with the other 3 institutions (P <0.01, for all comparisons). The overall proportion of diarrheic horses admitted to the VTH was 1.6% (300/18 481) and this was similar among all years of the study period.
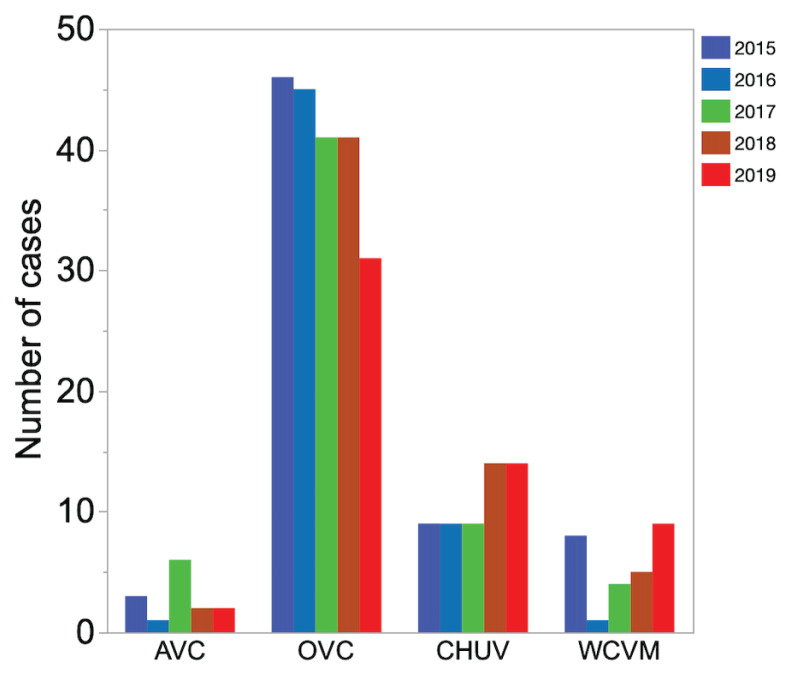
Distribution of cases was not statistically different between years, with 66 cases (22%) admitted in 2015, 56 cases (19%) in 2016, 60 cases (20%) in 2017, 62 cases (21%) in 2018, and 56 cases (18%) in 2019 (Figure 1). Most of the cases were admitted during the warmest months of the year (June to October) (Figure 2).
Figure 1.
Bar graph showing the number of horses with acute diarrhea admitted yearly to 4 Canadian teaching hospitals from 2015 to 2019. AVC — Atlantic Veterinary College; OVC — Ontario Veterinary College; CHUV — Centre hospitalier universitaire vétérinaire de l’Université de Montréal; WCVM — Western College of Veterinary Medicine.
Figure 2.
Bar graph showing the number of horses with acute diarrhea admitted monthly to 4 Canadian teaching hospitals from 2015 to 2019. AVC — Atlantic Veterinary College; OVC — Ontario Veterinary College; CHUV — Centre hospitalier universitaire vétérinaire de l’Université de Montréal; WCVM — Western College of Veterinary Medicine.
Demographic information
The median age was 8.5 y with a range between ≥1 to 26 y. Sex distribution was even between females (n = 140; 47%) and geldings (n = 142; 47%), and stallions accounted for a small number of the cases (n = 18; 6%). The distribution of sex did not statistically differ among hospitals. Twelve breeds were represented, although Quarter Horses, Thoroughbreds, and Standardbreds were most prevalent with 74 (25%), 51 (17%), and 29 cases (10%), respectively.
History
The most common clinical signs before admission were diarrhea, fever, and colic, in 205 (68%), 186 (62%), and 111 (37%) of the 300 horses, respectively. Ninety (30%) horses received antimicrobials before admission, but only 12 of those horses were treated for a disease different to the one leading to referral to the hospital (respiratory diseases, n = 6; disorders of the integumentary system, n = 4; orthopedic disorders, n = 2). A total of 213/300 (71%) horses received at least one dose of a non-steroidal anti-inflammatory drug before admission.
Clinicopathologic findings
Altered demeanor was observed in 73% (220/300) of the horses. The median rectal temperature was 38°C (range: 36 to 40°C) with 61/295 (21%) horses having a fever (T > 38.5°C) and 10/295 (3.5%) having hypothermia (T <36.5°C). The median HR was 52 bpm (range: 28 to 112 bpm) with 227/298 (76%) horses having tachycardia (HR > 40 bpm). The median respiratory rate was 20 rpm (range: 8 to 88 rpm) with 123/295 (42%) having tachypnea (RR > 20 rpm). The following clinical alterations were present on admission: diarrhea in 230/300 (77%), CRT > 2 s in 138/292 cases (47%), toxic line in 82/300 (27%), colic in 78/300 (26%), laminitis in 21/300 (7%) and reflux in 18/300 (6%). Nineteen of 21 horses with laminitis developed foot pain within 24 h before or after admission. Two horses had chronic laminitis associated with an endocrinologic disease.
A total of 124/264 horses (47%) had leukopenia (WBC <5300 cells/μL) and 14/264 (5%) had leukocytosis (WBC > 14 800 cells/μL); 123/263 (47%) had neutropenia (neutrophils <2300 cells/μL), and 23/263 (9%) had neutrophilia (neutrophils > 8500 cells/μL); 75/259 (29%) horses had azotemia (creatinine > 175 mmol/L); 34/257 horses (13%) had acidemia (pH <7.32), and 24/257 (9%) had alkalemia (pH > 7.45); 98/252 (39%) horses had low blood HCO3 − concentrations (HCO3 − <24 mmol/L), whereas 14/252 (6%) horses had high blood HCO3 − concentrations (HCO3 − > 30 mmol/L); 71/245 (29%) horses had hypocapnia (PCO2 <38 mmHg) and 13/245 (5%) had hypercapnia (PCO2 > 50 mmHg); 128/272 (47%) had hyponatremia (Na+ <132 mmol/L) and 2/272 (0.7%) had hypernatremia (Na+ > 148 mmol/L); 43/272 (16%) had hypokalemia (K+ <2.7 mmol/L) and 7/272 (3%) had hyperkalemia (K+ > 5 mmol/L); 158/268 (59%) had hypochloremia (Cl− <99 mmol/L) and 3/268 (1%) had hyperchloremia (Cl− > 108 mmol/L); 136/266 (51%) had hyperlactatemia (L-lac− > 2 mmol/L); 101/285 (35%) had hypoproteinemia (TP/TS <5.4 g/dL) and 13/285 (5%) had hyperproteinemia (TP/TS > 7.8 g/dL).
Etiologic agent investigation
Table 1 summarizes all the testing for Salmonella spp., ECoV, C. difficile, and N. risticii in each institution. Overall, an enteropathogen was identified in 14% (42/300) of the horses. The CHUV tested a greater proportion of horses for > 1 enteropathogen compared to the other 3 institutions with 20 (36%) horses tested for 4 pathogens, 16 (29%) for 3 pathogens, 10 (18%) for 2, and 8 (15%) for 1 pathogen and 1 (2%) for none. The CHUV had a greater detection rate of enteropathogens (29%, 16/55) compared to OVC (15%, 30/204) (P <0.05).
Table 1.
Number and type of samples submitted for detection of common enteropathogens in horses with acute diarrhea admitted to 4 Canadian teaching hospitals from 2015 to 2019.
| Enteropathogen | AVC n = 14 |
OVC n = 204 |
CHUV n = 55 |
WCVM n = 27 |
All N = 300 |
|---|---|---|---|---|---|
| Salmonella spp. | |||||
| Animals tested (n) | 13 (93%) | 164 (80%) | 49 (89%) | 15 (56%) | 241 (80%) |
| Positive culture 1 | 1/13 | 0/164 | 3/49 | 0/15 | 5/241 (2%) |
| Positive culture 2 | 1/12 | 0/71 | 2/43 | 0/13 | 3/136 (2%) |
| Positive culture 3 | 1/10 | 0/47 | 1/40 | 0/9 | 2/106 (2%) |
| Positive culture 4 | 0/0 | 1/36 | 0/1 | 0/0 | 1/38 |
| Positive culture 5 | 0/0 | 1/30 | 0/0 | 0/0 | 1/30 |
| Total animals positive | 1/13 (8%)a,b | 1/164 (0.6%)b | 3/49 (6%)a | 0/15 (0%)a,b | 5/245 (2%) |
| Neorickettsia risticii | |||||
| Animals tested (n) | 0 (0%) | 51 (25%) | 32 (58%) | 19 (70%) | 102 (34%) |
| Positive PCR blood | — | 13/51 | 4/29 | 1/4 | 18/84 |
| Positive PCR feces | — | 10/41 | 1/32 | 3/19 | 16/92 |
| Total animals positive | — | 23/51 (45%)a | 5/32 (16%)a | 4/19 (21%)a | 32/102 (31%) |
| Equine Coronavirus | |||||
| Animals tested (n) | 3 (21%) | 19 (9%) | 28 (51%) | 2 (7%) | 52 (17%) |
| Total animals positive | 1/3 (33%)a | 2/19 (11%)a | 6/28 (21%)a | 0/2 (0%)a | 9/52 (17%) |
| Clostridiodes difficile | |||||
| Animals tested | 0 (0%) | 39 (19%) | 37 (67%) | 1 (4%) | 77 (26%) |
| Tox. A/B ELISA | — | 4/36 | 2/37 | — | 6/73 |
| Tox. B PCR | — | 0/3 | — | 0/1 | 0/4 |
| Total animals positive | — | 4/39 (10%)a | 2//37 (5%)a | 0/1 (0%)a | 6/77 (7.8%) |
| Enteropathogen detected | 2 (14%)a,b | 30 (15%)b | 16 (29%)a | 4 (15%)a,b | 42 (14%) |
AVC — Hospital 1; OVC — Hospital 2; CHUV — Hospital 3; WCVM — Hospital 4.
Columns with different letters indicate statistical differences (P < 0.05) between groups.
The most frequently investigated etiologic agent was Salmonella spp. with 241/300 (80%) horses tested (fecal culture) at least once, 5/241 (2%) of which tested positive. Out of those 241 horses, 136 had a second fecal culture for Salmonella spp., 106 had 3 cultures, 38 had 4 cultures, and 30 had 5 cultures. Except for 1 horse, which tested positive only on culture 4 out of 5, all horses that were negative in the first culture were also negative in the subsequent ones. The proportion of horses positive for Salmonella spp. was higher in CHUV (n = 3/49; 6%) than OVC (1/164, 0.6%) (P = 0.04) (Table 1). At AVC and CHUV, the most common diagnostic approach for Salmonella spp. was 3 fecal cultures per horse (10/13 and 40/53, respectively). At OVC, the most common diagnostic approach for Salmonella spp. was 1 fecal culture.
Neorickettsia risticii was the second most frequently investigated etiologic agent. A total of 102/300 (34%) horses were tested either on feces or blood, or both. A total of 32/102 (31%) horses tested positive. No statistical difference (P > 0.05) in the number of positive horses for N. risticii, ECoV, or C. difficile among institutions was identified, although no horses were tested for N. risticii at the AVC. Seventy-seven horses (26%) were tested for C. difficile by ELISA for toxins A/B, 6 (8%) of which were positive. Equine coronavirus was the least frequently tested pathogen (n = 52/300; 17%) with 9/52 (17%) horses testing positive. Antimicrobial-associated diarrhea was suspected in 12/300 (4%) horses. Two of those horses were Salmonella spp positive.
Therapy
The number of cases receiving antimicrobial therapy was similar among institutions (P > 0.05 for all comparisons) with 171/300 cases (57%) starting therapy within the first 24 h of admission. Forty horses (23%) of the 171 that received antimicrobial therapy had a neutrophil count <1 K/μL and 43/171 (25%) had a neutrophil count > 1 K/μL and <2.3 K/μL. The median duration of antimicrobial therapy for all horses was 3 d (range: 1 to 14 d). The median duration of antimicrobial therapy was significantly greater at CHUV (median: 7 d, range: 1 to 14 d) than at AVC (4 d; range: 3 to 7 d), OVC (4 d; range: 1 to 14 d), and WCVM (3 d; range: 1 to 14 d). The median duration of antimicrobial therapy was 4 d (range: 1 to 14 d) for surviving horses and it was similar among institutions (P > 0.05, for all comparisons). The most common antimicrobial drug combination administered was penicillin and gentamicin in 64/171 horses (37%) and monotherapy with oxytetracycline in 47 cases (27%), whereas other single or polymicrobial therapies were used in the remaining 16% of horses. Penicillin and gentamicin were used as the most frequent antimicrobial therapy combination at AVC (5 cases, 62%) and OVC (52 cases, 43%), whereas the CHUV used oxytetracycline more frequently (13 cases, 37%). The WCVM had an equivalent frequency for penicillin and gentamicin (5 cases, 33%) and oxytetracycline (5 cases, 33%). Polymyxin B was used only at WCVM in 4 cases. Table 2 provides a complete description and comparison of therapies among institutions.
Table 2.
Selected therapies administered to horses with acute diarrhea admitted to 4 Canadian teaching hospitals from 2015 to 2019.
| Treatment | AVC n = 14 |
OVC n = 204 |
CHUV n = 55 |
WCVM n = 27 |
All n = 300 |
|---|---|---|---|---|---|
| Plasma transfusion | 0 (0%)a | 11 (5%)a | 10 (18%)b | 5 (19%)b | 25 (8%) |
| di- tri- octahedral Smectite | 0 (0%) | 0 (0%) | 0 (0%) | 4 (15%) | 4 (1%) |
| Antimicrobial drugs | 8 (57%)a | 121 (59%)a | 27 (49%)a | 15 (56%)a | 171 (57%) |
| Duration antimicrobial therapy (days, all horses) | 4 (3 to 7)a,c | 4 (1 to 14)a | 7 (1 to 14)a | 2 (1 to 7)c | 3 (1 to 14) |
| Duration of antimicrobial therapy (survivors) | 4 (3 to 6)a | 4 (1 to 9)a | 4 (2 to 14)a | 5 (3 to 7)a | 4 (1 to 14) |
| Antimicrobial therapy | |||||
| Ceftiofur sodium | 3 (38%)a | 2 (2%)b | 4 (15%)c | 1 (13%)b | 10 (6.5%) |
| Metronidazole | 2 (2%) | 2 (7%) | 4 (2.5%) | ||
| Oxytetracycline | 28 (23%)a | 13 (37%)a | 5 (33%)a | 47 (28%) | |
| Polymyxin B | 4 (13%) | 4 (2%) | |||
| TMS | 3 (2.5%)a | 1 (4%)a | 1 (8%)a | 5 | |
| Ceftiofur + Metro | 1 (1%)b | 3 (11%)a | 4 (2.5%) | ||
| Pen + Gen | 5 (62%)a,b | 52 (43%)b | 2 (7%)c | 5 (33%)b | 64 (37%) |
| Pen + TMS | 7 (6%) | 7 (4%) | |||
| Pen + Gen + Metro | 9 (7%) | 9 (5%) | |||
| Pen + Oxytetracycline | 6 (5%) | 6 (3.5%) | |||
| Other combinations | 11 (9%) | 2 (7%) | 11 (6%) | ||
AVC — Hospital 1; OVC — Hospital 2; CHUV — Hospital 3; WCVM — Hospital 4; Metro — Metronidazole; Pen — Penicillin; Gen — Gentamycin; TMS — Trimethoprim sulfonamide.
Columns with different letters indicate statistical differences (P < 0.05) between groups.
Plasma transfusions were infrequently administered overall, with only 25/300 cases (8%) receiving this treatment. These transfusions were more frequent at CHUV and WCVM, however, compared to other institutions (P <0.05). Ten out of 55 cases (18%) at CHUV and 5/27 cases (19%) at WCVM received plasma transfusions, whereas only 11/204 (5%) and 0/14 horses had a plasma transfusion at OVC and AVC, respectively. Di-tri-octahedral smectite was infrequently administered at all institutions with only 4/27 cases (15%) receiving this treatment at the WCVM.
Laminitis and phlebitis/thrombophlebitis
In addition to the 21 horses with acute laminitis identified on admission, 3/279 (1%) horses developed laminitis during hospitalization, for a total of 24/298 (8%). Seven (29%) of the 24 horses that developed acute laminitis were positive for N. risticii and 2 (8%) for Salmonella spp.; the cause of diarrhea was not identified in the remaining 15 horses. The proportion of horses positive for N. risticii (22%, 7/32) and Salmonella spp. (40%, 2/5) which developed laminitis was similar (P = 0.65). The CHUV had significantly (P <0.05, for all comparisons) higher rate of laminitis in diarrheic horses (18%, 10/55) than other institutions (0 in AVC, and 1/26 (4%) in WCVM and, 13/203 (6%) in OVC). Seven out of the 10 horses from the CHUV had signs of laminitis on admission. A total of 22/300 (7%) of the horses developed phlebitis/thrombophlebitis associated with acute diarrhea.
Outcome
The overall survival rate for acute diarrhea combining all institutions and years was 70% (212/300). The survival rates for each site were 63% for WCVM, 69% for OVC, and 78% at both AVC and CHUV. Among the 88 non-survivors, 7/25 (28%) of the N. risticii cases and 2/5 (40%) of the Salmonella spp. cases did not survive, and no antemortem diagnosis was reached in the remaining 79/88 (90%) horses. No significant differences on the overall survival proportion were identified among years and institutions (Table 3).
Table 3.
Survival rates of horses with acute diarrhea admitted to 4 Canadian teaching hospitals from 2015 and 2019.
| Year | AVC n = 14 |
OVC n = 204 |
CHUV n = 55 |
WCVM n = 27 |
ALL N = 300 |
|---|---|---|---|---|---|
| 2015 | 3 (100%)a | 33 (72%)a | 6 (67%)a | 4 (50%)a | 46 (70%)a |
| 2016 | 1 (100%)a | 30 (67%)a | 7 (78%)a | 0 (0%)a | 38 (68%)a |
| 2017 | 4 (67%)a | 29 (70%)a | 9 (100%)a | 3 (75%)a | 45 (75%)a |
| 2018 | 2 (100%)a | 26 (63%)a | 10 (72%)a | 4 (80%)a | 42 (68%)a |
| 2019 | 1 (50%)a | 23 (74%)a | 11 (78%)a | 6 (67%)a | 41 (73%)a |
| All | 11 (78%)b | 141 (69%)b | 43 (78%)b | 17 (63%)b | 212 (71%)b |
Percentage for that particular year.
Percentage of total for all years.
No statistical differences for all comparisons (P > 0.05) (e.g., years, institutions, year × institution).
AVC — Hospital 1; OVC — Hospital 2; CHUV — Hospital 3; WCVM — Hospital 4.
Factors associated with non-survival
Clinical admission findings more commonly detected in nonsurvivors compared to survivors included a higher percentage of dehydration, presence of a toxic line, colic, laminitis, and prolonged CRT (P <0.05). On admission, non-surviving horses had a significantly higher HR, RR, PCV, creatinine, K+ and L-lactate− concentrations, and lower TP/TS, Na+ and Cl− concentrations than non-surviving horses (P <0.05) (Table 4).
Table 4.
Demographic characteristics and clinicopathologic findings of surviving and non-surviving horses with acute diarrhea admitted to 4 Canadian teaching hospitals.
| Variable | Available data (surviving | non-surviving) | Survivors n = 212 |
Non-survivors n = 88 |
P-value |
|---|---|---|---|---|
| Sex | 212 | 88 | 0.331 | ||
| Female | 105/212 (50%) | 36/88 (41%) | ||
| Gelding | 96/212 (45%) | 45/88 (51%) | ||
| Stallion | 11/212 (5%) | 7/88 (8%) | ||
| Dehydration | 86 | 47 | < 0.001 | ||
| 5 to 7% | 56/86 (65%) | 10/47 (21%) | ||
| 8 to 10% | 18/86 (21%) | 18/47 (38%) | ||
| > 10% | 12/86 (14%) | 19/47 (40%) | ||
| Toxic line | 212 | 88 | 44/212 (21%) | 38/88 (43%) | < 0.001 |
| CRT > 2 seconds | 207 | 85 | 80/207 (39%) | 58/85 (68%) | < 0.001 |
| Reflux | 212 | 88 | 9/212 (4%) | 9/88 (10%) | 0.060 |
| Diarrhea | 212 | 88 | 167/212 (79%) | 63/88 (72%) | 0.186 |
| Colic | 212 | 88 | 47/212 (22%) | 31/88 (35%) | 0.021 |
| Laminitis | 212 | 88 | 10/212 (5%) | 11/88 (13%) | 0.016 |
| Age (years) | 212 | 87 | 8 (1 to 26) | 10 (1 to 26) | 0.654 |
| Temperature (°C) | 211 | 84 | 38 (35.7 to 40) | 38 (35.9 to 40) | 0.140 |
| Heart rate (bpm) | 211 | 87 | 48 (28 to 100) | 64 (40 to 112) | < 0.001 |
| Respiration (rpm) | 210 | 85 | 20 (8 to 80) | 24 (8 to 88) | 0.003 |
| PCV (%) | 203 | 83 | 40 (25 to 70) | 51 (28 to 75) | < 0.001 |
| TP (g/dL) | 202 | 83 | 6 (2.8 to 9.8) | 5.3 (2 to 9.2) | 0.012 |
| WBC (K/μL) | 194 | 70 | 5.5 (0.8 to 26) | 6 (1.4 to 28) | 0.890 |
| Neutrophils (K/μL) | 194 | 69 | 2.7 (0.1 to 24) | 2.3 (0.3 to 14) | 0.228 |
| Creatinine (mg/dL) | 191 | 68 | 125 (60 to 579) | 171 (46 to 915) | < 0.001 |
| Na+ (mmol/L) | 189 | 83 | 133(112 to 150) | 128 (110 to 139) | 0.049 |
| K+ (mmol/L) | 189 | 83 | 3.3 (1.5 to 5.8) | 3.4 (1.3 to 6) | 0.072 |
| Cl− (mmol/L) | 186 | 79 | 98 (70 to 117) | 94 (67 to 108) | 0.026 |
| L-lactate− (mmol/L) | 181 | 85 | 1.6 (0.4 to 13) | 3.2 (0.3 to 14) | < 0.001 |
| pH | 177 | 80 | 7.41 (7.2 to 7.52) | 7.39 (7.01 to 7.49) | 0.274 |
| PvCO2 (mmHg) | 167 | 78 | 41 (24 to 66) | 40 (26 to 60) | 0.675 |
| HCO3− (mmol/L) | 173 | 79 | 25 (11 to 36) | 24 (10 to 38) | 0.499 |
| AG (mmol/L) | 169 | 76 | 12 (0 to 38) | 14 (2 to 46) | 0.088 |
Data presented as proportion or median and lower and higher range. P-values were obtained using the Mann-Whitney test. PCV — Packed cell volume; TP — Total proteins; WBC — Total white blood cells; HCO3− — Bicarbonate; pvCO2 — Venous partial carbon dioxide pressure; AG — Anion gap.
AVC — Hospital 1; OVC — Hospital 2; CHUV — Hospital 3; WCVM — Hospital 4.
The multivariable logistic regression model used to evaluate the association between selected explanatory variables and the outcome of non-survival showed that every 20 bpm increase in HR at admission was associated with greater odds of nonsurvival (OR: 1.9; 95% CI: 1.13 to 3.17). The presence of colic signs on admission was also significantly associated with increased odds of non-survival (OR: 3.73; 95% CI: 1.67 to 8.31). Every 10% increase in PCV (OR: 2.40; 95% CI: 1.58 to 3.53), 50 μmol/L increase in creatinine concentration (OR: 1.2; 95% CI: 1.02 to 1.41), and 1 g/dL decrease in TP/TS (OR: 0.70; CI: 0.55 to 0.91) were also significantly associated with greater odds of non-survival (Table 5).
Table 5.
Results of a backward stepwise multivariable logistic model assessing the association between selected admission clinical and laboratory variables and outcome of horses with acute diarrhea admitted to 4 Canadian teaching hospitals from 2015 to 2019.
| Term | Unit of change | Coefficient (95% CI) | OR (95% CI) | VIF | P-value |
|---|---|---|---|---|---|
| Heart rate | 20 bpm | 0.032 (0.006 to 0.06) | 1.9 (1.13 to 3.17) | 1.35 | 0.014 |
| PCV | 10% | 0.086 (0.046 to 0.12) | 2.4 (1.58 to 3.53) | 1.28 | < 0.001 |
| Creatinine | 50 μmol/L | 0.003 (0.0005 to 0.007) | 1.2 (1.02 to 1.41) | 1.20 | 0.022 |
| TP/TS | 1 g/dL | −0.337 (−0.58 to 0.09) | 0.7 (0.55 to 0.91) | 1.09 | 0.007 |
| Colic | Absent | Referent | |||
| Present | 1.317 (0.52 to 2.12) | 3.7 (1.67 to 8.31) | 1.14 | 0.001 |
PCV — Packed cell volume; TP — total proteins; TS — Total solids; CI — Confidence interval; OR — Odds ratio; VIF — Variance insufflation factor; bpm — Beats per minute.
Discussion
This retrospective multicenter study describes the epidemiology, clinicopathological findings, diagnostic and treatment approaches, and factors associated with survival of diarrheic horses admitted to all 4 Canadian VTH. The proportion of hospitalized horses with acute diarrhea from 2015 to 2019 was 1.6%, although it varied significantly among hospitals. The survival proportion of diarrheic horses over the studied period was 70% and it was similar from year-to-year and institutions. We also demonstrated that the clinicopathological factors associated with non-survival of diarrheic horses were similar to those previously reported for colic and colitis cases as well as the complication rate associated with diarrhea. Finally, our study revealed differences in the diagnostic and treatment approaches to diarrheic horses in the different hospitals, especially those regarding investigation of the etiology of diarrhea.
Proportion of diarrheic horses admitted to Canadian teaching hospitals
The proportion of horses with acute diarrhea from 2015 to 2019 ranged from 0.7 to 3% of the total hospitalized horses, with the OVC having a significantly higher proportion compared to the other 3 institutions. Studies documenting the prevalence or incidence of acute diarrhea are scarce, although reports suggest that acute diarrhea is responsible for 5% of the total admissions to equine hospitals (1). The reasons for the differences in hospital prevalence are unknown, but in the authors’ experience acute GI diseases, and specifically diarrhea associated with N. risticii, have historically been an important cause of equine disease in Ontario (12,15–18). In our study, the number of horses diagnosed with Neorickettsiosis was higher at OVC than WCVM and CHUV. This was not statistically significant but could in part explain the higher proportion of diarrheic horses at OVC. Regional differences in other types of emergencies, proportion of elective cases, financial capabilities of owners, and isolation unit capacities can also explain the differences in the proportion of diarrheic horses among institutions.
Diagnostic approach and etiology
Enteropathogen investigation for the cause of acute diarrhea varies among Canadian VTH. In general, in most cases the etiologic testing was limited to a single fecal culture for Salmonella spp. Our results also demonstrated that institutions (i.e., CHUV) that tested for a greater number of enteropathogens had higher positive rates of pathogen detection than institutions with lower testing rates (i.e., OVC). These findings suggest that there currently is no standardized approach for pathogen detection in diarrheic horses among Canadian VTH. The experimental design of this study prevents us from determining the reason for the limited testing. Possible explanations include the epidemiology of the diseases, economic constraints, clinician attitudes regarding pathogen identification as they could consider that testing will not alter the treatment or prognosis, and test availability in the institution or regional laboratories. A recent report from the Havemeyer workshop on acute colitis stated that the etiology of acute colitis is undetermined in more than 50% of the cases (1). However, our results suggest that the high number of cases of acute diarrhea of undetermined cause can be explained, at least in part, by limited antemortem testing. In recent years, some of the Canadian veterinary hospitals (e.g., OVC) have implemented the use of an in-house GI pathogen panel to test for the presence of multiple pathogens in a fecal sample (i.e., ECoV, Salmonella spp., N. risticii and C. difficile, and C. perfringens). The GI pathogen panel also facilitates the detection of co-infections (more than one pathogen causing infection) and identifies microorganisms that can be missed with traditional methods. Finally, the GI pathogen panel results are usually available within hours, compared to days when using traditional methods (i.e., bacterial culture, virus isolation). Future studies investigating whether the implementation of GI pathogen panel improves the detection rates of pathogens and has an impact on the survival rates of diarrheic horses are warranted.
Most horses included in this study were tested for Salmonella spp. via fecal culture. Of interest, at AVC, CHUV and WCVM, 3 fecal cultures were performed in 60 to 75% of all the cases tested for Salmonella spp., whereas at OVC only 30% of the cases were tested. Currently, textbooks continue to recommend multiple, serial bacterial cultures (generally 5 cultures) based on studies published in the 1970’s and 80’s. However, new studies are needed to determine if serial cultures are indeed necessary for the diagnosis of Salmonellosis in diarrheic horses. Regarding Neorickettsiosis, 35% of the horses were tested for this bacterium, a percentage that is likely influenced by the seasonality of the disease (12,19). At AVC, none of the horses were tested for N. risticii probably because this disease is uncommonly reported in eastern Canada (11) and clinicians might consider it unnecessary to test for this pathogen. Testing for ECoV was limited; however, 17% of the tested horses were positive. This prevalence is higher than the 6% reported in the USA between 2010 and 2014 (20). The high prevalence of ECoV reported in our study strongly supports frequent testing for this pathogen in Canadian institutions. The prevalence of C. difficile was 10% and this is similar to the 8% prevalence recently reported in Switzerland (21) but lower than the 17.5% reported in Japan, 23% in Australia (22), and 22% at OVC 20 y ago (5).
Treatments
Medical management varied among institutions, although the number of horses receiving antimicrobial therapy was similar in all 4 institutions. Antimicrobial drugs were prescribed to 57% of horses in the first 24 h after admission. This proportion of diarrheic horses treated with antimicrobials was slightly higher than that recently reported in Switzerland (49%, 74/151) (21), although the antimicrobial treatment rates for diarrheic horses are largely unknown.
Antimicrobial treatment guidelines are lacking for these cases. However, antimicrobial therapy appears to be justified for treatment of a particular enteropathogen (i.e., N. risticii), or treatment of bacterial translocation and/or evidence of systemic disease (23). Broad-spectrum antimicrobial therapy is generally administered to cases with marked leukopenia (neutrophils <1000 cells/μL) because of the risk of secondary infection, and to horses with suspected or confirmed N. risticii infection. Administration of metronidazole is associated with survival in horses with C. difficile diarrhea (24). In our study, however, only 17 horses were treated with metronidazole, 14/17 of which were treated at OVC. Median duration of antimicrobial therapy for all horses was 3 d. However, in some institutions the median duration of antimicrobial therapy was 7 d. The ideal treatment duration in neutropenic diarrheic horses and horses with N. risticii is unknown. Most neutropenic and N. risticii cases were treated for 3 to 5 d. In our experience the neutropenia observed in diarrheic horses resolves within 3 to 4 d after commencing treatment (with or without antimicrobial drugs), whereas horses with neorickettsiosis improve markedly within 48 to 72 h after oxytetracycline therapy. Currently, the ideal length of antimicrobial treatment in these cases is unknown, and prospective studies are required to determine the ideal duration of antimicrobial therapy in diarrheic horses.
Plasma transfusion was infrequently performed with only 8% of the horses receiving a transfusion. In equine medicine, clinical studies determining the benefit or outcomes of such intervention in hypoproteinemic horses are lacking. A retrospective study revealed that the administration of plasma to horses with typhlocolitis/colitis did not improve the survival rate when compared to horses that did not receive a plasma transfusion (25). In another retrospective study on horses with colitis, plasma transfusion was associated with non-survival and higher cost of hospitalization (7). Similarly, administration of intraluminal intestinal binding agents to diarrheic horses was infrequent. Although administration of DTO smectite reduces the prevalence of post-operative diarrhea (26), the benefits of DTO smectite in diarrheic horses has not been evaluated. There is need for evidence-based medicine to support plasma transfusion and DTO smectite administration.
Laminitis and phlebitis/thrombophlebitis
The prevalence of laminitis in diarrheic horses was 8%, 22% (7/32) and 40% (2/5) of which were horses positive for N. risticii and Salmonella spp., respectively. The reported prevalence of laminitis in diarrheic horses ranges from 2 to 36% (6,27–29) and similar to our study, horses suffering from N. risticii infection appeared to have the same risk of developing laminitis as horses with Salmonella spp. (2). Phlebitis/thrombophlebitis was deterimned in 7% of our horses and it is similar to that reported for surgical colic cases (7.5%) (30). Risk factors associated with phlebitis/thrombophlebitis in hospitalized horses include fever, endotoxemia, hypoproteinemia, and infection with Salmonella spp. (31,32). All those factors were present in most horses included in our study and were presumed to have contributed to the development of phlebitis/thrombophlebitis.
Non-survival proportion and risk factors for non-survival
The survival proportion for all diarrheic horses included in this study was 70% and was similar among hospitals and among the years under study. This proportion of surviving horses is similar to those reported in 1990 (6) and recently in 2020 (27) in North America, suggesting that despite advances in the diagnosis and treatment of GI diseases over the years, no improvement in the survival proportion has occurred over time and results are similar among North American institutions. In our study, 72% and 67% of horses positive for N. risticii and Salmonella spp., respectively, survived to hospital discharge. This is in agreement with previous reports indicating that survival proportions of diarrheic horses can vary from 57 to 98% depending on the causative agent and geographic location (3,28,33,34). Reported survival rates for horses diagnosed with N. risticii range from 65 to 73% (27,29), and for Salmonella spp. from 70 to 92% in different areas of the USA (27,28). In Japan, the survival rates of diarrheic horses positive and negative for C. difficile is 17% and 66%, respectively (3). In Canada, 74% and 83% of C. difficile toxin-positive and toxin-negative survived, respectively, whereas in California (USA) 76% of horses with positive ELISA results for both C. difficile antigen and toxin A survived compared with 90% C. difficile negative horses (4). ECoV survival rates were reported to be 96% in USA and 100% in Japan (27,35,36) and are similar to the 100% survival proportion for ECoV reported in this study. Our conclusion regarding survival proportions by etiologic agent were limited by the small number of nonsurviving horses that had a definitive antemortem etiologic diagnosis for acute diarrhea.
Factors associated with non-survival
Factors associated with non-survival in the diarrheic horses enrolled in this study included presence of colic signs on admission, increased HR, PCV, and creatinine, and decreased TP/TS concentration. Except for the presence of colic signs on admission, these factors are also reported to be associated with non-survival in horses with colitis (6,27,29) and colic (37,38). Increase in HR, PCV, and creatinine concentrations likely reflects the severity of dehydration and the presence of endotoxemia. Horses experiencing acute diarrhea lose excessive quantities of sodium-containing fluids leading to a decrease in effective circulating volume (39,40), which in severe cases results in inadequate tissue perfusion and development of organ dysfunction (41). This organ dysfunction could be exacerbated by endotoxemia (42). Endotoxin concentration is not routinely measured in clinical cases, but it is suspected to occur in diarrheic horses with tachycardia, tachypnea, hypovolemia, presence of toxic line, and hyperlactatemia. In our study, all those clinicopathological factors were altered at greater magnitude in non-surviving than in surviving horses.
Hypoproteinemia, mostly due to hypoalbuminemia, is common in horses with acute diarrhea (40). Critically ill diarrheic horses develop a protein-losing enteropathy syndrome, but could also experience imbalance between synthesis and degradation as well as altered intravascular and tissue albumin distribution (43). In horses with acute diarrhea, intestinal mucosal inflammation and ulceration is the most important site and reason for protein loss, predominately albumin (44). It is likely that in our study, non-surviving horses had a more severe GI and systemic compromise leading to hypoalbuminemia and therefore the association between hypoproteinemia with non-survival.
Limitations of the study
This study had several limitations due to the retrospective design. Data collection and sample processing were not standardized within or between institutions, and categorization of clinical signs and treatments was not possible. The highly selected population of horses with acute diarrhea in some of the teaching hospitals included in this study, would tend to bias the study toward sicker patients. The number of cases meeting the inclusion criteria was large in one VTH and small in the other 3 institutions. This could be due in part to delayed referrals in some areas, excluding some relatively acute diarrhea cases from this study. However, the findings regarding predictive factors, mortality proportion, and treatments were similar among institutions. Nonetheless, this study provided a comprehensive analysis of the epidemiology, clinical presentation, diagnostic and treatment approaches, and factors associated with the survival of diarrheic horses admitted to the Canadian teaching hospitals. Similar multicenter studies on investigating differences between private and academic institutions from different latitudes are needed to establish new routes for clinical research.
Acknowledgments
The authors to thank Charlotte Paindaveine and Anna Henderson from University of Montreal and University of Saskatchewan for their contribution to data collection. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Arroyo L, Arnold CE, Barham M, et al. Science-in-brief: Report on the Havemeyer Foundation workshop on acute colitis of the adult horse. Equine Vet J. 2020;52:163–164. doi: 10.1111/evj.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luethy D, Feldman R, Stefanovski D, Aitken MR. Risk factors for laminitis and nonsurvival in acute colitis: Retrospective study of 85 hospitalized horses (2011–2019) J Vet Intern Med. 2021;35:2019–2025. doi: 10.1111/jvim.16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nomura M, Kuroda T, Tamura N, Muranaka M, Niwa H. Mortality, clinical findings, predisposing factors and treatment of Clostridioides difficile colitis in Japanese thoroughbred racehorses. Vet Rec. 2020;187:e14. doi: 10.1136/vr.105605. [DOI] [PubMed] [Google Scholar]
- 4.Ruby R, Magdesian KG, Kass PH. Comparison of clinical, microbiologic, and clinicopathologic findings in horses positive and negative for Clostridium difficile infection. J Am Vet Med Assoc. 2009;234:777–784. doi: 10.2460/javma.234.6.777. [DOI] [PubMed] [Google Scholar]
- 5.Weese JS, Staempfli HR, Prescott JF. A prospective study of the roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in equine diarrhoea. Equine Vet J. 2001;33:403–409. doi: 10.2746/042516401776249534. [DOI] [PubMed] [Google Scholar]
- 6.Cohen ND, Woods AM. Characteristics and risk factors for failure of horses with acute diarrhea to survive: 122 cases (1990–1996) J Am Vet Med Assoc. 1999;214:382–390. [PubMed] [Google Scholar]
- 7.Sage SE, Bedenice D, McKinney CA, et al. Assessment of the impact of age and of blood-derived inflammatory markers in horses with colitis. J Vet Emerg Crit Care (San Antonio) 2021;31:779–787. doi: 10.1111/vec.13099. [DOI] [PubMed] [Google Scholar]
- 8.Teymournejad O, Lin M, Bekebrede H, et al. Isolation and molecular analysis of a novel Neorickettsia species that causes Potomac Horse Fever. MBio. 2020;11:e03429–19. doi: 10.1128/mBio.03429-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gohari IM, Arroyo L, Macinnes JI, Timoney JF, Parreira VR, Prescott JF. Characterization of Clostridium perfringens in the feces of adult horses and foals with acute enterocolitis. Can J Vet Res. 2014;78:1–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Durán MC, Marqués FJ. Detection of Neorickettsia risticii, the agent of Potomac horse fever, in a Gypsy Vanner stallion from Manitoba. Can Vet J. 2016;57:293–295. [PMC free article] [PubMed] [Google Scholar]
- 11.Heller MC, McClure J, Pusterla N, Pusterla JB, Stahel S. Two cases of Neorickettsia (Ehrlichia) risticii infection in horses from Nova Scotia. Can Vet J. 2004;45:421–423. [PMC free article] [PubMed] [Google Scholar]
- 12.Arroyo LG, Moore A, Bedford S, et al. Potomac horse fever in Ontario: Clinical, geographic, and diagnostic aspects. Can Vet J. 2021;62:622–628. [PMC free article] [PubMed] [Google Scholar]
- 13.Arroyo LG, Staempfli H, Weese JS. Molecular analysis of Clostridium difficile isolates recovered from horses with diarrhea. Vet Microbiol. 2007;120:179–183. doi: 10.1016/j.vetmic.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Ravary B, Fecteau G, Higgins R, Paré J, Lavoie JP. (Prevalence of infections caused by Salmonella spp. in cattle and horses at the Veterinary Teaching Hospital of the Faculty of Veterinary Medicine of the University of Montreal) Can Vet J. 1998;39:566–572. [PMC free article] [PubMed] [Google Scholar]
- 15.Schofield F. An investigation into an endemic disease of horses (occurring chiefly in Kent and Essex counties of the Province of Ontario) Rep Ontario Vet Coll. 1924;49:41–49. [Google Scholar]
- 16.Baird JD, Arroyo LG. Historical aspects of Potomac horse fever in Ontario (1924–2010) Can Vet J. 2013;54:565–572. [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro J, Thomson G. Potomac horse fever in eastern Ontario. Can Vet J. 1995;36:448. [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin B, Gough J. Potomac horse fever in southwestern Ontario. Can Vet J. 1996;37:367–368. [PMC free article] [PubMed] [Google Scholar]
- 19.Willette JA, Kopper JJ, Kogan CJ, Seguin MA, Schott HC. Effect of season and geographic location in the United States on detection of potential enteric pathogens or toxin genes in horses ≥6-mo-old. J Vet diagnostic Investig. 2022;34:407–411. doi: 10.1177/10406387211056054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopper JJ, Willette JA, Kogan CJ, Seguin A, Bolin SR, Schott HC., 2nd Detection of pathogens in blood or feces of adult horses with enteric disease and association with outcome of colitis. J Vet Intern Med. 2021;35:2465–2472. doi: 10.1111/jvim.16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoster A, Kunz T, Lauper M, Graubner C, Schmitt S, Weese JS. Prevalence of Clostridium difficile and Clostridium perfringens in Swiss horses with and without gastrointestinal disease and microbiota composition in relation to Clostridium difficile shedding. Vet Microbiol. 2019;239:108433. doi: 10.1016/j.vetmic.2019.108433. [DOI] [PubMed] [Google Scholar]
- 22.Thean S, Elliott B, Riley TV. Clostridium difficile in horses in Australia — A preliminary study. J Med Microbiol. 2011;60:1188–1192. doi: 10.1099/jmm.0.030908-0. [DOI] [PubMed] [Google Scholar]
- 23.Dunkel B, Johns IC. Antimicrobial use in critically ill horses. J Vet Emerg Crit Care (San Antonio) 2015;25:89–100. doi: 10.1111/vec.12275. [DOI] [PubMed] [Google Scholar]
- 24.Weese JS, Toxopeus L, Arroyo L. Clostridium difficile associated diarrhoea in horses within the community: Predictors, clinical presentation and outcome. Equine Vet J. 2006;38:185–188. doi: 10.2746/042516406776563369. [DOI] [PubMed] [Google Scholar]
- 25.Arroyo LG, Sears W, Gomez DE. Plasma transfusions in horses with typhlocolitis/colitis. Can Vet J. 2019;60:193–196. [PMC free article] [PubMed] [Google Scholar]
- 26.Hassel DM, Smith PA, Nieto JE, Beldomenico P, Spier SJ. Di-tri-octahedral smectite for the prevention of post-operative diarrhea in equids with surgical disease of the large intestine: Results of a randomized clinical trial. Vet J. 2009;182:210–214. doi: 10.1016/j.tvjl.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Mattei DN, Kopper JJ, Sanz MG. Equine coronavirus-associated colitis in horses: A retrospective study. J Equine Vet Sci. 2020;87:102906. doi: 10.1016/j.jevs.2019.102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manship AJ, Blikslager AT, Elfenbein JR. Disease features of equine coronavirus and enteric salmonellosis are similar in horses. J Vet Intern Med. 2019;33:912–917. doi: 10.1111/jvim.15386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertin FR, Reising A, Slovis NM, Constable PD, Taylor SD. Clinical and clinicopathological factors associated with survival in 44 horses with equine neorickettsiosis (Potomac horse Fever) J Vet Intern Med. 2013;27:1528–1534. doi: 10.1111/jvim.12209. [DOI] [PubMed] [Google Scholar]
- 30.Mair TS, Smith LJ. Survival and complication rates in 300 horses undergoing surgical treatment of colic. Part 2: Short-term complications. Equine Vet J. 2005;37:303–309. doi: 10.2746/0425164054529364. [DOI] [PubMed] [Google Scholar]
- 31.Dolente BA, Beech J, Lindborg S, Smith G. Evaluation of risk factors for development of catheter-associated jugular thrombophlebitis in horses: 50 cases (1993–1998) J Am Vet Med Assoc. 2005;227:1134–1141. doi: 10.2460/javma.2005.227.1134. [DOI] [PubMed] [Google Scholar]
- 32.Geraghty TE, Love S, Taylor DJ, Heller J, Mellor DJ, Hughes KJ. Assessment of subclinical venous catheter-related diseases in horses and associated risk factors. Vet Rec. 2009;164:227–231. doi: 10.1136/vr.164.8.227. [DOI] [PubMed] [Google Scholar]
- 33.Staempfli HR, Townsend HG, Prescott JF. Prognostic features and clinical presentation of acute idiopathic enterocolitis in horses. Can Vet J. 1991;32:232–237. [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart MC, Hodgson JL, Kim H, Hutchins DR, Hodgson DR. Acute febrile diarrhoea in horses: 86 cases (1986–1991) Aust Vet J. 1995;72:41–44. doi: 10.1111/j.1751-0813.1995.tb15327.x. [DOI] [PubMed] [Google Scholar]
- 35.Berryhill EH, Magdesian KG, Aleman M, Pusterla N. Clinical presentation, diagnostic findings, and outcome of adult horses with equine coronavirus infection at a veterinary teaching hospital: 33 cases (2012–2018) Vet J. 2019;248:95–100. doi: 10.1016/j.tvjl.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kambayashi Y, Bannai H, Tsujimura K, Hirama A, Ohta M, Nemoto M. Outbreak of equine coronavirus infection among riding horses in Tokyo, Japan. Comp Immunol Microbiol Infect Dis. 2021;77:101668. doi: 10.1016/j.cimid.2021.101668. [DOI] [PubMed] [Google Scholar]
- 37.Hassel DM, Hill AE, Rorabeck RA. Association between hyperglycemia and survival in 228 horses with acute gastrointestinal disease. J Vet Intern Med. 2009;23:1261–1265. doi: 10.1111/j.1939-1676.2009.0395.x. [DOI] [PubMed] [Google Scholar]
- 38.Archer DC, Pinchbeck GL, Proudman CJ. Factors associated with survival of epiploic foramen entrapment colic: A multicentre, international study. Equine Vet J Suppl. 2011;39:56–62. doi: 10.1111/j.2042-3306.2011.00409.x. [DOI] [PubMed] [Google Scholar]
- 39.Ecke P, Hodgson DR, Rose RJ. Induced diarrhoea in horses. Part 1: Fluid and electrolyte balance. Vet J. 1998;155:149–159. doi: 10.1016/s1090-0233(98)80010-5. [DOI] [PubMed] [Google Scholar]
- 40.Gomez DE, Arroyo LG, Stämpfli HR, Cruz LE, Oliver OJ. Physicochemical interpretation of acid-base abnormalities in 54 adult horses with acute severe colitis and diarrhea. J Vet Intern Med. 2013;27:548–553. doi: 10.1111/jvim.12071. [DOI] [PubMed] [Google Scholar]
- 41.Carlson GP, Bruss M. Fluid, electrolyte, and acid-base balance. In: Kaneko J, Harvey J, Bruss M, editors. Clinical Biochemistry of Domestic Animals. 6th ed. New York, New York: Elsevier; 2008. pp. 529–559. [Google Scholar]
- 42.Porta F, Takala J, Weikert C, et al. Effects of prolonged endotoxemia on liver, skeletal muscle and kidney mitochondrial function. Crit Care. 2006;10:R118. doi: 10.1186/cc5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durward A, Mayer A, Skellett S, et al. Hypoalbuminaemia in critically ill children: Incidence, prognosis, and influence on the anion gap. Arch Dis Child. 2003;88:419–422. doi: 10.1136/adc.88.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins LG, Tyler DE. Experimentally induced phenylbutazone toxicosis in ponies: Description of the syndrome and its prevention with synthetic prostaglandin E2. Am J Vet Res. 1985;46:1605–1615. [PubMed] [Google Scholar]