Abstract
In this issue of Cell, Hulsmans et al. identify a subset of macrophages residing within the cardiac conduction system, which orchestrates cardiac rhythm. Macrophages directly couple with cardiomyocytes, and their perturbation alters cardiac conduction, suggesting that pharmacological manipulation of resident macrophages might represent a new strategy to combat cardiac arrhythmias.
Normal cardiac rhythm is essential for coordinating the critical pumping function of the heart. The cardiac conduction system, a specialized group of cardiomyocytes, is responsible for initiating and propagating cardiac electrical activity (van Weerd and Christoffels, 2016). Whereas the sinoatrial node (SAN) functions as the predominant pacemaker within the heart, the atrioventricular node (AVN) coordinates sequential contraction of the upper and lower chambers, the atria and ventricles, respectively. Any perturbation to this exquisitely regulated system can result in cardiac arrhythmias and subsequent dysfunction. Specific disruption of AVN conduction can cause varying degrees of atrioventricular (AV) block. In the extreme case of complete heart block, the atria and ventricles have no direct electrical communication, and patients with this condition require pacemaker implantation to avoid fatigue, decreased exercise capacity, or even circulatory collapse (Munshi and Olson, 2014). To date, it has been widely accepted that AV conduction is mediated exclusively by specialized cardiomyocytes within the AVN. However, findings reported in this issue of Cell by Nahrendorf and colleagues directly challenge this view (Hulsmans et al., 2017).
Macrophages have been classically described as professional phagocytes that function to clear the body of infectious agents (e.g., viruses, bacteria, and parasites) and cellular debris (e.g., necrotic tissue). As such, the canonical function of circulating and resident macrophages is to provide innate immune surveillance of individual organs, such as the heart (Figure 1). However, an appreciation for the heterogeneity of cardiac resident macrophages has only recently emerged. Indeed, prior studies have demonstrated that cardiac macrophages are derived from the bone marrow and embryonic yolk sac (Epelman et al., 2014). From a functional standpoint, cardiac macrophages participate in the body’s response to prolonged cardiac ischemia by regulating inflammation and repair during post-myocardial infarction remodeling (Hulsmans et al., 2016). Resident macrophages also play a key role during cardiac regeneration after myocardial injury in neonatal mice (Aurora et al., 2014; Lavine et al., 2014). Taken together, these studies highlight the heterogeneity of macrophage origin and function within the heart.
Figure 1. Macrophage Functions in the Heart.
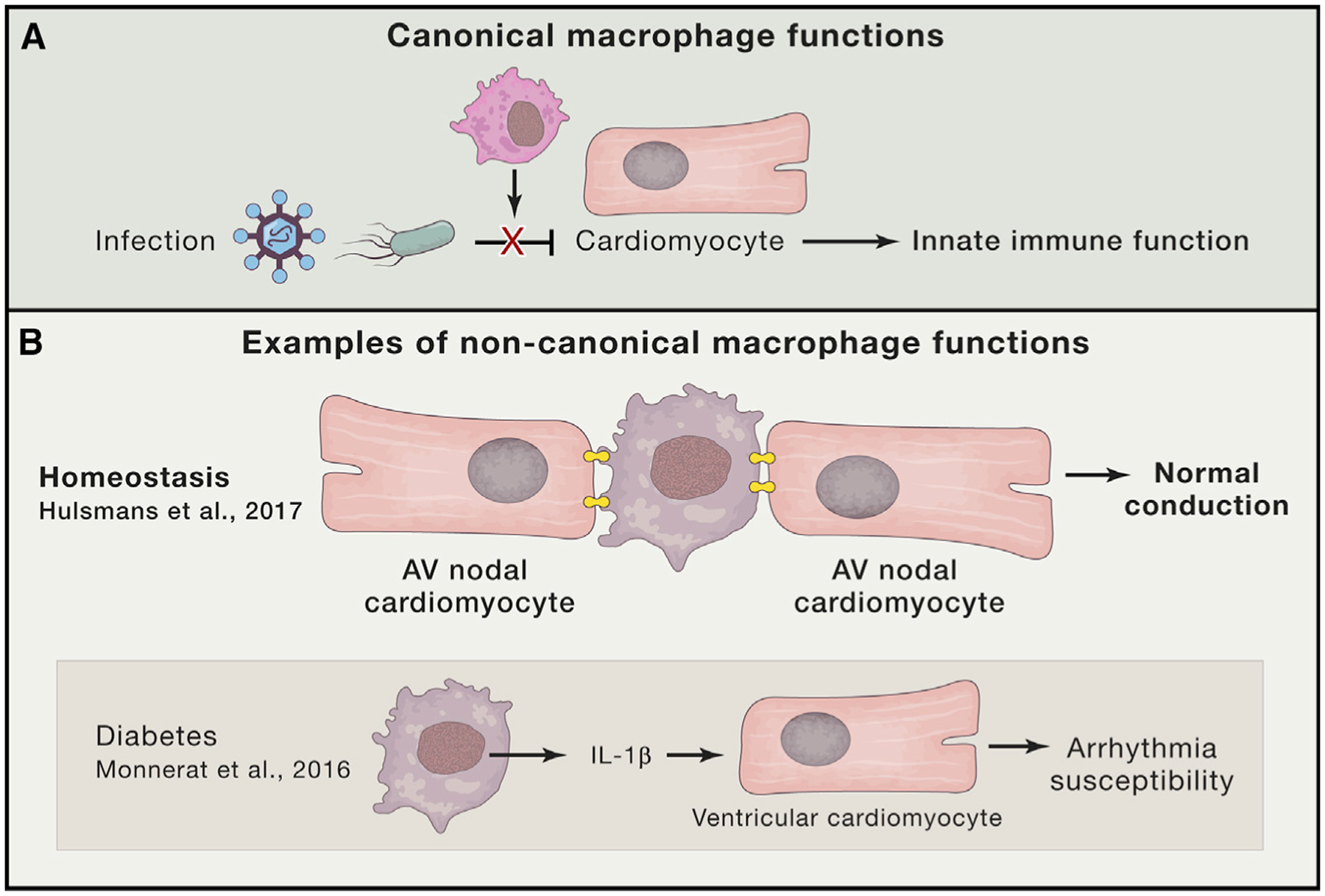
(A) The canonical function of macrophages in the heart includes innate immune surveillance. Resident macrophages (magenta) function to clear infectious agents (e.g. viruses and bacteria) in addition to necrotic cellular debris (not shown).
(B) Non-canonical functions for macrophages in the heart. Macrophages (purple) are shown to electrically couple with surrounding cardiomyocytes in the atrioventricular node (AVN) via connexin 43 gap junctions (shown in yellow) to mediate cardiac conduction. Resident macrophages (purple) have also been found to secrete interleukin-1β.
Building on these studies, Nahrendorf and colleagues sought to define additional functions for resident cardiac macrophages (Hulsmans et al., 2017). Using a macrophage reporter line in conjunction with optical clearing techniques and confocal microscopy, the authors find an abundance of macrophages residing within the distal AVN in mice and humans. Although resident macrophages have been well described in the ventricles, this is the first report of macrophages in the vicinity of the cardiac conduction system. The authors provide strong evidence that the identified cells are indeed resident macrophages that do not arise from the circulation. While anatomic enrichment of resident macrophages is remarkable, it does not by itself establish whether they are functionally important.
Given that macrophages have been shown to express gap junction proteins and that non-myocytes (i.e., fibroblasts) can form electrotonic connections with cardiomyocytes, the authors hypothesized that the newly identified resident macrophages couple directly with AVN cardiomyocytes. Consistent with this notion, the authors find that AVN macrophages specifically express the gap junction protein connexin 43 (Cx43) at contact points between macrophages and cardiomyocytes (Figure 1). Furthermore, direct intracellular recordings demonstrate that macrophages connected to cardiomyocytes are electrically active, and computational modeling accurately predicts that such heterologous coupling facilitates electrical conduction by promoting efficient repolarization. The authors go on to establish that resident macrophages function during AV conduction by using a series of elegant studies in mice involving macrophage-specific optogenetic stimulation and Cx43 deletion. Moreover, Hulsmans et al. genetically deplete macrophages to corroborate the functional connection between resident macrophages and cardiac impulse propagation. Collectively, these experiments define a novel, non-canonical role for resident macrophages in cardiac electrical activation (Figure 1).
What does this mean in terms of development and homeostasis of the cardiac conduction system and normal cardiac rhythm? Interestingly, a previous study demonstrated that resident macrophages in the brain actively prune synaptic connections during central nervous system development (Paolicelli et al., 2011). This raises the intriguing possibility that the newly identified cardiac macrophages might somehow affect morphogenesis of the cardiac conduction system and/or shape how it responds to injurious insults, such as hypoxia and infection. The authors also clearly note that macrophages take up residence in other components of the cardiac conduction system, such as the SAN. Given this observation, do macrophages similarly influence pacemaker development and/or function? It is also noteworthy that both the SAN and AVN are subject to age-dependent fibrotic replacement. Given that the distribution and function of macrophages can vary with age, it will be interesting to test whether such age-dependent changes influence the fibrosis commonly observed in the SAN and AVN of elderly individuals.
Perhaps the most exciting aspect of the current study is the potential for targeted treatment of resident macrophages in a variety of disease states. Given the critical immune function of macrophages, it will be important to evaluate the extent to which acute and chronic inflammatory conditions influence cardiac conduction. Interestingly, a separate study has described a novel function for alternatively activated adipose tissue macrophages in adaptive thermogenesis via catecholamine secretion (Nguyen et al., 2011). In this regard, recent work has demonstrated that diabetic inflammation causes resident macrophages to secrete interleukin-1β, which functions in a paracrine fashion to destabilize cardiomyocyte electrical activity in a manner that is known to promote ventricular arrhythmias (Monnerat et al., 2016) (Figure 1). Given the findings of this study, could there be other, as-yet-unidentified paracrine mechanisms by which resident macrophages influence cardiac rhythm in a non-canonical fashion? From a broader perspective, what role, if any, do non-canonical resident macrophage functions play during post-myocardial infarction remodeling and neonatal regeneration? Although many questions remain to be answered, what has become clear is that macrophages are likely to play a variety of critical functions within the heart to help maintain homeostasis.
REFERENCES
- Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, and Olson EN (2014). J. Clin. Invest 124, 1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. (2014). Immunity 40, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmans M, Sam F, and Nahrendorf M (2016).J. Mol. Cell. Cardiol 93, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seeman G, Courties G, et al. (2017). Cell 169, this issue, 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, and Mann DL (2014). Proc. Natl. Acad. Sci. USA 111, 16029–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnerat G, Alarcón ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, Casis O, Malan D, Travassos LH, Sepúlveda M, et al. (2016). Nat. Commun 7, 13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi NV, and Olson EN (2014). Science 345, 268–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, and Chawla A (2011). Nature 480, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. (2011). Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- van Weerd JH, and Christoffels VM (2016). Development 143, 197–210. [DOI] [PubMed] [Google Scholar]


