Abstract
BACKGROUND:
Brain injury progression after severe traumatic brain injury (TBI) is associated with worsening cerebral inflammation but it is unknown how a concomitant bone fracture (BF) affects this progression. Enoxaparin (ENX) decreases penumbral leukocyte mobilization after TBI and improves neurologic recovery. We hypothesized that a concomitant BF worsens learning/memory recovery weeks after TBI and that ENX improves this recovery.
METHODS:
CD1 male mice underwent controlled cortical impact or sham craniotomy with or without tibial fracture, receiving either daily ENX (0.8 mg/kg) or saline for 14 days after injury. Randomization defined four groups (Sham, TBI only, TBI + Fx, TBI + Fx + ENX, n = 5/each). Body weight loss and neurologic recovery (Garcia Neurologic Test, max score = 18) were assessed each day. Mouse learning (swimming time [s] and total distance [m] to reach the submerged platform Days 14 to 17 after TBI) and memory (swimming time [s] in platform quadrant after platform removed [probe]) was assessed by the Morris water maze. Ly-6G (cerebral neutrophil sequestration) and glial fibrillary acidic protein were evaluated by immunohistochemistry in brain tissue post mortem. Analysis of variance with Tukey’s post hoc test determined significance (p < 0.05).
RESULTS:
A concurrent BF worsened Garcia Neurologic Test scores post-TBI Days 2 to 4 (p < 0.01) as compared with TBI only, and ENX reversed this worsening on Day 4 (p < 0.01). Learning was significantly slower (greater swimming time and distance) in TBI + Fx versus TBI only on Day 17 (p < 0.01). This was despite similar swimming velocities in both groups, indicating intact extremity motor function. Memory was similar in isolated TBI and Sham which was significantly better than in TBI + Fx animals (p < 0.05). Glial fibrillary acidic protein–positive cells in penumbral cortex were most prevalent in TBI + Fx animals, significantly greater than in Sham (p < 0.05).
CONCLUSION:
A long BF accompanying TBI worsens early neurologic recovery and subsequent learning/memory. Enoxaparin may partially counter this and improve neurologic recovery.
Keywords: Traumatic brain injury, polytrauma, tibial fracture, enoxaparin, Morris Water Maze
Traumatic brain injury (TBI) is the principal ailment affecting the health of young adults, with over 1.7 million cases occurring annually in the United States.1 It results in more than 50,000 deaths, and 80,000 to 90,000 cases of long-term disability per year.2 Traumatic brain injury frequently occurs in the setting of polytrauma and remains a major obstacle in improving trauma outcomes: mortality is directly attributable to TBI in up to one third of patients who die after multiple injury.3,4 The most common concomitant injury that accompanies TBI is bone fractures (BFs).5 Although numerous human studies have demonstrated that TBI accelerates concomitant fracture healing in humans,6 few studies have evaluated the converse—the effect of BF on TBI outcomes.7–10
In moving toward therapies for TBI, there has been significant interest in the development of an agent that is effective in reducing such trauma-induced neuroinflammation and tissue edema. Enoxaparin (ENX) (a low-molecular-weight heparin) is often administered to injured patients to prevent deep vein thrombosis and venous thromboembolism (VTE). While this low molecular weight heparin (LMWH) may increase intracranial hemorrhage risk, it is also effective in reducing leukocyte-mediated tissue inflammation and swelling11,12 as well as improving cognitive recovery after isolated TBI.13–15
On the basis of the above data, we undertook a study on a dual injury TBI rodent model accompanied by a long BF and hypothesized that when TBI is accompanied by a concomitant BF, cognitive recovery is impaired. We further postulated that ENX can reverse this impaired recovery.
MATERIALS AND METHODS
Experimental Design and Study Groups
All investigational protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Adult male CD1 mice (25–30 g) (Charles River Laboratories, Wilmington, MA) were housed for 5 days to 7 days in standard facilities before experiments, with access to water and food ad libitum. Animals underwent sham craniotomy or TBI (controlled cortical impact [CCI]) with or without a concomitant tibial fracture (Fx), followed by daily subcutaneous injections of ENX (0.8 mg/kg, Winthrop, Sanofiaventis, NJ) or an equal volume of 0.9% normal saline (vehicle; 0.8 mL/kg, Baxter Deerfield, IL) for 14 days. Thereafter, Morris water maze (MWM) trials were conducted on Days 14 to 17 after injury. The 0.8-mg/kg dose of ENX was a reduced dose from that used in previously published studies13,15,16 to approach standard prophylactic human doses of 40 mg/d.
Twenty animals were randomized into four groups: sham craniotomy and vehicle (0.9% normal saline) without CCI or BF (Sham, n = 5), CCI and vehicle (TBI only, n = 5), CCI with BF and vehicle (TBI + Fx, n = 5), and CCI with BF and ENX (TBI + Fx + ENX, n = 5) (Fig. 1A).
Figure 1.
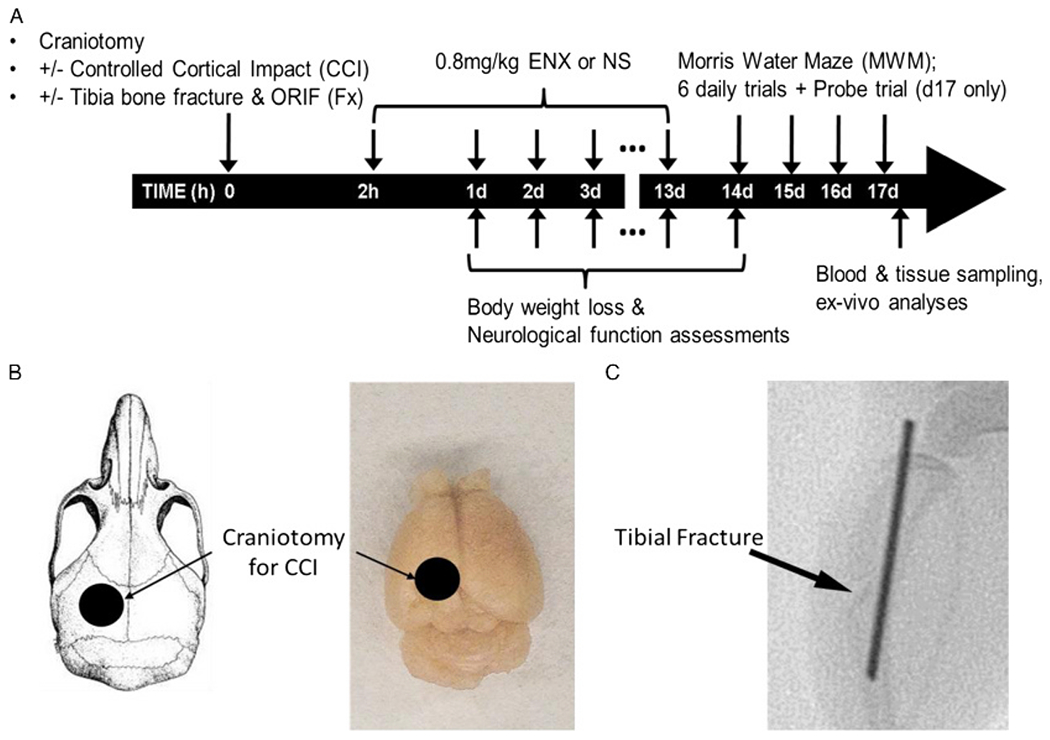
Experimental design and procedures. (A) Timeline of experimental protocol. (B) Schematic representation of localization of craniotomy. (C) A fluoroscopic image of tibial fracture with open reduction and internal fixation (ORIF) 2 days after the injury. The arrow indicates the tibial fracture site. The fracture was isolated to tibia (sparing the fibula), and an intermedullary pin was used for fixation.
Severe Traumatic Brain Injury—CCI
On Day 1, mice were anesthetized with intraperitoneal ketamine, xylazine, and acepromazine (KXA; 100, 10, 1 mg/kg, respectively) and placed prone in a stereotactic device. A left-sided 4-mm craniotomy centered between the sagittal suture, bregma, and lambda sutures was created with a trephine (Fig. 1B). The left parietotemporal cortex was exposed and was directly impacted using a CCI device (AMS 201; AmScien Instruments, Richmond, VA) using standard injury technique (3-mm diameter impactor tip, 6 m/sec velocity, 1.0-mm cortical deformation). This instrument and these settings are widely used in investigations of experimental severe TBI.17,18
Concomitant Open Tibial Diaphyseal Fracture With Intramedullary Fixation
A right-sided tibial Fx was added in certain animals to mimic a typical multi-injured TBI patient. The open fracture was immediately repaired with intramedullary fixation under aseptic conditions, as previously described.19 Briefly, within the same anesthetic setting used for CCI, a 2-mm skin incision was made on the proximal leg, and a small hole was drilled on the tibial plateau bone marrow cavity, using a 26-G needle. The soft tissues and vessels were separated from the periosteum, and a transverse osteotomy (fracture) was then performed with a surgical scissor without fracturing the adjacent fibula. The inner cylinder of a 22G spinal needle was pushed through the osteotomy to 7 mm and used as an intramedullary rod fixating the fracture internally. The protruding pin segment was severed using a wire cutter and discarded (Fig. 1C). Animals randomized to no fracture did not undergo a skin incision nor a fracture, but all animals received buprenorphine (0.1 mg/kg) as a postoperative analgesic every 6 hours for 72 hours after surgery.
Body Weight Loss and Hemoglobin Levels
Animal body weights were obtained before injury and daily for 14 days after craniotomy. Extent of weight loss was calculated as weight loss ratios [(W0h − W24h)/W0h × 100%].
All animals underwent cardiac puncture at the completion of MWM trials on Day 17, and 10 μL of whole blood were aspirated to measure hemoglobin concentration as a marker of surgical bleeding (i-STAT; Abbott Laboratories, Abbott Park, IL) in accordance to the manufacturer’s instructions.
Neurologic Recovery—The Garcia Neurologic Test
Neurologic recovery of animal daily living activities was assessed for 14 days after TBI using the validated modified Garcia neurologic test (GNT), which scores motor, sensory, reflex, and balance ability to a maximum sum of 18 points.
MWM Testing—Learning, Memory, and Swimming Motor Function
Based on previous analyses,20,21 on Day 14 after craniectomy, all animals were exposed to daily spatial learning and memory exercises using a MWM. A circular plastic tub (100 cm diameter, 50 cm high) served as a MWM pool (Fig. 5A, view from above) filled with 21°C water and fitted with a 23.5-cm clear cylindrical plexiglass platform placed 1.0 cm below water surface in the northwest (Target) quadrant (swim rest platform). Visual cues were displayed on the surrounding walls to help rodents with localization at four starting locations. All MWM exercises were conducted and scored by a single operator unaware of animal treatment group. This same operator scored all animals for all groups, for the entire study. Spatial learning acquisition was assessed in each mouse on Days 14 through 17, submitting each animal to six trials per day separated by 10-minute rest periods. With six daily trials to find the hidden submerged platform, animals with intact learning and memory are expected to find the platform faster each subsequent day. For each trial, the mouse was placed in the water facing outward in one of four starting locations (northeast, southwest, east, south). Animals were allowed a maximum of 60 seconds to independently find and mount the submerged platform, after which, the animal was lifted onto the platform by the operator and allowed to remain there for 15 seconds. Mice were then warmed under a heating lamp until the next trial.
Figure 5.
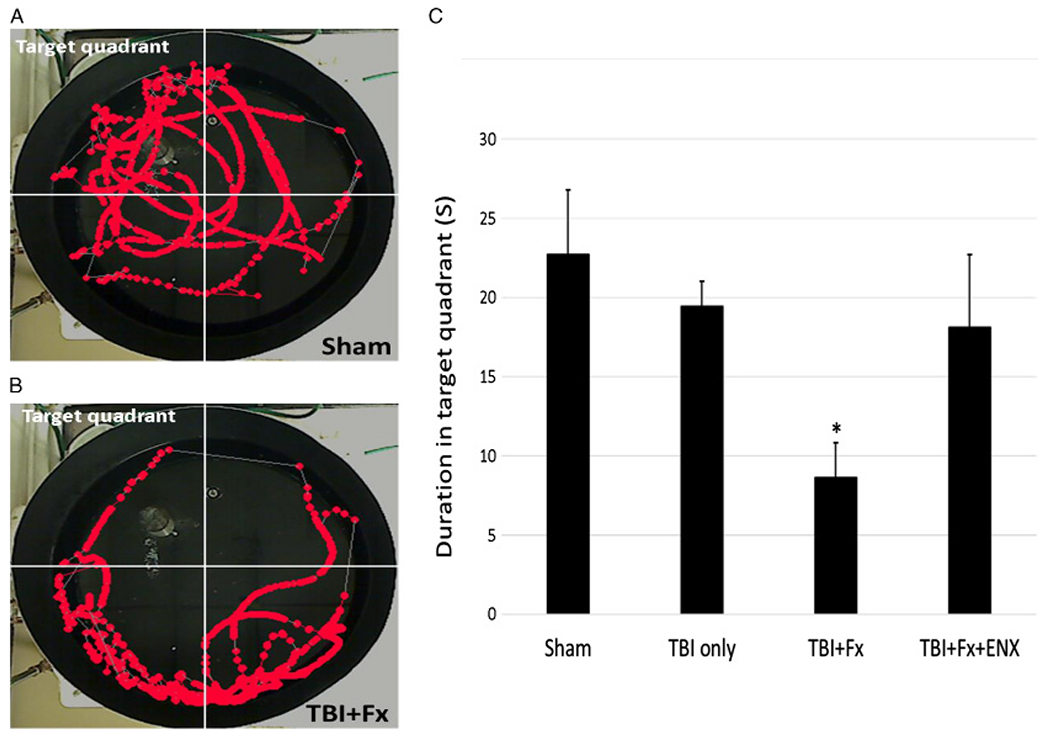
Memory ability (probe trial). (A) Representative image sequence of the geographical position of a sham animal in the 60-second observation period. (B) Representative image sequence of the geographical position of a TBI + Fx animal in the 60-second observation period. (C) Only TBI + Fx animals spent significantly less time in the target quadrant than sham. *p < 0.05 vs. sham.
Escape latency (time to reach the platform), total covered swimming distance, and swimming velocity (average speed in a maximum of 60 seconds – motor function) were recorded. On Day 17, after the final (sixth) trial, a probe trial was performed to assess spatial memory by removing the submerged platform and allowing mice to swim freely in the pool for 60 seconds. Spatial memory was gauged as the proportion of time animals spent in the target quadrant. All animal exercises were video recorded and analyzed using a commercial video-tracking system (Ethovision, Noldus, Leesburg, VA) mounted above the pool.
Cerebral Immunohistochemistry
Excised brains were postfixed in 10% neutral-buffered formalin (Sigma Aldrich) after transcardial perfusion with phosphate-buffered solution and 10% neutral-buffered formalin. Two-millimeter-thick coronal blocks of dissected brains were processed to paraffin using standard techniques. Staining procedures were performed on 8-μm-thick sections. After deparaffinization and rehydration, tissue sections were immersed in aqueous hydrogen peroxide (15 minutes) to quench endogenous peroxidase activity. Sections underwent immersion in preheated Tris EDTA buffer for antigen retrieval in a microwave pressure cooker. Subsequent blocking was achieved using 1% species-specific normal serum (Vector Labs, Burlingame, CA) in Optimax buffer (BioGenex, San Ramon, CA) for 30 minutes. The Ly-6G rat monoclonal primary antibody (1: 40K; BD Biosciences, San Jose, CA) or a monoclonal antibody specific for the protein glial fibrillary acidic protein (GFAP) (1: 5K; Abcam, Cambridge, MA) were applied overnight at four degree Celsius. Slides were subsequently rinsed and incubated with the appropriate secondary biotinylated antibody for 30 minutes at room temperature (Vector Labs). After further rinsing, the avidin biotin complex (Vectastain Universal Elite kit; Vector Labs) was applied for 30 minutes. Finally, visualization was achieved using 3,3′-diaminobenzidine (DAB) as per manufacturer’s instructions (Vector Labs). Counterstaining with haematoxylin was also performed.
Slides were visualized at 20× magnification and digitally scanned in Image Scope software (Leica Biosystems, Wetzlar, Germany). The immunopositive Ly-6G cells were considered neutrophils and were counted in whole brain sections. Glial fibrillary acidic protein–positive cells with a visible nucleus were counted by a blinded observer unaware of treatment group assignments in two penumbral sectors, the primary somatosensory (SSp) cortex and the hippocampal CA1 area, under high magnification (×400). All analyses were performed by an observer blind to the treatment group.
Statistical Analyses
The primary study endpoint was MWM performance on Days 14 through 17 after injury. A sample size of five animals per group was used as in a previous study (Nagata et al, Journal of Trauma 2018, accepted for publication) using the same MWM exercises demonstrated that four animals per group were sufficient in this model to elicit significant differences in both swimming velocity and probe trial latency. For all outcomes measured, six post hoc pairwise comparisons were conducted using analysis of variance with Tukey’s correction to determine significance between group means (corrected α = 0.008). Statistical analyses were performed using SPSS (version19, SPSS, Chicago, IL). All data are presented as means ± SEM. p Value less than 0.05 was considered statistically significant.
RESULTS
Body Weight Loss Ratio
On Days 2 to 5 after injury, TBI + Fx animals displayed significantly greater weight loss than Sham (p < 0.05 for Days 2–5) (Fig. 2A). TBI + Fx + ENX demonstrated higher weight loss than Sham on Day 2 (p < 0.01) and Day 4 (p < 0.05). After Day 6, there were no intergroup differences.
Figure 2.
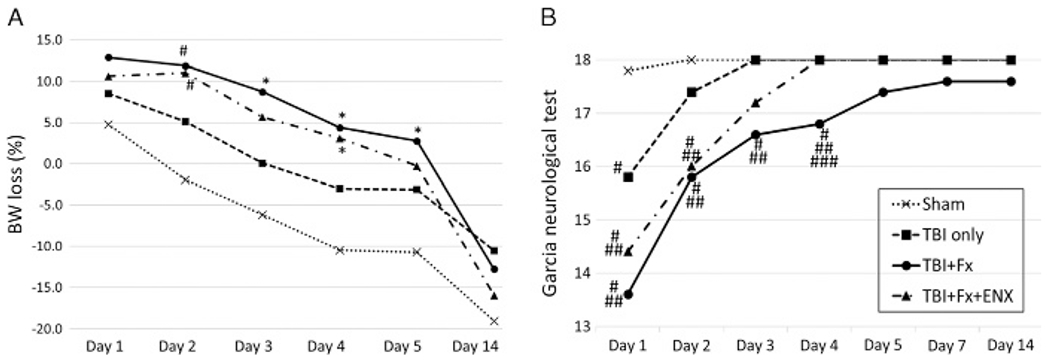
Body weight loss and neurological recovery. (A) TBI + Fx showed significant higher weight loss ratio than Sham on Days 2, 3, 4, and 5. TBI+ Fx + ENX demonstrated higher weight loss ratio significantly than Sham on Days 2 and 4. From Day 6 to Day 14, there were no significant differences among all groups. B, Neurological recovery evaluated with the GNT. Sham animals demonstrated perfect scores 48 hours after craniotomy. TBI + Fx demonstrated significantly lower GNT scores as compared with Sham and TBI only on Days 1, 2, 3, and 4. On Day 5 and later, there were no significant differences among all groups. *p < 0.05, #p < 0.01 vs. sham, ##p < 0.01 vs. TBI only, ###p < 0.01 vs. TBI + Fx + ENX.
Neurologic Recovery
On post injury Days 1 to 4, brain injured animals scored significantly worse on the GNT if CCI was accompanied by a BF (p < 0.01) (Fig. 2B). Enoxaparin administration improved GNT scores over untreated TBI + Fx only on Day 4 (p = 0.003). After Day 5, there were no intergroup differences.
Blood Hemoglobin Levels
Day 17 hemoglobin levels were similar between groups (Sham, 11.52 ± 0.5; TBI only, 11.8 ± 0.4; TBI + Fx, 11 ± 0.5; TBI + Fx + ENX, 11.1 ± 0.4 g/dL; p > 0.05 for all comparisons).
Morris Water Maze
Escape Latency and Distance Travelled (Spatial Learning)
Escape latency (swimming time needed for the animal to reach the platform) was greatest in TBI + Fx animals on Days 15 to 17 (Fig. 3A).
Figure 3.
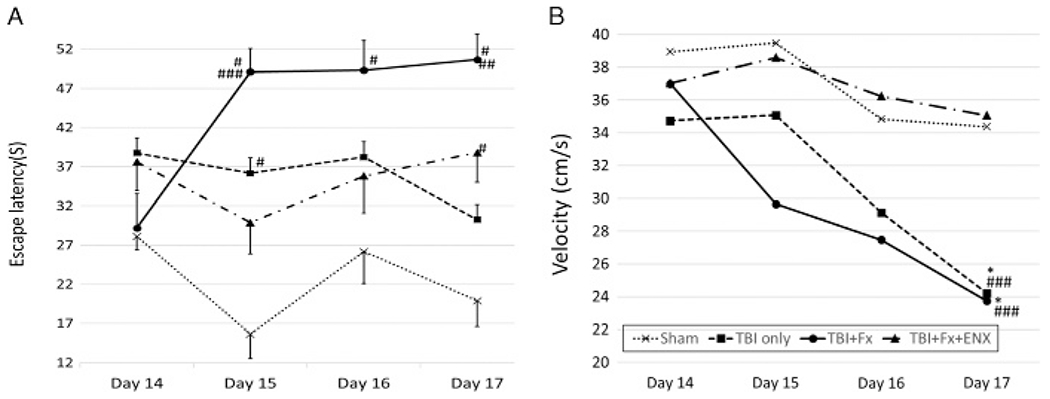
Spatial learning ability (escape latency) and motor function. (A) TBI + Fx demonstrated greater escape time to reach platform than sham on Days 15, 16, and 17. Also, TBI + Fx showed significant worsening on Day 17 as compared to isolated TBI, despite similar swimming velocities. ENX treatment corrected the latency time on Day 15. (B) Both TBI only and TBI+ Fx showed significantly slower swimming speed than Sham and ENX treated group on Day 17. *p < 0.05, #p < 0.01 vs. sham, ##p < 0.01 vs. TBI only, ###p < 0.01 vs. TBI + Fx + ENX.
In particular, on the final day, TBI + Fx(50.7±3.3 seconds) animals failed to quickly locate the platform, resulting in an escape latency that was significantly longer than in isolated TBI only (30.3 ± 3.8, p = 0.001) animals. ENX significantly improved TBI + Fx (49.1 ± 3.1 seconds) latency time significantly on Day 15 (TBI + Fx + ENX: 29.9 ± 4.0 seconds, p = 0.004). This observation did not reflect differences in swimming ability as measured by swim velocity (see below, Fig. 3B).
The swimming path taken to reach the platform was also shortest in Sham animals throughout—almost identical to that of TBI + Fx + ENX–treated animals (Fig. 4A). Controlled cortical impact animals with a BF (T) (TBI + Fx) fared worse in that they required a longer swimming distance to reach the submerged platform on Day 15 (7.3 ± 0.5 m) and Day 17 (6.2 ± 0.5 m), significantly greater than that without a concomitant Fx (d15: TBI only 5.0 ± 0.5 m, p = 0.03; d17: 3.7 ± 0.4 m, p = 0.009). Again, this was in the setting of identical swimming velocities, suggesting no impairment of the healing limb.
Figure 4.
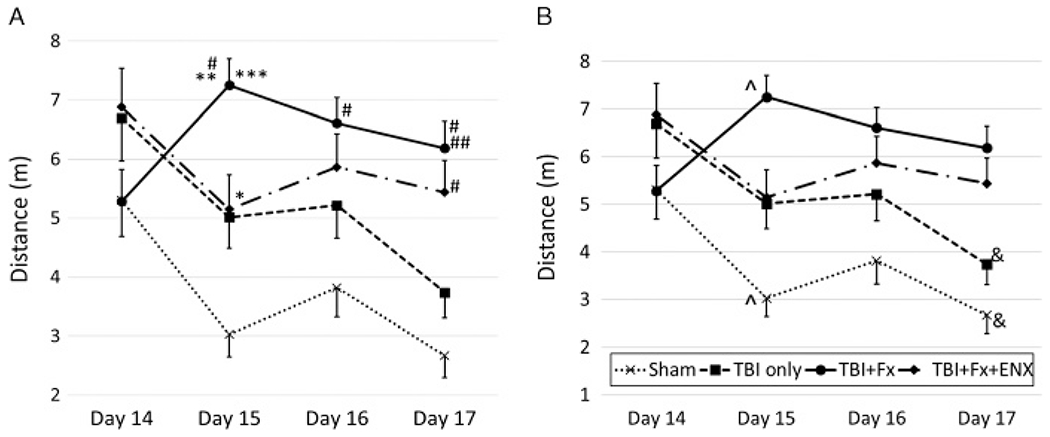
Spatial learning ability (traveled distance). (A) Comparison among each of the four groups. TBI + Fx demonstrated greater distance than sham on Days 15, 16, and 17. Also, TBI + Fx showed significant worsening compared to isolated TBI on Days 15 and 17. EXT treatment corrected the length on Day 15. *p < 0.05, #p < 0.01 vs. sham, **p < 0.05, ##p < 0.01 vs. TBI only, ***p < 0.05 vs. CCI + BF + ENX. (B) Comparison among each of the 4 days. Sham and isolated TBI demonstrated significant improvement of the distance, but TBI + Fx and ENX groups were similar throughout 4 day’s trajectory. ^p < 0.05, &p < 0.01 vs. Day 14.
Learning ability is particularly manifested in Figure 4B where, in each group, Day 17 distances are compared with those starting Day 14. Sham (2.7 ± 0.4 m) and TBI-only (3.7 ± 0.4 m) animals Day 17 distances were almost half of the distances observed on Day 14 (5.3 ± 0.6, p = 0.001; 6.7 ± 0.7, p = 0.002, respectively). On the other hand, TBI + Fx animals demonstrated poor to no spatial learning ability and remained static in the travel distance needed to reach the platform throughout the exercise days.
Swimming Velocity (Motor Function)
As a marker of motor function, swim velocity would be reduced if the repaired fractured limb was unable to function normally. On Day 17, the mean swimming velocity of TBI animals to reach the submerged platform was similar with or without Fx (TBI only: 24.2 ± 1.8 cm/s; TBI + Fx: 23.7 ± 1.3 cm/s, p > 0.05) (Fig. 3B). Both of these groups demonstrated a significantly slower swim velocity than Sham as well as that of dual injury animals treated with ENX (TBI+ Fx + ENX, 35.3 ± 10.1 cm/s). Importantly, TBI+ Fx + ENX animals swam as rapidly as their uninjured counterparts (Sham, 33.6 ± 3.1 cm/s; p > 0.05).
Probe trial (Memory Ability)
On Day 17, the amount of time the animal spent swimming in the target quadrant (where the platform had been previously located) was lowest in TBI + Fx animals (8.6 ± 2.2 seconds) and was significantly lower than that in uninjured Sham counterparts (22.7 ± 4.1 seconds, p = 0.047, Fig. 5). On the other hand, TBI-only animals (19.4 ± 1.6 seconds) demonstrated similar memory ability as Sham counterparts (p > 0.05)
Cerebral Immunohistochemistry—Neutrophil and Astrocyte Quantification
In coronal whole brain sections localized at the epicenter of the brain contusion, Ly-6G–positive cells (polymorphonuclear neutrophils) were counted independently. While no significant differences were found in the number of polymorphonuclear neutrophils (PMNs) counted in any of the groups, trends indicated the highest numbers in the TBI + Fx group. (Sham, 1.2 ± 0.7 cells; TBI only, 50 ± 24.3; TBI + Fx, 84.8 ± 53.2; TBI + Fx + ENX, 6.8 ± 3.1; p > 0.05 for all comparisons). Enoxaparin treatment showed a tendency of preventing PMN mobilization; however, this was not significant (p = 0.728 vs. TBI only, p = 0.275 vs. TBI + Fx).
Evaluation of discrete injury zones in the penumbrum involving the SSp cortex and the CA1 hippocampus (Fig. 6A + B) demonstrated that in both regions, the TBI + Fx group (SSp, 203 ± 39.5 cells/mm2; CA1, 248 ± 35.9 cells/mm2) localized a significantly greater number of GFAP-positive astrocytes than in Sham (SSp, 29 ± 8.3 cells/mm2; p = 0.001; CA1, 124 ± 29.2 cells/mm2; p = 0.035) (Fig. 6C). No statistically significant differences were observed between TBI only and TBI + Fx when analyzing other areas of the penumbral cortex. The TBI-only animals showed similar GFAP-positive cells to Sham, particularly in the hippocampal CA1 region.
Figure 6.
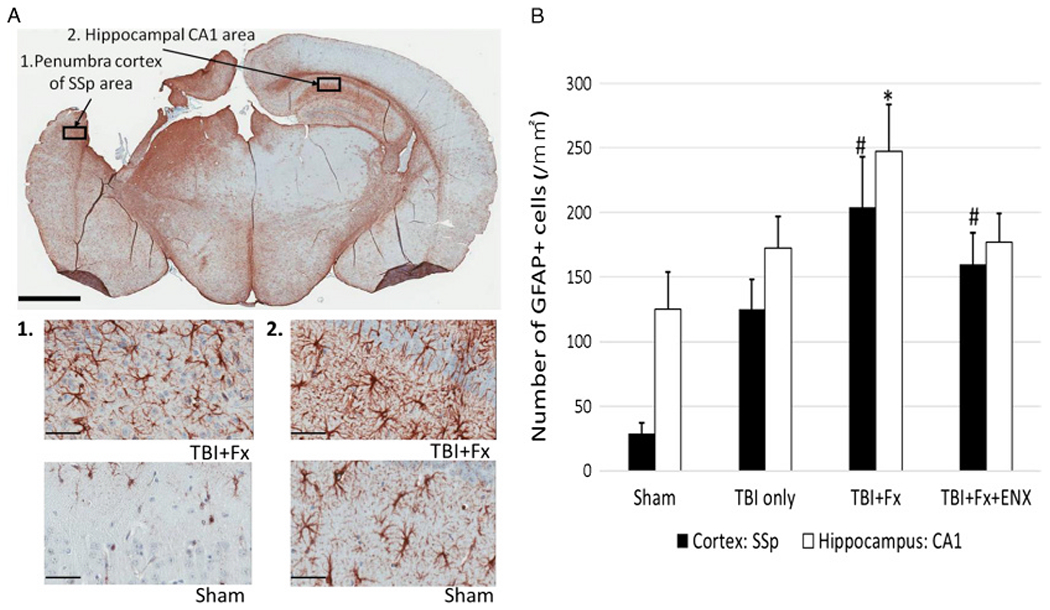
Immunohistochemical analysis for GFAP on 17 days after TBI. (A) Representative image (20× magnification; scale bar = 2 mm) demonstrating GFAP immunoreactive astrocytes. 1, Representative image (400× magnification; scale bar = 50 μm) of GFAP-positive cells in penumbra cortex of the SSp area. 2, Representative image (400× magnification; scale bar = 50 μm) of GFAP-positive cells in hippocampal CA1 area. (B) TBI + Fx demonstrated greater GFAP-positive astrocytes than Sham in cortex and hippocampus. *p < 0.05, #p < 0.05 vs. Sham.
DISCUSSION
In the current study, we sought to determine whether the early worsening in leukocyte-mediated cerebral inflammation and swelling encountered when TBI is accompanied by a long BF14 impairs subsequent neurologic recovery weeks later. Specifically, we found that the addition of a tibial fracture and immediate repair to rodent TBI impaired spatial learning ability and memory, but was unassociated with defective repaired limb motor function.
In previous studies, we highlighted how BBB disruption after TBI, and cerebral edema, may play a pivotal role in delayed neurologic recovery and that the developing cerebral edema may be modulated at 24 hours and 48 hours after TBI.13,14 Cerebral edema modulation depends on mitigating neutrophil sequestration in injured and penumbral cerebral tissue. After TBI, trafficked and sequestered neutrophils may directly impact loss of BBB integrity and exacerbate cerebral edema.22–24
Heparinoids, such as ENX, may reduce tissue inflammation after injury by blunting the activation of circulating leukocytes sequestered in the area of injury.14,15 In particular, ENX administration after TBI was shown to slow secondary brain injury progression—an effect that was linked to blocking microvascular leukocyte-endothelial interactions and resulting in improved BBB integrity.16,25 Our findings in this study support this previous work—we found that the addition of ENX to TBI + Fx animals markedly improved neurologic outcomes, body weight loss changes in early phase, and motor functional ability. While improved brain tissue healing in ENX animals may have accounted for their improved MWM scores, an alternative explanation could be analgesic effects by ENX, reducing limb pain and improving swimming ability.
Various other factors involving BF metabolism and acute healing have implicated neutrophils in the exacerbation of cerebral edema promoting secondary brain injury.26 For example, the addition of a BF to TBI resulted in greater brain edema and Evans blue dye extravasation 24 hours after injury.7 Our group also recently showed how brain edema was acutely increased 2 days after injury if a BF was concurrently present and correlated this with enhanced penumbral in vivo microvascular macromolecular leakage and leukocyte-endothelial interactions in the penumbral neurovasculature surrounding the brain injury.15 The current data also showed how TBI combined with a BF worsened body weight loss early on as compared with TBI alone.
Furthermore, when looking at early recovery of daily activities (GNT), TBI accompanied by a BF significantly worsened performance but was nearly completely reversed by ENX. Regardless, no significant intergroup differences were noted with regard to cerebral neutrophil sequestration 17 days after injury. This is in contrast to our previous results that found a greater PMN sequestration in TBI only than Sham animals. These seemingly conflicting results are potentially explained by noting the disparate timeframes between the two studies: the much earlier timeframe of 32 hours27 and 96 hours28 after CCI was used in the prior studies, in contrast to the more than 2 weeks postinjury timeframe in the current one. One possible reason for lack of difference in histological penumbral PMNs, otherwise seen in vivo at 48 hours in a previous study,15 was because of the 2-week time difference between studies where tissue clearance of these PMNs may have occurred. This different timeframe also highlights how the GNT results in both studies were only affected early on and perhaps suggest that GNT scoring in TBI may not be as sensitive 17 days after injury.
Water maze assessments presented in the current study are likely the most important results that serve as the clinical correlate of the earlier microscopic findings. Water mazes are commonly used as a learning evaluation when testing for congenital anomalies in behavioral and reproductive research.20,29 In this setting, avoidance of drowning is the animal’s primary objective, and learning ability is quantified by requiring progressively shorter swimming time and travel distance needed to reach a safety refuge (platform). Learning and memory abilities evaluated by the MWM are thought to reflect long-term potentiation to which N-methyl-d-aspartate receptor function is related, and are regarded as key outcomes in hippocampal circuit integrity.21,30 In our study, preservation of spatial positional learning acquisition and memory permanence appears preserved by ENX despite CCI and Fx, suggesting that ENX mitigates against impediments to new learning and recovery.
Traumatic brain injury often results in loss of memory as well as learning deficits in the short and long term.31,32 We elected to evaluate hippocampal and neocortical cerebral regions in the penumbrum to evaluate for astrocytosis. Hippocampal malfunction after mild TBI is recognized to be a particularly sensitive marker of this failure in both of clinical33 and animal34 cases. Severe TBI may also causes bilateral morphological changes in the hippocampus and ipsilateral neocortical cavitation, which has been directly correlated to impairment in MWM tasks.35 It is also widely understood that experimental TBI produces spatial learning acquisition deficits in MWM testing.36,37
Learning ability in MWM exercises can be most generally evaluated by the time taken to reach the platform (escape latency).38 However, the sum travelled distance required to reach the platform is also widely related to learning and has the advantage of not being affected by the swimming velocity of the animal.38,39 Here we were able to demonstrate superior learning in isolated TBI animals over that seen in counterparts with a concurrent BF—for both parameters. Importantly, using a model of fracture and immediate repair still triggered the decrement in learning associated with concomitant Fx despite rapid stabilization. Furthermore, the repair was sufficiently robust as to not impact swim velocity and may be discounted as a factor that could skew learning test performance results. This finding is consistent with reports of near complete limb motor recovery 5 weeks after injury in other models using this type of limb fracture.7
Reference memory, on the other hand, is generally evaluated by measuring how the animal searches for the absent platform (the probe trial) after the learning trials have been completed.38,40 Certainly, dysfunctional memory is closely interrelated to learning ability and demonstration of memory integration into performance-related tasks. Thus, impaired learning ability may also lead to poor performance in the probe trial because animals unable to correctly learn the position of the platform would then be unable to remember this position. In our experiments, TBI animals with a concomitant BF fared worst in the probe trial, indicating memory acquisition or integration failure as the underpinnings of failed cognitive recovery.
Pathological studies identify that morphologic changes in the cerebral cortex and hippocampus correlate closely with the results of MWM behavioral tests and that density of astrocyte expression in these territories may be one of the representative indicators.41 Astrocytes close to sites of brain injury upregulate vimentin and nestin production as well as cell division activities. There are also several other biochemical changes that occur locally, including the production of trophic factors, cytokines, proteases, and protease inhibitors.42
Glial fibrillary acidic protein is an established marker of activated astrocytes; greater numbers of GFAP positive cells are encountered in injured brain tissue. Greater GFAP activity has also been demonstrated in animals with progression of brain injury, worsening neurologic severity scores,43 and poor MWM outcomes.41 In clinical studies as well, TBI patients who died within 24 hours of injury demonstrated significantly higher blood GFAP concentrations at 6 hours, 12 hours, and 24 hours after injury.44 Our data similarly demonstrated increased GFAP immunoreactive cells in the penumbral cortex and hippocampus after TBI but was notably increased when TBI was accompanied by a BF. These findings directly paralleled the poor behavioral outcomes evaluated by the MWM in this model.
Interestingly, converse experiments evaluating bone remodeling and repair in the setting of a concomitant brain injury have also demonstrated synergistic effects on bone healing. Indeed, adding TBI to a rodent model of long BFs resulted in accelerated mineral density, gap bridging rate and greater bone callus and torsional strength two weeks after injury.45–47 Thus, interactions between brain and bone injuries appear to alter the local milieu that governs healing of each injury.
Our study is small and therefore has important limitations. First, it is in rodents which may possess different TBI physiology compared to humans. Second, while we only evaluated a subacute postinjury timeframe of 2 weeks, this time was chosen to provide clinical correlation for those with polytrauma. Two weeks is a common time at which decisions about patient trajectory are often rendered, and prognostication with regard to recovery potential is often entertained. Learning and memory abilities as observed by MWM or other testing months or a year after injury would yield better understanding of long-term effects of BF healing on neurologic recovery but may reflect other processes, such as brain remodeling, that are unlikely to be impacted by acute injury dynamics and anti-inflammatory therapeutics. Third, we only evaluated a single ENX dose, equivalent to slightly higher than a prophylactic dose in humans. Nonetheless, since therapeutic doses are uncommonly used in humans after TBI, using solely this dose seemed to have the greatest clinical relevance. The first 2-hour postinjury ENX dose is also not likely the clinical norm, though debate exists on when to give the first dose. Fourth, we did not include a Fx alone and a TBI + ENX group which would have better qualified mechanisms of ENX in brain injury recovery after a BF. Lastly, we did not document caloric intake and used weight gain or loss as a surrogate for catabolism. Given the herd nature of mice, we believe that isolation to accurately weigh food may have introduced individual stress that could have confounded evaluations of oral intake. Nonetheless, weight loss parallels commonly observed losses of lean body mass in the multiple-injured patient population despite adequate luminal or intravenous nutritional support and is, therefore, more likely related to catabolism rather than reduced caloric intake.
CONCLUSION
In conclusion, we demonstrated that a concomitant long BF worsens neurologic recovery in a rodent model of traumatic brain injury through evaluation of behavioral outcomes on MWM testing 2 weeks after injury. Administration of a low molecular weight heparin partially suppressed the impaired neurologic recovery improving some behavioral outcomes. Additional studies are appropriate to better understand the pathophysiologic mechanisms that impair cognitive recovery in the setting of TBI with a concomitant BF and to assess the optimal timing and therapeutic agent to enhance recovery without increasing the risk.
ACKNOWLEDGMENTS
We would like to relay our gratitude to Ms. Robin Armstrong for her technical and organizational assistance.
We would also like to thank Chao Xie, MD, Department of Orthopaedics and Rehabilitation, Center for Musculoskeletal Research, University of Rochester for his technical assistance in learning the validated closed tibial bone fracture model.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest and disclose no external funding.
Oral presentation at the 2018 Annual Meeting of the Eastern Association for Surgery of Trauma, January 9–13, 2018 at Lake Buena Vista, Florida.
REFERENCES
- 1.Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet. 2012;380(9847):1088–1098. [DOI] [PubMed] [Google Scholar]
- 2.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14(6):602–615. [DOI] [PubMed] [Google Scholar]
- 3.Robert AP. VHA handbook 1172.01. Washington, DC: 2013; March 20. [Google Scholar]
- 4.Lecky FE, Bouamra O, Woodford M, Alexandrescu R, O’Brien SJ. Epidemiology of polytrauma. Damage control management in polytrauma patient. 2010:13–24. [Google Scholar]
- 5.MacGregor AJ, Mayo JA, Dougherty AL, Girard PJ, Galarneau MR. Injuries sustained in noncombat motor vehicle accidents during Operation Iraqi Freedom. Injury. 2012;43:1551–1555. [DOI] [PubMed] [Google Scholar]
- 6.Hofman M, Koopmans G, Kobbe P, Poeze M, Andruszkow H, Brink PR, Pape HC. Improved fracture healing in patients with concomitant traumatic brain injury: proven or not? Mediators Inflamm. 2015;2015:204842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shultz SR, Sun M, Wright DK, Brady RD, Liu S, Beynon S, Schmidt SF, Kaye AH, Hamilton JA, O’Brien TJ. Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J Cereb Blood Flow Metab. 2015;35:1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weckbach S, Hohmann C, Braumueller S, Denk S, Klohs B, Stahel PF, Gebhard F, Huber-Lang MS, Perl M. Inflammatory and apoptotic alterations in serum and injured tissue after experimental polytrauma in mice: distinct early response compared with single trauma or “double-hit” injury. J Trauma Acute Care Surg. 2013;74(2):489–498. [DOI] [PubMed] [Google Scholar]
- 9.Degos V, Maze M, Vacas S, Hirsch J, Guo Y, Shen F, Jun K, Rooijen N, Gressens P, Young WL, et al. Bone fracture exacerbates murine ischemic cerebral injury. Anesthesiology. 2013;118(6):1362–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Guo Y, Wen D, Yang L, Chen Y, Zhang G, Fan Z. Bone fracture enhances trauma brain injury. Scand J Immunol. 2016;83(1):26–32. [DOI] [PubMed] [Google Scholar]
- 11.Borsig L Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb Res. 2010;125:S66–S71. [DOI] [PubMed] [Google Scholar]
- 12.Rao SV, Melloni C, Myles-DiMauro S, Broderick S, Kosinski AS, Kleiman NS, Dzavik V, Tanguay JF, Chandna H, Gammon R, et al. Evaluation of a new heparin agent in percutaneous coronary intervention. results of the phase 2 evaluation of M118 IN pErcutaNeous Coronary intErvention (EMINENCE) trial. Circulation. 2010;121:1713–1721. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Marks JA, Eisenstadt R, Kumasaka K, Samadi D, Johnson VE, Holena DN, Allen SR, Browne KD, Smith DH, et al. Enoxaparin ameliorates post-traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J Trauma Acute Care Surg. 2015;79(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata K, Kumasaka K, Browne KD, Li S, St-Pierre J, Cognetti J, Marks J, Johnson VE, Smith DH, Pascual JL. Unfractionated heparin after TBI reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J Trauma Acute Care Surg. 2016;81(6):1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suto Y, Nagata K, Syed MA, Browne KD, Cognetti J, Johnson VE, Ryan L, Kaplan LJ, Smith DH, Pascual JL. Cerebral edema and neurological recovery after traumatic brain injury (TBI) is worsened if it is accompanied by a concomitant bone fracture. In: Annual Scientific Session of the Western Surgical Association. November 4–7, 2017 Scottsdale, Arizona: 2017:40. [Google Scholar]
- 16.Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review. CNS Drug Rev. 2002;8(1):1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12(2):169–178. [DOI] [PubMed] [Google Scholar]
- 18.Pascual JL, Murcy MA, Li S, Gong W, Eisenstadt R, Kumasaka K, Sims C, Smith DH, Browne K, Allen S, et al. Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am J Surg. 2013;206(6):840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yukata K, Xie C, Li TF, Takahata M, Hoak D, Kondabolu S, Zhang X, Awad HA, Schwarz EM, Beck CA, et al. Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1-34 treatment. Bone. 2014;62:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. [DOI] [PubMed] [Google Scholar]
- 21.Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281(5835):2038–2042. [DOI] [PubMed] [Google Scholar]
- 22.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. [DOI] [PubMed] [Google Scholar]
- 23.Kubes P, Ward PA. Leukocyte recruitment and the acute inflammatory response. Brain Pathol. 2000;10(1):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual JL, Khwaja KA, Chaudhury P, Christou NV. Hypertonic saline and the microcirculation. J Trauma. 2003;54(5):S133–S140. [DOI] [PubMed] [Google Scholar]
- 25.Sen O, Sonmez E, Cekinmez M, Ozen O, Caner H. Antithrombin III and enoxaparin treatment inhibit contusion-triggered cell death, inflammation, hemorrhage and apoptosis after severe traumatic brain injury in rats. Turk Neurosurg. 2011;21(2):203–209. [DOI] [PubMed] [Google Scholar]
- 26.McDonald SJ, Sun M, Agoston DV, Shultz SR. The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J Neuroinflammation. 2016;13(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumasaka K, Marks JA, Eisenstadt R, Murcy MA, Samadi D, Li S, Johnson V, Browne KD, Smith DH, Schwab CW, et al. In vivo leukocyte-mediated brain microcirculatory inflammation: a comparison of osmotherapies and progesterone in severe traumatic brain injury. Am J Surg. 2014;208(6):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata K, Browne KD, Suto Y, Kumasaka K, Cognetti J, Johnson VE, Marks J, Smith DH, Pascual JL. Early heparin administration after traumatic brain injury: prolonged cognitive recovery associated with reduced cerebral edema and neutrophil sequestration. J Trauma Acute Care Surg. 2017;83(3):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams J Methods in behavioral teratology. In: Handbook of behavioral teratology. New York: Plenum Press; 1986:67–97. [Google Scholar]
- 30.Jeffery KJ, Morris RG. Cumulative long-term potentiation in the rat dentate gyrus correlates with, but does not modify, performance in the water maze. Hippocampus. 1993;3(2):133–140. [DOI] [PubMed] [Google Scholar]
- 31.Curtiss G, Vanderploeg RD, Spencer J, Salazar AM. Patterns of verbal learning and memory in traumatic brain injury. J Int Neuropsychol Soc. 2001;7(5):574–585. [DOI] [PubMed] [Google Scholar]
- 32.Kessels RP, de Haan EH, Kappelle LJ, Postma A. Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions. Brain Res Brain Res Rev. 2001;35(3):295–303. [DOI] [PubMed] [Google Scholar]
- 33.Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30(2):255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sajja VS, Galloway MP, Ghoddoussi F, Thiruthalinathan D, Kepsel A, Hay K, Bir CA, Vande Vord PJ. Blast-induced neurotrauma leads to neurochemical changes and neuronal degeneration in the rat hippocampus. NMR Biomed. 2012;25(12):1331–1339. [DOI] [PubMed] [Google Scholar]
- 35.Clausen F, Lewén A, Marklund N, Olsson Y, McArthur DL. Correlation of hippocampal morphological changes and Morris water maze performance after cortical contusion injury in rats. Neurosurgery. 2005;57(1):154–163. [DOI] [PubMed] [Google Scholar]
- 36.Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762(1–2):195–202. [DOI] [PubMed] [Google Scholar]
- 37.Dixon CE, Kraus MF, Kline AE, Ma X, Yan HQ, Griffith RG, Wolfson BM, Marion DW. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor Neurol Neurosci. 1999;14(4):285–294. [PubMed] [Google Scholar]
- 38.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S, Kaur H, Sandhir R. Fractal dimensions: a new paradigm to assess spatial memory and learning using Morris water maze. Behav Brain Res. 2016;299:141–146. [DOI] [PubMed] [Google Scholar]
- 40.Maei HR, Zaslavsky K, Wang AH, Yiu AP, Teixeira CM, Josselyn SA, Frankland PW. Development and validation of a sensitive entropy-based measure for the water maze. Front Integr Neurosci. 2009;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Zhang ZG, Chopp M, Meng Y, Zhang L, Mahmood A, Xiong Y. Treatment of traumatic brain injury in rats with N-acetyl-seryl-aspartyllysyl-proline. J Neurosurg. 2017;126(3):782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–391. [DOI] [PubMed] [Google Scholar]
- 43.Zhai PP, Xu LH, Yang JJ, Jiang ZL, Zhao GW, Sun L, Wang GH, Li X. Reduction of inflammatory responses by l-serine treatment leads to neuroprotection in mice after traumatic brain injury. Neuropharmacology. 2015;95:1–11. [DOI] [PubMed] [Google Scholar]
- 44.Di Battista AP, Buonora JE, Rhind SG, Hutchison MG, Baker AJ, Rizoli SB, Diaz-Arrastia R, Mueller GP. Blood biomarkers in moderate-to-severe traumatic brain injury: potential utility of a multi-marker approach in characterizing outcome. Front Neurol. 2015;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsitsilonis S, Seemann R, Misch M, Wichlas F, Haas NP, Schmidt-Bleek K, Kleber C, Schaser KD. The effect of traumatic brain injury on bone healing: an experimental study in a novel in vivo animal model. Injury. 2015;46(4):661–665. [DOI] [PubMed] [Google Scholar]
- 46.Locher RJ, Lunnemann T, Garbe A, Schaser K, Schmidt-Bleek K, Duda G, Tsitsilonis S. Traumatic brain injury and bone healing: radiographic and biomechanical analyses of bone formation and stability in a combined murine trauma model. J Musculoskelet Neuronal Interact. 2015;15(4):309–315. [PMC free article] [PubMed] [Google Scholar]
- 47.Brady RD, Grills BL, Church JE, Walsh NC, McDonald AC, Agoston DV, Sun M, O’Brien TJ, Shultz SR, McDonald SJ. Closed head experimental traumatic brain injury increases size and bone volume of callus in mice with concomitant tibial fracture. Sci Rep. 2016;6:34491. [DOI] [PMC free article] [PubMed] [Google Scholar]


