Abstract
Non-alcoholic steatohepatitis is becoming the most important aetiology for advanced liver disease. There has been important progress in the field in recent years and the complexity of the pathophysiology of NASH is better understood. Multiple non-invasive circulating and imaging biomarkers have been tested. The importance of lifestyle has been recognised and several drugs are being tested in clinical trials. This review addresses the challenges that healthcare professionals face in the management of NASH patients.
Introduction
Non-alcoholic steatohepatitis (NASH) is a fast-growing and highly prevalent threat to health, set to become a major cause of liver cancer and transplant.1 NASH, an advanced form of non-alcoholic fatty liver disease (NAFLD), is strongly linked to obesity and metabolic disorders.
There are no licensed therapies for NASH, despite its prevalence and clinical significance. While a number of drugs are in development, difficulties in staging and identification of accurate biomarkers have hampered the approval of effective therapeutics. The result is a reliance on lifestyle measures, primarily diet.
We brought together nine experts to discuss hot topics and current developments in NASH. Here, we provide an overview of the disease and consider how new technologies, from AI to novel biomarkers to gut microbiome signatures, could drive the introduction of new therapies.
We define the term NAFLD as representing the whole spectrum of fatty liver disease, including NAFL/steatosis and NASH/steatohepatitis.
Epidemiology of NAFLD/NASH
Key points
NAFLD is highly prevalent and increasing, with strong links to obesity and diabetes.
The impact of NAFLD is increasingly seen in severe liver disease and mortality.
NAFLD affects at least a quarter of the global population.2 This figure may be an underestimate, given that obesity rates globally have been rising since the 1970s.3 4
NAFLD is likely to have exceeded 30% prevalence in most middle-income and high-income countries. The most recent data are from Asia and suggest that NAFLD affects 30% of the Asian population, topping 40% in some territories.5
A case for screening?
NAFLD is closely linked with type 2 diabetes and obesity. In one study from China which used transient elastography to screen more than 1900 people with type 2 diabetes, 73% of people had NAFLD, rising to 95% of those who had a body mass index (BMI) of 30 or over.6 Some 35% of those with both risk factors had high liver stiffness suggestive of advanced fibrosis. This suggests a case for screening people with diabetes or obesity for NAFLD, as recommended by current Asian Pacific guidelines.7
NAFLD is not, however, confined to the obese population. In the USA, 43% of people with NAFLD are not obese, rising to 71% in Sweden.8
Figures on incidence of NAFLD are not easy to come by, but a study from Hong Kong using serial proton-MR spectroscopy to screen community participants with no liver disease at baseline found 13.8% developed fatty liver over 4 years, giving an incidence of 3.7 per 100 person-years.9 In contrast, a study using serial transient elastography examinations showed that 52% of patients with type 2 diabetes developed incident fatty liver in 3 years.10
Therefore, current European and Asian Pacific guidelines support screening people with diabetes or obesity for NAFLD.7 11 That said, the optimal screening strategy remains unclear and will depend on the local clinical practice, referral pathway and availability of different tests. Recently, a consensus paper commissioned by the American Gastroenterological Association recommends the use of the Fibrosis-4 index (FIB-4) followed by specific fibrosis tests to screen for advanced liver disease in patients at risk of NAFLD.12
Clinical impact
The impact of NAFLD should not be underestimated. It is the fastest-growing indication for liver transplantation in the USA, accounting for more than 25% of transplants, compared with around 5% in 2002.1 It is also the fastest-growing cause of liver cancer among candidates for liver transplant.13
Incidence of decompensated liver cirrhosis and liver-related deaths are projected to rise in coming decades.14 While the effect of NAFLD on mortality has been masked by cardiovascular disease, we can expect to see more people with severe disease dying of liver-related causes in future.15 That being said, cardiovascular disease and extrahepatic malignancies will remain the leading causes of death in the vast majority of patients with NAFLD in the foreseeable future. Clinicians taking care of patients with NAFLD should be aware of the association and provide proper care of the cardiometabolic conditions and screening for cancers according to current guidelines.
Across the world—but especially in South America, the Middle East and East Asia—childhood obesity has risen fast since 2000.16 Unless the trend can be reversed, these children are growing up with risk factors for serious liver disease as adults.
One hot question is whether the terminology of NAFLD should be changed, given that many people have liver damage both from fatty liver and alcohol. A new name of MAFLD—metabolic-associated liver disease—has been proposed, to include people with fatty liver and metabolic risk factors.17 The proposal is under discussion by professional bodies worldwide.
While it might include more people who have coexisting alcohol-related damage, it would exclude those with fatty liver who have not yet developed metabolic risk factors.18 As such, it is likely to affect understanding of the epidemiology of fatty liver disease.
Pathophysiology of NAFLD
Key points
NAFLD is a complex disease driven by lipotoxicity, insulin resistance and activation of inflammatory and immune pathways, closely linked to metabolic disorders.
There is increasing evidence of a link between the gut microbiome and development of both insulin resistance and NASH.
NAFLD is a complex disease which involves many of the systems of the body.
The development of NAFLD comes about through a process of multiple parallel ‘hits’ which put stress on the liver.19 External factors which raise the risk include unhealthy diet such as fructose overconsumption20 and lack of exercise, while genetic factors also affect people’s chances of developing the condition.21
Lipotoxicity and inflammatory pathways
Lipogenesis is a key factor in the early stage of development of NAFLD, driven mainly by increased delivery to the liver of free fatty acids (FFAs) from diet and derived from the adipose tissue and via increased de novo lipogenesis in the liver.22
An overload of FFAs and production of intermediaries such as ceramides and phospholipids in liver cells may result in apoptosis and liver damage. FFAs seem to be among the most important lipids involved in insulin signalling.23 Consequently, lipotoxicity might link metabolism with the development of inflammation and fibrosis in the liver. Unresolved inflammation frequently results in fibrosis and indeed fibrosis stage is by far the most relevant predictor of overall and disease-specific mortality.24 25
Inflammation is an important aspect of metabolic disorders. All adipose tissue contributes substantially to systemic and liver inflammation observed in NAFLD. Successful weight loss after bariatric surgery results in a massive reduction of IL-1 type cytokines, especially in the subcutaneous adipose tissue.26 In addition, we can see from animal studies that lack of interleukin-1 successfully inhibits the transformation of steatosis to steatohepatitis and liver fibrosis.27
Many diverse inflammatory hits are able to affect insulin signalling with receptor activator of nuclear factor28 and TNF and IL-6 just being some examples.23 Conversely, adiponectin has a protective effect in almost all metabolic pathways affecting organs.29
Many more pathways such as nuclear receptors, bile acids or fibroblast growth factors have evolved in the past decade as key pathophysiological players in NAFLD and related pathologies.30–32
In addition to inflammatory pathways, various genetic polymorphisms affect the development and progression of NAFLD.21 Almost all identified genetic polymorphisms in NAFLD affect lipid pathways, highlighting the crucial role of metabolic pathways in this disorder.
Gut microbiota and NAFLD
More recently, researchers have investigated the role of gut microbiota, trying to identify a specific microbial fingerprint in NAFLD. This research could pave the way for a non-invasive stool test to identify the progression from NAFLD to advanced fibrosis.33 Researchers have identified 8 species of gut microbes which are twice as abundant in advanced fibrosis, and 22 species which are twice as abundant in mild or moderate fibrosis. More recently, a universal gut microbiome signature in NAFLD cirrhosis has also been described.34 Besides the microbiome also the mycobiome might be involved in the pathogenesis especially in advanced NAFLD.35
Two types of bacteria, Prevotella copri and Bacteroides vulgatus, have been shown to drive the production of branched-chain amino acids (BCAA) in the gut. Increased levels of BCAA have been identified in people with insulin resistance, and animal experiments have shown that P. copri induces insulin resistance in mice.36 Whether similar pathways are relevant in human insulin resistance in NAFLD remains to be proven.
The pathophysiology of NASH and NAFLD explains its close correlation with type 2 diabetes and the metabolic syndrome. Presence of either type 2 diabetes or NAFLD at least doubles the risk of developing the other condition. In addition, both diabetes and NAFLD drive an increase in cardiovascular disease (up to double the risk), chronic kidney disease (up to 3.5 times the risk) and intrahepatic and extrahepatic malignancy.37 38
The histology of NASH
Key points
Liver biopsy remains the reference standard for diagnosis of NASH (steatosis vs steatohepatitis).
Diagnosis and scoring are distinct and separate steps in the evaluation of NAFLD.
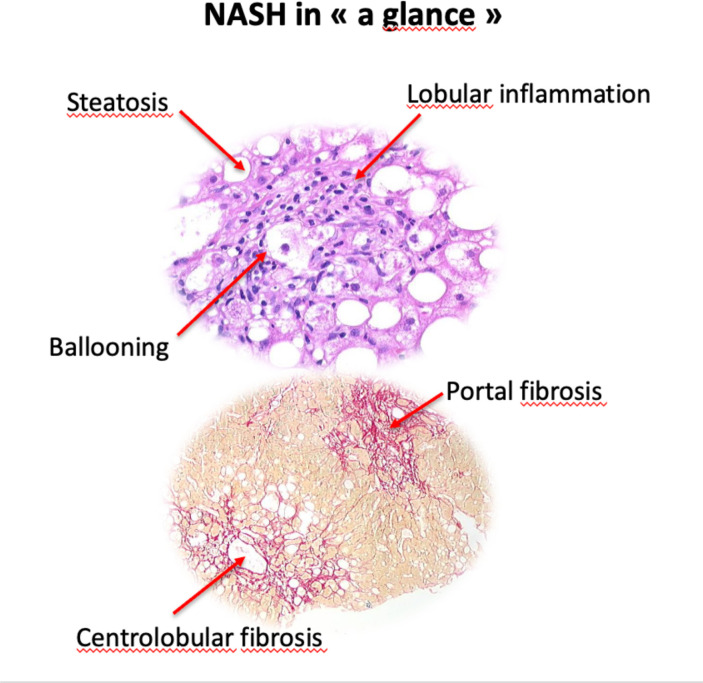
NAFLD encompasses a spectrum of liver lesions, from steatosis to cirrhosis, via NASH. The presence of 5% fat cells in the liver is a prerequisite for diagnosis, based on microscopic examination of liver tissue. Current European guidelines39 state that distinguishing NASH from simple steatosis requires biopsy (see figure 1), and the diagnosis cannot be made by clinical, biochemical or imaging measures.
Figure 1.
Histology of NASH illustrating steatosis, lobular inflammation, portal fibrosis and centrolobular fibrosis. NASH, non-alcoholic steatohepatitis.
Liver biopsy can also be used to assess severity (grade) and stage (fibrosis) of disease. This enables enrolment in clinical trials, evaluation of treatment response and may point to comorbidity risk factors. Among the usual limits of liver biopsy, especially its invasiveness, sampling variability is a challenging issue as it represents a tiny fraction (1/50 000) of the whole liver. This strongly supports the use of criteria of biopsy adequacy including length and width of liver specimen. Nevertheless, given the zonation of the disease (starting in centrolobular areas), we may expect less impact of sampling compared with viral hepatitis, for instance.
Scoring systems to identify NASH
Pathologists use two main types of scoring system to assess liver biopsy for NASH:
The NAS (NAFLD Activity Score),40 which sums together scores for degree of steatosis, ballooning of liver cells and lobular inflammation, giving a score of 0–8. The NAS is not diagnostic, and was designed to measure histological changes during clinical trials. Fibrosis may also be added, with a score of 0–4.
The Steatosis, Activity and Fibrosis (SAF) score,41 which separately assesses steatosis from activity based on ballooning, and lobular inflammation, enabling an algorithm to sort results into NAFLD or NASH.
The two systems are not interchangeable. An evaluation of 1000 biopsies found excellent concordance between the two in identification of definite NASH, while 84% of the biopsies identified by NAS as ‘borderline’ (NAS 3–4) were identified as NASH by the SAF.42
Reader agreement: a key challenge
While biopsy is the reference standard for diagnosis, reader agreement in interpretation of biopsies is a challenge, especially for less experienced readers. Use of the SAF system can decrease inter-reader variation.43 There is evidence of variation in intrareader agreement too.44 This raises the issue of the reliability of liver biopsy as the master tool for treatment response evaluation.
More use of digital pathology and automated quantification of individual features of NASH could begin to address this issue by providing an objective and accurate analysis, with better detection of subtle changes which might be quicker to detect benefits from treatment.
Through computerised image analysis, quantitative but also qualitative assessment of liver fibrosis may be achieved, allowing identification of specific patterns of fibrosis in adults and children.
The possibilities of digital pathology become apparent when combined with machine learning. Two studies45 46 have shown how machine learning allows quantitative analysis of steatosis, ballooning, inflammation and fibrosis in biopsy samples to a high degree of accuracy.
Unresolved issues in staging and diagnosis
Staging of fibrosis remains an issue—and an important one. Development of fibrosis may be the most relevant histological endpoint to monitor during clinical trials, yet the standard staging system lacks granularity.
In addition, the diagnosis of NASH itself could be refined, with the inclusion of additional key features such as centrilobular fibrosis and portal inflammation.
Circulating biomarkers of NASH
Key points
Non-invasive testing is useful to rule out people unlikely to have advanced liver disease, without need for biopsy.
Direct fibrosis biomarkers can be used in combination with simple scores such as FIB-4, but there remains a need for more sensitive, specific and better validated biomarkers in NAFLD.
While histology remains the reference standard for diagnosis, prognosis and monitoring treatment response in NAFLD, there are issues around accuracy, reader variation and safety—not to mention the need for less invasive tests.
When considering the performance of biomarkers, it is important to also consider the setting in which the biomarker will be used, as this affects target condition prevalence and therefore pretest probability.
In primary care or settings where pretest probability of severe disease is low, a test with high negative predictive value to rule out severe disease may be preferable. In secondary and tertiary care, probability of severe disease rises and a test needs higher positive predictive value to select cases for enhanced therapy or surveillance.
There are two clinically relevant liver disease categories in which high quality biomarkers are particularly needed.
Identification of at-risk NASH
Identifying at-risk NASH—where steatohepatitis is active and there is moderate fibrosis (NAS≥4, Fibrosis stage ≥2), suggesting that they are likely to progress to cirrhosis—is particularly important for identifying candidates for treatment.
To date, few blood-based biomarkers have been developed specifically targeting this condition. NIS-4 is a composite biomarker comprising microRNA 34A, alpha2 macroglobulin, HbA1c and YKL-40; representing information about insulin resistance, inflammation and fibrogenesis. Research published in 2020 showed it out-performed several other biomarkers to identify this cohort of patients, although these results need further validation.47
A study from 2021 by the LITMUS consortium compared the performance of 10 widely available biomarkers in prescreening patients for inclusion in a clinical trial.48 The researchers compared how many biopsies would be needed and how many eligible patients would be identified per 100 people tested. Performance varied, making it a trade-off decision as to whether to conduct more biopsies, but accept a marginally higher screen failure rate, to identify more eligible patients.
Some have suggested the use of combined MR elastography (MRE) with FIB-4 biomarkers (MEFIB). This is discussed further below in the section ‘imaging biomarkers’.
Identification of advanced fibrosis
People with advanced fibrosis—F3–F4—are likely to see outcomes worsen significantly.49
Some biomarkers are indirect measures of processes leading to fibrosis, such as markers of inflammation, necrosis and cell death, while others are direct serum markers of collagen components or factors regulating fibrogenesis.50
Risk stratification guides are currently used to direct patients along appropriate pathways. The FIB-4 combines three indirect markers (ALT, AST, platelets) with age, while the NAFLD Fibrosis score adds BMI, albumin and impaired fasting glucose or type 2 diabetes (see table 1). Both systems have a high sensitivity cut-off at the lower end of probability, and a high specificity cut-off at higher probability51–55 and exhibit a high negative predictive value to rule out advanced disease.
Table 1.
Circulating fibrosis biomarkers
| Test | Description | References |
| Indirect Fibrosis Biomarker Panels | ||
| AST:ALT ratio | AST (IU/L)/ALT (IU/L) | 128 |
| AST to platelet ratio index | AST (IU/L)/(ULN)/platelet count (x109/L) x 100 | 54 |
| BARD score | Weighted sum of BMI≥28 = 1 point, AST/ALT ratio ≥0.8 = 2 points, T2DM=1 | 129 |
| FIB-4 | Age x AST (IU/L)/platelet count (x109/L) x √ ALT (IU/L) | 130 131 |
| NAFLD fibrosis score | −1.675+0.037 x age (years)+0.094 x BMI (kg/m2)+1.13 x IFG or T2DM (yes=1, no=0)+0.99 x AST/ALT ratio − 0.013 x platelet (x109/L) − 0.66 x albumin (g/dL) | 53 |
| Direct fibrosis biomarker panels | ||
| ELF | ELF=−7.412 + (ln(HA)*0.681) + (ln(PIIINP)*0.775) + (ln(TIMP1)*0.494) | 60 132 |
| Fibro test | Patented algorithm combining total bilirubin, GGT, α2-macroglobulin, apolipoprotein A1, and haptoglobin, corrected for age and gender. | 133 134 |
| FibroMeter NAFLD | Patented algorithm combining age, body weight, glucose, AST, ALT, ferritin and platelet count | 135 136 |
| ADAPT | ADAPT=exp(log10((age x PRO-C3)/sqrt(Platelets)))+T2DM | 137–139 |
| FIBC3 | FIBC3=−5.939 + (0.053*age) + (0.076*BMI) + (1.614*T2DM) – (0.009*platelets) + (0.071*PRO-C3) | 138 139 |
| ABC3D | Age >50 = 1 point, BMI >30 = 1 point, platelet Count <200 = 1 point, PRO-C3 >15.5 = 1 point, T2DM=2 points | 138 139 |
AST:ALT, aspartate transaminase:alanine transaminase; BMI, body mass index; ELF, enhanced liver fibrosis; FIB-4, Fibrosis-4 index; IFG, impaired fasting glucose; NAFLD, non-alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus.
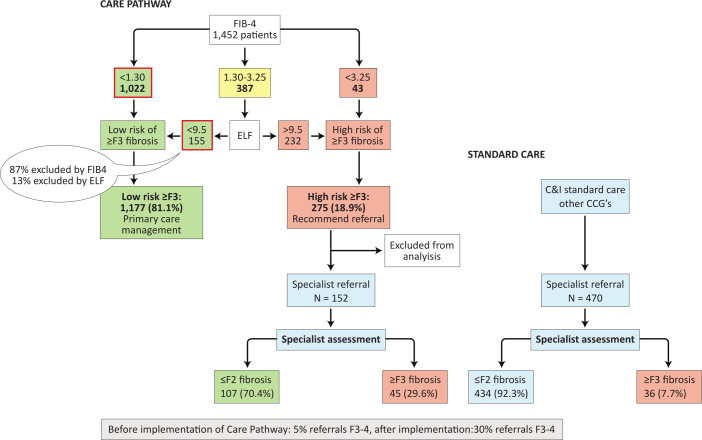
The most efficient route is to use biomarkers in combination within a care pathway, moving through clinical assessment to FIB-4 and then employing a second-line test such as ELF or transient elastography imaging, to minimise the number of patients that require biopsy (see figure 2).51 56–61
Figure 2.
Proposed primary care pathway versus standard care for patients with NAFLD, adapted from Srivastava et al.64 EFL, enhanced liver fibrosis; FIB-4, fibrosis-4 index; NAFLD, non-alcoholic fatty liver disease.
Direct biomarkers give more granular evidence of fibrosis development and so provide a tractable ‘second-line’ test, that might also aid monitoring of progression/response to treatment. Candidates include markers of extracellular matrix turnover, for example, the PRO-C3 Collagen Neoepitope, which has been incorporated into several risk scores62 63 and the ELF test (see table 1).
The ELF test (combining procollagen III N-terminal peptide, hyaluronic acid, tissue inhibitor of metalloproteinase 1) exhibits a high negative predictive value62 but more modest positive predictive value in most clinical settings. Its use in a care pathway following FIB-4 improved the proportion of patients with advanced fibrosis undergoing specialist referral from 5% to 30%, although it should be noted that 87% of cases with mild disease were eliminated by FIB-4 in that study (see figure 2).64
Comparative studies demonstrate that many fibrosis biomarkers offer only marginal improvement over the performance of FIB-4 in routine care.65
New markers on the horizon
Researchers continue to search for new biomarkers in the field. Current areas of interest include use of metabolomic profiling,66 which measures serum lipids and amino acids; transcriptomic profiling gene expression patterns in the liver to guide identification of circulating protein biomarkers67; and measurement of circulating proteins.
In addition, new technologies are allowing measurement of liver proteases, which do not get released into the circulation.68
Imaging biomarkers in NASH
Key points
Use of non-invasive imaging assessment is increasingly important to identify high risk NASH, to monitor patients for progression, and to identify response to treatment in clinical trials
Long-term studies are needed to assess the association between longitudinal change in biomarkers and long-term clinical outcomes.
Identification of NAFLD
While liver biopsy is the clinical standard for identifying NASH, non-invasive imaging is becoming more important for staging and quantifying disease.
Conventional ultrasound has low negative predictive value so is of limited use, especially for mild steatosis. CT scan lacks specificity and sensitivity, and exposes the patient to ionising radiation.
The controlled attenuation parameter provides a useful indication of NAFLD, but the de facto gold standard has become MRI-derived proton density fat fraction (MRI-PDFF), for its ability to quantify fat content. It is widely used for non-invasive screening for NAFLD in research studies.
Assessment of fibrosis relies on indirect biomarkers, primarily tissue stiffness.69 It is assessed by elastography, with the vibration-controlled transient elastography (FibroScan) imaging machine the most commonly used.70 Studies have shown that patients with higher liver stiffness also have higher liver-related mortality, suggesting that this is a prognostic biomarker.71 MRE has been shown to predict progression to advanced fibrosis (stage 3 or 4) with a higher degree of accuracy than FibroScan.72
Combination of various biomarkers in one imaging session could allow for assessment of degree of steatosis, inflammation and fibrosis, by using both MRE and PDFF.
‘At-risk’ NASH
As noted, a key challenge is identification of patients with fibrosis stage 2 or above, who are at greatly increased risk of disease progression and liver-related mortality.
The FAST score, which combines two imaging biomarkers (liver stiffness and CAP from the FibroScan, plus the circulating biomarker AST:ALT ratio) is being used to prescreen people for inclusion in clinical trials. A FAST score above 0.67 indicates biopsy, while below 0.35 would exclude people from the trial before biopsy.73 74 The main issue with FAST is low positive predictive value.
Combining MRE with FIB-4 biomarkers (MEFIB) may help. A FIB-4 score of 1.6 or above, combined with MRE of 3.3 kPa or above, yields a 97.1 positive predictive value that a patient has fibrosis stage 2 or above and is therefore a candidate for treatment.75 The investigators believe a PPV above 90 negates the need for a biopsy altogether in these populations. MEFIB has been shown to be better than FAST in detecting at-risk NASH patients, among a cohort with biopsy-proven NAFLD.76
The use of either vibration-controlled transient elastography or MRE, with FIB-4 where needed, could separate out patients with NAFLD into low risk fibrotic NAFLD and high risk for progressive fibrosis.77
What is a clinically significant treatment response?
Despite the clear need for treatment, there are no licensed treatments for NASH. However, many drugs are in development. A clinically significant biomarker for response to treatment is crucial to identify effective therapies.
An MRI-PDFF liver fat reduction of 30% from baseline has been shown to correlate with five times higher odds of NASH resolution, compared with those not achieving 30% MRI-PFF reduction.78
Researchers have not yet been able to identify a single clinically significant change in MRE that would indicate treatment response, but there are hints—for example, a 15% relative increase from baseline is associated with fibrosis progression.79
So far there is some evidence of histological response for four imaging biomarkers: MRI-PDFF, Multiscan cT1, FAST and 15%–20% reduction in MRE, although this needs further confirmation.
Multidisciplinary lifestyle intervention in NASH
Key points
The best evidence we have for lifestyle intervention in NASH is for weight reduction and the Mediterranean dietary pattern.
Improved dietary composition by itself, weight loss of 5%–10% of the initial body weight and moderate exercise can make a difference for NASH patients in terms of improvement of all histological features.
The lack of pharmacological interventions for NASH make lifestyle measures even more important (see figure 3). The challenge is adoption of these measures in the long term.
Figure 3.
Lifestyle recommendations for patients with NAFLD. BMI, body mass index; NAFLD, non-alcoholic fatty liver disease.
The best evidence of benefit comes from the Mediterranean diet—a diet characterised by plentiful intake of olive oil, vegetables, fruits and nuts, legumes, whole grains, fish and seafood, and a low intake of red meat and especially processed meat, along with reduced carbohydrates intake (40% of the calories vs 50%–60% in a typical low-fat diet), especially sugars.
A recent 18-month trial with 294 participants suggests the Mediterranean diet can be enhanced by additional green plants rich in polyphenols.80 The green-Mediterranean diet reduced hepatic fat by 39% compared with 20% for the Mediterranean diet, despite similar weight loss, and both diets did better than controls provided only with healthy dietary guidelines.
The importance of increased phenolic acid intake (from fruits and vegetables, nuts, green tea and coffee) is independently associated with lower prevalence of insulin resistance and NAFLD and fibrosis measured by fibrosis marker (FibroTest).81
Carbs or fats?
One question is over the role of fats and carbohydrates on liver fat content. Generally, low-carb weight reduction diets are not superior to low-fat diets for liver fat loss, and the patients can freely choose which diet they are capable of maintaining in the long term. A meta-analysis of 11 short-term small sample trials found no significant difference in the percentage change of hepatic fat reduction between low carb and low fat diets.82
Evidence is mounting on the importance of the type of fat eaten, pointing towards the specific harmful effect of saturated fat.
Short-term trials83 84 suggest that either low-fat +high carb or high-fat +low carb diets reduce liver fat on a weight-loss diet leading to weight reduction. However, for an isoenergetic or hyperenergic diet high-(mostly saturated) fat +low carb results in increased liver fat, while low-fat +high carb reduces liver fat (if isoenergetic) or increases liver fat to a much lower extent (if hyperenergic).
It can be concluded that the type of fat makes a difference to liver fat. Saturated fats consistently increase liver fat more than polyunsaturated or monounsaturated fat, when total energy intake is similar. This evidence supports the benefits of the Mediterranean diet which is low in saturated fat and high in polyunsaturated and monounsaturated fat.
Ultraprocessed food and drink is a major challenge
The consumption of ultraprocessed foods (UPF) has increased dramatically, accounting for 50%–60% of total daily energy intake in some countries.85 These foods are a major source of added sugar, saturated fats, are energy dense and have low nutritional value.
Consumption of UPFs increases cardiovascular disease, cancer incidence and all-cause mortality.85 103 In a UK Biobank study, including 21 730 participants, UPF consumption was associated with type 2 diabetes incidence.86
A meta-analysis of nine cross-sectional and three cohort studies found higher consumption of UPFs resulted in more overweight, obesity and abdominal obesity, with a clear dose–response association.87
The same relationship can be seen with metabolic syndrome both in the general population and among people with NAFLD. In a cross-sectional study of 789 people, among people with NAFLD, those getting 40% or more of their calories from UPFs had more than three times the chance of having metabolic syndrome.88
Higher consumption of UPFs is linked to food insecurity and low income, since healthy food is more expensive. Indeed, NAFLD is almost 10% more common among those with poor food security.89
One target to reduce NAFLD may be sugar-containing beverages such as fruit juice and sugar-added soft drinks. A cohort study of 1940 infants found that consumption of more than two sugar-containing beverages a day at 1 year of age increased the chances of MRI-assessed NAFLD at age 10 threefold, compared with those consuming fewer than one beverage a day.90
Looking beyond diet, physical activity protects from liver-related mortality and seems to lower the risk conveyed by adiposity.91 Both low physical activity and sedentary behaviour (8 hours and above of sitting time per day) raise the risk of NAFLD, independent of each other.92
NASH therapies in phase III
Key points
A plethora of drugs are in various stages of research, but only a few have currently entered in phase III.
Despite promising results so far, outcomes sufficient for full regulatory approval may still be years away.
Despite the rising prevalence and serious potential clinical consequences of NASH, there are currently no treatments licensed for the disease. Treatment relies on lifestyle changes, primarily weight loss.
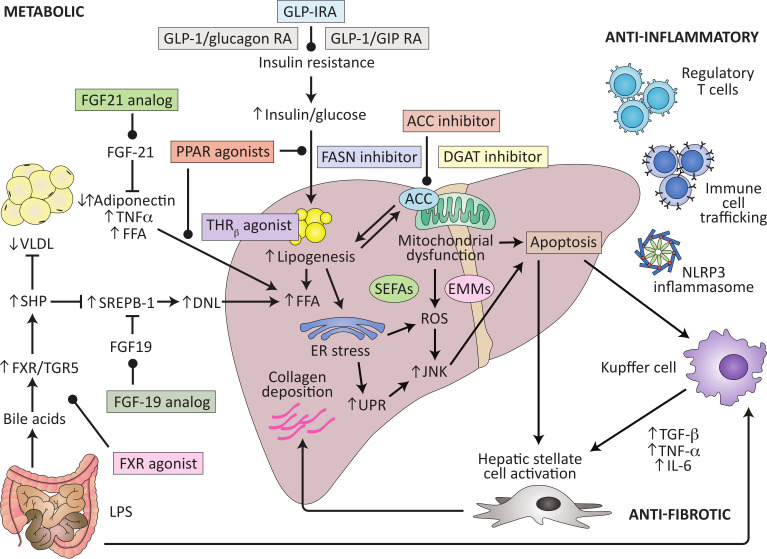
The complexity of the pathophysiology of the disease provide multiple potential target for drug treatment (figure 4). The FDA endpoints for NASH clinical trials in late stage development focus on the histological endpoints of NASH resolution without worsening of fibrosis or fibrosis improvement of at least one fibrosis stage without worsening of steatohepatitis. Thus, a repeat liver biopsy at entry and at the end of treatment is required.93
Figure 4.
Potential therapeutic targets for NASH, updated and adapted from Konerman et al.110
Drugs acting to restore metabolic homeostasis
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors of nuclear hormone receptor superfamily comprising of the following three subtypes: PPARα, PPARγ and PPARβ/δ. Activation of PPAR-α reduces triglyceride level and is involved in regulation of energy homeostasis, activation of PPAR-γ causes insulin sensitisation and enhances glucose metabolism, whereas activation of PPAR-β/δ enhances fatty acids metabolism.94
The pan-PPAR agonist lanifibranor has successfully completed a 24-week phase IIb trial with 247 participants, meeting its primary endpoint of a reduction of two points or more on the SAF activity score, with no increase in fibrosis, achieved by 49% of patients on 1200 mg compared with 27% on placebo.95 The drug also hit its secondary endpoint of reducing fibrosis by at least one stage without worsening NASH (42% on 1200 mg vs 24% on placebo).
Lanifibranor is generally well tolerated, although side effects include mild weight gain. It has been given FDA breakthrough therapy designation as the lead drug candidate in this class and a phase III study is underway.
GLP1-receptor agonists
GLP1-receptor agonists have multiple effects on several organs and systems, including the pancreas,96 the central nervous system and the liver.97 98
The GLP1 RA semaglutide has completed a 72-week phase II trial with 320 participants, showing resolution of NASH with no worsening of fibrosis for 56% of patients on 0.4 mg compared with 20% on placebo.99 It was unable to hit its secondary outcome of improvement of fibrosis with no worsening of NASH, although the number of patients with worsening fibrosis was lower in the active treatment arms. The drug induces a significant weight loss. Most common adverse events were gastrointestinal, while the safety in NASH was consistent with the observed profile in other trials and disease areas. A phase III trial has already started.
Drugs acting on the liver
Thyroid hormone receptor beta (THR-β) is crucial for liver homoeostasis, through multiple metabolic actions of thyroid hormones. THR-β agonists have been shown to improve lipid metabolism.100
The THR-β agonist resmetirom has completed a phase II trial and is in two phase III 52-week trials. The 36-week phase II study with 125 participants showed reduction of liver fat by an average 30% compared with baseline by 12 weeks, leading to significant resolution of NASH.101
Preliminary results from one of the phase III trials demonstrates positive results in imaging measurements of liver fat and liver stiffness, as well as reduction in LDL-cholesterol and ApoB.102
The farnesoid X receptor (FXR) is a ligand-activated transcription factor involved in the control of bile acid (BA) synthesis and is also central to a number of pathways in the liver, affecting inflammation, fibrosis, lipid metabolism and glucose metabolism.103
Obeticholic acid is a selective FXR agonist currently tested in a phase III trial of 1968 participants with NASH and fibrosis F2–F3.104 In an 18-month interim analysis, the drug met the endpoint of improvement by at least one stage in fibrosis with no worsening of NASH, but did not meet the endpoint of NASH resolution. The main adverse event was pruritus and increase in LDL cholesterol, responsive to statin therapy.
Aramchol, a partial inhibitor of hepatic stearoyl-CoA desaturase (SCD1) has been tested in a phase 2b trial including 247 patients with NASH.105 Aramchol at the highest dose of 600 mg did not reach the prespecified significance level for decrease in liver fat by MR spectroscopy at 52 weeks. However, post hoc analyses suggest a potential for improving liver histology in patients with high disease activity and precirrhotic stages of fibrosis.
The next 6 years
Five phase III studies are expected to complete the trial part having surrogate, histological endpoints before the end of 2024 (aramchol, resmetiron, obeticholic acid, belapectin, lanifibranor, efruxifermin). Completion of clinical outcomes trials are expected in September 2025 for obeticholic acid and in May 2028 for semaglutide. Clinicians will need to be ready to assess which patients to treat and how to treat and monitor treatment, when these drugs finally become available.
Historically, many drugs have failed to hit their histological endpoints.
NASH therapies in Phase II
Key points
Drugs under development rely on achieving two primary endpoints—resolution of NASH and reduced fibrosis—but many other targets are likely to be beneficial.
Combinations of drugs may allow targeting of both metabolic dysfunction and liver damage, with injectable or infusion drugs used to initiate therapy in more severe disease and oral drugs providing long-term maintenance therapy.
Key histological endpoints for clinical trials are resolution of NASH (without worsening fibrosis) and reduced fibrosis (without worsening NASH).106
Recent data prove that improvement in fibrosis stage translates into an improvement in clinical outcome.107 We know from studies of bariatric surgery that NASH is reversible, and that as NASH reverses, so fibrosis improves.108 109
The multifactorial nature of NASH means that there are a plethora of targets beyond histological resolution of NASH and reduction in fibrosis which could be beneficial. These could include reduction in lipotoxic fat, weight loss, atherogenic lipid improvement and glycaemic control. The question is whether one drug will be enough to target all these potential endpoints.
The routes that lead to NASH and fibrosis are complex, taking in metabolic and inflammatory pathways, meaning there is a multitude of targets.110 Compounds currently being tested in phase II can be divided into those that aim at five main targets:
Insulin resistance and lipid metabolism.
Lipotoxicity and oxidative stress.
Inflammation and immune activation.
Cell death.
Fibrogenesis and collagen turnover.
By far the majority of drugs in development aim at insulin and lipids, although some drugs have multiple effects and can be aimed at more than one target.
Many drugs are still in early phase II studies, looking at non-invasive endpoints, while there are a host of others in later phase II, collecting biopsies for histopathological endpoints that will allow them to progress to phase III. The ability of histopathology to accurately identify endpoints in clinical trials is likely to have a major impact on the future development of this field.
The majority of agents are oral, but there are a significant number of injectable/infusion agents under development, including all hormone therapies. Their place in therapy needs consideration.
Five of the compounds in phase II (efruxifermin(FGF-21), pegbelfirmin (FGF-21), aldafermin (FGF-19), pegozafermin (FGF-21) and BFK8588A (FGF-21)) achieved a reduction in ALT which has been shown to be associated with histological improvement in NAFLD, and these results are comparable to those seen in drugs now in phase III. Four phase II compounds (MET-409 (FXR agonist), pegozafermin, efruxifermin, VK2809 (THR-beta agonist)) show a 30% clinically significant reduction in MRI-PDFF. Two phase II drugs (efruxifermin, aldafermin) show some data indicating improvement in fibrosis, although in the case of aldafermin, later histopathology did not support this.
The same two drugs showed NASH resolution, although in the case of efruxifermin, this was undermined by NASH-resolving weight loss from one of only two patients in the placebo group who had a liver biopsy.
The combination of NASH resolution and fibrosis improvement gives a clearer picture: efruxifermin and aldafermin both demonstrate significant results, with 28% of efruxifermin111 and 22% of aldafermin112 patients achieving both endpoints, compared with no placebo patients. Minimising placebo response by use of duel endpoints may point a way forward.
Both efruxifermin and aldafermin are injectables, with potentially potent effects on histopathology and also metabolic parameters. Practical considerations may limit their use, including poor gastrointestinal tolerability, common to all injectable and infusion drugs in this area. Injection and infusion drugs might be best used for short-term induction therapy for patients with F3 or F4, or for patients with F2 showing risk factors for rapid fibrosis progression or significant metabolic comorbidity.
Better-tolerated oral drugs could then be used for long term treatment or maintenance therapy in people with F1–F3, perhaps in a fixed dose combination.
Combination therapy is likely the future. Some patients will benefit more from a focus on therapies to target metabolic syndrome, with secondary drugs targeting anti-inflammatory and antifibrotic endpoints, while for others with more advanced disease, the position will be reversed. A challenge will be to identify which patients are likely to respond best to which therapies, with the possibility of more personalised medicine in future.
Several phase II trials of combination therapies are in progress, offering the possibility for synergies between drugs.
Cancers and NASH
Key points
NASH increases the risk of hepatocellular carcinoma (HCC) and of other cancers.
About half of people with NASH-related HCC do not have cirrhosis and tend to be diagnosed at an advanced cancer stage.
Cardiovascular disease has long been the major cause of death for people with NAFLD,113 reflecting its close links to metabolic disorders. The second cause of death of NAFLD patients is cancer.
A Korean study found an incidence rate of 8.5 per 100 000 years for HCC, compared with 119.7 for breast cancer and 46.2 for colorectal cancer.114 However, HCC is more common among people with NAFLD—the same study showed an incidence rate of 23.1 for people with NAFLD, compared with 0.9 for people without. HCC is not the only cancer of concern. Studies have found people with NAFLD have an increased risk of colorectal cancers, cholangiocarcinoma and cancers of the breast, stomach, pancreas, prostate and oesophagus.115
After adjustment for risk factors, NAFLD conveyed a 17-fold increase in risk of HCC, and also a doubling of risk for breast cancer.116 This is not simply a reflection of obesity. A US case–control study of 4722 cases of NAFLD showed a significant increase in risk for people with NAFLD, even compared with controls with obesity.117
Researchers have identified a novel molecular signature found in HCC associated with NASH.118 This suggests a specific risk for this type of HCC, not found in non-NASH populations.
One meta-analysis calculated that each unit increase in liver stiffness was associated with an 11% increased risk of HCC, although this included all types of liver disease, not just NAFLD.119
While cirrhosis with NAFLD conveys the highest risk of HCC, that does not mean all NAFLD-related HCC patients have cirrhosis. One study of 4406 cases found only 46% had known cirrhosis at time of diagnosis.120 However, the absolute incidence in non-cirrhotic patients is still low compared with cirrhotic NAFLD patients: incidence for cirrhosis 13.55 or 4.82 per 1000 person-years vs non-cirrhosis 0.39 and 0.04 (each with and without high FIB-4).121
Patients with NAFLD are diagnosed at a later stage of HCC than patients with HCV.122 In a prospective observational study from Italy, 11% of HCV patients with HCC were diagnosed at BCLC stage 0, compared with none of the NAFLD patients. More NAFLD patients were diagnosed at stage B or C.
In post hoc analysis of phase 3 trials testing immunotherapy for HCC, patients with virus-related HCC seemed to fare better than patients with non-viral-related cancer.123 This was also observed in retrospective analysis of small cohorts.
In addition to the risk factors listed above, some medications lower the risk of developing liver cancer:
Metformin lowers risk.124
Statins (particularly lipophilic statins such as simvastatin, atorvastatin) decrease risk.125
Aspirin lowers risk.126
Lifestyles are also important to mitigate risk. Coffee consumption and regular physical activity are associated with a reduction in the risk of developing HCC. Can this risk be reversed, for example, in the case of bariatric surgery? A study of 98 090 newly diagnosed NAFLD patients with severe obesity, of whom 34% received bariatric surgery, suggests it can.127 The adjusted risk of any cancer was reduced by 18% after bariatric surgery, and obesity-related cancer risk fell by 25%.
Conclusions
The challenge of NASH remains to license a treatment for this increasingly prevalent and important condition. There is no shortage of candidate drugs, but difficulties in diagnosis, staging and monitoring the effects of treatment have added unprecedented complexity to the field.
Technologies to develop accurate, specific and meaningful imaging and circulating biomarkers, along with improvements in reading of biopsies, should allow for better interpretation of drug trial results and likely new drug licenses. Meantime, it is important not to forget the real differences that lifestyle measures can make to NASH. This is the reason why it is so important to screen patients at risk.
Acknowledgments
This paper was written following a virtual round table event organised by BMJ Journals, supported by an unrestricted educational grant from Intercept Pharmaceuticals and Siemens Healthineers. The sponsors had no control or influence over the selection of speakers, the round table or the writing of the paper. Anna Sayburn, an independent professional medical writer, facilitated the meeting and provided medical writing support.
Footnotes
Twitter: @dufour_jf
Correction notice: This article has been corrected since it published Online First. Typographical errors have been corrected in the abstract.
Contributors: J-FD elaboration and coordination, writing specific sections and critical review of the manuscript. All the others writing of specific sections and critical review of the manuscript.
Funding: RL receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019). Other authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: J-FD: Advisory committees : Abbvie, Alientis, Allergan, Axcella, Bayer, Bristol-Myers Squibb, Eisai, Enyo Pharma, Falk, Genfit, Gilead Sciences, HepaRegeniX, Intercept, Ipsen, Inventiva, Madrigal, Merck, Novartis, Pfizer, Roche, Trilliome DTx. Speaking and teaching : Bayer, Intercept, Genfit. Royalties : BMJ Journals, Springer, UpToDate. Quentin Anstee: Research Grant Funding: Abbvie, Allergan/Tobira, AstraZeneca, GlaxoSmithKline, GlympseBio, Novartis Pharma AG, Pfizer. Active Research Collaborations (including research supported through the EU IMI2 LITMUS Consortium*). Abbvie, Antaros Medical*, Allergan/Tobira, AstraZeneca, Boehringer Ingelheim International*, Ellegaard Gottingen Minipigs AS*, Eli Lilly & Company*, Exalenz Bioscience*, Genfit SA*, GlaxoSmithKline, HistoIndex, Intercept Pharma Europe*, iXscient*, Nordic Bioscience*, Novartis Pharma AG*, Novo Nordisk A/S*, One Way Liver Genomics SL*, Perspectum Diagnostics*, Pfizer.*, Sanofi-Aventis Deutschland*, SomaLogic*, Takeda Pharmaceuticals International SA*. Consultancy (undertaken on behalf of Newcastle University): Abbott Laboratories, Acuitas Medical, Allergan/Tobira, E3Bio, EcoR1, Eli Lilly & Company, Galmed, Genfit SA, Gilead, Grunthal, HistoIndex, Imperial Innovations, Intercept Pharma Europe, Inventiva, IQVIA, Janssen, Madrigal, MedImmune, NewGene, NGMBio, Novartis, Novo Nordisk A/S, Pfizer, Poxel, Raptor Pharma, Servier, Viking. Speaker: Abbott Laboratories, Allergan/Tobira, BMS, Clinical Care Options, Falk, Genfit SA, Gilead, Kenes. Elisabetta Bugianesi: Consultant: Gilead Sciences, Intercept, BMS, NovoNordisk, Pfeizer, Inventiva, Genfit, Lilly, MSD. Stephen Harrison: Scientific advisor or consultant for Akero, Alentis, Altimmune, Arrowhead, Axcella, Cirius, Cymabay, Echosens, Fibronostics, Forest Labs, Galectin, Genfit, Gilead, Hepagene, Hepion, HistoIndex, Intercept, Madrigal, Medpace, Metacrine, NGM Bio, Northsea, Novo Nordisk, PathAI, Poxel, Sagimet, Terns, Viking, 89 Bio. Stock options: Akero, Cirius, Galectin, Genfit, Hepion, HistoIndex, PathAI, Metacrine, NGM Bio, Northsea. Grant/Research support: Akero, Axcella, BMS, Cirius, CiVi Biopharma, Conatus, Cymabay, Enyo, Galectin, Genentech, Genfit, Gilead, Hepion, Hightide, Intercept, Madrigal, Metacrine, NGM Bio, Novartis, Novo Nordisk, Northsea, Pfizer, Sagimet, Viking. Rohit Loomba: Research supported by PI, R01DK106419, NIDDK, NIH; PI, R01DK121378, NIDDK, NIH; PI, R01 DK124318, NIDDK, NIH; PI, U01, NASH-CRN, NIDDK, NIH; PI, U01, AA029019, NIAAA, NIH; Project PI, P01HL147835, NHLBI; Investigator Initiated Research Grant, Astrazeneca; Investigator initiated Research Grant, Gilead Inc. Investigator initiated Research grant, Janssen Inc. Valérie Paradis: Consultant: Inventiva; Teaching: Novartis. Vincent Wong: Consultancy: 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Echosens, Gilead Sciences, Inventiva, Merck, Novartis, Novo Nordisk, Pfizer, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, Terns. Lectures: Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk. Research grants: Gilead Sciences Stock: Co-founder of Illuminatio Medical Technology.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Younossi ZM, Stepanova M, Ong J, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2021;19:580–9. 10.1016/j.cgh.2020.05.064 [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 3. NCD Risk Factor Collaboration (NCD-RisC) . Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–96. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dufour JF, Scherer R, Balp M-M. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors– a targeted literature review. Endocrine and Metabolic Science 2021;3:100089. [Google Scholar]
- 5. Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4:389–98. 10.1016/S2468-1253(19)30039-1 [DOI] [PubMed] [Google Scholar]
- 6. Kwok R, Choi KC, Wong GL-H, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016;65:1359–68. 10.1136/gutjnl-2015-309265 [DOI] [PubMed] [Google Scholar]
- 7. Wong VW-S, Chan W-K, Chitturi S, et al. Asia-Pacific Working Party on non-alcoholic fatty liver disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018;33:70–85. 10.1111/jgh.13857 [DOI] [PubMed] [Google Scholar]
- 8. Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:739–52. 10.1016/S2468-1253(20)30077-7 [DOI] [PubMed] [Google Scholar]
- 9. Wong VW-S, Wong GL-H, Yeung DK-W, et al. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol 2015;62:182–9. 10.1016/j.jhep.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 10. Lee HW, Wong GL-H, Kwok R, et al. Serial transient elastography examinations to monitor patients with type 2 diabetes: a prospective cohort study. Hepatology 2020;72:1230–41. 10.1002/hep.31142 [DOI] [PubMed] [Google Scholar]
- 11. European association for the study of the liver, European association for the study of diabetes, European association for the study of obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 12. Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657–69. 10.1053/j.gastro.2021.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Younossi Z, Stepanova M, Ong JP. Global nonalcoholic steatohepatitis Council. nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748–55. [DOI] [PubMed] [Google Scholar]
- 14. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896–904. 10.1016/j.jhep.2018.05.036 [DOI] [PubMed] [Google Scholar]
- 15. Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a Multi-National cohort study. Gastroenterology 2018;155:443–57. 10.1053/j.gastro.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 16. NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017;390:2627–42. 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–9. 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 18. Wong VW-S, Wong GL-H, Woo J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol 2021;19:2161–71. 10.1016/j.cgh.2020.10.046 [DOI] [PubMed] [Google Scholar]
- 19. Tilg, H. and Moschen, a. R., Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010;52:1836–46. [DOI] [PubMed] [Google Scholar]
- 20. Febbraio MA, Karin M. "Sweet death": Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab 2021;33:2316–28. 10.1016/j.cmet.2021.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trépo E, Valenti L. Update on NAFLD genetics: from new variants to the clinic. J Hepatol 2020;72:1196–209. 10.1016/j.jhep.2020.02.020 [DOI] [PubMed] [Google Scholar]
- 22. Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab 2008;19:371–9. 10.1016/j.tem.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 23. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 2017;14:32–42. 10.1038/nrgastro.2016.147 [DOI] [PubMed] [Google Scholar]
- 24. Dulai PS, Singh S, Patel J. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611–25. 10.1053/j.gastro.2020.01.043 [DOI] [PubMed] [Google Scholar]
- 26. Moschen AR, Molnar C, Enrich B, et al. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol Med 2011;17:840–5. 10.2119/molmed.2010.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamari Y, Shaish A, Vax E, et al. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol 2011;55:1086–94. 10.1016/j.jhep.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiechl S, Wittmann J, Giaccari A, et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med 2013;19:358–63. 10.1038/nm.3084 [DOI] [PubMed] [Google Scholar]
- 29. Straub LG, Scherer PE. Metabolic messengers: adiponectin. Nat Metab 2019;1:334–9. 10.1038/s42255-019-0041-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radun R, Trauner M. Role of FXR in bile acid and metabolic homeostasis in NASH: pathogenetic concepts and therapeutic opportunities. Semin Liver Dis 2021;41:461–75. 10.1055/s-0041-1731707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geier A, Tiniakos D, Denk H, et al. From the origin of NASH to the future of metabolic fatty liver disease. Gut 2021;70:1570–9. 10.1136/gutjnl-2020-323202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gadaleta RM, Moschetta A. Metabolic messengers: fibroblast growth factor 15/19. Nat Metab 2019;1:588–94. 10.1038/s42255-019-0074-3 [DOI] [PubMed] [Google Scholar]
- 33. Loomba R, Seguritan V, Li W, et al. Gut Microbiome-Based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017;25:1054–62. 10.1016/j.cmet.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. TG O, Kim SM, Caussy C. A universal Gut-Microbiome-Derived signature predicts cirrhosis. Cell Metab 2020;32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Demir M, Lang S, Hartmann P, et al. The fecal mycobiome in non-alcoholic fatty liver disease. J Hepatol 2022;76:788–99. 10.1016/j.jhep.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016;535:376–81. 10.1038/nature18646 [DOI] [PubMed] [Google Scholar]
- 37. Mantovani A, Petracca G, Beatrice G, et al. Non-Alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut 2022;71:778–88. 10.1136/gutjnl-2021-324191 [DOI] [PubMed] [Google Scholar]
- 38. Targher G, Tilg H, Byrne CD. Non-Alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol 2021;6:578–88. 10.1016/S2468-1253(21)00020-0 [DOI] [PubMed] [Google Scholar]
- 39. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 40. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 41. Bedossa P, Poitou C, Veyrie N, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012;56:1751–9. 10.1002/hep.25889 [DOI] [PubMed] [Google Scholar]
- 42. Rastogi A, Shasthry SM, Agarwal A, et al. Non-alcoholic fatty liver disease - histological scoring systems: a large cohort single-center, evaluation study. APMIS 2017;125:962–73. 10.1111/apm.12742 [DOI] [PubMed] [Google Scholar]
- 43. Bedossa P, FLIP Pathology Consortium . Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014;60:565–75. 10.1002/hep.27173 [DOI] [PubMed] [Google Scholar]
- 44. Davison BA, Harrison SA, Cotter G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol 2020;73:1322–32. 10.1016/j.jhep.2020.06.025 [DOI] [PubMed] [Google Scholar]
- 45. Forlano R, Mullish BH, Giannakeas N, et al. High-Throughput, machine Learning-Based quantification of steatosis, inflammation, ballooning, and fibrosis in biopsies from patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2020;18:2081–90. 10.1016/j.cgh.2019.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taylor-Weiner A, Pokkalla H, Han L, et al. A machine learning approach enables quantitative measurement of liver histology and disease monitoring in NASH. Hepatology 2021;74:133–47. 10.1002/hep.31750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harrison SA, Ratziu V, Boursier J, et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:970–85. 10.1016/S2468-1253(20)30252-1 [DOI] [PubMed] [Google Scholar]
- 48. Vali Y, et al. PO-919 comparative diagnostic accuracy of blood-based biomarkers for diagnosing NASH: phase 1 results of the litmus project. Easl Ilc 2021. [Google Scholar]
- 49. Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:e1612:1611–25. 10.1053/j.gastro.2020.01.043 [DOI] [PubMed] [Google Scholar]
- 50. Dyson JK, McPherson S, Anstee QM. Non-Alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol 2013;66:1033–45. 10.1136/jclinpath-2013-201620 [DOI] [PubMed] [Google Scholar]
- 51. McPherson S, Hardy T, Dufour J-F, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740–51. 10.1038/ajg.2016.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 53. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. 10.1002/hep.21496 [DOI] [PubMed] [Google Scholar]
- 54. McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. 10.1136/gut.2010.216077 [DOI] [PubMed] [Google Scholar]
- 55. Younossi ZM, et al. Poster presented at the annual meeting of American association for the study of liver disease. San Francisco, CA; November 9-13, 2018. Abstract 181.
- 56. Mózes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006-1019. 10.1136/gutjnl-2021-324243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong VW-S, Vergniol J, Wong GL-H, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454–62. 10.1002/hep.23312 [DOI] [PubMed] [Google Scholar]
- 58. Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-Controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:156–63. 10.1016/j.cgh.2018.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–30. 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 60. Vali Y, Lee J, Boursier J, et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J Hepatol 2020;73:252–62. 10.1016/j.jhep.2020.03.036 [DOI] [PubMed] [Google Scholar]
- 61. Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut 2018;67:6–19. 10.1136/gutjnl-2017-314924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boyle M, Tiniakos D, Schattenberg JM. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Reports 2019;1:188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Daniels SJ, Leeming DJ, Eslam M. ADAPT: an algorithm incorporating pro-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology 2019;69:1075–86. [DOI] [PubMed] [Google Scholar]
- 64. Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol 2019;71:371–8. 10.1016/j.jhep.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 65. Lee J, et al. OS-243 comparative diagnostic accuracy of blood-based biomarkers for staging fibrosis in NAFLD: phase 1 results of the litmus project. EASL 2021. [Google Scholar]
- 66. Mayo R, Crespo J, Martínez-Arranz I, et al. Metabolomic-Based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun 2018;2:807–8. 10.1002/hep4.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Govaere O, Cockell S, Tiniakos D, et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci Transl Med 2020;12:eaba4448. 10.1126/scitranslmed.aba4448 [DOI] [PubMed] [Google Scholar]
- 68. Cazanave SC, Warren AD, Pacula M, et al. Peptide-Based urinary monitoring of fibrotic nonalcoholic steatohepatitis by mass-barcoded activity-based sensors. Sci Transl Med 2021;13:eabe8939. 10.1126/scitranslmed.abe8939 [DOI] [PubMed] [Google Scholar]
- 69. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol 2016;65:1006–16. 10.1016/j.jhep.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol 2018;15:274–82. 10.1038/nrgastro.2018.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol 2016;65:570–8. 10.1016/j.jhep.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 72. Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014;60:1920–8. 10.1002/hep.27362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362–73. 10.1016/S2468-1253(19)30383-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Noureddin N, Alkhouri N, Brown KA, et al. Driving nonalcoholic steatohepatitis forward using the FibroScan aspartate aminotransferase score, but obey the traffic lights. Hepatology 2020;72:2228–30. 10.1002/hep.31498 [DOI] [PubMed] [Google Scholar]
- 75. Jung J, Loomba RR, Imajo K, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2021;70:1946–53. 10.1136/gutjnl-2020-322976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tamaki N, Higuchi M, Kurosaki M, et al. Risk difference of Liver-Related and cardiovascular events by liver fibrosis status in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2022;20:1171-1173.e2. 10.1016/j.cgh.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol 2019;17:630–7. 10.1016/j.cgh.2018.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stine JG, Munaganuru N, Barnard A, et al. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021;19:2274–83. 10.1016/j.cgh.2020.08.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2016;9:692–701. 10.1177/1756283X16656735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yaskolka Meir A, Rinott E, Tsaban G, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut 2021;70:2085–95. 10.1136/gutjnl-2020-323106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salomone F, Ivancovsky-Wajcman D, Fliss-Isakov N, et al. Higher phenolic acid intake independently associates with lower prevalence of insulin resistance and non-alcoholic fatty liver disease. JHEP Rep 2020;2:100069. 10.1016/j.jhepr.2020.100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ahn J, Jun DW, Lee HY, et al. Critical appraisal for low-carbohydrate diet in nonalcoholic fatty liver disease: review and meta-analyses. Clin Nutr 2019;38:2023–30. 10.1016/j.clnu.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 83. Yki-Järvinen H, Luukkonen PK, Hodson L, et al. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2021;18:770–86. 10.1038/s41575-021-00472-y [DOI] [PubMed] [Google Scholar]
- 84. Luukkonen PK, Sädevirta S, Zhou Y, et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 2018;41:1732–9. 10.2337/dc18-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Monteiro CA, Cannon G, Moubarac J-C, et al. The un decade of nutrition, the nova food classification and the trouble with ultra-processing. Public Health Nutr 2018;21:5–17. 10.1017/S1368980017000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Levy RB, Rauber F, Chang K, et al. Ultra-processed food consumption and type 2 diabetes incidence: A prospective cohort study. Clin Nutr 2021;40:3608–14. 10.1016/j.clnu.2020.12.018 [DOI] [PubMed] [Google Scholar]
- 87. Moradi S, Entezari MH, Mohammadi H, et al. Ultra-processed food consumption and adult obesity risk: a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr 2021:1–12. 10.1080/10408398.2021.1946005 [DOI] [PubMed] [Google Scholar]
- 88. Ivancovsky-Wajcman D, Fliss-Isakov N, Webb M, et al. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int 2021;41:2635–45. 10.1111/liv.14996 [DOI] [PubMed] [Google Scholar]
- 89. Golovaty I, Tien PC, Price JC, et al. Food insecurity may be an independent risk factor associated with nonalcoholic fatty liver disease among low-income adults in the United States. J Nutr 2020;150:91–8. 10.1093/jn/nxz212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Geurtsen ML, Santos S, Gaillard R, et al. Associations between intake of sugar-containing beverages in infancy with liver fat accumulation at school age. Hepatology 2021;73:560–70. 10.1002/hep.31611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Simon TG, Kim MN, Luo X, et al. Physical activity compared to adiposity and risk of liver-related mortality: results from two prospective, nationwide cohorts. J Hepatol 2020;72:1062–9. 10.1016/j.jhep.2019.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim D, Vazquez-Montesino LM, Li AA, et al. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology 2020;72:1556–68. 10.1002/hep.31158 [DOI] [PubMed] [Google Scholar]
- 93. Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011;54:344–53. 10.1002/hep.24376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang Y-X. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res 2010;20:124–37. 10.1038/cr.2010.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Francque SM, Bedossa P, Ratziu V, et al. A randomized, controlled trial of the pan-PPAR agonist Lanifibranor in NASH. N Engl J Med Overseas Ed 2021;385:1547–58. 10.1056/NEJMoa2036205 [DOI] [PubMed] [Google Scholar]
- 96. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013;17:819–37. 10.1016/j.cmet.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 97. Armstrong MJ, Hull D, Guo K, et al. Glucagon-Like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol 2016;64:399–408. 10.1016/j.jhep.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (lean): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. The Lancet 2016;387:679–90. 10.1016/S0140-6736(15)00803-X [DOI] [PubMed] [Google Scholar]
- 99. Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous Semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113–24. 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- 100. Sinha RA, Bruinstroop E, Singh BK, et al. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid 2019;29:1173–91. 10.1089/thy.2018.0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet 2019;394:2012–24. 10.1016/S0140-6736(19)32517-6 [DOI] [PubMed] [Google Scholar]
- 102. Harrison S, et al. MAESTRO-NAFLD-1: Resmetirom (100 Mg) reduces liver fat (MRI-PDFF) and liver stiffness (MRE). Abstract 2563, EASL 2021.
- 103. Sun L, Cai J, Gonzalez FJ. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol 2021;18:335–47. 10.1038/s41575-020-00404-2 [DOI] [PubMed] [Google Scholar]
- 104. Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184–96. 10.1016/S0140-6736(19)33041-7 [DOI] [PubMed] [Google Scholar]
- 105. Ratziu V, de Guevara L, Safadi R, et al. Aramchol in patients with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2B trial. Nat Med 2021;27:1825–35. 10.1038/s41591-021-01495-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. FDA . Draft guidance. noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment guidance for industry; 2018.
- 107. Sanyal. AASLD 2020. Abstr 90.
- 108. Lassailly G, Caiazzo R, Ntandja-Wandji L-C, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology 2020;159:1290–301. 10.1053/j.gastro.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 109. Kleiner DE, Brunt EM, Wilson LA, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2019;2:e1912565. 10.1001/jamanetworkopen.2019.12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: current and emerging. J Hepatol 2018;68:362–75. 10.1016/j.jhep.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 111. Harrison SA, Ruane PJ, Freilich BL, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2A trial. Nat Med 2021;27:1262–71. 10.1038/s41591-021-01425-3 [DOI] [PubMed] [Google Scholar]
- 112. Harrison SA, Neff G, Guy CD, et al. Efficacy and safety of Aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology 2021;160:219–31. 10.1053/j.gastro.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 113. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–97. 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kim G-A, Lee HC, Choe J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol 2017;S0168-8278:32294–8. 10.1016/j.jhep.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 115. Liu S-S, Ma X-F, Zhao J, et al. Association between nonalcoholic fatty liver disease and extrahepatic cancers: a systematic review and meta-analysis. Lipids Health Dis 2020;19:118. 10.1186/s12944-020-01288-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kim G-A, Lee HC, Choe J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol 2017. 10.1016/j.jhep.2017.09.012. [Epub ahead of print: 02 Nov 2017]. [DOI] [PubMed] [Google Scholar]
- 117. Allen AM, Hicks SB, Mara KC, et al. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - A longitudinal cohort study. J Hepatol 2019;71:1229–36. 10.1016/j.jhep.2019.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pinyol R, Torrecilla S, Wang H, et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol 2021;75:865–78. 10.1016/j.jhep.2021.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1573–84. 10.1016/j.cgh.2013.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sanyal A, Poklepovic A, Moyneur E, et al. Population-Based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 2010;26:2183–91. 10.1185/03007995.2010.506375 [DOI] [PubMed] [Google Scholar]
- 121. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018;155:1828–37. 10.1053/j.gastro.2018.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 2016;63:827–38. 10.1002/hep.28368 [DOI] [PubMed] [Google Scholar]
- 123. Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021;592:450–6. 10.1038/s41586-021-03362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Singh S, Singh PP, Singh AG, et al. Anti-Diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013;108:881–91. 10.1038/ajg.2013.5 [DOI] [PubMed] [Google Scholar]
- 125. Simon TG, Duberg A-S, Aleman S, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med 2019;171:318–27. 10.7326/M18-2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Simon TG, Duberg A-S, Aleman S, et al. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med Overseas Ed 2020;382:1018–28. 10.1056/NEJMoa1912035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rustgi VK, Li Y, Gupta K, et al. Bariatric surgery reduces cancer risk in adults with nonalcoholic fatty liver disease and severe obesity. Gastroenterology 2021;161:171–84. 10.1053/j.gastro.2021.03.021 [DOI] [PubMed] [Google Scholar]
- 128. Sheth SG, Flamm SL, Gordon FD, et al. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998;93:44–8. 10.1111/j.1572-0241.1998.044_c.x [DOI] [PubMed] [Google Scholar]
- 129. Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008;57:1441–7. 10.1136/gut.2007.146019 [DOI] [PubMed] [Google Scholar]
- 130. Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–12. 10.1016/j.cgh.2009.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mózes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006–19. 10.1136/gutjnl-2021-324243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology 2008;47:455–60. 10.1002/hep.21984 [DOI] [PubMed] [Google Scholar]
- 133. Ratziu V, Massard J, Charlotte F, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6:6. 10.1186/1471-230X-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vali Y, Lee J, Boursier J, et al. FibroTest for evaluating fibrosis in non-alcoholic fatty liver disease patients: a systematic review and meta-analysis. J Clin Med 2021;10. 10.3390/jcm10112415. [Epub ahead of print: 29 05 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009;50:165–73. 10.1016/j.jhep.2008.07.035 [DOI] [PubMed] [Google Scholar]
- 136. Van Dijk A-M, Vali Y, Mak AL, et al. Systematic review with meta-analyses: diagnostic accuracy of FibroMeter tests in patients with non-alcoholic fatty liver disease. J Clin Med 2021;10. 10.3390/jcm10132910. [Epub ahead of print: 29 06 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Daniels SJ, Leeming DJ, Eslam M, et al. ADAPT: an algorithm incorporating pro-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology 2019;69:1075–86. 10.1002/hep.30163 [DOI] [PubMed] [Google Scholar]
- 138. Boyle M, Tiniakos D, Schattenberg JM, et al. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Rep 2019;1:188–98. 10.1016/j.jhepr.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mak AL, Lee J, van Dijk A-M, et al. Systematic review with meta-analysis: diagnostic accuracy of pro-C3 for hepatic fibrosis in patients with non-alcoholic fatty liver disease. Biomedicines 2021;9. 10.3390/biomedicines9121920. [Epub ahead of print: 15 12 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]