Abstract
Objectives
To study pregnancy outcomes in a closely monitored, well-defined cohort of women with rheumatoid arthritis (RA). In particular, pregnancy outcomes of women that used a TNFi during pregnancy.
Methods
Patients were derived from a prospective study on pregnancy and RA (Preconception Counseling in Active RA study) and treated according to a treatment protocol aimed at minimal disease activity. Multivariate linear regression analysis was used to describe which variables influenced birth weight.
Results
188 patients were included, 92 (48.9%) patients with RA used a TNFi during pregnancy. Disease Activity Score in 28 joints C reactive protein (DAS28CRP) was low at all time points during pregnancy (DAS28CRP in the third trimester: 2.17 (SD 0.73). TNFi use was not associated with an increase of adverse pregnancy outcomes such as low birth weight (<2500 g), (emergency) caesarian section, hypertensive disorders or congenital malformations. TNFi use resulted in less children born small-for-gestational age (p=0.05), however, did not increase the risk of large-for-gestational age (p=0.73). Mean birth weight was 173 g higher in women that used a TNFi during pregnancy (3.344 kg vs 3.171 kg, p=0.03). In the multivariate analysis, maternal age (β −0.023, 95% CI −0.040 to –0.0065, p=0.007), TNFi use (β 0.20, 95% CI 0.066, 0.34, p=0.004), diabetes mellitus (β 0.37, 95% CI 0.12, 0.63, p=0.004) and gestational age (β 0.18, 95% CI 0.15, 0.2, p<0.001) were statistically significant associated with birth weight.
Conclusions
This is the first study to show that TNFi use during pregnancy is associated with increased birth weight of offspring of women with well-controlled RA. The underlying mechanism of TNF-inhibition on birth weight and the long-term consequences for the offspring should be explored in future research.
Keywords: Arthritis, Rheumatoid; Tumor Necrosis Factor Inhibitors; Certolizumab pegol
WHAT IS ALREADY KNOWN ON THIS TOPIC
Although tumour necrosis factor-inhibitors use (TNFi) during pregnancy is not associated with an increased risk of congenital malformations, data on other pregnancy outcomes is contradictory.
WHAT THIS STUDY ADDS
The current study is the first to show that the use of TNFi during pregnancy in women with rheumatoid arthritis (RA) is associated with increased birth weight of the offspring after correcting for all relevant confounders and less children with small for gestational age.
TNFi use during pregnancy in women with RA does not increase the risk of adverse pregnancy outcomes such as prematurity, low birth weight, hypertensive disorders and emergency caesarean section.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The exact mechanisms behind the increase in birth weight after TNFi use during pregnancy should be the focus of future studies.
TNFi might be a future therapeutic option in preventing and treatment of intrauterine growth restriction.
The long-term effects of the increase in birth weight on the offspring should be further explored.
Introduction
Rheumatoid arthritis (RA) is one of the most common chronic diseases in women in the reproductive age.1 Pregnancy outcomes, like small-for-gestational age (SGA) and hypertensive disorders, in women with RA are impaired compared with healthy women, especially in women with active disease during pregnancy.1 In recent years, more treatment options during pregnancy became available, including tumour necrosis factor (TNF)-inhibitors (TNFi), resulting in improved disease outcomes in women with RA during pregnancy.2 Therefore, TNFi are now important in the management of RA during pregnancy.2
Research on pregnancy outcomes after TNFi exposure during pregnancy have mainly focused on exposure to TNFi’s in the first trimester and congenital malformations.1 Multiple studies have shown that TNFi do not increase the risk of birth defects.3 However data on other pregnancy outcomes is contradictory, some studies report that TNFi are associated with increased risks of preterm birth, caesarean section (CS), low birth weight and SGA whereas others do not.2–5
These diverse findings across different studies may however indicate an association related to confounding variables, such as underlying disease, disease activity and the use of other certain medication (eg, glucocorticoids), rather than to the TNFi itself. Moreover, the use of a TNFi has an impact on these other variables by decreasing disease activity6 and preventing the need for use of glucocorticoids.7 A majority of previous studies that examined associations between TNFi use and pregnancy outcomes were performed in patients with different underlying diseases and without information on disease activity, making it difficult to interpret associations between TNFi use and pregnancy outcomes.
In the Preconception Counseling in Active RA (PreCARA) study, women with RA were prospectively followed-up, closely monitored and treated during pregnancy according to a modern treatment approach aimed at minimal disease activity including the use of TNFi.2 The objective of the current study was to describe pregnancy outcomes of offspring born to patients with RA included in the PreCARA-cohort, and in particular to describe pregnancy outcomes of women that used a TNFi during pregnancy when correcting for relevant confounders.
Methods
Patient population
Patients were included in the PreCARA-study (2011–ongoing).2 The PreCARA study is an ongoing, prospective cohort study on inflammatory rheumatic diseases before and during pregnancy. Data up to May 2021 was used for the current manuscript. The PreCARA study (ClinicalTrials.gov reference NCT01345071) is solely performed in the Erasmus MC, a tertiary referral hospital (Rotterdam, the Netherlands). All patients in this hospital are treated within a dedicated specialised healthcare pathway for rheumatic diseases during pregnancy.
PreCARA treatment protocol
The PreCARA-treatment protocol was extensively described previously.2 In brief, patients in the PreCARA cohort were treated according to a modified treat-to-target approach aimed at minimal disease activity. Treatment was, if needed, intensified at every study visit. If treatment was intensified in the PreCARA-protocol, first, sulfasalazine and/or hydroxychloroquine were started, followed by the addition of prednisone (preferably in a maximum daily dosage of 7.5 milligram) and/or a TNFi, preferably certolizumab-pegol. Patients could get pregnant using the TNFi which they used when they enrolled in the study. During pregnancy, TNFi was stopped at the gestational age (GA) as recommended by the European-Alliance-of-Associations-for-Rheumatology: adalimumab and infliximab were stopped at GA 20 weeks, etanercept at GA 28–32,8 certolizumab-pegol was discontinued at GA 38 weeks to prevent maternal infections during delivery, based on expert opinion. After stopping a TNFi a switch to certolizumab-pegol or prednisone was considered.
Data collection
Participants entered the PreCARA-study preferably before they got pregnant. Visits were scheduled every 3 months before conception, in the first, second and third trimester of pregnancy and at 6, 12 and 26 weeks after delivery. At every visit, patients were seen by their rheumatologist and a rheumatology nurse, they underwent joint examination, filled in questionnaires electronically (including on frequencies and dosages of conventional synthetic DMARDs and biologic DMARDs) and a blood sample was collected.
Information on characteristics such as smoking, body mass index (BMI), previous pregnancies, presence of rheumatoid factor (RF) or anticitrullinated protein antibodies (ACPA), medical history, education, race and previous medication use were collected at inclusion.
Data on pregnancy outcome included birth weight, GA at delivery (as calculated by their attending gynaecologist and/or midwife), sex of the child and mode and location of the delivery. Information on the mode of delivery was categorised to spontaneous birth, induced birth or CS. CSs were recorded as elective or emergency. Pregnancy complications were collected as well: premature birth (delivery at <37 weeks (259 days) of the GA, low birth weight (<2500 g), high birth weight (>4000 g), hypertensive disorders (gestational hypertension and preeclampsia, both as reported by their attending gynaecologist), Diabetes mellitus (DM) (analysed as both preexisting DM and gestational DM combined) and congenital malformations. These data was collected at various visits during pregnancy and postpartum (by interview, questionnaires and careful investigation of the electronic medical charts). If patients gave birth outside our tertiary centre, data on pregnancy outcomes was collected from their attending physicians.
Data analysis
Disease activity was calculated using the Disease Activity Score in 28 joints (DAS28)9 10: the DAS28 using three variables: the number of swollen joints, the number of tender joints, and the C reactive protein (CRP) level (DAS28CRP),11 this disease activity measure is validated for use during pregnancy.12
Birth weight SDS were calculated using the sex-specific formulas as previously described in literature,13 infants SGA (birth weight <p10) and large for GA (LGA) (birth weight >p90) were determined based on Dutch growth charts.14 For the current analysis, pregnancies carried beyond week 20 were included in the study, twin pregnancies and diagnosis other than RA were excluded.
Statistical analysis
Descriptive statistics are presented as numbers (n) and percentages (%). Continuous variables are given as mean±SD or median ±IQR as appropriate. We tested categorical data using χ2 and Fisher’s exact tests. Continuous data were checked for the distribution of the data and analysed using (paired) t-test and Wilcoxon-rank as appropriate. We considered a two-sided p<0.05 significant.
To describe which variables influenced birth weight, univariate and multivariate linear regression were performed. The following covariates were considered: disease activity (DAS28CRP), GA at delivery, maternal age, prednisone use during pregnancy, TNFi use during pregnancy, nulliparity, pregnancy through assisted reproductive technology (combined: ovulation induction, intrauterine insemination in vitro fertilisation and intracytoplasmic sperm injection), smoking, (gestational) DM. An interaction between TNFi use and disease activity and TNFi use and prednisone use were considered in the multivariate analysis separately.
Kaplan-Meier survival analysis was performed to visualise whether GA at delivery was dependent on TNFi use during pregnancy, significance was tested using the Wilcoxon-Gehan statistic.
Statistical analyses were performed using Stata V.17 by StataCorp. Patient involvement and ethics are provided in online supplemental file 1.
ard-2022-222679supp001.pdf (210.8KB, pdf)
Results
An overview of patients included in the PreCARA study (n=188) is presented in table 1. 48.9% of the women with RA used a TNFi during pregnancy. Disease activity was low at all pregnancy trimesters, for example DAS28CRP in the third trimester of pregnancy: 2.17 (SD 0.73). 119 (63.3%) women were included before there were pregnant.
Table 1.
Clinical and demographic features from patients with RA within the PreCARA cohort (n=188) who conceived
| PreCARA-cohort (n=188) | TNFi use during pregnancy (n=92) | No TNFi use during pregnancy (n=96) | P value, for difference yes/no TNFi use during pregnancy | |
| Mean age at delivery, years (SD) | 32.6 (4.0) | 33.3 (3.9) | 31.9 (4.1) | 0.023 |
| Median disease duration at first visit, years (IQR) | 6.8 (3.6–10.9) | 6.0 (2.9–10.7) | 7.3 (4.1–11.5) | 0.21 |
| Rheumatoid factor positive, n (%) | 130/186 (69.9) | 67/90 (74.4) | 63 (65.6) | 0.19 |
| ACPA positive, n (%) | 129/184 (70.1) | 70/90 (77.8) | 59/94 (62.8) | 0.026 |
| Caucasian race, n (%) | 157 (83.5) | 77 (83.7) | 80 (83.3) | 0.94 |
| Nulliparity, n (%) | 100 (53.2) | 42 (45.7) | 58 (60.4) | 0.043 |
| Education level, median no of years of education (IQR) | 16 (14–18) | 16 (14–18) | 17 (14–18) | 0.78 |
| Conception through assisted reproduction technique, n (%) | 26/185 (14.1) | 12/90 (13.3) | 14/95 (14.7) | 0.78 |
| DAS28CRP in the first trimester of pregnancy (SD)* | 2.21 (0.80) | 2.18 (0.81) | 2.24 (0.79) | 0.64 |
| DAS28CRP in the second trimester of pregnancy (SD)* | 2.30 (0.77) | 2.35 (0.85) | 2.24 (0.67) | 0.34 |
| DAS28CRP in the third trimester of pregnancy (SD)* | 2.17 (0.73) | 2.22 (0.70) | 2.14 (0.76) | 0.49 |
| DAS28CRP<2,6 1 st trimester, n (%) | 120/161 (74.5) | 59/79 (74.7) | 61/82 (74.4) | 0.96 |
| DAS28CRP<2,6 2 nd trimester, n (%) | 130/178 (73.0) | 63/89 (70.8) | 67/89 (75.3) | 0.49 |
| DAS28CRP<2,6 3 rd trimester, n (%) | 130/169 (80.7) | 59/84 (70.2) | 71/85 (83.5) | 0.04 |
| Smoking during pregnancy, n (%) | 6 (3.2) | 3 (3.3) | 3 (3.1) | 0.96 |
| Medication use during pregnancy (any use), n (%)†: | ||||
|
105 (55.9) 110 (58.5) 79 (42.0) 92 (48.9) |
45 (48.9) 52 (65.5) 42 (45.7) 92 (100) |
60 (62.5) 58 (60.4) 37 (38.5) |
0.061 0.58 0.32 |
|
62 (33.0) 8 (4.3) 25 (13.3) 12 (6.4) |
62 (67.4) 8 (8.7) 25 (27.2) 12 (13.0) |
Bold values denote statistical significance at the p < 0.05 level.
*Number of missing data for disease activity: 27/188 (14.4%) trimester 1, 10/188 (5.3%) trimester 2, 19/188 (10.1%) trimester 3.
†Either alone or in combination with other medication.
‡The sum of TNFi exceeds 100%, because some patients switched from etanercept, adalimumab or infliximab to certolizumab-pegol during pregnancy.
ACPA, anticitrullinated protein antibody; DAS28CRP, Disease Activity Score in 28 joints C reactive protein; PreCARA, Preconception Counseling in Active Rheumatoid Arthritis.
Maternal age, the number of patients with ACPA-antibodies and nulliparity were statistically significant different between women that used a TNFi during pregnancy and women who did not.
Pregnancy outcomes
Pregnancy outcomes are shown in table 2. Pregnancy cholestasis was observed in 2 (1.1%) women. One patient developed a villoglandular carcinoma of the cervix during pregnancy. Twelve out of a total of 49 CS (24.5%) were emergency procedures, children born via an emergency CS had a median APGAR score after 5 min of 9 (range: 2–10).
Table 2.
Pregnancy outcomes of women with RA included in the PreCARA cohort (n=188) that conceived stratified for (any) TNFi use during pregnancy
| PreCARA-cohort (n=188) |
TNFi use during pregnancy (n=92) | No TNFi use during pregnancy (n=96) | P value, for difference yes/no TNFi use during pregnancy | |
| Sex of the child (male), n (%) | 95 (50.8) | 53 (57.6) | 42/95 (44.2) | 0.07 |
| Birth weight, kg (SD) | 3.256 (0.56) | 3.344 (0.51) | 3.171 (0.59) | 0.03 |
| Gestational age at delivery, weeks (IQR) | 39.1 (37.8–40.1) | 39.0 (38–39.9) | 39.2 (37.7–40.4) | 0.53 |
| Birth weight SDS (SD) | - 0.096 (1.04) | 0.064 (0.99) | - 0.25 (1.06) | 0.04 |
| SGA (birth weight <p10), n (%) | 28/187 (15.0) | 9/92 (9.8) | 19/95 (20.0) | 0.05 |
| LGA (birth weight >p90), n (%) | 13/187 (7.0) | 7/92 (7.6) | 6/95 (6.3) | 0.73 |
| APGAR score (IQR) | 10 (9–10) | 10 (9–10) | 10 (9–10) | 0.99 |
| Location of delivery, n (%) | ||||
|
177/185 (95.7) 8/185 (4.3) |
89/91 (97.8) 2/91 (2.2) |
88/94 (93.6) 6/94 (6.4) |
0.16 0.16 |
| Mode of delivery, n (%) | ||||
|
77/185 (41.6) 59/185 (31.9) 49/185 (26.5) 12/49 (24.5) |
35/91 (38.5) 27/91 (29.7) 29/91 (31.9) 2/29 (6.9) |
42/94 (44.7) 32/94 (34.0) 20/94 (21.3) 10/20 (50.0) |
0.39 0.52 0.10 0.001 |
| Pregnancy complications, n (%) | ||||
|
23 (12.2) 15 (8.0) 10 (5.4) 14 (7.5) |
8 (8.7) 4 (4.4) 3 (3.3) 10 (10.9) |
15 (15.6) 11 (11.6) 7 (7.4) 4 (4.2) |
0.15 0.069 0.21 0.080 |
| Hypertensive disorders, n (%) | ||||
|
11 (5.9) 12 (6.4) |
5 (5.4) 4 (4.4) |
6 (6.3) 8 (8.3) |
0.81 0.26 |
| Congenital malformations, n (%) | 13 (6.9) | 7 (7.6) | 6 (6.3) | 0.71 |
Bold values denote statistical significance at the p < 0.05 level.
*Combined variable of both pre-existing diabetes mellitus and gestational diabetes mellitus.
APGAR, APGAR score; LGA, large for gestational age; PreCARA, Preconception Counseling in Active RA; RA, rheumatoid arthritis; SDS, standard deviation score; SGA, small for gestational age.
Pregnancy outcomes stratified for TNFi use during pregnancy showed that birth weight, birth weight SDS and the number of emergency CS were different between both groups (table 2). The absolute difference in mean birth weight compared between women that used a TNFi during pregnancy and women who did not was 173 g (3.344 kg vs 3.171 kg, p=0.03). Survival analysis showed that GA was not different between women that used a TNFi during pregnancy and women who did not (online supplemental figure 1). Information on SGA, prematurity, gestational hypertension and pre-eclampsia stratified for DAS28CRP <2.6 and TNFi use is presented in online supplemental table 1).
Influence of different factors on birth weight
In the univariate analysis, disease activity, GA at delivery, maternal age and TNFi use during pregnancy were significantly associated with birth weight (table 3).
Table 3.
Findings of univariate and multivariate regression analyses of actual birth weight (kilogram) of RA patients (n=188) included in the PreCARA cohort
| Univariate linear regression analysis for birth weight (kg) | Multivariate linear regression analysis for birth weight (kg)* | |||||
| Coefficient | 95% CI | P value | Coefficient | 95% CI | P value | |
| Disease activity in third trimester (DAS28CRP) | – 0.20 | –0.31 to 0.085 | 0.001 | –0.091 | –0.19 to 0.0058 | 0.065 |
| Gestational age at delivery (weeks) | 0.17 | 0.14 to 0.21 | <0.001 | 0.18 | 0.15 to 0.22 | <0.001 |
| Maternal age (years) | – 0.013 | – 0.033 to 0.0074 | 0.21 | –0.023 | –0.040 to 0.0065 | 0.007 |
| Parity (nulliparity) | – 0.21 | – 0.36 to 0.047 | 0.011 | –0.13 | –0.26 to 0.0042 | 0.057 |
| Sex of the newborn (female) | 0.034 | – 0.13 to 0.20 | 0.67 | 0.081 | –0.053 to 0.21 | 0.24 |
| Type of conception (assisted reproduction) | – 0.075 | – 0.31 to 0.16 | 0.53 | –0.051 | –0.26 to 0.15 | 0.62 |
| Diabetes Mellitus (yes)† | 0.22 | – 0.084 to 0.53 | 0.15 | 0.37 | 0.12 to 0.63 | 0.004 |
| TNF inhibitor use during pregnancy (yes) | 0.17 | 0.014 to 0.33 | 0.033 | 0.20 | 0.066 to 0.34 | 0.004 |
| Prednisone use during pregnancy (yes) | – 0.064 | – 0.23 to 0.99 | 0.44 | – 0.99 | – 0.23 to 0.039 | 0.16 |
| Smoking during pregnancy (yes) | – 0.17 | – 0.62 to 0.29 | 0.47 | – 0.028 | – 0.41 to 0.35 | 0.89 |
*Corrected for all the other variables listed in this table.
†Combined variable of both preexisting diabetes mellitus and gestational diabetes mellitus.
DAS28CRP, Disease Activity Score in 28 joints C reactive protein; preCARA, Preconception Counseling in Active RA; RA, rheumatoid arthritis.
In the multivariate analysis (table 3), maternal age had a significant negative effect on birth weight (kg). In addition, a positive effect on birth weight of TNFi use during pregnancy, DM and GA at delivery was observed. When the analysis were repeated with birth weight standard deviation score (SDS) instead of the actual birth weight as dependent variable, similar results were found (data not shown).
Since TNFi use and disease activity and TNFi use and the use of prednisone might have interaction, two additional analyses were performed, in which interaction terms (TNFi use and disease activity and TNFi use and prednisone use) were introduced into the model. These interaction terms showed no significant effect (interaction term TNFi use and disease activity (p=0.67), interaction term TNFi use and prednisone use (p=0.59).
To get more insight whether the effect of the use of TNFi during pregnancy on birth weight was depended on the trimester additional analysis were performed. When TNFi use was stratified for use per trimester, the following results for the outcome birth weight (corrected for DAS28CRP, GA at delivery, maternal age, nulliparity, sex of the newborn, type of conception, DM, prednisone use, and smoking) were observed: TNFi use in the first trimester β 0.081 (p=0.26), difference in mean birth weight 90.6 g. TNFi use in the second trimester β 0.16 (p=0.021), difference in mean birth weight 180.5 g. TNFi use in the third trimester β 0.22 (p=0.002); difference in mean birth weight 191.5 g.
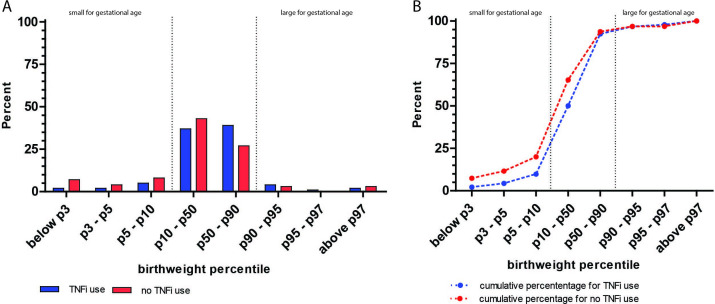
Figure 1 shows that maternal TNFi use during pregnancy results in a decreased number of children born SGA compared with no maternal use of TNFi during pregnancy.
Figure 1.
Bar charts showing the percentage of children born in different birth weight percentile groups stratified for maternal TNFi use during pregnancy (A). (B) shows the cumulative percentage of these birth weight percentiles. Small for gestational age (SGA) is defined as p<10, large of gestational age (LGA) as p>90, TNFi use during pregnancy is associated with less children born SGA (p=0.05), however the risk of LGA was not increased (p=0.73).
TNFi use during pregnancy and CSs
A total of 49/185 (26.5%) children were born via CS, 12/49 (24.5%) were emergency CS. Women that used a TNFi during pregnancy had a trend towards an increase in delivery via CS (TNFi use during pregnancy: CS in 29/91 (31.9%) women vs no TNFi use during pregnancy CS in 20/94 (21.3%) women, p=0.10), however, emergency CS was more common in women that did not use a TNFi during pregnancy: (TNFi use during pregnancy: emergency CS in 2/29 (6.9%) women versus no TNFi use during pregnancy emergency CS in 10/20 (50.0%) women, p=0.001).
Congenital malformations
In the PreCARA-cohort, 13 (6.9%) women had a child born with a congenital malformation, of which 7 (7.6% of the women that used a TNFi at any time point during pregnancy) used a TNFi during pregnancy. One child had two congenital malformations: both undescended testis and an umbilical hernia. The congenital malformations that were observed in women that used a TNFi during pregnancy were: umbilical hernia n=1, haemangioma n=1, pupil anomaly n=1, salmon patch n=1, epispadias n=1, port-wine stain n=1 and different stance of both ears and teeth which required further investigation by a geneticist n=1. Congenital malformations in women that did not use a TNFi during pregnancy included: clubfoot n=2, heel foot n=1, undescended testis n=2, umbilical hernia n=1 and polydactyly n=1.
Discussion
In the current manuscript, we describe pregnancy outcomes of women with RA that were prospectively followed up and treated according to a modern treatment approach, including the use of TNFi. We showed that the use of TNFi during pregnancy was associated with a clinically significant increased fetal birth weight even when corrected for major confounders such as disease activity and resulted in less children born SGA. The risk of adverse pregnancy outcomes did not differ between patients that used an TNFi during pregnancy and patients who did not. In addition, the use of TNFi during pregnancy showed a trend towards an increase in birth via CS, however, no increased risk for an emergency CS was observed.
Shimada et al reported in a small, retrospective study that birth weight is increased in women that used a TNFi during pregnancy.15 In their study, women that continued therapy with their TNFi (etanercept or certolizumab-pegol) during pregnancy had children with a higher birth weight compared with women that stopped their TNFi at conception. However, their study has several limitations; besides a low number of included patients, the authors were not able to perform additional analysis to correct for relevant confounders, such as disease activity. Our study is the first to show a strong association between TNFi use during pregnancy and an increased birth weight of the offspring when corrected for major confounders.
Increasing evidence shows that slight changes in birth weight (corrected for gestational-age) may have lifelong consequences for these children; both low and high birth weight are associated with complications throughout life.16 17 RA is for example associated with SGA, which itself is associated with an increased risk on metabolic and cardiovascular disease later in life.18 19 On the other hand, children born with a high birth weight have an increased risk of becoming overweight, on metabolic syndrome and giving birth to a child that is LGA as well. Our study shows a decreased risk of children born SGA and no increased risk of children born with high birth weight or LGA when a TNFi was by the mother used during pregnancy.
It is challenging to determine in which trimester the effect of TNFi was the largest, due to highly correlated exposure; women that used a TNFi in the third trimester most often used these biologics in the first and second trimester of pregnancy as well. Our study shows the largest effect in the third trimester of pregnancy, these results should be interpreted with caution and require replication by others research groups.
In RA pathology, high levels of circulating proinflammatory cytokines such as TNF alpha and interleukine (IL)-6 and a lower number of regulatory T-cells (Tregs) are observed in patients with active disease.20 Importantly, on treatment with a TNFi a decrease of these proinflammatory cytokines and increase the number and function of Tregs and IL-10 can be observed.21 22 The immune system is not only important in the pathogenesis of RA, but also for ensuring and maintaining a normal pregnancy. For pregnancy to avoid rejection of the semi allogenic fetal-placental unit and to ensure proper development of the placenta and hence fetal growth, local expansion of leukocytes with unique regulatory properties, including Tregs is required.23 24 In addition a tight balance between proinflammatory (eg, TNF and IL-6) and anti-inflammatory (eg, IL-10) cytokines is necessary, in which the anti-inflammatory cytokines prevail.24 Many disease of pregnancy, including recurrent miscarriages, intrauterine growth restriction, SGA and hypertensive disorders of pregnancy, like preeclampsia, are thought to arise from inadequate development and growth of the placenta and hence, if pregnancy continues, impaired fetal growth.23 24 Interestingly, in these conditions an increase in proinflammatory cytokines like TNF and IL-6 can be found.24 It is tempting to speculate that treatment with TNFi during pregnancy promotes placentation and thereby fetal growth and birth weight by changing the balance between proinflammatory and anti-inflammatory cytokines and by increasing the number and function of Tregs. As stated previously, several diseases of pregnancy are thought to arise from impaired placentation and are characterised by an immunological imbalance, whether treatment with TNFi is beneficial in these conditions should be the focus of future research.
An alternative, but not mutually exclusive, hypothesis could be that treatment with TNFi during pregnancy is able to induce epigenetic changes in the fetus, which positively influence fetal growth. In this respect, it has been shown that RA during pregnancy, either related to the disease itself, disease activity or medication, is associated with marked epigenetic changes in the offspring.25 In addition, in several populations of healthy mothers and their children, it has been shown that DNA-methylation in the newborn is associated with birth weight.26
Previous literature shows that TNFi use during pregnancy in patients with a chronic inflammatory conditions is associated with an increased risk of birth via elective and emergency CS.5 In the current study, patients that used a TNFi during pregnancy had trend towards a higher risk of an elective CS, but not for emergency CS compared with patients that did not use TNFi during pregnancy. The exact reasons behind this phenomenon are unknown. One possible explanation for this observation could be that gynaecologist/obstetricians sooner tend to plan a CS when patients are exposed to biologic DMARDs during pregnancy to avoid emergency situations in pregnancies at risk, however, this is speculation and should be further explored. The overall rate of CS in our study (26.5%) is probably comparable to the overall percentage of birth via CS in Europe (25.7%).27
In line with previous reported literature, we did not observe an association between TNFi use during pregnancy and an increased risk of congenital malformations.28 29 Although the risk of congenital malformations in infants born to women with RA is probably not increased,30 directly comparing percentages of congenital malformations observed in our study to that of safety studies that use different definitions, inclusion criteria and study designs is probably not appropriate.
Our study has several strengths: our study comprise a well-defined, large, prospectively followed up cohort of women with homogeneous disease making us able to correct for confounding factors when studying the effect of TNFi on pregnancy outcomes such as birth weight. Some limitations of the current study should however be acknowledged. Our study did not have sufficient power to perform multivariate analysis on outcomes such as CS. Furthermore, our study was not designed to detect congenital malformations, since not all confounders for these outcome were collected and children born to women included in this study were not examined by a paediatrician. In addition, we were not able to correct our multivariate analysis for pre-pregnancy BMI since women were both included before pregnancy and already pregnant. Separate multivariate analysis of women that were included before pregnancy showed that BMI was not a significantly associated with birth weight and did not affect the effect size of TNFi on birth weight (data not shown).
In conclusion, our study shows that the use TNFi during pregnancy is associated with increased birth weight of offspring of women with well-controlled RA. Interestingly TNFi use during pregnancy results in less children born SGA, however, it does not increase the risk of born LGA. Our results might pave the way towards new clinical indications for the use of TNFi during pregnancy such as intrauterine growth restriction. Future research should focus on understanding the underlying mechanism of TNF-inhibition on birth weight and the long-term consequences for the offspring.
Footnotes
Handling editor: Josef S Smolen
Contributors: All authors met the authorship criteria, they had a substantial contribution to the conception or design of the work (HTWS, ER, RJEMD) or the acquisition (RJEMD), analysis (HTWS, AGMGJM, RJEMD) or interpretation of data for the work (all authors) and were involved in revising a draft of this work, gave final approval of this version to be published, and are accountable for all aspects of the work in ensuring accuracy and integrity. The guarantor (RJEMD) accepts full responsibility for the work and controlled the decision to publish.
Funding: This work was supported by the Dutch Arthritis Foundation (ReumaNederland) (project number: LLP-26), a non-profit organisation. The PreCARA study is an Investigator Initiated Study and was financially supported by UCB.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants and was approved by MEC-2011-032. Participants gave informed consent to participate in the study before taking part.
References
- 1. Smeele HTW, Dolhain RJEM. Current perspectives on fertility, pregnancy and childbirth in patients with rheumatoid arthritis. Semin Arthritis Rheum 2019;49:S32–5. 10.1016/j.semarthrit.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 2. Smeele HT, Röder E, Wintjes HM, et al. Modern treatment approach results in low disease activity in 90% of pregnant rheumatoid arthritis patients: the PreCARA study. Ann Rheum Dis 2021;80:859–64. 10.1136/annrheumdis-2020-219547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsao NW, Rebic N, Lynd LD, et al. Maternal and neonatal outcomes associated with biologic exposure before and during pregnancy in women with inflammatory systemic diseases: a systematic review and meta-analysis of observational studies. Rheumatology 2020;59:1808–17. 10.1093/rheumatology/keaa064 [DOI] [PubMed] [Google Scholar]
- 4. Kammerlander H, Nielsen J, Knudsen T, et al. Anti-TNF-α Use During the Third Trimester of Pregnancy in Women with Moderate-severe Inflammatory Bowel Disease and the Risk of Preterm Birth and Low Birth Weight. Inflamm Bowel Dis 2017;23:1916–23. 10.1097/MIB.0000000000001234 [DOI] [PubMed] [Google Scholar]
- 5. Bröms G, Kieler H, Ekbom A, et al. Anti-Tnf treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf 2020;29:316–27. 10.1002/pds.4930 [DOI] [PubMed] [Google Scholar]
- 6. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 7. Sokal A, Elefant E, Leturcq T, et al. Pregnancy and newborn outcomes after exposure to bisphosphonates: a case-control study. Osteoporos Int 2019;30:221–9. 10.1007/s00198-018-4672-9 [DOI] [PubMed] [Google Scholar]
- 8. Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. 10.1136/annrheumdis-2015-208840 [DOI] [PubMed] [Google Scholar]
- 9. Andreoli L, Gerardi MC, Fernandes M, et al. Disease activity assessment of rheumatic diseases during pregnancy: a comprehensive review of indices used in clinical studies. Autoimmun Rev 2019;18:164–76. 10.1016/j.autrev.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 10. de Man YA, Hazes JMW, van de Geijn FE, et al. Measuring disease activity and functionality during pregnancy in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:716–22. 10.1002/art.22773 [DOI] [PubMed] [Google Scholar]
- 11. Fransen J, van Riel PLCM, PLCM vanR. Outcome measures in inflammatory rheumatic diseases. Arthritis Res Ther 2009;11:244. 10.1186/ar2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andreoli L, Gerardi MC, Fernandes M, et al. Disease activity assessment of rheumatic diseases during pregnancy: a comprehensive review of indices used in clinical studies. Autoimmun Rev 2019;18:164–76. 10.1016/j.autrev.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 13. Niklasson A, Ericson A, Fryer JG, et al. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981). Acta Paediatr Scand 1991;80:756–62. 10.1111/j.1651-2227.1991.tb11945.x [DOI] [PubMed] [Google Scholar]
- 14. Hoftiezer L, Hof MHP, Dijs-Elsinga J, et al. From population reference to national standard: new and improved birthweight charts. Am J Obstet Gynecol 2019;220:383.e1–383.e17. 10.1016/j.ajog.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 15. Shimada H, Kameda T, Kanenishi K, et al. Effect of biologic disease-modifying anti-rheumatic drugs for patients with rheumatoid arthritis who hope to become mothers. Clin Rheumatol 2019;38:1453–8. 10.1007/s10067-019-04450-3 [DOI] [PubMed] [Google Scholar]
- 16. Hong YH, Lee J-E. Large for gestational age and obesity-related comorbidities. J Obes Metab Syndr 2021;30:124–31. 10.7570/jomes20130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnsson IW, Haglund B, Ahlsson F, et al. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes 2015;10:77–83. 10.1111/ijpo.230 [DOI] [PubMed] [Google Scholar]
- 18. de Steenwinkel FDO, Hokken-Koelega ACS, de Ridder MAJ, et al. Rheumatoid arthritis during pregnancy and postnatal catch-up growth in the offspring. Arthritis Rheumatol 2014;66:1705–11. 10.1002/art.38519 [DOI] [PubMed] [Google Scholar]
- 19. Chiavaroli V, Marcovecchio ML, de Giorgis T, et al. Progression of cardio-metabolic risk factors in subjects born small and large for gestational age. PLoS One 2014;9:e104278. 10.1371/journal.pone.0104278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bystrom J, Clanchy FI, Taher TE, et al. Tnfα in the regulation of Treg and Th17 cells in rheumatoid arthritis and other autoimmune inflammatory diseases. Cytokine 2018;101:4–13. 10.1016/j.cyto.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 21. Nguyen DX, Ehrenstein MR. Anti-Tnf drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. J Exp Med 2016;213:1241–53. 10.1084/jem.20151255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans HG, Roostalu U, Walter GJ, et al. TNF-α blockade induces IL-10 expression in human CD4+ T cells. Nat Commun 2014;5:3199. 10.1038/ncomms4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yuan J, Li J, Huang S-Y, et al. Characterization of the subsets of human NKT-like cells and the expression of Th1/Th2 cytokines in patients with unexplained recurrent spontaneous abortion. J Reprod Immunol 2015;110:81–8. 10.1016/j.jri.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 24. Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, et al. Tumor necrosis factor-alpha and pregnancy: focus on biologics. An updated and comprehensive review. Clin Rev Allergy Immunol 2017;53:40–53. 10.1007/s12016-016-8596-x [DOI] [PubMed] [Google Scholar]
- 25. Ince-Askan H, Mandaviya PR, Felix JF, et al. Altered DNA methylation in children born to mothers with rheumatoid arthritis during pregnancy. Ann Rheum Dis 2019;78:1198–204. 10.1136/annrheumdis-2018-214930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Küpers LK, Monnereau C, Sharp GC, et al. Meta-Analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat Commun 2019;10:1893. 10.1038/s41467-019-09671-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Betran AP, Ye J, Moller A-B, et al. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health 2021;6:e005671. 10.1136/bmjgh-2021-005671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chambers CD, Johnson DL, Xu R, et al. Birth outcomes in women who have taken adalimumab in pregnancy: a prospective cohort study. PLoS One 2019;14:e0223603. 10.1371/journal.pone.0223603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol 2016;14:234–41. 10.1016/j.cgh.2015.08.039 [DOI] [PubMed] [Google Scholar]
- 30. de Jong PHP, Dolhain RJEM, Fertility DRJ. Fertility, pregnancy, and lactation in rheumatoid arthritis. Rheum Dis Clin North Am 2017;43:227–37. 10.1016/j.rdc.2016.12.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-222679supp001.pdf (210.8KB, pdf)
Data Availability Statement
No data are available.