Abstract
Preconditioning is such a paradigm that a stimulus below the threshold of causing harm makes the brain stronger and resilient to subsequent injury. Preconditioning affords a vigorous tolerance to the brain against neurodegeneration. Numerous efforts have tried to identify the molecular targets involved in preconditioning-induced protective responses and interestingly many of those diverse mechanisms posit mitochondria as a master regulator of preconditioning. Therefore, in this review, we will critically discuss recent and emerging evidence for the involvement of mitochondria within the preconditioning paradigm. We will introduce the crucial targets and signaling cascades by which mitochondria exert preconditioning with a focus on white matter mitochondria and whether and how mechanisms for preconditioning differ in neurons and glial cells. In this aspect, we will evaluate the role of mitochondrial shaping proteins to establish structure-function interdependence for fusion-fission balance, motility, ATP production, Ca+2, and ROS scavenging. We will also discuss how aging impacts mitochondria and the consequences of mitochondrial aging on preconditioning mechanisms. We will concentrate on the regulation of mitochondrial DNA content and quantification specifically for its value as a biomarker to monitor disease conditions. The identification of these mitochondrial preconditioning mechanisms can be translated to potential pharmacological interventions to increase intrinsic resilience of the brain to injury and to develop novel approaches to neurodegenerative diseases. Moreover, mitochondria dynamics can be used as a memory or biomarker of preconditioning.
Keywords: Mitochondria, White matter, Aging, Preconditioning, Biomarker
Introduction
Since its first introduction in 1964 (Janoff, 1964) the concept of preconditioning in the brain has been widely accepted as a small dose of noxious stimulus to afford neuroprotection responses against subsequent injury (Dirnagl et al., 2003). In other words “Was mich nicht umbringt macht mich starker” (Friedrich Nietzsche’s Twilight of the Idols 1888), “what does not kill me makes me stronger. In fact, hypoxia, ischemia, small doses of endotoxins, intermittent fasting, caloric restriction, exercise, stem cells, and drugs have all been shown to evoke tolerance to future injuries (Cadet and Krasnova, 2009; Bernstock et al., 2017; Li et al., 2017; Zhang et al., 2019; Cozene et al., 2020; Kinoshita et al., 2021; Vemuganti and Arumugam, 2021). Surprisingly adaptation to one stimulus provides resilience to the other, which underlies the essence of “cross-tolerance”. Depending on the onset of the effect, three different phases of preconditioning are defined as: a) immediate preconditioning occurs within minutes after the preconditioning stimulus and involves cellular changes related to the activity of function of the enzymes, secondary messengers, and ion channels, b) delayed preconditioning takes several hours or days to generate new gene expression and protein synthesis (Barone et al., 1998; Kirino, 2002; Gidday, 2006) and a recent addition is c) chronic conditioning, which is characteristic of remote ischemic conditioning (RIC). Alternatively, depending on the timing of the treatment, three types of preconditioning protection can be described: 1) preconditioning when applied before the onset of the injury, 2) per-conditioning when the treatment with sub lethal ischemia occurs during ischemia and before the reperfusion (Hahn et al., 2011), and finally 3) post conditioning if the conditioning occurs during the reperfusion (Zhao et al., 2003). Trials in stroke in which single neuroprotective pathways were targeted for activation have been unsuccessful, so alternative strategies that target multiple pathways are attractive, and preconditioning the brain with RIC is one such feasible approach. Ideally, the conditioning stimulus provides protection not only when triggered before ischemia but also when applied during ischemia or reperfusion after ischemia. Note that, preconditioning may be limited to clinical conditions in which ischemia is predictable such as before surgery, high-risk patients with a history of transient ischemic attack, or cardiac problems prone to emboli. However, the protective effects of ischemic preconditioning spans across many species and organs including heart, lung, brain, intestines, and kidneys (Murry et al., 1986; Kitagawa et al., 1991; Ates et al., 2002; Waldow et al., 2005; Chen et al., 2008; Abu-Amara et al., 2011).
Almost after 60 years (Janoff, 1964), our information on the complex molecular mechanisms for the induction and maintenance of preconditioning induced brain tolerance remains largely undefined. However, mitochondrial-mediated mechanisms seem to mediate an important portion of the preconditioning response. Increasing evidence support the proposal that brief exposure to physiological or pathological stimuli, cellular events, pharmacological applications, and remote interventions induce mitochondrial changes that ultimately protect neurons against a variety of lethal insults (Duchen, 2004; Perez-Pinzon et al., 2005; Hess et al., 2015). A precise sequence of events occur where a change in mitochondrial function leads to energy preservation and manifests itself as preconditioning induced neuroprotection. This sequence of events has been successfully observed in vitro and in vivo cerebral ischemia models (Duchen, 2004; Perez-Pinzon et al., 2005). These experiments demonstrated that antioxidants and mitochondrial adenosine triphosphate (ATP)-sensitive potassium channel (mito-KATP) blockers abolish preconditioning induced protection in cerebral ischemia, and an important role for mitochondrial reactive oxygen species (ROS) generation and mito-KATP channel activation were among the first pathways established in the preconditioning phenomenon (Vanden Hoek et al., 1998; Oldenburg et al., 2002). Indeed, these findings initiated the investigation of numerous approaches to test and characterize preconditioning mechanisms.
Mitochondria proved to be a merging integrator of preconditioning mediated endogenous neuroprotection. Therefore, this review summarizes the structural and functional characteristics of “preconditioning mitochondria” with a focus on necessary vs. sufficient conditions to precondition that were presented and discussed during the 2021 Virtual Conditioning Medicine Workshop by the American Association of Conditioning Medicine. We will also highlight whether preconditioning mitochondria show the location and age-related specificity by comparing neuronal, axonal, and glial preconditioning requirements taking into account the impact of age by considering aging mitochondria. Finally, we will discuss whether preconditioning mitochondria and their mitochondrial DNA content can be used as a biomarker to predict disease onset, prognosis, and the effect of treatment on disease course. We propose that long-lasting changes in mitochondria structure and function serve as a memory of preconditioning and as a result, a better description and understanding of the role of preconditioning mitochondria will help in the development of novel, effective, and more targeted therapeutic strategies against neurodegenerative diseases.
Preconditioning Mitochondria
Mitochondria are dynamic organelles that produce ~90% of the ATP via the tricarboxylic acid cycle and oxidative phosphorylation, regulate intracellular Ca2+ and redox signaling, and command apoptosis (Green and Kroemer, 2004; Beal, 2005; Mattson et al., 2008). Consequently, mitochondria are considered as the “gatekeeper” for cell viability and death. Therefore, it is not surprising that mitochondria play a pivotal role in the preconditioning phenomenon for many pathological conditions, foremost neurodegeneration (Cho et al., 2010) and stroke (Piquereau et al., 2013).
As dynamic organelles, mitochondria continuously undergo fission and fusion while carefully maintaining their structural integrity and motility. Mitochondrial length is determined by the balance between the rates of mitochondrial fission and fusion and is important for controlling the spatiotemporal properties of mitochondrial responses during physiological and pathophysiological processes (Szabadkai and Duchen, 2008). The precise balance between fission and fusion is equally an important process for mitochondria to adapt their shape specific to cellular compartments within a cell (Figure 1, A to D). For instance, in neurons with their nerve processes mitochondria assume an elongated tubular structure in axons (Figure 1B, red arrows) while in neuronal cell bodies they switch to a rounder smaller shape (Figure 1C, yellow arrows). This rapid transient change in shape is mostly dictated by cellular activity and metabolic demand so that ATP production, Ca2+, and ROS regulation can be efficiently controlled under physiological conditions. The processes of fusion and fission also contribute to the response of mammalian cells to stress such that fusion is stimulated by energy demand and stress, while fission generates new organelles and facilitates quality control (Frank et al., 2001; Skulachev, 2001; Tondera et al., 2009). Mitochondrial fission is facilitated by the translocation of dynamin-related protein 1 (Drp-1) from cytosol to mitochondria (Reddy et al., 2011; Hatch et al., 2014; Flippo and Strack, 2017; Bastian et al., 2018), while mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) on the mitochondrial outer membrane, and optic atrophy gene 1 (Opa1) on the mitochondrial inner membrane are essential for mitochondrial fusion. A pathological event such as stroke causes extensive fission and fragmentation, leading to smaller-sized dysfunctional mitochondria (Figure 2) that generate excessive amounts of ROS and Ca2+, with reduced levels of ATP leading to irreversible neuronal and axonal structural and functional loss. Note that mitochondrial structural disintegration is a continuous process that allows an opportunity to interrupt and improve mitochondrial fate (Figure 2). For instance, pharmacological blockade of Drp-1 with mitochondrial division inhibitor 1 (Mdivi-1) is shown to be protective in several tissues such as heart, kidney, retinal ganglion cells, spinal cord, and in cerebral ischemia-reperfusion models (Brooks et al., 2009; Ong et al., 2010; Park et al., 2011a; Grohm et al., 2012; Liu et al., 2015). Therefore, whether regulation of mitochondrial fusion-fission balance underlies mitochondria-mediated preconditioning has been of great interest. Indeed, studies in neuronal cells established the concept that Mdivi-1, which prevents translocation of cytosolic Drp-1 into mitochondria and thus inhibits fission, (Kim et al., 2017; Valenti et al., 2017) confers preconditioning to neuronal function in gray matter (GM) (Ravati et al., 2000; Ravati et al., 2001; Dirnagl and Meisel, 2008; Jou, 2008; Dirnagl et al., 2009). These studies also set the expectation for the visual description of preconditioning mitochondria that is “dressed for preconditioning” with a size and shape appropriate for the cellular anatomic location.
Figure 1.

(A, C) Mitochondria in neuronal cell bodies (yellow arrow heads) and (B) nerve processes (red arrows); bar = 10 μm. (D) Combining cyan fluorescent protein (CFP) with microtubule associated protein 2 creates useful images in which to contrast mitochondrial morphology in the perinuclear region of cell bodies as compared with neuronal processes. A “preconditioned mitochondria” is expected to conform to the size and shape of its cellular location.
Figure 2.

Three-dimensional construction of control axons (yellow, left top) with their elongated green mitochondria (middle top) and the two-dimensional electron micrographs of myelinated axons with numerous elongated mitochondria with their cristea (red arrows). One hour after oxygen-glucose deprivation (OGD 1h after, middle left) axons are swollen and mitochondria are fragmented (midline, middle, in colors) as seen as circular swollen hollow structures with loss of cristae. The OGD induced mitochondrial destruction progresses with time such that axons become visibly deformed with bulging sections (OGD 5h after, bottom left) characterized with mitochondria further disintegrating into smaller fragments (bottom middle and left). A “preconditioned mitochondria” is expected to remain elongated and tubular in axons akin to control conditions. Scale bar = 1 μm.
Identification of recent molecular mechanisms of preconditioning from the cancer and cardiac field independently confirmed the concept that inhibition of mitochondrial fission is a merging target that underlies preconditioning. For instance, studies by Dr. Jurgen Bernhagen (2021 Virtual Conditioning Medicine Workshop) provided compelling evidence that macrophage migration inhibitory factor (MIF) could be a key player mediating ischemic preconditioning. Following the expression and localization of mitochondrial dynamics associated proteins after MIF inhibition showed excessive fission due to upregulation of Drp-1 translocation into mitochondria, correlated with robust downregulation of Opa-1 and Mfn-1 levels (De et al., 2018) resulting in extensive mitochondrial fission. These structural alterations resulted in lower levels of ATP production, increased mitochondrial pore opening, and depolarization of transmembrane potential leading to mitochondrial dysfunction.
A more recent paradigm of self-protection proposed by Drs. Xunming Ji, Davis Hess, and Derek Hauser (2021 Virtual Conditioning Medicine Workshop) revealed that remote ischemic conditioning (RIC) can recruit neuroprotective and cardioprotective pathways with high potential for a wide variety of patients suffering from cerebral ischemia, cerebral hemorrhage, and cardiac ischemia. An exciting aspect of this preconditioning paradigm is that the RIC can involve the third phase of chronic conditioning, which implies protection when applied, before during, or after the injury. The leading candidates for this remote signaling to the brain include stromal cell-derived factor (SDF-1), which is activated by hypoxia and involved in stem cell trafficking, interleukin 10 (IL-10), micro ribonucleic acid (miRNA)-144, and nitrite. Most pathways that are triggered by RIC converge on the mitochondria such that blood-borne factors stimulate G-protein coupled receptors on the cell surface, which induce intracellular kinase signaling that leads to the opening of KATP preventing the formation of the mitochondrial permeability transition pore. Subsequently, inhibition of KATP abrogates the RIC-induced preconditioning. The mitochondria are also a subcellular target of nitrite, which is involved in key nitrosylation of key mitochondrial proteins such as complex I and IV. As a result, the generation of mitochondrial ROS declines to protect mitochondrial structure and function. Clinical trials testing the role of RIC in patients with subarachnoid hemorrhage, intracranial atherosclerosis, and ischemic heart conditions will also help identify the targets for pharmacological manipulations for preconditioning (Hess et al., 2015).
Since studies that were performed in white matter (WM) provided interesting results emphasizing the differences in the role of neuronal, axonal, and glial mitochondria in preconditioning (see below), whether RIC offers mitochondria-induced preconditioning in WM components remain to be investigated
Preconditioning Mitochondria in WM
Mechanisms of ischemic preconditioning have been extensively studied in GM. An ischemic episode affects both the GM and WM portions of the brain (Mohr et al., 2011) and faithful axon conduction is crucial for signaling among neuronal cell bodies to connect GM and WM to achieve and maintain proper function. However, the effect of preconditioning on WM axons, myelin, and glial cells has received much less attention compared to neurons, presumably assuming that preconditioning of neuronal cells bodies may extend to axons and suffice to protect the entire brain. Although axons are anatomical extensions of neuronal cell bodies, injury mechanisms differ between GM and WM such that approaches for neuronal protection fail to improve, or even impede, axon function recovery after ischemia (Tekkok et al., 2007; Baltan, 2009, 2012; Baltan et al., 2014). In addition, axons are independent of their neuronal soma in terms of their energy status; consequently to assume that preconditioning of neuronal soma spreads and induces similar tolerance in axons is a misleading extrapolation. Inhibition of mitochondrial fission has been shown to induce preconditioning tolerance to neurons in vivo (Park et al., 2011b; Xie et al., 2013; Zhang et al., 2013a; Zuo et al., 2014; Jin et al., 2016; Kim et al., 2016; Deryagin et al., 2017) and in vitro (Correia et al., 2010; Wang et al., 2014). Ischemic preconditioning has been reported to protect WM against ischemic injury, (Hamner et al., 2015) however whether preservation of mitochondria can precondition WM against ischemic injury remains to be investigated.
The optic nerve is a pure isolated WM tract and provides a compelling in vitro preparation to tests injury mechanisms specific to WM components where axons, myelin, and glial cells in their three-dimensional configuration maintain their cell to cell signaling. This in vitro preparation addresses the concern that rodent brains contain only 10% WM by volume, as opposed to the human brain (50% WM by volume) (Zhang and Sejnowski, 2000) and thus, the response to ischemia is dominated by neuronal injury in rodent brain. Similar to neuronal cell bodies, mitochondria in myelinated axons undergo rapid and profuse fission during oxygen-glucose deprivation (OGD) that is mediated by translocation of cytoplasmic Drp-1 to mitochondria (Bastian et al., 2018). OGD-induced mitochondrial fission correlates with reduced mitochondrial motility and loss of axon function. Mitochondrial fragmentation and loss of motility become permanent during the recovery period (Bastian et al., 2018). Mdivi-1 is a small molecule that is readily permeant through the blood-brain barrier and it provides ischemic tolerance to neurons by maintaining mitochondrial integrity and function. Expectedly, administering Mdivi-1 to WM during OGD preserves mitochondrial shape and motility and promotes axon functional recovery. However, despite the Mdivi-1 effect on preconditioning neuronal cell bodies (Grohm et al., 2012; Zhang et al., 2013b; Wang et al., 2014; Cui et al., 2016), in WM Drp-1 inhibition fails to offer ischemic preconditioning tolerance to myelinated axons and fails to benefit axon function (Bastian et al., 2018). These findings suggest that inhibition of mitochondrial fission during ischemia is necessary to promote axon functional recovery but is not sufficient to precondition myelinated axons against ischemia. These results raise caution in that approaches to preconditioning neuronal cell bodies may not successfully translate into functional improvement following ischemia.
The Mdivi-1 application also modifies mitochondrial dynamics as evidenced by the enhanced mitochondrial motility in myelinated axons (Bastian et al., 2018). This increase in motility is preserved during OGD, albeit to a smaller extent compared to Mdivi-1 application during ischemia. However, the enhanced motility of mitochondria vanishes during the recovery period. This suggests that mechanisms of fission and mitochondrial motility are interconnected. Mitochondrial motility along microtubules is regulated by protein complexes, of which ATP and the Ca2+-dependent protein mitochondrial Rho GTPase-2 (Miro-2) play a major role (Guo et al., 2005; Saotome et al., 2008; Russo et al., 2009; Melkov et al., 2016) and is intricately linked to the mitochondrial fission protein Drp-1 (Saotome et al., 2008). Miro-2 forms complexes with kinesin for anterograde mitochondrial transport (Guo et al., 2005; Russo et al., 2009) and dynein for retrograde mitochondrial transport (Russo et al., 2009; Morlino et al., 2014; Melkov et al., 2016) along microtubules. Miro-2 is increasingly associated with neurodegenerative diseases that are characterized by mitochondrial dysfunction (Tang, 2015). Dysregulation of Miro-2 leads to mitochondrial arrest in movement and clearance (Wang et al., 2011). Miro-2 also affects both anterograde (Macaskill et al., 2009; Wang and Schwarz, 2009) and retrograde motility (Morlino et al., 2014) and the fusion-fission dynamics of mitochondria (Misko et al., 2010; Tang, 2015). Live imaging studies of mitochondria in optic nerves show that axonal mitochondria move bi-directionally, change direction, or become stationary in response to OGD (Bastian et al., 2018). Mitochondria are dynamic organelles whose coordinated motility ensures that metabolically active areas of axons are adequately supplied with ATP. Moreover, injured mitochondria are replaced with healthy ones following injury. Kymograph analysis of mitochondrial motility illustrates that under control conditions, most mitochondria are still, and those that move maintain a stable speed. The onset of OGD stalls mitochondrial motility to 50% of baseline levels both in the anterograde and retrograde directions and preservation of mitochondrial motility against OGD is an important predictor of axon function recovery (Bastian et al., 2018). For instance, electrophysiology and live mitochondrial imaging studies propose that nitric oxide synthase 3 inhibition promotes axon functional recovery by preventing mitochondrial fission and by preserving mitochondrial structure and motility during ischemia by conserving Miro-2 levels. Based on the interplay between mitochondrial motility and mitochondrial fission, it is plausible that motile mitochondria are an indicator of better functional recovery. In particular, the extent of mitochondrial motility during recovery guides functional recovery. The downstream molecular mechanisms of this protection are currently under investigation such as whether Miro-2 and Drp-1 interact to precondition mitochondria in WM axons. In summary, conserving mitochondrial structure sustains mitochondrial integrity and motility during ischemia, however, this approach fails to precondition axon function against ischemia, in contrast to its protective effects on neuronal survival. These findings support the notion that manipulations to precondition the brain should consider interventions to be beneficial for both the gray and WM portions of the brain.
The protective effect of astrocytes on neurons has been shown to include mitochondrial transfer, although the protective role of preconditioning astrocytes and astrocyte mitochondria has been reported more recently (Wu et al., 2021). In astrocyte and neuron co-culture systems, prior hypoxic preconditioning of astrocytes improves astrocyte mitochondrial structure and function through peroxisome proliferator-activator receptor 1 alpha (PGC-1α)/hypoxia-inducible factor (HIF) signaling, leading to increased astrocyte viability, reduced oxidative stress, and greater neuroprotection. The proposed mechanism is that the hypoxia-activated PGC-1α/HIF signaling improves mitochondrial biogenesis in astrocytes, which subsequently lowers neuronal apoptosis after OGD (Wu et al., 2021). Another interesting approach to preconditioning astrocytes is the use of resveratrol (RPC), which activates nuclear erythroid 2 related factors (Nrf2). Nrf2 localizes to the outer membrane in astrocyte mitochondria and its activation increases mitochondrial abundance, glycolysis, and mitochondrial respiration efficiency. Therefore, the contribution of Nrf2 to RPC-induced neuroprotection through maintaining astrocyte mitochondrial coupling and anti-oxidant protein expression exerts neuroprotection during cerebral ischemia (Narayanan et al., 2015; Khoury et al., 2019). Expectedly, in transgenic mice without Nrf2 (Nrf2−/−) the loss of neuroprotection correlates with induction of altered supercomplex formation in mitochondria and injury to neurons (Narayanan et al., 2018) while transgenic mice overexpressing Nrf2 is more resilient to neurotoxicity (Calkins et al., 2010). Preconditioning using a combination of mild oxidative stress with glucose deprivation (OSGD), further confirms that astrocyte survival and astrocyte-mediated neuroprotection is activated via the Nrf2 pathway. Nrf2 can enhance IL-10 expression, therefore mild OSGD preconditioning of astrocytes confer neuroprotection, with the participation of IL-10 to manipulate mitochondria and reduce oxidative injury (Segev-Amzaleg et al., 2013).
Nrf2 is also expressed in oligodendrocytes (Licht-Mayer et al., 2015) and ischemic preconditioning (medial cerebral artery occlusion for 12 min) promotes the generation of oligodendrocyte precursors (OPCs) and oligodendrogenesis by differentiation of OPCs in the striatum, corpus callosum, and external capsule, mediated by Nrf2 signaling. Knocking down Nrf2 blocks oligodendrogenesis induced by ischemic preconditioing (Li et al., 2021). In oligodendrocytes, sodium azide treatment inhibits mitochondria metabolism, results in depolarization of mitochondria membrane, decreases metabolic activity, and increases cytotoxicity. Endoplasmic reticulum stress induces Nrf2 hyperactivation, which partially rescues the mitochondrial metabolism inhibition induced by sodium azide. Furthermore, knocking down Nrf2 expression in oligodendrocytes worsens mitochondrial dysfunction regulating oligodendrocyte survival against injury (Liessem-Schmitz et al., 2018). Surprisingly, cell culture studies using primary OPCs reveal that prior exposure of OPCs to sublethal OGD as a mode of preconditioning results in enhanced vulnerability to subsequent excitotoxicity or to OGD mediated by down-regulation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunit glutamate receptor 2 on the cell surface that subsequently increases Ca2+ permeability (Deng et al., 2003). In neonatal rat brains, hypoxic injury reduces the amount of myelin basic protein without affecting the early or late OPCs in the corpus callosum. Interestingly, hypoxic preconditioning prevents the loss of myelin caused by a hypoxic injury without affecting the density of OPCs, suggesting that hypoxic preconditioning directly protects myelin or by promoting the maturation of pre oligodendrocytes to regenerate the damaged myelin (Suryana and Jones, 2014). Further studies investigating preconditioning of oligodendrocytes and their mitochondria and whether preconditioning oligodendrocytes support myelin and axon function are yet to be designed.
Aging Mitochondria and Preconditioning
Age-related reduction of preconditioning was first demonstrated in the isolated and perfused rat heart from 24-month-old rats subjected to an ischemic preconditioning with a short period of ischemia (2 minutes) followed by 10 minutes of reperfusion. Interestingly, caloric restriction and physical activity can restore preconditioning in aged hearts in both animals and humans (Abete et al., 2001). Reduction of “brain” preconditioning mediated protection in the aged brain may be due to the composite mechanisms that characterize the aged brain, ie, loss in many neurons, impairment in mitochondrial function with an increase in ROS production, alteration in gene expression and metabolic regulation, and alteration in intracellular Ca2+ homeostasis. All these modifications make the organ more susceptible to stress such as ischemia (Shankar, 2010). In particular, a gene expression profile following middle cerebral anterior occlusion shows reduced transcriptional activity, proapoptotic genes, and downregulation of axonogenesis and neurogenesis in the periinfarct area, which is more pronounced in aged versus young animals (Budas et al., 2007). These findings suggest that an aging brain is capable of upregulating gene expression, but the response is often blunted and temporally uncoordinated in response to cerebral ischemia (Della-Morte et al., 2012).
The impact of aging on central nervous system (CNS) WM is of particular interest because it appears that the global effects of aging on myelinated axons are more complex and profound than those in GM (Peters, 1999). Myelinated nerve fibers in WM deteriorate structurally and functionally with age, leading to impaired communication between different parts of the CNS. The underlying reasons for increased susceptibility with age to stroke, dementia, Alzheimer’s disease, or other neurodegenerative diseases are just beginning to be explored; therefore, it is important to separate between WM structural and functional changes that are due to natural aging and pathological changes associated with neurodegenerative diseases. Mitochondrial dysfunction and oxidative damage with age are gaining increasing interest as merging targets that underlie increased risk for stroke, AD, PD, and different forms of dementia. Indeed fewer, but longer and larger mitochondria produce increased oxidative stress markers and less ATP in aging axons. The processes of fusion and fission contribute to mitochondrial quality control and the response of mammalian cells to stress such that fusion is stimulated by energy demand and stress, while fission generates new organelles and facilitates quality control (Frank et al., 2001; Skulachev, 2001; Tondera et al., 2009). In WM, aging axons have fewer mitochondria than young axons, but they are thicker and longer (Baltan, 2014; Stahon et al., 2016) (Figure 3). This age-dependent expansion of mitochondrial morphology correlates with a mismatch among mitochondrial shaping proteins such that increases in levels of fusion proteins, for example Mfn-1 and Mfn-2, and decreases in fission protein Drp-1 levels result in conditions that favor mitochondrial fusion in aging axons (Baltan, 2014; Stahon et al., 2016). Fused mitochondria is an adaptation in response to the lower ATP levels observed in aging axons and results in shared components, thereby helping to maintain energy output during stress (Westermann, 2010). However, this also results in reduced mitochondrial numbers, which when combined with increased axonal volume results in parts of the axon being without mitochondria (Stahon et al., 2016) (Figure 3). The number of mitochondria directly correlates with the level of cellular metabolic activity. An interruption in mitochondrial dynamics due to a mismatch in mitochondrial shaping proteins results in reduced ATP production in aging axons. In addition, aging axons also show increased levels of oxidative stress markers (4-hydroxynonenal, 3-nitrotyrosine, and nitric oxide), which can lead to mitochondrial impairment and resulting stress (Stahon et al., 2016). Morphological alterations compromise mitochondrial function and the resultant reduced energy production and increased oxidative stress underlie the increased vulnerability of WM to an ischemic attack. Yet, aging axons adapt to transmit signals faithfully under control conditions as reported previously (Baltan et al., 2008; Baltan et al., 2010). Then the question of whether it is possible to precondition the aging brain raises substantial interest, particularly in the face of a rise in the aging population and how to support brain function in health and disease. Agents able to mimic the “cerebral” preconditioning effect may represent a new and powerful therapeutic option for the treatment of acute ischemic stroke in the elderly. Further studies are needed to establish the age-related reduction of brain preconditioning in the aging brain and translate these discoveries to clinical practice.
Figure 3.
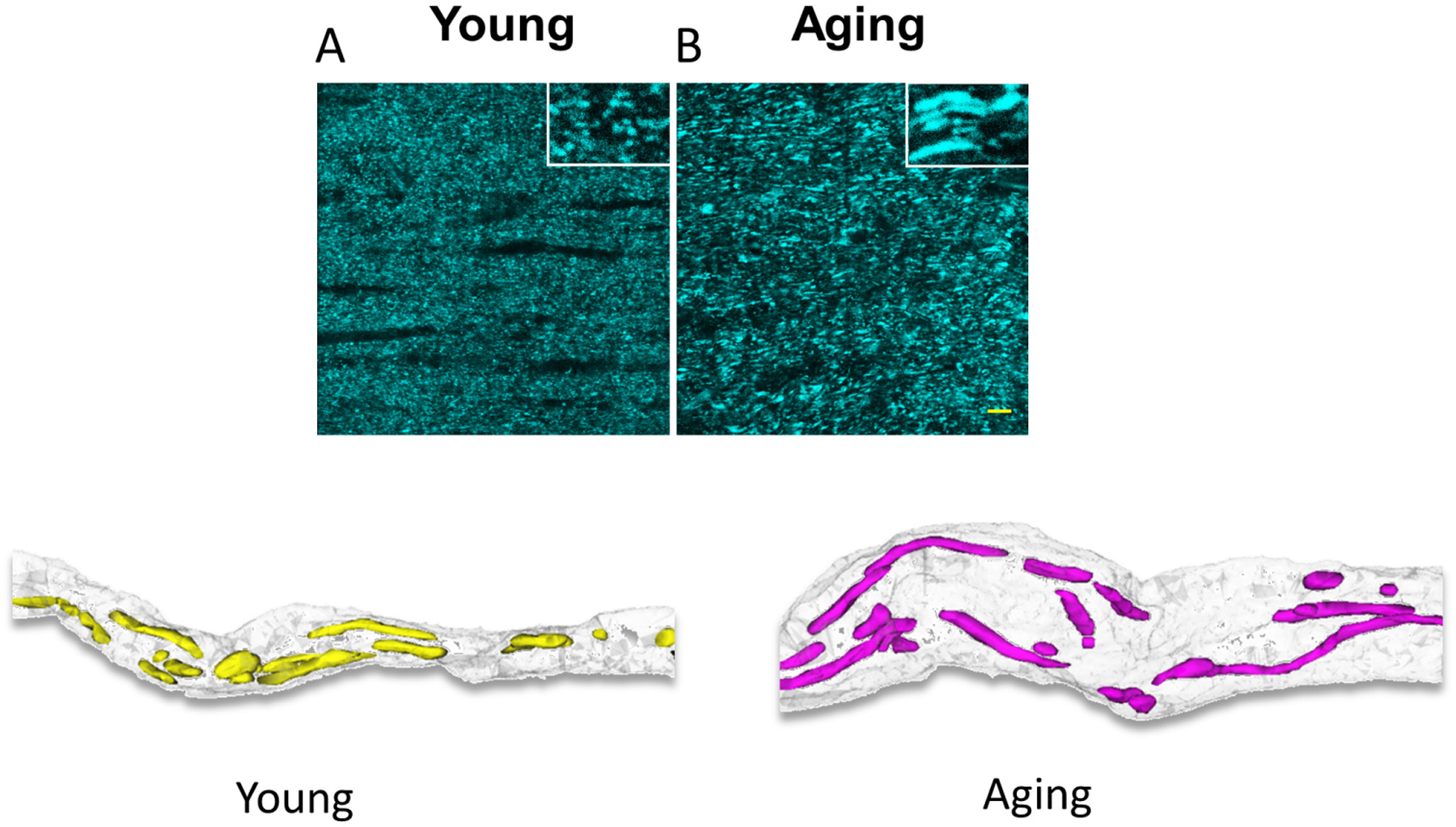
Aging increases the length and thickness of mitochondria as seen in CFP (+) mitochondria in optic nerves obtained from Thy-1 mito-CFP mice (upper two panels). Three-dimensional construction of electron micrograph of young and aging axons demonstrate that age causes a prominent increase in axon diameter correlated with a drastic increase in mitochondrial length, thickness, and volume. Note the areas devoid of mitochondrial coverage in aging axons. A “preconditioned aging mitochondria” is speculated to maintain size and shape comparable to normal aging conditions. Scale bar = 1 μm.
Mitochondria as a Biomarker for Preconditioning
Each mitochondrion can contain several copies of the mitochondrial genome (Veltri et al., 1990; Cavelier et al., 2000; Navratil et al., 2008), and changes in mitochondrial DNA (MtDNA) content are observed in physiological and pathological conditions, for instance in aging brain increase in MtDNA content has been reported (Barrientos et al., 1997). Under normal conditions, the maternally inherited mitochondrial genome in an individual will be the same in all cell types unless it has been damaged (Chinnery, 1993; Moraes et al., 2003). The number of mitochondria in a cell varies depending on the energetic requirements of the cell, for example, a brain cell may have around 2000 mitochondria (Uranova et al., 2001), a white blood cell may have less than a hundred (Selak et al., 2011), whereas oocytes may contain several hundred thousand mitochondria (Piko and Matsumoto, 1976; Duran et al., 2011). The number of mitochondria in a particular cell type can also vary depending on many factors, including the stage in the cell cycle, the environment and redox balance of the cell, the stage of differentiation, and several cell signaling mechanisms (Rodriguez-Enriquez et al., 2009; Michel et al., 2012).
As mitochondria contain their own DNA outside of the nuclear genome, a convenient way to measure mitochondrial DNA content in a cell is to measure mitochondrial versus nuclear genome ratio, called Mt/N (Malik et al., 2009; Malik et al., 2011). This approach is attractive, as the methodology for quantifying nucleic acids with real-time quantitative polymerase chain reaction (RT-qPCR) has become conventional (Gourlain et al., 2003; Andreu et al., 2009; Malik et al., 2011). Changes in MtDNA content could be used as a biomarker to detect mitochondrial dysfunction. To determine if Mt/N is a consistent biomarker of mitochondrial dysfunction, it is important to validate methods for accurate and reproducible measurement of cellular MtDNA content. Previous studies using RT-qPCR have tended to use primers for estimation of nuclear genome content from control genes previously used for mRNA quantification, such as β-actin and 18S rRNA. The rationale for using these genes for mRNA quantification is that they are presumed to have “housekeeping” functions and are expected to be expressed at equal levels in all cells. However, when quantifying copy numbers from genomic DNA these genes are not the best choice as many of the regions being amplified are not single copies (Zhang and Gerstein, 2003; Malik et al., 2011). The currently recommended stable gene that shows a low level of variability is β−2 microglobulin (Malik and Czajka, 2013).
Mt/N is attractive as a putative biomarker because it can be measured in as little as 1 pg of genomic DNA (Malik et al., 2011), thereby requiring very little clinical sample. Subsequently, MtDNA content changes have been reported using DNA isolated from various body fluids such as circulating blood cells, cell-free serum, saliva, sperm, urine, and cerebrospinal fluid and also in tissue samples such as tumor tissue and various organs and biopsy materials indicating common interest in measuring Mt/N in different body fluids and tissues in numerous human diseases, and during development, and aging. The use of body fluids as surrogate markers for changes in organs is a feasible option as tissues and organs are not easily accessible. As blood cells are in contact with the whole body and organs they may reflect changes in mitochondrial function/dysfunction.
Despite the growing interest in MtDNA content as a putative biomarker in numerous diseases, there are several conflicting studies and there is little consensus on how to interpret the results due to significant problems associated with the accuracy of measurement of MtDNA with current methodology. Furthermore, the development of assays for measuring the integrity of MtDNA is needed as current methods for Mt/N quantification do not distinguish between intact mitochondrial genomes and damaged MtDNA fragments. Emerging quantification systems such as digital PCR may offer the accuracy needed for Mt/N determination (Baker, 2012). Therefore, in conclusion, MtDNA could be a novel biomarker for mitochondrial dysfunction. In the presence of oxidative stress, ROS would lead to increased mitochondrial biogenesis resulting in increased MtDNA throughout the whole body and this change would be reflected in circulating cells. Accumulation of damaged MtDNA may directly contribute to pathology by eliciting a cellular anti-inflammatory response. Therefore, according to this hypothesis, an increase in MtDNA may precede mitochondrial dysfunction as an adaptive response and could therefore be a predictive biomarker. Hence, utilizing the changes in MtDNA after induction of preconditioning as a biomarker for preconditioning is a novel concept and warrants of further investigation. These studies are yet to be designed.
Acknowledgments
This work was supported by grants from the National Institute of Aging (NIA, AG033720) and the National Institute of Neurological Diseases (NINDS, NS094881) to S.B. We thank Dr. Chris Nelson for help editing this paper.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Abete P, Ferrara N, Cacciatore F, Sagnelli E, Manzi M, Carnovale V, Calabrese C, de Santis D, Testa G, Longobardi G, Napoli C, Rengo F (2001) High level of physical activity preserves the cardioprotective effect of preinfarction angina in elderly patients. J Am Coll Cardiol 38:1357–1365. [DOI] [PubMed] [Google Scholar]
- Abu-Amara M, Yang SY, Quaglia A, Rowley P, Tapuria N, Seifalian AM, Fuller BJ, Davidson BR (2011) Effect of remote ischemic preconditioning on liver ischemia/reperfusion injury using a new mouse model. Liver Transpl 17:70–82. [DOI] [PubMed] [Google Scholar]
- Andreu AL, Martinez R, Marti R, Garcia-Arumi E (2009) Quantification of mitochondrial DNA copy number: pre-analytical factors. Mitochondrion 9:242–246. [DOI] [PubMed] [Google Scholar]
- Ates E, Genc E, Erkasap N, Erkasap S, Akman S, Firat P, Emre S, Kiper H (2002) Renal protection by brief liver ischemia in rats. Transplantation 74:1247–1251. [DOI] [PubMed] [Google Scholar]
- Baker M (2012) Digital PCR hits its stride. Nature Methods 9:541–544. [Google Scholar]
- Baltan S (2009) Ischemic injury to white matter: an age-dependent process. Neuroscientist 15:126–133. [DOI] [PubMed] [Google Scholar]
- Baltan S (2012) Histone deacetylase inhibitors preserve function in aging axons. J Neurochem 123 Suppl 2:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S (2014) Excitotoxicity and mitochondrial dysfunction underlie age-dependent ischemic white matter injury. Adv Neurobiol 11:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S, Carmichael ST, Matute C, Xi G, Zhang JH (2014) White matter injury in stroke and CNS disease: Springer. [Google Scholar]
- Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR (2008) White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci 28:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S, Inman DM, Danilov CA, Morrison RS, Calkins DJ, Horner PJ (2010) Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci 30:5644–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ (1998) Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke 29:1937–1950; discussion 1950–1931. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Casademont J, Cardellach F, Ardite E, Estivill X, Urbano-Marquez A, Fernandez-Checa JC, Nunes V (1997) Qualitative and quantitative changes in skeletal muscle mtDNA and expression of mitochondrial-encoded genes in the human aging process. Biochem Mol Med 62:165–171. [DOI] [PubMed] [Google Scholar]
- Bastian C, Politano S, Day J, McCray A, Brunet S, Baltan S (2018) Mitochondrial dynamics and preconditioning in white matter. Cond Med 1:64–72. [PMC free article] [PubMed] [Google Scholar]
- Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58:495–505. [DOI] [PubMed] [Google Scholar]
- Bernstock JD, Peruzzotti-Jametti L, Ye D, Gessler FA, Maric D, Vicario N, Lee YJ, Pluchino S, Hallenbeck JM (2017) Neural stem cell transplantation in ischemic stroke: A role for preconditioning and cellular engineering. J Cereb Blood Flow Metab 37:2314–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Cho SG, Dong Z (2009) Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Mochly-Rosen D (2007) Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res 55:523–536. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN (2009) Cellular and molecular neurobiology of brain preconditioning. Mol Neurobiol 39:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Vargas MR, Johnson DA, Johnson JA (2010) Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci 115:557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier L, Johannisson A, Gyllensten U (2000) Analysis of mtDNA copy number and composition of single mitochondrial particles using flow cytometry and PCR. Exp Cell Res 259:79–85. [DOI] [PubMed] [Google Scholar]
- Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu H, Chen Z (2008) Ozone oxidative preconditioning inhibits inflammation and apoptosis in a rat model of renal ischemia/reperfusion injury. Eur J Pharmacol 581:306–314. [DOI] [PubMed] [Google Scholar]
- Chinnery PF (1993) Mitochondrial Disorders Overview. In: GeneReviews((R)) (Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A, eds). Seattle (WA). [Google Scholar]
- Cho DH, Nakamura T, Lipton SA (2010) Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci 67:3435–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SC, Carvalho C, Cardoso S, Santos RX, Santos MS, Oliveira CR, Perry G, Zhu X, Smith MA, Moreira PI (2010) Mitochondrial preconditioning: a potential neuroprotective strategy. Front Aging Neurosci 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozene B, Sadanandan N, Gonzales-Portillo B, Saft M, Cho J, Park YJ, Borlongan CV (2020) An Extra Breath of Fresh Air: Hyperbaric Oxygenation as a Stroke Therapeutic. Biomolecules 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Ding H, Chen F, Zhao Y, Yang Q, Dong Q (2016) Mdivi-1 protects against ischemic brain injury via elevating extracellular adenosine in a cAMP/CREB-CD39-dependent manner. Mol Neurobiol 53:240–253. [DOI] [PubMed] [Google Scholar]
- De R, Sarkar S, Mazumder S, Debsharma S, Siddiqui AA, Saha SJ, Banerjee C, Nag S, Saha D, Pramanik S, Bandyopadhyay U (2018) Macrophage migration inhibitory factor regulates mitochondrial dynamics and cell growth of human cancer cell lines through CD74-NF-kappaB signaling. J Biol Chem 293:19740–19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Morte D, Guadagni F, Palmirotta R, Testa G, Caso V, Paciaroni M, Abete P, Rengo F, Ferroni P, Sacco RL, Rundek T (2012) Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics 13:595–613. [DOI] [PubMed] [Google Scholar]
- Deng W, Rosenberg PA, Volpe JJ, Jensen FE (2003) Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci U S A 100:6801–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryagin OG, Gavrilova SA, Gainutdinov KL, Golubeva AV, Andrianov VV, Yafarova GG, Buravkov SV, Koshelev VB (2017) Molecular bases of brain preconditioning. Front Neurosci 11:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Meisel A (2008) Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology 55:334–344. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26:248–254. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A (2009) Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 8:398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR (2004) Roles of mitochondria in health and disease. Diabetes 53 Suppl 1:S96–102. [DOI] [PubMed] [Google Scholar]
- Duran HE, Simsek-Duran F, Oehninger SC, Jones HW Jr., Castora FJ (2011) The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil Steril 96:384–388. [DOI] [PubMed] [Google Scholar]
- Flippo KH, Strack S (2017) Mitochondrial dynamics in neuronal injury, development and plasticity. J Cell Sci 130:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1:515–525. [DOI] [PubMed] [Google Scholar]
- Gidday JM (2006) Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7:437–448. [DOI] [PubMed] [Google Scholar]
- Gourlain K, Amellal B, Ait Arkoub Z, Dupin N, Katlama C, Calvez V (2003) Quantitative analysis of human mitochondrial DNA using a real-time PCR assay. HIV Med 4:287–292. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629. [DOI] [PubMed] [Google Scholar]
- Grohm J, Kim SW, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J, Plesnila N, Culmsee C (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19:1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE (2005) The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron 47:379–393. [DOI] [PubMed] [Google Scholar]
- Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN (2011) Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke 42:2960–2962. [DOI] [PubMed] [Google Scholar]
- Hamner MA, Ye Z, Lee RV, Colman JR, Le T, Gong DC, Ransom BR, Weinstein JR (2015) Ischemic preconditioning in white matter: magnitude and mechanism. J Neurosci 35:15599–15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch AL, Gurel PS, Higgs HN (2014) Novel roles for actin in mitochondrial fission. J Cell Sci 127:4549–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, Ji X (2015) Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol 11:698–710. [DOI] [PubMed] [Google Scholar]
- Janoff A (1964) Alterations in lysosomes (intracellular enzymes) during shock; effects of preconditioning (tolerance) and protective drugs. Int Anesthesiol Clin 2:251–269. [DOI] [PubMed] [Google Scholar]
- Jin Z, Wu J, Yan LJ (2016) Chemical conditioning as an approach to ischemic stroke tolerance: mitochondria as the target. Int J Mol Sci 17:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou MJ (2008) Pathophysiological and pharmacological implications of mitochondria-targeted reactive oxygen species generation in astrocytes. Adv Drug Deliv Rev 60:1512–1526. [DOI] [PubMed] [Google Scholar]
- Khoury N, Xu J, Stegelmann SD, Jackson CW, Koronowski KB, Dave KR, Young JI, Perez-Pinzon MA (2019) Resveratrol preconditioning induces genomic and metabolic adaptations within the long-term window of cerebral ischemic tolerance leading to bioenergetic efficiency. Mol Neurobiol 56:4549–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee JY, Park KJ, Kim WH, Roh GS (2016) A mitochondrial division inhibitor, Mdivi-1, inhibits mitochondrial fragmentation and attenuates kainic acid-induced hippocampal cell death. BMC Neurosci 17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim C, Park S (2017) Mdivi-1 protects adult rat hippocampal neural stem cells against palmitate-induced oxidative stress and apoptosis. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Hamanaka G, Ohtomo R, Takase H, Chung KK, Lok J, Lo EH, Katsuki H, Arai K (2021) Mature adult mice with exercise-preconditioning show better recovery after intracerebral hemorrhage. Stroke 52:1861–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T (2002) Ischemic tolerance. J Cereb Blood Flow Metab 22:1283–1296. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T (1991) ‘Ischemic tolerance’ phenomenon detected in various brain regions. Brain Res 561:203–211. [DOI] [PubMed] [Google Scholar]
- Li Q, Lou J, Yang T, Wei Z, Li S, Zhang F (2021) Ischemic preconditioning induces oligodendrogenesis in mouse brain: effects of Nrf2 deficiency. Cell Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Hafeez A, Noorulla F, Geng X, Shao G, Ren C, Lu G, Zhao H, Ding Y, Ji X (2017) Preconditioning in neuroprotection: From hypoxia to ischemia. Prog Neurobiol 157:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht-Mayer S, Wimmer I, Traffehn S, Metz I, Bruck W, Bauer J, Bradl M, Lassmann H (2015) Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta Neuropathol 130:263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liessem-Schmitz A, Teske N, Scheld M, Nyamoya S, Zendedel A, Beyer C, Clarner T, Fragoulis A (2018) Nrf2 signaling in sodium azide-treated oligodendrocytes restores mitochondrial functions. J Mol Neurosci 66:229–237. [DOI] [PubMed] [Google Scholar]
- Liu JM, Yi Z, Liu SZ, Chang JH, Dang XB, Li QY, Zhang YL (2015) The mitochondrial division inhibitor mdivi-1 attenuates spinal cord ischemia-reperfusion injury both in vitro and in vivo: Involvement of BK channels. Brain Res 1619:155–165. [DOI] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61:541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AN, Czajka A (2013) Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 13:481–492. [DOI] [PubMed] [Google Scholar]
- Malik AN, Shahni R, Iqbal MM (2009) Increased peripheral blood mitochondrial DNA in type 2 diabetic patients with nephropathy. Diabetes Res Clin Pract 86:e22–24. [DOI] [PubMed] [Google Scholar]
- Malik AN, Shahni R, Rodriguez-de-Ledesma A, Laftah A, Cunningham P (2011) Mitochondrial DNA as a non-invasive biomarker: accurate quantification using real time quantitative PCR without co-amplification of pseudogenes and dilution bias. Biochem Biophys Res Commun 412:1–7. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A (2008) Mitochondria in neuroplasticity and neurological disorders. Neuron 60:748–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkov A, Baskar R, Alcalay Y, Abdu U (2016) A new mode of mitochondrial transport and polarized sorting regulated by Dynein, Milton and Miro. Development 143:4203–4213. [DOI] [PubMed] [Google Scholar]
- Michel S, Wanet A, De Pauw A, Rommelaere G, Arnould T, Renard P (2012) Crosstalk between mitochondrial (dys) function and mitochondrial abundance. J Cell Physiol 227:2297–2310. [DOI] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30:4232–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr J, Wolf PA, Moskowitz MA, Mayberg MR, Von Kummer R (2011) Stroke E-Book: Pathophysiology, diagnosis, and management: Elsevier Health Sciences. [Google Scholar]
- Moraes CT, Atencio DP, Oca-Cossio J, Diaz F (2003) Techniques and pitfalls in the detection of pathogenic mitochondrial DNA mutations. J Mol Diagn 5:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlino G, Barreiro O, Baixauli F, Robles-Valero J, Gonzalez-Granado JM, Villa-Bellosta R, Cuenca J, Sanchez-Sorzano CO, Veiga E, Martin-Cofreces NB, Sanchez-Madrid F (2014) Miro-1 links mitochondria and microtubule Dynein motors to control lymphocyte migration and polarity. Mol Cell Biol 34:1412–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136. [DOI] [PubMed] [Google Scholar]
- Narayanan SV, Dave KR, Perez-Pinzon MA (2018) Ischemic preconditioning protects astrocytes against oxygen glucose deprivation via the nuclear erythroid 2-related factor 2 pathway. Transl Stroke Res 9:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan SV, Dave KR, Saul I, Perez-Pinzon MA (2015) Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke 46:1626–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratil M, Terman A, Arriaga EA (2008) Giant mitochondria do not fuse and exchange their contents with normal mitochondria. Exp Cell Res 314:164–172. [DOI] [PubMed] [Google Scholar]
- Oldenburg O, Cohen MV, Yellon DM, Downey JM (2002) Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res 55:429–437. [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121:2012–2022. [DOI] [PubMed] [Google Scholar]
- Park SJ, Park YJ, Shin JH, Kim ES, Hwang JJ, Jin DH, Kim JC, Cho DH (2011a) A receptor tyrosine kinase inhibitor, Tyrphostin A9 induces cancer cell death through Drp1 dependent mitochondria fragmentation. Biochem Biophys Res Commun 408:465–470. [DOI] [PubMed] [Google Scholar]
- Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, Ellisman MH, Weinreb RN, Ju WK (2011b) A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Ophthalmol Vis Sci 52:2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Dave KR, Raval AP (2005) Role of reactive oxygen species and protein kinase C in ischemic tolerance in the brain. Antioxid Redox Signal 7:1150–1157. [DOI] [PubMed] [Google Scholar]
- Peters A (1999) Normal aging in the cerebral cortex of primates. In: Cerebral cortex, pp 49–80: Springer. [Google Scholar]
- Piko L, Matsumoto L (1976) Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol 49:1–10. [DOI] [PubMed] [Google Scholar]
- Piquereau J, Caffin F, Novotova M, Lemaire C, Veksler V, Garnier A, Ventura-Clapier R, Joubert F (2013) Mitochondrial dynamics in the adult cardiomyocytes: which roles for a highly specialized cell? Front Physiol 4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravati A, Ahlemeyer B, Becker A, Krieglstein J (2000) Preconditioning-induced neuroprotection is mediated by reactive oxygen species. Brain Res 866:23–32. [DOI] [PubMed] [Google Scholar]
- Ravati A, Ahlemeyer B, Becker A, Klumpp S, Krieglstein J (2001) Preconditioning-induced neuroprotection is mediated by reactive oxygen species and activation of the transcription factor nuclear factor-kappaB. J Neurochem 78:909–919. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P (2011) Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67:103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Enriquez S, Kai Y, Maldonado E, Currin RT, Lemasters JJ (2009) Roles of mitophagy and the mitochondrial permeability transition in remodeling of cultured rat hepatocytes. Autophagy 5:1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE (2009) Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J Neurosci 29:5443–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G (2008) Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A 105:20728–20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev - Amzaleg N, Trudler D, Frenkel D (2013) Preconditioning to mild oxidative stress mediates astroglial neuroprotection in an IL-10-dependent manner. Brain Behav Immun 30:176–185. [DOI] [PubMed] [Google Scholar]
- Selak MA, Lyver E, Micklow E, Deutsch EC, Onder O, Selamoglu N, Yager C, Knight S, Carroll M, Daldal F, Dancis A, Lynch DR, Sarry JE (2011) Blood cells from Friedreich ataxia patients harbor frataxin deficiency without a loss of mitochondrial function. Mitochondrion 11:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SK (2010) Biology of aging brain. Indian J Pathol Microbiol 53:595–604. [DOI] [PubMed] [Google Scholar]
- Skulachev VP (2001) Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 26:23–29. [DOI] [PubMed] [Google Scholar]
- Stahon KE, Bastian C, Griffith S, Kidd GJ, Brunet S, Baltan S (2016) Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J Neurosci 36:9990–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryana E, Jones NM (2014) The effects of hypoxic preconditioning on white matter damage following hypoxic-ischaemic injury in the neonatal rat brain. Int J Dev Neurosci 37:69–75. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Duchen MR (2008) Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 23:84–94. [DOI] [PubMed] [Google Scholar]
- Tang BL (2015) MIRO GTPases in Mitochondrial Transport, Homeostasis and Pathology. Cells 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkok SB, Ye Z, Ransom BR (2007) Excitotoxic mechanisms of ischemic injury in myelinated white matter. J Cereb Blood Flow Metab 27:1540–1552. [DOI] [PubMed] [Google Scholar]
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC (2009) SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 28:1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V (2001) Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 55:597–610. [DOI] [PubMed] [Google Scholar]
- Valenti D, Rossi L, Marzulli D, Bellomo F, De Rasmo D, Signorile A, Vacca RA (2017) Inhibition of Drp1-mediated mitochondrial fission improves mitochondrial dynamics and bioenergetics stimulating neurogenesis in hippocampal progenitor cells from a Down syndrome mouse model. Biochim Biophys Acta Mol Basis Dis 1863:3117–3127. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT (1998) Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273:18092–18098. [DOI] [PubMed] [Google Scholar]
- Veltri KL, Espiritu M, Singh G (1990) Distinct genomic copy number in mitochondria of different mammalian organs. J Cell Physiol 143:160–164. [DOI] [PubMed] [Google Scholar]
- Vemuganti R, Arumugam TV (2021) Much ado about eating: Intermittent fasting and post-stroke neuroprotection. J Cereb Blood Flow Metab 41:1791–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldow T, Alexiou K, Witt W, Albrecht S, Wagner F, Knaut M, Matschke K (2005) Protection against acute porcine lung ischemia/reperfusion injury by systemic preconditioning via hind limb ischemia. Transpl Int 18:198–205. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang P, Li S, Wang S, Li Y, Liang N, Wang M (2014) Mdivi-1 prevents apoptosis induced by ischemia-reperfusion injury in primary hippocampal cells via inhibition of reactive oxygen species-activated mitochondrial pathway. J Stroke Cerebrovasc Dis 23:1491–1499. [DOI] [PubMed] [Google Scholar]
- Wang X, Schwarz TL (2009) The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147:893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11:872–884. [DOI] [PubMed] [Google Scholar]
- Wu Y, Gu C, Huang LU, Zhao Y, Tang Y, Zhao H, Wu Q (2021) Hypoxia preconditioning improves structure and function of astrocytes mitochondria via PGC-1alpha/HIF signal. J Biosci 46. [PubMed] [Google Scholar]
- Xie H, Wu Y, Jia J, Liu G, Zhang F, Zhang Q, Yu K, Hu Y, Bai Y, Hu R (2013) Enriched environment preconditioning induced brain ischemic tolerance without reducing infarct volume and edema: the possible role of enrichment-related physical activity increase. Brain Res 1508:63–72. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang W, Gao X, Zhao Y, Chen D, Xu N, Pu H, Stetler RA, Gao Y (2019) Preconditioning with partial caloric restriction confers long-term protection against grey and white matter injury after transient focal ischemia. J Cereb Blood Flow Metab 39:1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ (2000) A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A 97:5621–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wang S, Li Y, Che L, Zhao Q (2013a) A selective inhibitor of Drp1, mdivi-1, acts against cerebral ischemia/reperfusion injury via an anti-apoptotic pathway in rats. Neurosci Lett 535:104–109. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, Wang G, Chen Z (2013b) Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9:1321–1333. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gerstein M (2003) Identification and characterization of over 100 mitochondrial ribosomal protein pseudogenes in the human genome. Genomics 81:468–480. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285:H579–588. [DOI] [PubMed] [Google Scholar]
- Zuo W, Zhang S, Xia CY, Guo XF, He WB, Chen NH (2014) Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: the role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology 86:103–115. [DOI] [PubMed] [Google Scholar]


