Abstract
Purpose
While recreational physical activity (RPA) has been associated with reduced mortality in breast, colorectal, and prostate cancers, evidence for epithelial ovarian cancer (EOC) is limited. Most EOC studies have been in predominantly white populations, although inactivity is more prevalent and survival is poorer among African-American (AA) women. We examined RPA before and after EOC diagnosis and associations with survival among AA women.
Methods
We analyzed data from 264 EOC survivors enrolled in a population-based, case–control study who completed surveys that included questions about pre- and post-diagnosis RPA. Data were collected on RPA frequency, intensity, and duration before diagnosis and approximately 1 year after the baseline interview. We calculated metabolic equivalent of task (MET)-hours/week for pre- and post-diagnosis RPA, and evaluated associations with risk of mortality using Cox proportional hazards models.
Results
RPA before diagnosis was not associated with mortality. Hazard ratios (HRs) for post-diagnosis RPA were < 1.0 but not statistically significant after adjustment for covariates; HRs were 0.94 (95% CI 0.58, 1.54) for > 0–9 MET-hours/week and 0.53 (95% CI 0.21, 1.35) for > 9 MET-hours/week.
Conclusions
Our results suggest that RPA may be inversely associated with mortality among AA women with ovarian cancer, although it is possible that the present study was underpowered to detect an association. There is a clear need for more studies of RPA after diagnosis in EOC survivors with attention to potential differences by race.
Keywords: Physical activity, African-American, Ovarian cancer, Cancer survival
Introduction
Ovarian cancer is the fifth leading cause of cancer death in women, with an estimated 14,080 deaths in the United States in 2017 [1]. While the incidence of epithelial ovarian cancer (EOC) is lower in African-American (AA) than in European-American (EA) women [2], 5-year relative survival is poorer in AA women (36 vs. 46%) [1] and the causes of this disparity are not well understood.
Physical activity, a modifiable lifestyle factor, has been associated with reduced mortality [3–5] and reduced risk of recurrence [3] in breast, colorectal, and prostate cancers. However, evidence among non-white minority populations for a relationship between physical activity and other cancers, including ovarian cancer, is limited. As inactivity is prevalent among cancer survivors [6], there is a need for a better understanding of potential effects of regular exercise before and after EOC diagnosis on mortality and other outcomes. A recent pooled analysis of 12 studies participating in the Ovarian Cancer Association Consortium (OCAC) found that EOC survivors who were inactive before diagnosis had a 34% greater risk of mortality compared with women reporting any regular physical activity [7]. A recent review [8] identified only one prospective [9] and two case–control [10, 11] studies of recreational physical activity (RPA) and survival in women with EOC; one additional study [12] was prospective but had a small number of EOC cases. All four studies found no overall association between RPA before diagnosis and survival [9–12], although statistically significant associations were reported for various subgroups. Notably, the Women’s Health Initiative [9] reported a 26% lower risk of EOC-specific mortality associated with vigorous-intensity RPA. Other subgroup analyses suggestive of a relationship between RPA and EOC survival include an inverse association between physical activity and mortality among women with a BMI < 30 kg/m2 in the North Carolina Ovarain Cancer Study [10] and a reported benefit from physical activity among those diagnosed with early-stage disease in Sweden [11].
To our knowledge, no study to date has examined RPA after EOC diagnosis in association with EOC survival. Additionally, the majority of literature on RPA and EOC has been in predominantly white populations: the OCAC pooled analysis included mostly white participants (88.6%) and only 2% (n = 135) were black [7], while the North Carolina Ovarian Cancer Study included the second greatest number of black EOC survivors with 80 African-American (AA) cases [10]. However, survival is poorer [1] and the prevalence of RPA is lower [13, 14] in AA women. To address these knowledge gaps, we analyzed the associations of RPA before and after EOC diagnosis with survival in the largest study of EOC in AA women to date.
Methods
Study population
The African American Cancer Epidemiology Study (AACES) is a population-based, case–control study of AA women. AACES includes 11 participating sites (Alabama, Georgia, Illinois, Louisiana, Michigan, New Jersey, North Carolina, Ohio, South Carolina, Tennessee, and Texas). Institutional review board approval was obtained from all participating institutions and informed consent was obtained from all study participants. Methods have been described previously in detail [15]. Briefly, cases are AA women aged 20–79 with newly diagnosed EOC between December 2010 and December 2015. Participants completed a baseline telephone interview including questions on demographic characteristics, reproductive and medical history, family history, and lifestyle characteristics including smoking and physical activity. To improve participation, a short form of the questionnaire was offered to women who would otherwise have refused; however, the short form did not include physical activity questions. Cases were followed annually and a follow-up telephone survey including questions on physical activity after diagnosis and other factors potentially related to outcome was administered.
A total of 601 cases have been enrolled, 549 of whom completed the full questionnaire at baseline. Of these, 420 were enrolled prior to the last year of the study, including 50 who died less than a year after the baseline interview, making 370 eligible to complete a first annual follow-up survey (see Fig. 1). A total of 267 women completed a first annual follow-up survey approximately 1 year after completion of the baseline interview, with a mean time since diagnosis of 22.4 months. Analyses were restricted to cases who completed a follow-up survey. Cases missing BMI (n = 1) or income (n = 2) were excluded from analyses, resulting in a sample size of 264 (71.4% of eligible).
Fig. 1.
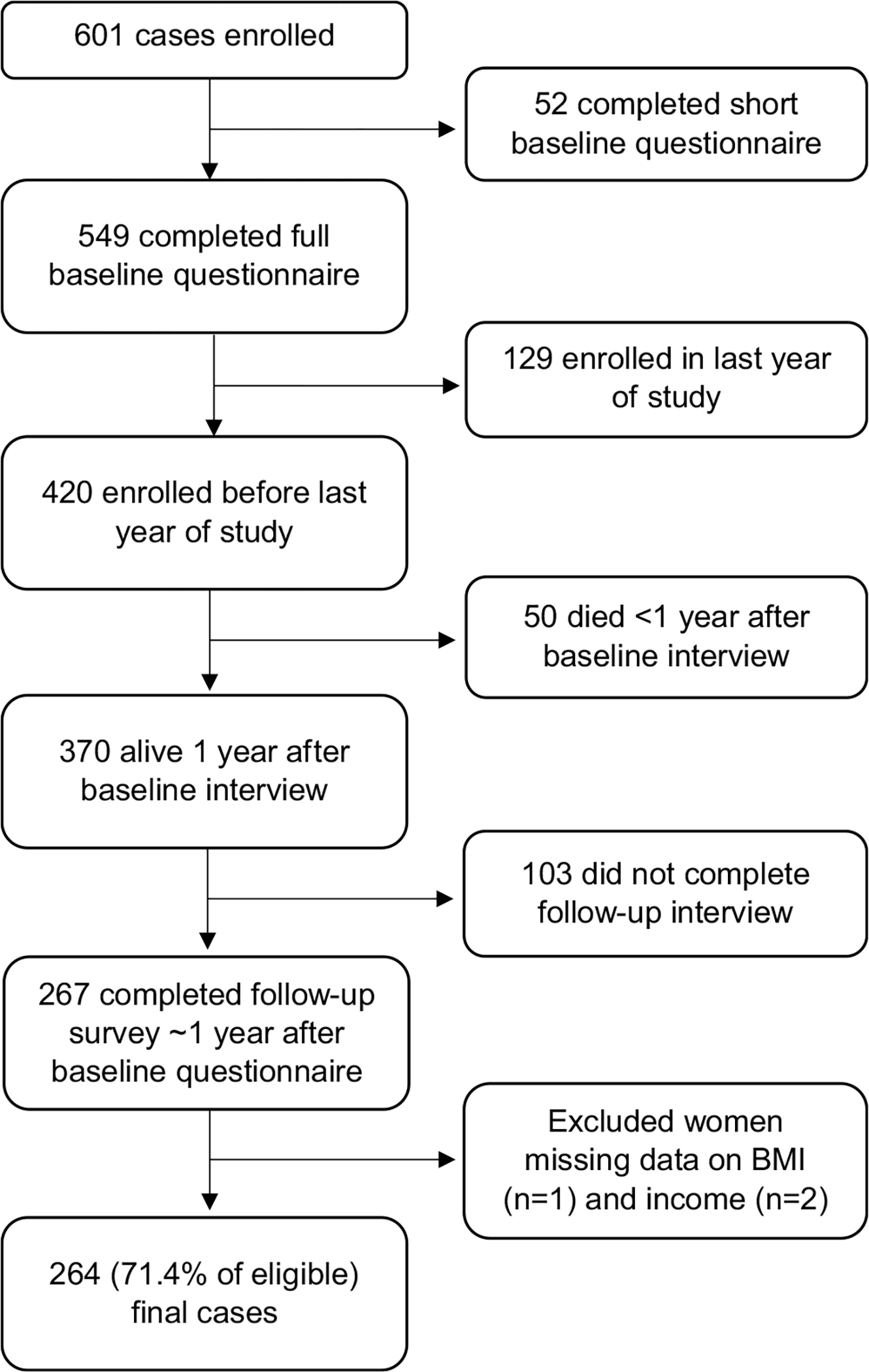
Flow chart of case selection for analysis of recreational physical activity and survival
Recreational physical activity
At baseline, participants reported their average weekly RPA (frequency, duration, and intensity) 1 year before diagnosis; in the follow-up interview, they reported their average weekly RPA during the prior year. The questions, which have been described previously [16], were adapted from the International Physical Activity Questionnaire (IPAQ) [17] and assessed mild-, moderate-, and strenuous-intensity activity. Average total weekly hours were calculated by multiplying the number of times per week and the average length of each exercise session at each intensity. Each intensity level was assigned a metabolic equivalent of task (MET) score according to IPAQ guidelines [17] and MET-hours/week were calculated using the following equation:
The number of MET-hours/week was categorized into three levels: 0, > 0–9, and > 9 MET-hours/week. These cut points were chosen because 9 MET-hours/week is approximately the amount recommended by the 2008 U.S. Department of Health and Human Services Physical Activity Guidelines for Americans (PAG) [18], defined as performing at least 150 min/week moderate-intensity RPA, 75 min/week vigorous-intensity RPA, or an equivalent combination. The PAG do not include a recommendation for mild-intensity RPA. The PAG are the currently recommended guidelines for cancer survivors [19]. Inactivity before and after diagnosis was defined as 0 MET-hours/week.
We additionally classified RPA according to the intensity for which participants reported at least 50% of their total RPA time per week, the predominant RPA intensity. In the event of a tie, participants were classified according to the higher intensity. Moderate and strenuous intensity were combined due to the small number of participants reporting predominantly strenuous RPA.
Statistical analysis
Distributions of demographic, lifestyle, and clinical characteristics were determined for cases by categories of MET-hours/week before diagnosis (0, > 0–9, and > 9 MET-hours/week). Chi-square tests (for categorical variables) or Kruskal–Wallis tests (for continuous variables) were performed to compare these groups.
Survival was assessed with time-to-event analyses. Vital status and date of death/date of last contact were obtained from LexisNexis Accurint [20], state cancer registries, medical records, and follow-up interview dates. Kaplan–Meier survival curves were used to compare survival by category of RPA before and after EOC diagnosis. Log-rank tests were performed to compare survival between groups. Cox proportional hazards (PH) models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between all-cause mortality and RPA before and after diagnosis, adjusting for covariates. We further evaluated the association between MET-hours/week and ovarian cancer mortality within predominant RPA intensity categories (mild and moderate/strenuous). In these models, time from diagnosis was used as the underlying time variable with delayed entry at baseline interview for associations before diagnosis and at follow-up interview for associations after diagnosis. Delayed entry was used to avoid biasing time-dependent exposures on future events [21]. Cox PH models were stratified by covariates which may modify the association between RPA and risk of mortality, including histology (serous, non-serous), stage (I–II, III–IV), and body mass index (BMI < 30, 30+ kg/m2).
Covariates included in the survival analyses were age at diagnosis, region (south and mid-Atlantic [Georgia, New Jersey, North Carolina, South Carolina]; south central [Alabama, Louisiana, Tennessee, Texas]; Midwest [Illinois, Michigan, Ohio]), stage at diagnosis (I–II; III–IV; unstaged), number of comorbid conditions using a modified Charlson comorbidity index [22] (0, 1, 2+), education (high school or less, some post-high school training, college or graduate degree), and family income (< $25,000, ≥ $25,000). The following were considered for inclusion as covariates in the adjusted models: family history of breast/ovarian cancer (yes/no), histology (serous/non-serous), BMI approximately 1 year before diagnosis (< 25, 25–29.9, 30+ kg/m2), parity (0, 1, 2+ live births), menopausal status (pre-/post-menopause), smoking (ever/never), and occupational physical activity at baseline (mainly sitting, mainly standing or walking, mainly active, do not work outside the home). However, none of these changed the HR by ≥ 10% and therefore were not included in the final adjusted models.
All included participants underwent primary debulking surgery. Although residual disease after primary debulking surgery is an established prognostic factor for EOC [23, 24], information on residual disease was only available for a subset of participants (n = 158). Therefore, we performed a sensitivity analysis restricted to cases with residual disease data and adjusted for this variable in our analyses (optimal debulking: residual disease < 1 cm; suboptimal debulking: residual disease ≥ 1 cm). For participants missing residual disease data, we used a CA 125 of > 35 U/mL after the first round of adjuvant chemotherapy as a proxy for suboptimal debulking [25]. All analyses were performed using SAS version 9.4 (Cary, NC, USA).
Results
Characteristics of participants who completed a follow-up survey are presented in Table 1 according to their RPA category before diagnosis. Participants who were inactive (0 MET-hours/week) before diagnosis had lower educational attainment than participants (p = 0.02); otherwise, participant characteristics were not statistically significantly different between these groups (Table 1). Mean follow-up time was 42.7 months since diagnosis and 80 deaths were recorded.
Table 1.
Characteristics of African-American ovarian cancer survivors by recreational physical activity (MET-hours/week) before diagnosis (n = 264)
| Inactive (0 MET-hours/week)a
n = 90 |
> 0–9 MET-hours/week n = 94 |
> 9 MET-hours/week n = 80 |
p value | ||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
|
| |||||||
| Mean age at diagnosis (years) (SD) | 58.9 | (10.2) | 57.8 | (9.6) | 57.4 | (11.1) | 0.72 |
| Median age at diagnosis (years) | 58.0 | 56.0 | 58.5 | ||||
| Region | 0.35 | ||||||
| South and mid-Atlantic | 48 | (53.3) | 60 | (63.8) | 41 | (51.3) | |
| South central | 28 | (31.1) | 25 | (26.6) | 24 | (30.0) | |
| Midwest | 14 | (15.6) | 9 | (9.6) | 15 | (18.8) | |
| Education | 0.02 | ||||||
| High school or less | 51 | (56.7) | 38 | (40.4) | 28 | (35.0) | |
| Some post-high school training | 21 | (23.3) | 26 | (27.7) | 19 | (23.8) | |
| College or graduate degree | 18 | (20.0) | 30 | (31.9) | 33 | (41.3) | |
| Family income | 0.41 | ||||||
| < $25,000 | 45 | (50.0) | 39 | (41.5) | 33 | (41.3) | |
| ≥ $25,000 | 45 | (50.0) | 55 | (58.5) | 47 | (58.8) | |
| Number of comorbid conditionsb | 0.11 | ||||||
| 0 | 25 | (27.8) | 39 | (41.5) | 38 | (47.5) | |
| 1 | 23 | (25.6) | 21 | (22.3) | 15 | (18.8) | |
| 2+ | 42 | (46.7) | 34 | (36.2) | 27 | (33.8) | |
| Body Mass Index (BMI) (kg/m2) | 0.42 | ||||||
| <25 (underweight or normal weight) | 11 | (12.2) | 13 | (13.8) | 18 | (22.5) | |
| 25–29.9 (overweight) | 24 | (26.7) | 26 | (27.7) | 20 | (25.0) | |
| 30+ (obese) | 55 | (61.1) | 55 | (58.5) | 42 | (52.5) | |
| Parity (number of live births) | 0.37 | ||||||
| 0 | 11 | (12.2) | 19 | (20.2) | 20 | (25.0) | |
| 1 | 13 | (14.4) | 18 | (19.2) | 10 | (12.5) | |
| 2 | 25 | (27.8) | 21 | (22.3) | 20 | (25.0) | |
| 3+ | 41 | (45.6) | 36 | (38.3) | 30 | (37.5) | |
| Family history of breast or ovarian cancer | 0.14 | ||||||
| No | 71 | (78.9) | 63 | (67.0) | 54 | (67.5) | |
| Yes | 19 | (21.1) | 31 | (33.0) | 26 | (32.5) | |
| Menopausal status | 0.49 | ||||||
| Premenopausal | 20 | (22.2) | 28 | (29.8) | 20 | (25.0) | |
| Postmenopausal | 70 | (77.8) | 66 | (70.2) | 60 | (75.0) | |
| Smoking status | 0.31 | ||||||
| Never | 49 | (54.4) | 60 | (63.8) | 43 | (53.8) | |
| Ever | 41 | (45.6) | 34 | (36.2) | 37 | (46.3) | |
| Occupational physical activity | 0.40 | ||||||
| Mainly sitting | 16 | (17.8) | 16 | (17.0) | 13 | (16.3) | |
| Mainly standing or walking | 18 | (20.0) | 15 | (16.0) | 22 | (27.5) | |
| Mainly active | 10 | (11.1) | 18 | (19.2) | 14 | (17.5) | |
| Do not work outside the home | 46 | (51.1) | 45 | (47.9) | 31 | (38.8) | |
| Stage | 0.79 | ||||||
| I–II | 30 | (33.3) | 32 | (34.0) | 32 | (40.0) | |
| III–IV | 55 | (61.1) | 58 | (61.7) | 46 | (57.5) | |
| Unstaged | 5 | (5.6) | 4 | (4.3) | 2 | (2.5) | |
| Histology | 0.93 | ||||||
| Serous | 63 | (70.0) | 65 | (69.2) | 54 | (67.5) | |
| Mucinous | 1 | (1.1) | 7 | (7.5) | 2 | (2.5) | |
| Endometrioid | 15 | (16.7) | 12 | (12.8) | 10 | (12.5) | |
| Clear cell | 2 | (2.2) | 5 | (5.3) | 4 | (5.0) | |
| Other | 7 | (7.8) | 5 | (5.3) | 8 | (10.0) | |
| Missing | 2 | (2.2) | 0 | (0.0) | 2 | (2.5) | |
| Residual disease | 0.09 | ||||||
| Optimal debulking | 31 | (34.4) | 39 | (41.5) | 43 | (53.8) | |
| Suboptimal debulking | 20 | (22.2) | 14 | (14.9) | 11 | (13.8) | |
| Missing residual disease | 39 | (43.3) | 41 | (43.6) | 26 | (32.5) | |
Metabolic equivalent of task (MET)-hours/week
Number of comorbid conditions was determined using a modified Charlson comorbidity index [22]
cp value is for serous versus non-serous cases due to small numbers of non-serous histotypes
Before diagnosis, 24.6% of cases met the PAG for aerobic activity [18], compared with only 9.1% meeting the PAG after diagnosis (p < 0.01; Table 2). Approximately one-third of the cases (34.1%) were inactive before diagnosis, which increased to 49.2% after diagnosis (p < 0.01).
Table 2.
Recreational physical activity (RPA) before and after ovarian cancer diagnosis
| Before diagnosis |
After diagnosis |
p value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
|
| |||||
| MET-hours/week | < 0.01 | ||||
| Inactive (0 MET-hours/week) | 90 | (34.1) | 130 | (49.2) | |
| >0–9 MET-hours/week | 94 | (35.6) | 90 | (34.1) | |
| >9 MET-hours/week | 80 | (30.3) | 44 | (16.7) | |
| Physical Activity Guidelines for Americans (PAG)a | < 0.01 | ||||
| Meeting PAG | 65 | (24.6) | 24 | (9.1) | |
| Not meeting PAG | 199 | (75.4) | 240 | (90.9) | |
Defined as at least 150 min/week moderate-intensity RPA, 75 min/ week vigorous-intensity RPA, or an equivalent combination
Unadjusted Kaplan–Meier survival curves are presented in Fig. 2. Survival did not differ by pre-diagnosis RPA. Improved survival was observed among participants reporting 9 MET-hours/week after diagnosis (p = 0.02) and those meeting the PAG after diagnosis (p = 0.04).
Fig. 2.
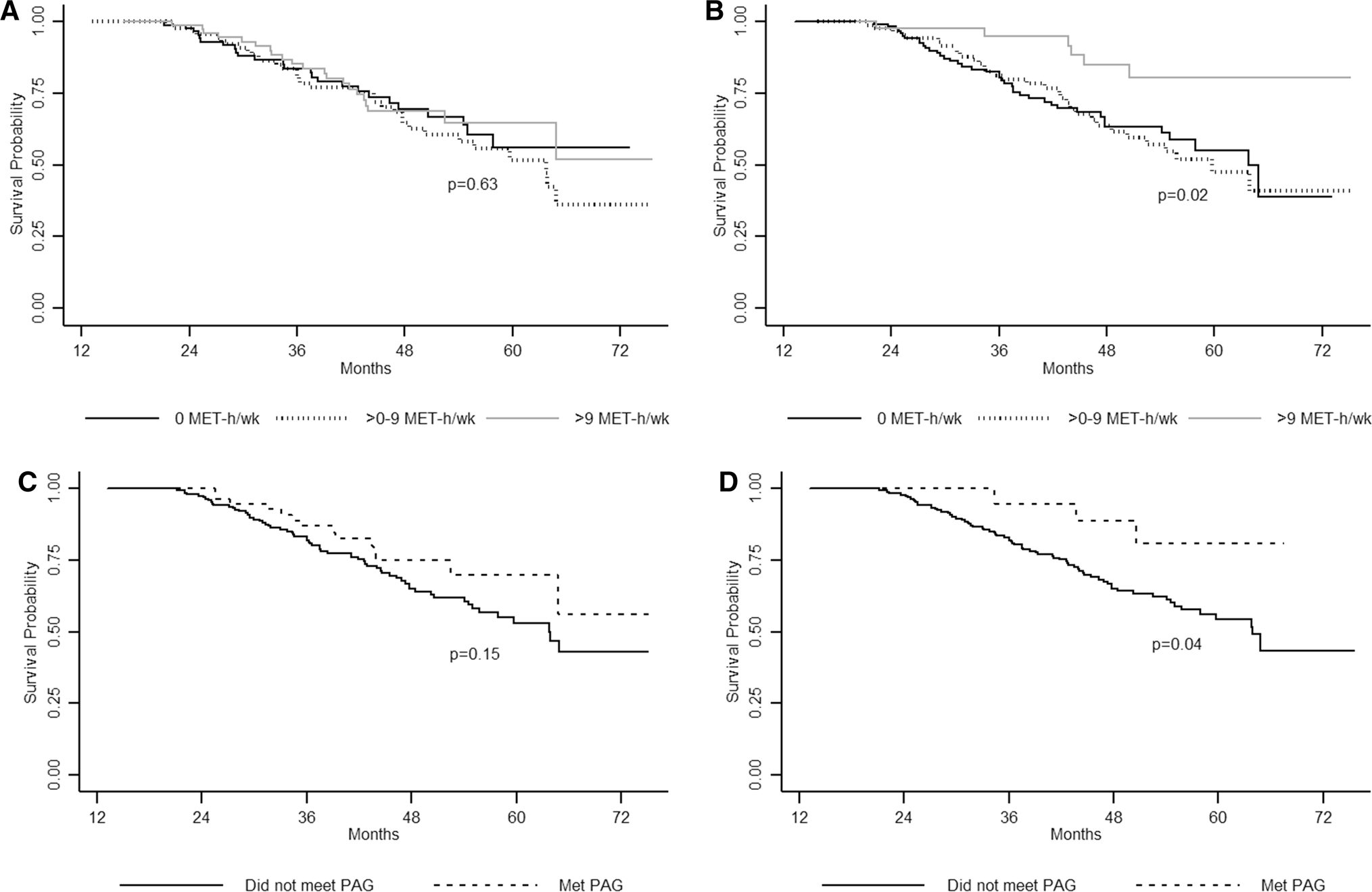
Kaplan–Meier survival curves of overall survival by physical activity in MET-hours/week a 1 year before diagnosis and b after diagnosis; and overall survival according to whether participants met the Physical Activity Guidelines for Americans (PAG) c 1 year before diagnosis and d after diagnosis
In multivariable analyses, RPA before diagnosis was not associated with mortality (Table 3). In the fully adjusted model, HRs for RPA after diagnosis were < 1.0, with HR = 0.94 (95% CI 0.58, 1.54) and HR = 0.53 (95% CI 0.21, 1.35) for > 0–9 MET-hours/week and > 9 MET-hours/week, respectively. The highest category of activity (> 9 MET-hours/week) was statistically significant in the minimally adjusted model (adjusted for age, stage, and region), but was slightly attenuated and not significant in the fully adjusted model. We also did not observe an association between RPA and mortality for women reporting mild or moderate/strenuous RPA as their predominant RPA intensity (data not shown).
Table 3.
Association between all-cause mortality and recreational physical activity before and after diagnosis
| n | (%) | Minimally adjusteda |
Fully adjustedb |
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
|
| ||||||
| RPA before diagnosis | ||||||
| Inactive (0 MET-hours/week) | 90 | (34.1) | 1.00 | Referent | 1.00 | Referent |
| > 0–9 MET-hours/week | 94 | (35.6) | 1.02 | 0.60, 1.73 | 1.04 | 0.61, 1.79 |
| > 9 MET-hours/week | 80 | (30.3) | 0.94 | 0.52, 1.68 | 1.01 | 0.55, 1.83 |
| RPA after diagnosis | ||||||
| Inactive (0 MET-hours/week) | 130 | (49.2) | 1.00 | Referent | 1.00 | Referent |
| > 0–9 MET-hours/week | 90 | (34.1) | 0.92 | 0.58, 1.48 | 0.94 | 0.58, 1.54 |
| > 9 MET-hours/week | 44 | (16.7) | 0.40 | 0.17, 0.96 | 0.53 | 0.21, 1.35 |
| PAG before diagnosisc | ||||||
| Not meeting PAG | 199 | (75.4) | 1.00 | Referent | 1.00 | Referent |
| Meeting PAG | 65 | (24.6) | 0.66 | 0.37, 1.17 | 0.70 | 0.39, 1.26 |
| PAG after diagnosisc | ||||||
| Not meeting PAG | 240 | (90.9) | 1.00 | Referent | 1.00 | Referent |
| Meeting PAG | 24 | (9.1) | 0.35 | 0.11, 1.12 | 0.46 | 0.14, 1.56 |
Adjusted for age, stage, and geographic region
Adjusted for age, stage, geographic region, number of comorbid conditions, education, and income. RPA after diagnosis is additionally adjusted for pre-diagnosis RPA (0, > 0–9, > 9 MET-hours/week)
Physical Activity Guidelines for Americans, defined as at least 150 min/week moderate-intensity RPA, 75 min/week vigorous-intensity RPA, or an equivalent combination
Survival analyses were also performed according to whether participants met the PAG before and after diagnosis. Women meeting the PAG before diagnosis had non-statistically significantly better prognosis than those not meeting the PAG, with HR = 0.70 (95% CI 0.39, 1.26) (Table 3). The HR for women meeting the PAG 1 year after diagnosis was more pronounced yet not significant, with HR = 0.46 (95% CI 0.14, 1.56).
Stratification by stage, histology (serous vs. non-serous histotypes), and BMI did not yield any notable differences in mortality (data not shown) and samples sizes in some strata were small and inadequate. For RPA after diagnosis, HRs for serous-only cases were 0.89 (95% CI 0.51, 1.54) and 0.49 (95% CI 0.18, 1.35) for > 0–9 MET-hours/week and > 9 MET-hours/week, respectively. Cases diagnosed at a later stage had HRs that were slightly more pronounced yet similar to the overall HRs, with HR = 0.83 (95% CI 0.48, 1.42) and HR = 0.36 (95% CI 0.11, 1.14) for > 0–9 MET-hours/week and > 9 MET-hours/week, respectively. It was not possible to assess differences by histology for non-serous histotypes and stage at diagnosis due to small numbers of non-serous cases and cases diagnosed at an earlier stage.
We also evaluated the HRs and 95% CIs for the associations between inactivity before and after diagnosis and mortality for comparison to the OCAC pooled analysis. For inactivity before diagnosis, the minimally adjusted HR was 1.01 (95% CI 0.63, 1.64) and the fully adjusted HR was 0.97 (95% CI 0.60, 1.59); for inactivity after diagnosis, the minimally adjusted HR was 1.30 (95% CI 0.83, 2.04) and the fully adjusted HR was 1.17 (95% CI 0.72, 1.89) (data not shown).
In a sensitivity analysis restricted to cases with information on residual disease, adjustment for residual disease (optimal/suboptimal) did not change the HRs by ≥ 10%. In the fully adjusted model for pre-diagnosis RPA, HRs were 1.13 (95% CI 0.55, 2.31) and 0.89 (95% CI 0.40, 1.99) for > 0–9 MET-hours/week and > 9 MET-hours/week, respectively, before adjustment for residual disease; after adjustment for residual disease, HRs were 1.16 (95% CI 0.57, 2.39) and 0.93 (95% CI 0.41, 2.12) for > 0–9 MET-hours/week and > 9 MET-hours/week, respectively. For post-diagnosis RPA, fully adjusted HRs were 0.85 (95% CI 0.42, 1.69) and 0.64 (95% CI 0.20, 2.07) before adjustment for residual disease and 0.83 (95% CI 0.42, 1.66) and 0.63 (95% CI 0.19, 2.04) after adjustment for residual disease, for > 0–9 MET-hours/week and > 9 MET-hours/week, respectively (data not shown).
Discussion
In the present study, we observed no association between RPA 1 year before diagnosis and survival, although meeting the PAG 1 year before diagnosis was not statistically significantly associated with improved survival. The HRs for the association between RPA after diagnosis and mortality among EOC survivors were in the inverse direction, as was the HR for participants meeting the PAG after diagnosis, although these associations were not statistically significant. The direction and magnitude of these associations persisted among serous cases and women diagnosed with later stage disease, although small numbers of non-serous cases and women with early-stage disease limited our ability to determine whether there were differences by histology and stage.
The prevalence of inactivity before diagnosis in AACES (34.1%) is similar to that of AA women in the general population (36.1% according to a Centers for Disease Control and Prevention report) [13]. The prevalence of inactivity approximately 1 year after EOC diagnosis was statistically significantly higher (49.2%; p < 0.01) and just 9.1% of survivors were meeting the PAG for aerobic activity. While the five previous reports of RPA and survival did not have information on RPA after diagnosis [7, 9–12], a study of RPA and quality of life in EOC survivors reported that 31.1% of participants were meeting physical activity guidelines [26]. Blanchard et al. [6] reported that the prevalence of physical activity among cancer survivors in general is low and varies by cancer site, ranging from 29.6 to 47.3% meeting recommendations. Both of these reports [6, 26] used the 2006 American Cancer Society (ACS) guidelines for strenuous-intensity RPA [27] (60 vs. 75 min/week in the current guidelines). For comparison to these reports, we also determined the prevalence of participants meeting the 2006 guidelines; the prevalence of AACES participants meeting the 2006 guidelines was identical to the prevalence meeting the 2008 guidelines (9.1%) (data not shown), indicating that a lower prevalence of AACES survivors met the guidelines compared to these reports. It is possible that this difference is due to differences in race, cancer site, or gender.
AACES is the first study to examine the associations of RPA after diagnosis with EOC survival. Our findings for inactivity after diagnosis (HR = 1.17, 95% CI 0.72, 1.89) and meeting the PAG after diagnosis (HR = 0.46, 95% CI 0.14, 1.56) suggest that inactivity or less than the recommended amount of activity after diagnosis may be associated with increased risk of mortality among EOC survivors, although these results were not statistically significant and must therefore be interpreted with caution. It is possible that physical activity plays a smaller role in EOC survival than in other cancers; the present study may also not have had adequate power to detect an association, primarily due to the low prevalence of RPA within this population.
Our finding of no overall association between RPA before diagnosis and survival (for inactivity as well as for categories of MET-hours/week) is consistent with four previous studies of RPA and ovarian cancer survival [9–12] but is not consistent with the OCAC pooled analysis, the largest study to date and the only study to use inactivity as the exposure [7]. The OCAC found a 34% increased risk of mortality among EOC survivors who were inactive before diagnosis [7]. However, it is worth noting that the association for participants meeting the PAG before diagnosis in the present study (HR = 0.70, 95% CI 0.39, 1.26), while not statistically significant, is similar in magnitude to the 34% increased risk associated with inactivity reported by the OCAC pooled analysis.
Several potential biological mechanisms for effects of exercise on cancer risk or prognosis have been proposed. RPA may affect carcinogenesis or prognosis through effects on obesity, such as changes in levels of sex hormones, adipokines, and cytokines, and blood insulin levels and insulin resistance [28]. Effects could also occur through pathways independent of obesity [7, 28, 29]. For instance, physical activity reduces insulin resistance and hyperinsulinemia independently of changes in body composition [28]. It is also possible that RPA is indicative of better overall health or of other lifestyle behaviors such as healthier diet [28].
A limitation of this study is the retrospective collection of self-reported pre-diagnosis RPA as the baseline survey was administered after diagnosis. However, cases were asked to report their typical activity 1 year prior to diagnosis to reduce potential effects of undetected disease on exercise habits. Aside from the reliance on self-report, another limitation is the lack of more detailed information about types of physical activity, which would have allowed for more precise assignment of MET values. Such misclassification of MET values would have likely led to attenuation of the associations between RPA and mortality. Post-diagnosis RPA was an average over the span of a year and therefore did not capture fluctuations in activity level during that time period. The low response rate to the follow-up survey introduces the possibility of selection bias among cases with post-diagnosis RPA data; therefore, we compared the characteristics of follow-up survey respondents to those of AACES participants who did not complete the follow-up survey (Supplemental Table 1). Stage was statistically significantly different between cases with and without a follow-up survey (p = 0.03). However, this was mainly due to a higher prevalence of missing stage among cases without a follow-up survey and we found no other significant differences between the two groups, suggesting that selection bias is unlikely. Although there is a possibility of residual confounding as we were unable to adjust for residual disease in the full sample due to missing data, we did not find strong evidence for confounding by debulking status. We also examined whether there were systematic differences between cases with and without residual disease data by several covariates, only noting that cases with residual disease data were more likely to reside in the South and mid-Atlantic region (p < 0.01). Vital status data were limited to overall survival, so we could not evaluate disease-specific or progression-free survival, although as EOC is rapidly fatal it was likely the cause of death for the majority of those deceased. Finally, sample size and length of follow-up time (mean: 42.7 months since diagnosis) were limitations and power may have been inadequate to detect associations.
Despite these limitations, this study is an important contribution to the literature for several reasons. First, while most studies of EOC have been in majority white populations, this is the first to examine associations of RPA and survival in exclusively AA women, who have a lower prevalence of RPA [13, 14] and poorer survival [1]. Second, although many studies of other cancer types have analyzed RPA after diagnosis in association with survival, this is the first study of EOC to do so. Additionally, there have only been four previous studies and a large pooled analysis of RPA and survival in women with EOC. While our results suggest that RPA may not play as large a role in EOC survival as it does in breast, colorectal, and prostate cancers, it is possible that exercise has other favorable outcomes in EOC survivors. Two studies have reported the associations between physical activity after EOC diagnosis and improved quality of life [26], happiness, sleep quality, and sleep efficiency [30], and inverse associations with peripheral neuropathy, fatigue, depression, and anxiety [30].
There is a clear need for a better understanding of associations of physical activity both before and after diagnosis with mortality and other outcomes in EOC survivors. Due to poorer survival among AA women, attention to potential differences by race in these associations is also warranted.
Supplementary Material
Acknowledgments
We would like to acknowledge the AACES interviewers, Christine Bard, LaTonda Briggs, Whitney Franz (North Carolina), and Robin Gold (Detroit). We also acknowledge the individuals responsible for facilitating case ascertainment across the ten sites including Christie McCullum-Hill (Alabama); the Metropolitan Detroit Cancer Surveillance System staff (Detroit); Rana Bayakly, Vicki Bennett, Judy Andrews, and Debbie Chambers (Georgia); the Louisiana Tumor Registry; Lisa Paddock and Manisha Narang (New Jersey); Diana Slone, Yingli Wolinsky, Steven Waggoner, Anne Heugel, Nancy Fusco, Kelly Ferguson, Peter Rose, Deb Strater, Taryn Ferber, Donna White, Lynn Borzi, Eric Jenison, Nairmeen Haller, Debbie Thomas, Vivian von Gruenigen, Michele McCarroll, Joyce Neading, John Geisler, Stephanie Smiddy, David Cohn, Michele Vaughan, Luis Vaccarello, Elayna Freese, James Pavelka, Pam Plummer, William Nahhas, Ellen Cato, John Moroney, Mark Wysong, Tonia Combs, Marci Bowling, and Brandon Fletcher, (Ohio); Susan Bolick, Donna Acosta, and Catherine Flanagan (South Carolina); Martin Whiteside (Tennessee) and Georgina Armstrong and the Texas Registry, Cancer Epidemiology and Surveillance Branch, Department of State Health Services.
Funding
This study was supported by the National Cancer Institute (R01CA142081). Additional support was provided by the Metropolitan Detroit Cancer Surveillance System with funding from the National Cancer Institute, National Institute of Health, and the Department of Health and Human Services (Contract HHSN261201000028C), and the Epidemiology Research Core, supported in part by the National Cancer Institute (P30CA22453) to the Karmanos Cancer Institute, Wayne State University School of Medicine. The New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey Department of Health, is funded by the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute under Contract HHSN261201300021I, the National Program of Cancer Registries (NPCR), Centers for Disease Control and Prevention under Grant 5U58DP003931-02 as well as the State of New Jersey and the Rutgers Cancer Institute of New Jersey.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Research involving human and animal participants This article does not contain any studies with animals performed by any of the authors.
Ethical approval All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10552-017-0986-8) contains supplementary material, which is available to authorized users.
References
- 1.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Naishadham D, Jemal A (2013) Cancer statistics for African Americans, 2013. CA Cancer J Clin 63:151–166. 10.3322/caac.21173 [DOI] [PubMed] [Google Scholar]
- 3.Friedenreich CM, Neilson HK, Farris MS, Courneya KS (2016) Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res 22:4766–4775. 10.1158/1078-0432.CCR-16-0067 [DOI] [PubMed] [Google Scholar]
- 4.Ballard-Barbash R, Friedenreich CM, Courneya KS et al. (2012) Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 104:815–840. 10.1093/jnci/djs207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin ML, Smith AW, McTiernan A et al. (2008) Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol 26:3958–3964. 10.1200/JCO.2007.15.9822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard CM, Courneya KS, Stein K, American Cancer Society’s SCS-II (2008) Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol 26:2198–2204. 10.1200/JCO.2007.14.6217 [DOI] [PubMed] [Google Scholar]
- 7.Cannioto RA, LaMonte MJ, Kelemen LE et al. (2016) Recreational physical inactivity and mortality in women with invasive epithelial ovarian cancer: evidence from the Ovarian Cancer Association Consortium. Br J Cancer 115:95–101. 10.1038/bjc.2016.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannioto RA, Moysich KB (2015) Epithelial ovarian cancer and recreational physical activity: a review of the epidemiological literature and implications for exercise prescription. Gynecol Oncol 137:559–573. 10.1016/j.ygyno.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Chlebowski R, LaMonte MJ et al. (2014) Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: results from the Women’s Health Initiative. Gynecol Oncol 133:4–10. 10.1016/j.ygyno.2014.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moorman PG, Jones LW, Akushevich L, Schildkraut JM (2011) Recreational physical activity and ovarian cancer risk and survival. Ann Epidemiol 21:178–187. 10.1016/j.annepidem.2010.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Klint Å, Lambe M et al. (2008) Predictors of ovarian cancer survival: a population-based prospective study in Sweden. Int J Cancer 123:672–679. 10.1002/ijc.23429 [DOI] [PubMed] [Google Scholar]
- 12.Sakauchi F, Khan MMH, Mori M et al. (2007) Dietary habits and risk of ovarian cancer death in a large-scale cohort study (JACC study) in Japan. Nutr Cancer 57:138–145. 10.1080/01635580701274178 [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) (2007) Prevalence of regular physical activity among adults—United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep 56:1209–1212 [PubMed] [Google Scholar]
- 14.Tucker JM, Welk GJ, Beyler NK (2011) Physical activity in U.S.: adults compliance with the physical activity guidelines for Americans. Am J Prev Med 40:454–461. 10.1016/j.amepre.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 15.Schildkraut JM, Alberg AJ, Bandera EV et al. (2014) A multicenter population-based case–control study of ovarian cancer in African-American women: the African American Cancer Epidemiology Study (AACES). BMC Cancer 14:688. 10.1186/1471-2407-14-688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott SE, Bandera EV, Qin B et al. (2016) Recreational physical activity and ovarian cancer risk in African American women. Cancer Med 5:1319–1327. 10.1002/cam4.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Physical Activity Questionnaire (2016) https://sites.google.com/site/theipaq/. Accessed 15 Jan 2016 [Google Scholar]
- 18.Office of Disease Prevention and Health Promotion (2017) Physical activity guidelines. https://health.gov/paguidelines/. Accessed 27 Mar 2017
- 19.Schmitz KH, Courneya KS, Matthews C et al. (2010) American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42:1409–1426. 10.1249/MSS.0b013e3181e0c112 [DOI] [PubMed] [Google Scholar]
- 20.LexisNexis (2017) LexisNexis® Accurint®. http://www.accurint.com/. Accessed 7 Aug 2017
- 21.Therneau T, Crowson C, Atkinson E, Clinic M (2017) Using time dependent covariates and time dependent coefficients in the cox model. ftp://ftp.br.debian.org/CRAN/web/packages/survival/vignettes/timedep.pdf
- 22.Chaudhry S, Jin L, Meltzer D (2005) Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care 43(6):607–615. http://www.ncbi.nlm.nih.gov/pubmed/15908856. Accessed 6 April 2016 [DOI] [PubMed] [Google Scholar]
- 23.Chang S-J, Hodeib M, Chang J, Bristow RE (2013) Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol 130:493–498. 10.1016/j.ygyno.2013.05.040 [DOI] [PubMed] [Google Scholar]
- 24.Randall TC, Rubin SC (2001) Cytoreductive surgery for ovarian cancer. Surg Clin North Am 81:871–883. 10.1016/S0039-6109(05)70171-7 [DOI] [PubMed] [Google Scholar]
- 25.Gupta D, Lis CG (2009) Role of CA125 in predicting ovarian cancer survival—a review of the epidemiological literature. J Ovarian Res 2:13. 10.1186/1757-2215-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevinson C, Faught W, Steed H et al. (2007) Associations between physical activity and quality of life in ovarian cancer survivors. Gynecol Oncol 106:244–250. 10.1016/j.ygyno.2007.03.033 [DOI] [PubMed] [Google Scholar]
- 27.Doyle C, Kushi LH, Byers T et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin 56:323–353 [DOI] [PubMed] [Google Scholar]
- 28.McTiernan A (2008) Mechanisms linking physical activity with cancer. Nat Rev Cancer 8:205–211. 10.1038/nrc2325 [DOI] [PubMed] [Google Scholar]
- 29.Sanchis-Gomar F, Lucia A, Yvert T et al. (2015) Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer Prev Res 8:105–110. 10.1158/1940-6207.CAPR-14-0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevinson C, Steed H, Faught W et al. (2009) Physical activity in ovarian cancer survivors: associations with fatigue, sleep, and psychosocial functioning. Int J Gynecol Cancer 19:73–78. 10.1111/IGC.0b013e31819902ec [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


