Abstract
OprM is the outer membrane component of the MexA-MexB-OprM efflux system of Pseudomonas aeruginosa. Multiple-sequence alignment of this protein and its homologues identified several regions of high sequence conservation that were targeted for site-directed mutagenesis. Of several deletions which were stably expressed, two, spanning residues G199 to A209 and A278 to N286 of the mature protein, were unable to restore antibiotic resistance in OprM-deficient strains of P. aeruginosa. Still, mutation of several conserved residues within these regions did not adversely affect OprM function. Mutation of the highly conserved N-terminal cysteine residue, site of acylation of this presumed lipoprotein, also did not affect expression or activity of OprM. Similarly, substitution of the OprM lipoprotein signal, including consensus lipoprotein box, with the signal peptide of OprF, the major porin of this organism, failed to impact on expression or activity. Apparently, acylation is not essential for OprM function. A large deletion at the N terminus, from A12 to R98, compromised OprM expression to some extent, although the deletion derivative did retain some activity. Several deletions failed to yield an OprM protein, including one lacking an absolutely conserved LGGGW sequence near the C terminus of the protein. The pattern of permissive and nonpermissive deletions was used to test a topology model for OprM based on the recently published crystal structure of the OprM homologue, TolC (V. Koronakis, A. Sharff, E. Koronakis, B. Luisi, and C. Hughes, Nature 405:914–919, 2000). The data are consistent with OprM monomer existing as a substantially periplasmic protein with four outer membrane-spanning regions.
Pseudomonas aeruginosa is an opportunistic human pathogen characterized by high-level intrinsic and mutationally acquired resistance to multiple antibiotics, for which multidrug efflux systems have emerged as key mechanisms. A number of multidrug efflux systems, including MexAB-OprM (12, 35, 36), MexCD-OprJ (34), MexEF-OprN (18), and MexXY-OprM (1, 27, 46), have been identified in this organism; two of these, MexAB-OprM and MexXY-OprM, contribute to intrinsic resistance in wild-type cells (1, 21, 46). Hyperexpression of MexAB-OprM occurs in nalB-type multidrug-resistant mutants, while overexpression of MexXY (AmrAB) correlates with acquired aminoglycoside resistance in this organism (1, 21, 46). Expression of MexCD-OprJ and MexEF-OprN is so far restricted to nfxB (34)- and nfxC (18)-type multidrug-resistant mutants, respectively. These tripartite efflux systems include an inner membrane, presumed drug-proton antiporter of the resistance-nodulation-cell division efflux family (39) (MexB, MexD, MexF, and MexY), a periplasmic but cytoplasmic membrane-anchored membrane fusion protein (15) (MexA, MexC, MexE, and MexX), and an outer membrane efflux protein (15) (OprM, OprJ, and OprN) (31). Apparently, antibiotics entering the cytoplasm or, perhaps, the cytoplasmic membrane are captured by the pump and extruded directly into the medium (31).
The OprM protein was first reported in 1992 to be associated with acquired multiple antibiotic resistance in P. aeruginosa (26). A component both of the MexAB-OprM and MexXY-OprM multidrug efflux systems, the protein plays a role in resistance to several antibiotics, including aminoglycosides (as part of MexXY-OprM) (1), tetracycline, chloramphenicol, quinolones, β-lactams, novobiocin, macrolides, trimethoprim, and organic solvents (as part of MexAB-OprM) (19, 21–23, 26). Hybridization studies with an oprM probe have revealed that OprM is highly conserved in all serotypes of P. aeruginosa (3), highlighting its probable significance vis-à-vis intrinsic and acquired antibiotic resistance in this organism. Expected to function in the extrusion of drugs across the outer membrane, OprM has been implicated as a channel-forming protein. Recently, channel-forming activity has been demonstrated in planar lipid bilayer membranes, although the channels observed did not seem compatible with export of the diversity and size of compounds known to be substrates of the MexAB-OprM pump (47). Still, the probable involvement of TonB, a cytoplasmic membrane energy-coupling protein, in MexAB-OprM-mediated drug efflux and resistance (51) may facilitate channel opening. This protein is known, for example, to interact with outer membrane receptors for ferric siderophore complexes, promoting conformational changes necessary for ligand passage through the receptor channel (5, 28).
OprM homologues have been identified in a variety of gram-negative bacteria (33) and include OprJ and OprN of P. aeruginosa (18, 34), SrpC of the Pseudomonas putida SrpABC pump (17), OpcM of the Burkholderia cepacia CeoAB-OpcM pump (6), and SmeC of the Stenotrophomonas maltophilia SmeABC pump (GenBank accession number AF173226; X.-Z. Li, L. Zhang, and K. Poole, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. A-33, 2000). Predicted to be lipoproteins, these all possess a characteristic lipoprotein box; indeed, acylation of OpcM has been demonstrated (6).
This study was undertaken to examine the significance of acylation for OprM function, as well as the functional importance of the highly conserved regions of the OprM family. The numerous deletions constructed as part of this process were then used to assess the accuracy of a topology model for OprM based on the recently solved crystal structure of TolC, an OprM homologue (20). This trimeric protein is comprised of monomers that span that membrane four times, producing a 12-membrane-spanning β-barrel within the outer membrane with an extensive periplasmic α-helical barrel (20).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
Bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were routinely cultured in Luria-Bertani (LB) broth (1% [wt/vol] Difco tryptone, 0.5% [wt/vol] Difco yeast extract, 0.5% [wt/vol] NaCl) at 37°C with shaking (180 rpm), except for susceptibility testing, for which cultures were not shaken. In some instances, 2× TY broth (1.6% [wt/vol] Difco tryptone, 1% [wt/vol] Difco yeast extract, 0.5% [wt/vol] NaCl) was used as the growth medium. Escherichia coli strains carrying plasmids were cultivated in the presence of the appropriate antibiotic: ampicillin (100 μg/ml), chloramphenicol (30 μg/ml), kanamycin (50 μg/ml), or tetracycline (10 μg/ml). P. aeruginosa strains harboring pVLT31 and its derivatives were cultivated in the presence of tetracycline (10 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | endA hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15] | Gibco-BRL |
| S17-1 | thi pro hsdR recA Tra+ | 43 |
| MV1190 | Δ(lac-proAB) thi supE Δ(srl-recA)306::Tn10 (Tcr)[F′ traD36 proAB lacIqZΔM15] | Bio-Rad |
| CJ236 | dut-1 ung-1 thi-1 relA1; pCJ105, Cmr | Bio-Rad |
| P. aeruginosa | ||
| PAO1 | Prototroph | 22 |
| ML5087 | ilv-220 thr-9001 leu-9001 met-9011 pur-67 aphA | 32 |
| K1110 | ML5087 ΔoprM | This study |
| K1112 | ML5087 nalB | 23 |
| K1113 | K1112 ΔoprM | This study |
| Plasmids | ||
| pK18mobsacB | Gene replacement vector derived from plasmid pK18, Mob+sacB Kmr | 41 |
| pRK415 | Broad-host-range cloning vector; plac MCS Tcr, 10.5 kb | 16 |
| pT7-7 | pBR322 derivative carrying an MCS downstream of a strong phage T7 gene 10 promoter and a ribosome-binding site; Apr | 45 |
| pTZ19U | pUC-derived phagemid vector; MCS Apr | Bio-Rad |
| pVLT31 | Broad-host-range expression vector; lacI plac MCS Tcr | 9 |
| pKPM-1 | pT7-7::oprMb | 48 |
| pXZL04 | pK18mobsacB::ΔoprM | 44 |
| pXZL11 | pRK415::oprM* (C1G) | This study |
| pXZL13 | pT7-7::oprM* (C1G) | This study |
| pXZL33 | pT7-7::oprM wtc | This study |
| pXZL34 | pVLT31::oprM wtc | This study |
| pXZL40 | pTZ19U::oprM wtc | This study |
| pXZL48 | pTZ19U::oprM* (ΔS93-G107) | This study |
| pXZL51 | pXZL33::oprM* (HA)d | This study |
| pXZL57 | pVLT31::oprM* (ΔS93-G107) | This study |
| pXZL58 | pVLT31::oprM* (HA)d | This study |
| pXZL64 | pT7-7::oprM* (ΔL446-A468) | This study |
| pXZL65 | pT7-7::oprM* (ΔN451-A468) | This study |
| pXZL66 | pVLT31::oprM* (ΔL446-A468) | This study |
| pXZL67 | pVLT31::oprM* (ΔN451-A468) | This study |
| pXZL68 | pTZ19U::oprM* (ΔA233-G241) | This study |
| pXZL69 | pTZ19U::oprM* (ΔP5-P10) | This study |
| pXZL70 | pTZ19U::oprM* (E125-G130) | This study |
| pXZL71 | pTZ19U::oprM* (ΔA445-W450) | This study |
| pXZL72 | pTZ19U::oprM* (ΔA278-N286) | This study |
| pXZL73 | pVLT31::oprM* (ΔA233-G241) | This study |
| pXZL74 | pVLT31::oprM* (ΔP5-P10) | This study |
| pXZL75 | pVLT31::oprM* (E125-G130) | This study |
| pXZL76 | pVLT31::oprM* (ΔA445-W450) | This study |
| pXZL77 | pVLT31::oprM* (ΔA278-N286) | This study |
| pXZL78 | pTZ19U::oprM* (S2D) | This study |
| pXZL80 | pTZ19U::oprM* (ΔT28-D37) | This study |
| pXZL81 | pTZ19U::oprM* (ΔA145-A153) | This study |
| pXZL82 | pTZ19U::oprM* (ΔG199-A209)e | This study |
| pXZL83 | pTZ19U::oprM* (ΔF317-F326) | This study |
| pXZL84 | pTZ19U::oprM* (ΔR74-I79)e | This study |
| pXZL85 | pTZ19U::oprM* (ΔR341-I349) | This study |
| pXZL86 | pVLT31::oprM* (S2D) | This study |
| pXZL88 | pVLT31::oprM* (ΔT28-D37) | This study |
| pXZL89 | pVLT31::oprM* (ΔA145-A153) | This study |
| pXZL90 | pVLT31::oprM* (ΔG199-A209)e | This study |
| pXZL91 | pVLT31::oprM* (ΔF317-F326) | This study |
| pXZL92 | pVLT31::oprM* (ΔD91-Q96)e | This study |
| pXZL93 | pVLT31::oprM* (ΔR341-I349) | This study |
| pXZL95 | pTZ19U::oprM* (E125K) | This study |
| pXZL96 | pTZ19U::oprM* (F129A) | This study |
| pXZL97 | pTZ19U::oprM* (F129Y) | This study |
| pXZL98 | pTZ19U::oprM* (L446V) | This study |
| pXZL99 | pTZ19U::oprM* (L446Q) | This study |
| pXZL100 | pTZ19U::oprM* (G448T) | This study |
| pXZL101 | pTZ19U::oprM* (G448A) | This study |
| pXZL102 | pTZ19U::oprM* (E279D) | This study |
| pXZL103 | pTZ19U::oprM* (E279K) | This study |
| pXZL104 | pVLT31::oprM* (F129A) | This study |
| pXZL105 | pVLT31::oprM* (F129Y) | This study |
| pXZL106 | pVLT31::oprM* (L446V) | This study |
| pXZL107 | pVLT31::oprM* (G448A) | This study |
| pXZL108 | pVLT31::oprM* (E279K) | This study |
| pXZL109 | pVLT31::oprM* (E125K) | This study |
| pXZL110 | pVLT31::oprM* (L446Q) | This study |
| pXZL111 | pVLT31::oprM* (E279D) | This study |
| pXZL112 | pVLT31::oprM* (G448T) | This study |
| pXZL114 | pTZ19U::oprM* (L128R) | This study |
| pXZL117 | pTZ19U::oprM* (R131E) | This study |
| pXZL119 | pTZ19U::oprM* (A284F) | This study |
| pXZL120 | pTZ19U::oprM* (A284Y) | This study |
| pXZL122 | pTZ19U::oprM* (W450Y) | This study |
| pXZL125 | pVLT31::oprM* (L128R) | This study |
| pXZL126 | pVLT31::oprM* (A284F) | This study |
| pXZL127 | pVLT31::oprM* (W450Y) | This study |
| pXZL128 | pVLT31::oprM* (R131E) | This study |
| pXZL129 | pVLT31::oprM* (A284Y) | This study |
| pXZL130 | pTZ19U::oprM* (ΔL257-L264) | This study |
| pXZL133 | pVLT31::oprM* (ΔL257-L264) | This study |
| pXZL134 | pTZ19U::oprM* (ΔA12-R98) | This study |
| pXZL137 | pVLT31::oprM* (ΔA12-R98) | This study |
| pXZL142 | pTZ19U::oprF-oprMf | This study |
| pXZL146 | pVLT31::oprM* (C1G) | This study |
| pXZL147 | pVLT31::oprF-oprM | This study |
Mutated plasmid-borne oprM genes are indicated with asterisks, and the nature of the mutation is indicated in parentheses. Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance; MCS, multiple cloning site.
The originally cloned oprM gene had an incorrect 3′ end which yielded a functional OprM protein in which the final 22 amino acids of the protein were replaced with 14 amino acids derived from the phagemid used to clone the gene.
Using the available P. aeruginosa genome sequence, the correct 3′ end of oprM was engineered into the originally cloned oprM gene to yield the wild-type gene.
The oprM gene carries the coding sequence for a nine-amino-acid HA tag inserted between bp 366 and 367 (i.e., between T106 and T107 of the mature OprM protein).
As a consequence of the deletion construction, an additional leucine codon was introduced into oprM at the position of the deletion.
Hybrid oprF-oprM gene in which the coding sequence for putative lipoprotein signal sequence of OprM is replaced with the coding sequence for the OprF signal sequence.
DNA methodology.
Basic DNA procedures, including restriction endonuclease digestions, ligations, transformations, and agarose gel electrophoresis, were performed as described by Sambrook et al. (40). The alkaline lysis method (40) or a plasmid midi kit (Qiagen Inc., Missassauga, Ontario, Canada) was used to isolate plasmids from E. coli DH5α. The genomic DNA of P. aeruginosa was extracted by the method of Barcak et al. (2). DNA fragments used in cloning were extracted from agarose gels using Prep-A-Gene (Bio-Rad Laboratories, Richmond, Calif.) according to the manufacturer's instructions. E. coli cells were made competent by the CaCl2 method (40) or, when supercompetent cells of E. coli were required, the method of Inoue et al. (14). Oligonucleotides used in this study (Table 2) were designed and assessed using a Primer Design software package (version 2.01; Scientific & Educational Software, Durham, N.C.) to minimize secondary structure and dimer formation. All oligonucleotides were chemically synthesized by Cortec DNA Services Inc., Queen's University, with the exception of oprm68xz and oprm69xz, which were synthesized by MWG Biotech Inc. (High Point, N.C.). Nucleotide sequencing was carried out using universal or custom primers by Cortec DNA Services (Queen's University) or the Laboratory Services Division, University of Guelph, Guelph, Ontario, Canada). Nucleotide sequence was analyzed using the PCGene software package (Intelligenetics Inc., Mountain View, Calif.) and DNAMAN (Lynnon Biosoftware, Vaudreuil, Quebec, Canada).
TABLE 2.
Oligonucleotides used in the mutagenesis of oprM
| Name | Descriptiona |
|---|---|
| oprm1xz | 5′-CGACTCACTATAGGGAGACC-3′ |
| Anneals upstream of XbaI site in vector pT7-7; XbaI site underlined | |
| oprm2xz | 5′-AGTCAAGCTTTCCCGCCCTCTTTTGGCAG-3′ |
| Anneals at the 3′ end of oprM on pKPM-1; HindIII site underlined | |
| oprm6xz | 5′-TTAACTGCAGACAGCGGCTACCGCCAGG-3′ |
| Anneals ca. 100 bp downstream of the oprM start codon; PstI site underlined | |
| oprm7xz | 5′-AATTCTGCAGTTCTGTCCGGCGGCTCGCTGATCC-3′ |
| Mutagenic primer for C1G substitution in OprM; Gly codon italicized and altered base bolded; anneals downstream of oprM start codon; PstI site underlined | |
| oprm10xz | 5′-AATTGAGCTCTCGCTGATCCCCGACTACCA-3′ |
| Anneals near the 5′ end of oprM, downstream of start codon; SstI site underlined | |
| oprm14xz | 5′-GTTGCAGCTGACCAAGGACA-3′ |
| Anneals upstream of oprM | |
| oprm21xz | 5′-ATGTAAGCTTCTGACCCGCAACCGCTAAGG-3′ |
| Anneals downstream of the wild-type oprM gene present in the P. aeruginosa chromosomeb; HindIII site underlined | |
| oprm23xz | 5′-GGATCGGCGTGGACGGTAGTCCGGCGATTTCCAG-3′ |
| Mutagenic primer used to construct OprM ΔS93-G107 (pXZL48) | |
| oprm24xz | 5′-GCGACCTGTCGACCTACCCATATGACGTTCCAGACTACGCGTATACCGGCAGTCCGGCGATTTCCA-3′ |
| Mutagenic primer used to insert a 9-amino-acid HA (coding sequence bolded) into OprM; SalI and MluI sites underlined; additional Tyr codon (italics) created as a result of MluI site (pXZL51) | |
| oprm27xz | 5′-TCGAAAGCTTCAGGCCTTGTACAGGTTGACCA-3′ |
| Anneals near the 3′ end of oprM and used to construct an OprM deletion derivative lacking the last 23 amino acids (L446–A468; pXZL64) | |
| oprm28xz | 5′-TCGAAAGCTTCACCAGCCGCCGCCGCCGCCGAGG-3′ |
| Anneals near the 3′ end of oprM and used to construct an OprM deletion derivative lacking the last 18 amino acids (N451–A468; pXZL65) | |
| oprm29xz | 5′-CGCCCGGACATCCTCGAAGCCAGCATCGGCGCCGC-3′ |
| Mutagenic primer used to construct OprM ΔA278-N286 (pXZL72) | |
| oprm30xz | 5′-GTAGCCCAGGACCAGAATATCCCGGCGAACCTGCCG-3′ |
| Mutagenic primer used to construct OprM ΔA233-G241 (pXZL68) | |
| oprm31xz | 5′-GAGGTCAACCTGTACAAGAACCAGCAGACCGTGACC-3′ |
| Mutagenic primer used to construct OprM ΔA445-W460 (pXZL71) | |
| oprm32xz | 5′-TCCGGCTGCTCGCTGATCGAGGCGCCGGTAGCCGCG-3′ |
| Mutagenic primer used to construct OprM ΔP5-P10 (pXZL69) | |
| oprm33xz | 5′-TGGGCACTACCGCCTGGCTGCGCAGCCTGCGCGAC-3′ |
| Mutagenic primer used to construct OprM ΔE125-G130 (pXZL70) | |
| oprm34xz | 5′-TCGTTCTGTCCGGCTGCGACCTGATCCCCGACTACCA-3′ |
| Mutagenic primer used to construct OprM S2D (pXZL78) | |
| oprm36xz | 5′-GCCTACGGGCAGAACACCGACATCGGCTGGCGCGAGT-3′ |
| Mutagenic primer used to construct OprM ΔT28-D37 (pXZL80) | |
| oprm37xz | 5′-GCCCTGGAGCAGTACCTGCAGACCACCCTGGTGGCCAG-3′ |
| Mutagenic primer used to construct OprM ΔA145-A153 (pXZL81); PstI site underlined | |
| oprm38xz | 5′-ACCCAGCGCAGCTACGACGTCCTGCAGACCGCCGTGGAAGG-3′ |
| Mutagenic primer used to construct OprM ΔG199-A209 (pXZL82); PstI site underlined | |
| oprm39xz | 5′-CGCCAACTGTCCGGCCTGCAGCCGTCGATCAACCT-3′ |
| Mutagenic primer used to construct OprM ΔF317-F326 (pXZL83); PstI site underlined | |
| oprm40xz | 5′-TGAACGTCGAGGCCTTCCTGCAGCGGGCCGACCTGTTC-3′ |
| Mutagenic primer used to construct OprM ΔD91-Q96 (pXZL84); PstI site underlined | |
| oprm41xz | 5′-ATCTTCACCGCCGGCAGCCTGCAGAAGGACATCAACGTCG-3′ |
| Mutagenic primer used to construct OprM ΔR341-I349 (pXZL85); PstI site underlined | |
| oprm42xz | 5′-CTGGGACCTCGATCTCTTC-3′ |
| Mutagenic primer used to construct OprM E125D (pXZL94) | |
| oprm43xz | 5′-GCCTGGAAGCTCGATCTCTTCGG-3′ |
| Mutagenic primer used to construct OprM E125K (pXZL95) | |
| oprm45xz | 5′-CGATCTCTACGGCCGCCTG-3′ |
| Mutagenic primer used to construct OprM F129Y (pXZL97) | |
| oprm46xz | 5′-ACAAGGCGGTCGGCGGCGGCT-3′ |
| Mutagenic primer used to construct OprM L446V (pXZL98) | |
| oprm47xz | 5′-CAAGGCCCAGGGCGGCGGCT-3′ |
| Mutagenic primer used to construct OprM L446Q (pXZL99) | |
| oprm48xz | 5′-CTCGGCACCGGCTGGAACC-3′ |
| Mutagenic primer used to construct OprM G448T (pXZL100) | |
| oprm49xz | 5′-CCTCGGCGCTGGCTGGAACC-3′ |
| Mutagenic primer used to construct OprM G448A (pXZL101) | |
| oprm50xz | 5′-GAGGCCGACCACCAGCTCA-3′ |
| Mutagenic primer used to construct OprM E279D (pXZL102) | |
| oprm51xz | 5′-GAGGCCAAGCACCAGCTCA-3′ |
| Mutagenic primer used to construct OprM E279K (pXZL103) | |
| oprm57xz | 5′-CTGGGAACTCGATCGCTTCGGCCGCCTG-3′ |
| Mutagenic primer used to construct OprM L128R (pXZL114) | |
| oprm60xz | 5′-GATCTCTTCGGCGACCTGCGCAGCCT-3′ |
| Mutagenic primer used to construct OprM R131E (pXZL117) | |
| oprm62xz | 5′-CACACGCTCATGTTCGCCAACGCCAGCA-3′ |
| Mutagenic primer used to construct OprM A284F (pXZL119) | |
| oprm63xz | 5′-AGCTCATGGATGCCAACGCCA-3′ |
| Mutagenic primer used to construct OprM A284Y (pXZL120) | |
| oprm65xz | 5′-TCGGCGGCGGCTACAACCAGCAGACC-3′ |
| Mutagenic primer used to construct OprM W450Y (pXZL122) | |
| oprm67xz | 5′-TGGACCAGACCCTGCCGTCGGACCTGCTGCAA-3′ |
| Mutagenic primer used to construct OprM ΔL257-L264 (pXZL130) | |
| oprm68xz | 5′-AATTGAGCTCGGGGCGCTGGTAGTC-3′ |
| Used to amplify the 5′ end of oprM in constructing a OprM ΔA12-R98 derivative; SstI site underlined | |
| oprm69xz | 5′-AATTGAGCTCCCGGGCGACCTGTCGACCA-3′ |
| Used to amplify the 3′ end of oprM in constructing a OprM ΔA12-R98 derivative; SstI site underlined | |
| oprf2xz | 5′-TATGAAACTGAAGAACACCTTAGGCGTTGTCATCGGCTCGCTGGTTGCCGCTTCGGCAATGAACGCCTTTGCCCAGGGCCAG AACTCGGAGCT-3′ |
| Complementary to oprf3xz and encodes the OprF signal peptide; oprF start codon italicized; NdeI (TA) and SstI (AGCT) overhangs produced following annealing with oprf3xz underlined | |
| oprf3xz | 5′-CCGAGTTCTGGCCCTGGGCAAAGGCGTTCATTGCCGAAGCGGCAACCAGCGAGCCGATGACAACGCCTAAGGTGTTCTTCAGTTTCA-3′ |
| Complementary to oprf2xz and encodes the OprF signal peptide |
Primer sequences are shown followed by the plasmid (in parentheses) carrying the mutated oprM gene that was constructed with the primer. Primers used in in vitro phagemid-based mutagenesis (oprm23xz to oprm67xz) were 5′ phosphorylated.
The originally cloned oprM gene had an incorrect 3′ end; therefore, it was necessary to amplify the correct sequence from the oprM gene on the P. aeruginosa chromosome.
Construction of the oprM deletion mutants.
To delete oprM in P. aeruginosa ML5087 and its derivatives, the gene replacement suicide vector pK18mobsacB carrying an oprM deletion (pXZL04) was constructed as previously described (44). Following transformation of E. coli S17-1, pXZL04 was mobilized into P. aeruginosa ML5087 via conjugation (34). Briefly, E. coli S17-1 carrying pXZL04 was grown overnight in LB broth in the presence of kanamycin with shaking at 37°C, and the P. aeruginosa strains were grown in LB broth overnight without shaking at 42°C. Overnight donor and recipient cultures (100 μl of each) were mixed, pelleted by centrifugation (3 min in a microcentrifuge), and resuspended in 50 μl of LB broth. The cell suspensions were spotted on LB agar and incubated overnight at 37°C. The bacterial cells on LB agar were resuspended with 1 ml of LB broth, and dilutions were plated onto agar plates supplemented with kanamycin and tetracycline (to counterselect E. coli). Transconjugants carrying a copy of pXZL04 in the chromosome were recovered from kanamycin-tetracycline-containing LB agar plates and streaked onto LB agar containing 10% (wt/vol) sucrose. Sucrose-resistant colonies, which had lost pK18mobsacB sequences (kanamycin sensitive) and carried either an unaltered wild-type copy of oprM or the oprM deletion, were thus recovered. Potential oprM deletion mutants were initially screened for antibiotic susceptibility, a phenotype typical of OprM-deficient P. aeruginosa. The presence of the oprM deletion was then confirmed using PCR as described previously (52) with genomic DNA and primers oprmp1 and oprm21xz (Table 2). The absence of OprM in these mutants was also confirmed by Western immunoblotting with an antiserum specific to OprM (see below).
Cloning of the native oprM gene.
The wild-type oprM gene (GenBank accession number L23839) of ca. 1.5 kb was previously cloned into pT7-7 and pVLT31, yielding pKPM-1 and pKPM-2, respectively (48). Examination of the genomic oprM sequence of P. aeruginosa (Pseudomonas genome project [http://www.pseudomonas.com]) revealed that the cloned oprM gene of pKPM-1 and pKPM-2 differed from the chromosomal counterpart at the 3′ end, with the last 66 bp of native oprM (corresponds to 22 residues in OprM) replaced by 42 bp (14 residues in OprM) from the phagemid originally used to clone the gene. Still, the OprM protein encoded by pKPM-2 (48) was fully functionally and restored drug resistance to OprM-deficient strains (44, 47). To obtain a clone of the wild-type oprM gene, PCR was used to amplify 800 bp at the 3′ end of the native oprM gene from the chromosome of P. aeruginosa PAO1 (or ML5087), which was then used to replace the corresponding segment of oprM on pKPM-1. Amplification was carried out using primers oprm14xz (anneals upstream of the BamHI site in oprM) and oprm21xz (contains a HindIII site) (Table 2) in a reaction mixture containing 50 ng of chromosomal DNA, 40 pmol of each primer, 0.2 mM each deoxynucleoside triphosphate, 2 mM MgSO4, 10% (vol/vol) dimethyl sulfoxide, and 2 U of Vent DNA polymerase in 1× Thermo reaction buffer (New England Biolabs; NEB). The mixture was heated for 2 min at 94°C and then amplified by 30 cycles of 1 min at 94°C, 1 min at 56°C, and 2 min at 72°C. The PCR products were purified using a Qiaquick PCR purification kit (Qiagen) according to the manufacturer's instructions. After digestion with BamHI and HindIII, the PCR product was cloned into BamHI-HindIII-restricted pKPM-1, which removed the incorrect 3′ sequence of oprM, yielding plasmid pXZL33. Following confirmation of the correct nucleotide sequence and expression of OprM from pXZL33, an oprM-containing XbaI-HindIII fragment of pXZL33 was cloned into XbaI-HindIII-restricted-pVLT31 and -pTZ19U, yielding pXZL34 and pXZL40, respectively.
PCR-based site-directed mutagenesis of oprM.
A number of deletion, insertion, and point mutations were introduced into oprM by using an approach based on PCR. To construct the A12-R98 deletion derivative, two PCRs were carried out, resulting in the amplification of sequences upstream (using primers oprm1xz and oprm68xz) and downstream (using primers oprm69xz and oprm21xz) of the deletion endpoints. The PCR mixtures were formulated as above except that 10 ng of pXZL33 was used as the template. The mixtures were heated for 2 min at 94°C and then subjected to 30 cycles of 1 min at 94°C, 1 min at 52°C, and 0.5 min (for the 100-bp upstream product) or 1.2 min (for the 1.2-kb downstream product) at 72°C. Following purification and digestion with XbaI and SstI (100-bp product) or SstI and HindIII (1.2-kb product), the PCR products were cloned into XbaI-HindIII-restricted pTZ19U via a three-piece ligation procedure to yield pXZL134. The deletion was confirmed by nucleotide sequencing, liberated on a ca. 1.3-kb XbaI-HindIII fragment, and cloned into XbaI-HindIII-restricted pVLT31 to yield pXZL137. The C1G OprM mutation was also constructed by amplifying oprM in two parts. The region upstream of the mutation site was amplified with primers oprm1xz and oprm6xz (Table 2), and that downstream was amplified with primers oprm7xz (a mutagenic primer that changes the TGC cysteine codon to the GGC glycine codon) and oprm2xz (Table 2), using reaction conditions described above. The 140-bp upstream fragment was digested with XbaI and PstI and cloned into XbaI-PstIII-restricted pRK415. This construct was then digested with PstI-HindIII to permit cloning of the PstI-HindIII-digested 1.4-kb downstream product. The resultant vector, which carries an intact oprM gene with a PstI site near the glycine codon, was digested with PstI and treated with T4 DNA polymerase (NEB) at 12°C for 20 min (40). Vector recircularization with T4 DNA ligase yielded pXZL11, which carried the desired C1G oprM mutation. The C1G mutant oprM gene of pXZL11 was then recovered on a 1.5-kb XbaI-HindIII fragment and cloned into XbaI-HindIII-digested pT7-7 to produce pXZL13. Since the oprM gene used here originated from pKPM-1, in which the 3′ sequence of oprM differed from that of the native oprM (above), the C1G oprM-containing XbaI-SalI fragment (ca. 300 bp at the 5′ end of oprM) of pXZL13 was used to replace the XbaI-SalI fragment of the native oprM gene of pXZL40, resulting in pXZL141. The C1G mutated oprM gene of pXZL141 was then liberated on an XbaI-HindIII fragment and cloned into XbaI-HindIII-restricted pVLT31 to yield pXZL146.
Truncation of oprM at the 3′ end of the gene, yielding OprM derivatives lacking the last 23 (ΔL446-A468) and 18 (ΔN451-A468) amino acids, was achieved following amplification of oprM using primer pairs oprm14xz-oprm27xz (ΔL446-A468) and oprm14xz-oprm28xz (ΔN451-A468), respectively. Reaction mixtures were formulated as described above and heated using the same parameters. PCR products were digested with BamHI and HindIII and cloned into BamHI-HindIII-restricted pXZL33 to yield pXZL64 (ΔN451-A468) and pXZL65 (ΔN451-A468). The oprM-containing XbaI-HindIII oprM fragments of pXZL64 and pXZL65 were then cloned into XbaI-HindIII-digested pVLT31 to produce pXZL66 and pXZL67, respectively.
To obtain an OprM derivative which could be processed to target the outer membrane but which lacks the N-terminal consensus lipoprotein box, the signal sequence of the major outer membrane protein OprF of P. aeruginosa was fused to the N terminus of OprM. To achieve this, PCR was used to amplify the oprM gene downstream of the cysteine codon that corresponds to the first amino acids of the mature protein. A 1.4-kb oprM-containing fragment was amplified with primers oprm10xz and oprm2xz (Table 2), using conditions described above. Following digestion with SstI and HindIII, the PCR product was cloned into SstI-HindIII-restricted pT7-7, yielding pXZL14. The OprF signal peptide-encoding sequence (MKLKNTLGVVIGSLVAASAMNAFAQG) was provided by two complementary oligonucleotides (oprf2xz [93-mer] and oprf2xz [87-mer] [Table 2]) derived from the 5′ sequence of the oprF gene of P. aeruginosa (GenBank accession number AF027290). Annealing of the two nucleotides (100 pmol of each) was carried out in 6 μl of H2O at 85°C for 3 min and then room temperature (23°C) for 30 min. The annealed oligonucleotides (ca. 5 μl), which possessed NdeI and SstI overhangs at either end, were ligated to NdeI-SstI-digested pXZL14, producing pXZL17. The resultant oprF-oprM chimera thus fused the first 27 amino acids of OprF plus two additional amino acids (derived from the SstI site) to the N terminus of OprM beginning at Ser2. As the oprM sequences of pXZL17 originated from pKPM-1, which carries an incorrect 3′ end of the gene, a 300-bp XbaI-SalI fragment carrying the 5′ end of the oprF-oprM fusion was isolated and used to replace the corresponding region of the wild-type oprM gene of pXZL40. Nucleotide sequencing confirmed that the resultant vector, pXZL142, contained the expected sequence for the OprF-OprM fusion. The oprF-oprM fusion was subsequently recovered on a 1.5-kb XbaI-HindIII fragment from pXZL142 and cloned into XbaI-HindIII-restricted pVLT31 to yield pXZL147.
A nine-residue hemagglutinin epitope (HA; YPYPDVPDY) was inserted between residues T106 and T107 of mature OprM by PCR with primers oprm21xz and oprm24xz (Table 2) and plasmid pXZL33 as template. Using conditions described above, a ca. 1.2-kb PCR fragment comprising the 3′ end of oprM, complete with epitope-encoding sequence, was amplified. Following digestions with SalI and BamHI, a 700-bp fragment carrying the epitope-encoding sequence and surrounding region of oprM was liberated from the PCR product and cloned into SalI-BamHI-digested pXZL33, yielding pXZL51. This served to replace a portion of the wild-type oprM gene on pXZL33 with the epitope-tagged sequence. Plasmids carrying HA-tagged oprM genes were identified by virtue of their cutting with MluI, as a MluI site was engineered into the HA-tagged mutagenic primer oprm24xz. The HA-tagged oprM gene was subsequently liberated on an XbaI-HindIII fragment and cloned into XbaI-HindIII restricted-pVLT31 to produce pXZL58.
Phagemid-based site-directed mutagenesis of oprM.
Several deletion and most substitution mutations were constructed using a Muta-Gene phagemid in vitro mutagenesis kit (Bio-Rad) according to the manufacturer's instructions, with some modifications. To obtain a single-stranded copy of oprM as required for the mutagenesis protocol, E. coli CJ236 cells harboring the oprM-containing phagemid pXZL40 (pTZ19U::oprM) were cultured in chloramphenicol- and ampicillin-supplemented 2× TY broth to an optical density at 600 nm of 0.3. The helper phage M13K07 was then added at a multiplicity of infection of ca. 20; the cells were incubated for an additional 1 h, at which time kanamycin (70 μg/ml) was added. Following incubation overnight, bacteria were removed by centrifugation (17,000 × g for 30 min at 4°C), and the supernatant was recovered and recentrifuged. DNase-free RNase A (Sigma) was added to the supernatant at a final concentration of 10 μg/ml, and the supernatant was incubated at room temperature for 30 min. Then 15 ml of 3.5 M ammonium acetate–20% (wt/vol) polyethylene glycol 8000 was added to 45 ml of the phage-containing supernatant, and the mixture was incubated on ice for 30 min. Phage particles containing single-stranded pXZL40 were recovered by centrifugation (17,000 × g for 15 min at 4°C) and resuspended in high-salt buffer (300 mM NaCl, 100 mM Tris-HCl [pH 8.0], 1 mM Na2EDTA). Single-stranded phagemid DNA was prepared from the phage particles as described elsewhere (40) and dissolved in 10 mM Tris-HCl–1 mM EDTA (pH 8.0).
In vitro oprM mutagenesis was performed using single-stranded pXZL40 prepared from E. coli CJ236 as the template and mutagenic oligonucleotides (Table 2) as described below. All oligonucleotides were chemically phosphorylated at their 5′ ends and purified by thin-layer chromatography (Cortec DNA Services). Annealing of the mutagenic oligonucleotides to the template was carried out in a 10-μl mixture of 0.2 μg of the single-stranded pXZL40 template and 20 pmol of a mutagenic oligonucleotide in 1× annealing buffer (20 mM Tris-HCl [pH 7.4], 2 mM MgCl2, 50 mM NaCl). The annealing mixtures were placed in a 70°C water bath and cooled to 30°C at a rate of approximately 1°C per min. The mixtures were then placed in an ice-water bath, and 1 μl of 10× synthesis buffer (5 mM each deoxynucleoside triphosphate, 10 mM ATP, 100 mM Tris-HCl [pH 7.9], 50 mM MgCl2, 15 mM dithiothreitol), 1 μl of T4 DNA ligase (3 U/μl), and 1 μl of unmodified T7 DNA polymerase (0.5 U/μl; Bio-Rad or NEB) was added. The mixtures were incubated on ice for 5 min, at room temperature for 5 min, and finally at 37°C for 40 min, at which time 30 μl of TE stop buffer (10 mM Tris-HCl [pH 8.0], 10 mM EDTA) was added. The reaction products were analyzed on 1% (wt/vol) agarose gels and used to transform competent E. coli MV1190. Plasmid DNA was then extracted from ampicillin-resistant E. coli MV1190 transformants and screened for the appropriate mutations by restriction analysis (when applicable) and by nucleotide sequencing. Mutated oprM genes were recovered from their pTZ19U derivatives on 1.5-kb XbaI-HindIII fragments and cloned into XbaI-HindIII-restricted pVLT31.
OprM expression in P. aeruginosa.
To assess expression of mutated oprM genes in P. aeruginosa, the oprM-containing pVLT31 derivatives were mobilized from E. coli DH5α into OprM-deficient P. aeruginosa strains K1110 and K1113 via triparental mating (44). The P. aeruginosa transconjugants were selected on LB agar containing either tetracycline (10 μg/ml) and chloramphenicol (15 to 25 μg/ml) (for K1110 or K1113 derivatives) or tetracycline (10 μg/ml) and imipenem (0.5 μg/ml) (for K1113 derivatives only). For the preparation of whole cell extracts, cells were grown overnight at 37°C in LB broth in the presence of the appropriate antibiotics, diluted 1:20 in 5 ml of LB broth, and incubated for 4 to 5 h with shaking. One milliliter of cell culture was then harvested by centrifugation (15,000 × g for 3 min), and the cell pellet was resuspended in 100 μl of 50 mM sodium (or potassium) phosphate buffer (pH 7.2). Inner and outer membranes were prepared from cell envelopes following their separation on sucrose density gradients or by differential sarkosyl solubilization as described (30, 50). Expression of OprM derivatives in E. coli and P. aeruginosa was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (25) and Western immunoblotting using a polyclonal anti-OprM antiserum (23, 52).
Antibiotic susceptibility assay.
The susceptibility of P. aeruginosa strains harboring mutant oprM plasmids (see above) to various antimicrobial agents was tested by a twofold serial broth dilution method, with an inoculum of 5 × 105 bacteria/ml. The MIC was reported as the lowest antimicrobial concentration inhibiting visible growth after 18 to 20 h of incubation at 37°C.
Protein secondary structure prediction and sequence analysis.
Prediction of OprM and TolC secondary structure involved the use of the Jpred2 server (http://jura.ebi.ac.uk:8888/) (7, 8). Alignment of the OprM and TolC amino acid sequences was carried out using the PCGene (Intelligenetics) PALIGN program and the DNAMAN sequence analysis software (Lynnon Biosoft). Multiple alignment of the OprM homologues was carried out using DNAMAN.
RESULTS
Operation of mutant OprM proteins in P. aeruginosa.
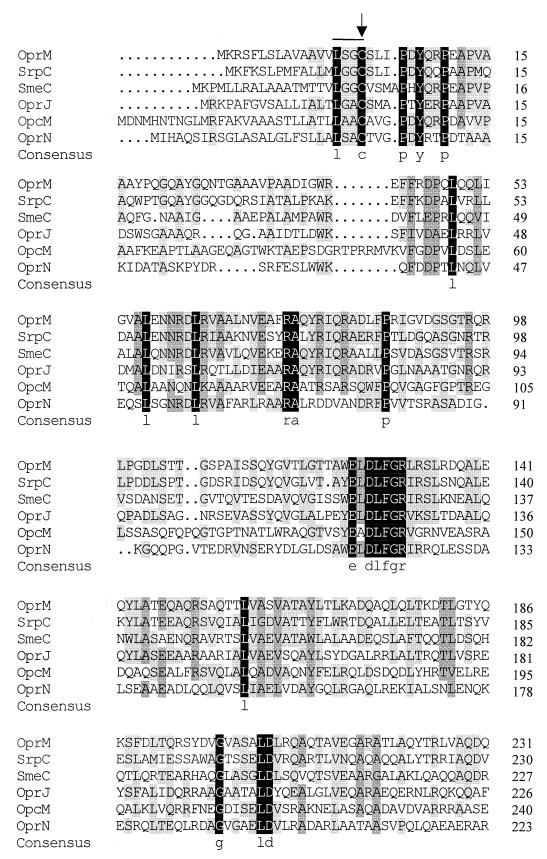
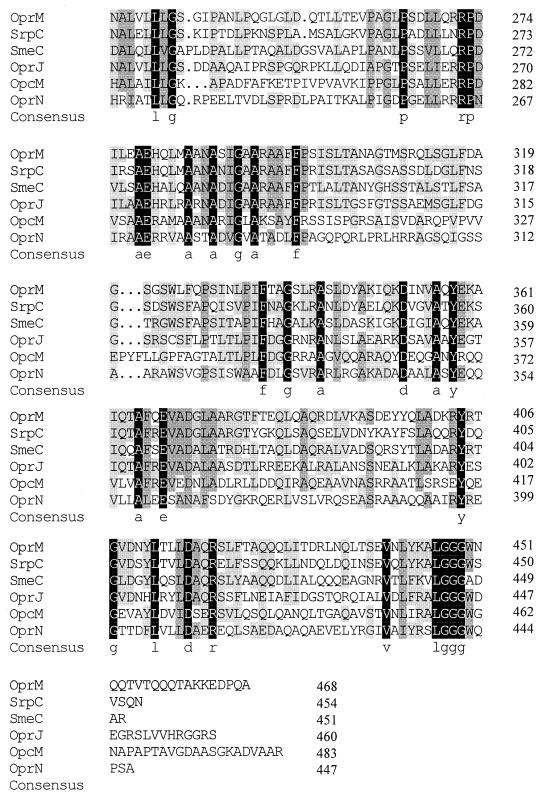
Alignment of OprM with its homologues in P. aeruginosa, P. putida, B. cepacia, and S. maltophilia identified a number of regions with a high degree of sequence conservation (Fig. 1). To assess their importance, if any, for OprM function, we deleted several of these conserved regions and assessed the effect on activity in the OprM-deficient strains K1110 and K1113. The latter strain is a nalB derivative that hyperexpresses MexA and MexB and thus provides for a greater enhancement of antibiotic resistance (compared to K1110) in the presence of functional OprM. One of the more striking examples of sequence conservation involved an LGGGW sequence near the extreme C terminus of OprM and its homologues (Fig. 1). C-terminal truncations which removed this and all downstream OprM sequence (pXZL66) abrogated OprM expression (Fig. 2, lane 19), as did an in-frame deletion of the LGGGW (residues 446 to 450) sequence only (pXZL76; Δ13 in Fig. 3) (Fig. 2, lane 18). In contrast, deletion of sequence downstream of LGGGW (N451 to A468; pXZL67; Δ14 in Fig. 3) yielded a protein (Fig. 2, lane 20) which restored the antibiotic resistance of P. aeruginosa strains K1110 (Table 3) and K1113 (Table 4). Still, mutations in individual residues within this region, including L446 (Fig. 2, lanes 33 and 34), G448 (Fig. 2, lanes 35 and 36), and W450 (Fig. 2, lane 37) failed to affect OprM expression or activity (Tables 3 and 4).
FIG. 1.
Multiple alignment of OprM and its homologues in Pseudomonas spp. and related organisms. Residues conserved in all proteins are highlighted in black, while those conserved in 75 and 50% of the indicated proteins are highlighted in dark and light gray, respectively. Proteins examined include SrpC of the SrpABC efflux system of P. putida (accession number AF029405), SmeC of the SmeABC efflux system of S. maltophilia (accession number AF173226), OprJ of the MexCD-OprJ efflux system of P. aeruginosa (accession number U57969), OpcM of the CeoAB-OprcM efflux system of B. cepacia (accession number U38944), and OprN of the MexEF-OprN efflux system of P. aeruginosa (accession number X99514). The lipoprotein box is overlined and occurs downstream of putative lipoprotein signal sequence. Numbers at the right show the position of the last residue in each line within the mature protein sequences, where the Cys residue within the lipoprotein box (indicated by an arrow) is the first amino acid of the mature proteins. Alignment was carried out using the DNAMAN software package (Lynnon Biosoft).
FIG. 2.
Immunodetection of OprM proteins expressed by ΔoprM P. aeruginosa strain K1110 (A) and nalB ΔoprM P. aeruginosa strain K1113 (B) harboring the indicated plasmids. Whole cell extracts were separated by SDS-PAGE, electroblotted, and developed with antibodies to OprM. The OprM derivative encoded by each of the plasmids is indicated in parentheses.
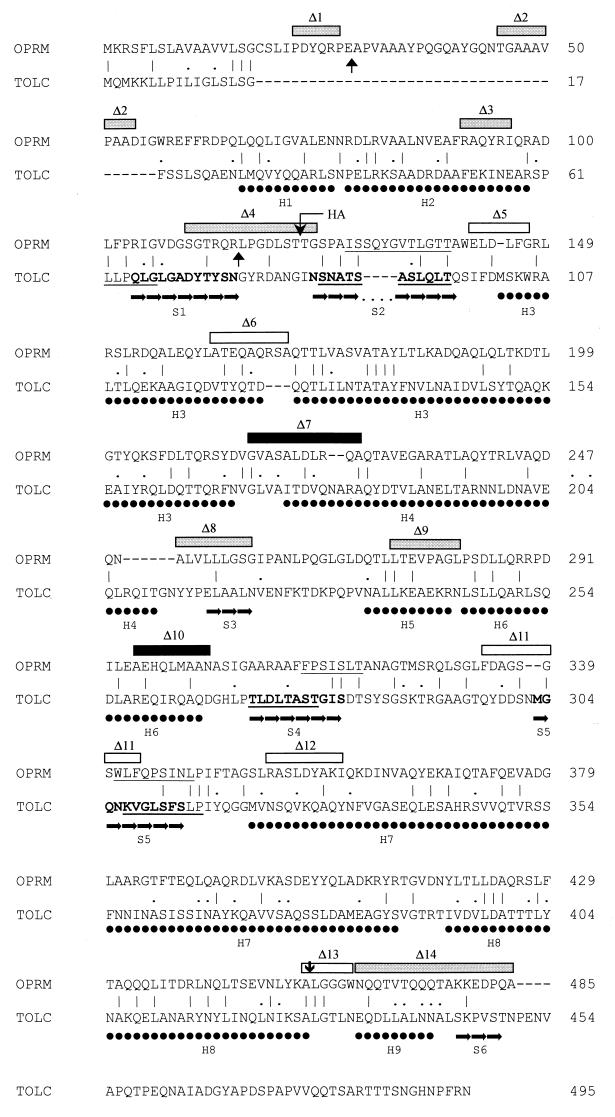
FIG. 3.
Alignment of OprM and TolC, highlighting the structural features of TolC and putative membrane-spanning regions of OprM. Exact matches (|) and conserved changes (.) are indicated. Regions of TolC existing as β-sheet (➞) and α-helix (●) are highlighted below the TolC sequence (derived from and enumerated as described in reference 20), and the membrane-spanning β-sheets are highlighted in bold. Regions of both TolC and OprM predicted to be β-sheet by secondary structure prediction programs on the Jpred2 server are underlined. OprM deletions constructed and tested in this study are highlighted above the OprM sequence, with shaded boxes representing deletions which yielded a functional protein, filled boxes representing deletions which yielded a nonfunctional protein, and open boxes representing deletions which failed to yield an OprM protein. The endpoints of the large but partially functional N-terminal deletion (ΔA12-R98; pXZL137) are indicated with vertical arrows directed upward, and a nonfunctional C-terminal truncation (ΔL446-A468; pXZL66) is indicated with a vertical arrow directed downward. The site of insertion of a nine-amino-acid HA tag is also highlighted. Numbers at the right represent the position of the last amino acid residue in each line within the mature OprM sequence, where the acylated Cys residue is residue 1. Numbering of TolC residues is relative to the precursor form of the protein.
TABLE 3.
Antibiotic resistance of ΔoprM P. aeruginosa K1110 expressing mutant OprM proteinsa
| Strain | Plasmid | OprM derivative | OprM expressionb | MIC (μg/ml) ofc:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| CAR | CPZ | CIP | NOR | CAM | NOV | ||||
| ML5087 | Wild type | + | 32–64 | 8 | 0.2 | 1 | 64 | 128 | |
| K1110 | − | 0.5 | 1 | 0.1 | 0.5 | 64 | 16 | ||
| K1110 | pVLT31 | − | 1 | 1 | 0.1 | 0.5 | 64 | 16 | |
| pXZL34 | Wild type | ++ | 64 | 8 | 0.2 | 1 | 128 | 128 | |
| pXZL74 | ΔP5-P10 | ++ | 32 | 8 | 0.2 | 0.5 | 128 | 128 | |
| pXZL88 | ΔT28-D37 | ++ | 64 | 8 | 0.1 | 1 | 64 | 128 | |
| pXZL92 | ΔR74-I79 | ++ | 32 | 4 | 0.2 | 1 | 64 | 128 | |
| pXZL57 | ΔS93-G107 | + | 64 | 4 | 0.1 | 1 | 64 | 64 | |
| pXZL137 | ΔA12-R98 | −de | 8–16 | 1 | 0.1 | 0.5 | 64 | 16 | |
| pXZL58 | T106-HA-T107 | ++ | 64 | 4 | 0.2 | 0.5 | 128 | 128 | |
| pXZL75 | ΔE125-R131 | − | 0.5 | 1 | 0.1 | 0.5 | 32 | 16 | |
| pXZL89 | ΔA145-A153 | −e | 1 | 0.5 | 0.05 | 0.125 | 32 | 8 | |
| pXZL90 | ΔG199-A209 | + | 0.5 | 1 | 0.05 | 0.25 | 64 | 16 | |
| pXZL73 | ΔA233-G241 | +/− | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL133 | ΔL257-L264 | + | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL77 | ΔA278-N286 | +/− | 0.5 | 0.5 | 0.05 | 0.25 | 32 | 16 | |
| pXZL91 | ΔF317-F326 | −e | 0.5 | 0.5 | 0.2 | 0.5 | 64 | 16 | |
| pXZL93 | ΔR341-I349 | − | 0.5 | 0.5 | 0.05 | 0.25 | 32 | 16 | |
| pXZL76 | ΔA445-W450 | −de | 2 | 2 | 0.05 | 0.125 | 32 | 16 | |
| pXZL66 | ΔL446-A468 | −e | 0.5 | 1 | 0.1 | 0.25 | 32 | 16 | |
| pXZL67 | ΔA451-A468 | + | 16 | 2 | 0.05 | 0.5 | 64 | 64 | |
| pXZL146 | C1G | + | 64 | 4 | 0.2 | 1 | 128 | 128 | |
| pXZL86 | S2D | ++ | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL147 | OprF-OprM fusion | + | 64 | 4 | 0.2 | 1 | 128 | 128 | |
| pXZL109 | E125K | + | 64 | 8 | 0.2 | 0.5 | 64 | 128 | |
| pXZL125 | L128R | + | 32 | 8 | 0.2 | 1 | 64 | 64 | |
| pXZL104 | F129A | + | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL105 | F129Y | + | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL128 | R131E | + | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL111 | E279D | ++ | 64 | 8 | 0.2 | 0.5 | 128 | 128 | |
| pXZL108 | E279K | + | 64 | 8 | 0.2 | 1 | 128 | 128 | |
| pXZL126 | A284F | ++ | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL129 | A284Y | ++ | 64 | 8 | 0.2 | 1 | 128 | 128 | |
| pXZL106 | L446V | + | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL110 | L446Q | + | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL112 | G448T | + | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL107 | G448A | + | 64 | 8 | 0.2 | 1 | 64 | 128 | |
| pXZL127 | W450Y | ++ | 64 | 8 | 0.2 | 1 | 64 | 128 | |
The antibiotic susceptibility of the ΔoprM strain K1110 harboring plasmids encoding the indicated OprM derivatives was determined as described in Materials and Methods. Susceptibility of the wild type (with respect to MexAB-OprM) parent strain ML5087 is shown for comparison purposes.
Relative expression (taken from Fig. 2) indicated as ++ (high), + (moderate), +/− (weak), or − (none).
Values in italics represent MICs substantially below that provided by the wild-type OprM protein. CAR, carbenicillin; CPZ, cefoperazone; CIP, ciprofloxacin; NOR, norfloxacin; CAM, chloramphenicol; NOV, novobiocin.
OprM expression was detectable in cells following induction with IPTG.
Weakly expressed in E. coli DH5α.
TABLE 4.
Antibiotic resistance of nalB ΔoprM P. aeruginosa K1113 expressing mutant OprM proteinsa
| Strain | Plasmid | OprM derivative | OprM expressionb | MIC (μg/ml) ofc:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| CAR | CPZ | CIP | NOR | CAM | NOV | ||||
| ML5087 | Wild type | + | 32–64 | 8 | 0.1–0.2 | 0.5–1 | 64 | 128 | |
| K1112 | Wild type | ++ | 512 | 32 | 0.8 | 2 | 256 | >512 | |
| K1113 | − | 1 | 0.25 | 0.0125 | 0.06 | 2 | 16 | ||
| K1113 | pVLT31 | − | 1 | 0.25 | 0.0125 | 0.06 | 2 | 16 | |
| pXZL34 | Wild type | ++ | 128–256 | 8 | 0.2 | 0.5 | 64–128 | 128 | |
| pXZL74 | ΔP5-P10 | ++ | 128 | 8 | 0.4 | 0.5 | 128 | 128 | |
| pXZL88 | ΔT28-D37 | ++ | 128 | 8 | 0.4 | 0.5 | 128 | 64 | |
| pXZL92 | ΔR74-I79 | ++ | 64 | 4 | 0.2 | 0.5 | 64 | 128 | |
| pXZL137 | ΔA12-R98 | +/− | 32–64 | 2 | 0.05 | 0.5 | 8 | 16 | |
| pXZL57 | ΔS93-G107 | + | 128 | 8 | 0.2 | 0.5 | 8 | 32 | |
| pXZL58 | T106-HA-T107 | ++ | 256 | 8 | 0.2 | 0.5 | 32 | 64 | |
| pXZL75 | ΔE125-R131 | − | 1 | 0.25 | 0.0125 | 0.06 | 4 | 16 | |
| pXZL89 | ΔA145-A153 | −d | 1 | 0.25 | 0.0125 | 0.06 | 8 | 8 | |
| pXZL90 | ΔG199-A209 | ++ | 1 | 0.25 | 0.0125 | 0.125 | 4 | 8 | |
| pXZL73 | ΔA233-G241 | + | 256 | 16 | 0.4 | 1 | 64 | 256 | |
| pXZL133 | ΔL257-L264 | ++ | 256 | 16 | 0.2 | 1 | 128 | 256 | |
| pXZL77 | ΔA278-N286 | + | 1 | 0.25 | 0.0125 | 0.125 | 4 | 16 | |
| pXZL91 | ΔF317-F326 | −d | 1 | 0.25 | 0.0125 | 0.125 | 2 | 8 | |
| pXZL93 | ΔR341-I34 | − | 1 | 0.25 | 0.0125 | 0.125 | 4 | 8 | |
| pXZL76 | ΔA445-W450 | − | 8 | 1 | 0.025 | 0.125 | 8 | 16 | |
| pXZL66 | ΔL446-A468 | −e | 1 | 0.25 | 0.0125 | 0.06 | 8 | 8 | |
| pXZL67 | ΔN451-A468 | + | 128 | 8 | 0.05 | 0.25 | 64 | 256 | |
| pXZL146 | C1G | ++ | 128 | 4 | 0.1 | 0.25 | 64 | 128 | |
| pXZL86 | S2D | + | 128 | 8 | 0.2 | 0.5 | 128 | 256 | |
| pXZL147 | OprF-OprM fusion | ++ | 128 | 4 | 0.1 | 0.5 | 64 | 128 | |
| pXZL109 | E125K | ++ | 64 | 4 | 0.1 | 0.5 | 64 | 128 | |
| pXZL125 | L128R | ++ | 128 | 16 | 0.2 | 0.25 | 128 | 256 | |
| pXZL104 | F129A | + | 128 | 16 | 0.2 | 0.5 | 64 | 256 | |
| pXZL105 | F129Y | + | 128 | 16 | 0.2 | 0.5 | 128 | 256 | |
| pXZL128 | R131E | ++ | 128 | 16 | 0.2 | 0.5 | 128 | 256 | |
| pXZL111 | E279D | ++ | 128 | 8 | 0.1 | 0.5 | 64 | 128 | |
| pXZL108 | E279K | + | 128 | 16 | 0.2 | 0.5 | 128 | 256 | |
| pXZL126 | A284F | + | 128 | 8 | 0.1 | 0.5 | 128 | 128 | |
| pXZL129 | A284Y | + | 128 | 16 | 0.2 | 0.5 | 128 | 256 | |
| pXZL106 | L446V | + | 128 | 16 | 0.2 | 0.5 | 128 | 256 | |
| pXZL110 | L446Q | + | 128 | 8 | 0.2 | 0.5 | 128 | 256 | |
| pXZL112 | G448T | + | 128 | 16 | 0.1 | 0.5 | 128 | 256 | |
| pXZL107 | G448A | + | 256 | 8 | 0.2 | 0.5 | 32 | 256 | |
| pXZL127 | W450Y | + | 128 | 8 | 0.2 | 0.5 | 256 | 128 | |
The antibiotic susceptibility of the nalB ΔoprM strain K1113 harboring plasmids encoding the indicated OprM derivatives was determined as described in Materials and Methods. Susceptibility of the wild type (with respect to MexAB-OprM) strain ML5087 and its nalB derivative K1112 (the parent of K1113) is shown for comparison purposes.
Relative expression (taken from Fig. 2) indicated as ++ (high), + (moderate), +/− (weak), or − (none).
Values in italics represent MICs substantially below that provided by the wild-type OprM protein. CAR, carbenicillin; CPZ, cefoperazone; CIP, ciprofloxacin; NOR, norfloxacin; CAM, chloramphenicol; NOV, novobiocin.
OprM expression detectable in E. coli DH5α.
OprM expression was detectable in cells following induction with IPTG.
A second highly conserved sequence, ELDLFGR, occurs nearer the N terminus (residues 125 to 131 in OprM). Deletion of this region (pXZL75; Δ5 in Fig. 3) also abrogated OprM expression (Fig. 2, lane 14) and thus activity (Tables 3 and 4). Despite the conservation of this sequence in OprM and its homologues, substitutions at E125 (Fig. 2, lane 24), L128 (Fig. 2, lane 25), F129 (Fig. 2, lanes 26 and 27), and R131 (Fig. 2, lane 28) did not adversely affect expression (Fig. 2) or activity (Tables 3 and 4) of the mutant OprM proteins.
Although additional regions of shared similarity exist in OprM and its homologues (Fig. 1), strict sequence conservation was rather limited in the remainder of the proteins. Near the extreme N terminus of each of these proteins, from P5 to P10 of OprM, lies the sequence PDYQRP, of which P5, Y7, and P10 are strictly conserved in all OprM homologues (Fig. 1). Still, deletion of this region (pXZL74; Δ1 in Fig. 3) had no impact on OprM expression (Fig. 2, lane 4) or activity (Tables 3 and 4). The region encompassing A278 to P298 of OprM also showed some sequence conservation among the OprM homologues (Fig. 1), and deletion of residues in this region (A278 to N286; pXZL77; Δ10 in Fig. 3), while severely compromising OprM expression in P. aeruginosa K1110 (Fig. 2A, lane 16), had no impact on OprM expression in P. aeruginosa K1113 (Fig. 2B, lane 16). Moreover, the OprM ΔA278-N286 protein was inactive in both K1110 (Table 3) and K1113 (Table 4). Still, substitutions at the strictly conserved E279 (Fig. 2, lanes 29 and 30) and A284 (Fig. 2, lanes 31 and 32) residues yielded well-expressed (Fig. 2) and functional (Tables 3 and 4) OprM proteins.
Influence of N-terminal acylation on OprM activity.
OprM and its homologues possess a region at the N terminus, termed the lipoprotein box, immediately downstream of an N-terminal signal sequence (Fig. 1). A conserved Cys residue within this box is the first amino acid of the mature proteins and the deduced site of acylation for this family of presumed lipoproteins; indeed, acylation of OprM has been demonstrated (N. Bianco and K. Poole, unpublished data; T. Nakae, A. Nakajima, Y. Sugimoto, and H. Yoneyama, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. A-28, 2000). To assess the importance of acylation for OprM expression and function, we mutated the Cys residue (to Gly) and examined expression and activity of the C1G mutant OprM (pXZL146) in K1110 and K1113. P. aeruginosa harboring pXZL146 produced a readily detectable OprM protein (Fig. 2, lane 21), and this plasmid enhanced the antibiotic resistance of K1110 and K1113 strains carrying it. To further assess the significance of OprM acylation, the lipoprotein signal (including lipoprotein box) of OprM was replaced by the type I signal sequence of OprF, the major outer membrane protein of P. aeruginosa(pXZL147). Again, the OprM derivative carrying the OprF signal was well expressed (Fig. 2, lane 22), particularly in K1113 (Fig. 2B, lane 22), and highly active in both K1110 (Table 3) and K1113 (Table 4). Cell fractionation studies also confirmed that the C1G and OprF-OprM proteins were present in the outer membrane (data not shown). Thus, loss of the acylation site of OprM did not obviate OprM activity.
Lipoproteins occur in both the inner and outer membranes, and there is some indication in E. coli, at least, that the penultimate (in the mature proteins) residue helps define membrane localization, with aspartate favoring an inner membrane location and serine favoring an outer membrane location (37, 42). Intriguingly, the second residue in mature OprM is the expected serine residue. To assess if this plays any role in location and thus expression or function of OprM, this residue was mutated to aspartate (S2D; pXZL86). The resultant OprM protein was well expressed (Fig. 2, lane 23) and active (Tables 3 and 4) and retained its predominantly outer membrane location (data not shown).
Topological model of OprM.
The recently proposed topological model that describes OprM as a β-barrel of 16 transmembrane domains (47) is likely incorrect, in light of the recently solved crystal structure of TolC (20), an OprM homologue (ca. 20% identical [Fig. 3]). Accordingly, OprM likely spans the outer membrane only four times, with both the N and C termini occurring within the periplasm, and displays a substantial periplasmic component. To assess the accuracy of such a model for OprM, the Jpred2 secondary structure prediction server was used to assess the secondary structure of this protein. Initially, however, the server was applied to TolC, to determine how closely the predicted secondary structure matched the crystal structure. Intriguingly, the various prediction programs of Jpred2 correctly identified TolC as a protein that was substantially α-helical (data not shown) and accurately identified three of the four membrane-spanning β-sheets (S2, S4, and S5 [Fig. 3]). The first membrane-spanning β-sheet (S1) was only weakly predicted (Fig. 3). Similarly, Jpred2 identified OprM as a predominantly α-helical protein (data not shown) with three putative membrane-spanning β-sheets located roughly where they would be predicted following alignment of TolC and OprM (opposite S2, S4 and S5 [Fig. 3]). Alignment of the N- and C-terminal halves of OprM, which confirmed the homology of these two halves (23.7% identity and 9.8% conserved changes) and the likely gene duplication involved, also confirmed that the putative membrane-spanning regions S2 and S5 were equivalently placed within the N- and C-terminal halves of the protein (Fig. 4). Moreover, the region within the N-terminal portion of OprM which aligns with S4 of the C-terminal half is bracketed by sequences whose deletion (Δ3 and Δ4 [Fig. 3]) is tolerated (see below) and thus unlikely to correspond to membrane-spanning regions. As this region also overlaps sequences of OprM that align with S1 of TolC (Fig. 3), it is the only reasonable candidate for the first membrane-spanning β-sheet of OprM. In light of the internal homology, the equivalent placement of the putative membrane-spanning regions within the N- and C-terminal halves of OprM supports the existence of these regions as membrane-spanning domains in OprM (Fig. 4) and support OprM adopting a structure reminiscent of TolC.
FIG. 4.
Internal alignment of OprM, highlighting the homology between the N-terminal (OPRMN) and C-terminal (OPRMC) halves of the protein. Exact matches (|) and conserved changes (.) are indicated. Putative membrane-spanning β-sheet regions are underlined in bold and enumerated as for TolC. Numbers at the right represent the position of the last amino acid residue in each line within the mature OprM sequence, where the acylated Cys residue is residue 1.
To assess the accuracy of such a structural model and to assess the functional importance of defined regions of OprM, we engineered several deletions into oprM and determined the effect on OprM expression and activity. While several of these deletions were originally designed with the β-barrel model (47) in mind, the phenotype of several deletions actually provided support for the TolC model. Deletion of residues F317 to F326 (pXZL91; Δ11 in Fig. 3), which overlapped the fourth putative membrane-spanning β-sheet (S5 in the TolC model), for example, failed to yield a protein (Fig. 2A and B, lanes 12), and it is well known that membrane-spanning domains are not tolerant of deletions (13, 29, 38). Several deletions, including ΔP5-P10 (pXZL74; Δ1 in Fig. 3), ΔT28-D37 (pXZL88; Δ2 in Fig. 3), ΔR74-I79 (pXZL92; Δ3 in Fig. 3), ΔS93-G107 (pXZL57; Δ4 in Fig. 3), ΔG199-A209 (pXZL90; Δ7 in Fig. 3), ΔA233-G241 (pXZL73; Δ8 in Fig. 3), and ΔL257-L264 (pXZL133; Δ9 in Fig. 3), yielded OprM products in both K1110 (Fig. 2A, lanes 4, 5, 13, 6, 10, 15, and 11) and K1113 (Fig. 2B, lanes 4, 5, 13, 6, 10, 15, and 11), consistent with these regions lying outside the membrane, as predicted by the TolC model (Fig. 3). All of these deletions were functional, with the exception of the pXZL90-encoded OprM ΔG199-A209 derivative (Tables 3 and 4), which might yield a shortened H4 α-helix (Fig. 3). Insertion of the nine-amino-acid HA tag between the putative S1 and S2 membrane-spanning domains (Fig. 3) also yielded a well-expressed OprM protein (Fig. 2, lane 7) that was active (Tables 3 and 4), again suggesting that this region lies outside of the membrane.
Not all nonpermissive deletions occurred within putative membrane-spanning regions, however. Deletions of E125 to G130 (pXZL75; Δ5 in Fig. 3), A145 to A153 (pXZL89; Δ6 in Fig. 3), R341 to I349 (pXZL93; Δ12 in Fig. 3), and A445 to W450 (pXZL76; Δ13 in Fig. 3) failed to yield an OprM protein (Fig. 2A and B, lanes 14, 9, 17, and 18). According to the TolC model 20, Δ5 and Δ6 would truncate H3, one of two particularly lengthy α-helices that extend into the periplasm, while Δ12 would truncate H7, the other lengthy periplasmic α-helix (Fig. 3). The nonpermissive nature of Δ13 is surprising in that it occurs between putative helices H8 and H9 (Fig. 3).
Four different deletions within the first 108 amino acids of mature OprM were expressed and functional, raising questions as to the functional significance of this portion of the protein. Deletion of much of the N terminus of OprM (A12 to R98; pXZL137) did compromise expression of OprM in P. aeruginosa K1110 (Fig. 2A, lane 8) although the protein was detectable in P. aeruginosa K1113 (Fig. 2B, lane 8). Moreover, the protein, though less active than wild-type OprM in K1113, did increase the resistance of this OprM-deficient strain to most antibiotics that were tested (Table 4).
DISCUSSION
The OprM family of outer membrane multidrug efflux proteins shares a number of regions of sequence conservation, several of which were targeted here for mutagenesis. While the expectation was that these would be important for function, only two of nine permissive deletions (ΔG199-A209 and ΔA278-N286) impaired OprM function, and none of the highly conserved residues that were mutated in these regions was essential for activity. Still the very highly conserved LGGGW sequence near the extreme C terminus of OprM appears to be especially critical for expression and, thus activity of this protein. Consistent with these results, deletion of the final 70 amino acids of OprM was previously shown to obviate expression of the protein (47). Intriguingly, the originally reported oprM gene possessed an anomalous sequence at its 3′ end provided by the phagemid vector into which oprM had been cloned, although the resultant OprM protein was fully functional. This protein lacked the final 22 amino acids of native OprM (replaced with 14 phagemid-derived amino acids), confirming results presented here that the extreme C terminus of OprM is dispensable for activity. Perhaps importantly, however, the nature of the LGGGW sequence was retained, being changed to LWGG in this altered oprM gene. The conservation of a tryptophan residue in this region may be of some importance. A conservative substitution (W450Y) failed to alter expression or activity of OprM, while additional substitutions were unsuccessful.
Lipoproteins typically possess a signature lipoprotein box near the N terminus, immediately following a signal sequence (37). An absolutely conserved cysteine residue within this box in OprM and its homologues, though the site of acylation of these lipoproteins, was dispensable, suggesting that acylation is not a prerequisite for function. Similarly, MexA, though also a lipoprotein, need not be acylated to be functionally active (49). The role of these lipid tails, then, in the assembly or activity of the MexAB-OprM efflux pump is unclear.
In light of the recently solved crystal structure of TolC, which describes the protein as a trimeric “channel-tunnel” that spans both the outer membrane (as a β-barrel) and the periplasm (as a α-helical barrel), it is reasonable to assume that OprM, a TolC homologue, adopts much the same conformation. Such a structure contrasts with the recently published model for OprM, which describes it a monomeric β-barrel comprised of 16 membrane-spanning segments (47). Consistent with the TolC model and in contrast to the monomeric β-barrel model, however, recent analysis of the OprM sequence using a neural network approach predicted that a large portion of the protein would be periplasmic (11; A. Ferguson, personal communication). Using a variety of secondary structure prediction tools, the predominantly α-helical nature of OprM (and TolC) was confirmed, as was the placement of putative β-sheet regions that could form membrane-spanning domains. These agreed quite nicely with predictions based on alignment of the TolC and OprM sequences (Fig. 3). Interestingly, too, OprM, like TolC (20), displays internal homology, further supporting OprM adopting a TolC-like conformation. Using the TolC model, then, it was possible to assess the implications of the various deletions constructed in this study and to use this information to validate the TolC model for OprM. Surprisingly, the highly conserved LGGGW encompassed by Δ13 does not correspond to any significant structural feature in TolC, occurring as it does in a region of OprM that aligns with TolC between α-helices H8 and H9. Although one cannot be sure about the exact placement of these helices in OprM, much of the region encompassed by Δ13 is conserved between OprM and TolC, suggesting that it does not lie within OprM helix H8 or H9. While the reason for the nonpermissive nature of this deletion is unknown, the fact that deletion of downstream residues does not affect expression (or function) of OprM indicates that an H9 is unlikely to be important for OprM function or assembly. As such, the lack of expression of a Δ13 OprM derivative cannot be due to an effect on topology of this downstream helix.
The region from G199 to A209 of OprM (encompassed by Δ7 in Fig. 3) corresponds to the beginning of H4 in TolC, although the proximity of this region to H3 and the possibility of some variation regarding the exact positioning of comparable helices in OprM suggests that either of these α-helices may be affected in OprM ΔG199-A209. The fact, however, that other deletions in the putative H3 α-helix (e.g., Δ5 and Δ6 in Fig. 3) are nonpermissive suggests that Δ7 may well affect H4. Why this would abrogate function is unclear, although the placement of this region of TolC at the extreme periplasmic end of the protein (facing the cytoplasmic membrane) suggests it may, in OprM, be important for interaction with MexA or MexB. Alternatively, since the N-terminal end of H4 contacts H7 in forming the periplasmic α-helical barrel of TolC (20), Δ7 could disrupt this contact in OprM, perhaps affecting proper barrel formation or assembly.
Δ10, encompassing A278 to N286 of OprM, corresponds to the C terminus of H6 in TolC (Fig. 3) which, together with the downstream α-helix H8, forms a pseudocontinuous helix that spans the length of the α-helical barrel of TolC (20). Intriguingly, the region encompassed by this deletion corresponds to a region in the TolC α-helix H6 that contacts α-helix H7 (20). Quite possibly, then, this deletion disrupts such an interaction in OprM, again compromising proper barrel formation.
With the exception of Δ11 (F317 to F326), which likely disrupts a membrane-spanning domain of OprM and thus is expected to be nonpermissive, the nonpermissive deletions identified in this study do not correspond to expected membrane-spanning regions of the protein. Interestingly, however, they all target the long helices, dubbed H3 and H7 in TolC. Given their importance in formation of the periplasmic α-helical barrel of TolC (they extend the length of the barrel), their truncation might prevent assembly of the periplasmic barrel domain, yielding an improperly folded and highly unstable protein. Moreover, in contrast to the permissive deletions in H4 and H6, which likely affect only one set of contacts, truncations in H3 and H7 would, by virtue of their shortening these helices, disrupt the positioning of residues downstream of the deletion, perhaps impacting on multiple contacts with other helices. This, too, might render the protein unstable.
The ability to delete the first ca. 100 amino acids of OprM and retain some function, though surprising, was reminiscent of the ferrichrome receptor FhuA (4). It is now known that the N terminus of this and other ferric siderophore receptors encompasses a TonB-responsive cork domain that blocks the receptor channel from the periplasmic side of the protein (10, 24). As MexAB-OprM is apparently dependent on TonB (51), it may be that the N terminus of OprM similarly functions as a TonB-responsive cork. Given the unique structure predicted for OprM, however, it likely looks and functions somewhat differently than it does in FhuA. This would explain both the lack of a TonB box (the site on traditional TonB-dependent outer membrane receptors [e.g., FhuA] that interacts with TonB) in OprM and its homologues and the differential effect of TonB mutations on drug efflux versus iron transport activity in P. aeruginosa (Q. Zhao and K. Poole, submitted for publication), since TonB would have to interact differently with OprM than with a ferric siderophore receptor. It is also interesting that OprM possesses an N-terminal extension not shared by TolC (Fig. 3) and TolC appears not to be TonB dependent (at least antibiotic resistance, and therefore AcrAB-TolC-mediated resistance, is not compromised in an E. coli tonB mutant [Q. Zhao and K. Poole, unpublished data]). Whether the additional N-terminal residues are associated with a TonB dependence of MexAB-OprM activity or simply relate to the acylation and membrane anchoring of the N terminus of OprM (TolC is not a lipoprotein) is, however, uncertain. The results of our study are in consonance with those of Hancock's group (46a).
ACKNOWLEDGMENTS
This work was supported by an operating grant from the Canadian Cystic Fibrosis Foundation (CCFF). X.-Z.L. holds a CCFF studentship. K.P. is a CCFF Martha Morton Scholar.
REFERENCES
- 1.Aires J R, Köhler T, Nikaido H, Plésiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcak G J, Chandler M S, Redfield R J, Tomb J-F. Genetic systems in Haemophilus influenzae. Methods Enzymol. 1991;204:321–337. doi: 10.1016/0076-6879(91)04016-h. [DOI] [PubMed] [Google Scholar]
- 3.Bianco N, Neshat S, Poole K. Conservation of the multidrug resistance efflux gene oprM in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:853–856. doi: 10.1128/aac.41.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun M, Killmann H, Braun V. The β-barrel domain of FhuAΔ5-160 is sufficient for TonB-dependent FhuA activity of Escherichia coli. Mol Microbiol. 1999;33:1037–1049. doi: 10.1046/j.1365-2958.1999.01546.x. [DOI] [PubMed] [Google Scholar]
- 5.Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:293–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 6.Burns J L, Wadsworth C D, Barry J J, Goodall C P. Nucleotide sequence analysis of a gene from Burkholderia (Pseudomonas) cepacia encoding an outer membrane lipoprotein involved in multiple antibiotic resistance. Antimicrob Agents Chemother. 1996;40:307–313. doi: 10.1128/aac.40.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuff J A, Clamp M E, Siddiqui A S, Finlay M, Barton G J. Jpred: a consensus secondary structure prediction server. Bioinformatics. 1998;14:892–893. doi: 10.1093/bioinformatics/14.10.892. [DOI] [PubMed] [Google Scholar]
- 8.Cuff J A, Barton G J. Evaluation and improvement of multiple sequence methods for protein secondary structure prediction. Proteins Struct Func Genet. 1999;34:508–529. doi: 10.1002/(sici)1097-0134(19990301)34:4<508::aid-prot10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.de Lorenzo V, Eltis L, Kessler B, Timmis K. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson A D, Hofmann E, Coulton J W, Diederichs K, Weltes W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2225. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 11.Fiederichs K, Preigang J, Umhau S, Zeth K. Prediction by a neural network of outer membrane β-strand protein topology. Protein Sci. 1988;7:2413–2420. doi: 10.1002/pro.5560071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotoh N, Tsujimoto H, Poole K, Yamagishi J-I, Nishino T. The outer membrane protein OprM of Pseudomonas aeruginosa is encoded by oprK of the mexA-mexB-oprK multidrug resistance operon. Antimicrob Agents Chemother. 1995;39:2567–2569. doi: 10.1128/aac.39.11.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H, Jeanteur D, Pattus F, Hancock R E W. Membrane topology and site-specific mutagenesis of Pseudomonas aeruginosa porin OprD. Mol Microbiol. 1995;16:931–941. doi: 10.1111/j.1365-2958.1995.tb02319.x. [DOI] [PubMed] [Google Scholar]
- 14.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1991;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J M, Church G M. Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J Mol Biol. 1999;287:695–715. doi: 10.1006/jmbi.1999.2630. [DOI] [PubMed] [Google Scholar]
- 16.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 17.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1998;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- 18.Köhler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 19.Köhler T, Kok M, Michea-Hamzehpour M, Plesiat P, Gotoh N, Nishino T, Kocjanici Curty L, Pechere J-C. Multidrug efflux in intrinsic resistance to trimethoprim and sulfamethoxazole in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:2288–2290. doi: 10.1128/aac.40.10.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 21.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X-Z, Zhang L, Poole K. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol. 1998;180:2987–2991. doi: 10.1128/jb.180.11.2987-2991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X-Z, Zhang L, Srikumar R, Poole K. β-Lactamase inhibitors are substrates of the multidrug efflux pumps of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:399–403. doi: 10.1128/aac.42.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locher K P, Rees B, Koebnik R, Mitscher A, Moulinier L, Rosenbush J P, Moras D. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. [DOI] [PubMed] [Google Scholar]
- 25.Lugtenberg B, Meijiers R, Peters R, van der Hock P, van Alphen L. Electrophoretic resolution of the “major outer membrane protein” of Escherichia coli K-12 into four bands. FEBS Lett. 1975;58:254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- 26.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–417. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moeck G S, Coulton J W, Postle K. Cell envelope signaling in Escherichia coli. Ligand binding to the ferrichrome-iron receptor FhuA promotes interaction with the energy-transducing protein TonB. J Biol Chem. 1997;272:28391–28397. doi: 10.1074/jbc.272.45.28391. [DOI] [PubMed] [Google Scholar]
- 29.Newton S M C, Klebba P E, Michel V, Hofnung M, Charbit A. Topology of the membrane protein LamB by epitope tagging and a comparison with the X-ray model. J Bacteriol. 1996;178:3447–3456. doi: 10.1128/jb.178.12.3447-3456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikaido H. Isolation of outer membrane. Methods Enzymol. 1994;235:225–234. doi: 10.1016/0076-6879(94)35143-0. [DOI] [PubMed] [Google Scholar]
- 31.Nikaido H. Multiple antibiotic resistance and efflux. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 32.Okii M, Iyobe S, Mitsuhashi S. Mapping of the gene specifying aminoglycoside 3′-phosphotransferase II on the Pseudomonas aeruginosa chromosome. J Bacteriol. 1983;155:643–649. doi: 10.1128/jb.155.2.643-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen I T, Park J H, Choi P S, Saier M H., Jr A family of gram-negative bacterial outer membrane factors that function in the export of proteins, carbohydrates, drugs and heavy metals from gram-negative bacteria. FEMS Microbiol Lett. 1997;156:1–8. doi: 10.1111/j.1574-6968.1997.tb12697.x. [DOI] [PubMed] [Google Scholar]
- 34.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J-I, Li X-Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB multidrug resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 35.Poole K, Heinrichs D E, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole K, Krebes K, McNally C, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 37.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehm B H, Hancock R E W. Membrane topology of the outer membrane protein OprH from Pseudomonas aeruginosa: PCR-mediated insertion and deletion mutagenesis. J Bacteriol. 1996;178:3346–3349. doi: 10.1128/jb.178.11.3346-3349.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saier M H, Jr, Tam R, Reizer A, Reizer J. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol Microbiol. 1994;11:841–847. doi: 10.1111/j.1365-2958.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 41.Schaefer, A., A. Tauch, W. Jaeger, J. Kalinowski, G. Thierbach, and A. Puehler. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. [DOI] [PubMed]
- 42.Seydel A, Gounon P, Pugsley A P. Testing the ‘+2 role’ for the lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol Microbiol. 1999;34:810–821. doi: 10.1046/j.1365-2958.1999.01647.x. [DOI] [PubMed] [Google Scholar]
- 43.Simon, R., U. Priefer, and A. Puehler. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784–791.
- 44.Srikumar R, Li X-Z, Poole K. The inner membrane efflux components are responsible for the β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 46.Westbrock-Wadman S, Sherman D R, Hickey M J, Coulter S N, Zhu Y Q, Warrener P, Nguen L Y, Sharwar R M, Folger K R, Stover C K. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob Agents Chemother. 1999;43:2975–2983. doi: 10.1128/aac.43.12.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Wong K K Y, Brinkman F S L, Benz R S, Hancock R E W. Evaluation of a structural model of Pseudomonas aeruginosa outer membrane protein OprM, an efflux component involved in intrinsic antibiotic resistance. J Bacteriol. 2001;183:367–374. doi: 10.1128/JB.183.1.367-374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong K K Y, Hancock R E W. Insertion mutagenesis and membrane topology model of the Pseudomonas aeruginosa outer membrane protein OprM. J Bacteriol. 2000;182:2402–2410. doi: 10.1128/jb.182.9.2402-2410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong K K Y, Poole K, Gotoh N, Hancock R E W. Influence of OprM expression on multiple antibiotic resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2009–2012. doi: 10.1128/aac.41.9.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoneyama H, Maseda H, Kamiguchi H, Nakae T. Function of the membrane fusion protein, MexA, of the MexA, B-OprM efflux pump in Pseudomonas aeruginosa without an anchoring membrane. J Biol Chem. 2000;275:4628–4634. doi: 10.1074/jbc.275.7.4628. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Li X-Z, Poole K. Multiple antibiotic resistance in Stenotrophomonas maltophilia: involvement of a multidrug efflux system. Antimicrob Agents Chemother. 2000;44:287–293. doi: 10.1128/aac.44.2.287-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Q, Li X-Z, Mistry A, Srikumar R, Zhang L, Lomovskaya O, Poole K. Influence of the TonB energy-coupling protein on efflux-mediated multidrug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:2225–2231. doi: 10.1128/aac.42.9.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Q, Li X-Z, Srikumar R, Poole K. Contribution of outer membrane efflux protein OprM on antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob Agents Chemother. 1998;42:1682–1688. doi: 10.1128/aac.42.7.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]