Abstract
Background
Complicated urinary tract infection (cUTI) is the one which is associated with structural and functional abnormalities of the urinary tract, thus increasing the risk of infection and failure of therapy.
Aim:
This study aims to determine the risk factors, changing trends in etiology, current treatment options, and outcomes in cUTI.
Materials and Methods:
This prospective observational study was done on patients presenting with cUTI. Hematological, biochemical workup, urine routine, urine culture, blood culture, ultrasonography, and wherever necessary computerized tomography of the genitourinary tract was done. The medical/surgical interventions and outcomes in these patients were recorded.
Results:
A total of 100 patients were enrolled in the study. Diabetes mellitus was the most common risk factor present in 53%. The most common organism isolated in urine culture was Escherichia coli (48%) followed by Klebsiella pneumoniae (19%) and similar trend but lesser positive yield was there in blood culture (Escherichia coli - 26% followed by Klebsiella pneumoniae - 3%). The organisms were most susceptible to colistin/polymyxin (100%) followed by carbapenems (88%), and later were the most commonly used empirical antibiotics in our study, yielding 95% survival rate. Surgical interventions (percutaneous/endourological) were required in 28%, renal replacement therapy in 14%, intensive care in 40% and mechanical ventilation in 10%, with 4% overall mortality at the end of 1-month follow-up. The mean duration of hospital stay was 9.1 ± 2.7 days.
Conclusion
Escherichia coli was the most common organism causing cUTI, with diabetes being the most common risk factor. Most of the patients were treated with carbapenems with excellent survival outcomes.
Keywords: Clinical profile, complicated urinary tract infection, etiology, outcome, risk factors
Introduction
Complicated urinary tract infections (cUTI) occur in individuals with functional or structural abnormalities of the genitourinary tract such as obstruction, neurological impairment, urological abnormalities/devices, immunological impairment, and congenital diseases.[1] cUTI may be caused by a wide variety of organisms and many of them maybe resistant to broad-spectrum antimicrobial agents. Escherichia coli is the most common organism causing complicated UTI, but Citrobacter, Enterobacter, Pseudomonas, Staphylococcus aureus, and others are also seen in higher frequency (6%–20%) in complicated UTI as compared to uncomplicated UTI.[1,2] The prevalence of fungal infection like candida is also higher.[3] Patients of UTI may have myriad presentations varying from burning micturition and fever to severe sepsis and septic shock. It may also present as delirium or confusion in the elderly.[4] The present study intended to study the spectrum of cUTI with respect to its presenting features, risk factors, etiology, and outcomes.
Materials and Methods
The study was conducted in a tertiary care hospital in North India from January 2018 to June 2019. Adult patients (more than 18 years) with one or more symptoms suggestive of UTI such as fever, dysuria, increased frequency of micturition, flank pain, renal angle tenderness, suprapubic pain, and investigational evidence suggestive of complicated UTI were included in the study. A total of 100 patients were enrolled and followed up for 1 month or discharge whichever was later. Patients that were excluded were pregnant females, children <18 years, acute uncomplicated UTI, and recurrent acute uncomplicated cystitis in young women. The patient’s detailed history and physical examination were recorded. Complete blood counts, renal function tests, urine routine, urine cultures, and blood cultures were done in all at baseline and relevant investigations monitored regularly. Ultrasound abdomen was done in all the patients and noncontrast computed tomography of the kidneys and urinary bladder (NCCT-KUB) wherever required. The details of empirical antibiotics used, the need for change of antibiotics following culture reports, any surgical intervention required, the need for renal replacement therapy, intensive care, duration of hospital stay, and outcome were noted. The study was approved by the institutional and medical university ethical committee and a written informed consent was taken from each patient before inclusion in the study.
Statistical analysis
Data were described in terms of range; mean ± standard deviation, frequencies (number of cases), and relative frequencies (percentages) as appropriate. All statistical calculations were done using (Statistical Package for the Social Science) SPSS 21 version (SPSS Inc., Chicago, IL, USA) statistical program for Microsoft Windows.
Results
A total of 100 cases were enrolled in the study, 64 males and 36 females. Majority of the patients were aged between 51 and 70 years (49%), with a mean age of 58.8 ± 16 years (range 18–100). Fever was the most common presenting complaint (90%) followed by flank pain (34%) [Figure 1]. Diabetes mellitus was the most common risk factor present in 53% of our patients besides multitude of comorbidities such as hypertension, chronic kidney disease, and coronary artery diseases. Other risk factors included obstructive uropathy, indwelling urinary catheter, history of urological procedure, and renal stones [Figure 2]. Most of the patients were initiated on broad-spectrum empirical antibiotics including meropenem and teicoplanin (52%). Urine culture yielded growth in 77% of the cases, Escherichia coli being the most common organism (48%) followed by Klebsiella pneumoniae (19%) [Figure 3]. The organisms were 100% susceptible to Colistin and Polymixin B followed by carbapenems (Imipenem- 88%, Meropenem - 84%, and Ertapenem - 62%) [Figure 4]. Blood cultures yielded growth in 31% of cases, majority of them Escherichia coli (26 out of 31). Antibiotics needed revision based on culture results and clinical response in 4%. Ultrasonography revealed obstructive drainage in the urinary system as the cause in 76% cases. NCCT-KUB was done in 58% of cases to make a diagnosis or plan interventions. Surgical interventions (radiologically guided percutaneous or endourological) were required in 28% cases, including percutaneous nephrostomy, radiopaque polyurethane double-J stenting. The mean duration of hospital stay in our study was 9.1 ± 2.7 days, with majority patients (52%) staying from 6 to 10 days. Intensive care was needed in 40% of cases, at some point in their hospital course, with 10% of these requiring invasive ventilatory supports. Renal replacement therapy was required in 14% cases. Recovery was seen in 95% of cases at the end of 1 month of follow-up. In hospital mortality was 4%, whereas one patient was discharged against medical advice and lost to follow-up.
Figure 1.
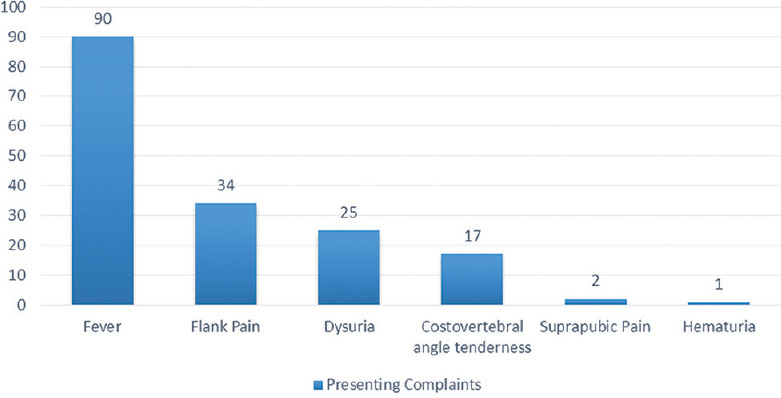
Clinical features at presentation in complicated urinary tract infection
Figure 2.
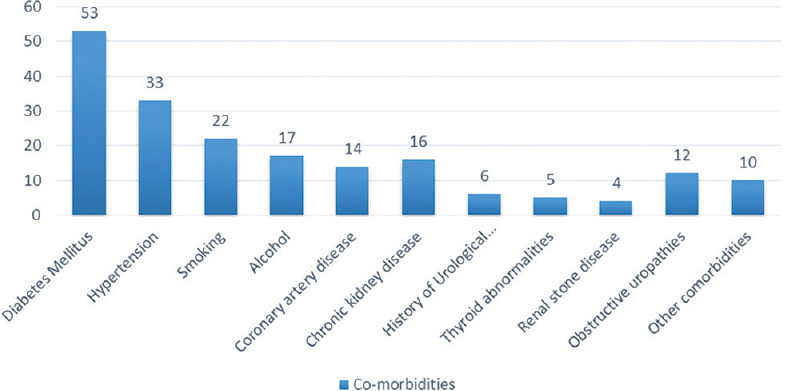
Risk factors and co-morbidities in complicated urinary tract infection
Figure 3.
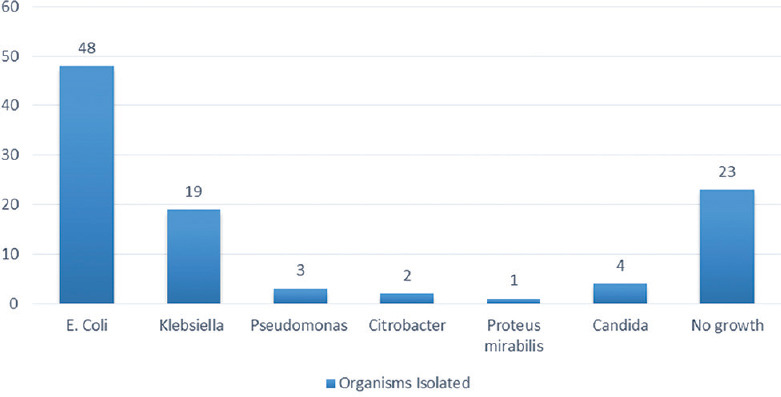
Etiological organisms on urine culture sensitivity in patients with complicated urinary tract infection
Figure 4.
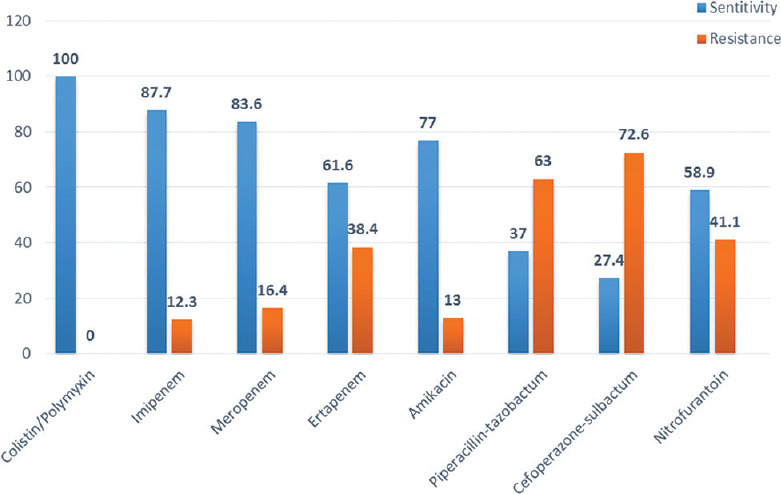
Antibiotic sensitivity pattern on urine culture
Discussion
Complicated UTI is a poorly recognized condition in view of masquerading presentations. Early identification and aggressive management is the key to a successful outcome. The high prevalence of antimicrobial resistance is a big challenge in its treatment in developing countries like India. The Rampart over-the-counter abuse of many antimicrobials like fluoroquinolones and penicillins has led to the loss of their effectiveness. Currently, the antibiotic sensitivity pattern varies in different regions for different organisms and an updated knowledge is important for rational use and effectiveness of empirical therapy in life-threatening cUTI.[1,2]
This single-center prospective observational study was done with the intend to guide effective management of cUTI by early diagnosis based on clinical presentation, identification of the risk factors, and implicating organisms with their sensitivity patterns thus aiding in the choice of empirical antibiotics, estimating the severity of disease, identifying the need of timely surgical interventions, and studying the ultimate outcomes.
The patients in our study were mainly in the age group of 51–70 years, with a mean age of 58.8 ± 16 years, with males outnumbering females. Two other Indian studies were having similar demographics with 51–60 years comprising 25.4% of cases and majority males in one study.[5] and the mean age of 53.6 ± 17.55 years in another.[6] The male preponderance is possibly linked to the fact that male UTI is always considered complicated, besides higher incidence of obstructive pathologies in males. Females on the other hand have higher instances of uncomplicated cystitis.[1,7]
Diabetes mellitus was the most common risk factor present in 53% of our patients. The diabetics have higher propensity of UTI as glycosuria acts as a harbinger for infection.[8] Two other Indian studies in cUTI have also implicated diabetes as the single most important risk factor, reported in 40%[5] and 47.13%[6] cases, respectively.
Urine culture yielded growth in 77% of cases, out of which Escherichia coli was the most common etiological organism, followed by Klebsiella pneumoniae. Candida growth was also seen in 4%. Negative growth in remaining could be due to antibiotic use, either over the counter or during prior admission outside before presenting to us. In some cases, it could be due to late sample collection after initiation of antibiotics, especially in self-voiding patients. Escherichia coli being the most common cause of ascending UTI in general, similar observations were reported by many other studies.[1,2,5,9,10,11]
The organisms were highly susceptible to colistin/polymyxin, imipenem, and meropenem. There was moderate sensitivity to aminoglycosides, ertapenem, and nitrofurantoin. However, increasing level of resistance was seen for piperacillin-tazobactam (63%) and cefoperazone- sulbactam (73%). Carbapenems were the preferred empirical antibiotics in our patients, and need for revision was there in only 4%. A comparable pattern has been reported by a South Indian study that found good sensitivity to carbapenems (96%), and comparable resistance to amikacin (28.0%) and nitrofurantoin (28.6%).[6] Another study reported good sensitivity to carbapenems and amikacin and high rates of resistance to broad-spectrum penicillins such as penicillins (56%–94%), cephalosporins (≥45%), aztreonam and ciprofloxacin. In this study, third-generation cephalosporins were used as first-line treatment and the need of revision was seen in 41.78% of cases.[12]
The mean duration of hospital stay in our study was being 9.1 ± 2.7 days. Many patients needed ICU care (40%), with a few of them needing invasive ventilation (10%). At 1 month of follow-up, most of the patients recovered well (94%), whereas mortality of 4% was seen. A Chinese study has reported an average hospital stay of 13 days, but the mortality rate was comparable (2.5%).[12]
This study had its own limitations. It was a single-center study and the number of patients was small. Being a referral center normally catering to relatively sicker patients, there was a tendency to use higher generation antimicrobials at the onset, rather than a gradual escalation approach.
Conclusion
Complicated UTI mostly affects males with comorbidities, diabetics being at the highest risk. Escherichia coli is the most common etiological organism and the overall antimicrobial resistance is very high. Most of the patients were treated with carbapenems show excellent recovery.
Ethical clearance
The study was approved by the ethical committee of Dayanand Medical College and Hospital, Ludhiana, and Baba Farid University of Health Sciences, Faridkot, Punjab, India, vide approval no. BFUHS/2k18p-TH/14223.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nicolle LE AMMI Canada Guidelines Committee*. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16:349–60. doi: 10.1155/2005/385768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karve S, Ryan K, Peeters P, Baelen E, Rojas-Farreras S, Potter D, et al. The impact of initial antibiotic treatment failure:Real-world insights in patients with complicated urinary tract infection. J Infect. 2018;76:121–31. doi: 10.1016/j.jinf.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Nicolle LE. A practical guide to antimicrobial management of complicated urinary tract infection. Drugs Aging. 2001;18:243–54. doi: 10.2165/00002512-200118040-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sabih A, Leslie SW. Complicated urinary tract infections. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [Last updated on 2021 Aug 12]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK436013/ [Google Scholar]
- 5.Karishetti MS, Shaik HB. Clinicomicrobial assessment of urinary tract infections in a tertiary care hospital. Indian J Health Sci Biomed Res (KLEU) 2019;12:69–74. [Google Scholar]
- 6.Eshwarappa M, Dosegowda R, Aprameya IV, Khan MW, Kumar PS, Kempegowda P. Clinico-microbiological profile of urinary tract infection in south India. Indian J Nephrol. 2011;21:30–6. doi: 10.4103/0971-4065.75226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan R, Saif Q, Fatima K, Meher R, Shahzad HF, Anwar KS. Clinical and bacteriological profile of UTI patients attending a North Indian tertiary care center. J Integr Nephrol Androl. 2015;2:29–34. [Google Scholar]
- 8.Nicolle LE. Asymptomatic bacteriuria in diabetic women. Diabetes Care. 2000;23:722–3. doi: 10.2337/diacare.23.6.722. [DOI] [PubMed] [Google Scholar]
- 9.Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotechnol. 2006;5:1562–5. [Google Scholar]
- 10.Stefaniuk E, Suchocka U, Bosacka K, Hryniewicz W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur J Clin Microbiol Infect Dis. 2016;35:1363–9. doi: 10.1007/s10096-016-2673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghadage DP, Muley VA, Sharma J, Bhore AV. Bacteriological profile and antibiogram of urinary tract infections at a tertiary care hospital. Natl J Lab Med. 2016;5:20–4. [Google Scholar]
- 12.Li X, Chen Y, Gao W, Ye H, Shen Z, Wen Z, et al. A 6-year study of complicated urinary tract infections in southern China:Prevalence, antibiotic resistance, clinical and economic outcomes. Ther Clin Risk Manag. 2017;13:1479–87. doi: 10.2147/TCRM.S143358. [DOI] [PMC free article] [PubMed] [Google Scholar]


