Abstract
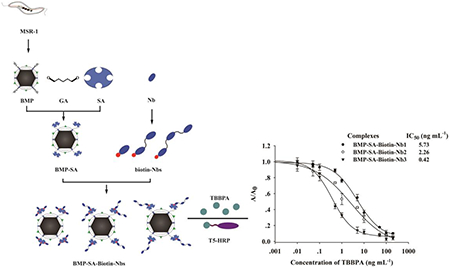
Bacterial magnetic particles (BMPs) are an attractive carrier material for immunoassays because of their nanoscale size, dispersal ability, and membrane-bound structure. Antitetrabromobisphenol-A (TBBPA) nanobodies (Nbs) in the form of monovalence (Nb1), bivalence (Nb2), and trivalence (Nb3) were biotinylated and immobilized onto streptavidin (SA)-derivatized BMPs to construct the complexes of BMP-SA-Biotin-Nb1, -Nb2, and -Nb3, respectively. An increasing order of binding capability of BMP-SA-Biotin-Nb1, -Nb2, and -Nb3 to TBBPA was observed. These complexes showed high resilience to temperature (90 °C), methanol (100%), high pH (12), and strong ionic strength (1.37 M NaCl). A BMP-SA-Biotin-Nb3-based enzyme linked immunosorbent assay (ELISA) for TBBPA dissolved in methanol was developed, showing a half-maximum inhibition concentration (IC50) of 0.42 ng mL−1. TBBPA residues in landfill leachate, sewage and sludge samples determined by this assay were in a range of <LOD–1.17 ng mL−1, <LOD–0.75 ng mL−1, and <LOD–3.65 ng g−1 (dw), respectively, correlating well with the results by liquid chromatography tandem mass spectrometry. The BMP-SA-Biotin-Nb3 was reusable at least three times without significant loss of the binding capability. The BMP-SA-Biotin-Nb3-based ELISA, with a total assay time of less than 30 min, is promising for the rapid monitoring of TBBPA in the environment.
Graphical Abstract
Tetrabromobisphenol-A (TBBPA) is a well-known brominated flame retardant (BFR). TBBPA can be released from different processes and sources into the environment. TBBPA has been found in a variety of biotic and abiotic samples.1–4 Studies have suggested that TBBPA can induce hepatotoxicity, cytotoxicity, and immunotoxicity in human beings.5 The International Agency for Research on Cancer (IARC) has recently upgraded TBBPA to group 2A on the basis of sufficient evidence of carcinogenicity in experimental animals and strong mechanistic evidence in humans.6 TBBPA is commonly detected by instrumental methods, such as liquid chromatography tandem mass spectrometry (LC–MS/MS),7 which is highly sensitive and selective but technically connected to well-trained analysts and expensive instruments. Immunoassays proved to be an efficient method for the high-throughput detection of environmental chemicals in complicated matrices. Nonetheless, the majority of antibodies are susceptible to harsh conditions such as solvents, heat, extreme pH, and strong ionic strength, which restrict the wide application of immunoassays to environmental monitoring.
Camelid variable domain of heavy chain antibodies, also known as nanobodies (Nbs), are increasingly attractive in the detection of small molecules from various matrices due to their high resilience to solvent and temperature. For example, the sensitivity of a Nb-based enzyme-linked immunosorbent assay (ELISA) for 3-phenoxybenzenemethanol could be improved in buffer with 50% of methanol (MeOH),8 and the antiaflatoxin Nb did not lose any binding ability in 80% of MeOH.9 The Nb against caffeine retained more than 90% of its binding activity after incubation at 90 °C for 20 min.10 Nbs have been viewed as potentially superior alternatives to conventional antibodies (e.g., poly- and monoclonal antibodies) in analysis, research, and therapeutic applications. In addition, Nbs have been notably regarded for their ease of production in microbial systems, their potential ability to target cryptic epitopes that are inaccessible to conventional antibodies, and the fact that they can be readily formatted into more complex molecules.11 However, multiple studies showed that the sensitivities of the Nb-based immunoassays are lower than those of conventional antibody-based assays for small molecules.12 This is because the antigen-binding site of Nbs, with just three CDRs in their variable domains, has a more limited structural diversity than that of conventional antibodies.13 Moreover, unlike conventional antibodies, Nbs do not have the ideal surface, that is, a pocket, in which a small molecule can be bound with high affinity.14,15 Nevertheless, Nbs could be genetically reformatted into multivalent reagents to enhance the binding affinity to targets. For instance, after generation of pentavalence, the affinity of a Nb to parathyroid hormone was increased from 1000- to 10000-fold higher than its monomeric counterpart.16 As compared to the parental Nb, the corresponding pentabodies against 15-acetyl-deoxynivalenol boosted 2.4-fold affinity in a fluorescence polarization assay.17
Bacterial magnetic particles (BMPs), synthesized by magnetotactic bacteria (MTB), are composed of membrane-enclosed single-domain ferrimagnetic iron oxide (magnetite, Fe3O4) or iron sulfide (greigite, Fe3S4) crystals.18 BMPs-based immunoassays have some unique merits for applications. An abundant amount of BMPs could be produced by fermentation of MTB with a high yield. Their nanoscale size (20–100 nm) endows BMPs with a high surface-to-volume ratio to display more capture antibodies. The well-dispersed BMPs tend to have fast kinetics. It has been reported that immobilization of antibodies onto BMPs usually has higher activities than those linked to chemically synthesized magnetic particles.19,20 Recently, BMPs showed a promise in immunoassays for the detection of small molecules in the environment,4,21 but the binding affinity and resilience of the constructed immunomagnetic particles to stringent conditions need to be improved.
It is known that site-directed immobilization of antibodies onto support materials can result in a considerably higher binding capacity of antibodies than that by random coupling.22 Site-directed immobilization of conventional antibodies commonly includes the formation of covalent bonds between the carbohydrate chains in their Fc region and the support material, or noncovalent binding of the immunoglobulin to immobilized antibody-binding proteins such as protein A and protein G.23 Another efficient site-directed immobilization can be achieved by using an antibody first biotinylated at its C-terminus and then noncovalently bound to a streptavidin (SA)-derivatized support material.24 As recombinant proteins, Nbs can be easily biotinylated via an AviTag/BirA technology, in which a Nb is modified with the AviTag peptide (GLNDIFEAQKIEWHE), and a biotin residue is enzymatically incorporated at the C-terminus using the E. coli biotin protein ligase BirA.25 Site-directed immobilization of the genetically engineered antimethotrexate Nb via AviTag/BirA technology significantly increased the binding capacity of immunoaffinity columns.26
Currently, there remains a lack of highly stable and effective methods to monitor TBBPA contamination in different environmental matrices. The BMP-based immunoassays might provide an alternative for the high throughput detection of TBBPA in the environment. Herein, we constructed novel immunomagnetic particles with high stability in stringent conditions by immobilizing simultaneously multimerized and biotinylated anti-TBBPA Nbs onto SA-derivatized BMPs in a site-directed pattern. The affinity to TBBPA and resilience to harsh conditions were investigated. The immunomagnetic particles were applied to the rapid and sensitive detection of TBBPA in environmental samples including landfill leachate, sewage, and sludge.
Material and methods
Chemicals and Reagents
The TBBPA standard was purchased from TCI Co., Ltd. (Tokyo, Japan). All other chemicals were from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China) unless otherwise specified. Ring-13C12 labeled TBBPA (99% purity) was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). Isopropyl-β-D-thiogalactopyranoside (IPTG), 3,3’,5,5’-tetramethylbenzidine (TMB), D-biotin, and imidazole were purchased from Sigma−Aldrich Chemical Co. (St. Louis, MO). All restriction enzymes and T4 DNA ligase were purchased from New England Biolabs, Inc. (Ipswich, MA). HisPur Ni-NTA resin, Halt protease inhibitor cocktail, and Nunc MaxiSorp flat-bottom 96-well plates were purchased from Corning (Rockford, IL). SA was purchased from Pangu Gene Co., Ltd. (Nanjing, China). The mouse anti-SA monoclonal antibody and goat antimouse IgG labeled with horseradish peroxidase (HRP) were purchased from Abcam (Cambridge, MA). The alpaca-derived Nb1 (T3–15) with high selectivity to TBBPA and the tracer T5-HRP were available from our previous studies.4,27
Preparation of Biotinylated Nbs with Various Valences
The gene of Nb1 in the pComb3X was amplified by polymerase chain reaction (PCR). Overlap PCR was performed to assemble the bi- and trivalence Nbs (Nb2 and Nb3) in tandem with linker (G4S)3 by using primers including SacI and HindIII restriction enzyme sites (see Table S1). The gene fragments of Nb1, Nb2, and Nb3 were cloned into pAC6 and the recombinant plasmids (pAC6-Nb1, -Nb2, and -Nb3) were transferred into E. coli CVB101. A 1 mL aliquot of overnight culture was pipetted into 100 mL of Luria−Bertani liquid medium supplemented with ampicillin (100 μg mL−1) and incubated in a shaker at 37 °C until the OD600 reached 0.8. The culture was then induced with 0.5 mM IPTG in the presence of 50 μM D-biotin for 5 h. The cells were harvested by centrifugation (8000g, 15 min) and lysed by sonication (10 s each, 10 cycles with 5 s interval) in an ice water bath. After another centrifugation (8000g, 10 min), the supernatant of the lysate was collected, and the fusion proteins biotin-Nb1, -Nb2, and -Nb3 were purified with Ni-NTA column (Invitrogen, U.S.). The purity and size of biotin-Nbs were analyzed on 12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE).
Preparation of SA-Derivatized BMPs
The Magnetospirillum gryphiswaldense strain (MSR-1, DSM 6361) was fermented for the production of BMPs.4,28 The membrane proteins of BMPs were conjugated to SA by glutaraldehyde with the method described previously.29 Briefly, 10 mg of fresh BMPs was suspended in 1 mL of 0.01 M phosphate buffered saline (PBS, 7.4) and mixed with 1 mL of SA solution (4 mg mL−1). The mixture was dispersed under ultrasonic bath for 5 min, followed by the addition of 0.2 mL of glutaraldehyde (25%). After incubation at room temperature for 2 h, SA-derivatized BMPs were collected by magnetic absorption and washed with PBS five times. The conjugate of BMP-SA was identified by a Western blotting analysis. The amount of SA conjugated to BMPs was evaluated by a BCA protein assay kit (Pierce, Rockford, IL). After being blocked with 1% skimmed milk, BMP-SA complexes were stored at 4 °C until use.
Site-Directed Immobilization of Nbs onto BMPs
A 10 mg aliquot of SA-derivatized BMPs (in 1 mL of PBS) was mixed with biotin-Nb1 (3 mg), -Nb2 (2 mg), and -Nb3 (2 mg) separately, and the mixtures were sonicated at room temperature for 30 min. The constructed BMP-SA-Biotin-Nbs were collected and washed with PBS three times to remove biotin-Nbs adsorbed nonspecifically. The amount of biotin-Nbs immobilized onto the BMP-SA was also evaluated by a BCA protein assay kit. The purity and size of BMPs, BMP-SA, and BMP-SA-Biotin-Nbs were evaluated by transmission electron microscopy (TEM) (JEM-1400, Japan; CCD: Gatan 832, 4k×3.7k, U.S.). Their hydrated radii, zeta potentials, and polydispersity were analyzed by a zeta potential analyzer (Brookhaven Instruments Corp., Long Island, NY). For a comparative experiment, biotin-Nbs were also immobilized onto commercial SA-derivatized magnetic nanoparticles (MNP-SA) with a size of approximately 2.8 μm in diameter (Dynabeads M-280 Streptavidin, Invitrogen).
Resilience of BMP-SA-Biotin-Nbs to Stringent Conditions
For the thermal stability study, BMP-SA-Biotin-Nbs were incubated at 90 °C for variable times (5–60 min), followed by cooling to room temperature. Their binding capability to T5-HRP was determined by ELISA, and the signal was compared to that of untreated BMP-SA-Biotin-Nbs. Additionally, BMP-SA-Biotin-Nbs were stored in other stringent conditions including high pH (12), MeOH (100%), and high ionic strength (1.37 M NaCl) for different times. After the samples was washed with PBS, the binding capability of recovered BMP-SA-Biotin-Nbs was also evaluated by ELISA.
BMP-SA-Biotin-Nbs-Based ELISA for TBBPA
The performance of BMP-SA-Biotin-Nbs-based ELISAs for TBBPA was available in our previous study with a slight modification.4 Briefly, a 96-well microtiter plate was blocked with 1% gelatin (300 μL per well) in carbonate−bicarbonate buffer (pH 9.6) at 4°C overnight and washed with PBST (PBS containing 0.05% Tween-20). The BMP-SA-Biotin-Nbs were blocked with 1% gelatin overnight, and the particle suspension was added to the blocked plate above (100 μL per well). This plate was fastened to a 96-well magnetic frame and washed with PBST three times. A 50-μL aliquot of TBBPA in MeOH was added to the well holding BMP-SA-Biotin-Nbs and incubated on an oscillator (150 rpm min−1) for 10 min, followed by the addition of 50 μL of T5-HRP. After incubation for 5 min, the particles in the plate were washed three times, and 100 μL of TMB solution (400 μL of 0.6% TMB and 100 μL of 1% H2O2 dilution in 25 mL of citrate buffer, pH 5.5) was added into the plate. The reaction was stopped approximately 8 min later by the addition of 50 μL of 2 M H2SO4. The absorbance was read at 450 nm on a microtiter plate reader (ELx800, BioTek, USA). The inhibition concentration of half-maximum signal (IC50), linear range (IC20–IC80), and limit of detection (LOD, IC10) were obtained from a four-parameter logistic equation generated by SigmaPlot 10.
The effects of pH (5–12), MeOH (0–100%, v/v), and NaCl (0–2.188 M) on the BMP-SA-Biotin-Nb3-based ELISA for TBBPA (IC50 and A0, signal in the absence of TBBPA) were studied at ambient temperature. Except for the single variable, the rest of the assay conditions were performed in PBS (pH 7.4, NaCl 0.137 M) containing 10% MeOH.
Analysis of TBBPA in Environmental Samples
The BMP-SA-Biotin-Nb3-based ELISA was employed to detect TBBPA in landfill leachate samples and sewage and sludge samples collected from sewage treatment plants in the Beijing area. For recovery studies, samples free of TBBPA identified by LC–MS/MS were fortified with TBBPA at the concentrations of 0.1, 0.5, and 2 ng mL−1 in both leachate and sewage samples and 0.5, 2 and 5 ng g−1 dry weight (dw) in sludge. Leachate and sewage samples (each 5 mL) were extracted with 5 mL of ethyl acetate (2% formic acid) in an ultrasonic water bath at room temperature for 20 min (×2). The solvent layer was collected and evaporated to dryness under a gentle stream of nitrogen. The residue was reconstituted with at least 4 mL of MeOH. A 2.0-g aliquot of dry sludge was weighed in a 20 mL glass tube and ultrasonically extracted with 5 mL of ethyl acetate at room temperature for 20 min (×2). The slurry was centrifuged at 8000g for 10 min, and the supernatant was treated according to the method used for leachate and sewage samples. The extracts with or without dilution were subjected to ELISA. TBBPA levels in the samples were also detected by a LC−MS/MS method described by Yang et al.30
Regeneration of the BMP-SA-Biotin-Nb3
Acidic medium was used to dissociate the binding of Nb3 with TBBPA/T5-HRP. Following an ELISA, the complexes of BMP-SA-Biotin-Nb3-TBBPA/T5-HRP were washed with PBS under magnetic absorption, and 100 μL of H2SO4 solution (pH 1–4) was added into the wells. The mixture was incubated by shaking at 37 °C for variable times (1–10 min). After washing with PBS (pH 7.4) five times, the regenerated BMP-SA-Biotin-Nb3 were employed in an ELISA for TBBPA. Afterward, the complexes of BMP-SA-Biotin-Nb3-TBBPA/T5-HRP were shifted to the next round of regeneration and ELISA performance. These processes were repeated 10 times, and the IC50 and A0 of ELISAs for TBBPA using regenerated BMP-SA-Biotin-Nb3 were compared to those using fresh BMP-SA-Biotin-Nb3.
Results and discussion
Multimerization and Biotinylation of Anti-TBBPA Nbs
Nbs are amenable to genetic manipulations such as multimerization and biotinylation.31 Multimerization of Nbs is possible to increase their binding affinity to targets, and biotinylation will facilitate the site-directed conjugation of Nbs to SA-derivatized support materials, thereby increasing their binding capacity. In an attempt to produce robust immunomagnetic particles with high binding affinity to TBBPA, both Nbs and BMPs were modified; that is, two or three Nbs specific for TBBPA were genetically linked in tandem, and the C-terminus of the Nb chain was biotinylated; meanwhile, BMPs were chemically coupled with SA. A series of primers were designed to amplify the monomeric Nb (Nb1) gene (441 bp) and assemble bivalent Nb2 (840 bp) and trivalent Nb3 (1251 bp) fragments (Figure 1a). The pAC6 vector containing Nb genes (Figure 1b) was transferred into E. coli CVB101, which contained an engineered pACYC184 plasmid with an IPTG inducible BirA gene to overexpress the biotin ligase. The fusion proteins biotin-Nb1, -Nb2, and -Nb3 were purified and identified by SDS-PAGE analysis, showing the expected molecular weight (MW) of ~18, ~32, and ~47 kDa, respectively (Figure 1c). It was deduced that the multimerization of Nbs restricted the expression of proteins because the yields of biotin-Nb1, -Nb2, and -Nb3 were 18.3, 8.3, and 3.5 mg L–1, respectively, in a decreasing order.
Figure 1.
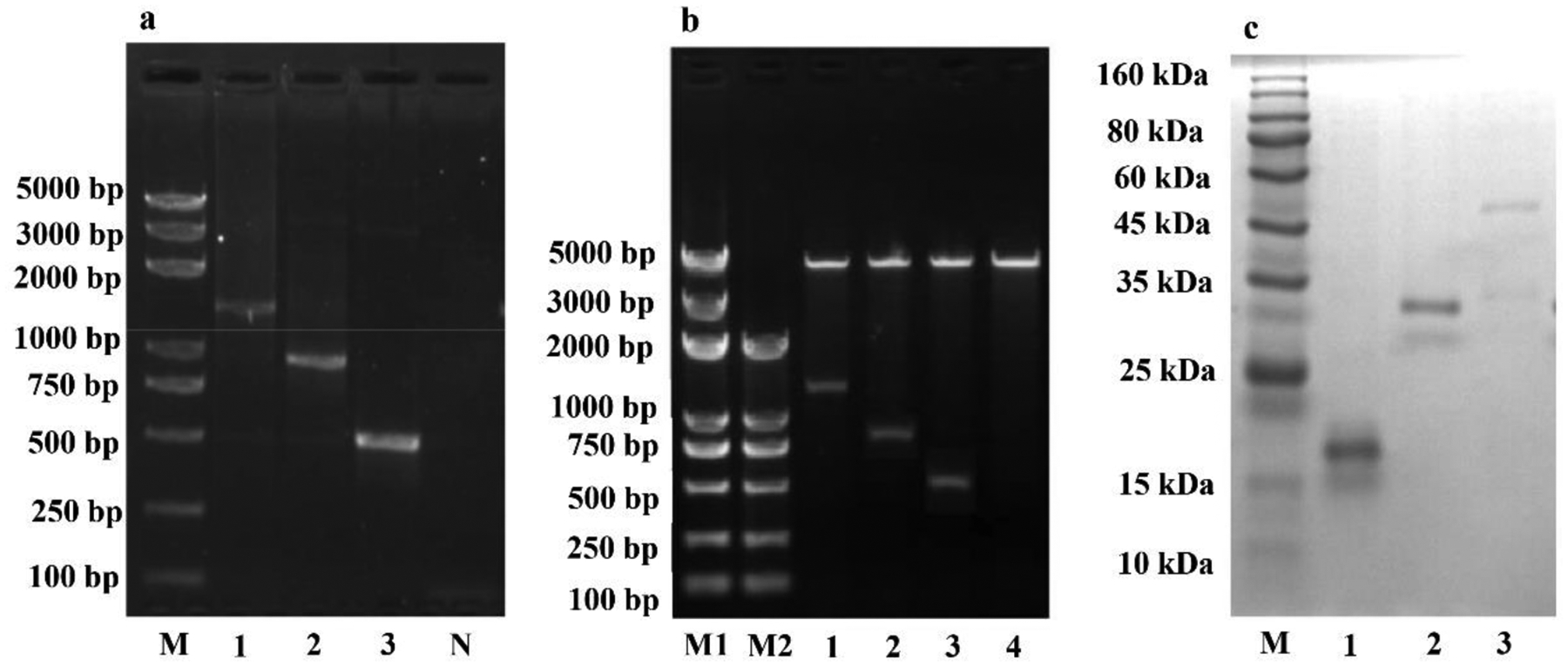
Preparation of biotinylated anti-TBBPA Nbs with variable valence. (a) Agarose gel of Nb gene fragments by PCR. Lane M, DNA marker; Lane 1, Nb3; Lane 2, Nb2; Lane 3, Nb1; Lane N, negative control. (b) Agarose gel of pAC6-Nbs plasmid digested by restriction enzyme Sac I and Hind III; Lane M1 and M2, DNA marker; Lane 1, pAC6-Nb3; Lane 2, pAC6-Nb2; Lane 3, pAC6-Nb1; Lane 4, pAC6. (c) SDS-PAGE analysis of purified Nbs; Lane 1, biotin-Nb1; Lane 2, biotin-Nb2; Lane 3, biotin-Nb3.
Construction of BMP-SA-Biotin-Nbs
Fermentation of MSR-1 for 48 h gave an excellent yield of BMPs (Fe3O4) approximately 400 mg L–1 by dry weight. An abundance of amino groups in the external membrane of BMPs makes it possible to couple bioactive substances in the presence of cross-linking reagents, such as glutaraldehyde,28 bis(sulfosuccinimidyl)suberate29 and succinimidyl-3-(2-pyridyldithiol)propionate.4 In the present study, glutaraldehyde was used to couple SA (66 kDa, homotetramers) to membrane proteins of BMPs, and the maximum amount of SA bound with 1 mg BMPs was about 287 μg under an optimized condition. The complex of BMP-SA was confirmed by Western blotting, as an obvious reaction occurred at MW ≈16 kDa for monomeric SA (see Figure S1).
The maximum amount of biotin-Nb1, -Nb2, and -Nb3 immobilized onto 1 mg of BMP-SA was 160, 92, and 80 μg, respectively (see Figure S2). The least amount of Nb3 on BMPs might be attributed to the steric hindrance of Nb3 that has a larger size than Nb1 and Nb2. The values of hydrated radii and zeta potential of BMP-SA-Biotin-Nbs were higher than those of BMPs, while the polydispersity values were the reverse (see Table S2). The possible reason is that glutaraldehyde may lead to cross-linking among BMPs. The TEM image of BMPs, BMP-SA, and BMP-SA-Biotin-Nbs revealed that these particles have a narrow size distribution of 20–60 nm in diameter (see Figures S3 and S4).
The binding affinity of the resulting BMP-SA-Biotin-Nbs to TBBPA was evaluated by a competitive ELISA for TBBPA in PBS (pH 7.4, 10% MeOH). The BMP-SA-Biotin-Nb1, -Nb2, and -Nb3-based ELISA have an IC50 value of 1.85, 0.96, and 0.66 ng mL–1, respectively (see Figure S5a). The affinity constants (Kaff) of BMP-SA-Biotin-Nb1, -Nb2 and -Nb3 with TBBPA, estimated by using the law of mass action and considering the antibody-antigen binding pattern at OD 100% and OD 50% points,32 were 1.75 × 109, 5.88 × 109, and 2.0 × 1010 moL–1, respectively. The results indicated that the binding affinity of Nbs to TBBPA was increased with the multimerization (Nb3 > Nb2 > Nb1), which is in agreement with previous studies.33,34
As compared to BMP-SA, commercial MNP-SA was able to bind a smaller amount of biotin-Nbs, because the maximum amount of biotin-Nb1, -Nb2, and -Nb3 immobilized onto 1 mg of MNP-SA was about 96, 44, and 37 μg, respectively. One possible explanation was that the nanoscale of BMP-SA with a high surface-to-volume ratio could provide more binding sites than MNP-SA with a size of 2.8 μm in diameter. In consequence, MNP-SA-Biotin-Nb1, -Nb2, and -Nb3-based ELISAs for TBBPA, with an IC50 of 10, 7 and 4.3 ng mL–1, respectively (see Figure S5b), showed much lower sensitivities than did BMP-SA-Biotin-Nbs-based ELISAs.
Stability of BMP-SA-Biotin-Nbs in Stringent Conditions
Nb1 has shown high thermal stability in our previous study,27 whereas BMP-SA-Biotin-Nbs exhibited variable thermal stability (Figure 2a). After being heated at 90 °C for 10 min, the binding capability of BMP-SA-Biotin-Nb1, -Nb2, and -Nb3 to T5-HRP remained approximately 34%, 54%, and 76% of the unheated control (Figure 2a), indicating that the thermal stability increased with the multimerization of Nbs. No remarkable change was observed among the binding activities of BMP-SA-Biotin-Nbs that were kept in a harsh solution with pH 12 or 1.37 M NaCl for 60 d (Figure 2 b and c). In 100% MeOH, the binding capability of BMP-SA-Biotin-Nb1, -Nb2, and -Nb3 was steady for approximately 1, 2, and 5 d, respectively (Figure 2d). The multimerization of Nb appeared to enhance its resilience to temperature and MeOH with the following order of Nb3 > Nb2 > Nb1, similar to its binding affinity aforementioned. The BMP-SA-Biotin-Nb3, having the highest binding affinity and stability in harsh conditions among the immunecomplexes, was selected for the development of an immunomagnetic particle-based assay for the detection of TBBPA in the environment.
Figure 2.
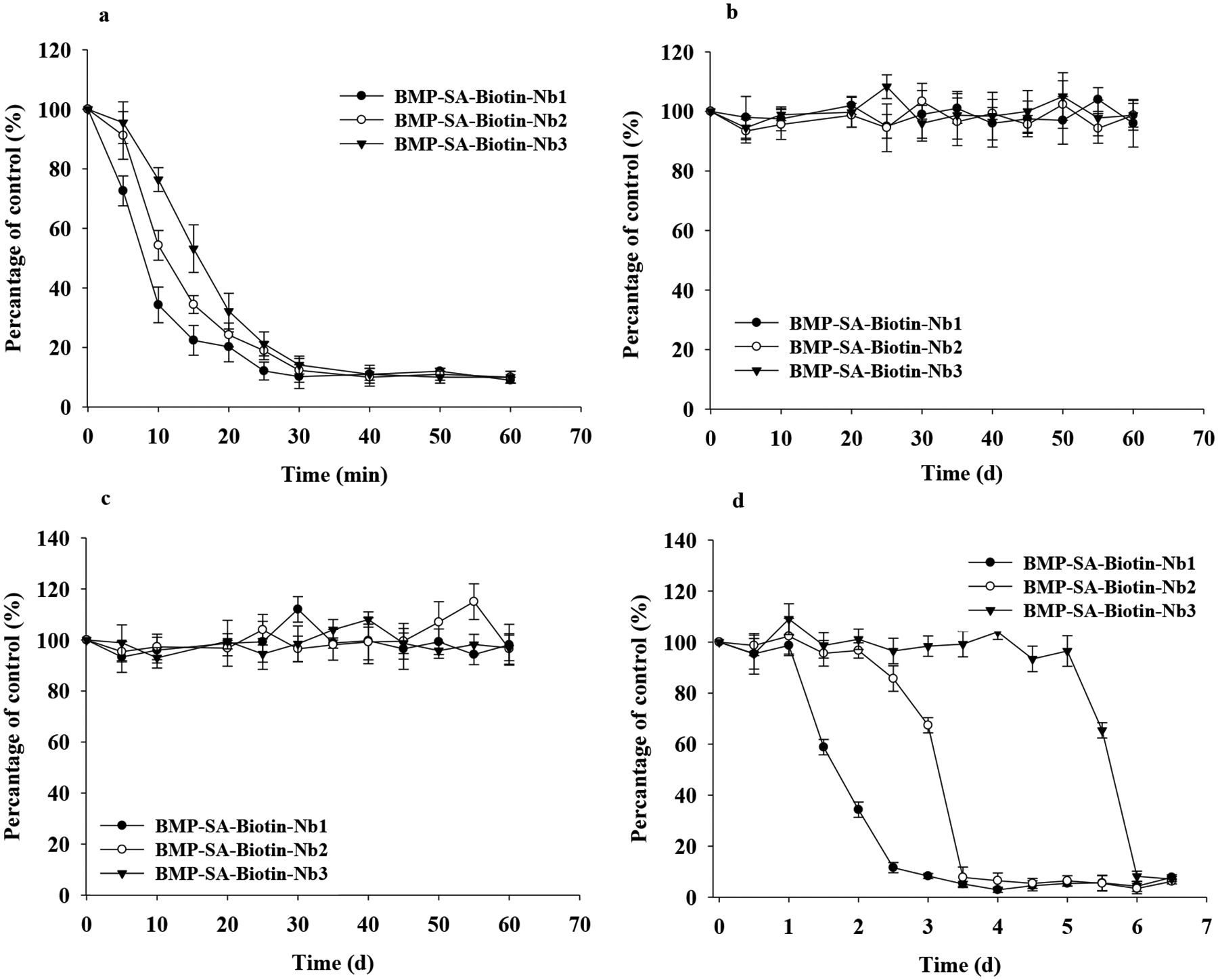
The binding activities of BMP-SA-Biotin-Nb3 to T5-HRP after the treatment in stringent conditions: (a) heat at 90 °C; (b) pH 12; (c) 1.37 M NaCl; and (d) 100% MeOH. The data shown are the average of triplicate.
Optimization of BMP-SA-Biotin-Nb3-Based ELISA for TBBPA
Determined by a checkerboard method, 20 μg of BMP-SA-Biotin-Nb3 and 1.5 ng of T5-HRP per well were employed in the assay for TBBPA. Although the BMP-SA-Biotin-Nb3 turned out to be highly stable in extreme pH, strong ionic strength, and 100% MeOH, these factors may affect the BMP-SA-Biotin-Nb3-based ELISA for TBBPA. As shown in Figure 3 a, the sensitivity was improved with pH value rising from 5 to 12, and the highest sensitivity was observed at pH 12 (IC50 = 0.62 ng mL–1 and A 0 = 1.05), suggesting that the basic solution facilitated the interaction of immunomagnetic particles and TBBPA rather than the acidic one. It was noteworthy that the BMP-based ELISA showed good sensitivity even in high levels of MeOH (60–100%) and the excellent sensitivity to TBBPA was obtained in 100% MeOH (IC50 = 0.44 ng mL–1 and A 0 = 1.07) (Figure 3 b). This assay is adaptable to a wide range of ionic strength (0–2.18 M NaCl) and the optimal assay was observed in PBS with 0.137 moL L–1 NaCl (IC50 = 0.71 ng mL–1 and A 0 = 0.95) (Figure 3 c).
Figure 3.
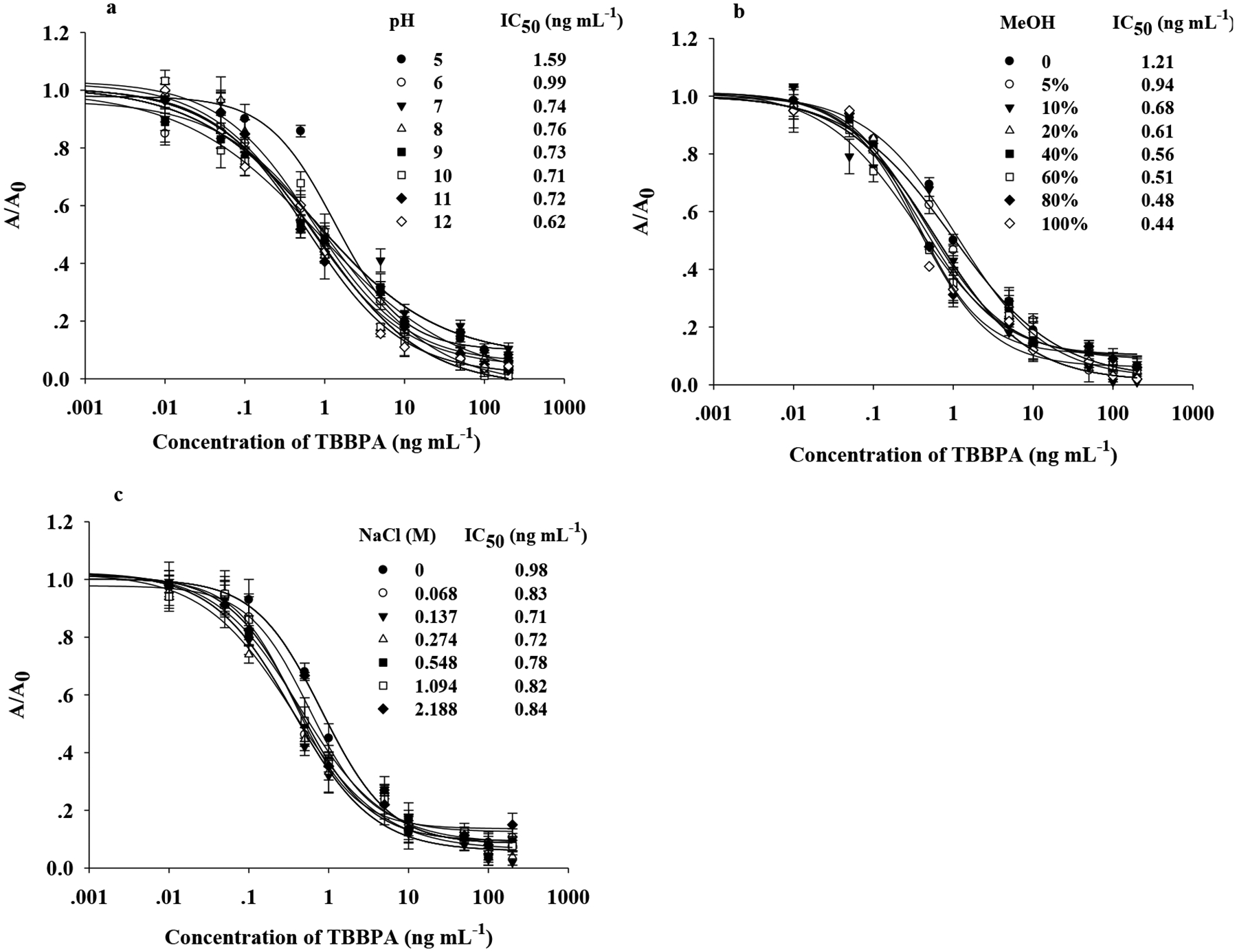
Effects of pH (a), MeOH (b), and NaCl (c) on the performance of the BMP-SA-Biotin-Nb3-based ELISA for TBBPA at ambient temperature. Except for the single variable, ELISA was carried out in PBS (pH 7.4; NaCl 0.137 M) containing 10% MeOH. The data shown are the average of triplicate.
In brief, even though the BMP-SA-Biotin-Nb3-based assay showed rational dose–response curves in different harsh conditions, the best sensitivity came from the assay performed in 100% MeOH. Because TBBPA is a lipophilic compound and methanol is commonly used in sample pretreatment, this solvent was selected to develop an immunomagnetic particle-based assay for TBBPA. The high resilience of BMP-SA-Biotin-Nb3 to temperature could extend its application, storage, and shipment. However, the BMP-SA-Biotin-Nb3-based ELISA was carried out at room temperature that proved to be optimal for the interaction of Nb and TBBPA (data not shown). A typical calibration curve of BMP-SA-Biotin-Nb3-based ELISA for TBBPA was generated in 100% MeOH (Figure 4). This assay had a linear range of 0.11–2.1 ng mL–1 (IC20–IC80), an IC50 value of 0.42 ng mL–1, and a LOD of 0.03 ng mL–1, which are comparable to those of an Nb1-based ELISA performed in 10% MeOH.27 The BMP-SA-Biotin-Nb1/2-based ELISA was also suitable for performance in 100% MeOH but showed less sensitivity than the BMP-SA-Biotin-Nb3-based assay (Figure 4).
Figure 4.
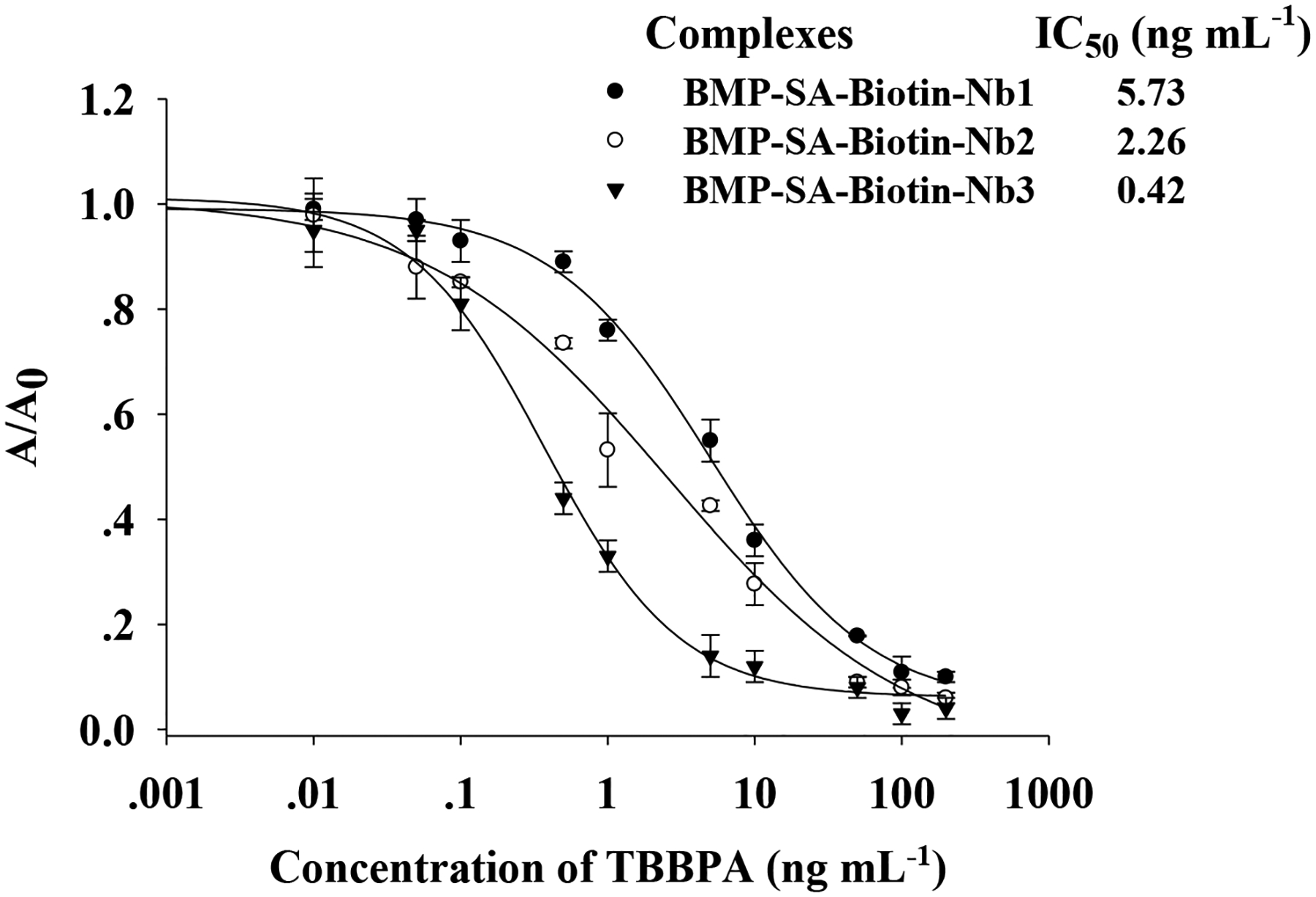
Calibration curves of BMP-SA-Biotin-Nb-based ELISAs for TBBPA in 100% MeOH. Each value is the average of three replicates and the standard deviations.
Sample Analysis
Even though the BMP-SA-Biotin-Nb3-based assay is highly specific for TBBPA (data not shown), the effects of complicated matrices on this assay are inevitable. Generally, matrix effects can be circumvented by simple dilution with assay buffer, but the assay limit of detection would be sacrificed. In the present study, matrix effects of environmental samples on the BMP-SA-Biotin-Nb3-based ELISA were evaluated by comparing the dose–response curve generated in the blank extract, which was constituted by dissolving residues with different volumes of MeOH, with that generated in pure MeOH. The matrix interference from swage, landfill leachate, and sludge was minimized by dissolving their residues with at least 4, 5, and 6 mL of MeOH, respectively (see Figure S6).
By the BMP-SA-Biotin-Nb3-based ELISA, the recoveries of TBBPA from spiked landfill leachate, sewage, and sludge samples were in a range of 72.3–85.4%, 91.3–95.4% and 96.4–127%, respectively (Table 1). This assay was also applied to detect TBBPA residues in real samples collected from Beijing area, having TBBPA levels in a range of <LOD–1.17 ng mL–1, <LOD–0.75 ng mL–1 and <LOD–3.65 ng g–1 (dw) from landfill leachate, sewage, and sludge, respectively (see Table S3). The average level of TBBPA in sludge was higher than that in sewage and landfill leachate. The results of ELISA were compared to those of an instrumental method LC–MS/MS and both methods correlated well with each other (see Figure S7). Therefore, the resulting immunomagnetic particle-based assay was demonstrated to be an efficient method to detect TBBPA in landfill leachate, sewage, and sludge.
Table 1.
Determination of TBBPA spiked in landfill leachate, sewage and sludge by the BMP-SA-Biotin-Nb3-based ELISA and LC-MS/MS.
| Samples | Spiked level (ng mL−1 or ng g−1 (dw)) | Average recovery (%) ± CV (n= 3) | |
|---|---|---|---|
| ELISA | LC-MS/MS | ||
| Landfill leachate | 0 | <LODa | <LODb |
| 0.1 | 72.3 ± 5.4 | 86.7 ± 4.6 | |
| 0.5 | 80.6 ± 6.2 | 92.4 ± 3.9 | |
| 2 | 85.4 ± 7.1 | 93.3 ± 6.8 | |
| Sewage | 0 | <LODa | <LODb |
| 0.1 | 91.3 ±7.3 | 87.4 ± 6.2 | |
| 0.5 | 95.4 ± 5.2 | 95.6 ± 2.4 | |
| 2 | 91.3 ± 3.1 | 98.8 ± 4.4 | |
| Sludge | 0 | <LODa | <LODb |
| 0.5 | 127 ± 13.7 | 88.7 ± 7.4 | |
| 2 | 125 ± 11.9 | 92.5 ± 6.6 | |
| 5 | 96.4 ± 4.3 | 93.3 ± 4.1 | |
The LOD of the BMP-SA-Biotin-Nb3-based ELISA for TBBPA in leachate and sewage was 0.03 and 0.024 ng mL−1, respectively, and in sludge was 0.075 ng g−1 (dw).
The LOD of LC-MS/MS for TBBPA in leachate and sewage was 0.04 and 0.05 ng mL−1, respectively, and in sludge was 0.06 ng g−1 (dw).
TBBPA has the largest market among all of the BFRs in China.35 Trace concentrations have been detected in a wide variety of environmental samples.36 The residues of TBBPA found in this study indicated that this compound has been transferred from different processes and sources to the environment. The average levels of TBBPA in landfill leachate, sewage, and sludge from the Beijing area are comparable to those found in other places worldwide.5
Up to now, multiple techniques with LC–MS/MS and immunoassays in majority have been developed to detect TBBPA in various complicated matrices (see Table S4). Immunoassays are well-known to be rapid, cost-effective, and in situ applicable, as compared to instrumental methods. The BMP-SA-Biotin-Nb3-based ELISA for TBBPA showed some advantages over previous microtiter plate-based immunoassays using either conventional antibodies or Nbs.27,37 For instance, the immunomagnetic particle-based assay has higher sensitivity to TBBPA and resilience to MeOH, pH, and ionic strength than does a polyclonal antibody-based ELISA.37 By substituting the polyclonal antibody with a Nb specific for TBBPA, the assay sensitivity and resilience to solvent were improved, but the optimal assay was conducted in requirement with 10% MeOH, which might restrict the application of this method to some complicated matrices.27 Because of the multimerization of Nbs, the BMP-SA-Biotin-Nb3-based assay exhibited the highest resilience to harsh conditions of pH, solvent, and ionic strength among all of the developed immunoassays for TBBPA. This study demonstrated the utility of 100% MeOH in the detection of TBBPA in environmental samples. Probably MeOH could accelerate the separation of TBBPA from complicated matrices and thus facilitate the detection of trace TBBPA after a preconcentration step (usually dissolved or eluted using MeOH). Furthermore, the site-directed immobilization of Nb3 and the high surface-to-volume ratio of immunomagnetic particles allowed a relatively high probability of their interaction with TBBPA, and consequently increased the assay efficiency in comparison with conventional ELISAs. Except for the application in the environmental monitoring, the BMP-SA-Biotin-Nb3 could potentially be used as a cleaning tool for the purification of TBBPA-contaminated water.
Reusability of BMP-SA-Biotin-Nb3
Noncovalent interaction between antibody and antigen could be dissociated rapidly under harsh conditions (e.g., high concentration of solvents and extreme pH) and restored by neutralization. Variable acidic solutions were evaluated to dissociate TBBPA from Nbs, given that the BMP-SA-Biotin-Nb3 exhibited high stability in both MeOH (100%) and basic solution (pH 12). The dissociation of BMP-SA-Biotin-Nb3 with TBBPA/T5-HRP could be achieved by incubating the complexes of BMP-SA-Biotin-Nb3-TBBPA/T5-HRP in the acidic solution (pH 3) for 1 min. After neutralization, the BMP-SA-Biotin-Nb3 was employed for the detection of TBBPA and no statistic differences were observed between the performance of ELISA (A0 and IC50) using the fresh immunomagnetic particles and that using the first through third regenerated ones (see Table S5), indicating the robust BMP-SA-Biotin-Nb3 could be reused at least three times.
In this study, the site-directed immobilization of simultaneously multimerized and biotinylated anti-TBBPA Nbs onto SA-derivatized BMPs obviously increased the binding capability of BMP-SA-Biotin-Nbs to TBBPA and their resilience to temperature and MeOH, exhibiting the following order of BMP-SA-Biotin-Nb3 > -Nb2 > -Nb1. BMP-SA-Biotin-Nbs showed higher binding activity to TBBPA than did a commercial MNP-SA-based counterpart. The BMP-SA-Biotin-Nb3-based ELISA was applied to the rapid detection of TBBPA in landfill leachate, sewage, and sludge samples, in which TBBPA residue was detectable, and the result was confirmed by LC–MS/MS, indicating the ubiquitous distribution of this compound. The BMP-SA-Biotin-Nb3 appeared to be a creative and attractive material suitable for the environmental monitoring of TBBPA, especially in the case of a large screening campaign. High quality of BMPs could be readily produced in MTB with an excellent yield (~400 mg L–1). The usage of 100% MeOH in the BMP-based assay improved the sensitivity and reduced the analysis time (<30 min). Finally, the easy availability and regeneration of BMP-SA-Biotin-Nb3 reduced the cost of analysis.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Project of the National Natural Science Foundation of China (21577170 and 31570037), the Key Project of Inter-Governmental International Scientific and Technological Innovation Cooperation (2016YFE0108900), the National Key Research and Development Program of China (2016YFD0800606), and the National Institute of Environmental Health Sciences Superfund Research Program, P42ES04699.
Footnotes
The Supporting Information is available free of charge at ttps://pubs.acs.org/doi/10.1021/acs.analchem.9b04177.
REFENCES
- (1).BSEF. TBBPA factsheet: http://www.bsef.com/our-substances/tbbpa/about-tbbpa. 2012.
- (2).Harrad S; Abdallah MAE; Rose NL; Turner SD; Davidson TA Environ Sci Technol. 2009, 43, 9077–9083. [DOI] [PubMed] [Google Scholar]
- (3).Zhang Z; Zhu N; Huang M; Liang Y; Zeng K; Wu X; Wu X; Liu Z; Ma Q; Qu G; Shi J Environ Pollut. 2017, 229, 431–438 [DOI] [PubMed] [Google Scholar]
- (4).He J; Tian J; Xu J; Wang K; Li J; Gee SJ; Hammock BD; Li QX; Xu T Anal Bioanal Chem. 2018, 410, 6633–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Abdallah MAE Environ Int. 2016, 94, 235–250. [DOI] [PubMed] [Google Scholar]
- (6).Grosse Y; Loomis D; Guyton KZ; El Ghissassi F; Bouvard V; Benbrahim-Tallaa L; Mattock H; Straif K Lancet Oncol. 2016, 17, 419–420. [DOI] [PubMed] [Google Scholar]
- (7).Morris S; Allchin CR; Zegers BN; Haftka JJH; Boon JP; Belpaire C; Leonards PEG; Van Leeuwen SPJ; De Boer J Environ. Sci. Technol 2004, 38, 5497–5504. [DOI] [PubMed] [Google Scholar]
- (8).Kim HJ; McCoy MR; Majkova Z; Dechant JE; Gee SJ; Tabares-da Rosa S; González-Sapienza GG; Hammock BD Anal Chem. 2012, 84, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).He T; Wang Y; Li P; Zhang Q; Lei J; Zhang Z; Ding X; Zhou H; Zhang W Anal Chem. 2014, 86, 8873–8880. [DOI] [PubMed] [Google Scholar]
- (10).Ladenson RC; Crimmins DL; Landt Y; Ladenson JH Anal Chem. 2006, 78, 4501–4508. [DOI] [PubMed] [Google Scholar]
- (11).Steeland S; Vandenbroucke RE; Libert C Drug Discov Today. 2016, 21, 1076–113. [DOI] [PubMed] [Google Scholar]
- (12).He T; Zhu J; Nie Y; Hu R; Wang T; Li P; Zhang Q; Yang Y Toxins (Basel). 2018, 10, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Muyldermans S Annu Rev Biochem. 2013, 82, 775–797. [DOI] [PubMed] [Google Scholar]
- (14).De Simone EA; Saccodossi N; Ferrari A; Leoni J Vet Immunol Immunopathol. 2008, 126, 64–73. [DOI] [PubMed] [Google Scholar]
- (15).Gonzalez-Sapienza G; Rossotti MA; Tabares-da Rosa S Front Immunol. 2017, 8, 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Zhang JB; Li QG; Nguyen TD; Tremblay TL; Stone E; To R; Kelly J; Roger MacKenzie C J Mol Biol. 2004, 341, 161–169. [DOI] [PubMed] [Google Scholar]
- (17).Doyle PJ; Arbabi-Ghahroudi M; Gaudette N; Furzer G; Savard ME; Gleddie S; McLean MD; Mackenzie CR; Hall JC Mol Immunol. 2008, 45, 3703–3713. [DOI] [PubMed] [Google Scholar]
- (18).Schüler D Int Microbiol. 2002, 5, 209–214. [DOI] [PubMed] [Google Scholar]
- (19).Nakamura N; Hashimoto K; Matsunaga T Anal Chem. 1991, 63, 268–272. [DOI] [PubMed] [Google Scholar]
- (20).Tanaka T; Matsunaga T Anal Chem. 2000, 72, 3518–3522. [DOI] [PubMed] [Google Scholar]
- (21).Xu J; Liu L; He J; Ma S; Li S; Wang Z; Xu T; Jiang W; Wen Y; Li Y; Tian J; Li F J Nanobiotechnology. 2019, 17, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Franco EJ; Hofstetter H; Hofstetter O J Sep Sci. 2006, 29, 1458–1469. [DOI] [PubMed] [Google Scholar]
- (23).Makaraviciute A; Ramanaviciene A Biosens Bioelectron. 2013, 50, 460–471. [DOI] [PubMed] [Google Scholar]
- (24).Cho IH; Paek EH; Lee H; Kang JY; Kim TS; Paek SH Anal Biochem. 2007, 365, 14–23. [DOI] [PubMed] [Google Scholar]
- (25).Chapman-Smith A; Cronan JE Jr. J Nutr. 1999, 129, 477S–484S. [DOI] [PubMed] [Google Scholar]
- (26).Davenport KR; Smith CA; Hofstetter H; Horn JR; Hofstetter O J Chromatogr B Analyt Technol Biomed Life Sci. 2016, 1021, 114–121. [DOI] [PubMed] [Google Scholar]
- (27).Wang J; Bever CR; Majkova Z; Dechant JE; Yang J; Gee SJ; Xu T; Hammock BD Anal Chem. 2014, 86, 8296–8302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Li A; Zhang H; Zhang X; Wang Q; Tian JS; Li Y; Li J J Sep Sci. 2010, 33, 3437–3443. [DOI] [PubMed] [Google Scholar]
- (29).Sun JB; Duan JH; Dai SL; Ren J; Guo L; Jiang W; Li Y Biotechnol Bioeng. 2008, 101, 1313–1320. [DOI] [PubMed] [Google Scholar]
- (30).Yang Y; Lu L; Zhang J; Yang Y; Wu Y; Shao B; J Chromatogr A. 2014, 1328, 26–34. [DOI] [PubMed] [Google Scholar]
- (31).Bever CS; Dong JX; Vasylieva N; Barnych B; Cui Y; Xu ZL; Hammock BD; Gee SJ Anal Bioanal Chem. 2016, 408, 5985–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Safdari Y; Farajnia S; Asgharzadeh M; Khalili M; Jaliani HZ Monoclon Antib Immunodiagn Immunother. 2014, 33, 13–19. [DOI] [PubMed] [Google Scholar]
- (33).Desmyter A; Spinelli S; Boutton C; Saunders M; Blachetot C; de Haard H; Denecker G; Van Roy M; Cambillau C; Rommelaere H Front Immunol. 2017, 8, 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wang L; Liu X; Zhu X; Wang L; Wang W; Liu C; Cui H; Sun M; Gao B Protein Eng Des Sel. 2013, 26, 417–423. [DOI] [PubMed] [Google Scholar]
- (35).Ma J; Qiu X; Zhang J; Duan X; Zhu T Chemosphere. 2012, 88, 769–778. [DOI] [PubMed] [Google Scholar]
- (36).Liu K; Li J; Yan S; Zhang W; Li Y; Han D Chemosphere. 2016, 148, 8–20. [DOI] [PubMed] [Google Scholar]
- (37).Xu T; Wang J; Liu SZ; Lü C; Shelver WL; Li QX; Li J Anal Chim Acta. 2012, 751, 119–127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


