Abstract
BACKGROUND:
TB is a major cause of mortality worldwide, with the highest risk in people living with HIV/AIDS (PLWHA). Isoniazid preventive therapy (IPT), in combination with antiretroviral therapy (ART), reduces the overall incidence and mortality from TB by up to 90% among PLWHA. Tanzania has limited published data on IPT coverage among PLWHA.
OBJECTIVE:
To investigate coverage and determinants of IPT among PLWHA receiving care in selected care and treatment clinics in Dar es Salaam, Tanzania.
METHODS:
An analytical cross-sectional design to study 31,480 HIV-positive adults. Proportions and comparisons were obtained using χ2 tests, while determinants for IPT were assessed using adjusted multivariable analysis.
RESULTS:
The IPT coverage among eligible PLWHA was generally low (28.9%), with increased coverage over time. The determinants for IPT coverage included age >36 years, having WHO Clinical Stages 1 and 2 compared to 3 and 4, and having normal weight, or being overweight and obesity compared to underweight.
CONCLUSION:
IPT coverage in Dar es Salaam is very low; individuals with minor HIV disease severity were more likely to initiate IPT. This shows a possible gap in the prescribing practices among healthcare providers. More efforts to ensure IPT coverage implementation in Dar es Salaam are required.
Keywords: HIV/AIDs, tuberculosis, TB, Dar es Salaam
Abstract
CONTEXTE :
La TB est une cause majeure de mortalité dans le monde, le risque étant le plus élevé chez les personnes vivant avec le VIH/SIDA (PLWHA). Le traitement préventif à l’isoniazide (TPI), associé au traitement antirétroviral (ART), réduit l’incidence globale et la mortalité de la TB jusqu’à 90% chez les PLWHA. La Tanzanie dispose de peu de données publiées sur la couverture du TPI chez les PLWHA.
OBJECTIF :
Étudier la couverture et les déterminants du TPI chez les PLWHA recevant des soins dans des cliniques de soins et de traitement sélectionnées à Dar es Salaam, en Tanzanie.
MÉTHODES :
Une conception analytique transversale pour étudier 31 480 adultes séropositifs. Les proportions et les comparaisons ont été obtenues à l’aide de tests χ2, tandis que les déterminants du TPI ont été évalués à l’aide d’une analyse multivariable ajustée.
RÉSULTATS :
La couverture du TPI parmi les PLWHA admissibles était généralement faible (28,9%), avec une augmentation de la couverture au fil du temps. Les déterminants de la couverture du TPI comprenaient l’âge >36 ans, les stades cliniques 1 et 2 de l’OMS par rapport aux stades 3 et 4, et un poids normal ou un surpoids et une obésité par rapport à un poids insuffisant.
CONCLUSION :
La couverture du TPI à Dar es Salaam est très faible ; les personnes dont la gravité de la maladie VIH était mineure étaient plus susceptibles d’initier un TPI. Cela montre une possible lacune dans les pratiques de prescription parmi les prestataires de soins de santé. Des efforts supplémentaires sont nécessaires pour assurer la mise en œuvre de la couverture TPI à Dar es Salaam.
TB accounts for more than 10 million new cases per year worldwide.1,2 Almost a quarter of the world’s population is said to have TB infection (LTBI). People with compromised immune systems, such as people living with HIV/AIDS (PLWHA), have a higher risk of progressing from TBI to active TB.3 TB is the leading cause of death among PLWHA, accounting for around one in three HIV-related deaths.4 Isoniazid preventive therapy (IPT) reduces the risk of developing TB by 60–70%, and the combination of IPT and antiretroviral treatment (ART) reduces the overall incidence and mortality from TB by up to 90% in PLWHA.5,6
There are approximately 1.7 million PLWHA in Tanzania, with about 1.3 million on ART. The Dar es Salaam Region contributes about 11% of PLWHA on ART in Tanzania. HIV/AIDS-related deaths in Tanzania are estimated at 27,000 deaths annually.7,8 Tanzania notifies about 133,000 TB cases and about 26,000 TB-related deaths annually. Dar es Salaam region contributes about 20% of all notified TB cases in Tanzania.9 The Tanzanian national guidelines for the management of HIV and AIDs emphasise the importance of including TB preventive therapy (TPT), which includes the use of IPT, as part of the care package for PLWHA.10–12
Reliable data on IPT coverage among PLWHA in Tanzania are currently limited. It is important to get reliable data on the prevalence of TB and coverage of IPT for the Dar es Salaam region and the whole country. In this study, we investigated the coverage and determinants of IPT among PLWHA receiving care in selected care and treatment clinics (CTCs) in Dar es Salaam, Tanzania, from 1 June 2014 to 31 May 2019 using record review methods.
METHODS
Study design and setting
We used an analytical cross-sectional design to study de-identified secondary data of adult HIV-positive clients enrolled in CTCs of 17 health facilities in Dar es Salaam. The study period was from 1 June 2014 to 31 May 2019. The selected sites covered all levels of health facilities in Tanzania and were part of the RESPOND-AFRICA (Research Partnership for the Control of Chronic Diseases in Africa) projects. The sites offered an environment for smooth data collection with fewer resources required. These included Mwananyamala Referral Hospital, Shree Hindu Mandal Hospital, Sinza Hospital, Kimara Health Centre, Tandale Health Centre, Mnazi Mmoja Hospital, Amana Referral Hospital, Buguruni Health Centre, Tabata A Dispensary, Temeke Referral Hospital, Mbagala Rangi Tatu Hospital, Kigamboni Health Centre, Yombo Vituka Health Centre, Bunju Health Centre, Mbezi Health Centre, Tambukareli Dispensary and Vingunguti Health Centre.
Eligibility criteria
All HIV-positive adults (⩾18 years) enrolled in CTCs of the Dar es Salaam Region for the first time between 1 June 2014 to 31 May 2019 were included.
Exclusion criteria
All HIV-positive adults diagnosed with TB, those on anti-TB medications, those with a history of hepatitis, or those transferred in from other clinics were excluded.
Data collection and management
The study involved retrospective data collection. The data were abstracted from the HIV programme (CTC-2) electronic database into Microsoft Excel 2019 (MicroSoft, Redmond, WA, USA) and exported to Stata v14.1 (Stata Corp Inc, College Station, TX, USA) for data management and analysis. Data were checked for consistency, i.e., all observations not meeting eligibility criteria and duplicates were removed from the dataset.
The national guidelines for the management of HIV and AIDS implemented before 2018 proposed the following: daily isoniazid (INH) 300 mg for 6 months for PLWHA in care and treatment comprising one complete cycle of IPT. IPT should then be repeated after 2 years from the first dose of the last IPT cycle. The eligibility criteria for IPT/TPT included all HIV-positive individuals with no signs or symptoms suggestive of active TB. Patients who had active TB in the past 2 years from the time of screening were not considered for TPT.11,13 Although post-2018 guidelines for the management of HIV and AIDs recommended the administration of 6 months of daily INH 300 mg to complete one cycle of IPT in PLWHA in care and treatment, only one IPT cycle was administered in a patient’s life time, and no repeat cycle was needed. Eligibility criteria included HIV-positive individuals who screened negative for active TB with no history of TB treatment. PLWHA with a history of TB treatment who completed their TB treatment were to immediately receive TPT for 6 months.12 Exclusion criteria for IPT/TPT in all available guidelines included alcohol abuse, non-adherence to long-term treatment, current or past history of hepatitis or medical contra-indication to INH.10–12
Derived variables such as BMI were generated, and variables such as age and WHO Clinical Stage that were collected at baseline were categorized into groups. Health facilities were classified into low, moderate, and high volumes depending on the number of clients that received care in the entire study duration, i.e., low <1000, moderate 1000 to <2000 and high 2000+. Coding of variables was also done to ensure conformity with models.
Sampling and sample size estimation
We observed records of all (31,480) HIV-positive patients who enrolled in care and treatment clinics in the Dar es Salaam Region between 1 June 2014, and 31 May 2019. We observed that the minimum time for ART initiation was one day with median time from enrolment to IPT intake 150 days (interquartile range 75–240). Among the 31,480 observations, 2,425 (7.7%) records were dropped because of duplication (n = 444), registration before or after the study period (n = 24), being HIV-negative (n = 2), death at the date of enrolment (n = 85), transfer at the date of enrolment (n = 169) and not meeting the eligibility criteria (n = 1,701). Of the remaining records, 2,915 (3.2%) were excluded as they were either diagnosed with TB or were on anti-TB medications during the study period. The process of obtaining the final sample is shown in the flow diagram (Figure).
FIGURE.
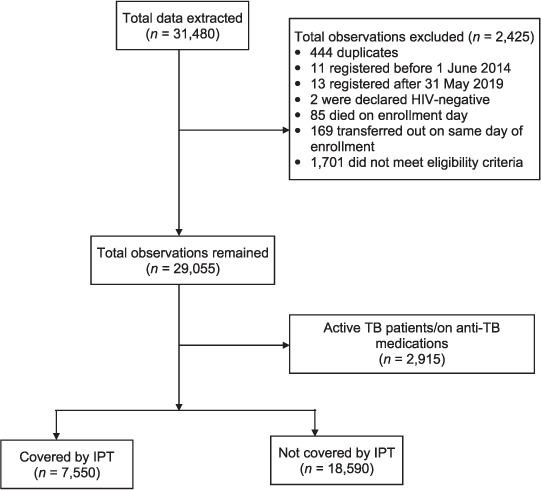
Data flow chart. IPT = isoniazid preventive therapy.
Data analysis
The data were categorised into two time periods, 2014–2017 and 2018–2019, to reflect major changes in the national guidelines for the management of HIV/AIDS in Tanzania. The definition used for the purposes of this study was, “initiation of IPT within 12 months of enrolment”.
Proportions of IPT coverage were calculated and stratified by facility size, WHO Clinical Stage, patient category, CD4 count, age groups and body mass index (BMI). Factors for receiving IPT were also determined using adjusted multiple logistic regression analysis, odds ratios (ORs) with their respective 95% confidence intervals (CIs) were reported, and P < 0.05 was considered significant.
Ethical considerations and informed consent
This protocol was submitted for ethical clearance to the National Health Research Ethics Committee (NatHREC) of the Tanzania Medical Research Coordinating Committee at the National Institute for Medical Research, Dar es Salaam, Tanzania (NIMR/HQ/R.8a/Vol.IX/3312). Consent was also obtained from administrative authorities of all the included clinics and health facility chiefs.
RESULTS
Baseline characteristics by IPT coverage
Overall IPT coverage during 12 months of enrolment was low, at about 28.9%, for eligible PLWHA for the entire study period from 2014 to 2019. We observed increased IPT coverage over time from 25.0% in 2014–2017 to 43.0% in 2018–2019.
IPT coverage was very low (23.5%) in the 18–24 years’ age group. This increased with age: respectively 27.3%, 31.5% and 32.2% in the 25–34, 35–44 and 45–55 years’ age groups. The 55–64 years’ age group experienced a decline in coverage to about 29.1%. The lowest coverage of 19.6% was observed in the >65 years’ age group.
IPT coverage increased with an increase in BMI, with 22.5% in the underweight, 29.1% in the normal weight, 31.4% in the overweight and 32.2% in the obese categories. The medium-volume health facilities showed higher coverage of IPT (31.8%) than low-volume (28.6%) and higher-volume (26.7%) facilities (Table 1).
TABLE 1.
Baseline characteristics of study participants, 2014–2019
| Characteristics | Year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 2014–2017 | 2018–2019 | Overall (2014–2019) | ||||||||||
|
|
|
|
||||||||||
| Total | IPT | No IPT | P value | Total | IPT | No IPT | P value | Total | IPT | No IPT | P value | |
| Overall coverage | 20,554 | 5,149 (25.0) | 15,405 (75.0) | — | 5,586 | 2,401 (43.0) | 3,185 (57.0) | — | 26,140 | 7,550 (28.9) | 18,590 (71.1) | — |
| Sex | ||||||||||||
| Female | 14,821 | 3,716 (25.1) | 11,105 (74.9) | 0.909 | 3,741 | 1,636 (43.7) | 2,105 (56.3) | 0.107 | 18,562 | 5,352 (28.8) | 13,210 (71.2) | 0.781 |
| Male | 5,733 | 1,433 (25.0) | 4,300 (75.0) | 1,845 | 765 (41.5) | 1,080 (58.5) | 7,578 | 2,198 (29.0) | 5,380 (71.0) | |||
| Age groups, years | ||||||||||||
| 18–24 | 2,450 | 479 (19.6) | 1,971 (80.4) | <0.001 | 705 | 262 (37.2) | 443 (62.8) | <0.001 | 3,155 | 741 (23.5) | 2,414 (76.5) | <0.001 |
| 25–34 | 7,167 | 1,698 (23.7) | 5,469 (76.3) | 1,919 | 782 (40.8) | 1137 (59.2) | 9,086 | 2,480 (27.3) | 6,606 (72.7) | |||
| 35–44 | 6,599 | 1,802 (27.3) | 4,797 (72.7) | 1,783 | 839 (47.1) | 944 (52.9) | 8,382 | 2,641 (31.5) | 5,741 (68.5) | |||
| 45–54 | 3,057 | 881 (28.8) | 4,797 (71.2) | 842 | 374 (44.4) | 468 (55.6) | 3,899 | 1,255 (32.2) | 2,644 (67.8) | |||
| 55–64 | 959 | 239 (24.9) | 720 (75.1) | 256 | 115 (44.9) | 141 (55.1) | 1,215 | 354 (29.1) | 861 (70.9) | |||
| 65+ | 322 | 50 (15.5) | 272 (84.5) | 81 | 29 (35.8) | 52 (64.2) | 403 | 70 (19.6) | 324 (80.4) | |||
| Binary age group, years | ||||||||||||
| 18–35 | 10,393 | 2,387 (23.0) | 8,006 (77.0) | <0.001 | 2,828 | 1,134 (40.1) | 1,694 (59.9) | <0.001 | 13,221 | 3,521 (26.6) | 9,700 (73.4) | <0.001 |
| >36 | 10,161 | 2,762 (27.2) | 7,399 (72.8) | 2,760 | 1,267 (45.9) | 1,491 (54.1) | 12,919 | 4,029 (31.2) | 8,890 (68.8) | |||
| BMI, kg/m2 | ||||||||||||
| <18.5 (under-weight) | 2,754 | 563 (12.4) | 2,191 (16.2) | <0.001 | 574 | 187 (32.6) | 387 (67.4) | <0.001 | 3,328 | 750 (22.5) | 2,578 (77.5) | <0.001 |
| 18.5–24.9 (normal weight) | 9,839 | 2,524 (55.4) | 7,315 (54.0) | 2,723 | 1,135 (41.7) | 1,588 (58.3) | 12,562 | 3,659 (29.1) | 8,903 (70.9) | |||
| 25.0–29.9 (overweight) | 3,643 | 971 (21.3) | 2,672 (19.7) | 953 | 471 (49.4) | 482 (50.6) | 4,596 | 1,442 (31.4) | 3,154 (68.6) | |||
| >30 (obese) | 1,854 | 494 (10.9) | 1,360 (10.0) | 573 | 287 (50.1) | 286 (49.9) | 2,427 | 781 (32.2) | 1,646 (67.8) | |||
| Size of health facility | ||||||||||||
| Low volume | 4,724 | 1,172 (24.8) | 3,552 (75.2) | 0.001 | 1,431 | 590 (41.2) | 841 (58.8) | <0.001 | 6,255 | 1,762 (28.6 | 4,393 (71.4) | <0.001 |
| Medium volume | 6,966 | 1,850 (26.6) | 5,116 (73.4) | 1,930 | 980 (50.8) | 950 (49.2) | 8,896 | 2,830 (31.8) | 6,066 (68.2) | |||
| High volume | 8,874 | 2,132 (24.0) | 6,742 (76.0) | 2,218 | 827 (37.3) | 1,391 (62.7) | 11,092 | 2,959 (26.7) | 8,133 (73.3) | |||
IPT = isoniazid preventive therapy; BMI = body mass index.
IPT coverage and clinical features
In 2014–2019, patients with WHO Stages 1 and 2 had higher IPT coverage than those with WHO Stages 3 and 4 (31.4% vs. 24.4%; P < 0.001). The same groups had higher coverage in all study periods. In terms of CD4 count, those with CD4 ⩾200 cells/μL had slightly higher coverage (32.3%) than those with CD4 <200 cells/ μL (30.1%) in all study periods (Table 2).
TABLE 2.
Association of IPT coverage with clinical HIV stage and CD4 count
| Variable | 2014–2016 | 2017–2019 | Overall (2014–2019) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Total | IPT | No IPT | P value | Total | IPT | No IPT | P value | Total | IPT | No IPT | P value | |
| WHO clinical stage |
||||||||||||
| Stage 1 and 2 | 12,229 | 3,291 (26.9) | 8,938 (73.1) | <0.001 | 4,369 | 1,923 (44.0) | 2,446 (56.0) | 0.002 | 16,598 | 5,214 (31.4) | 11,384 (68.6) | <0.001 |
| Stage 3 and 4 | 8,295 | 1,854 (22.4) | 6,441 (77.6) | 1,202 | 467 (38.9) | 733 (61.1) | 9,544 | 2,321 (24.4) | 7,174 (75.6) | |||
| CD4 count at enrolment, cells/mm3 | ||||||||||||
| <200 | 3,330 | 900 (27.0) | 2,430 (73.0) | 0.498 | 424 | 229 (54.0) | 195 (46.0) | 0.926 | 3,754 | 1,129 (30.1) | 2,425 (69.9) | 0.018 |
| >200 | 5,986 | 1,657 (27.7) | 4,329 (72.3) | 1,253 | 680 (54.3) | 573 (45.7) | 7,239 | 2,337 (32.3) | 4,902 (67.7) | |||
IPT = isoniazid preventive therapy.
IPT coverage by health facilities
We observed heterogeneity across health facilities included in this study. The Kigamboni Health Centre had the highest IPT coverage (50.5%) within 12 months of enrolment during the entire study duration. while Bunju Health Centre had the lowest (15.3%) during the study entire study period (Table 3).
TABLE 3.
IPT coverage by health facility, Tanzania
| Facility | Total | IPT | No IPT | P value |
|---|---|---|---|---|
| Amana Referral Hospital | 1892 | 647 (34.2) | 1245 (65.8) | <0.001 |
| Buguruni Health Centre | 437 | 155 (45.5) | 282 (64.5) | |
| Bunju Health Centre | 844 | 129 (15.3) | 715 (84.7) | |
| Kigamboni Health Centre | 931 | 470 (50.5) | 461 (49.5) | |
| Kimara Health Centre | 645 | 136 (21.1) | 509 (78.9) | |
| Mbagala Rangi Tatu Hospital | 3972 | 895 (22.5) | 3077 (77.5) | |
| Mbezi Health Centre | 1518 | 493 (32.5) | 10245 (67.5) | |
| Mnazi Mmoja Hospital | 2273 | 1038 (45.7) | 1235 (54.3) | |
| Mwananyamala Referral Hospital | 3057 | 796 (26.0) | 2261 (74.0) | |
| Shree Hindu Mandal Hospital | 304 | 80 (26.3) | 224 (73.7) | |
| Sinza Hospital | 2172 | 622 (28.6) | 1550 (71.4) | |
| Tabata A Dispensary | 1691 | 323 (19.1) | 1368 (80.9) | |
| Tambukareli Dispensary | 1186 | 320 (27.0) | 866 (73.0) | |
| Tandale Health Centre | 1770 | 541 (30.6) | 1229 (69.4) | |
| Temeke Referral Hospital | 1648 | 439 (26.6) | 1209 (73.4) | |
| Vingunguti Health Centre | 1083 | 220 (20.3) | 863 (79.7) | |
| Yombo Vituka Health | 723 | 245 (33.9) | 478 (66.1) |
IPT = isoniazid preventive therapy.
Determinants of IPT coverage
PLWHA aged ⩾36 years were 1.37 (95% CI 1.25–1.50; P < 0.001) times more likely to have IPT coverage than those aged ⩽35 years after adjusting for other factors. PLWHA with WHO Clinical Stages 1 and 2 were 1.48 (95% CI 1.35–1.63; P < 0.001) times more likely to have IPT coverage than those with WHO clinical staging 3 and 4. Those with normal weight were 1.3 (95% CI 1.14–1.50; P < 0.001) times more likely to have IPT coverage than underweight patients, and overweight patients were 1.34 (95% CI 1.14–1.57; P < 0.001) times more likely to have coverage than underweight patients (Table 4).
TABLE 4.
Multivariable analysis of factors for IPT coverage in CTCs in Dar es Salaam
| Variables | Cumulative total | ||||||
|---|---|---|---|---|---|---|---|
| IPT | No IPT | P value | OR (95%CI) | P value | aOR (95%CI) | P value | |
| Sex |
|||||||
| Female | 5,352 (70.9) | 13,210 (71.1) | 0.781 | Reference | Reference | ||
| Male | 2,198 (29.1) | 5,380 (28.9) | 1.01 (0.95–1.07) | 0.781 | 0.94 (0.85–1.03) | 0.191 | |
| Age, years | |||||||
| 18–35 | 3,521 (46.6) | 9,700 (52.2) | <0.001 | Reference | Reference | ||
| ⩾36 | 4,029 (53.4) | 8,890 (47.8) | 1.25 (1.18–1.32) | <0.001 | 1.37 (1.25–1.50) | <0.001 | |
| WHO HIV clinical stage | |||||||
| Stage 1 and 2 | 5,214 (69.2) | 11,384 (61.3) | <0.001 | 1.42 (1.34–1.50) | <0.001 | 1.48 (1.35–1.63) | <0.001 |
| Stage 3 and 4 | 2,321 (30.8) | 7,174 (38.7) | Reference | Reference | |||
| CD4 count at enrolment, cells/mm3 | |||||||
| <200 | 1,129 (32.6) | 2,425 (34.9) | 0.018 | Reference | Reference | ||
| >200 | 2,337 (67.4) | 4,902 (65.1) | 1.11 (1.02–1.21) | 0.018 | 1.04 (0.94–1.14) | 0.466 | |
| BMI, kg/m2 | |||||||
| <18.5 (underweight) | 750 (11.3) | 2,578 (15.8) | <0.001 | Reference | Reference | ||
| 18.5–24.9 (normal weight) | 3,659 (55.2) | 8,903 (54.7) | 1.41 (1.29–1.55) | <0.001 | 1.30 (1.14–1.50) | <0.001 | |
| 25.0–29.9 (overweight) | 1,442 (21.7) | 3,154 (19.4) | 1.57 (1.42–1.74) | <0.001 | 1.34 (1.14–1.57) | <0.001 | |
| >30 (obese) | 781 (11.9) | 1,646 (10.1) | 1.63 (1.45–1.74) | <0.001 | 1.17 (0.97–1.41) | 0.101 | |
| Size of health facility | |||||||
| Low volume | 1,762 (28.6 | 4,393 (71.4) | <0.001 | 1.10 (1.02–1.18) | 0.009 | 0.84 (0.74–0.96) | 0.011 |
| Medium volume. | 2,830 (31.8) | 6,066 (68.2) | 1.28 (1.21–1.36) | <0.001 | 1.09 (0.99–1.19) | 0.083 | |
| High volume | 2,959 (26.7) | 8,133 (73.3) | Reference | ||||
IPT = isoniazid preventive therapy; CTC = care and treatment clinic; OR = odds ratio; CI = confidence interval; aOR = adjusted OR; BMI = body mass index.
DISCUSSION
The IPT coverage in this study was generally low, with an overall coverage of 28.9% among 26,140 potentially eligible patients. The period 2014 to 2017 was important as the national guidelines recommended the administration of 6 months of daily INH 300 mg to complete one cycle of IPT, with the cycle repeated after 2 years.11,14 IPT coverage during this period was the lowest, with only 25% of eligible patients. IPT coverage during the period 2018–2019, when the guidelines recommended only one cycle of daily INH 300 mg for 6 months, was 43% of eligible patients. The increase in IPT coverage observed in these two study periods can be explained by the changes in policy guidelines that occurred between the two periods. These changes were accompanied by the promotion of the guidelines and improvements in supply chain which may have contributed to the observed increase.10,12 The low coverage of IPT in this study is similar to those reported in other countries in sub-Saharan Africa.15–17 The low IPT coverage has been attributed to several reasons, including interruptions in supply chains, and resulting in drug stockouts, ineffective TB screening, inefficient health service delivery, increasing pill burden, and inadequate high-level commitment and support for the IPT programme by programme managers and policymakers.5,18–20
The findings in this study showed increased IPT coverage with age, from age 18 to 54, and a decline in coverage in older patients of ⩾55 years. Also, multivariable analysis showed that those aged ⩾36 years were more likely to receive IPT than those aged <36 years. The findings of this study are not different from other studies in sub-Saharan Africa that reported higher IPT coverage among middle-aged patients.21,22 However, some studies have reported no association between IPT coverage and age.23,24 Relative high coverage among middle-aged patients reported in our study can be explained by the tendencies of young people to be more active in seeking healthcare than older patients who mostly depend on assistance from other people to access healthcare services, as shown in other studies.25,26
Having a normal weight (BMI 18.5–24.9 kg/m2) was significantly associated with higher coverage of IPT in the study population. In the multivariable analysis, we established that, participants with normal weight and those who were overweight were more likely to have IPT coverage than those with low BMI. This is similar to the findings of a study conducted in Tanzania showing an association between higher BMI and IPT initiation.21
We found that PLWHA with WHO Clinical Stages 1 and 2 had better IPT coverage than those with Clinical Stages 3 and 4. This finding is concurrent with other studies conducted in sub-Saharan Africa.22–24 Others have suggested that the low level of IPT coverage in WHO Clinical Stages 3 and 4 could be because clinicians are avoiding IPT in severely ill PLWHA due to the difficulty in ruling out TB disease in this population.23 This finding is alarming with regard to TB control, as PLWHA with WHO Clinical Stages 3 and 4 are at higher risk of developing TB. Other studies have reported that even with IPT provision, some PLWHA develop TB.6,27 The lack of IPT coverage among high-risk patients therefore undermines the efforts to control TB among PLWHA.
We observed variations in IPT coverage across health facilities such that coverage ranged from 15.3% at Bunju Heath Centre to 50.5% at Kigamboni Health Centre. Health facility volume tends to influence IPT stock requisitions. However, we found that medium-volume health facilities had higher IPT coverage than high-volume facilities, but this was not significant when adjusted for other factors. The observed variations may be due to difference in service delivery between medium-volume and high-volume health facilities. Other factors such as health facility ownership and location of the facilities may explain variations in IPT coverage across health facilities. The study findings are similar to another study that reported variation in IPT completion across health facilities.17
Strengths and limitations
This is one of the few studies in Tanzania to report on IPT coverage using routine HIV programme data. This study also had a large number of patients to review from different sites. The use of HIV programme data from the CTC 2 database, which is updated daily, provided us with greater access to data and ensured improved data collection and more reliable findings. However, this study also had some limitations. Information on IPT used in this study did not include details on treatment completion and default or adherence levels during treatment.
Generalisability
As this study focused only on selected health facilities using non-probability sampling techniques, results cannot be generalised. However, the selected facilities covered all levels of primary health care facilities in Tanzania, including district hospitals, health centres, dispensaries and not-for-profit health facilities.
CONCLUSION AND RECOMMENDATIONS
IPT coverage among eligible PLWHA is low (28.9%), with individuals with lower HIV disease severity more likely to initiate IPT. However, PLWHA with advanced HIV or at higher WHO Clinical Stages are more vulnerable to TB. Our study shows a possible gap in the prescribing practices among healthcare providers. Access to TB diagnosis and diagnostic capability before IPT may also be an area for further investigation. More research is needed to further understand the facilitators of and barriers to implementing IPT coverage. In addition, policy interventions and peer influences may influence IPT coverage scale up among vulnerable groups in routine settings.
ACKNOWLEDGMENTS
This study would not have been possible without the assistance of individuals and organisations that supported this study. The authors wish to thank the National Institute for Medical Research (NIMR), Dar es Salaam, Tanzania, for taking a leading role, the East African Consortium for Clinical Research (EACCR) for technical and financial facilitation, the leadership of RESPOND AFRICA projects for logistical support at the study sites, the President’s Office, Regional Administration and Local Government Tanzania for permission to use the sites for the study, health facility staff at the study sites which supported the study, and all people who willingly helped us to the best of their abilities.
Funding Statement
This research was funded by the TB node of EACCR. The consortium is funded by the EDCTP2 Programme supported by the European Union (RegNet2015-1104). The views expressed in this report are those of the investigator(s) and not necessarily those of the EDCTP.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization Latent tuberculosis infection updated and consolidated guidelines for programmatic management. Geneva, Switzerland: WHO; 2018. [PubMed] [Google Scholar]
- 2.World Health Organization Global tuberculosis report, 2019. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 3.World Health Organization Tuberculosis facts. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 4.UNAIDS UNAIDS data, 2018. Geneva, Switzerland: UNAIDS; 2018. [Google Scholar]
- 5.Kagujje M, et al. Implementation of isoniazid preventive therapy in people living with HIV in Zambia: challenges and lessons. BMC Public Health. 2019;19(1):1329. doi: 10.1186/s12889-019-7652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabasaba A, et al. Effect of isoniazid preventive therapy on tuberculosis incidence and associated risk factors among HIV infected adults in Tanzania: a retrospective cohort study. BMC Infect Dis. 2019;19(1):62. doi: 10.1186/s12879-019-3696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS Global AIDS update. Geneva, Switzerland: UNAIDS; 2020. [Google Scholar]
- 8.United Nations Childrens’ Fund Tanzania HIV Programme Fact Sheet. New York, NY, USA: UNICEF; 2019. [Google Scholar]
- 9.National Tuberculosis and Leprosy Programme Annual Report for 2018 National TB and Leprosy Programme (NTLP) Dar es Salaam, Tanzania: Department of Preventive Services; 2018. [Google Scholar]
- 10.United Republic of Tanzania, National AIDS Control Programme National Guidelines for the Management of HIV and AIDS. Dar es Salaam, Tanzania: NACP; 2019. [Google Scholar]
- 11.United Republic of Tanzania, National AIDS Control Programme National guidelines for the management of HIV and AIDS. Dar es Salaam, Tanzania: NACP; 2015. [Google Scholar]
- 12.United Republic of Tanzania National Guidelines for the Management of HIV and AIDS. Dar es Salaam, Tanzania: United Republic of Tanzania; 2017. [Google Scholar]
- 13.United Republic of Tanzania Ministry of Health and Social Welfare National policy guidelines for collaborative TB/HIV activities. Dar es Salaam, Tanzania: MoHSW; 2016. [Google Scholar]
- 14.United Republic of Tanzania, Ministry of Health and Social Welfare, National AIDS Control Programme. National Guidelines for the Management of HIV and AIDS. Dar es Salaam, Tanzania: MoHSW; 2012. [Google Scholar]
- 15.Takarinda KC, et al. Trend analysis of tuberculosis case notifications with scale-up of antiretroviral therapy and roll-out of isoniazid preventive therapy in Zimbabwe, 2000–2018. BMJ Open. 2020;10(4):e034721. doi: 10.1136/bmjopen-2019-034721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roscoe C, et al. Evaluation of the uptake of tuberculosis preventative therapy for people living with HIV in Namibia: a multiple methods analysis. BMC Public Health. 2020;20(1):1838. doi: 10.1186/s12889-020-09902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert M, et al. Determinants of isoniazid preventive therapy completion among people living with HIV attending care and treatment clinics from 2013 to 2017 in Dar es Salaam Region, Tanzania. A cross-sectional analytical study. BMC Infect Dis. 2020;20(1):276. doi: 10.1186/s12879-020-04997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugomeri E, Olivier D, van den Heever WM. Health system challenges affecting the implementation of isoniazid preventive therapy in people living with HIV in Lesotho. HIV AIDS Rev. 2018;17(4):299–307. [Google Scholar]
- 19.Wambiya EOA, et al. Factors affecting the acceptability of isoniazid preventive therapy among healthcare providers in selected HIV clinics in Nairobi County, Kenya: qualitative study. BMJ Open. 2018;8(12):e024286. doi: 10.1136/bmjopen-2018-024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thindwa D, et al. Completion of isoniazid preventive therapy among human immunodeficiency virus positive adults in urban Malawi. Int J Tuberc Lung Dis. 2018;22(3):273–279. doi: 10.5588/ijtld.17.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maokola W, et al. Coverage of isoniazid preventive therapy among people living with HIV: a retrospective cohort study in Tanzania (2012–2016) Int J Infect Dis. 2021;103:562–567. doi: 10.1016/j.ijid.2020.11.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhungana GP, et al. Initiation and completion rates of isoniazid preventive therapy among people living with HIV in far-western region of Nepal: a retrospective cohort study. BMJ Open. 2019;9(5):e029058. doi: 10.1136/bmjopen-2019-029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyathi S, et al. Isoniazid preventive therapy: Uptake, incidence of tuberculosis and survival among people living with HIV in Bulawayo, Zimbabwe. PLoS One. 2019;14(10):e0223076. doi: 10.1371/journal.pone.0223076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legese H, et al. Utilization of isoniazid prophylaxis therapy and its associated factors among HIV positive clients taking antiretroviral therapy at Fre Semaetat primary hospital, Hawzien districts, Tigrai, Northern Ethiopia. Trop Dis Travel Med Vaccines. 2020;6(1):11. doi: 10.1186/s40794-020-00106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latunji OO, Akinyemi OO. Factors influencing health-seeking behaviour among civil servants in Ibadan, Nigeria. 2018. Ann Ib Postgrad Med. 2018;16(1):52–60. [PMC free article] [PubMed] [Google Scholar]
- 26.Lawson D. Manchester, UK: Global Development Institute, University of Manchester; 2004. Determinants of health seeking behaviour in Uganda: is it just income and user fees that are important? Development Economics and Public Policy Working Paper Series. Paper no 6; pp. 13–17. [Google Scholar]
- 27.Dravid A, et al. Incidence of tuberculosis among HIV infected individuals on long term antiretroviral therapy in private healthcare sector in Pune, Western India. BMC Infect Dis. 2019;19(1):714. doi: 10.1186/s12879-019-4361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


